Abstract
Aims
To study all‐cause mortality in patients hospitalized with COVID‐19 with or without chronic heart failure (CHF) during hospitalization and at 3 and 6 months of follow‐up.
Methods and results
The international registry Analysis of Comorbid Disease Dynamics in Patients with SARS‐CoV‐2 Infection (ACTIV) was conducted at 26 centres in seven countries: Armenia, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Russian Federation, and Uzbekistan. The primary endpoints were in‐hospital all‐cause mortality and all‐cause mortality at 3 and 6 months of follow‐up.
Of the 5616 patients hospitalized with COVID‐19, 917 (16.3%) had CHF. Total in‐hospital mortality was 7.6%. In‐hospital mortality was higher in patients with CHF than in patients without a history of CHF [17.7% vs. 4.0%, P < 0.001; odds ratio (OR) 4.614, 95% confidence interval (CI) 3.633–5.859; P < 0.001]. The risk of in‐hospital all‐cause mortality correlated significantly with the severity of CHF; specifically, the risk of in‐hospital all‐cause mortality was greater for patients in New York Heart Association functional classes III and IV (OR 6.124, 95% CI 4.538–8.266; P < 0.001 vs. patients without CHF) than for patients in functional classes I and II (OR 2.446, 95% CI 1.831–3.267, P < 0.001 vs. patients without CHF). The risk of mortality in patients with ischemic CHF was 58% higher than in patients with non‐ischaemic CHF [OR 1.58 (95% CI 1.05–2.45), P = 0.030]. In the first 3 months of follow‐up, the all‐cause mortality rate in patients with CHF was 10.32%, compared with 1.83% in patients without CHF (P < 0.001). At 6 months of follow‐up, NYHA classes II–IV was a strong risk factor for all‐cause mortality [OR 5.343 (95% CI 2.717–10.508); P < 0.001].
Conclusions
Hospitalized COVID‐19 patients with CHF have an increased risk of in‐hospital all‐cause mortality, which remains high 6 months after discharge.
Keywords: Coronavirus disease 2019, Cardiovascular disease, Chronic heart failure, SARS‐CoV‐2
Introduction
Coronavirus disease 2019 (COVID‐19), a new coronavirus infection caused by the SARS‐COV‐2 virus, resulted in a rapidly spreading worldwide pandemic associated with very considerable morbidity and mortality for more than 2 years. The association between cardiovascular diseases (CVD) and a negative prognosis in COVID‐19 is well established. 1 However, there have been limited data on the prevalence and prognostic significance of chronic heart failure (CHF) in a population of patients with COVID‐19 in the acute period of infection, nor of the course of CHF in the post‐discharge period after COVID‐19‐related hospitalization.
In the Eurasian region (total population >200 million people), there were no clinical registries to collect and analyse information on the interplay between COVID‐19 and co‐morbid conditions. To address this deficit, the international registry Analysis of Comorbid Disease Dynamics in Patients with SARS‐CoV‐2 Infection (ACTIV) 2 was established. We now report our findings on the effect of CHF on all‐cause mortality in patients enrolled in the ACTIV registry.
Methods
A detailed description of the study design and statistical data processing methods has been published previously. 3 Further information about the ACTIV registry is available on the website of the Eurasian Association of Therapists or via a direct link (https://ACTIV.euat.ru).
The registry is organized and overseen by three committees: an organizational committee, a supervisory committee, and a committee for endpoint analysis and control of completion of individual registration cards (IRCs). IRCs and document turnover were in electronic format only.
Healthcare professionals from seven Euroasian countries participated in the ACTIV registry: Armenia (one centre), Belarus (one centre), Kazakhstan (one centre), Kyrgyzstan (one centre), Moldova (one centre), Russian Federation (20 centres), and Uzbekistan (one centre).
Study population
The registry included hospitalized men and women aged ≥18 years with a confirmed diagnosis of COVID‐19 [positive result by polymerase chain reaction testing of a nasopharyngeal sample, a positive blood antigen test, typical computerized tomography (CT) image]. Exclusion criteria for the registry were age ≤18 years at hospitalization and outpatients.
Enrolled patients were divided into two cohorts according to their CHF status:
Patients with a history of CHF, including heart failure (HF) with preserved ejection fraction (EF), mildly reduced EF, or reduced EF. The diagnosis of HF was based on the national clinical guidelines of each participating country.
Patients without a history of CHF.
Study procedures
Demographic data (age, sex, and ethnicity), clinical data (medical history, medications taken at admission, cardiac manifestations of COVID‐19 at admission, signs and symptoms of COVID‐19 at admission, and physical examination at admission), laboratory findings, chest X‐ray and/or CT scan data, electrocardiographic and echocardiographic data, and data on clinical course in hospital and COVID‐19 complications were extracted from electronic medical records using a standardized data collection form on three visits: Visit 1 (day of admission); Visit 2 (7 days after admission); and Visit 3 (day of discharge).
Further data on the post‐discharge status of patients were obtained via telephone interviews using a standardized questionnaire at 3 and 6 months after recovery from COVID‐19. Telephone calls were planned only for the patients who gave their consent at discharge to take part in the procedure. The questionnaire for interviewing patients by telephone is available at https://activ.euat.ru/documents.
Data acquisition, pooling, and standardization
Patient recruitment commenced on 29 June 2020 and ended on 29 October 2020. Data were collected from 26 health centres in the seven participating countries. A total of 188 physicians took part in the registry. Details on medical contributors are provided in Appendix S1, along with details of members of the Data Monitoring and the Endpoint Committees.
Inclusion of patients was limited by the number of COVID‐19 cases and local COVID‐19 triage rules. Whenever centres provided a set of eligible patients, we attempted to obtain consecutive patients. Each IRC was checked by reviewers operating as part of the central structure of the registry. The medical diagnosis was established on the basis of ICD‐10 criteria.
We developed a standardized data collection form to control for the fact that definitions of clinical manifestations may vary from country to country and from centre to centre. Registry documentation was maintained in Russian.
Pseudo‐anonymized forms (with protected keys stored at the local centres) were collected by the core working group and merged into a common database, and a common identifier was created for each patient.
Data quality was checked for all variables. For categorical variables, numbers that did not correspond to any of the predefined categories were excluded. For continuous variables, temporal variables (expressed in number of days) were excluded as follows: negative values (<0 days) and values >365 days.
Data quality for continuous variables was further checked by systematically estimating the mean, median, minimum, maximum, and range of values for each centre and comparing them with the total values for the entire registry. We identified outliers and implausible values and, if necessary, questioned participants to address any issues that arose.
Laboratory measurements
Laboratory parameters comprised erythrocytes, haemoglobin, white blood cells (lymphocytes and neutrophils), platelets, high‐sensitivity cardiac troponin T or I, C‐reactive protein (CRP), procalcitonin, arterial blood gases (partial pressures of CO2 and O2), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, glucose, albumin, creatinine (for use in Cockroft–Gault estimates), serum potassium and sodium levels, D‐dimer, ferritin, international normalized ratio, and fibrinogen.
Endpoints
The primary endpoints were in‐hospital all‐cause mortality and all‐cause mortality within 6 months after hospital discharge.
Statistical analysis
The registry data were processed using the IBM SPSS Statistics 25 statistical package. Categorical variables are represented as numbers of patients, with percentages in parentheses. Continuous variables are described as medians with lower and upper quartiles. Intergroup differences were tested using Student's t‐test for normally distributed data and the Mann–Whitney U test for non‐normally distributed data. Proportions were compared using the χ2 test or Fisher's exact test where appropriate. Logistic regression (univariate regression followed by multivariate stepwise regression) identified variables that most significantly influenced mortality and the need for hospital admission. Correlation matrices and multi‐input frequency tables were constructed to avoid multi‐collinearity and incorrect interpretation of relationships.
Ethics
The study was conducted in accordance with the provisions of the Declaration of Helsinki, approved by the Ethics Committee of the Federal State Autonomous Educational Institution of Higher Education ‘Pirogov Russian National Research Medical University’ of the Ministry of Healthcare of the Russian Federation, and registered in the ClinicalTrials.gov database as Analysis of Chronic Non‐infectious Diseases Dynamics After COVID‐19 Infection in Adult Patients (ACTIV) (identifier NCT04492384).
