Abstract
Objective
This research aims to study structural brain changes in patients with persistent olfactory dysfunctions after coronavirus disease 2019 (COVID‐19).
Methods
COVID‐19 patients were evaluated using T1‐weighted and diffusion tensor imaging (DTI) on a 3T MRI scanner, 9.94 ± 3.83 months after COVID‐19 diagnosis. Gray matter (GM) voxel‐based morphometry was performed using FSL‐VBM. Voxelwise statistical analysis of the fractional anisotropy, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity was carried out with the tract‐based spatial statistics in the olfactory system. The smell identification test (UPSIT) was used to classify patients as normal olfaction or olfactory dysfunction groups. Intergroup comparisons between GM and DTI measures were computed, as well as correlations with the UPSIT scores.
Results
Forty‐eight COVID‐19 patients were included in the study. Twenty‐three were classified as olfactory dysfunction, and 25 as normal olfaction. The olfactory dysfunction group had lower GM volume in a cluster involving the left amygdala, insular cortex, parahippocampal gyrus, frontal superior and inferior orbital gyri, gyrus rectus, olfactory cortex, caudate, and putamen. This group also showed higher MD values in the genu of the corpus callosum, the orbitofrontal area, the anterior thalamic radiation, and the forceps minor; and higher RD values in the anterior corona radiata, the genu of the corpus callosum, and uncinate fasciculus compared with the normal olfaction group. The UPSIT scores for the whole sample were negatively associated with both MD and RD values (p‐value ≤0.05 FWE‐corrected).
Interpretation
There is decreased GM volume and increased MD in olfactory‐related regions explaining prolonged olfactory deficits in post‐acute COVID‐19 patients.
Introduction
Coronavirus disease 2019 (COVID‐19) is an infectious disease caused by the SARS‐CoV‐2 virus that has resulted in a worldwide pandemic with high rates of mortality. The major clinical manifestation that has been systematically described include respiratory complications, fever, cough, headache, diarrhea, and myalgia. 1 Other early symptoms, like olfactory and central nervous system dysfunctions, have been increasingly reported. In particular, the incidence of smell loss according to subjective complains is up to >80%. 2 According to a recent meta‐analysis the prevalence of smell loss is 35–36% when measuring olfaction with validated tools. 3 Although most patients tend to recover the olfactory dysfunction within few months, some report smell impairment after one month, or even after 6 months. A follow‐up with 1462 participants suggests that ~60% of women and ~48% of men recover <80% of their pre‐illness olfactory ability multiple months (6 months median) since COVID‐19 infection. 4
Despite the increasing interest in this matter, limited literature has focused on olfactory dysfunction's neural correlates throughout neuroimaging data. Early case reports revealed changes in the olfactory system of COVID‐19 patients with subjective complaints of smell dysfunction, such as the presence of microhemorrhages at the olfactory bulb 5 and olfactory bulb edema. 6 Cortical involvement has been evidenced in acute patients by means of magnetic resonance imaging (MRI) and computed tomography (CT) presenting alterations in the olfactory gyrus, such as intracranial hemorrhage 7 and cortical hyperintensities. 8 Later on, a study with a sample of 23 participants reported several cleft and olfactory bulb abnormalities (i.e., olfactory opacification and reduced olfactory bulb volume) evidenced by conventional radiological analyses after one month of infection but also a signal abnormality in the olfactory cortex in 22% of the cases assessed with quantitative measurements. 9 A systematic review including 31 studies involving 305 patients, identified normal morphology and signal intensity in the olfactory bulb, but opacification of the olfactory cleft as the most prevalent abnormality in COVID‐19 olfactory dysfunction. 10 This result suggested that obstruction is the likely mechanism for olfactory dysfunction in COVID‐19. However, in the same review, increase in the frequency of abnormalities in signal intensity and morphology of olfactory bulb on late versus early imaging studies indicate that central mechanisms for anosmia may also play a role.
