Abstract
How frequently autoantibodies against angiotensin‐converting enzyme 2 (ACE2) occur in patients infected by SARS‐CoV‐2 is understudied and limited to investigations on a small sample size. The presence of these antibodies may contribute to the long‐lasting effects of COVID‐19 observed in some individuals, particularly if IgG‐class antibodies would emerge in patients. This study assessed the prevalence of IgG autoantibodies against ACE2 in 1139 patients infected with SARS‐CoV‐2 and examined their relationship with severity, demographic characteristics, and status of vaccination against influenza. The overall prevalence of anti‐ACE IgG antibodies in our cohort was 1.5%. Most of these individuals were men (76.5%) and underwent mild COVID‐19, but some severe and asymptomatic cases were also observed. Patients with severe infection had twofold higher titers than mild and asymptomatic cases. Age, comorbidities, and influenza vaccination status were not related to antibody prevalence. The prevalence of IgG anti‐SARS‐CoV‐2 antibodies (against nucleocapsid protein and S2 subunit, but not against receptor‐binding domain) was higher in the subset with ACE2 autoantibodies. Further research is required to understand the potential spectrum and duration of effects of IgG autoantibodies against ACE2 in patients after SARS‐CoV‐2 infection, particularly concerning long COVID‐19.
Keywords: autoantibody, autoimmunity, COVID‐19, renin–angiotensin system
1. INTRODUCTION
There is mounting evidence that a range of persistent symptoms can remain after SARS‐CoV‐2 infection, a condition coined long COVID‐19, post‐COVID‐19 syndrome, or post‐acute sequelae of SARS‐CoV‐2. Although the most common symptom is fatigue, long COVID‐19 can affect the sensory, neurologic, and cardiorespiratory systems and mental health. 1 It also has a substantial prevalence, globally estimated at 43% of all SARS‐CoV‐2 infections, higher in hospitalized patients, and more common in Asia and Europe than in North America. 2 Altogether, long COVID‐19 is generating additional healthcare burden and economic costs. 3
There are numerous hypotheses put forward to explain the mechanisms behind long COVID‐19. Current evidence indicates that it is most likely a multifaceted phenomenon, advocating the need to pursue research to confirm and exclude different factors that could be involved in its emergence. 4 Some investigations point toward the involvement of autoantibodies that arise in some patients infected with SARS‐CoV‐2 and may be responsible for some of the reported long COVID‐19 symptoms. For example, one study found functionally active autoantibodies targeting G‐protein coupled receptors such as β2‐ and α1‐adrenoceptors, angiotensin II AT1‐, muscarinic M2‐, MAS‐, nociceptin‐ and ETA‐receptors in some COVID‐19 convalescent individuals who displayed persistent neurological and cardiological symptoms, or a combination of both. 5 Other research demonstrated the presence of IgG antibodies against interleukin‐2, CD8B, and thyroglobulin in more than 10% of analyzed patients and anti‐interferons antibodies in 5%–10% of individuals. 6 All in all, the findings suggest that autoimmunity may be a hallmark in long COVID‐19 and is triggered by SARS‐CoV‐2 infection through overstimulation of the immune system and molecular resemblance between components of the virus and the host. 7
Small sample size studies indicate that patients who underwent SARS‐CoV‐2 infection may have detectable levels of autoantibodies against angiotensin‐converting enzyme 2 (ACE2). 8 , 9 Some speculate that they may represent anti‐idiotypic antibodies, which are specific to the antigen‐binding region of a host antibody that recognizes a foreign protein. 10 It is also suggested that a subset of these antibodies, known as homobodies, 11 could recognize the binding partner of the original viral protein, which in the case of the receptor‐binding domain (RBD) of spike protein is ACE2. It can also be speculated that anti‐ACE2 antibodies may arise as immune mechanisms leading to the suppression of the viral spread in the host, although the exact pathways through which this process could constitute are unknown.
