Abstract
The worldwide spreading of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has posed a serious threat to health, economic, environmental, and social aspects of human lives. Currently, there are no approved treatments that can effectively block the virus although several existing antimalarial and antiviral agents have been repurposed and allowed use during the pandemic under the emergency use authorization (EUA) status. This review gives an updated overview of the antiviral effects of phytochemicals including alkaloids, flavonoids, and terpenoids against the COVID‐19 virus and their mechanisms of action. Search for natural lead molecules against SARS‐CoV‐2 has been focusing on virtual screening and in vitro studies on phytochemicals that have shown great promise against other coronaviruses such as SARS‐CoV. Until now, there is limited data on in vivo investigations to examine the antiviral activity of plants in SARS‐CoV‐2‐infected animal models and the studies were performed using crude extracts. Further experimental and preclinical investigations on the in vivo effects of phytochemicals have to be performed to provide sufficient efficacy and safety data before clinical studies can be performed to develop them into COVID‐19 drugs. Phytochemicals are potential sources of new chemical leads for the development of safe and potent anti‐SARS‐CoV‐2 agents.
Keywords: antiviral effects, COVID‐19, in vitro studies, phytochemicals, SARS‐CoV‐2, virtual screening
1. INTRODUCTION
The extensive and rapid spreading of the coronavirus disease 2019 (COVID‐19) globally is due to the great transmissibility of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). This pandemic respiratory disease is a serious threat to the health, economic, environmental, and social aspects of human lives globally. The number of mortality has exceeded 6 million people from over 629 million cases recorded globally as of October 2022. Available, effective, and affordable prevention and treatment to control and eradicate the virus is urgently needed. Vaccination has successfully lowered the risk of people infected and spreading the virus. Vaccines have helped to decrease the risk of severe illnesses and deaths from COVID‐19 infections among people who are fully vaccinated by creating an antibody response. Although vaccination programs are ongoing globally and vaccination is expected to provide herd immunity to the virus in the near future, there is still an urgent need to search for efficient antivirals against COVID‐19 infection. More so due to the continued emergence of new and more virulent and contagious SARS‐CoV‐2 variants of concern (VOC). Research in various fields to battle the virus has been pursued including basic research to identify leads for the development of antiviral agents that can effectively fight the disease. Numerous antiviral strategies have been employed against the virus and already more than 400 clinical trials have been registered on ClinicalTrials.gov (Ahidjo et al., 2020).
The drug repurposing approach is a strategy that has been initially used to accelerate the discovery and approval of COVID‐19 drugs during the pandemic. Repurposing of known drugs has been used as an approach to substantially accelerate the deployment of new antiviral and antiinflammatory therapies for COVID‐19. The known drugs were assessed in COVID‐19 patients based on assumption that they have been tested in human patients and proved to be safe and effective against a variety of diseases related to novel coronavirus disease. Initially, the US Food and Drug Administration (FDA) and World Health Organization (WHO) allowed the emergency use of some investigational drugs such as the antimalarial agents, chloroquine, and hydroxychloroquine, to fight the COVID‐19 outbreak even before going through the formal approval process but based on in vitro studies against the virus and limited human studies. However, emergency use of these antimalarial drugs and antiviral agents under compassionate use protocols and a clinical trial has now been discontinued as clinical trials showed serious side effects, little benefit or little effect in reducing COVID‐19 death (Oren et al., 2020).
Among the earliest drug repurposing effort to search for antiviral agents against SARS‐CoV‐2 was on in vitro antiviral effects of FDA‐approved antiviral drugs (remdesivir, penciclovir, favipiravir, ribavirin nitazoxanide, chloroquine, and nafamostat) against SARS‐CoV‐2 clinical isolate (Wang, Cao, et al., 2020). They reported that chloroquine and remdesivir demonstrated strong antiviral effects against the virus. Currently, there is no full FDA approval for a specific antiviral treatment for COVID‐19 except for remdesivir which is indicated for COVID‐19 treatment requiring hospitalization or a mild‐to‐moderate infection and at high risk for progression to severe condition, including hospitalization or death. An emergency use authorization (EUA) has been issued by FDA for oral antiviral drugs, molnupiravir, and nirmatrelvir/ritonavir. The former is for the treatment of adult outpatients with mild‐to‐moderate infection who are at high risk for progression to severe condition, including hospitalization or death, while the latter is for treatment of adult and pediatric outpatients with the mild‐to‐moderate condition and who are at high risk for progression to hospitalization or death (Bergman et al., 2022; Rizk et al., 2021). Camostat mesylate, an orally available type II transmembrane serine protease inhibitor is currently undergoing clinical trials for development into a safe and potent COVID‐19 drug (Kitagawa et al., 2021).
Human coronaviruses have been in the research spotlight since the emergence of highly pathogenic Middle East respiratory syndrome coronavirus (MERS‐CoV) and SARS‐CoV in humans. Several studies have revealed that many plant‐based products possessed strong antiviral effects against coronaviruses (Remali & Aizat, 2021). Many reviews were on the antiviral effects of phytochemicals against these coronaviruses and their potential as anti‐SARS‐CoV‐2 agents, but information on their antiviral effects against SARS‐CoV‐2‐infected cells and animal models is limited (Merarchi et al., 2021; Wang & Yang, 2020; Wijayasinghe et al., 2021). Most of the recent reviews were discussing on the prospect of natural products or phytochemicals as potential antiviral agents against SARS‐CoV‐2 due to their reported broad‐spectrum antiviral activities, particularly against other coronaviruses (Boozari & Hosseinzadeh, 2021; Verma et al., 2020). For example, Wang and Yang (2020) reviewed the broad‐spectrum antiviral activities of several phytochemicals (emetine, homoharringtonine, cepharanthine, and carolacton) and other compounds (ivermectin, ebselen, remdesivir, and molnupiravir) against SARS‐CoV and MERS‐CoV. They suggested that some of the compounds show great promise as candidates for the development of COVID‐19 drugs. Search for natural antiviral agents against SARS‐CoV‐2 has been focusing on virtual screening of phytochemicals which have previously shown promising results against other coronaviruses (MERS‐CoV and SARS‐CoV) and in vitro antiviral assay of the phytochemicals against clinically isolated SARS‐‐2. In vivo studies on the antiviral effects of phytochemicals against SARS‐CoV‐2‐infected animal models are still limited.
The present review documents and discusses the in silico, in vitro, in vivo, and clinical studies on the inhibitory effects of phytochemicals including alkaloids, flavonoids, and terpenoids against SARS‐CoV‐2 and their mechanisms of action that have been reported in the literature since the emergence of the virus in December 2019.
2. SARS‐COV‐2 ENTRY MECHANISMS AND PATHOGENESIS
Like other coronavirus infections, COVID‐19 is a syndrome characterized by viral replication in concert with an inflammatory response of the host. After entering the respiratory tract, the virus initially binds to epithelial cells, replicates, and migrates down the airways and eventually inflaming the lungs by infecting the alveolar epithelial cells. Uncontrolled and rapid viral replication in the airways may induce a strong immune response, resulting in cytokine storm syndrome, and may lead to the rapid onset of acute respiratory distress syndrome (ARDS), respiratory failure, and possible progression to death (Shang et al., 2020). Human angiotensin‐converting enzyme 2 (ACE2) serves as the entry receptor for the virus, and human proteases serve as the entry activators. The virus enters the host cell through specific interaction of spike (S) glycoprotein and human angiotensin‐converting enzyme 2 (hACE2). The interaction is via the virus receptor‐binding domain (RBD) (S1 and S2 subunits) to form a binding complex that is activated proteolytically by human proteases. To activate the endocytic route in SARS‐CoV‐2, the S protein must be proteolytically processed. Three host proteases (cathepsin L, transmembrane protease serine protease 2 [TMPRSS2], and furin) have been identified as essential in the splitting of the S protein and activating the virus entry (Jackson et al., 2022). Viral genome RNA release into the host cytoplasm occurs after membrane fusion. The uncoated RNA, open reading frames (ORF), that is, ORF1a and ORF1b, translate into viral replicase proteins which are then cleaved into several non‐structural proteins (nsps), leading to the formation of a replication‐transcription complex (RTC) in a double‐membrane vesicle (DMV). Viral RNA synthesis occurs after the viral replicase complexes are translated and assembled. The RTC replicates and synthesizes a nested set of sub‐genomic RNAs encoding nucleoside‐sparing polypeptides and structural proteins. For viral genomic replication, the nsp12 derived from ORF1b forms the RNA‐dependent RNA polymerases (RdRp) (Sawicki & Sawicki, 2005). A DMV derived from the endoplasmic reticulum (ER) forms convoluted membranes upon integration. Sarcoplasmic reticulum (S), M protein, E protein, and membrane‐bound structural proteins are positioned into the ER and then move to the endoplasmic reticulum–Golgi intermediate compartment (ERGIC). When progeny genomes are encapsidated by the N protein, nucleocapsids are formed and they coalesce with membrane‐bound components to form virions via budding into the ERGIC. Finally, the vesicles containing new virions will merge with the plasma membrane for virus release (Masters, 2006). Figure 1 depicts the entry mechanisms and pathogenesis of SARS‐CoV‐2 in the host cells and possible target sites of some antiviral phytochemicals.