A recent work used diffusion tensor imaging (DTI) data to study white matter (WM) integrity in post COVID‐19 patients with persistent olfactory dysfunction compared to post‐infectious olfactory loss related to other pathogens. 11 This work points toward damage in the olfactory bulb and shows higher quantitative anisotropy in the orbitofrontal and entorhinal cortices but does not elucidate if this damage is also present in COVID‐19 patients who have recovered their sense of smell. In the same line, structural connectivity derived from diffusion MRI data was found to be higher in COVID‐19 patients than in healthy control subjects with no history of COVID‐19 infection in the medial orbitofrontal subdivision. 12
A recent MRI longitudinal work analyzed a large sample from the UK Biobank, revealing greater changes in limbic and olfactory brain regions in COVID‐19 patients compared to healthy subjects suggesting that these changes may underpin smell dysfunction in COVID‐19. 13 Nonetheless, this hypothesis has not been tested since they did not include specific olfactory measures.
In the light of this evidence, we aim to investigate whether structural brain MRI abnormalities in the olfactory system are present in post‐acute COVID‐19 patients with persistent smell dysfunction compared to those with normal olfaction established using validated measures.
Methods
Subjects
The cohort of this study was recruited at the Alzheimer's disease and other cognitive disorders Unit, Neurology Service, Hospital Clinic de Barcelona, Spain (April 2021–November 2021) referring cognitive complains. Forty‐eight COVID‐19 patients were included in the current work. The interval between the infection and the assessment in months was as follows: mean = 9.94; SD = 3.83.
Inclusion criteria were (1) COVID‐19 diagnosis, based on biological diagnosis or medical report; (2) ≥8 weeks after the onset of COVID‐19 symptoms; (3) fluent in Spanish and having at least 6 years of formal education; (4) age 35–65 years old. Exclusion criteria consisted of (1) previous diagnosis of any neurological, psychiatric, or medical condition that could affect the baseline cognitive performance; (2) any severe medical condition or visual or auditory problems that prevent the completion of the cognitive assessment; (3) imaging findings on MRI other than mild WM hyperintensities in the FLAIR sequence, and MRI artifacts. For the DTI analyses, the final sample consisted of 47 patients: one patient was excluded due to MRI artifacts.
Standard protocol approvals and patient consents
Written informed consent was obtained from all study participants after full explanation of the procedures. The study was approved by the Institutional Ethics Committee of the Hospital Clinic de Barcelona (HCB/2020/1483). The study conforms with the World Medical Association Declaration of Helsinki (https://jamanetwork.com/journals/jama/fullarticle/1760318).
Olfactory assessment
Olfaction was assessed at the time of neuroimaging, using the Spanish version of the smell identification test (UPSIT). 14 The UPSIT is a standardized multiple‐choice scratch‐and‐sniff test consisting of four test booklets with 10 items each. In accordance with normative instructions, subjects scratch the impregnated area and are asked to select one of four possible answers for each item. All hyposmic/anosmic patients have the persistence of olfactory dysfunction according to the World Health Organization criteria, 15 since they have an interval between infection of 4–19 months.
Sociodemographic and clinical data
Age, sex, years of education, and time from the acute phase of the COVID‐19 diagnosis were registered. The acute phase of the COVID‐19 was classified as mild (patients who were not admitted in hospital), moderate (patients admitted in hospital conventional care), and severe (intensive care admission).
MRI acquisition
Magnetic resonance images (MRI) were acquired with a 3T scanner (MAGNETOM Prisma, Siemens, Germany). The scanning protocol included high‐resolution 3‐dimensional T1‐weighted images acquired in the sagittal plane (TR = 2400 ms, TE = 2.22 ms, TI = 1000 ms, 208 slices, FOV = 256 mm, 0.8 mm isotropic voxel), an axial FLAIR sequence (TR = 6000 ms, TE = 397 ms) and two diffusion‐weighted imaging acquisitions with identical parameters (TR = 3230 ms, TE = 89.20 ms, diffusion‐encoding in 99 directions at b = 0 and 3000 s/mm2) but reversed phase‐encoding direction (anterior–posterior and posterior–anterior) were obtained for each subject. The two‐weighted imaging acquisitions are used to estimate and correct susceptibility induced distortions with the Topup tool from the FSL https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/topup/TopupUsersGuide/.