ACE2 plays a role in the renin–angiotensin hormone system by transforming angiotensin II into protective angiotensin (1–7). Moreover, it regulates blood volume, stem cell maintenance and differentiation, hematopoiesis, erythropoiesis, myeloid differentiation, inflammation, and innate and adaptive immunity. 12 This considered, ACE2 autoantibodies may adversely affect numerous physiological processes, particularly if one considers its wide expression encompassing cardiomyocytes, brain, intestines, kidneys, and male reproductive tract. 13 The observations of anti‐ACE2 antibodies in COVID‐19 convalescent underscored the need for further studies that would encompass a larger sample size and attempt to identify potential factors that could influence the emergence of such autoantibodies. Moreover, the IgG class of anti‐ACE2 antibodies was not a primary focus of studies in patients after SARS‐CoV‐2 infection. If generated, their extended half‐life may be more deleterious and potentially contribute to some symptoms observed in long COVID‐19, mainly related, but not limited, to cardiovascular function.
Therefore, the present study assessed the frequency of IgG autoantibodies against ACE2 in the cohort of 1139 individuals who underwent SARS‐CoV‐2 infection and evaluated whether their presence may be related to COVID‐19 severity, demographic characteristics, and prevalence of anti‐SARS‐CoV‐2 antibodies. Because influenza virus infection may increase ACE2 expression, 14 we also aimed to evaluate whether the presence of autoantibodies may be differentiated by the status of influenza vaccination in the studied cohort.
2. METHODS
2.1. Sample collection and patients characteristics
The residual serum samples for this study were purchased from the eight Regional Blood Donation and Blood Treatment Centers in Poland. The research project was approved by the Bioethical Committee of the Institute of Public Health—National Research Institute and the Bioethics Committee at Poznan University of Medical Sciences. All samples were originally collected in September–December 2020 from patients who underwent RT‐PCR‐confirmed SARS‐CoV‐2 infection and were 1 month after the resolution of COVID‐19 symptoms/end of the isolation period. Overall, samples from 1139 patients were purchased and analyzed. The patient's age, sex, comorbidities (present/not present), COVID‐19 severity, and status of influenza vaccination in the 2019–2020 epidemic season were available for each sample. None of the studied subjects were vaccinated against COVID‐19 since the samples were collected in the last quarter of 2020.
2.2. Determination of IgG antibodies against ACE2 and anti‐SARS‐CoV‐2 antibodies
The IgG ACE‐2 autoantibodies in serum samples were determined using the CE‐IVD certified Microblot‐Array COVID‐19 IgG assay (TestLine Clinical Diagnostics). In addition, the IgG antibodies against SARS‐CoV‐2 against RBD of the spike protein (anti‐RBD), subunit S2 of the spike protein (anti‐S2), and nucleocapsid protein (anti‐N) were determined. The concentration of antibodies was reported as U/ml and considered positive if above 210 U/ml according to the manufacturer's instructions.
2.3. Statistical analyses
Data were analyzed with Statistica v.13.3 (StatSoft Inc.). Comparing patients' characteristics between groups presenting and not presenting IgG autoantibodies against ACE2 was performed with Pearson's χ2 test. The difference in age between these groups and differences in anti‐ACE2 titers in relation to the severity of patients with anti‐ACE2 antibodies were analyzed using the nonparametric Mann–Whitney U test. Differences were deemed statistically significant when p < 0.05.