FIGURE 1.
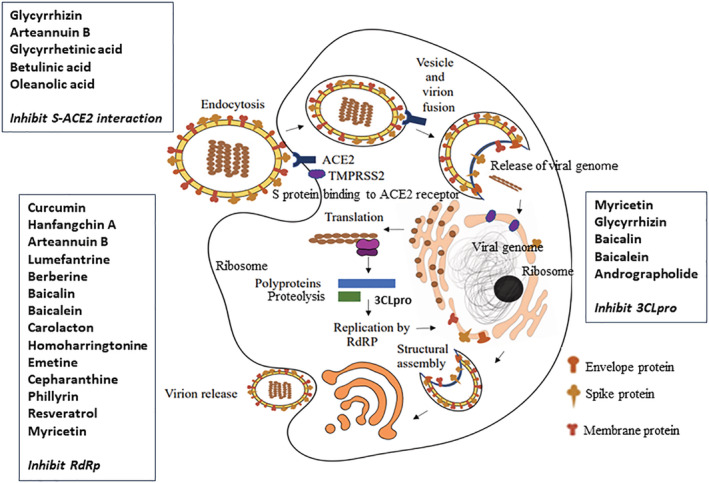
The entry mechanisms and pathogenesis of SARS‐CoV‐2 in host cell and possible target sites of antiviral phytochemicals. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3. MECHANISMS OF ANTIVIRAL ACTION OF PHYTOCHEMICALS AGAINST CORONAVIRUSES
The inhibition of coronaviruses by antiviral agents may occur at different steps of coronavirus replication, ranging from binding with ACE2 receptor, entry, and fusion to viral replication. It has been known that there are several molecular targets of compounds against the coronaviruses which include coronavirus S glycoprotein, 3C‐like protease (3CLpro) or main protease (Mpro), papain‐like protease (PLpro) and RdRp (Xian et al., 2020).
3.1. Inhibition of S glycoprotein binding to the ACE2 receptor
The S protein expressed on the viral surface is the primary antigen and target of vaccine development. Additionally, the S protein participates in host–cell attachment and facilitates the fusion of host cell and viral membranes during infection (Walls et al., 2020). The antimalarial drugs, chloroquine, and hydroxychloroquine, block SARS‐CoV‐2 entry into the host cells. They inhibit membrane fusion by inhibiting virus–host receptor binding, suppressing the glycosylation of cellular receptors, and increasing the endosomal pH (Mahmoud et al., 2020). Umifenovir, an anti‐influenza drug, can also inhibit membrane fusion by suppressing the virus–host receptor binding. Umifenovir has a strong in vitro antiviral effect against SARS‐CoV‐2, and a recent clinical study suggested that it was more effective in treating COVID‐19 than ritonavir and lopinavir (Huang, Yu, et al., 2021). Camostat mesylate, which has been approved for the treatment of postoperative reflux oesophagitis and pancreatitis, was able to inhibit TMPRSS2 activity, thereby preventing SARS‐CoV infection of host cells. Additionally, it protected mice in a pathogenic mouse model from serious infection with the virus. The entry of SARS‐CoV‐2 into human lung cells may also be inhibited by the compound, but its clinical efficacy is still being investigated (Kitagawa et al., 2021).
Numerous phytochemicals have been reported to be antiviral due to their interaction with the S protein. Eucalyptus globulus and Lonicera japonica extracts were reported to exhibit their antiviral effects against the coronaviruses by disrupting the envelope S glycoprotein (Wu et al., 2004). Isolated compounds from medicinal plants also demonstrated potent antiviral activity against coronaviruses. It was reported that the anthraquinone, emodin, a major compound of the Rhei Radix dose dependently inhibited the S protein‐ACE2 binding (Ho et al., 2007). Saikosaponins isolated from Bupleuri Radix were found to be capable of inhibiting the viral attachment process and disrupting viral S glycoproteins (Yuan et al., 2017). By targeting the S protein, bisbenzylisoquinoline alkaloids such as fangchinoline, cepharanthine, and tetrandrine isolated from Stephaniae tetrandrae significantly inhibited replication of human coronavirus, HCoVOC43 (Kim et al., 2020).
3.2. Inhibition of 3C‐like protease and papain‐like protease
Other targets for antiviral agents against coronavirus are 3CLpro and PLpro. It is well established that 3CLpro and PLpro are involved in the conversion of the polypeptide translation product from genomic RNA to structural and nsp components. These processes are critical for virus replication and packaging (Xian et al., 2020). Moreover, 3CLpro or Mpro is a vital part of the polyprotein and becomes the target of drug action due to its crucial activity, viral survival, and replication (Durai et al., 2015). On the other hand, the PLpro enzyme is important in regulating and processing polyproteins into individual proteins which are important for viral replication. This enzyme is also important to help viruses in avoiding immune cells (Ratia et al., 2014). Due to their importance for viral replication and controlling the host cells, these proteins have become targets of antiviral action. Replication inhibitors include remdesivir, favipiravir, ribavirin, molnupiravir, ritonavir, and lopinavir. While the four earlier mentioned inhibitors target RdRp, ritonavir and lopinavir suppress MERS‐CoV and SARS‐CoV replication by inhibiting 3CLpro. A randomized, controlled clinical trial evaluating the combination of lopinavir and ritonavir in patients with COVID‐19 found no benefit. The WHO discontinued the usage of these drugs for COVID‐19 infection due to their lack of clinical benefit and adverse pharmacodynamics. Ivermectin, an FDA‐approved antiparasitic agent, was revealed to be able to suppress replication of a clinical isolate of SARS‐CoV‐2 in vitro, most likely by inhibiting IMP/1/mediated nuclear import of viral proteins (Caly et al., 2020).
Several studies have documented promising natural products that can combat coronaviruses by targeting PLpro and 3CLpro. For instance, flavanones, chalcones, and coumarins isolated from the radix of Angelicae sinensis demonstrated antiviral activity by significantly inhibiting 3CLpro activity (Xian et al., 2020). Emodin and hesperetin, two compounds isolated from Isatis indigotica, significantly inhibited the 3CLpro activity in a dose‐dependent pattern (Lin et al., 2005). Another study reported the antiviral activity of other quercetin derivatives including quercetin‐3‐b‐galactoside against SARS‐CoV by specifically inhibiting its 3CLpro (Chen et al., 2006). Among the nine diarylheptanoids isolated from Alnus japonica, hirsutenone showed the strongest anti‐SAR‐CoV activity by interfering with and specifically targeting PLpro. Structure–activity analysis indicated that the unsaturated carbonyl and catechol moieties of hirsutenone were determined to be the critical requirement for the inhibition of SARS‐CoV cysteine protease (Park et al., 2012). Molecular docking and molecular dynamics (MD) study revealed that tomatidine and patchouli alcohol could dock into the active sites of SARS‐CoV‐2 3CLpro, PLpro, and nsp15, and demonstrated excellent pharmacokinetic properties with good bioavailability, absorption, and devoid of toxicity in in silico absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies (Zrieq et al., 2021).