Subsequent preprocessing and analyses were performed at the Neuroimaging Laboratory of the Medical Psychology Unit, Department of Medicine, University of Barcelona, Spain.
Voxel‐based morphometry
Structural images were brain‐extracted and gray‐matter‐segmented using SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Then, the optimized FSL‐VBM pipeline was followed 16 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM) using FSL tools, 17 starting with the non‐linear registration to the MNI 152 standard. The next step was the creation of a study‐specific template by averaging the resulting images. Subsequently, the native images were non‐linearly pre‐registered to this template with resampling to a voxel size of 2 × 2 × 2 mm3 and modulation to correct local expansion or contraction. The modulated segmented images were then smoothed with an isotropic Gaussian kernel (9.42 mm).
Tract‐based spatial statistics analysis
Preprocessed diffusion MRI images were analyzed with FDT software from FSL, starting with the eddy current distortions and subject motion. Individual FA maps were obtained using a diffusion tensor model fit (DTIFIT), and the voxel‐wise statistical analysis of FA was carried through with TBSS. 18 TBSS performs nonlinear registration (using nonlinear image registration tool [FMRIB]) of FA images from DTIFIT to the MNI standard space and generates a mean FA skeleton that represents the center of all WM tracts common to the whole group. Each subject's FA image was projected onto the skeleton and the resulting FA skeleton images were fed into a general linear model (GLM) modeling the two groups of patients to find vertex‐wise differences in FA skeleton maps. The same steps were used to obtain the mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) maps. FSL's randomize 19 was used to compute group and correlation analysis.
Definition of regions of interest
For the gray matter (GM) VBM analyses, a mask that defined the olfactory system was created including the amygdalae hippocampi; thalami; insular cortices; parahippocampal gyri; superior frontal gyrus, orbital part; middle frontal gyrus, orbital part; gyrus rectus; olfactory cortex; and the limbic subregion of the striatum. The Automated Anatomical Labeling atlas was used to create the corresponding masks, except the limbic striatum mask, which was obtained from the Oxford‐ GSK‐Imanova striatal connectivity atlas (available in FSL). This mask was previously used in Campabadal et al. 2019. 20
The olfactory mask used for the TBSS was created including the hippocampi, amygdalae, insular cortices, parahippocampal gyri, olfactory cortex, gyrus rectus, external capsule, the genu of corpus callosum, the anterior thalamic radiation, and the uncinate fasciculus. The Automated Anatomical Labeling atlas, the Johns Hopkins University (JHU) White‐Matter Tractography atlas and the JHU ICBM‐DTI‐81 White‐Matter Labels atlas (available in FSL) were used.
Statistical analyses
Olfactory system masks encompassing the structures of interest were created to define a search volume for subsequent VBM and TBSS data statistical testing. The statistical analyses consisted of a voxel‐wise general linear model with non‐parametric permutation tests (5000 permutations) besides threshold‐free cluster enhancement (TFCE) for statistical inference. Voxel‐wise intergroup comparisons, as well as multiple regression analyses between imaging measures and the UPSIT score were computed. For multiple testing corrections, the family‐wise error rate (FWE) correction was applied, with a reporting criterion of FWE‐corrected p‐value ≤0.05.
Statistical analyses of demographic and clinical data variables were carried out using the statistical package SPSS Version 27.0 (2020; Armonk, NY: IBM Corp.). Student's t and Mann–Whitney U tests were used to assess between‐group differences. Pearson's chi‐squared and Fisher's exact tests were applied to assess contingencies between qualitative variables.
Results
Sociodemographic and clinical data
Patients were infected between February 2020 and May 2021. Figure S1 shows the average weekly mutations counts in Spain and Catalonia for this period according to World Health Organization variant classification. In addition, Table S1 shows mutation's relative frequencies in Spain for each patient's period of infection. Twenty‐five patients were classified as normal or mild hyposmia and twenty‐three as moderate, severe hyposmia or anosmia according to the UPSIT cut‐off scores (from now on, “normal olfaction” and “olfactory dysfunction” groups, respectively). For the DTI analyses, one patient with olfactory dysfunction was excluded due to MRI artifact in the DWI sequence. Demographic and clinical data of the participants are summarized in Table 1 (see also Table S2). Following the aforementioned criteria, 38 patients were classified as mild (23 had normal olfaction and 15 olfactory dysfunction), 8 as moderate (1 with normal olfaction and 7 with olfactory dysfunction), and 2 as severe (1 with normal olfaction and 1 with olfactory dysfunction). Groups did not differ in age, years of education, or sex. There were no differences in the interval between COVID‐19 diagnosis and MRI acquisition and the percentages of admission in the intensive care unit. The group of patients with olfactory dysfunction had significantly more hospitalization cases than the normal olfaction group.