3. RESULTS AND DISCUSSION
3.1. Prevalence of IgG autoantibodies against ACE2
Overall serum samples of 1139 COVID‐19 patients, collected 1 month after the resolution of symptoms/end of the isolation period, were analyzed in the present study. The prevalence of IgG autoantibodies against ACE2 was rare and occurred in 17 individuals (1.5%) with the mean ± SD serum concentration 344 ± 158 U/ml, on average 60% over the threshold level. This finding contradicts previous research on a small group of patients, which reported a very high occurrence of ACE2 autoantibodies in the majority—14/15 inpatients (93%) and 26/32 of convalescents after SARS‐CoV‐2 infection (81%). However, the assay employed in this study did not distinguish the class of immunoglobulins. 8 Since the samples for this research were collected 2 weeks after the resolution of COVID‐19 symptoms, it is plausible that the detected antibodies were IgM. Indeed, IgM autoantibodies were previously detected in patients undergoing COVID‐19 and were common in severe cases. 9 It has been shown that they play a role in the angiocentric pathology of COVID‐19 by complement‐binding and functional changes in endothelial cells in microvessels. Surprisingly though, these antibodies did not undergo class‐switching to IgG. 9 Here, we evidence that some patients may also develop IgG autoantibodies against ACE2 that are present at least 1 month after the resolution of symptoms. Considering their extended half‐life compared to IgM, it is plausible that they may exert a prolonged effect on the cardiovascular system by inhibiting the ACE2 function. This may result, among others, in reduced levels of vasoprotective angiotensin (1–7) and contribute to vasculopathy in patients who underwent SARS‐CoV‐2 infection similar to those observed previously in patients with connective tissue diseases whose serum contained antibodies suppressing ACE2 activity. 15
3.2. Factors influencing the prevalence of IgG autoantibodies against ACE2
The characteristics of the group with detectable IgG autoantibodies against ACE2 are summarized in Table 1. The majority of these individuals were men (76.5%). In turn, symptoms of long COVID‐19, including palpitation, dyspnea, weakness, or thoracic pain, were more commonly reported in women. 16 However, research indicates that undergoing COVID‐19 can result in later infertility in some men due to testicular damage due to either direct invasion of SARS‐CoV‐2 and interaction with ACE2 receptor in the male reproductive tract, or as a result of secondary immunological response. 17 Whether ACE2‐targeting autoantibodies can contribute to male infertility remains to be elucidated. The age of positive and negative patients was similar, and there was no difference in the frequency of comorbidities between the groups (Table 1).
Table 1.
Main characteristics of patients with IgG autoantibodies against ACE2 (n = 17) and without them (n = 1122)
| Parameter | Group | p Value | |
|---|---|---|---|
| ACE2 IgG antibodies | No ACE2 IgG antibodies | ||
| Age (years), mean ± SD | 35.2 ± 5.9 | 36.9 ± 9.9 | >0.05 |
| Women/men, % (n) | 23.6 (4)/76.4 (13) | 20.8 (233)/79.2 (889) | >0.05 |
| Comorbidities, % (n) | 5.9 (1) | 6.8 (76) | >0.05 |
| Influenza‐vaccinated in the 2019–2020 epidemic season, % (n) | 52.9 (9)/8 (47.1) | 57.9 (650)/42.1 (472) | >0.05 |
Most patients with IgG autoantibodies against ACE2 had mild COVID‐19 (47.1%), and only 5.9% required hospitalization (Figure 1A). This is an important finding indicating that the generation of this class of autoantibodies may not be, contrary to IgM, linked to greater disease severity. 9 However, as shown in Figure 1B, their titers were higher in hospitalized patients, with approx. twofold difference when compared to asymptomatic and mild cases.
Figure 1.
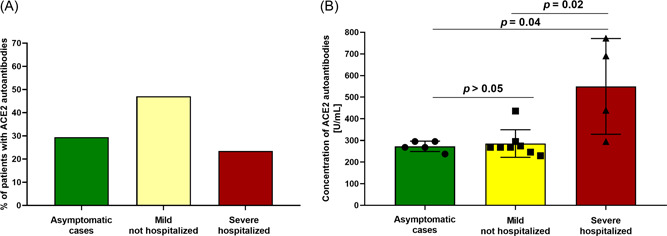
(A) Distribution of patients with IgG autoantibodies against ACE2 (n = 17) in relation to the severity of SARS‐CoV‐2 infection. (B) Titers of IgG autoantibodies against ACE2 I in relation to the severity of SARS‐CoV‐2 infection.