3.3. Inhibition of RNA‐dependent RNA polymerases
Another antiviral target is RdRp or RNA replicases. It is well established that this protein is a significant protease capable of catalyzing RNA replication from an RNA template. Additionally, this protein is a critical component of all RNA‐containing virus genomes that lack a DNA stage. The RNA replicase is found in a diverse array of RNA viruses. It participates in a variety of viral life cycle events, including replication of the genome, synthesis of mRNA, and RNA recombination (Ganeshpurkar et al., 2019). Due to its important role in the development of the virus, RdRp has become the promising antiviral target of action against coronaviruses. Through inhibition of RdRp, molnupiravir has been shown to increase the frequency of viral RNA mutations and impair SARS‐CoV‐2 replication in animal models and humans. Remdesivir demonstrated in vitro and in vivo antiviral effects against SARS‐CoV‐2 by targeting RNA‐RdRp (Agrawal & Goyal, 2022). Several studies have reported that many natural compounds showed their antiviral activities by targeting RdRp. Lung et al. (2020) evaluated the antiviral effects of theaflavin (TF) against several coronaviruses including SARS‐CoV‐2 using in silico study which targeted RNA replicases. It was demonstrated that the idock score for TF was low in the catalytic pocket of RdRp of all coronaviruses, suggesting that it inhibited SARS‐CoV‐2 replication by suppressing RdRp.
3.4. Inhibition of the nucleocapsid (N) protein
Host‐virus interaction studies have indicated that 3CLpro, PLpro, RdRp and S proteins are the major drug targets for coronaviruses including SARS‐CoV‐2. However, there are compounds with antiviral activity that can also target the nucleocapsid (N) protein. The N protein is known to be associated with RTCs. The binding of the N protein to the gRNA is necessary for the virus to encapsulate its genetic material within coronavirus particles. Additionally, the N protein is a major component of the RNA complex in virion cores, and it plays a critical role in virus particle formation via interactions with other proteins such as M protein, gRNA, and other N molecules. Besides encapsulating the viral genome, the N protein also plays a critical role in viral assembly, replication, and release of virus particles in the case of coronavirus (Cong et al., 2017). Due to these roles N protein has been identified as a significant target of action for natural antiviral compounds. Similar to another target of the action, several natural products have also shown their potency in interfering with this protein. For instance, resveratrol besides exhibiting antiinflammatory effects by the decreasing production of nitric oxide (NO) also suppressed nucleocapsid (N) protein expression in MERS‐CoV, which is necessary for its replication. The results showed that resveratrol at concentrations of 250–125 μM remarkedly and dose‐dependently inhibited MERS nucleocapsid protein translation (Lin et al., 2017).
4. SEARCH FOR NATURAL ANTIVIRAL AGENTS AGAINST SARS‐COV‐2
Currently, there are no approved treatments that can effectively block the virus although several antiviral agents have been given EUA status during the pandemic. There is still an inadequate of well‐tolerated and efficacious antiviral agents for the treatment of COVID‐19 patients. Several natural products such as silvestrol, scutellarein, saikosaponin B2, tryptanthrin, psoralidin, isobavachalcone, myricetin, quercetin, caffeic acid, and lectins such as griffithsin were reported to inhibit the coronaviruses SARS or MERS in humans. It was reported that the most promising coronavirus inhibitors were mostly polyphenols containing a conjugated fused ring structure (Mani et al., 2020). Some natural products (7‐methoxycryptopleurine, lycorine, ouabain, homoharringtonine, tylophorine, and silvestrol) which are the main components of dietary supplements, exhibited significant inhibitory activity against coronaviruses at nanomolar concentrations. They have the potential to be used as a model for antiviral drug design or as chemical leads for antiviral drug development (Islam et al., 2020). Strong antiviral effects of polyphenols (kazinol, kazinol A, kazinol B, papyriflavonol A, kazinol F, broussochalcone A, broussochalcone B, 4‐hydroxyisolonchocarpin, 3′‐[3‐methylbut‐2‐enyl]‐3′,4,7‐trihydroxyflavane, and broussoflavan A) isolated from Broussonetia papyrifera against 3CLpro and PLpro of coronavirus have been reported. The polyphenols exhibited stronger inhibition against PLpro and among them, papyriflavonol A exhibited the strongest inhibition against PLpro, with an IC50 value of 3.7 μM. The two phenyl groups of the molecules may be responsible for the virus protease's proteolytic activity being inhibited (Park et al., 2017). Antiviral activity of many polyphenols such as resveratrol, curcumin, epigallocatechin gallate, indigo, betulinic acid, aloe‐emodine, quinomethyl triterpenoids, luteolin, quercetin or gallates has been determined in in silico and in vitro studies using cell‐free polyphenol–protein interaction and cell‐based virus infection and some of them have great promise as leads for the development of COVID‐19 drugs (Chojnacka et al., 2020; Horne & Vohl, 2020; Mehany et al., 2021; Paraiso et al., 2020). However, the in silico and in vitro screening methods used to test the effectiveness of the polyphenols against the human viral disease were mostly not validated. Although it has been suggested that polyphenols control the COVID‐19 virus by inhibiting ACE2 in vivo, their effects on SARS‐CoV‐2‐infected animal models have not been much investigated.
4.1. In silico study on antiviral effect of phytochemicals against SARS‐CoV‐2
Table 1. shows the antiviral effects of various phytochemicals against protein targets of SARS‐CoV‐2 by using in silico methods. Most of the compounds have a high negative binding energy score, indicating better stability of the compound‐protein complex. The lower the energy the higher the stability of the complex. The high stability of the complexes implied that the compounds have high potential to be developed into effective antiviral drugs against the coronavirus. Virtual screening of 10,870 ligands which were made up of different types of functional foods (carbohydrates, vitamins, fatty acids, flavonoids, phospholipids, nordihydroguaiaretic acid, betulinic acid, nootkatone, curcumin, tincturoid, β‐pinene, β‐sitosterol, and their isomers/analogs/derivatives) revealed that 60 compounds have good docking affinity for SARS‐CoV‐2 Mpro. Quercetrin and its isomers/analogs/derivatives have the best affinity for the target protein. According to MD simulations, quercetrin‐protease and quercetrin‐analog‐protease exhibited remarkable stability (Wang, Zhang, et al., 2020). In another molecular docking study, quercetin exhibited low score values for S docking (−6.48 kcal/mol) and Mpro docking (−6.23 kcal/mol). The binding energies were found to be lower than that of the reference standard, hydroxychloroquine (Kandeil et al., 2021). Based on the results of these studies, quercetrin has the potential for further development into a COVID‐19 drug. An integrated molecular modeling approach was carried out to show that oleanolic acid, ursolic acid, and carvacrol demonstrated strong inhibition against SARS‐CoV‐2 3CLpro. During 50 ns of MD simulations, the three compounds were found to bind to the protease. The compounds were found to be stable with favorable energies using molecular mechanics Poisson–Boltzmann surface area (MM‐PBSA) free energy calculations, resulting in robust interaction with the receptor site. The molecules have passed Lipinski's rule of five as well as the Absorption, Distribution, Metabolism and Excretion, (ADME) properties. The results indicate that these natural products could be potential inhibitors of viral replication (Kumar et al., 2021).
TABLE 1.