Informed consent was obtained from all patients for the use of clinical, laboratory, and instrumental data from medical documentation in the IRCs on condition of anonymity, as well as for receipt of telephone calls 3 and 6 months after discharge. Further information on the principles and practical aspects of informed consent in the ACTIV registry is available at https://activ.euat.ru/documents (in Russian only).
Results
The registry included 5616 hospitalized patients with laboratory‐confirmed COVID‐19. The average hospital stay was 14 ± 4.5 days [median 14.0 days, interquartile range (IQR) 11.0;17.0]. Table 1 shows baseline characteristics of COVID‐19 patients with and without pre‐existing CHF. A total of 917 (16.3%) patients in the ACTIV registry had incident CHF. Of those patients, 10.6% were in New York Heart Association (NYHA) functional classes I and II, and 5.6% were in NYHA classes III and IV. The number of patients with non‐ischaemic CHF was 264 (28.8%), and the number of patients with ischaemic CHF was 653 (71.2%).
Table 1.
Comparative baseline characteristics of COVID‐19 patients with and without CHF
| Total cohort | HF subgroup | Non‐HF subgroup | P age‐adjusted | |
|---|---|---|---|---|
| N = 5616 | n = 917 | n = 4699 | ‐ | |
| Age (years); median (IQR) | 58 [48–68] | 69 [63–79] | 56 [45–65] | 0.001 |
| Men, n (%) | 2561 (45.6) | 444 (48.4) | 2119 (45.1) | 0.001 |
| Died, n (%) | 348 (6.2) | 161 (17.7) | 187 (4.0) | 0.001 |
| CT 3–4, n (%) | 1100 (19.6) | 253 (27.4) | 847 (18.0) | 0.001 |
| SpO2 75–94%, n (%) | 2302 (41.0) | 568 (62.0) | 1734 (36.9) | 0.001 |
| SpO2 < 75%, n (%) | 72 (1.3) | 38 (4.1) | 34 (0.7) | 0.001 |
| Glucose ≥7 mmol/L in patients with diabetes mellitus, n (%) | 711/984 (72.3) | 228/298 (76.5) | 483/686 (70.5) | 0.04 |
| Arterial hypertension, n (%) | 3111 (55.4) | 809 (88.1) | 2302 (49.0) | 0.001 |
| Obesity (body mass index ≥30 kg/m2), n (%) | 1952 (34.8) | 367 (39.9) | 1585 (33.7) | 0.001 |
| Coronary heart disease, n (%) | 1156 (20.6) | 653 (71.2) | 503 (10.7) | 0.001 |
| Previous myocardial infarction, n (%) | 321 (5.7) | 213 (23.2) | 108 (2.3) | 0.001 |
| Stroke, n (%) | 240 (4.3) | 107 (11.7) | 133 (2.8) | 0.001 |
| Diabetes mellitus type 2, n (%) | 984 (17.5) | 298 (32.6) | 686 (14.6) | 0.001 |
| Atrial fibrillation, n (%) | 383 (6.8) | 242 (26.4) | 141 (3.0) | 0.001 |
| Chronic kidney disease, n (%) | 422 (7.5) | 188 (20.6) | 234 (5.0) | 0.001 |
| COPD, n (%) | 259 (4.6) | 99 (10.8) | 160 (3.4) | 0.001 |
| Anaemia, n (%) | 1504 (26.8) | 369 (40.1) | 1135 (24.1) | 0.001 |
| SpO2(%); median [IQR] | 95 [93–97] | 93 [90–95] | 95 [93–97] | 0.001 |
| Haemoglobin (g/L); median [IQR] | 130 [118–141] | 125 [111–136] | 131 [120–142] | 0.001 |
| Leukocytes (×109/L); median [IQR] | 7 [5.4–9.72] | 8.3 [6.1–11.7] | 6.9 [5.3–9.2] | 0.001 |
| Lymphocytes (%);median [IQR] | 18.8 [10–28] | 14.55 [7.4–21.9] | 20 [10.3–29.4] | 0.001 |
| C‐reactive protein (mg/L); median [IQR] | 34.3 [12–90] | 45.05 [18.1–102.0] | 31.1 [10.61–86.0] | 0.001 |
| D‐dimer (μg FEU/mL); median [IQR] | 0.77 [0.35–1.81] | 1.1 [0.35–2.18] | 0.74 [0.35–1.61] | 0.04 |
| Glomerular filtration rate (mL/min/1.73 m2); median [IQR] | 70.07 [54.46–86.51] | 56.26 [39.84–73.95] | 72.55 [58.19–89.09] | 0.001 |
| Alanine aminotransferase (units/L); median [IQR] | 34 [24.0–52.0] | 37 [25.3–55.0] | 34 [23.5–51.0] | 0.001 |
| Alanine aminotransferase (units/L); median [IQR] | 33 [21.0–58.4] | 31.65 [20.0–51.0] | 33.5 [21.0–59.0] | 0.02 |
| Glucose in non‐diabetic patients (mmol/L); median [IQR] | 5.6 [5.0–6.5] | 5.98 [5.0–7.0] | 5.6 [5.0–6.5] | 0.001 |
| Procalcitonin (ng/mL); median [IQR] | 0.16 [0.06–0.40] | 0.21 [0.11–0.50] | 0.13 [0.05–0.36] | 0.001 |
| Troponin I (ng/mL); median [IQR] | 0.03 [0.0–0.1] | 0.08 [0.02–0.13] | 0.01 [0.00–0.06] | 0.001 |
| Complications | ||||
| Deep vein thrombosis, n (%) | 23 (0.4) | 10 (1.1) | 13 (0.3) | 0.03 |
| Pulmonary embolism, n (%) | 34 (0.6) | 14 (1.5) | 20 (0.4) | 0.12 |
| Stroke, n (%) | 26 (0.5) | 13 (1.3) | 13 (0.3) | 0.22 |
| Bacterial pneumonia, n (%) | 552 (9.8) | 183 (20.1) | 369 (7.9) | 0.001 |
| ARDS, n (%) | 331 (5.9) | 148 (16.2) | 183 (3.9) | 0.001 |
| Cytokine storm, n (%) | 1306 (23.3) | 294 (31.8) | 1012 (21.5) | 0.001 |
| Acute kidney injury, n (%) | 490 (8.7) | 152 (16.6) | 338 (7.2) | 0.001 |
| Myocarditis, n (%) | 17 (0.3) | 4 (0.4) | 13 (0.3) | 0.63 |
| Sepsis, n (%) | 17 (0.3) | 10 (1.1) | 7 (0.1) | 0.01 |
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; CT, computerized tomography; CT 4, 75–100% lesion; CT 3, 50–75% lesion; FEU, fibrinogen equivalent units; IQR, interquartile range; SpO2, saturated partial pressure of oxygen.
Patients with left ventricular EF ≤ 40%, 41–49%, and ≥50% accounted for 9.78%, 30.33%, and 59.89% of the total cohort, respectively.
Patients with CHF were characterized by a higher level of co‐morbidity and greater abnormalities in laboratory and instrumental markers of the severity of COVID‐19 than patients who did not have CHF. Patients with CHF also differed from those without CHF with regard to the incidence of acute infectious complications. For example, deep vein thrombosis (DVT), strokes, bacterial pneumonia, acute respiratory distress syndrome (ARDS), cytokine storm, acute kidney injury (AKI), and sepsis were more frequent in patients with CHF.
Patients with CHF were, on average, older than those without CHF [median 69 (IQR 63–79) vs. 56 (45–65) years, P < 0.001]. For this reason, further comparison was made using logistic regression after controlling for age.
The overall in‐hospital all‐cause mortality rate was 7.6%. Patients with CHF had significantly higher all‐cause mortality than those without CHF (17.7% vs. 4.0%, P < 0.001).
