Abstract
Introduction/Aims
Due to muscular weakness and cardiopulmonary dysfunction, patients with muscular dystrophy (MD) have an increased risk of serious complications from coronavirus disease‐2019 (COVID‐19). Although vaccination is recommended, COVID‐19 vaccination safety and immunogenicity in these patients are unknown. We investigated reaction frequency, post‐vaccine antibody titers after two mRNA COVID‐19 vaccine doses, and clinical predictors of antibody response among patients with MD.
Methods
We recruited 171 inpatients with MD receiving two BNT162b2 mRNA COVID‐19 vaccine doses from seven hospitals. Blood samples were obtained from 53 inpatients before the first dose and 28 to 30 days after the second dose, and antibody titers were measured.
Results
Overall, 104 (60.8%) and 115 (67.6%) patients had side effects after the first and second doses, respectively. These were generally mild and self‐limited. Multiple logistic regression analysis showed that a bedridden state was associated with reduced side effects (odds ratio [OR] = 0.29; 95% confidence interval [CI], 0.12 to 0.71). The antibody titers of all participants changed from negative to positive after two vaccine doses. The geometric mean titer (GMT) of the inpatients was 239 (95% CI, 159.3 to 358.7). Older age (relative risk [RR] = 0.97; 95% CI, 0.95 to 0.99) and bedridden state (RR = 0.27; 95% CI, 0.14 to 0.51) were associated with a lower antibody titer. Patients with myotonic dystrophy type 1 (DM1) had a lower GMT than patients with other MDs (RR = 0.42; 95% CI, 0.21 to 0.85).
Discussion
COVID‐19 vaccination is safe and immunogenic in inpatients with MD. Patients with DM1 appear to have a poorer COVID‐19 antibody response than those with other MDs.
Keywords: ambulatory status, COVID‐19 vaccination, muscular dystrophy, myotonic dystrophy type 1, serum antibody titers
Abbreviations
- ADL
activities of daily living
- CI
confidence interval
- COVID‐19
coronavirus disease‐2019
- CTCAE
Common Terminology Criteria for Adverse Events
- DM1
myotonic dystrophy type 1
- DMD/BMD
Duchenne/Becker muscular dystrophy
- FCMD
Fukuyama congenital muscular dystrophy
- FSHD
facioscapulohumeral muscular dystrophy
- GMT
geometric mean titer
- IgG
immunoglobulin G
- LGMD
limb‐girdle muscular dystrophy
- MD
muscular dystrophy
- NPPV
noninvasive positive pressure ventilation
- OR
odds ratio
- RR
relative ratio
- SARS‐CoV‐2
severe acute respiratory syndrome–coronavirus‐2
- TPPV
tracheostomy positive pressure ventilation
1. INTRODUCTION
Patients with neuromuscular disorders, including muscular dystrophy (MD), have an increased risk of serious complications from respiratory infections due to respiratory muscle weakness or cardiopulmonary dysfunction. 1 The Japanese government started its coronavirus disease‐2019 (COVID‐19) vaccination program in February 2021 and subsequently prioritized high‐risk groups, including patients with MD. However, concerns about the newly adopted mRNA vaccine arose among patients with MD and their physicians. This is because details of safety and immune response remain unclear in the specific context of disease characteristics, such as the MD type, respiratory function, activities of daily living (ADL), underlying complications (such as heart failure), and treatment with steroids. 2 , 3 Previous studies on COVID‐19 vaccination in healthy populations reported mild to moderate side effects in up to 70% to 90% of participants. 4 , 5 , 6 In addition, infrequent but severe side effects, including myocarditis, have been reported. 7 Another potential concern for patients with MD is whether the vaccine can be safely injected into their atrophied muscles and an immune response successfully mounted.
Thus, knowledge is needed on the disease‐specific factors of MD that may be associated with vaccine safety and immunogenicity. In this study we aimed to provide information on the side effects and antibody response after two doses of mRNA COVID‐19 vaccines in patients with MD.
