Abstract
The aim of this study is to evaluate development of side effects, thrombotic or obstetric complications in our antiphospholipid syndrome (APS) patient group, after vaccination against coronavirus disease 2019 (COVID‐19). A cohort was formed from patients who have previously been followed up with a diagnosis of APS. The patients of the cohort were evaluated retrospectively to find out if they were vaccinated with CoronaVac and/or BNT162b2 vaccines which are being used in our country. To evaluate the side effects seen after the vaccination, the information was collected by the patients in their outpatient appointments or making a phone call. Thirty‐five APS patients who had received at least 1 dose of any of the COVID‐19 vaccines were included in the study. Median (min–max) number of vaccine doses per patient was 2 (1–3). Eleven patients had a booster dose after primary vaccination. Twenty patients were ever vaccinated with BNT162b2 and 18 with CoronaVac. Among BNT162b2 recipients, 9 (45.0%) and among CoronaVac recipients 15 (42.9%) reported an adverse event after a vaccine administration. The most common adverse events were myalgia and malaise after any dose of both vaccines. No vaccine‐related new thrombotic events or APS flares were observed. Our results were comparable with those reported in the literature. Comprehensive large‐scale studies are needed for more accurate results on the evaluation of side effects after COVID‐19 vaccination in APS patients.
Keywords: antiphospholipid syndrome, COVID‐19, vaccination
1. INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by presence of antiphospholipid antibodies (aPL) comprising lupus anticoagulant (LAC), anti‐β2‐glycoprotein I (anti‐β2GPI) and/or anti‐cardiolipin (aCL) antibodies together with venous/arterial thrombosis and/or obstetric comorbidity. 1
COVID‐19 is a highly contagious disease, caused by the RNA virus severe acute respiratory syndrome coronavirus 2 (SARS CoV 2). 2 The course of the disease ranges from asymptomatic illness to severe disease with life‐threatening complications such as acute respiratory distress syndrome (ARDS). 3 In addition to this, in considerable number of cases, the clinical scenario is complicated by coagulopathy. 4 Tendency to hypercoagulation worsens the prognosis and is thought to be related with development of cytokine storms.
Infections are acknowledged as one of the precipitating factors for multifactorial pathogenetic processes of APS via triggering proinflammatory cascades that promote the clinical manifestations in individuals with pre‐existing aPLs. 5 Likewise, development of aPL positivity has been reported in COVID‐19. 6
SARS CoV 2 is thought to trigger hypercoagulation and formation of aPLs via host immune response to viral antigens. Therefore, hypothetically, a similar reaction may occur after vaccination against SARS CoV 2. Concerns regarding COVID‐19 vaccines started to occur several months after the mass COVID‐19 vaccination campaign began, upon seeing unexpected thromboembolic events. 7 , 8 , 9 In a patient group like APS, in which the individual is already prone to thrombotic events, the tolerability of COVID‐19 vaccines is intriguing in this manner.
The aim of the study was the evaluation of APS flares as well as adverse events of COVID‐19 vaccination in APS patients.
2. METHODS
2.1. Study design
The study was designed as a cross‐sectional, retrospective cohort study with approval by our institutional ethics committee and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. An official permission was obtained from the Ministry of Health to conduct this study, dated February 20, 2021.
The medical records of the patients who were followed up in our clinic with a diagnosis of APS, were investigated for the presence of vaccine‐related adverse events and APS disease activation (thrombosis, embolism or pregnancy complications) after any dose of a COVID‐19 vaccine. Data were confirmed from patients either via telephone calls or during follow‐ups. A questionnaire form was prepared for the patients. In this questionnaire, the date of APS diagnosis, the symptoms/findings at the time of diagnosis, history of medications for APS, COVID‐19 infection, data regarding COVID‐19 vaccines if they were vaccinated and adherence to APS treatment during the vaccination period and occurrence of any new thrombotic event and/or obstetric complication within 3 months after a COVID‐19 vaccine were questioned. Patient‐reported adverse events related to COVID‐19 vaccines occurring during the 1‐month period after vaccination were recorded. Verbal or written consent was obtained from the patients.