The presence of CHF correlated strongly with in‐hospital all‐cause mortality risk [odds ratio (OR) 4.614, 95% confidence interval (CI) 3.633–5.859, P < 0.001]. The risk of death was related to the severity of CHF and was greater for patients with NYHA class III–IV CHF (OR 6.124, 95% CI 4.538–8.266, P < 0.001) than those with NYHA class I–II CHF (OR 2.446, 95% CI 1.831–3.267, P < 0.001). CHF was a recurring feature of disease clusters associated with increased mortality risk, including CHF + arterial hypertension (AH) (OR 3.963, 95% CI 3.022–5.197); CHF + AH + coronary heart disease (CHD) (OR 4.082, 95% CI 3.054–5.455); and CHF + AH + CHD + type 2 diabetes mellitus (OR 4.215, 95% CI 2.784–6.382) (P < 0.001 for all comparisons with patients who did not have the respective disease cluster).
Information on demographic, clinical‐instrumental, and laboratory characteristics was available for 681 surviving and 146 deceased patients with CHF (Table 2 ). Comparison of mortality rates between the ischemic CHF and non‐ischaemic CHF groups showed that 114 patients died in the ischaemic CHF group (78.1% of all deaths) and 32 died in the non‐ischaemic CHF group (21.9% of all deaths). Mortality risk was 58% higher in patients with ischemic CHF [OR 1.58 (95% CI 1.05–2.45), P = 0.030]. Inspections within the CHF subgroup based on survival status established that deceased patients were older and that the combination of male sex and age ≥60 years was associated with a markedly increased risk of all‐cause mortality (Table 2 ). Other risk markers for all‐cause mortality in this subgroup were severity of lung injury on CT scan, CRP > 40 mg/L, and glucose ≥7 mmol/L in patients without diabetes mellitus (Table 2 ).
Table 2.
Comparison of characteristics of patients in the ACTIV registry with COVID‐19 plus CHF according to survival status
| Patients who survived | Patients who died | P for differences | ||
|---|---|---|---|---|
| n = 681 | n = 146 | ‐ | Odds ratio (95% confidence interval) | |
| Age (years), median [IQR] | 68 [62–76] | 77 [66–83] | 0.001 | ‐ |
| Men, n (%) | 322 (47.4) | 77 (52.7) | 0.24 | 1.237 (0.865–1.769) |
| Men aged ≥60 years, n (%) | 254 (37.3) | 68 (46.9) | 0.03 | 1.483 (1.034–2.129) |
| Computerized tomography Grade 1 (0–25% lung lesion) or Grade 2 (26–50% lung lesion), n (%) | 512 (75.1) | 86 (58.9) | 0.001 | 2.105 (1.372–3.228) |
| Computerized tomography Grade 3 (51–75% lung lesion) or Grade 4 (76–100% lung lesion), n (%) | 169 (24.9) | 60 (41.1) | ||
| C‐reactive protein >40 mg/L, n (%) | 341 (50.1) | 92 (63.1) | 0.01 | 1.701 (1.117–2.590) |
| Glucose ≥7 mmol/L in non‐diabetic patients, n (%) | 114/467 (24.4) | 37/83 (45.1) | 0.001 | 2.545 (1.513–4.281) |
| Glucose ≥ 7 mmol/L in patients with diabetes, n (%) | 157/214 (73.2) | 53/63 (84.1%) | 0.08 | 1.998 (0.916–4.357) |
| Arterial hypertension and aged ≥60 years, n (%) | 495 (72.7) | 126 (86.2) | 0.01 | 2.345 (1.421–3.872) |
| Obesity and aged ≥60 years, n (%) | 187 (27.5) | 58 (39.7) | 0.01 | 1.732 (1.114–2.621) |
| Coronary artery disease, n (%) | 471 (69.2) | 114 (78.1) | 0.03 | 1.588 (1.039–2.428) |
| Coronary artery disease and aged ≥60 years, n (%) | 411 (60.3) | 107 (73.1) | 0.001 | 1.788 (1.201–2.661) |
| Stroke, n (%) | 65 (9.5) | 33 (22.6) | 0.001 | 2.768 (1.739–4.404) |
| Type 2 diabetes mellitus, n (%) | 214 (31.4) | 63 (43.2) | 0.01 | 1.656 (1.150–2.387) |
| Type 2 diabetes mellitus and aged ≥60 years, n (%) | 173 (25.4) | 57 (39.3) | 0.001 | 1.906 (1.309–2.774) |
| Atrial fibrillation, n (%) | 163 (23.9) | 57 (39.0) | 0.001 | 2.035 (1.397–2.695) |
| Atrial fibrillation and aged ≥60 years, n (%) | 144 (21.1) | 53 (36.6) | 0.001 | 2.155 (1.466–3.168) |
| Chronic kidney disease, n (%) | 121 (17.8) | 45 (30.8) | 0.001 | 2.062 (1.379–3.084) |
| Chronic kidney disease and aged ≥60 years, n (%) | 102 (15.0) | 39 (26.9) | 0.001 | 2.078 (1.361–3.172) |
Medians and interquartile ranges reported for quantitative variables; proportion of categories reported for categorical variables. Results are presented as n (%) or as median with interquartile range (IQR). Comparisons based on Mann–Whitney or χ2 tests.
Demographic features associated with increased all‐cause mortality included the combination of age ≥60 years plus AH or obesity. Factors most strongly associated with increased all‐cause mortality risk in patients with CHF were (in descending order) history of stroke, glucose ≥7 mmol/L in patients without diabetes, advanced (Grade 3–4) CT evidence of lung damage, chronic kidney disease (CKD), atrial fibrillation (AF), CRP > 40 mg/L, type 2 diabetes mellitus, and CHD.
A comparative analysis of the severity of laboratory markers of infection in deceased and surviving patients with CHF demonstrated that patients who died had lower saturated partial oxygen pressure (SpO2), partial oxygen pressure (pO2), haemoglobin, lymphocytes, platelets and glomerular filtration rate (GFR) (Table S1 ). Compared with surviving patients in the CHF subgroup, those who died also had higher white blood cell count, CRP, D‐dimer, AST, blood glucose levels (regardless of diabetes status), and procalcitonin level.
Examination of pre‐admission therapies relevant to the management of CHF established that 45% of the CHF subgroup patients were taking angiotensin‐converting enzyme inhibitors (ACEIs) and 23.1% were taking angiotensin receptor blockers (ARBs); beta‐blockers (BBs) were prescribed to 63.4% of patients, mineralocorticoid receptor antagonists (MRAs) to 4.0% of patients (but 12.6% of HF patients with left ventricular EF < 40%), and diuretics to 54.5% of patients. None of the included patients was reported to be taking sacubitril/valsartan or SGLT2 inhibitors, and only nine were taking eplerenone. A comparison of dead and surviving patients with CHF demonstrated that prior use of BBs, statins, oral antiplatelet drugs, and oral antihyperglycemic medications was associated with a reduction in all‐cause mortality (Table 3 ). A non‐significant trend (P = 0.054) was identified for a reduced risk of death in patients prescribed ACEI therapy. Prior MRA treatment was associated with a non‐significant trend for increased risk of all‐cause mortality [OR 2.342 (95% CI 0.921–4.543), P = 0.07] (Table 3 ).
Table 3.