In silico antiviral activity of various phytochemicals against SARS‐CoV‐2 protein targets
| Name of the compound | Source | Protein target | Docking binding energy (kcal/Mol) | References |
|---|---|---|---|---|
| Fisetin, quercetin, and kampferol | Medicinal plants | S protein | −8.5, −8.5, −7.4 | Pandey et al. (2021) |
| Saikosaponins U and V | Chinese herbs | S protein | −7.272, −8.358 | Sinha et al. (2021) |
| Stilbenoid analogs and resveratrol | Medicinal plants | S protein | > −7.0 | Wahedi et al. (2021) |
| Artelinic acid, artesunate, artemisinin, and artenimol | Artemisia annua | S protein | −7.1 | Sehailia and Chemat (2021), Cao et al. (2020) |
| Phycocyanobilin, Phycoerythrobilin, Phycourobilin, and folic acid | Arthrospira | S protein |
Autodock Vina; − 7.25, −7.45, −7.1, − 6.95 Swissdock; −9.355, −9.37, −9.285, −10.35 |
Petit et al. (2021) |
| Ichangin, β‐amyrin, and deacetylnomilin | Various plants | S protein 3CLpro | −8.40, − 8.79, −8.35 | Giofrè et al. (2021) |
| Coumarylquinic acid and stigmasterol | Various herbs | S protein 3CLpro | −7.349, −7.195 | Kamaz et al. (2020) |
| Quercetrin and quercetin | Functional foods |
3CLpro S protein 3CLpro |
Not available −6.48, −6.23 |
Wang and Yang (2020), Kandeil et al. (2021) |
| Curcumin | Curcuma longa | S protein 3CLpro | −7.02, −7.28 | Kandeil et al. (2021) |
| Andrographolide | Andrographis paniculata | 3CLpro | −9.72 | Shi et al. (2020) |
| Oleanolic acid, ursolic acid, and carvacrol | Various plants | 3CLpro | Not available | Kumar et al. (2021) |
| Glycyrrhizic acid and liquiritigenin, Glabridin | Glycyrrhiza glabra | 3CLpro | −8.0 | Srivastava et al. (2022) |
| Hesperidin | Flavone glycoside | 3CLpro | −8.99 | Basu et al. (2020) |
| Momordiciode F2 and momordicine | Momordica charantia | 3CLpro | −41.1, −43.4 | Ogidigo et al. (2022) |
| Pavetannin C1 and tenufolin | Cinnamon | 3CLpro | −7.3, −8.8 | Prasanth et al. (2021) |
| Betulinic acid, quercetin, kaempferol, lignan, sugiol, coumaroyltyramine, cryptotanshinone, moupinamide, desmethoxyreserpine, dihomo‐c‐linolenic acid, dihydrotanshinone I, N‐cis‐feruloyltyramine, and tanshinone IIa | TCM | 3CLpro | Not available | Zhang et al. (2020) |
| Ellagic acid, p‐coumaric acid, kaempferol, and quercetin | Plants source | 3CLpro | −7.5, −5.6 ‐7.8, −7.4 | Shaldam et al. (2021) |
| Phlorotannins | Ecklonia cava | 3CLpro | −13.5, −15.8, −12.9 | Gentile et al. (2020) |
| (4Z,6E)‐1,5‐Dihydroxy‐1,7‐bis(4‐hydroxyphenyl)hepta‐4,6‐dien‐3‐one and (1 E,6 E)‐1,2,6,7‐Tetrahydroxy‐1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)hepta‐1,6‐diene‐3,5‐dione) | C. longa | 3CLpro | −9.08, −8.07 | Gupta et al. (2021) |
| Violanthin, acetoside, rutin, chebulagic acid, acanthoside, andrographidine, syrigaresinol, myricetin, gingerenone‐A, tinosporinone, luteolin 7‐rutinoside, geraniol, nootkatone, γ‐sitosterol, and asarianin | Kabasura kudineer | 3CLpro | −153.06, −134.6, −133.06, −124.3, −120.03, −122.21, −114.9, −101.8, −99.96, −93.9, −83.42, −62.87, −62.4, −79.94, −81.94 | Vincent et al. (2020) |
| Epigallocatechin gallate, epicatechin gallate, and gallocatechin‐3‐gallate | Camellia sinensis | 3CLpro | −7.6, −7.2, −7.1 | Ghosh, Chakraborty, et al. (2020) |
| Taxifolin, pectolinarigenin, tangeretin, gardenin B, and hispidulin | Kickxia aegyptiaca Citrus reticulata Anastatica hierochuntica | 3CLpro | −6.61 to −5.74 | Al‐Karmalawy et al. (2021) |
| Gallocatechin gallate and amentoflavone | Various plants | 3CLpro, PLpro | −8.8, −9.4 | Swargiary et al. (2022) |
| Piperine | Medicinal plants | S protein, 3CLpro, PLpro | −5.93, −7.8, −6.17 | Ghosh, Palbag, and Ghosh (2020) |
| Ursolic acid and zingiberene | Medicinal plants | S protein, 3CLpro, PLpro | −5.82, −6.72, −5.76, −5.4, −6.65, −4.99 | Ghosh, Palbag, and Ghosh (2020) |
| Curcumin | Medicinal plants | S protein, PLpro, 3CLpro | −6.99, −7.31, −5.64 | Ghosh, Palbag, and Ghosh (2020) |
| Withacoagin, ashwagandhanolide, withaferin, withanolide‐A, withanolide‐B, withanone, withanoside‐IV, withanoside‐V, 12‐deoxywithastramonolide, and 27‐hydroxywithanone | Withania somnifera, | S protein, 3CLpro, RdRp | −8.8, −8.8, −8.5, −8.3, −8.8, −5.6, −6.1, −8.1, −8.6 | Borse et al. (2021) |
| Syringin, berberine, columbamine, columbin, 20‐hydroxy ecdysone, menisperine, tinocordiside, tinosporide, and tinosporaside | Tinospora cordifolia | S protein, 3CLpro, RdRp | −5.3, −6.3, −5.9, −7.9, −6.4, −6.9, −8.1, −7.9, −4.3 | Borse et al. (2021) |
| Asparanin‐A, asparagamine‐A, shatavarin‐I, shatavarin‐IV, shatavarin‐IX, shatavarin‐VI, shatavarin‐VII, shatavarin‐X, isoagatharesinol, muzanzagenin, and rutin, | Asparagus racemosus | S protein, 3CLpro, RdRp | −7.1, −6.3, −1.1, −2.7, −0.6, −4.9, −6.6, −0.4, −5.8, −7.6, −5.3 | Borse et al. (2021) |
| phycocyanobilin | Cyanobacteria | 3CLpro, PLpro | −8.6, −9.8 | Pendyala et al. (2021) |
| TF2‐ TF3 | Green tea and black tea | 3CLpro | −9.8 ‐10.0 | Mhatre et al. (2021) |
| TF2 TF | Black tea | 3CLpro | −48.02 ‐11.21 | Chauhan et al. (2022) |
| TF | TMC | RdRp | −8.8 | Lung et al. (2020) |
Abbreviations: 3CLpro, 3C‐like protease; PLpro, papain‐like protease; RdRp, RNA‐dependent RNA polymerases; S protien, spike protien; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TF, theaflavin; TF2, theaflavin‐3‐gallate; TF3, Theaflavin‐3,3′ ‐digallate; TMC, traditional Chinese medicine.
In MD simulations of 14 phytochemicals (limonoids and terpenoids) against SARS‐CoV‐2 S and Mpro targets, ichangin, β‐amyrin, deacetylnomilin, and nomilin showed good interaction energies with SARS‐CoV‐2 3CLpro. The phytochemicals, on the other hand, were ineffective at interfering with the virus binding to the ACE2 receptor. In silico assessments of binding energies and docking scores, as well as projected pharmacokinetic profiles, suggested that the compounds could be used as protease inhibitors of SARS‐CoV‐2 (Giofrè et al., 2021). In another molecular docking study, glycyrrhizic acid, liquiritigenin, and glabridin from Glycyrrhiza glabra inhibited the enzyme Mpro active sites strongly by exhibiting strong noncovalent binding with some of the amino acid residues (His41, Gly143, Cys 145, Cys44, Thr45 and pro168, Thr25, Asn142, Gln189, Glu 166, and Met49). According to the ADMET attributes prediction, the compounds are non‐carcinogenic and non‐toxic with good solubility, permeation, and absorption properties. The findings revealed that the three constituents of G. glabra have the potential to be developed into effective inhibitors for SARS‐CoV2 Mpro. However, among the three compounds, glycyrrhizic acid has the highest binding affinity and the best ADMET properties (Srivastava et al., 2022).
