This letter to the editor aims to summarize our novel findings, which show the important biological pathways underpinning fatigue, depression, and anxiety symptoms in patients with acute and long coronavirus disease 2019 (COVID). 1 , 2 , 3 , 4 , 5 We present a brief description of the novel model we developed using precision medicine methods, which shows that immune‐inflammatory and oxidative pathways and lowered antioxidant defenses may explain, at least in part, some of the neuropsychiatric symptoms seen in acute and long COVID. 1 , 2 , 3 , 4 , 5
Individuals with acute COVID‐19 infection frequently experience neuropsychiatric manifestations, mainly fatigue, anxiety, and depressive symptoms, along with systemic symptoms. 2 Interestingly, during the acute phase of infection, we were able to extract a single latent factor from fatigue, psychosomatic, depressive, and anxiety symptoms as assessed with the Hamilton Depression (HAMD) and Anxiety (HAMA) Rating Scales and Fibromylagia and Fatigue Rating Scale (FF) scores. 2 This indicates that fatigue, (psycho)somatic, and affective symptoms are manifestations of a shared core, which we named the physio‐affective phenome 2 and that a common pathophysiology should underpin these manifestations. In this respect, we found that a large part of the variance in the physio‐affective phenome was explained by the combined effects of lowered oxygen saturation (SpO2) and elevated peak body temperature (PBT) resulting from the immune‐inflammatory response and lung injuries during the acute infectious phase. 2 Increased PBT and lowered SpO2 during acute infection are indicants of the severity of the immune‐inflammatory response and predict critical disease and even increased mortality. 1 , 2 Therefore, the severity of the immune‐inflammatory response during acute infection largely determines the physio‐affective phenome of acute COVID‐19. Since immune‐inflammatory pathways may mechanistically explain affective symptoms, chronic fatigue, and psychosomatic symptoms, 6 , 7 it is safe to conclude that the immune‐inflammatory response causes the physio‐affective phenome of acute infection.
A significant part of recovered COVID‐19 patients show, 2 to 6 months after the acute infection, a cluster of psychiatric and somatic manifestations, including fatigue, depressed mood, anxiety, cognitive impairments, and insomnia, along with somatic symptoms such as autonomic disturbances, muscle pain, muscle tension, headache, a flu‐like malaise, gastrointestinal symptoms, dyspnea, persistent cough, and chest pain. 8 , 9 , 10 Importantly, we discovered that 3 to 4 months after acute infection, some people with long COVID experienced chronic fatigue, psychosomatic, and affective symptoms, and that a common core could be extracted from such assessments using factor analysis, indicating that long COVID is characterized by high levels of physio‐affective symptoms. 1 , 3 Importantly, the physio‐affective phenome of long COVID was strongly predicted by decreased SpO2 and increased PBT, 3 indicating that the physio‐affective phenome of long COVID is the result of immune‐inflammatory processes during acute infection. 1 , 3 , 4 , 5
In addition, we discovered that the effects of low SpO2 and high PBT are partially mediated by immune‐inflammatory and nitro‐oxidative processes under prolonged COVID. 1 Thus, we found that increased C‐reactive protein and nitro‐oxidative toxicity, as assessed by increased protein carbonyls, malondialdehyde, myeloperoxidase, and nitric oxide, and decreased antioxidant defenses, including decreased glutathione peroxidase, zinc, and total antioxidant capacity, were strongly associated with the HAMD, HAMA, and FF scores as well as the physio‐affective core. 1 In addition, aberrations in C‐reactive protein, total antioxidant capacity, and zinc were predicted by elevated PBT during acute COVID‐19 infection, whereas decreased glutathione peroxidase and nitric oxide production were predicted by a decreased SpO2 during acute infection. 1
In a second study performed on a different study sample, we found that the long COVID physio‐affective core was again predicted by SpO2 and PBT during the acute phase and that the effects were mediated by a highly significant increase in a neurotoxicity index computed based on interleukin 1 and 18 and caspase‐1 (indicating elevated activity of the nucleotide‐binding domain, leucine‐rich repeat, and pyrin domain‐containing protein 3 [NLRP3] inflammasome), C‐reactive protein, myeloperoxidase, advanced oxidative protein product, and increased insulin resistance. 5 Moreover, the above biomarkers and pathways also predict lowered health‐related quality of life in long COVID. 4
The connections between the infectious‐immune‐inflammatory response during acute infection and the onset of physio‐affective symptoms of acute and chronic COVID are depicted in Fig. 1. Our results show that the effects of the acute phase on long COVID are partly mediated by neurotoxic pathways attributed to increased NLRP3 activity, inflammation, nitro‐oxidative stress, increased insulin resistance, and lowered calcium. The results indicate that the inflammatory response during acute infection and increased nitro‐oxidative stress, a mild chronic inflammatory response, decreased antioxidant defenses, and calcium levels are new drug targets to prevent the development of physio‐affective symptoms during both the acute and chronic phases.