Characteristics of survivors and deceased patients with CHF in the ACTIV registry according to therapy preceding hospital admission
| Background therapy | Overall cohort | Patients who survived | Patients who died | P for differences | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|
| n = 827 | n = 681 | n = 146 | ‐ | ‐ | |
| Angiotensin‐converting enzyme inhibitors, n (%) | 372 (45.0) | 317 (46.5) | 55 (37.5) | 0.054 | 0.689 (0.471–1.007) |
| Angiotensin blockers, n (%) | 191 (23.1) | 163 (24.0) | 28 (19.1) | 0.22 | 0.75 (0.472–1.193) |
| Beta blockers, n (%) | 524 (63.4) | 455 (66.8) | 69 (47.1) | 0.001 | 0.441 (0.304–0.642) |
| Mineralocorticoid receptor antagonists, n (%) | 37 (4.6) | 23 (3.4) | 14 (9.6) | 0.07 | 2.342 (0.921–4. 543) |
| Statins, n (%) | 341 (41.2) | 299 (43.9) | 42 (27.9) | 0.001 | 0.495 (0.330–0.742) |
| Ticagrelor, clopidogrel, prasugrel, n (%) | 101 (12.2) | 91 (13.4) | 10 (6.6) | 0.03 | 0.459 (0.225–0.937) |
| Oral antidiabetic therapy for type 2 diabetes mellitus, n (%) | 260 (31.2) | 231 (34.3) | 29 (20.3) | 0.04 | 0.489 (0.244–0.981) |
Post‐discharge status of COVID‐19 patients with CHF
For follow‐up data, 3007 patients were contacted by telephone 3 months after discharge from hospital and 2011 patients after 6 months. At 3 months, 432 patients (14.4%) did not respond to the follow‐up call, and 390 responses (12.9%) were considered incomplete. At 6 months, 409 patients (20.3%) did not respond to the follow‐up call, and there were 394 (19.7%) incomplete responses. Analysis was thus based on data from 2185 telephone interviews at 3 months (including 174 patients who answered the telephone but refused to continue collaboration) and 1208 interviews at 6 months.
In the general cohort of ACTIV patients, deterioration of at least one symptom or appearance of at least one new symptom occurred in 38.2% of patients during the first 3 months of follow‐up and in 27.7% after 6 months of follow‐up. Patients with CHF were more likely than patients without CHF to have a new symptom or deterioration of symptoms that were typical for them before COVID‐19 (Figure 1 ). Dyspnoea markedly worse than before index hospitalization was recorded during the first 3 months post‐discharge in 44.17% of patients with CHF and 26.8% of patients without CHF (P < 0.0001) and in 45.45% of patients with CHF and 17.11% without CHF at 4–6 months post‐discharge (P < 0.0001).
Figure 1.
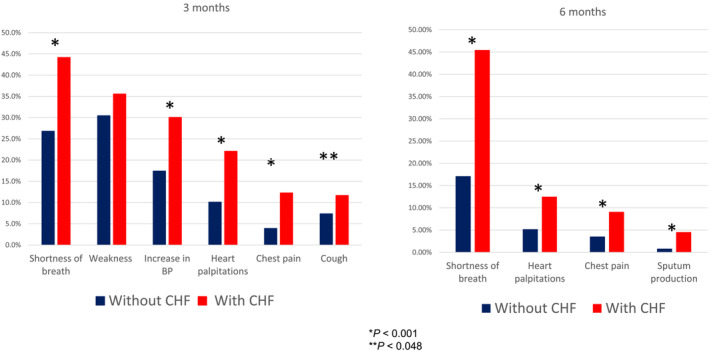
Comparative analysis of symptomatology in COVID‐19 patients in the ACTIV registry with or without CHF (NYHA classes II–IV) at 3 and 6 months of follow‐up. BP, blood pressure. *P < 0.001. **P < 0.048.
Another widespread finding in patients with CHF was AH no longer controlled by standard‐of‐care therapies. This was observed more frequently in patients with CHF than in those without CHF in the first 3 months post‐discharge (30.06% vs. 17.41%, P < 0.0001) (Figure 1 ). Patients with CHF were also significantly more likely than those without CHF to have palpitations, chest pain, and cough (in descending order of frequency) in the first 3 months post‐discharge and to have palpitations, chest pain, and sputum production at 4–6 months (Figure 1 ).
Almost one‐third (29.2%) of the total ACTIV population sought unplanned medical care in the post‐hospital period, and at least twice during the first 3 months. Patients with CHF were significantly more likely than those without CHF to seek unplanned care in the first 3 months for (in decreasing frequency) CHD worsening, decompensation of CHF, and onset of AF and at 4–6 months for (in decreasing frequency) CHD worsening, decompensation of CHF, AF, and stroke (Figure 2 ).
Figure 2.
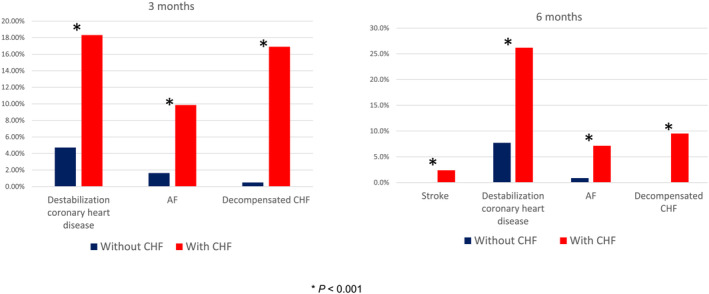
Comparative analysis of the frequency and causes of healthcare seeking in COVID‐19 patients in the ACTIV registry with or without CHF (NYHA classes II–IV) at 3 and 6 months of follow‐up. AF, atrial fibrillation. *P < 0.001.
Newly diagnosed diseases in the general ACTIV patient population were reported in 5.6% and 6.4% of patients in the post‐hospital period at 3 and 6 months, respectively. These included AH (3%), CHD (1.4%), type 2 diabetes mellitus (1.4%), AF (0.3%), and arthritis (0.3%). Patients with CHF were more likely than those without CHF to have a first diagnosis (in descending order of frequency) of AF, COPD, stroke, DVT, or CHD in the initial 3 months post‐discharge, and, in the period 4–6 months post‐discharge, patients with CHF were more likely to experience stroke (Table S2 ).
All‐cause mortality in the general population of the ACTIV registry in the post‐hospital period was 1.9% during the first 3 months of follow‐up and 0.2% during months 4–6 of follow‐up. CHF severity corresponding to NYHA classes II–IV was found to be a strong predictor of all‐cause mortality in the post‐hospital period (OR 5.343, 95% CI 2.717–10.508, P < 0.001). The highest rate of all‐cause mortality was observed in the first 3 months in the group of patients with NYHA class II–IV CHF (10.32%); the corresponding rate in patients without CHF was 1.83% (Figure S1 ). Cardiovascular complications (38.46%) were prominent among all causes of death in the post‐hospital period in patients with CHF: acute decompensation of CHF (23.08%) was the single most common cause of death, with additional contributions from acute MI and stroke (7.69% each).
Discussion
The main findings of our study suggest that pre‐existing CHF in hospitalized patients with COVID‐19 was associated with high all‐cause mortality and more frequent complications both in hospital and after discharge from the hospital. To our knowledge, this is the first registry investigating the interplay between COVID‐19 and pre‐existing morbidities, which has included patients with CHF in the Eurasian region. Some comparison with other studies is therefore instructive.
In a Cochrane systematic review, Pellicori et al. 1 examined the interplay between COVID‐19, cardiovascular co‐morbidities, and cardiovascular complications. It should be noted that almost 90% of the studies included in that meta‐analysis were of a retrospective nature. Among the 20 prospective investigations identified, only the study by Petrilli et al. 4 recruited a patient population similar in size to that of ACTIV (n = 5279 vs. n = 5616) and did so in a single centre in New York.
The average incidence of CHF in patients with COVID‐19 in the Cochrane meta‐analysis averaged 6.5% 1 but ranged from 0% to 28%. Substantial variation in incidence of CHF has been recorded: for example, Petrilli et al. 4 reported a CHF incidence of 7% in patients with COVID‐19; a retrospective analysis by Alvarez‐Garcia et al. 5 (n = 6439) reported a 6.6% prevalence of CHF among hospitalized patients with COVID‐19; and Palmieri et al. in Italy 6 reported an incidence of 16% in patients with COVID‐19. Our own finding of a CHF incidence of 16.3% in patients hospitalized with COVID‐19 is thus towards the upper end of the range established in other research. This may reflect particular demographic features of our population that we have not yet explored.