Coumatylquinic acid and stigmasterol were revealed as strong inhibitors of SARS‐COV‐2 after an in silico screening of 13 chemicals present in herbs recognized for their antioxidants and antiviral activities. Both the S protein and Mpro of SARS‐COV‐2 were significantly complexed by these drugs. Coumarylquinic acid formed a strong hydrogen connection with both the S protein and Mpro, but stigmasterol only had a strong bond with the S protein (Kamaz et al., 2020). A molecular docking study on the five anthraquinones and flavonoids on the binding of S protein of SARS‐CoV‐2 with ACE2 molecules demonstrated that hesperidin, emodin, and chrysin have S protein inhibiting efficacy. Hesperidin may bind noncompetitively to the binding structures of the ACE2 protein and the SARS‐CoV2 S protein. Molecular docking and MD studies confirmed that hesperidin destabilized the binding of hACE2 receptors with the virus S protein (Basu et al., 2020). A molecular docking experiment on andrographolide from Andrographis paniculata showed that the diterpenoid entered the catalytic pockets of SARS‐CoV‐2 and exhibited a binding affinity of estimated −9.72 kcal/mol (ΔG) and the distance between Cys145 and the acceptor carbon of Michael reaction (C12 of andrographolide) was 3.7 Å. The results suggest andrographolide‐mediated inhibition and covalent linkage of Mpro of SARS‐CoV‐2 (Shi et al., 2020).
In silico screening, molecular dynamics (MD) simulation, and ADME properties prediction on inhibition of Mpro of SARS‐CoV‐2 were conducted on 86 compounds from Azadirachta indica and Momordica charantia. Of all compounds studied, momordicine, momordiciode F2, margolonone, deacetylnimninene, 17‐hydroxyazadiradione and nimbandiol demonstrated stable and significant binding with SARS‐CoV‐2 Mpro via the active site amino acid residues, similar to the reference drugs (remdesivir, hydroxychloroquine, and ribavirin). Momordiciode F2 and momordicine demonstrated strong inhibition potential with molecular mechanics with generalized Born and surface area (MM/GBSA)‐binding energies of −41.1 and −43.4 kcal/mol, respectively, against Mpro of SARS‐CoV‐2 when compared with FDA reference anti‐viral drugs. According to per‐residue analysis, solvent‐accessible surface area, and root mean square deviation, the compounds bound to key amino acid residues of the active sites of the enzyme and displayed good system stability (Ogidigo et al., 2022). In a more recent in silico study of the antiviral activity of 16 compounds of M. charantia against SARS‐CoV‐2, momordicoside B was found to have the lowest binding energy compared to other compounds. Based on Lipinski's rule of five, kuguaglycoside A and cucurbitadienol demonstrated better profiles for drug‐like properties. The result indicates that these molecules have the potential to be developed into candidates against SARS‐CoV‐2 (Evary et al., 2022). A molecular docking study of 48 isolates from Cinnamon against SARS‐CoV‐2 Mpro revealed that nine compounds were highly active inhibitors. ADME and Lipinski's rule of five analyses and further MD simulations identified pavetannin C1 and tenufolin as potential lead molecules for further development into antiviral agents (Prasanth et al., 2021).
Virtual screening approaches were carried out to investigate the interaction of 32 phytochemicals with SARS‐CoV‐2 3CLpro and PLpro. Their antiviral activity was studied using molecular docking and MD simulation, as well as ADME and drug similarity. Out of the compounds investigated, amentoflavone and gallocatechin gallate possessed promising drug‐likeness properties and exhibited the best binding affinity to PLpro and 3CLpro. Other compounds showing strong affinity were kazinol‐A, savinin, and theaflavin‐3,3‐digallate (TF3). The study suggested that gallocatechin gallate and amentoflavone were potent inhibitors of PLpro and 3CLpro proteins and could be developed as effective COVID‐19 drug (Swargiary et al., 2022). The inhibitory effect of 10 natural flavonoids (fisetin, quercetin, and kampferol) and non‐flavonoids on the virus S protein was studied using molecular docking and found to be comparable to hydroxychloroquine. According to MD simulations and energy landscape studies, the compounds bound with the ACE2‐S protein complex with low binding free energy, indicating that they may have the ability to inhibit the ACE2‐S protein complex. Because of their good pharmacokinetic qualities, as demonstrated by ADME studies, these compounds can be considered as good candidates for development into anti‐SARS‐CoV‐2 drugs (Pandey et al., 2021).
The efficacy of oleanane derivatives, saikosaponins, found in Chinese herbs against several binding proteins of SARS‐CoV‐2 was predicted using molecular docking simulations, binding energy and interaction investigations. Of all the compounds evaluated, saikosaponins U and V exhibited strong inhibition on nsp15, which is involved in the binding of virus S glycoprotein with ACE2 and RNA replication (Sinha et al., 2021). Molecular docking studies carried out on curcumin against both S and Mpro receptors of SARS‐CoV‐2 revealed that for S docking, the binding score of curcumin at −7.02 kcal/mol was found to be better than that of hydroxychloroquine (− 6.60 kcal/mol). However, the binding score of curcumin for Mpro docking was −7.28 kcal/mol which were higher than that of hydroxychloroquine (−7.05 kcal/mol) (Kandeil et al., 2021). Molecular docking, MD simulations and scoring functions analysis of 267 compounds from Curcuma longa to determine their interactions with Mpro of SARS‐CoV‐2 Mpro revealed only two compounds, that is, (4Z,6E)‐1,5‐dihydroxy‐1,7‐bis(4‐hydroxyphenyl)hepta‐4,6‐dien‐3‐one and (1E,6E)‐1,2,6,7‐tetrahydroxy‐1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)hepta‐1,6‐diene‐3,5‐dione showed strong inhibition to the Mpro binding. The binding score for both compounds against Mpro protein were comparable to standard Mpro inhibitors, shikonin and lopinavir. Based on free energy landscape, main component analysis and protein‐ligand energy calculation, both compounds exhibited stronger inhibition against the binding to Mpro catalytic centre than lopinavir. The findings suggested that these phytochemicals could be used to produce antiviral agents against the COVID‐19 virus (Gupta et al., 2021).
A total of 145 compounds from Kabasura kudineer, an Ayurvedic poly‐herbal formulation, were submitted to a bioinformatic approach for therapeutic repurposing to search for strong inhibitors of the SARS‐CoV‐2 3CLpro. This formulation has been recommended for COVID‐19 treatment since it is effective against fever, cough, shortness of breath and sore throat, which are symptoms comparable to SARS‐CoV‐2. Molecular docking showed that violanthin, andrographidine, acetoside, chebulagic acid, acanthoside, tinosporinone, syrigaresinol, rutin myricetin, geraniol, gingerenone‐A, luteolin 7‐rutinoside, nootkatone, γ‐sitosterol and asarianin exhibited low binding energies indicating that they could inhibit COVID‐19 virus by exhibiting lower binding energy score compared to synthetic drugs. These compounds have a higher negative binding energy score, implying that they could be developed as effective antiviral medicines against the coronavirus (Vincent et al., 2020). Six active compounds chosen from Ayurvedic medicinal plant resources were evaluated for their antiviral activity using molecular docking and ADME analysis with the three viral proteins, PLpro, 3CLpro and S proteins. Piperine was thought to interact with the virus's 3CLpro and S proteins, preventing it from entering and growing within the host body. Ursolic acid and zingiberene might be able to halt viral replication by interacting with the 3CLpro protein. Curcumin might suppress viral efficacy against host immunity and growth inside the host cell by inhibiting the 3CLpro and PLpro proteins (Ghosh, Palbag, & Ghosh, 2020).