2. METHODS
2.1. Study participants
The participants of this study were inpatients with MD at seven hospitals belonging to the National Hospital Organization in northern, eastern, and western Japan. Patients with MD who were at least 20 years of age and wished to receive COVID‐19 vaccines were invited to participate in a vaccine safety study. In addition, inpatients with MD at the Osaka Toneyama Medical Center were invited to participate in an immunogenicity survey. The definition of MD included Duchenne/Becker MD (DMD/BMD), myotonic dystrophy type 1 (DM1), Fukuyama congenital MD (FCMD), facioscapulohumeral MD (FSHD), limb‐girdle MD (LGMD), oculopharyngeal MD, pathologically diagnosed distal myopathy with rimmed vacuoles, and congenital MD not classified more specifically. Patients with previous COVID‐19 were excluded. We also excluded those with a history of anaphylaxis due to vaccine components, or other vaccine contraindications.
All study participants provided written informed consent. Informed consent was obtained from parents or siblings if patients could not consent due to cognitive impairment. The study protocol was approved by the review board of the Osaka Toneyama Medical Center (No. TNH‐R‐2021009‐5).
2.2. Information collection
Before vaccination, the attending physician of each patient recorded information on sex; age; weight; comorbid diseases, such as asthma, diabetes mellitus, atopic dermatitis, pulmonary and cardiac diseases, and malignancy; medication, including steroid therapy; ambulatory status (ambulatory, wheelchair user, or bedridden); and use of mechanical ventilation (none, use of noninvasive positive pressure ventilation [NPPV], or tracheostomy positive pressure ventilation [TPPV]).
Body temperature, blood pressure, oxygen saturation, and pulse were recorded daily from the day of vaccination until 8 days after vaccination. During the same period, local and systemic reactions were recorded by nurses as grades 1 (mild), 2 (moderate), and 3 or higher (severe), based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. 8 Local reactions included redness, swelling, induration, warmth, itching, and pain. Systemic reactions included fever (axillary temperature at least 37.5°C), malaise, myalgia, headache, and rhinorrhea. We also collected information on any severe events, including admission to the intensive care unit or death.
2.3. Vaccine
Participants received two vaccinations, 21 days apart, of the BNT162b2 mRNA COVID‐19 vaccine (Pfizer). Attending physicians injected the vaccine into the deltoid muscle and observed patients for 30 minutes afterward for any acute reactions.
2.4. Measurement of antibody titer
Blood samples were collected before the first vaccination and 28 to 30 days after the second vaccination. The serum titers of antibodies to the spike protein were measured using anti–S‐protein‐receptor‐binding domain antibodies using an anti–SARS‐CoV‐2 S‐antibody test (Elecsys; Roche Diagnostics, Rotkreuz, Switzerland) on an automated platform (Cobas e801; Roche). Samples less than 0.8 units/mL (U/mL) were considered negative, whereas those at least 0.8 U/mL were considered positive according to the manufacturer's instructions. The lower limit of quantification was 0.4 U/mL.
2.5. Statistical analyses
Data are presented as percentage or as median with range. We evaluated the associations between several factors and vaccine side effects using the odds ratio (OR) and 95% confidence interval (CI) obtained from a logistic regression model. We subsequently constructed a multivariate model using factors with values <.10 in the univariate analysis to determine factors that were independently related to side effects in patients with MD. Because antibody titers usually follow a log‐normal distribution, we summarized these values using the geometric mean, that is, geometric mean titer (GMT). For data with a sample size of n, the geometric mean is defined as the nth root of the product of these “n” numbers. To identify the factors associated with immunogenicity, we used linear regression analyses with the log‐transformed antibody titer as the outcome. To facilitate interpretation of the results of the regression analysis, the estimated values were exponentially transformed into relative ratios (RRs). We divided the MD types into DM1 and other MDs, as DM1‐associated hypogammaglobulinemia is well known, 9 , 10 and poor outcomes from COVID‐19 in patients with DM1 has been reported. 11 To analyze the side effects, we performed an additional analysis excluding patients with cognitive impairment because they may not be able to describe their symptoms properly, which could result in an underestimation.