Being vaccinated against influenza in the 2019–2020 epidemic season did not affect the frequency of ACE2 autoantibodies (p > 0.05). We have speculated that since influenza virus infections can increase ACE2 expression and COVID‐19 and influenza waves overlap, 14 the vaccination against influenza may exert some protective effect against predisposing subjects infected with SARS‐CoV‐2 to the generation of anti‐ACE2 antibodies. However, this seems not to be the case, at least in our cohort.
3.3. IgG autoantibodies against ACE2 and the presence of anti‐SARS‐CoV‐2 antibodies
The majority, but not all, individuals with detectable IgG anti‐ACE2 antibodies were positive for anti‐RBD, anti‐N, and anti‐S2 antibodies. The prevalence of anti‐S2 and anti‐N was significantly higher in this group than in patients presenting no anti‐ACE2 antibodies—by 2‐fold and 1.4‐fold, respectively (Table 2). Whether the humoral response to the N protein and S2 subunit of the spike protein of SARS‐CoV‐2 play a specific role in the risk of developing autoantibodies against ACE2 remains to be elucidated in further research.
Table 2.
The prevalence of anti‐SARS‐CoV‐2 IgG antibodies in patients with IgG autoantibodies against ACE2 (n = 17) and without them (n = 1122)
| Anti‐SARS‐CoV‐2 | Group | p Value (Pearson's χ2 test) | |
|---|---|---|---|
| IgG antibody | ACE2 IgG antibodies | No ACE2 IgG antibodies | |
| Anti‐RBD (% of patients) | 88.2 | 76.3 | >0.05 (χ2 = 1.3) |
| Anti‐S2 (% of patients) | 82.4 | 38.7 | 0.0003 (χ2 = 13.4) |
| Anti‐N (% of patients) | 88.2 | 65.2 | 0.04 (χ2 = 3.9) |
3.4. Study limitations
One should consider the limitations of our study. It should be highlighted that due to the residual availability of serum samples, we could not study functional aspects of detected autoantibodies against ACE2. However, previous studies have shown that such immunoglobulins decrease ACE2 activities and induce vasculopathy. 8 , 9 , 15 Moreover, the presence of anti‐ACE2 antibodies was not determined before SARS‐CoV‐2 infection. Thus, we cannot unequivocally state that they developed after acquiring the virus. At the same time, the study did not include a control group of healthy individuals with no history of SARS‐CoV‐2 infection to establish the prevalence of anti‐ACE2 IgG antibodies in the general population. Additionally, to the best of our knowledge, no studies have reported the prevalence of these autoantibodies in representative population samples. The presence of anti‐ACE2 antibodies was also not determined before SARS‐CoV‐2 infection. Thus, we cannot unequivocally state that they developed after acquiring the virus. However, one should note that ACE2 autoantibodies tend to be more common in patients suffering from particular conditions such as constrictive vasculopathy or neurological disorders. 15 , 18 In turn, our study did not find any difference in the frequency of comorbidities between groups testing positive and negative on IgG anti‐ACE‐2 antibodies. Therefore, we suggest that the observed antibodies are likely a result of SARS‐CoV‐2 infection. It should also be noted that the present study consisted of samples collected between September and December 2020, a period dominated by Nextstrain SARS‐CoV‐2 clades 20A, 20B, and 20C. 19 It remains unknown whether other variants that differed in pathogenicities, such as Delta or Omicron, may influence the prevalence of autoantibodies against ACE2 Last but not least, it should be highlighted that as long as this study focused on autoantibodies against ACE2 that serve as a receptor for cellular entry of the SARS‐CoV‐2, this virus can alternatively utilize neuropilin 1 for this purpose. 20 Whether autoreactivity can also emerge in the realm of neuropilin 1 in COVID‐19 remains yet to be explored.
4. CONCLUSIONS
In summary, this study shows that IgG autoantibodies against ACE2 can be present in individuals who underwent SARS‐CoV‐2 infection. These antibodies appear rare, although we show that they can also occur in mild COVID‐19 and asymptomatic individuals. Further research is required to understand the potential spectrum and duration of effects of IgG autoantibodies against ACE2 in patients after SARS‐CoV‐2 infection, particularly in relation to long COVID‐19.