Table 1.
Sociodemographic and clinical variables of the normal olfaction and olfactory dysfunction groups.
| Normal olfaction (n = 25) | Olfactory dysfunction (n = 23) | Test stat (p‐value) | |
|---|---|---|---|
| Age, y, mean (SD) | 48.04 (7.59) | 51.96 (7.92) | −1.749 (0.087) 1 |
| Sex, n male (%) | 7 (28%) | 3 (13%) | 1.625 (0.292) 2 |
| Education, y, mean (SD) | 14.72 (2.59) | 14.57 (2.57) | 283.500 (0.931) 3 |
| Interval COVID‐19 – UPSIT, m, mean (SD) | 8.92 (3.76) | 11.04 (3.82) | −1.939 (0.59) 1 |
| Interval COVID‐19 – MRI, m, mean (SD) | 8.92 (3.72) | 11.04 (3.72) | −1.975 (0.054) 1 |
| UPSIT total, mean (SD) | 32.92 (1.53) | 25.83 (3.35) | 0.000 (<0.001) 3 |
| Hospitalization, n (%) | 2 (8%) | 8 (34.8%) | 5.210 (0.033) 2 |
| ICU admission, n (%) | 1 (4%) | 1 (4.3%) | 0.004 (1.000) 2 |
Bold text indicates a statistically significant difference with a p‐value <0.05.
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; m, months; UPSIT, the smell identification test; y, years.
t‐test was used.
Fisher's exact test was used.
Mann–Whitney U test was used.
VBM comparison between groups
The COVID‐19 patients with olfactory dysfunction had less volume in gray matter volume compared with the normal olfaction patients in a cluster involving the left amygdala, insular cortex, parahippocampal gyrus, frontal superior and inferior orbital gyri, gyrus rectus, olfactory cortex, caudate, and putamen (378 voxels; MNI coordinates of cluster maximum: x = −22, y = 10, z = −28; t stat = 4.669; p‐value = 0.0082) (Fig. 1).
Figure 1.
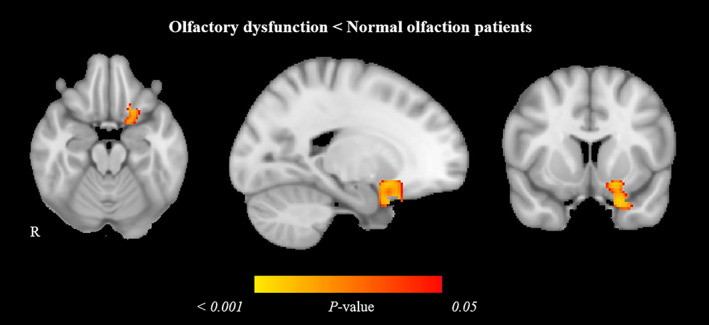
Gray matter volume reduction in olfactory dysfunction patients compared with normal olfaction COVID‐19 patients. Significant voxel‐wise difference is marked in warm colors. Results are displayed over the sagittal, coronal, and axial sections of the MNI 152 standard brain at p‐value ≤0.05 FWE‐corrected. MNI, Montreal Neurosciences Institute; R, right.
DTI comparison between groups
Groups did not differ in FA or AD maps. Patients with olfactory dysfunction showed higher MD values (t stat = 4.711; p‐value = 0.031) in the genu of the corpus callosum, orbitofrontal WM tracts, the anterior thalamic radiation, and the forceps minor compared to those with normal olfaction (Fig. 2A and Table S3) and higher RD values (t stat = 4.759; −value p = 0.043) in the anterior corona radiata, genu of the corpus callosum, and uncinate fasciculus (Fig. 2B and Table S3).