Fig. 1.
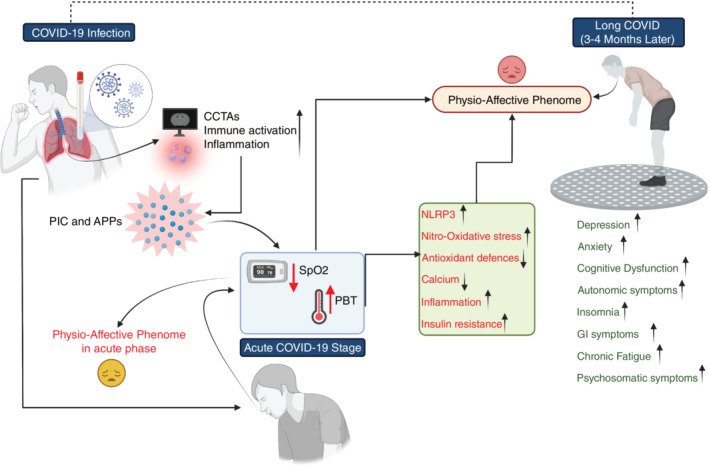
Associations between the infectious‐immune‐inflammatory response to acute coronavirus disease 2019 (COVID‐19) infection and the onset of physio‐affective symptoms in acute and long COVID stages. APPs, acute phase proteins; CCTAs, chest computed tomography abnormalities; GI, gastrointestinal; NLRP3, nucleotide‐binding domain, leucine‐rich repeat and pyrin domain‐containing protein 3; PBT, peak body temperature; PIC, proinflammatory cytokine; SpO2, oxygen saturation.
Disclosure statement
We have no conflict of interest with any commercial or other association connected with the submitted article.
Supporting information
Appendix S1. Supporting information
References
- 1. Al‐Hakeim HK, Al‐Rubaye HT, Al‐Hadrawi DS, Almulla AF, Maes M. Long‐COVID post‐viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2022; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al‐Jassas HK, Al‐Hakeim HK, Maes M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome‐like symptoms due to COVID‐19: A nomothetic network approach. J. Affect. Disord. 2022; 297: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Hadrawi DS, Al‐Rubaye HT, Almulla AF, Al‐Hakeim HK, Maes M. Lowered oxygen saturation and increased body temperature in acute COVID‐19 largely predict chronic fatigue syndrome and affective symptoms due to long COVID: A precision nomothetic approach. Acta Neuropsychiatr. 2022. 10.1017/neu.2022.21. [DOI] [PubMed] [Google Scholar]
- 4. Maes M, Al‐Rubaye HT, Almulla AF et al. Lowered quality of life in long COVID is predicted by affective symptoms, chronic fatigue syndrome, inflammation and Neuroimmunotoxic pathways. Int. J. Environ. Res. Public Health 2022; 19: 10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Hakeim HK, Al‐Rubaye HT, Almulla AF, Al‐Hadrawi DS, Maes M. Chronic fatigue, depression and anxiety symptoms in long COVID are strongly predicted by neuroimmune and neuro‐oxidative pathways which are caused by the inflammation during acute infection. medRxiv 2022. 10.1101/2022.06.29.22277056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leonard B, Maes M. Mechanistic explanations how cell‐mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012; 36: 764–785. [DOI] [PubMed] [Google Scholar]
- 7. Maes M, Kubera M, Kotańska M. Aberrations in the cross‐talks among redox, nuclear factor‐κB, and Wnt/β‐catenin pathway signaling underpin Myalgic encephalomyelitis and chronic fatigue syndrome. Front. Psych. 2022; 13: 822382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID‐19 in non‐hospitalised subjects: A population‐based cohort study. Thorax 2021; 76: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C et al. More than 50 long‐term effects of COVID‐19: A systematic review and meta‐analysis. Sci. Rep. 2021; 11: 16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