In our study, patients with CHF were on average 13 years older than the comparator subgroup and exhibited more co‐morbidities. Our CHF cohort exhibited a high prevalence of hypertension, CVD or cerebrovascular disease, and type 2 diabetes mellitus, much in accordance with the aggregate findings of Pellicori et al. 1 and Petrilli et al. 4 In contrast to these two studies, we did not identify a marked prevalence of obesity in our CHF patients compared with the non‐CHF subgroup, but obesity was a risk marker for worse outcomes in our CHF patients, a finding in line with general experience with COVID‐19. 7 , 8 , 9
Our patients with CHF experienced a more severe course of COVID‐19 and more frequent complications in the acute period of infection than the non‐CHF comparators. These findings are consistent with those from a range of other studies. 5 , 10 , 11 , 12 , 13 , 14
Mortality among our patients with CHF was four times higher that than in those without CHF (17.7%vs. 4.0%, P < 0.001). Similarly, other authors have reported higher mortality in patients with COVID‐19 and CHF compared with those without CHF. 4 , 10 , 11
The major factors associated with increased mortality risk in ACTIV patients with CHF were NYHA class (more severe CHF conferred greater risk); laboratory and instrumental markers of severe infection; and the following co‐morbidities (in descending order of their influence on mortality risk): history of stroke, CKD, AF, type 2 diabetes mellitus, and CHD. Age ≥60 years had a consistently strong adverse influence on prognosis, and there was a notable association between age ≥60 years and male sex. These findings are compatible with those in various other reports, including those of Saleh et al., 15 Alvarez‐Garcia et al., 16 the SEMI‐COVID‐19 registry 17 (which included 1718 patients and reported an overall mortality rate of 47.6%), and Belarte‐Tornero et al. 11 Also of note is the study by Bhatt et al., 18 whose retrospective analysis of 8383 HF patients hospitalized with COVID‐19 identified male sex, morbid obesity, and greater age as being associated with higher risk of mortality. Mortality in that database analysis of patients with HF plus COVID‐19 approached 25%, a value even higher than that in our registry. This may reflect growing experience in the management of COVID‐19 in the second and third quarters of 2020, a possibility identified by the authors of that investigation.
Based on the ACTIV registry data, deceased patients with or without diabetes mellitus tended to have higher blood glucose levels than patients who survived. This finding is consistent with the findings of the study by Long et al., 19 which showed that both diabetes mellitus and hyperglycaemia without diabetes mellitus were independently associated with adverse outcomes of COVID‐19 with risk coefficients of 10.41 and 3.58, respectively. Elsewhere, Wang et al. have reported that a plasma glucose level ≥7.0 mmol/L at hospitalization is an independent predictor of 28‐day mortality in patients with COVID‐19 without a prior diagnosis of diabetes mellitus. 20 This phenomenon may have a number of explanations. For example, patients with COVID‐19 may suffer from stress‐induced hyperglycaemia. 21 Patients in a critical condition may develop acute insulin resistance, which manifests as hyperglycaemia and hyperinsulinaemia. 22 Separately or perhaps simultaneously, drugs such as antibiotics and corticosteroids can also increase serum glucose levels. 23 , 24
Continuation of guideline‐directed medical therapy in chronic CHF patients is considered of primary importance for effective management in the era of COVID‐19. 25 In‐hospital withdrawal of drug therapy, including ACEIs, ARBs, BBs, and MRAs, is associated with higher mortality in acute decompensated heart failure. 10 Only 12.6% of CHF patients included in the ACTIV registry were pre‐treated with MRAs, and none of the included patients was reported to take sacubitril/valsartan or SGLT2 inhibitors. Notwithstanding differences in the registration of the above‐mentioned medications in each country participating in the registry, we believe that it is necessary to make additional efforts to optimize the drug management of patients with CHF and COVID‐19 in the Eurasian region. In our study, pre‐treatment of patients with CHF and COVID‐19 with statins, oral antiplatelet, and anti‐hyperglycaemic medications was associated with a reduction in mortality. Identification of reasons for this are outside the scope of the present report, but we speculate that prescription of several of these classes of drugs might be considered as a part of medical therapy for CHF patients who develop COVID‐19. Further investigation of these possibilities may be instructive for the medical management of COVID‐19 in patients with CHF.
Throughout the post‐discharge period, patients with CHF were more likely than CHF‐free patients to experience (in descending order of frequency) breathlessness, weakness, uncontrolled hypertension, palpitations, cough, and chest pain. Patients with CHF were also significantly more likely to seek unscheduled care in the first 3 months for (in descending order of frequency) deterioration of ischaemic heart disease and decompensation of CHF and AF and, during months 4–6 post‐discharge, for those three complications plus stroke. The scope and prospective nature of the ACTIV registry make it one of the first large reports on the post‐discharge status of COVID‐19 patients with CHF.
According to our data, 5.6% and 6.4% of patients in the ACTIV registry population were diagnosed with a ‘new’ disease, including ‘new’ CHF, in the post‐discharge period during 3 and 6 months of follow‐up, respectively. A number of other reports also point to the possibility of ‘new’ HF, in both the acute and post‐hospitalization periods of COVID‐19. 16 , 26 Signs of myocardial inflammation and fibrosis on magnetic resonance images have been reported in patients discharged from hospital, and these may persist for several months after discharge. 27 , 28 , 29 The significance of these signs of cardiac involvement is not fully known, but one evident possibility is that the SARS‐COV‐2 infection may be a risk factor for the development of CHF, either by direct myocardial effects or the propagation of AF or the destabilization of recognized co‐morbidity risk factors such as AH, kidney disease, or diabetes. This may extend also to HF with preserved ejection fraction. 30 , 31
The mortality rate during the first 3 months after discharge in patients with NYHA class II–IV CHF was 10.32%, making CHF functional class one of the strongest identifiers (and, we suppose, determinants) of fatal outcome in the early post‐hospitalization period. Cardiovascular events (acute CHF, acute MI, and stroke) were recorded causes of death in 38.5% of cases. A high rate of post‐discharge mortality, especially in patients with CVD, has been reported by various authors. 32 , 33 , 34 , 35
Viral infections are known to be a potential cause of decompensation of CHF. 36 , 37 That general premise is affirmed for the specific situation of SARS‐CoV‐2 by the new data from the ACTIV registry: CHF had a negative impact on the prognosis of patients with COVID‐19, increasing the risk of death in both the acute and post‐hospitalization periods. COVID‐19, in turn, increased the risk of decompensation of existing CHF and the development of new HF. Various other recent publications have either corroborated the effect of this detrimental relationship between COVID‐19 and CHF on patient prognosis or outlined some possible mechanistic explanations for these adverse effects. 38 , 39 , 40 , 41 The negative correlation between COVID‐19 and CHF needs to be taken into account when deciding whether to prioritize hospital admission for patients with a history of CHF.
Limitations of the study
Although the ACTIV registry nominally included all patients who were admitted to hospital with a diagnosis of COVID‐19 during the registry timeline, the potential for selection bias must be acknowledged. As with other multicentre registries, there were practical limits to our ability to verify data for every patient. Echocardiography was performed in the context of routine clinical practice, the assessment of left ventricular EF was not standardized, and the information on the levels natriuretic peptides was not available in many patients. Taken together, these factors may have led to misclassification of some patients, and this needs to be considered when interpreting the data. As regards mortality rates and post‐discharge complications, our findings may have been influenced by differences in the management of patients with CHF in the different participating countries.
Deteriorating patient condition, whether due to progression of CHF, late complications of COVID‐19, the combination of both conditions, or other illnesses, may have created a selection bias in our follow‐up cohort by excluding the most severely affected participants. The implication of such a bias could be that outcomes in the whole ACTIV CHF cohort were even worse than revealed by our investigations.
Conclusions
ACTIV is currently the only registry that generates data on the COVID‐19 patient population in the Eurasian region and the course of COVID‐19 and co‐morbid disease dynamics over a 6‐month post‐discharge period. Our findings show that CHF appears to be encountered more often in COVID‐19 patients in the Eurasian region than in some other countries and territories. Advanced CHF (NYHA classes III and IV) is the strongest risk predictor of fatal outcome for COVID‐19, and its negative impact on prognosis extends into the early post‐hospitalization period, when COVID‐19 patients with CHF feel worse than those without it, are more likely to seek unplanned medical care, and are more likely to develop de novo disease. This suggests the need to develop optimal rehabilitation regimens and a multidisciplinary approach to the management of patients with CHF following COVID‐19 infection.