Two‐sided P < .05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Profiles of participants
A total of 171 inpatients with MD were enrolled in the study. Table 1 summarizes the baseline characteristics of the participants. The participants were inpatients in long‐term care units, and most of them had advanced MD, with low levels of ADL; only five patients were ambulatory and 82.5% of the participants were dependent on NPPV or TPPV. Most patients had DM1 (42.1%) or DMD/BMD (40.9%) (Table 1). Three patients with DMD, one with DM1 and one with LGMD, were receiving oral steroid therapy at the time of the study.
TABLE 1.
Baseline characteristics of the study participants
| Characteristics | Safety study (N = 171) | Immune response study (N = 53) |
|---|---|---|
| Sex: male, n (%) | 128 (74.9%) | 38 (71.7%) |
| Age (years), median (range) | 46.0 (20‐89) | 41.7 (20‐78) |
| Body weight (kg), median (range) | 42.3 (18‐81) | 38.6 (18‐81) |
| MD type, n (%) | ||
| Duchenne/Becker MD | 70 (40.9%) | 24 (45.3%) |
| Myotonic dystrophy type 1 | 72 (42.1%) | 20 (37.7%) |
| Fukuyama congenital MD | 7 (4.1%) | 4 (7.5%) |
| Facioscapulohumeral MD | 5 (2.8%) | 1 (1.9%) |
| Limb‐girdle MD | 10 (5.8%) | 1 (1.9%) |
| Other | 7 (4.1%) | 3 (5.7%) |
| Underlying disease, n (%) | ||
| Heart failure | 89 (52.0%) | 29 (54.7%) |
| Hypertension | 7 (4.1%) | 0 |
| Diabetes mellitus | 19 (11.1%) | 2 (3.8%) |
| Hyperlipidemia | 23 (13.5%) | 3 (5.7%) |
| Any allergic disease a | 5 (2.9%) | 3 (5.7%) |
| Ambulatory status, n (%) | ||
| Ambulatory | 5 (2.9%) | 2 (3.8%) |
| Wheelchair user | 80 (46.8%) | 10 (18.8%) |
| Bedridden | 86 (50.3%) | 41 (77.4%) |
| Respiratory status, n (%) | ||
| None | 30 (17.5%) | 2 (3.8%) |
| NPPV | 77 (45.0%) | 23 (43.4%) |
| TPPV | 64 (37.4%) | 28 (52.8%) |
|
Did he/she need informed consent by proxy (parents or siblings)? | ||
| Yes, n (%) | 39 (22.8%) | 16 (30.2%) |
Abbreviations: MD, muscular dystrophy; NPPV, noninvasive positive pressure ventilation; TPPV, tracheostomy positive pressure ventilation.
Any allergic diseases include atopic dermatitis and asthma.
3.2. Safety of the vaccine
No anaphylaxis occurred after either the first or the second vaccination. The local and systemic side effects of the first and second vaccinations are presented in Table 2. Fewer than 70% of patients experienced any side effects after either vaccination.
TABLE 2.
Summary of all side effects
| Category | First dose (N = 171) | Second dose (N = 171) |
|---|---|---|
| Symptoms | ||
| Any reactions | 104 (60.8%) | 115 (67.6%) |
| Local reactions | ||
| Skin reaction a | 17 (9.9%) | 39 (22.9%) |
| Warmth | 13 (7.6%) | 42 23.5%) |
| Itching | 5 (2.9%) | 12 (7.1%) |
| Pain | 84 (49.1%) | 85 (50.0%) |
| Systemic reactions | ||
| Fever (>37.5°C) | 5 (2.9%) | 8 (4.8%) |
| Malaise | 16 (9.4%) | 31 (18.2%) |
| Headache | 9 (5.3%) | 20 (11.8%) |
| Joint pain | 4 (2.3%) | 5 (2.5%) |
| Rhinorrhea | 3 (1.8%) | 6 (2.9%) |
| Rash | 1 (2.0%) | 0 (0%) |
Skin reactions include redness, swelling, and induration at the injection site.