Figure 2.
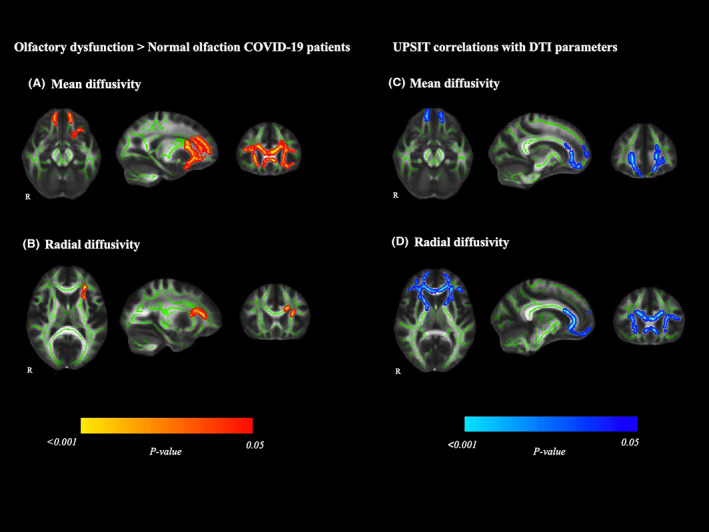
White matter abnormalities in olfactory dysfunction patients compared with normal olfaction COVID‐19 patients. Images on the left show tract‐based spatial statistics differences between patients with olfactory dysfunction and those with normal olfactory function in mean diffusivity (A) and radial diffusivity (B). Voxelwise group differences (olfactory dysfunction > Normal olfaction) are marked in warm colors. Images on the right depict Tract‐based spatial statistics correlation in the whole sample of COVID‐19 patients between mean diffusivity (C), radial diffusivity (D) and the UPSIT. Significant group negative correlations are marked in cold colors. Results are overlaid on the white matter skeleton (green) and displayed over the sagittal, coronal, and axial sections of the MNI 152 standard brain at p < .05 FWE‐corrected. Image shows significant clusters >10 voxels. DTI, diffusion tensor imaging; MNI, Montreal Neurosciences Institute; R, right; UPSIT, the smell identification test.
Correlations between olfactory function and structural data
There were no significant correlations between GM volume of the olfactory system and the UPSIT.
The UPSIT was negatively associated with both, MD (t stat = 0.607; p‐value = 0.040), and RD (t stat = 0.781; p‐value = 0.011) values for the whole sample, in orbitofrontal WM tracts, the anterior thalamic radiation, the forceps minor, and the genu of the corpus callosum, (Fig. 2C,D, respectively; and Table S3 and Fig. S2).
Discussion
The olfactory deficit is a frequent clinical manifestation of COVID‐19; some of these patients present persistent smell loss that lasts even beyond months after symptoms onset. 3 As far as we know, this is the first work to investigate whether COVID‐19 patients with prolonged olfactory dysfunction have structural brain changes compared to those with normal olfaction. Our data point toward GM volume decreases and MD increase in the olfactory system, which might explain why some COVID‐19 patients have not recovered their sense of smell.
The results of the VBM comparison analysis showed less GM volume in the olfactory dysfunction group compared with the normal olfaction group. These differences involved left regions of the amygdala, insular cortex, parahippocampal gyrus, frontal superior and inferior orbital gyri, gyrus rectus, olfactory cortex, caudate, and putamen. This is the first evidence of GM reductions in the olfactory system directly related to the persistent olfactory dysfunction using a group comparison approach on MRI data. Initial case studies in this field suggested the brain implication of olfactory loss. 5 , 7 , 8 Subsequently, Kandemirli et al. reported high frequency of olfactory clefts opacification on CT, MRI volume reductions in 10/23 cases and a negative correlation between olfactory sulcus depth and olfaction. 9 Moreover, using visual inspection, signal intensity of the bulb was seen in two cases. However, in this interesting study, the associative regions were not analyzed.