This detrimental interplay of CHF and COVID‐19 requires careful monitoring, as there is a high likelihood of an increased burden of CHF and a change in the course of this condition due to COVID‐19 infection. Patients with CHF should be a priority group in national or population‐wide vaccination programmes. Further analysis of the ACTIV database and similar resources may help to guide the medical response to this continuing challenge.
Conflict of interest
The authors and investigators declare no conflicts of interest in respect of their work on the ACTIV registry.
Funding
The Eurasian Association of Therapists (EAT) bore all the costs of the ACTIV registry. The EAT is an independent association that provides opportunities for its members to share experience and discuss problems of evidence‐based medicine and clinical practice.
Supporting information
Appendix S1. Supporting information.
Acknowledgements
The authors and investigators thank all patients and relatives who agreed to participate in the ACTIV registry. Translation and editorial services were provided by Information International Ltd, Oxford, UK.
Arutyunov, G. P. , Tarlovskaya, E. I. , Arutyunov, A. G. , Lopatin, Y. M. , and ACTIV Investigators (2023) Impact of heart failure on all‐cause mortality in COVID‐19: findings from the Eurasian International Registry. ESC Heart Failure, 10: 1013–1024. 10.1002/ehf2.14243.
References
- 1. Cochrane Heart Group , Pellicori P, Doolub G, Wong CM, Lee KS, Mangion K, Ahmad M, Berry C, Squire I, Lambiase PD, Lyon A, McConnachie A, Taylor RS, Cleland JGF. COVID‐19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev 2022; 2022: CD013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arutyunov GP, Tarlovskaya EI, Arutyunov AG, Belenkov YN, Konradi AO, Lopatin YM, Tereshchenko SN, Rebrov AP, Chesnikova AI, Fomin IV, Grigorieva NU, Boldina VM, Vaisberg AR, Blagonravova AS, Makarova EV, Shaposhnik II, Kuznetsova TY, Malchikova SV, Protsenko DN, Evzerikhina AV, Petrova MM, Demko IV, Saphonov DV, Hayrapetyan HG, Galyavich AS, Kim ZF, Sugraliev AB, Nedogoda SV, Tsoma VV, Sayganov SA, Gomonova VV, Gubareva IV, Sarybaev AS, Ruzanau DY, Majseenko VI, Babin AP, Kamilova UK, Koroleva EV, Vilkova OE, Fomina IY, Pudova IA, Soloveva DV, Doshchannikov DA, Kiseleva NV, Zelyaeva NV, Kouranova IM, Pogrebetskaya VA, Muradova FN, Omarova YV, Badina OY, Kovalishena OV, Gаlova AE, Plastinina SS, Grigorovich MS, Lyubavina NA, Vezikova NN, Levankova VI, Ivanova SY, Ermilova AN, Muradyan RG, Gostishev RV, Tikhonova EP, Kuzmina TY, Soloveva IA, Kraposhina AY, Kolyadich MI, Kolchinskaya TP, Genkel VV, Kuznetsova AS, Kazakovtseva MV, Odegova AA, Chudinovskikh TI, Baramzina SV, Rozanova NA, Kerimova AS, Krivosheina NA, Chukhlova SY, Levchenko AA, Avoyan HG, Azarian KK, Musaelian SN, Avetisian SA, Levin ME, Karpov OV, Sokhova FM, Burygina LA, Sheshina TV, Tiurin AA, Dolgikh OY, Kazymova EV, Konstantinov DY, Chumakova OA, Kondriakova OV, Shishkov KY, Fil ST, Prokofeva NA, Konoval MP, Simonov AA, Bitieva AM, Trostianetckaia NA, Cholponbaeva MB, Kerimbekova ZB, Duyshobayev MY, Akunov AC, Kushubakova NA, Melnikov ES, Kim ES, Sherbakov SY, Trofimov DA, Evdokimov DS, Ayipova DA, Duvanov IA, Abdrahmanova AK, Aimakhanova GT, Ospanova SO, Dabylova GM, Tursunova AT, Kaskaeva DS, Tulichev AA, Ashina EY, Kordukova VA, Barisheva OY, Egorova KE, Varlamova DD, Kuprina TV, Pahomova EV, Kurchugina NY, Frolova IA, Mazalov KV, Subbotin AK, Kamardina NA, Zarechnova NV, Mamutova EM, Smirnova LA, Klimova AV, Shakhgildyan LD, Tokmin DS, Tupitsin DI, Kriukova TV, Polyakov DS, Karoli NA, Grigorieva EV, Magdeyeva NA, Aparkina AV, Nikitina NM, Petrov LD, Budu AM, Rasulova ZD, Tagayeva DR, Fatenkov OV, Gubareva EY, Demchenko AI, Klimenko DA, Serikbolkyzy S, Zheldybayeva AE. International register “analysis of chronic non‐infectious diseases dynamics after COVID‐19 infection in adult patients (ACTIV SARS‐CoV‐2)”. Kardiologiia 2020; 60: 30–34. [PubMed] [Google Scholar]
- 3. Arutyunov GP, Tarlovskaya EI, Arutyunov AG, Belenkov YN, Konradi AO, Lopatin YM, Rebrov AP, Tereshchenko SN, Chesnikova AI, Hayrapetyan HG, Babin AP, Bakulin IG, Bakulina NV, Balykova LA, Blagonravova AS, Boldina MV, Vaisberg AR, Galyavich AS, Gomonova VV, Grigorieva NU, Gubareva IV, Demko IV, Evzerikhina AV, Zharkov AV, Kamilova UK, Kim ZF, Kuznetsova TY, Lareva NV, Makarova EV, Malchikova SV, Nedogoda SV, Petrova MM, Pochinka IG, Protasov KV, Protsenko DN, Ruzanov DY, Sayganov SA, Sarybaev AS, Selezneva NM, Sugraliev AB, Fomin IV, Khlynova OV, Chizhova OY, Shaposhnik II, Sсhukarev DA, Abdrahmanova AK, Avetisian SA, Avoyan HG, Azarian KK, Aimakhanova GT, Ayipova DA, Akunov AC, Alieva MK, Aparkina AV, Aruslanova OR, Ashina EY, Badina OY, Barisheva OY, Batchayeva AS, Bitieva AM, Bikhteyev IU, Borodulina NA, Bragin MV, Budu AM, Burygina LA, Bykova GA, Varlamova DD, Vezikova NN, Verbitskaya EA, Vilkova OE, Vinnikova EA, Vustina VV, Gаlova EA, Genkel VV, Gorshenina EI, Grigorieva EV, Gubareva EY, Dabylova GM, Demchenko AI, Dolgikh OY, Duvanov IA, Duyshobayev MY, Evdokimov DS, Egorova KE, Ermilova AN, Zheldybayeva AE, Zarechnova NV, Ivanova SY, Ivanchenko EY, Ilina MV, Kazakovtseva MV, Kazymova EV, Kalinina YS, Kamardina NA, Karachenova AM, Karetnikov IA, Karoli NA, Karpov OV, Karsiev MK, Кaskaeva DS, Kasymova KF, Kerimbekova ZB, Kerimova AS, Kim ES, Kiseleva NV, Klimenko DA, Klimova AV, Kovalishena OV, Kolmakova EV, Kolchinskaya TP, Kolyadich MI, Kondriakova OV, Konoval MP, Konstantinov DY, Konstantinova EA, Kordukova VA, Koroleva EV, Kraposhina AY, Kriukova TV, Kuznetsova AS, Kuzmina TY, Kuzmichev KV, Kulchoroeva CK, Kuprina TV, Kouranova IM, Kurenkova LV, Kurchugina NY, Kushubakova NA, Levankova VI, Levin ME, Lyubavina NA, Magdeyeva NA, Mazalov KV, Majseenko VI, Makarova AS, Maripov AM, Marusina AA, Melnikov ES, Moiseenko NB, Muradova FN, Muradyan RG, Musaelian SN, Nikitina NM, Ogurlieva BB, Odegova AA, Omarova YM, Omurzakova NA, Ospanova SO, Pahomova EV, Petrov LD, Plastinina SS, Pogrebetskaya VA, Polyakov DS, Ponomarenko EV, Popova LL, Prokofeva NA, Pudova IA, Rakov NA, Rakhimov AN, Rozanova NA, Serikbolkyzy S, Simonov AA, Skachkova VV, Smirnova LA, Soloveva DV, Soloveva IA, Sokhova FM, Subbotin AK, Sukhomlinova IM, Sushilova AG, Tagayeva DR, Titojkina YV, Tikhonova EP, Tokmin DS, Torgunakova MS, Trenogina KV, Trostianetckaia NA, Trofimov DA, Tulichev AA, Tupitsin DI, Tursunova AT, Tiurin AA, Ulanova ND, Fatenkov OV, Fedorishina OV, Fil TS, Fomina IY, Fominova IS, Frolova IA, Tsvinger SM, Tsoma VV, Cholponbaeva MB, Chudinovskikh TI, Shakhgildyan LD, Shevchenko OA, Sheshina TV, Shishkina EA, Shishkov KY, Sherbakov SY, Yausheva EA. International register “dynamics analysis of comorbidities in SARS‐CoV‐2 survivors” (AСTIV) and the register “analysis of hospitalizations of comorbid patients infected during the second wave of SARS‐CoV‐2 outbreak” (AСTIV 2). Russ J Cardiol 2021; 26: 4358. [Google Scholar]