2.2. Patients
The patients who meet the Sapporo classification criteria for APS (also named as Sydney Criteria), who are followed up in our clinic, 18 years and over, diagnosed with APS and vaccinated with any of the COVID‐19 vaccines, were included into the study (Figure 1).
FIGURE 1.
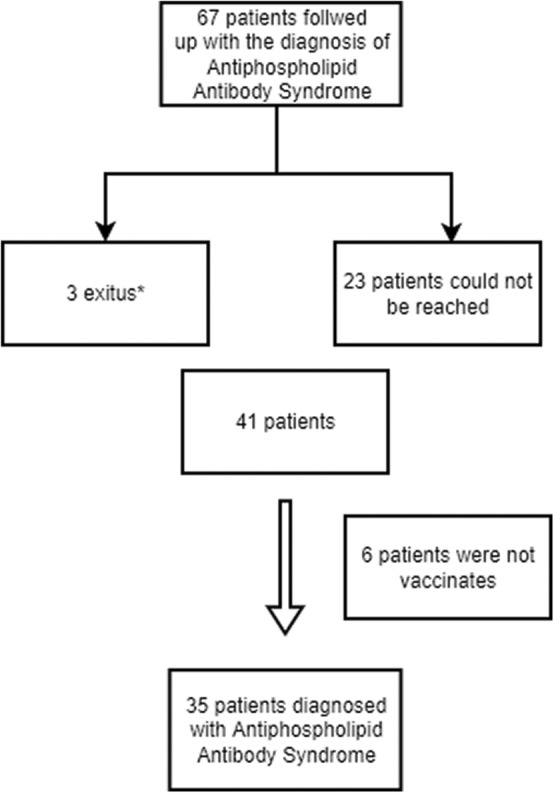
Selection of the patients participating in the study. *The reason of the exitus is not COVID‐19 infection or any of the SARS‐CoV‐2 vaccines administrated.
2.3. Main outcomes and other variables
Occurrence of an APS disease flare after COVID‐19 vaccination (CoronaVac or BNT162b2) or an adverse event due to the vaccines were selected as main outcome variables and collected from medical records and patient questionnaires. Any event related by the patient with vaccine administration within 24 hours to 1 month after vaccination were recorded as an adverse event. APS activation was defined as new thromboembolic event and/or obstetric complication. Data regarding demographics, comorbidities and medical treatment administered for APS were collected from the hospital database.
2.4. Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) 22.0 software. Normality of continuous variables was evaluated with Shapiro–Wilk test and visually with plots and histograms. Continuous variables were presented either with median (minimum–maximum) or mean ± standard deviation, according to normality. Categorical variables are presented with numbers and percentages.
3. RESULTS
Thirty‐five patients who had received at least 1 dose of any of the COVID‐19 vaccines were included in the study. Demographics, comorbid diseases and APS characteristics, medications and adherence to treatment during vaccination are presented in Table 1.
TABLE 1.
Demographic and clinical characteristics of patients with APS who received COVID‐19 vaccine
| N = 35 | |
|---|---|
| Age, y, median (min–max) | 43 (28–63) |
| Gender, female, number (%) | 33 (94.3) |
| Active smokers, number (%) | 2 (5.7) |
| Comorbidities, number (%) | |
| Hypertension | 6 (17.1) |
| Chronic kidney disease | 2 (5.7) |
| Sjögren's syndrome | 2 (5.7) |
| Diabetes mellitus | 0 |
| Coronary artery disease | 0 |
| Other | 4 (11.4) |
| Time from diagnosis, y, median (min–max) | 7.0 (1.0–27.0) |
| APS characteristics, n (%) | |
| Thrombotic APS | 22 (62.9) |
| Cerebrovascular event | 11 (52.4) |
| Deep vein thrombosis | 10 (47.7) |
| Pulmonary thromboembolism | 2 (9.6) |
| Obstetric APS | 21 (63.6) |
| Treatment agents, n (%) | |
| Hydroxychloroquine | 28 (82.4) |
| Acetylsalicylic acid | 16 (59.3) |
| Warfarin | 16 (48.5) |
| Corticosteroids | 15 (50) |
| Adherence to APS drugs during vaccination | 32 (97) |
Abbreviations: APS, antiphospholipid antibody syndrome.