Generally, the reactions were mild in severity and resolved within 1 to 3 days; no side effects were observed more than 7 days after vaccination. The most common symptom was local pain (Table 2). Other frequently reported local reactions were skin reactions and warmth at the injection site after the second dose. Regarding systemic reactions, a minority of patients reported malaise after the first and second doses, respectively. Headache was infrequent (Table 2). Severe side effects (of grade 3 or above according to the CTCAE) were reported in two and three patients after the first and second doses, respectively (Table 3). After excluding the patients with informed consent by proxy due to cognitive impairment, the results remained largely unchanged (Table 3).
TABLE 3.
Summary of side effects according to severity in all patients and after excluding those who needed IC by proxy
| Category | All patients, n (%) | Excluding those who needed IC by proxy a , n (%) |
|---|---|---|
| Any reaction after either dose | 138 (80.7%) | 109 (82.6%) |
| First dose | (N = 171) | (N = 132) |
| All | 104 (60.8%) | 85 (64.4%) |
| Grade 1 | 79 (46.2%) | 69 (52.3%) |
| Grade 2 | 20 (11.7%) | 13 (9.8%) |
| Grade ≥3 | 2 (1.2%) | 2 (1.5%) |
| Fever (>38.5°C) | 3 (1.8%) | 2 (1.5%) |
| Second dose | (N = 170) | (N = 132) |
| All | 115 (67.6%) | 93 (70.5%) |
| Grade 1 | 83 (48.8%) | 71 (53.8%) |
| Grade 2 | 27 (15.9%) | 20 (15.2%) |
| Grade ≥3 | 3 (1.8%) | 2 (1.5%) |
| Fever (>38.5°C) | 5 (2.9%) | 2 (1.5%) |
Severity was defined according to the Common Terminology Criteria for Adverse Events version 5.0.
Abbreviation: IC, informed consent (parents or siblings).
In the multiple logistic regression model adjusted for age, sex, and ambulatory status, bedridden patients were found to encounter significantly fewer side effects (Table 4).
TABLE 4.
Association between selected characteristics and side effects in patients with MD
| Category | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex (male vs female) | 0.35 (0.12‐1.06) | .064 | 0.53 (0.16‐1.70) | .283 |
| Age (increase by 1 year) | 1.03 (1.00‐1.06) | .037 | 1.02 (0.99‐1.06) | .195 |
| DM1 (vs other MDs) | 1.88 (0.83‐4.24) | .130 | ‐‐‐ | ‐‐‐ |
| Ambulatory status (bedridden vs other status b ) | 0.25 (0.11‐0.60) | .002 | 0.29 (0.12‐0.71) | .006 |
Abbreviations: CI, confidence interval; DM1, myotonic dystrophy type 1; MD, muscular dystrophy; OR, odds ratio.
Other status: wheelchair user or ambulatory.
Adjusted for age, sex, and ambulatory status.
Two deaths occurred during the study period. A 52‐year‐old woman with DM1 died of cardiac arrest 7 days after the first vaccination. She had a history of arrhythmias. The day after the vaccination, she developed a fever of 38.1°C and nausea, but the symptoms resolved 2 days later. Another woman with DM1, who was 61 years old and had a history of fatty liver, repeated ileus, colonic perforation, and peritonitis, developed peritonitis 10 days after the second vaccination and died of sepsis 11 days later. The patient did not note any side effects after the two vaccinations.
3.3. Immunogenicity
A total of 55 inpatients with MD at Osaka Toneyama Medical Center agreed to participate in the immunogenicity study. The participants' characteristics are presented in Table 1. Patients with advanced MD comprised the majority of the sample, with bedridden patients accounting for 77%, and 96% of the participants needed mechanical ventilation (Table 1). The anti–severe acute respiratory syndrome–coronavirus‐2 (SARS‐CoV‐2) S‐antibody levels of all participants were confirmed negative at baseline. Blood samples were collected from 53 patients after the second dose of the vaccine. Figure 1 shows the distribution of the antibody titers of the 53 participants. The GMT was 239 (95% confidence interval [CI], 159.3 to 358.7), and the antibody titer was positive in all patients. The GMT of patients with DM1 was 123.5 (95% CI, 59.1 to 258.0), and that of those with other MDs was 356.8 (95% CI, 227.4 to 559.7).
FIGURE 1.