- 4. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5,279 people with coronavirus disease 2019 in New York City: prospective cohort study. Br Med J 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvarez‐Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas‐Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, Brunjes D, Glicksberg BS, Levin MA, Nadkarni G, Fayad Z, Fuster V, Mancini D, Lala A. Prognostic impact of prior heart failure in patients hospitalized with COVID‐19. J Am Coll Cardiol 2020; 76: 2334–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, Raparelli V, Pezzotti P, Riccardo F, Bella A, Fabiani M, D'Ancona FP, Vaianella L, Tiple D, Colaizzo E, Palmer K, Rezza G, Piccioli A, Brusaferro S, Onder G, Italian National Institute of Health COVID‐19 Mortality Group , Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, Bertinato L, Boros S, Brambilla G, Calcagnini G, Canevelli M, Rita Castrucci M, Censi F, Ciervo A, Colaizzo E, D'Ancona F, del Manso M, Donfrancesco C, Fabiani M, Facchiano F, Filia A, Floridia M, Galati F, Giuliano M, Grisetti T, Kodra Y, Langer M, Lega I, Lo Noce C, Maiozzi P, Malchiodi Albedi F, Manno V, Martini M, Mateo Urdiales A, Mattei E, Meduri C, Meli P, Minelli G, Nebuloni M, Nisticò L, Nonis M, Onder G, Palmisano L, Petrosillo N, Pezzotti P, Pricci F, Punzo O, Puro V, Raparelli V, Rezza G, Riccardo F, Cristina Rota M, Salerno P, Serra D, Siddu A, Stefanelli P, de Bella MT, Tiple D, Unim B, Vaianella L, Vanacore N, Vichi M, Rocco Villani E, Zona A, Brusaferro S, Italian National Institute of Health COVID‐19 Mortality Group , Italian National Institute of Health COVID‐19 Mortality Group . Clinical characteristics of hospitalized individuals dying with COVID‐19 by age group in Italy. J Gerontol A Biol Sci Med Sci 2020; 75: 1796–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finer N, Garnett SP, Bruun JM. COVID‐19 and obesity. Clin Obes 2020; 10: e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwok S, Adam S, Ho JH, Iqbal Z, Turkington P, Razvi S, le Roux CW, Soran H, Syed AA. Obesity: A critical risk factor in the COVID‐19 pandemic. Clin Obes 2020; 10: e12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos D, Tsatsakis A. Obesity ‐ a risk factor for increased COVID‐19 prevalence, severity and lethality. Mol Med Rep 2020; 22: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rey JR, Caro‐Codón J, Rosillo SO, Iniesta ÁM, Castrejón‐Castrejón S, Marco‐Clement I, Martín‐Polo L, Merino‐Argos C, Rodríguez‐Sotelo L, García‐Veas JM, Martínez‐Marín LA, Martínez‐Cossiani M, Buño A, Gonzalez‐Valle L, Herrero A, López‐Sendón JL, Merino JL, for the CARD‐COVID Investigators , Merino JL, Caro‐Codon J, Castrejon‐Castrejon S, Iniesta AM, Martinez‐Cossiani M, Merino C, Martin‐Polo L, Martinez LA, Marco I, Garcia‐Veas JM, Rodriguez‐Sotelo L, Rosillo SO, Lopez‐Sendon JL, Rey JR, Rios JJ, Arribas JR, Arnalich F, Prados C, Alvarez‐Sala R, Quintana M, García de Lorenzo A, Reinoso F, Rivera A, Torres RM, Garcia‐Rodriguez J, Gonzalez‐Valle L, Herrero A, Borobia A, Buño A, CARD‐COVID Investigators , CARD‐COVID Investigators . Heart failure in COVID‐19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020; 22: 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belarte‐Tornero LC, Valdivielso‐Moré S, Vicente Elcano M, Solé‐González E, Ruíz‐Bustillo S, Calvo‐Fernández A, Subinara I, Cabero P, Soler C, Cubero‐Gallego H, Vaquerizo B, Farré N. Prognostic implications of chronic heart failure and utility of NT‐proBNP levels in heart failure patients with SARS‐CoV‐2 infection. J Clin Med 2021; 10: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, Danilov T, Kukar N, Shaban N, Kini A, Camaj A, Bienstock SW, Rashed ER, Rahman K, Oates CP, Buckley S, Elbaum LS, Arkonac D, Fiter R, Singh R, Li E, Razuk V, Robinson SE, Miller M, Bier B, Donghi V, Pisaniello M, Mantovani R, Pinto G, Rota I, Baggio S, Chiarito M, Fazzari F, Cusmano I, Curzi M, Ro R, Malick W, Kamran M, Kohli‐Seth R, Bassily‐Marcus AM, Neibart E, Serrao G, Perk G, Mancini D, Reddy VY, Pinney SP, Dangas G, Blasi F, Sharma SK, Mehran R, Condorelli G, Stone GW, Fuster V, Lerakis S, Goldman ME. Characterization of myocardial injury in patients with COVID‐19. J Am Coll Cardiol 2020; 76: 2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M. COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020; 22: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID‐19. Heart Fail Rev 2021; 26: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saleh KB, Hafiz A, Alsulaiman K, Aljuhani O, Alharbi S, Alharbi A, Vishwakarma R, Albekairy A, Alkathiri A, Alanazi F, Almujarri G, Alobathani S, Alharbi Y, Zowawi HM, Badreldin HA. Clinical characteristics and outcomes of patients with heart failure admitted to the intensive care unit with coronavirus disease 2019 (COVID‐19): A multicenter cohort study. Am Heart J Plus 2021; 7: 100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez‐Garcia J, Jaladanki S, Rivas‐Lasarte M, Cagliostro M, Gupta A, Joshi A, Ting P, Mitter SS, Bagiella E, Mancini D, Lala A. New heart failure diagnoses among patients hospitalized for COVID‐19. J Am Coll Cardiol 2021; 77: 2260–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salinas‐Botrán A, Sanz‐Cánovas J, Pérez‐Somarriba J, Pérez‐Belmonte LM, Cobos‐Palacios L, Rubio‐Rivas M, de‐Cossío‐Tejido S, Ramos‐Rincón JM, Méndez‐Bailón M, Gómez‐Huelgas R, en nombre del grupo SEMI‐COVID‐19 , SEMI‐COVID‐19 group . Clinical characteristics and risk factors for mortality upon admission in patients with heart failure hospitalized due to COVID‐19 in Spain. Rev Clin Esp (Barc) 2022; 222: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, Signorovitch J, Thune JJ, Vardeny O, Solomon SD. Clinical outcomes in patients with heart failure hospitalized with COVID‐19. JACC Heart Fail 2021; 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long H, Li J, Li R, Zhang H, Ge H, Zeng H, Chen X, Lu Q, Jiang W, Zeng H, Che T, Ye X, Fang L, Qin Y, Wang Q, Wu Q, Li H, Liu W. Plasma glucose levels and diabetes are independent predictors for mortality in patients with COVID‐19. Epidemiol Infect 2022; 150: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, Xu J, Wu F, Duan L, Yin Z, Luo H, Xiong N, Xu M, Zeng T, Jin Y. Fasting blood glucose at admission is an independent predictor for 28‐day mortality in patients with COVID‐19 without previous diagnosis of diabetes: a multi‐centre retrospective study. Diabetologia 2020; 63: 2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia. Diabetes Care 2005; 28: 810–815. [DOI] [PubMed] [Google Scholar]