3.1. Outcomes
The median (min–max) age of the patients was 43 (28–63) years. The number of the female patients was 33 (94.7%). Four (11.4%) of the patients were active smokers. The most common comorbidity was hypertension (17.1%) (Table 1).
In 22 (62.9%) of the patients there was a history of a thrombotic event at the time of diagnosis or during the course of the disease. Eleven (52.4%) of these patients had a history of cerebrovascular disease, 10 (47.7%) of them had a history of deep vein thrombosis, 2 (9.6%) of them had a history of pulmonary thromboembolism. Twenty‐one (63.6%) of the patients had experienced an obstetric complication (history of abortion, stillbirth). All of these thromboembolic events and obstetric complications occurred prior to vaccination. Most common active APS medications were hydroxychloroquine (28 patients [82.4%]), acetylsalicylic acid (16 patients [59.3%]), warfarin (16 patients [48.5%]), and corticosteroids (15 patients [50%]) (Table 1). Thirty‐two (97%) of the patients adhered to treatment during the vaccination period.
Vaccination characteristics and adverse events are presented in Table 2. Median (min–max) number of vaccine doses per patient was 2 (1–3). Eleven patients had a booster dose after primary vaccination. Twenty patients were ever vaccinated with BNT162b2 and 18 ever with CoronaVac. Among BNT162b2 recipients, 9 (45.0%) reported an adverse event after a vaccine administration and among CoronaVac recipients 15 (42.9%) reported an adverse event after a vaccine administration. In patients with adverse events, the most common adverse events after a CoronaVac dose were myalgia (33.3%), malaise (22.2%) and local pain/arm pain (16.6%). In BNT162b2 recipients most common adverse events were myalgia (30%), malaise (20%) and fever (15%). In 1 patient, nosebleed due to increased international normalized ratio (INR) and need for hospitalization was observed after a dose of BNT162b2 vaccine. APS flare was observed in none of the patients within the 3‐month period after any vaccine dose. No patient became pregnant or gave birth during the follow‐up.
TABLE 2.
Vaccination properties and side effects of the patients
| N = 35 | ||
|---|---|---|
| BNT162b2 | CoronaVac | |
| Patients ever vaccinated with BNT162b2 or CoronaVac, n | 20 | 18 |
| Vaccine distribution of patients, n | ||
| 3 doses of CoronaVac | 7 | |
| 2 doses of CoronaVac | 7 | |
| 1 dose of CoronaVac | 1 | |
| 3 doses of BNT162b2 | 1 | |
| 2 doses of BNT162b2 | 16 | |
| 3 doses of CoronaVac + single dose of BNT162b2 | 3 | |
| Patients vaccinated with BNT162b2 or CoronaVac alone, n (%) | 17 (48.6) | 15 (42.9) |
| Patients with an adverse effect after a dose of BNT162b2 or CoronaVac, n (%) | 9/20 (45.0) | 7/18 (38.9) |
| Adverse reactions, n (%) a | ||
| Fever | 3 (15) | 0 (0) |
| Malaise | 4 (20) | 4 (22.2) |
| Local pain/arm pain | 1 (5) | 3 (16.6) |
| Myalgia | 6 (30) | 6 (33.3) |
| Patients with an adverse effect after 1st dose of BNT162b2 or CoronaVac, n (%) | 7 (35) | 2 (11.1) |
| Adverse reactions, n (%) a | ||
| Fever | 3 (15) | 0 |
| Malaise | 4 (20) | 2 (11.1) |
| Local pain/arm pain | 2 (10) | 0 |
| Myalgia | 3 (15) | 1 (5.5) |
| Patients with an adverse effect after 2nd dose of BNT162b2 or CoronaVac, n (%) | 6/20 (30) | 5/18 (27.8) |
| Adverse reactions, n (%) a | ||
| Fever | 3 (15) | 0 |
| Malaise | 1 (5) | 3 (16.6) |
| Local pain/arm pain | 1 (5) | 0 |
| Myalgia | 5 (25) | 3 (16.6) |
| Patients with an adverse effect after a booster dose with BNT162b2 or CoronaVac, n (%) b | 1/4 (25) | 4/7 (57.1) |
| Adverse reactions, n (%) a | ||
| Fever | 0 | 0 |
| Malaise | 1 (25) | 3 (42.8) |
| Local pain/arm pain | 0 | 2 (28.5) |
| Myalgia | 1 (25) | 3 (42.8) |
Out of patients with adverse events.