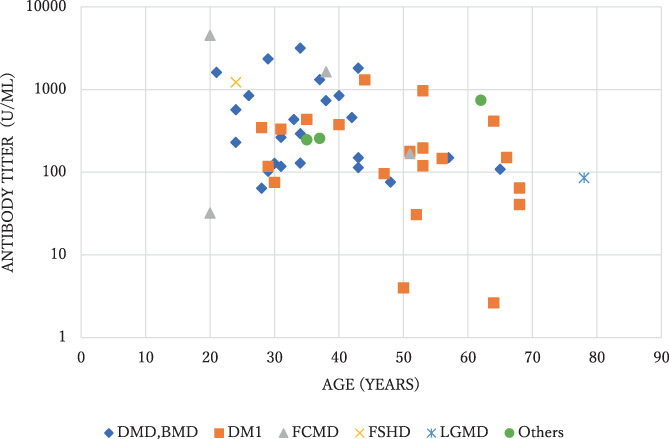
Scatterplot of antibody titers after the second COVID‐19 vaccination according to age. BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; DM1, myotonic dystrophy type 1; FCMD, Fukuyama congenital muscular dystrophy; FSHD, facioscapulohumeral muscular dystrophy; LGMD, limb‐girdle muscular dystrophy.
In the univariate analysis, age and weight were inversely associated with antibody response. Type of MD and ambulatory status also showed significant associations with antibody response (Table 5). We subsequently constructed a multivariate model that included age, type of MD, and ambulatory status. Weight was not included because obesity is a well‐known complication of DM1 12 and the type of MD and weight showed the strongest correlation among these factors (correlation coefficient = 0.51). Older age and a bedridden state were significantly associated with a low GMT. DM1 was also an independent factor associated with a lower antibody titer (Table 5).
TABLE 5.
Association between selected characteristics and immune response in patients with MD
| Category | Univariate analysis | Multivariate analysis b | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Male (vs female) | 0.77 (0.38‐1.56) | .463 | ‐‐‐ | ‐‐‐ |
| Age (increase by 1 year) | 0.96 (0.94‐0.99) | .005 | 0.97 (0.95‐0.99) | .004 |
| Weight (increase by 1 kg) | 0.95 (0.91‐0.98) | .007 | ‐‐‐ | ‐‐‐ |
| DM1 (vs other MDs) | 0.35 (0.15‐0.78) | .012 | 0.42 (0.21‐0.85) | .017 |
| Bedridden (vs others a ) | 0.32 (0.14‐0.72) | .007 | 0.27 (0.14‐0.51) | <.001 |
| TPPV (vs NPPV or none) | 1.31 (0.59‐2.93) | .499 | ‐‐‐ | ‐‐‐ |
| Underlying disease | ||||
| Heart failure | 1.73 (0.79‐3.78) | .163 | ‐‐‐ | ‐‐‐ |
| Diabetes mellitus | 0.15 (0.01‐3.90) | .249 | ‐‐‐ | ‐‐‐ |
| Allergic disease | 0.20 (0.01‐3.03) | .241 | ‐‐‐ | ‐‐‐ |
|
Side effect of COVID‐19 vaccine (any vs none) | ||||
| After first dose | 1.62 (0.70‐3.75) | .255 | ‐‐‐ | ‐‐‐ |
| After second dose | 1.38 (0.62‐3.04) | .422 | ‐‐‐ | ‐‐‐ |
| After either of the two doses | 1.13 (0.55‐2.31) | .740 | ‐‐‐ | ‐‐‐ |
Abbreviations: CI, confidence interval; MD, muscular dystrophy; NPPV, noninvasive positive pressure ventilation; RR, relative ratio; TPPV, tracheostomy positive pressure ventilation.
Others: wheelchair user or ambulatory.
Adjusted for age, type of MD, and ambulatory status.