TBSS results showed that COVID‐19 patients with persistent olfactory dysfunction had increased MD in the genu of the corpus callosum, orbitofrontal WM tracts, the anterior thalamic radiation, and the forceps minor compared to those with normal olfaction and higher RD values in the anterior corona radiata, genu of the corpus callosum, and uncinate fasciculus. Remarkably, these DTI parameters were negatively associated with the UPSIT scores when the analysis was computed for the whole sample, which provides additional support for the relationship between the WM integrity and the sense of smell in these patients. Structural changes in some of these structures have been reported elsewhere. 7 , 11 , 13 , 21 Some of these findings were located in regions functionally connected with the piriform olfactory cortex and anterior olfactory nucleus compared to healthy subjects. 13 However, this later work did not include an objective measure of olfaction, which does not allow the study of the relationship between olfactory dysfunction and underlying structural brain damage.
Contrarily to our findings, one VBM study reported volume increments in the olfactory cortex, among other regions, in post‐infected patients compared with controls. The gray matter increments were also seen in olfactory cortices, hippocampi, insulas, left rolandic operculum, left Heschl's gyrus, and right cingulate gyrus. 21 These increments of GM are accompanied by WM changes such as MD decrements and FA increments from DTI data. In our opinion, altogether their findings are suggestive of inflammatory conditions present in the initial period post‐infection. There are three main mechanisms hypothesized to explain subsequent effects on the brain and the initial loss of smell: inflammatory response 22 ; infection of supporting cells 23 ; and direct invasion of olfactory neurons via the olfactory bulb or the mucosa. 24 , 25 Also, anterograde neurodegeneration from the olfactory neurons cannot be ruled out. 13 These factors alone or in an interaction could cause the permanence of the symptom. Our sample was in a late‐post‐acute phase of the disease (mean 10 months) and our MRI findings are suggestive of neuronal loss. The structural changes that we have found agree with previous functional neuroimaging data. In this sense, one FDG‐PET study showed hypometabolism in the bilateral orbital region, 26 another FDG‐PET study showed a pattern of hypo‐ and hypermetabolism probably depending on the structural brain damage and evolution time since the sample was studied only one month on average after symptoms onset. 27 Olfactory dysfunction has also been associated with lower tissue perfusion in the orbital and medial frontal regions in the arterial spin labeling sequence. 28
The main strength of the current research is the use of MRI quantitative data and the inclusion of an objective tool to evaluate olfaction in subjects at mid‐term follow‐up of post‐acute COVID‐19 condition. These allow characterizing with high accuracy the smell impairment based on a validated measure, as well as studying its relationship with MRI quantitative data. With this work, we confirm the presence of structural brain abnormalities in olfactory‐related regions in subjects with persistent smell loss when compared to normal olfaction months after acute COVID‐19. Furthermore, although the lack of a control group could be a limitation, our design has advantages since we can identify bran regions specifically related with olfactory dysfunction in COVID‐19. A previous study with a larger sample including patients with and without anosmia also found cortical thinning in orbitofrontal and parahippocampal regions. 13 In that particular study, the differences with controls could be related to unspecific brain damage associated with the direct effects of the virus. In our design, both groups with and without olfactory dysfunction have similar clinical characteristics. Thus, we demonstrated that structural damage in olfactory regions might explain specifically the presence of olfactory dysfunction as well as its severity. Future longitudinal investigations should focus on olfactory impairment as a potential biomarker of subsequent neurodegeneration, with a particular interest in those COVID‐19 patients presenting persistent smell loss.
In our work, we were interested in those COVID‐19 patients with clinically relevant smell alterations affecting daily life. Hyposmia has been reported in 20% of participants in non‐clinical population. 29 When using UPSIT, 14 mild hyposmia is distributed similarly in healthy controls and in a sample of patients with COVID‐19, with percentages of 18% and 13%, respectively. 30 Altogether, this would indicate that the mild hyposmia category does not discriminate the olfactory deficit between COVID‐19 patients and healthy controls, for that reason we grouped patients with mild hyposmia together with patients with normosmia and compared them to those with severe olfactory impairment.