Out of 11 patients who had a booster dose, 7 with CoronaVac, 4 with BNT162b2.
When the frequency of adverse events after first, second and booster doses were evaluated, it was observed that adverse events were more common after the first dose in BNT162b2 recipients and after the booster dose in CoronaVac recipients (Table 2).
SARS CoV 2 polymerized chain reaction (PCR) test was positive in 8 (22.9%) of the patients. No serious disease courses or death were observed in any of these patients. None of them required hospitalization. There were 71.4% of the patients infected prior to vaccination. In those who were positive for COVID‐19 after vaccination, it was determined that the disease was seen 2 months after the last dose.
4. DISCUSSION
Our results demonstrated that out of 35 APS patients with COVID‐19 vaccination, 45.0% of BNT162b2 and 42.9% of CoronaVac recipients reported an adverse event. Only a single patient had an event requiring hospitalization. No mortality was observed. None of the patients suffered from an APS flare. The majority of patients adhered to APS treatment during the vaccination period.
APS is characterized by thrombotic events, microangiopathy and obstetric complications. While deep venous thrombosis is the most frequently reported manifestation, cerebrovascular events are the most common arterial involvement. Early or late fetal loss, prematurity and preeclampsia are the most common fetal and obstetric signs. 10 Clinical manifestations may not always occur despite presence of aPLs. Therefore, a “two‐hit theory” has been proposed, which requires a second trigger in addition to the aPLs in circulation, in order to develop clinical manifestations. 11 In some studies, it has been thought that antibodies lose their normal function under abnormal vascular conditions such as oxidant stress, and cause autoimmunity. 12
The presence of coagulopathy as part of the systemic inflammatory response syndrome is a common feature in severe cases of COVID‐19. Changes in coagulation parameters (elevated d‐dimer, prolonged prothrombin time, thrombocytopenia, and/or low fibrinogen levels) were detected in approximately 20%–50% of the hospitalized COVID‐19 patients. 13 COVID‐19 is related to thrombotic events (especially venous thromboembolism [VTE]) rather than hemorrhagic events.
Infectious agents can cause aPL production by molecular mimicry. 14 It has been reported that aPL antibodies can be synthesized by B cell clones as a result of a molecular mimicry between exogenous molecules and β2GPI. 15 This molecular similarity between the β2GPI epitope and the pathogens such as Haemophilus influenzae, Neisseria gonorrhoeae, cytomegalovirus and tetanus toxoid, has been demonstrated in previous studies. 15 In addition; detection of these antibodies has also been reported in patients with COVID‐19. 16 A systematic review and meta‐analysis of the literature examining the presence of acute ischemic stroke in individuals infected with SARS CoV 2 found a high prevalence of aPL in these patients, with LAC positivity in 41.7% of cases. 17 In a cohort of 122 COVID‐19 patients, 16 of whom had major thrombotic events, immunoglobulin G (IgG)/IgA/IgM of anti‐β2GPI were detected in 15.6%/6.6%/9.0% of patients, respectively. 18 However, interestingly, no significant correlation was observed between the risk of thrombotic events and aPL positivity. It was stated that attention should be paid to the anti‐β2GPI antibodies identified in these patients exhibiting a different epitope specificity compared to the aPL antibodies elicited during APS.