- 22. Bar‐Or D, Rael LT, Madayag RM, Banton KL, Tanner A 2nd, Acuna DL, Lieser MJ, Marshall GT, Mains CW, Brody E. Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front Med (Lausanne) 2020; 62: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burcelin R, Amar J. Diabetes: antibiotics or prodiabetics? Nat Rev Endocrinol 2015; 11: 385–386. [DOI] [PubMed] [Google Scholar]
- 24. Johns EC, Reynolds RM. Topical glucocorticoids and risk of type 2 diabetes mellitus. Nat Rev Endocrinol 2019; 15: 379–380. [DOI] [PubMed] [Google Scholar]
- 25. Task Force for the management of COVID‐19 of the European Society of Cardiology . ESC guidance for the diagnosis and management of cardiovascular disease during the COVID‐19 pandemic: part 2‐care pathways, treatment, and follow‐up. Eur Heart J 2022; 43: 1059–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, Barbieri L, Bellasi A, Camporotondo R, Canale C, Carubelli V, Carugo S, Catagnano F, Dalla Vecchia LA, Danzi GB, di Pasquale M, Gaudenzi M, Giovinazzo S, Gnecchi M, Iorio A, la Rovere MT, Leonardi S, Maccagni G, Mapelli M, Margonato D, Merlo M, Monzo L, Mortara A, Nuzzi V, Piepoli M, Porto I, Pozzi A, Sarullo F, Sinagra G, Volterrani M, Zaccone G, Guazzi M, Senni M, Metra M. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID‐19. Results of the cardio‐COVID‐Italy multicentre study. Eur J Heart Fail 2020; 22: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 27. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID‐19 infection. JAMA Cardiol 2021; 6: 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, Leith D, Shetye A, Ariff B, Bell R, Captur G, Coleman M, Goldring J, Gopalan D, Heightman M, Hillman T, Howard L, Jacobs M, Jeetley PS, Kanagaratnam P, Kon OM, Lamb LE, Manisty CH, Mathurdas P, Mayet J, Negus R, Patel N, Pierce I, Russell G, Wolff A, Xue H, Kellman P, Moon JC, Treibel TA, Cole GD, Fontana M. Patterns of myocardial injury in recovered troponin‐positive COVID‐19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021; 42: 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, Kovacs R, Kovan J, Rifat SF, Albrecht J, Bento AI, Albers L, Bernhardt D, Day C, Hecht S, Hipskind A, Mjaanes J, Olson D, Rooks YL, Somers EC, Tong MS, Wisinski J, Womack J, Esopenko C, Kratochvil CJ, Rink LD, Big Ten COVID‐19 Cardiac Registry Investigators , Simonetti O, Zareba K, Bhatti S, Addison D, Obarski T, Daoud E, Granger M, Smart S, Mayercin‐Johnson J, Subramanian P, Glitt J, Mitchell D, Chumita R, Mumford A, Garcia A, Garris L, Liu H, Hatfield B, Zhang Y, Boersma D, Schlader Z, Goodwin S, Port N, Zuidema T, Maldonado J, Eckhardt L, Reeder S, Baker M, Sebastianelli W, Wadlinger R, Millard R, Bosha P, Sunday H, Steele D, Chaudhry A, Smith S, Pfeiffer M, Kellerman J, Billy G, Krystofiak J, Eimer M, Big Ten COVID‐19 Cardiac Registry Investigators . Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS‐CoV‐2 infection: results from the big ten COVID‐19 cardiac registry. JAMA Cardiol 2021; 6: 1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaccone G, Tomasoni D, Italia L, Lombardi CM, Metra M. Myocardial involvement in COVID‐19: an interaction between comorbidities and heart failure with preserved ejection fraction. A further indication of the role of inflammation. Curr Heart Fail Rep 2021; 18: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freaney PM, Shah SJ, Khan SS. COVID‐19 and heart failure with preserved ejection fraction. JAMA 2020; 324: 1499–1500. [DOI] [PubMed] [Google Scholar]
- 32. Günster C, Busse R, Spoden M, Rombey T, Schillinger G, Hoffmann W, Weber‐Carstens S, Schuppert A, Karagiannidis C. 6‐month mortality and readmissions of hospitalized COVID‐19 patients: A nationwide cohort study of 8,679 patients in Germany. PLoS ONE 2021; 16: e0255427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty‐day outcomes among patients hospitalized with COVID‐19. Ann Intern Med 2021; 174: 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leijte WT, Wagemaker NMM, van Kraaij TDA, de Kruif MD, Mostard GJM, Leers MPG, Mostard RL, Buijs J, van Twist DJ. Sterfte en heropname na ziekenhuisopname met covid‐19 [Mortality and re‐admission after hospitalization with COVID‐19]. Ned TijdschrGeneeskd 2020; 164: D5423 [Dutch]. [PubMed] [Google Scholar]
- 35. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: Recurrent hospitalizations and mortality. Eur J Heart Fail 2018; 20: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Yancy CW, Fonarow GC, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail 2016; 4: 464–472. [DOI] [PubMed] [Google Scholar]
- 37. Bocchi EA, Lima IGCV, Biselli B, Salemi VMC, Ferreira SMA, Chizzola PR, Munhoz RT, Pessoa RS, Cardoso FAM, Bello MVO, Hajjar LA, Gomes BR. Worsening of heart failure by coronavirus disease 2019 is associated with high mortality. ESC Heart Fail 2021; 8: 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirkegaard C, Falcó‐Roget A, Sánchez‐Montalvá A, Valls Á, Clofent D, Campos‐Varela I, García‐García S, Leguízamo LM, Sellarès‐Nadal J, Eremiev S, Villamarín M, Marzo B, Almirante B, Len Ò. Incidence and risk factors for early readmission after hospitalization for SARS‐CoV‐2 infection: results from a retrospective cohort study. Infection 2022; 50: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sisti N, Valente S, Mandoli GE, Santoro C, Sciaccaluga C, Franchi F, Cameli P, Mondillo S, Cameli M. COVID‐19 in patients with heart failure: the new and the old epidemic. Postgrad Med J 2021; 97: 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goha A, Mezue K, Edwards P, Nunura F, Baugh D, Madu E. COVID‐19 and the heart: An update for clinicians. Clin Cardiol 2020; 43: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sokolski M, Trenson S, Sokolska JM, D'Amario D, Meyer P, Poku NK, Biering‐Sørensen T, Højbjerg Lassen MC, Skaarup KG, Barge‐Caballero E, Pouleur AC, Stolfo D, Sinagra G, Ablasser K, Muster V, Rainer PP, Wallner M, Chiodini A, Heiniger PS, Mikulicic F, Schwaiger J, Winnik S, Cakmak HA, Gaudenzi M, Mapelli M, Mattavelli I, Paul M, Cabac‐Pogorevici I, Bouleti C, Lilliu M, Minoia C, Dauw J, Costa J, Celik A, Mewton N, Montenegro CEL, Matsue Y, Loncar G, Marchel M, Bechlioulis A, Michalis L, Dörr M, Prihadi E, Schoenrath F, Messroghli DR, Mullens W, Lund LH, Rosano GMC, Ponikowski P, Ruschitzka F, Flammer AJ. Heart failure in COVID‐19: the multicentre, multinational PCHF‐COVICAV registry. ESC Heart Fail 2021; 8: 4955–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