In a virtual screening of eight polyphenols from green tea (Camellia sinensis), known for their antiviral activity against many RNA viruses, three polyphenols (gallocatechin‐3‐gallate, epicatechingallate and epigallocatechin gallate) markedly inhibited one or both catalytic residues (His41 and Cys145) of SARS‐CoV‐2 Mpro. The binding modes and binding affinities between the polyphenols were determined by molecular docking studies. The three polyphenol‐Mpro complexes were very stable, have fewer conformational changes, and have a similar degree of compactness, according to MD simulations. Estimation of the total number of intermolecular H‐bonds and MM/GBSA analysis were used to confirm the stability of these three polyphenol‐Mpro complexes. These polyphenols exhibit outstanding drug‐like characteristics based on pharmacokinetic studies. The results of the study suggested that the three drugs are suitable COVID‐19 therapy options (Ghosh, Chakraborty, et al., 2020). Molecular docking study on artemisinin and its derivatives resulted in better Vina docking score than hydroxychloroquine. Artelinic acid (−7.1 kcal/mol) demonstrated lower score than hydroxychloroquine (−5.5 kcal/mol). Artemisinin, artenimol and artesunate exhibited interactions with Lys31 and Lys353 binding of the S protein. Furthermore, MD studies validated the generated complexes' capacity to engage and remain stable in the active sites of their respective targets. Artemisinin and its derivatives have potential for repurposing as antiviral agents for the treatment of COVID‐19 (Sehailia & Chemat, 2021).
A total of 31 constituents of Withania somnifera, Asparagus racemosus, and Tinospora cordifolia extracts were investigated for their anti‐SARS‐CoV‐2 and immunomodulatory potential using in silico network pharmacology model and molecular docking. Furthermore, W. somnifera extract contained withacoagin, ashwagandhanolide, withanolide‐A, withaferin, withanolide‐B, withanoside‐IV, withanone, withanoside‐V, 12‐deoxywithastramonolide, and 27‐hydroxywithanone. T. cordifolia extract revealed the presence of magnoflorine, syringin, berberine, columbamine, columbin, 20‐hydroxy ecdysone, menisperine, tinocordiside, tinosporide, and tinosporaside while asparanin‐A, asparagamine‐A, shatavarin‐I, shatavarin‐IV, shatavarin‐IX, shatavarin‐VI, shatavarin‐VII, shatavarin‐X, isoagatharesinol, muzanzagenin, and rutin, were identified in A. racemosus extract. Prediction of the potential of the phytochemicals to stimulate or inhibit several drug targets in immune pathways was performed by using a network pharmacology model while prediction of antiviral activity against the virus S protein, Mpro and RdRp was by molecular docking. Several of these compounds had high affinity for the three protein targets. They are potential candidates for further development into COVID‐19 drugs (Borse et al., 2021). Virtual screening, molecular docking, molecular dynamics modelling, and the MM/GBSA estimation were employed for screening of several Peruvian plants for their antiviral activity against three therapeutic targets of SARS‐CoV‐2. Among the compounds tested, it was found that rutin was the most effective in binding to all three SARS‐CoV‐2 targets. The receptor–ligand complexes were exceptionally stable during the simulation, according to the MD simulation. Based on MM/GBSA analysis, the Mpro–Rutin system has the highest negative binding free energy of −40.29 kcal/mol, followed by N‐Rutin (−27.01 kcal/mol) and PLpro‐Rutin (−11.70 kcal/mol) systems (Goyzueta‐Mamani et al., 2021).
Molecular docking and redocking studies indicated that folic acid, phycoerythrobilin, phycocyanobilin and phycourobilin found in the algae, Arthrospira have antiviral effect by inhibiting SARS‐CoV‐2 S protein. The binding energies of the compounds were between −9.285 and −10.35 kcal/mol in SwissDock and −6.95 and − 7.45 kcal/mol in Autodock Vina (Petit et al., 2021). In another in silico study, phycobilins (phycoerythrobilin, phycourobilin, phycoerythrobilin and phycoviolobilin) isolated from a cynobacteria showed similar binding affinity to SARS‐CoV‐2 Mpro and PLpro. Phycocyanobilin showed binding free energies of −8.6 and −9.8 kcal/mol against Mpro and PLpro, respectively (Pendyala et al., 2021).
Molecular binding experiments were used to assess the binding affinities of 14 compounds which were made up of phenolics and terpenes, found in honey and propolis (bees glue) against the Mpro and RdRp of SARS‐CoV‐2. Among the compounds tested, quercetin, ellagic acid, kaempferol and p‐coumaric acid demonstrated the strongest interaction with the virus target enzymes (Shaldam et al., 2021). Molecular docking studies on five flavonoid aglycones (taxifolin, pectolinarigenin, tangeretin, gardenin B, and hispidulin) isolated from Kickxia aegyptiaca (L.) Nabelek, Citrus reticulata Blanco, Anastatica hierochuntica L. against Mpro of SARS‐CoV‐2 showed that the binding energy scores of the compounds were close to each other (from −6.61 to −5.74 kcal/mol) and in the following order: Tangeretin > taxifolin > gardenin B > hispidulin > pectolinarigenin. Tangeretin exhibited the best binding score among all isolates (−6.61 kcal/mol) compared to α‐ketoamide inhibitor, the docked co‐crystallized inhibitor of Mpro inhibitor (−8.17 kcal/mol) (Al‐Karmalawy et al., 2021).
Virtual screening of 83 compounds commonly found in the Chinese traditional medicine for antiviral activity revealed that TF could effectively inhibit RdRp of SARS‐CoV, MERS‐CoV and SARS‐CoV‐2. Furthermore, TF was found to have the lowest idock score (−9.11 kcal/mol) in the catalytic pocket of SARS‐CoV‐2 RdRp compared to those of the other coronaviruses. Also, TF also demonstrated a low binding energy (−8.8 kcal/mol) when it docks in the catalytic pocket of RdRp of SARS‐CoV‐2, binding significantly through hydrophobic interactions with additional hydrogen bonds between TF and RdRp. The findings suggest that TF could be further studied to be a potential SARS‐CoV‐2 RdRp inhibitor (Lung et al., 2020). In another molecular docking study on TF and TF derivatives, theaflavin‐3‐gallate (TF2) and TF3 showed docking scores of −9.8 and − 10 kcal/mol on the 3CLpro receptor of SARS‐CoV‐2, respectively (Mhatre et al., 2021). Moreover, MD simulations analyses indicated that TF2 has stronger interactions with the active site of Mpro compared to TF. Also, TF2 formed a higher number of H‐bonds throughout the entire simulation run and showed a low binding energy of −48.02 kcal/ mol, compared to TF (−11.21 kcal/mol) (Chauhan et al., 2022).
Among the 115 compounds found in Chinese traditional medicines, which have been confirmed as biologically active against SARS‐CoV and MERC‐CoV, 13 compounds (betulinic acid, quercetin, kaempferol, lignan, sugiol, cryptotanshinone, coumaroyltyramine, moupinamide, dihydrotanshinone I, dihomo‐c‐linolenic acid, desmethoxyreserpine, tanshinone IIa, and N‐cis‐feruloyltyramine) were subjected to docking analysis as inhibitors of SARS‐CoV‐2. ADMET analysis suggested that these compounds possessed favorable drug‐likeness characteristics. The compounds were evaluated for their ability to inhibit 3CLpro, PLpro, and S proteins. Finally, network pharmacology analysis was used to predict the general in vivo effects of each selected herb (Zhang et al., 2020). The Chinese traditional medicine, Jinhua Qinggan granules which contained 11 medicinal herbs has been used to treat COVID‐19. The bioactive compounds of the preparation responsible for its antiviral activity were determined using network pharmacology and molecular docking. Several main compounds including anhydroicaritin, formononetin, β‐sitosterol, and stigmasterol of the preparation were shown to interact with 3CLpro of SARS‐CoV‐2 and ACE2. The effective constituents of the traditional medicine were reported to act on targets, such as HSP90AB1, PTGS2, HSP90AA1, NCOA2, and PTGS1, and regulate multiple signaling pathways via binding ACE2 for the prevention of COVID‐19 (Gong, 2020).