Hypothetically, it is possible to observe similar presentations after vaccination. Several studies have shown that aPLs can be detected in the sera of the individuals who were vaccinated with tetanus toxoid, seasonal influenza, and human papillomavirus vaccines, with or without causing clinical manifestations of APS. 19 , 20 A similar situation was detected following COVID‐19 vaccines. 21 Induction of experimental APS was accomplished by hyperimmunization with different adjuvants (glycerol or aluminum hydroxide) or different adjuvant pretreatments (glycerol or complete Freund adjuvant). There is varying evidence that these adjuvants induce autoantibody production. 22
Several concerns that COVID‐19 vaccines may cause hypercoagulation were raised several months after the mass COVID‐19 vaccination campaign began, upon reports of unexpected thromboembolic events. Currently available COVID‐19 vaccination strategies include messenger RNA (mRNA)‐based (BNT162b2 and mRNA‐1273), adenoviral vector‐based (ChAdOx1‐S and Ad26.COV2) and inactive (CoronaVac) formulations. The safety profile of ChAdOx1‐S was recently reviewed by the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) due to several reports of coagulopathy events developing days to weeks after the first or second injection of the vaccine. 23 Given the numerical imbalance in clinical trials, venous thromboembolism was also included among the safety concerns of the Risk Management Plan (RMP) of the adenoviral vector‐based COVID‐19 vaccine Ad26.COV2. Recently, several experimental studies have been conducted to investigate the mechanism of vaccine‐induced thrombosis development, and as a result, a new possible mechanism has been proposed. 7 Accordingly, it is hypothesized that the development of thrombosis is associated with a soluble adenoviral spike protein variant that binds to angiotensin converting enzyme 2 (ACE2) expressing endothelial cells. This gives an idea why thrombosis is more common with administration of adenovirus‐based vaccines compared to mRNA‐based vaccines. However, the relationship of COVID‐19 vaccines with thrombotic events is still unclear, but it is thought that an immune‐mediated mechanism similar to that in APS may occur. 5
COVID‐19 vaccines being administrated at the time of this study in our country are inactivated CoronaVac and mRNA‐based BNT162b2 vaccines. In a study of 406 healthcare workers who received CoronaVac, it was stated there was no significant difference in the presence of aPL autoantibodies before and after vaccination, and no thrombosis was observed in this study during follow‐up. 24 Also in our study, no post‐vaccine thrombotic complication was observed in any patient who received the CoronaVac vaccine in accordance with these results. A recent study comprising 39 APS patients vaccinated with inactive COVID‐19 vaccines reported no induction of thrombotic events, similar to our results. 25
The impact of COVID‐19 mRNA‐based vaccines on the thrombotic process is still being discussed. In a study in which safety and tolerability of mRNA‐based vaccine were evaluated among patients with APS diagnosis and/or aPL positivity, no vaccine‐related new thrombotic event was reported. 26 Although no safety issues emerged in clinical trials compared to the placebo group, a case report described the development of deep venous thrombosis 1 day after receiving a second dose of BNT162b2 in a 66‐year‐old woman with no prior coagulation defects. 9 , 27 Similarly, only a single vaccine‐related thrombotic event was reported among 161 APS patients: deep vein thrombosis which occurred 39 days after the second dose of an adenovirus‐based COVID‐19 vaccine, without requiring hospitalization. 28 A descriptive analysis examining the relationship between administration of BNT162b2, mRNA‐1273 and ChAdOx1 nCov‐19 vaccines with the development of thrombotic events, including cerebral vein thrombosis, reported finding 0.21 cases of thrombotic events per 1 million vaccinated people, between December 13, 2020 and March 16, 2021, to The Health Organization Global Database for Individual Case Safety Reports (VigiBase). 29 These thrombotic events were reported in association with both the mRNA vaccines (BNT162b2 and mRNA‐1273) and the analyzed DNA vaccine (ChAdOx1 nCov‐19). In the details of the analysis, it was stated that thrombotic events were reported in 4 female patients (4/1197, 0.4%) after BNT162b2. In our study, none of the BNT162b2 recipients suffered from a thrombosis or an APS flare.