4. DISCUSSION
The frequency of side effects in patients with MD in this study was lower than that previously reported in the general population. Research by the Japanese Ministry of Health, Labour and Welfare of the same vaccine under the same protocol, but focusing on a population of healthcare workers, showed more frequent side effects; approximately 90% of the participants had local pain. 5 That previous study also reported that some of the participants had headaches, malaise, and fever, which require sick leave, but we did not find such severe reactions in inpatients with MD. In this study, a bedridden state was associated with fewer reported side effects. Previously, we reported that pandemic influenza vaccination was associated with fewer adverse reactions in patients with DMD than in healthy controls; the rate of any local side effect was 32% in the patients vs 51% in controls, and the rate of any systemic side effect was 7% in the patients vs 29% in controls. 13 Some patients may not accurately describe their symptoms because of intellectual disability, which may result in an apparently lower incidence of side effects. However, the frequency of side effects did not change remarkably after excluding patients who could not provide consent for participation in the study because of cognitive impairment. Severe reactions occurred in less than 2% of the patients, and most reactions resolved without medical treatment.
In our study, older age was associated with a reduced immune response, in accordance with previously reported results. 6 , 14 In addition to age, we identified two factors specific to patients with MD, namely ambulatory status and DM1. Here, we should consider the involvement of immobility and the consequently decreased blood flow, which reduces the ability of mRNA vaccines to induce an immune response. Rich blood flow allows efficient processing of antigens. 15 , 16 Therefore, we hypothesize that the immobility was related to infrequent side effects and low immunogenicity.
DM1 was another significant risk factor for a blunted immune response. Patients with DM1 showed significantly lower antibody titers than those with other types of MD. Low levels of serum immunoglobulin G (IgG) and abnormalities in cellular and humoral immunity in patients with DM1 have been reported previously. 9 , 10 Furthermore, a study on immunity in response to the same vaccine in hematology and oncology patients reported a significant association between low IgG levels and low immunity. 17 The effects of hypogammaglobulinemia are unclear because IgG concentrations were not measured in our study. Future studies should address the possible association between serum levels of IgG and immune response in patients with DM1.
There were two deaths during the study period. The attending physicians assumed that the deaths were unlikely related to the vaccine because the patients had medical histories of other conditions, such as arrhythmia, repeated ileus, and peritonitis. We found one case report of a patient who developed bradyarrhythmia soon after BBIBP‐CorV (Sino‐pharm) vaccination and recovered, 18 but we found no case reports of peritonitis induced by COVID‐19 vaccination. In our study, both patients had advanced DM1. This result may indicate that careful observation is needed after COVID‐19 vaccination, especially in patients with advanced MD.
This study has some limitations. First, it did not include a control group. A preliminary study that included 14 patients with neuromuscular disease reported that immunity was comparable between the patients and controls, but the result was not statistically significant because of the sample size. 3 Second, in our study only a few patients were on long‐term steroid treatment, and we were unable to evaluate the influence of immunosuppressive therapy on vaccine efficacy. Third, all participants were inpatients, and most had advanced disease, which limits the generalizability of our findings. Further studies of outpatients with MD, including a greater number of ambulatory patients, and those receiving steroid therapy are needed.
In conclusion, we found that the mRNA COVID‐19 vaccine safely induced an immune response in patients with advanced MD. Patients with DM1 or low levels of physical activity, including those who are bedridden, should take all necessary precautions to prevent SARS‐CoV‐2 infection in addition to being vaccinated. Our results provide useful information for clinical decisions, further research, and future public health policy regarding vaccination in patients with MD.
AUTHOR CONTRIBUTIONS
Tomoko Saito: Conceptualization; data curation; investigation; methodology; project administration; validation; visualization; writing – original draft; writing – review and editing. Toshio Saito: Conceptualization; investigation; methodology; project administration; validation; writing – review and editing. Hiroya Hashimoto: Formal analysis; software; visualization. Katsuhisa Ogata: Investigation. Michio Kobayashi: Investigation. Hiroto Takada: Investigation. Satoshi Kuru: Investigation. Takashi Kimura: Investigation. Akinori Nakamura: Investigation. Tsuyoshi Matsumura: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – review and editing.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors are grateful to the patients and staff at the seven national hospitals for their understanding and cooperation throughout this study.