Molecular docking and a hyphenated pharmacophore model followed by MD and redocking were used to screen a marine natural product library for potent SARS‐CoV‐2 Mpro inhibitors. A total of 17 potential inhibitors were identified. It was revealed that phlorotannins (8,8′‐bieckol, 6,6′‐bieckol, dieckol) from Ecklonia cava (brown algae) inhibited the major protease showing hydrophobic interaction with Leu27, Leu167, Met41, Met49, and Met165 and H‐bonds with His41 and Cys145 (Gentile et al., 2020). Four stilbenoid analogues were investigated for their inhibitory activity against SARS‐CoV‐2 S protein and hACE2 receptor complex by using molecular docking and MD simulation and binding free energy analysis. All the stilbenoids demonstrated good affinity (> −7 kcal/mol) and resveratrol exhibited very stable bound conformation to the ACE2 receptor–viral protein complex based on 50 ns MD simulation in aqueous solution. Also, MM‐PBSA was used to confirm the resveratrol–protein complex stability by its net free energy of binding. The results revealed that the stilbenoid analogs particularly resveratrol could be further developed into promising antiviral candidates against the virus (Wahedi et al., 2021).
Computational strategies such as molecular docking, MD simulation, drug similarity prediction, and pharmacokinetic properties calculation may reveal that some of these natural products could emerge as promising therapeutic inhibitors for molecular targets which include SARS‐CoV‐2 S protein, 3CLpro, PLpro, RdRp, and N proteins. The ADMET analysis performed on the compounds provide useful information on drug‐likeness properties of the ligands including toxicity prediction and oral bioavailability. The results of these in silico investigations should be validated in in vitro experiments, which include examining the active compounds' capacity to suppress viral entrance and replication in virally infected human lung cells. To understand the underlying mechanisms of action and confirmation of their inhibitory potential, the predictive in silico findings should also be supported by in vivo studies using SARS‐CoV‐2‐infected animal models. However, currently very few of the active compounds identified by in silico studies have been reported for their effects in in vitro cells and in vivo animal models for SARS‐CoV‐2.
4.2. In vitro antiviral effects of phytochemicals against SARS‐CoV‐2
In vitro antiviral studies against SARS‐CoV‐2 are currently carried out using cells and organoids. Replication and isolation of the COVID‐19 virus and its infection experiments can be carried out using several proliferating cell lines including Vero E6, HEK293T, Huh7, Calu‐3, and Caco‐2. These cell lines are used in vitro studies as they provide important information on virus replication and infection, although they produce low titer of infectious SARS‐CoV‐2 and do not imitate the physiological conditions in humans accurately. Correspondingly, Vero E6 cells are the most often utilized cells to isolate and replicate SARS‐CoV‐2 because they have been proven to be very permissive to SARS‐CoV‐2 infection and exhibit high levels of the SARS‐CoV‐2 entry receptors, ACE2 and TMPRSS2. They are kidney epithelial cells obtained from an African green monkey. The antiviral activity of compounds is assessed in the the cells through determination of viral‐induced cytopathogenic effect (Kim et al., 2020; Ou et al., 2020). Organoids are made up of multiple cell types and have been used in big‐scale drug screening programs. Organoids can self‐replicate and are used as models of human organ physiological conditions. They can reproduce the pathology of COVID‐19 in specific tissues and thus can be utilized in examining the properties of SARS‐CoV‐2 infection on definite human tissues. The antiviral effects of compounds can be evaluated by using organoids as they are permissive to SARS‐CoV‐2 infection (Takayama, 2020).
Several natural products including clinically available drugs were screened virtually and subjected to molecular in vitro testing to screen or repurpose them as potential antiviral agents to fight the COVID‐19 virus. Several groups of compounds including alkaloids, flavonoids, and terpenoids have displayed antiviral activity against SARS‐CoV‐2 in vitro (Table 2).
TABLE 2.
In vitro antiviral effect of phytochemicals against SARS‐CoV‐2
| Name of compounds | Source | Mechanisms of action | IC50/EC50 values | References |
|---|---|---|---|---|
| Alkaloids | ||||
| Berberine | Berberis vulgaris | Inhibits the viral life cycle at the late stage and induce the formation of non‐infectious virus particles | IC50 = 10.6 Μm | Pizzorno et al. (2020) |
| Homoharringtonine | Cephalotaxus harringtonii | Inhibits the replication of SARS‐CoV‐2 in Vero E6 cells | IC50 = 2.55 mM | Choy et al. (2020) |
| Emetine | Psychotria ipecacuanha | Inhibits the replication of SARS‐CoV‐2 in Vero E6 cells | IC50 = 0.46 mM | Choy et al. (2020) |
| Cepharanthine | Stephania cephalantha | Inhibits SARS‐CoV‐2 replication in vitro | IC50 = 0.35 mM | Ohashi et al. (2020) |
| Berbamine | Berberis amurensis | Inhibits lysosomal Ca2+ channel activity of the SARS‐CoV‐2 E protein to compromise the trafficking of ACE2 via inhibition of TRPMLs, thereby preventing the entry of the virus. | EC50 = ~2.4 μM | Huang, Yu, et al. (2021) |
| Bromhexine reserpine Hydroquinidine quinidine | FDA‐approved drugs | Antiviral activity was measured based on the immunofluorescent staining of infected cells using anti‐dsRNA antibody | Not available | Ku et al. (2020) |
| Quinine | Cinchona bark | Inhibits SARS‐CoV‐2 infection in Vero E6 cells, human Caco‐2 colon epithelial cells and lung cell line A549. | ~3.7 to ~50 μM | Große et al. (2021) |
| Quinacrine | Antimalarial drug | Reduces SARS‐CoV‐2 virus replication in Vero E6 cells by inhibition of viral ensemble | 6.5 μM | Salas Rojas et al. (2021) |
| Hanfangchin A | Stephania tetrandra | Exhibits appreciable synergistic effects with remdesivir in inhibiting SARS‐CoV‐2 replication in Vero E6 cells | Not available | Riva et al. (2020) |
| Flavonoids | ||||
| Baicalin | Scutellaria baicalensis | Inhibits 3 CLpro and RNA polymerization activity of SARS‐CoV‐2 3CLpro | IC50 = 9 μM | Zandi et al. (2021) |
| Baicalein | S. baicalensis | Inhibits 3 CLpro and RNA polymerization activity of SARS‐CoV‐2 RdRp | IC50 = 4.5 Μm | Zandi et al. (2021) |
| Myricetin | Diospyros kaki | Inhibits the enzymatic activity of SARS‐CoV‐2 3CLpro and interfere the replication of SARS‐CoV‐2 in Vero E6 cells | IC50 = 0.63 μM | Su et al. (2021) |
| Terpenoids | ||||
| Betulinic acid | Betula pubescens | Inhibits the binding of SARS‐CoV‐2 S protein RBD to ACE2 of host cell | IC50 = 0.1 μM | Carino et al. (2020) |
| Oleanolic acid | B. pubescens | Inhibits the binding of SARS‐CoV‐2 S protein RBD to ACE2 of host cell | IC50 = 1 μM | Carino et al. (2020) |
| Glycyrrhetinic acid | Glycyrrhiza glabra | Inhibits S protein‐ACE2 binding between SARS‐CoV‐2 and host cell | IC50 = 10 μM | Carino et al. (2020) |
| Tretinoin | FDA‐approved drug |
Inhibits SARS‐CoV‐2 3CLpro activity Inhibits SARS‐CoV‐2 replication in Vero E6/TMPRSS2 and Calu‐3 cells |
IC50 = 24.7 μM IC50 = 2.69 μM IC50 = 0.82 μM |
Morita et al. (2021) |