Our study has several limitations. The study has the typical limitations of a single‐center retrospective case study. The number of the evaluated patients was few. Different results may be obtained by examining more patients. The other important limitation is that the data regarding vaccine experience of our subjects collected via telephone survey and mainly based on subject self‐reports could not be confirmed by clinicians. Lastly, since this is a real‐life study, vaccine types and doses administered to our patients were highly heterogeneous.
5. CONCLUSION
Our results suggest that CoronaVac and BNT162b2 vaccination did not develop thrombotic events and the vaccination‐related adverse events were comparable with those reported in the registry studies. However, due to the inadequate literature data, comprehensive large‐scale studies are needed for more accurate results on the evaluation of side effects after COVID‐19 vaccination in APS patients.
AUTHOR CONTRIBUTIONS
All the authors made a substantial contribution to this research. All members contributed to the study design, data collection, writing the paper and approved the final form.
CONFLICT OF INTEREST
The authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Karakaş Ö, Erden A, Armağan B, et al. Evaluation of patients with antiphospholipid syndrome subsequently COVID‐19 vaccinations: A retrospective cohort study. Int J Rheum Dis. 2022;00:1‐6. doi: 10.1111/1756-185X.14490
REFERENCES
- 1. Rodziewicz M, D’Cruz DP. An update on the management of antiphospholipid syndrome. Ther Adv Musculoskeletal Dis. 2020;12:1759720X20910855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Hanses F. Epidemiologie von SARS‐CoV‐2‐Infektion und COVID‐19. Internist. 2020;61(8):782‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang N, Li D, Wang X, Sun Z. Des paramètres de coagulation anormaux sont associés à un mauvais pronostic chez les patients atteints de pneumonie à nouveau coronavirus. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Talotta R, Robertson ES. Antiphospholipid antibodies and risk of post‐COVID‐19 vaccination thrombophilia: the straw that breaks the camel's back? Cytokine Growth Factor Rev. 2021;60:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atalar E, Erden A, Guven SC, et al. The clinical significance of antiphospholipid antibodies in COVID‐19 infection. J Infect Dev Ctries. 2022;16(02):276‐282. [DOI] [PubMed] [Google Scholar]
- 7. Kowarz E, Krutzke L, Külp M, et al. Vaccine‐induced COVID‐19 mimicry syndrome. Elife. 2022;11:e74974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novak N, Tordesillas L, Cabanillas B. Adverse rare events to vaccines for COVID‐19: from hypersensitivity reactions to thrombosis and thrombocytopenia. Int Rev Immunol. 2022;41(4):438‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS‐CoV‐2 vaccine. Intern Emerg Med. 2021;16(3):803‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vega‐Ostertag M, Harris EN, Pierangeli SS. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50(9):2911‐2919. [DOI] [PubMed] [Google Scholar]
- 11. Meroni PL, Riboldi P. Pathogenic mechanisms of antiphospholipid syndrome: a new autoimmune disease. Drug Discov Today Dis Mech. 2004;1(3):309‐314. [Google Scholar]
- 12. McIntyre JA, Wagenknecht DR, Faulk WP. Redox‐reactive autoantibodies: detection and physiological relevance. Autoimmun Rev. 2006;5(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 13. Gomez‐Mesa JE, Galindo‐Coral S, Montes MC, Muñoz Martin AJ. Thrombosis and coagulopathy in COVID‐19. Curr Probl Cardiol. 2021;46(3):100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espinosa G, Cervera R. Antiphospholipid syndrome. In: Asherson RA, Font J, Manuel RC, Juan R, (eds). Handbook of Systemic Autoimmune Diseases. Vol 8; Netherlands: Elsevier; 2008:39‐49. [Google Scholar]