Saito T, Saito T, Hashimoto H, et al. Safety and immunogenicity of mRNA COVID‐19 vaccine in inpatients with muscular dystrophy. Muscle & Nerve. 2023;67(2):117‐123. doi: 10.1002/mus.27761
Funding information Ministry of Health, Labour, and Welfare of Japan, Grant/Award Number: 21FC1006
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request
REFERENCES
- 1. World Muscle Society . Covid‐19 and people with neuromuscular disorders: World Muscle Society position and advice. https://www.worldmusclesociety.org/file/ec4c36f3-8ce1-4762-981 e-a9b81823fd8a/WMS%20covid-19%20advice%2028-03-2020%201800.pdf. Accessed October 1, 2021.
- 2. Wasilewska E, Sobierajska‐Rek A, Śledzińska K, Małgorzewicz S, Jassem E, Wierzba J. Morbidity, clinical course and vaccination against SARS‐CoV‐2 virus in patients with Duchenne muscular dystrophy: a patient reported survey. Int J Environ Res Public Health. 2021;19:406. doi: 10.3390/ijerph19010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demonbreun AR, Velez MP, Saber R, et al. mRNA intramuscular vaccination produces a robust IgG antibody response in advanced neuromuscular disease. Neuromuscul Disord. 2022;32:33‐35. doi: 10.1016/j.nmd.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ministry of Health, Labour and Welfare. Health status survey after the first inoculation of the new corona vaccine. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_kenkoujoukyoutyousa.html. Accessed October 1, 2021.
- 6. Izumo T, Kuse N, Awano N, et al. Side effects and antibody titer transition of the BNT162b2 messenger ribonucleic acid coronavirus disease 2019 vaccine in Japan. Respir Investig. 2021;59:635‐642. doi: 10.1016/j.resinv.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu Mouch S, Roguin A, Hellou E, et al. Myocarditis following COVID‐19 mRNA vaccination. Vaccine. 2021;39:3790‐3793. doi: 10.1016/j.vaccine.2021.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Cancer Institute . Adverse events/CTCAE. https://ctep.cancer.gov/protocoldevelopment/adverse_effects.htm. Updated April 30, 2021. Accessed May 26, 2021.
- 9. Suzumura A, Yamada H, Matsuoka Y, Sobue I. Immunoglobulin abnormalities in patients with myotonic dystrophy. Acta Neurol Scand. 1986;74:132‐139. doi: 10.1111/j.1600-0404.1986.tb04639.x [DOI] [PubMed] [Google Scholar]
- 10. Walker GL, Mastaglia FL, Lane RJ, Karagol U. Immunological studies in myotonic dystrophy. Clin Exp Neurol. 1983;19:29‐36. [PubMed] [Google Scholar]
- 11. Dhont S, Callens R, Stevens D, et al. Myotonic dystrophy type 1 as a major risk factor for severe COVID‐19. Acta Neurol Belg. 2021;121:1761‐1765. doi: 10.1007/s13760-020-01514-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peric S, Bozovic I, Nisic T, et al. Body composition analysis in patients with myotonic dystrophy types 1 and 2. Neurol Sci. 2019;40:1035‐1040. doi: 10.1007/s10072-019-03763-0 [DOI] [PubMed] [Google Scholar]
- 13. Saito T, Ohfuji S, Matsumura T, et al. Safety of a pandemic influenza vaccine and the immune response in patients with Duchenne muscular dystrophy. Intern Med. 2015;54:1199‐1205. doi: 10.2169/internalmedicine.54.1186 [DOI] [PubMed] [Google Scholar]
- 14. Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID‐19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27:1861. doi: 10.1016/j.cmi.2021.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heinz FX, Stiasny K. Distinguishing features of current COVID‐19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benda M, Mutschlechner B, Ulmer H, et al. Serological SARS‐CoV‐2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID‐19 vaccine in haematological and oncological patients. Br J Haematol. 2021;195:523‐531. doi: 10.1111/bjh.17743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shams P, Ali J, Saadia S, Khan AH, Sultan FAT, Tai J. COVID‐19 BBIBP‐CorV vaccine and transient heart block‐‐‐a phenomenon by chance or a possible correlation‐‐‐a case report. Ann Med Surg. 2021;71:102956. doi: 10.1016/j.amsu.2021.102956 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request