Finally, although we have included information about the frequency of cases and mutations in our region by the time this sample was infected, this information was not available for each participant. The local epidemiological data indicated that most participants were probably infected with the alpha variant. Even so, with our data we cannot answer this intriguing question, further studies will elucidate the effect of different variants in brain changes associated to COVID‐19 sequalae.
Although mechanisms leading the neuronal injury are unknown, our data suggest the presence of brain damage; evidenced by increased mean diffusivity and gray matter loss in regions of the olfactory system in COVID‐19 patients with long olfactory dysfunction pointing out the central nervous system involvement in the persistent COVID symptoms.
Author Contributions
CJ, RSV, and BS contributed to the design of the study. AC, JO, and BS contributed to the analysis of the data. JO, NG, GL, MAB, RSV, contributed to the acquisition of data. AC, JO, CJ, and BS contributed to the interpretation of the data. AC, JO, CJ, and BS contributed to the draft of the article. AC, JO, CJ, NG, MAB, RSL, GCMR, GL, NB, LR, RSV, and BS revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Conflict of Interest
The authors report no conflicts of interest relevant to this study.
Supporting information
Figure S1. Average weekly mutations counts in Spain and Catalonia from February 2020 to May 2021. Frequency and counts by variant is reported. Variant classification according to World Health Organization. Data and figures obtained from the work of Català, F. and Noguera, M. (July 25, 2022). CovidTag http://covidtag.paseq.org (Enabled by Data from GISAID https://www.gisaid.org/).
Figure S2. Scatterplot of the linear association between the UPSIT and mean diffusivity (figure on the left), and radial diffusivity (figure on the right).
Table S1. Mutation's relative frequencies in Spain for each patient's period of infection (from February 2020 to May 2021). Frequency by variant is reported. Variant classification according to World Health Organization. Data obtained from the work of Català, F. and Noguera, M. CovidTag http://covidtag.paseq.org (Enabled by data from GISAID https://www.gisaid.org/). UPSIT, Spanish version of the smell identification test (Doty RL. The Smell Identification Test. Administration Manual. 3rd ed. Haddon Hts: Sensonics; 1995).
Table S2. Demographic and clinical data of the participants for the DTI sample.
Table S3. Significant clusters showing tract‐based spatial statistics differences between groups (COVID‐19 patients with olfactory dysfunction vs normal olfaction); and correlations between DTI parameters and the UPSIT for the whole sample.
Acknowledgment
The study was partially supported by a research agreement with Sage therapeutics. AC, CJ, RSL, and BS were supported by the PANDÈMIES 2020 program of the Agència de Gestió D'Ajuts Universitaris I de Recerca (AGAUR) (2020PANDE00053). AC was supported by a Margarita Salas (2021‐2023) postdoctoral fellowship.
Funding Statement
This work was funded by PANDÈMIES 2020 Program of the Agència de Gestió D'Ajuts Universitaris I de Recerca (AGAUR) grant 2020PANDE00053; Ministerio de Universidades, Gobierno de España grant MargaritaSalas(2021‐2023); Sage therapeutics .
Contributor Information
Raquel Sánchez‐Valle, Email: rsanchez@clinic.cat.