- 15. Blank M, Krause I, Fridkin M, et al. Bacterial induction of autoantibodies to β2‐glycoprotein‐I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109(6):797‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Linden J, Almskog L, Liliequist A, et al. Thromboembolism, hypercoagulopathy, and antiphospholipid antibodies in critically ill coronavirus disease 2019 patients: a before and after study of enhanced anticoagulation. Crit Care Explor. 2020;2(12):e0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan Y‐K, Goh C, Leow AST, et al. COVID‐19 and ischemic stroke: a systematic review and meta‐summary of the literature. J Thromb Thrombolysis. 2020;50(3):587‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borghi MO, Beltagy A, Garrafa E, et al. Anti‐phospholipid antibodies in COVID‐19 are different from those detectable in the anti‐phospholipid syndrome. Front Immunol. 2020;11:2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inic‐Kanada A, Stojanovic M, Zivkovic I, et al. Murine monoclonal antibody 26 raised against tetanus toxoid cross‐reacts with β2‐glycoprotein I: its characteristics and role in molecular mimicry. Am J Reprod Immunol. 2009;61(1):39‐51. [DOI] [PubMed] [Google Scholar]
- 20. Bizjak M, Bruck O, Kanduc D, Praprotnik S, Shoenfeld Y. Vaccinations and secondary immune thrombocytopenia with antiphospholipid antibodies by human papillomavirus vaccine. Semin Hematol. 2016;53:S48‐S50. [DOI] [PubMed] [Google Scholar]
- 21. Cruz‐Tapias P, Blank M, Anaya JM, Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24(4):389‐393. [DOI] [PubMed] [Google Scholar]
- 22. Katzav A, Kivity S, Blank M, Shoenfeld Y, Chapman J. Adjuvant immunization induces high levels of pathogenic antiphospholipid antibodies in genetically prone mice: another facet of the ASIA syndrome. Lupus. 2012;21(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 23. Committee EMAPRA Signal assessment report on embolic and thrombotic events (SMQ) with COVID‐19 Vaccine (ChAdOx1‐S [recombinant])–Vaxzevria (previously COVID‐19 Vaccine AstraZeneca)(Other viral vaccines). 2021, European Medicine Agency Amsterdam, The Netherlands.
- 24. Liu T, Dai J, Yang Z, et al. Inactivated SARS‐CoV‐2 vaccine does not influence the profile of prothrombotic antibody nor increase the risk of thrombosis in a prospective Chinese cohort. Science Bulletin. 2021;66(22):2312‐2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan H, Tang Z, Teng J, et al. COVID‐19 vaccine affects neither prothrombotic antibody profile nor thrombosis in primary anti‐phospholipid syndrome: a prospective study. Rheumatology (Oxford). 2022;keac400. 10.1093/rheumatology/keac400. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sciascia S, Costanzo P, Radin M, et al. Safety and tolerability of mRNA COVID‐19 vaccines in people with antiphospholipid antibodies. Lancet Rheumatol. 2021;3(12):e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baden LR, el Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pengo V, del Ross T, Tonello M, et al. Impact of COVID‐19 and COVID‐19 vaccination on high‐risk patients with antiphospholipid syndrome: a nationwide survey. Rheumatology. 2022;61:SI136‐SI142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo‐le Louet A. Vaccination against COVID‐19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58:2100956. [DOI] [PMC free article] [PubMed] [Google Scholar]