AUTHOR CONTRIBUTIONS
Conceptualization: Piotr Rzymski and Lidia Brydak. Methodology: Piotr Rzymski and Barbara Poniedziałek. Investigations: Piotr Rzymski, Dominika Sikora, Barbara Poniedziałek, Karol Szymański, Katarzyna Kondratiuk, Jakub Żurawski, and Barbara Poniedziałek. Writing – original draft: Piotr Rzymski and Barbara Poniedziałek. Writing – review and editing: Lidia Brydak.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
This work was financially supported by the National Institute of Public Health NIH—National Research Institute (Grant number 1BIBW/2022).
Hallmann E, Sikora D, Poniedziałek B, et al. IgG autoantibodies against ACE2 in SARS‐CoV‐2 infected patients. J Med Virol. 2022;95:e28273. 10.1002/jmv.28273
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID‐19 condition or long COVID: a meta‐analysis and systematic review. J Infect Dis. 2022;226:1593‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cutler DM. The costs of long COVID. JAMA Health Forum. 2022;3(5):e221809. [DOI] [PubMed] [Google Scholar]
- 4. Norton A, Olliaro P, Sigfrid L, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis. 2021;21(5):601‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G‐protein coupled receptors in patients with persistent Long‐COVID‐19 symptoms. J Transl Autoimmun. 2021;4:100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rojas M, Rodríguez Y, Acosta‐Ampudia Y, et al. Autoimmunity is a hallmark of post‐COVID syndrome. J Transl Med. 2022;20(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS‐CoV‐2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4):102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arthur JM, Forrest JC, Boehme KW, et al. Development of ACE2 autoantibodies after SARS‐CoV‐2 infection. PLoS One. 2021;16(9):e0257016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casciola‐Rosen L, Thiemann DR, Andrade F, et al. IgM anti‐ACE2 autoantibodies in severe COVID‐19 activate complement and perturb vascular endothelial function. JCI Insight. 2022;7(9):e158362. 10.1172/jci.insight.158362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ladjemi MZ. Anti‐idiotypic antibodies as cancer vaccines: achievements and future improvements. Front Oncol. 2012;2:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindenmann J. Speculations on idiotypes and homobodies. Ann Immunol. 1973;124(2):171‐184. [PubMed] [Google Scholar]
- 12. Turner AJ. ACE2 cell biology, regulation, and physiological functions. The Protective Arm of the Renin Angiotensin System (RAS). Elsevier; 2015:185‐189. [Google Scholar]
- 13. Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS‐COV‐2. Front Cell Infect Microbiol. 2020;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schweitzer KS, Crue T, Nall JM, et al. Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells. Eur Respir J. 2021;58(1):2003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takahashi Y, Haga S, Ishizaka Y, Mimori A. Autoantibodies to angiotensin‐converting enzyme 2 in patients with connective tissue diseases. Arthritis Res Ther. 2010;12(3):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pelà G, Goldoni M, Solinas E, et al. Sex‐related differences in long‐COVID‐19 syndrome. J Womens Health. 2022;31(5):620‐630. [DOI] [PubMed] [Google Scholar]
- 17. Malki MI. COVID‐19 and male infertility: an overview of the disease. Medicine. 2022;101(27):e29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miziołek B, Sieńczyk M, Grzywa R, et al. The prevalence and role of functional autoantibodies to angiotensin‐converting‐enzyme‐2 in patients with systemic sclerosis. Autoimmunity. 2021;54(4):181‐186. [DOI] [PubMed] [Google Scholar]
- 19.Genomic Epidemiology of SARS‐CoV‐2 with Subsampling Focused Globally since Pandemic Start. Accessed October 3, 2022. https://nextstrain.org/ncov/gisaid/global/
- 20. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370(6518):856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