Bàrbara Segura, Email: bsegura@ub.edu.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID‐19): a meta‐analysis of 27,492 patients. Laryngoscope. 2021;131:865‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohla K, Veldhuizen MG, Green T, et al. A follow‐up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID‐19 smell loss. Rhinol J. 2022;60(3):207‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aragão MFVV, Leal MC, Cartaxo Filho OQ, Fonseca TM, Valença MM. Anosmia in COVID‐19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol. 2020;41:1703‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID‐19‐related anosmia. Neurology. 2020;95:224‐225. [DOI] [PubMed] [Google Scholar]
- 7. Thu SS, Matin N, Levine SR. Olfactory gyrus intracerebral hemorrhage in a patient with COVID‐19 infection. J Clin Neurosci. 2020;79:275‐276. https://linkinghub.elsevier.com/retrieve/pii/S0967586820313710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID‐19) and anosmia. JAMA Neurol. 2020;77:1028‐1029. [DOI] [PubMed] [Google Scholar]
- 9. Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID‐19 anosmia. Acad Radiol. 2021;28:28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keshavarz P, Haseli S, Yazdanpanah F, Bagheri F, Raygani N, Karimi‐Galougahi M. A systematic review of imaging studies in olfactory dysfunction secondary to COVID‐19. Acad Radiol. 2021;28:1530‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yildirim D, Kandemirli SG, Tekcan Sanli DE, Akinci O, Altundag A. A comparative olfactory MRI, DTI and fMRI study of COVID‐19 related anosmia and post viral olfactory dysfunction. Acad Radiol. 2022;29:31‐41. https://linkinghub.elsevier.com/retrieve/pii/S1076633221004906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esposito F, Cirillo M, de Micco R, et al. Olfactory loss and brain connectivity after COVID‐19. Hum Brain Mapp. 2022;43:1548‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douaud G, Lee S, Alfaro‐Almagro F, et al. SARS‐CoV‐2 is associated with changes in brain structure in UK biobank. Nature. 2022;604(7907):697‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doty RL. The Smell Identification Test. Administration Manual. 3rd ed. Sensonics; 1995. [Google Scholar]
- 15. World Health Organization . A Clinical Case Definition of Post COVID‐19 Condition by a Delphi Consensus. World Health Organization; 2021. [Google Scholar]
- 16. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21‐36. [DOI] [PubMed] [Google Scholar]
- 17. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208‐S219. [DOI] [PubMed] [Google Scholar]
- 18. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage. 2006;31:1487‐1505. [DOI] [PubMed] [Google Scholar]
- 19. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campabadal A, Segura B, Junque C, et al. Comparing the accuracy and neuroanatomical correlates of the UPSIT‐40 and the Sniffin’ sticks test in REM sleep behavior disorder. Parkinsonism Relat Disord. 2019;65:197‐202. [DOI] [PubMed] [Google Scholar]
- 21. Lu Y, Li X, Geng D, et al. Cerebral micro‐structural changes in COVID‐19 patients – an MRI‐based 3‐month follow‐up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID‐19. Nature. 2021;595:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butowt R, von Bartheld CS. Anosmia in COVID‐19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2021;27:582‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butowt R, Bilinska K. SARS‐CoV‐2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Nerosci. 2020;11:1200‐1203. [DOI] [PubMed] [Google Scholar]
- 25. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2021;24:168‐175. [DOI] [PubMed] [Google Scholar]
- 26. Guedj E, Campion JY, Dudouet P, et al. (18)F‐FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823‐2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain. 2021;144:1263‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yus M, Matias‐Guiu JA, Gil‐Martínez L, et al. Persistent olfactory dysfunction after COVID‐19 is associated with reduced perfusion in the frontal lobe. Acta Neurol Scand. 2022;146:194‐198. doi: 10.1111/ane.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255:1121‐1126. doi: 10.1007/s00415-008-0807-9 [DOI] [PubMed] [Google Scholar]
- 30. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944‐950. doi: 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Average weekly mutations counts in Spain and Catalonia from February 2020 to May 2021. Frequency and counts by variant is reported. Variant classification according to World Health Organization. Data and figures obtained from the work of Català, F. and Noguera, M. (July 25, 2022). CovidTag http://covidtag.paseq.org (Enabled by Data from GISAID https://www.gisaid.org/).
Figure S2. Scatterplot of the linear association between the UPSIT and mean diffusivity (figure on the left), and radial diffusivity (figure on the right).
Table S1. Mutation's relative frequencies in Spain for each patient's period of infection (from February 2020 to May 2021). Frequency by variant is reported. Variant classification according to World Health Organization. Data obtained from the work of Català, F. and Noguera, M. CovidTag http://covidtag.paseq.org (Enabled by data from GISAID https://www.gisaid.org/). UPSIT, Spanish version of the smell identification test (Doty RL. The Smell Identification Test. Administration Manual. 3rd ed. Haddon Hts: Sensonics; 1995).
Table S2. Demographic and clinical data of the participants for the DTI sample.
Table S3. Significant clusters showing tract‐based spatial statistics differences between groups (COVID‐19 patients with olfactory dysfunction vs normal olfaction); and correlations between DTI parameters and the UPSIT for the whole sample.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