| Glycyrrhizin | G. glabra | Blocks the viral replication by inhibiting the SARS‐CoV‐2 Mpro and targets the S‐RBD‐ACE2 complex | EC50 = 0.44 μg/ml | van de Sand et al. (2021), Luo et al. (2020) |
| Arteannuin B | Artemisia annua | Inhibits the extracellular viral RNA production along with intracellular viral protein expression at the postentry step of SARS‐CoV‐2 infection | IC50 = 25 μM | Cao et al. (2020) |
| Andrographolide | Andrographis paniculata | Inhibits SARS‐CoV‐2 Mpro by forming a covalent bond with the active site Cys145 of Mpro. Inhibits virus replication in Calu‐3 cells | IC50 = 0.034 μM | Sa‐Ngiamsuntorn et al. (2021) |
| Other phytochemicals | ||||
| Lumefantrine | A. annua | Blocks the extracellular viral RNA production and intracellular viral protein expression during full‐time of SARS‐CoV‐2 infection process | IC50 = 100 μM | Cao et al. (2020) |
| Curcumin | Curcuma longa | Reduces SAR‐Cov‐2 RNA levels in Vero E6 and human Calu‐3 cells | EC50 = 7.9 μg/ml | Bormann et al. (2021) |
| Carolacton | Sorangium cellulosum |
Inhibits the MTHFD1 which involve in viral metabolism pathways. Blocks replication of SARS‐CoV‐2 |
IC50 = 0.14 mM | Anderson et al. (2020) |
| Resveratrol | Vitis vinifera and various members of spermatophytes such as grapes, mulberry, and peanuts | Inhibits replication of SARS‐CoV‐2 replication in Vero E6 cells | IC50 = 25 μM | Pasquereau et al. (2021), Yang et al. (2021) |
| Phillyrin | Forsythia suspensa | Inhibits replication of SARS‐CoV‐2 replication in Vero E6 cells and reduce the production of pro‐inflammatory cytokines (TNF‐α, IL‐6, IL‐1β, MCP‐1, and IP‐10) at the mRNA levels | IC50 = 63.90 μg/ml | Ma, Pan, et al. (2020) |
Abbreviations: 3CLpro, 3C‐like protease; ACE2, angiotensin‐converting enzyme 2; Mpro, main protease; MTHFD1, methylenetetrahydrofolate dehydrogenase; RBD, receptor‐binding domain; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; S‐RBD, spike protein receptor‐binding domain; TRPML, transient receptor potential mucolipin channels.
4.2.1. Alkaloids
Berberine, an alkaloid isolated from Berberis vulgaris L. has been intensively investigated for its pharmacological effects and has potential applications in a variety of therapeutic areas, including cancer. Antiviral action of berberine against alphaviruses and influenza has been reported (Warowicka et al., 2020). Antiviral effects of berberine together with chloroquine, remdesivir, lopinavir, cyclosporine A and umifenovir against the COVID‐19 virus were evaluated in Vero E6 cells. Berberine exhibited a strong antiviral effect with an IC50 value of 10.6 μM. However, in a virus‐directed plus host‐directed drug combination study, a combination of remdesivir and berberine exhibited strong antagonism compared to the remdesivir/diltiazem combination which has a high level of synergy, with peak values above 50 and mean Loewe synergy scores of 12. The combination of host‐directed drugs with direct‐acting antivirals provides interesting avenues to treat COVID‐19 (Pizzorno et al., 2020). It was recently demonstrated that the alkaloids homoharringtonine and emetine isolated from Cephalotaxus harringtonii and Psychotria ipecacuanha, respectively, could strongly inhibit replication of SARS‐CoV‐2 in Vero E6 cells, with EC50 values of 2.55 and 0.46 mM, respectively. Interestingly, there was a synergistic suppressive effect against the replication of the virus when emetine was combined with a C nucleoside analog GS‐5734 (Choy et al., 2020). In an in vitro screening of 306 FDA/EMA/PMDA‐approved drugs against SARS‐CoV‐2 infected Vero E6/TMPRSS2 cells, nelfinavir (HIV protease inhibitor) and cepharanthine (antiinflammatory drug) demonstrated strong inhibition against the virus. Cepharanthine a bisbenzylisoquinoline alkaloid isolated from Stephania cephalantha could strongly inhibit SARS‐CoV‐2 replication with minimum toxicity (selectivity index above 70) and an EC50 value of 0.35 mM. These results were consistent with a previous in silico study which showed that cepharanthine inhibited viral attachment and entry into cells, whilst nelfinavir could bind to the SARS‐CoV‐2 Mpro. Consistent with this predicted different mechanisms of action, the nelfinavir/cepharanthine combination demonstrated a synergistic effect in limiting SARS‐CoV‐2 replication in vitro (Ohashi et al., 2020). This study reveals another example of a new multidrug combination that has great potential to battle both the progression and risk of transmission of COVID‐19.
Berbamine, a bis‐benzylisoquinoline alkaloid exhibited strong anti‐SARS‐CoV‐2 activity in Vero E6 cells by significantly inhibiting viral yield, as quantified by qRT‐PCR assays (EC50 = ~2.4 μM). It inhibited lysosomal Ca2+ channel activity of the SARS‐CoV‐2 E protein to compromise the trafficking of ACE2, thereby preventing the entry of the virus. Berbamine also reduced infectious virus production and reduced genome replication (Huang, Yuen, et al., 2021; Xia et al., 2021). Xia et al. (2021) demonstrated that a derivative of berbamine, BE‐33, was more active than berbamine against SARS‐CoV‐2 infection by exhibiting an EC50 of 0.94 μM and higher binding affinity to the E protein ion channel. This derivative also reduced viral loads in the lungs and reduced inflammatory cytokines in hACE2‐transgenic mice, suggesting antiinflammatory properties that may be beneficial to severe COVID‐19 cases. In a cell‐based screening of 1,473 FDA‐approved drugs using an anti‐dsRNA antibodies to identify inhibitors of SARS‐CoV‐2 infection, 29 drugs including the alkaloids, bromhexine hydrochloride, reserpine, hydroquinidine, and quinidine showed antiviral activity against SARS‐CoV‐2. The antiviral activity of each compound was measured based on the immunofluorescent staining of infected cells using an anti‐dsRNA antibody (Ku et al., 2020). Quinine, an antimalarial alkaloid, was shown to be more effective in inhibiting SARS‐CoV‐2 infection in Vero E6 cells compared to chloroquine and hydroxychloroquine. It also demonstrated antiviral activity in human Caco‐2 colon epithelial cells and lung cell line A549 (Große et al., 2021). Another antimalarial alkaloid, quinacrine reduces SARS‐CoV‐2 virus replication in Vero E6 cells by inhibition of viral ensemble (Salas Rojas et al., 2021). Celgosivir, a prodrug of castanospermine, and a deoxynojirimycin derivative (UV‐4), are iminosugars that have been shown to exhibit effective inhibition of SARS‐CoV‐2 viral replication and reduced S protein levels in a dose‐dependent manner in Vero E6 cells. The lowering of S protein levels was probably due to its ability to inhibit α‐glucosidases, leading to improper S protein folding (Clarke et al., 2021).
Riva et al. (2020) performed a large‐scale repurposing investigation via high‐throughput analysis of around 12,000 FDA‐approved or clinical‐stage compounds including natural products for activity against SARS‐CoV‐2 HKU‐001a replication in Vero E6 cells. A total of 100 compounds could suppress the viral replication and 21 of them dose‐dependently inhibited the virus. Thirteen of these compounds including apilimod, a PIKfyve kinase inhibitor and four cysteine protease inhibitors (Z LVG CHN2, ONO 5334, VBY‐825, and MDL‐28170) showed EC50 values less than 500 nM in at least one cell line, corresponding with credible achievable therapeutic dosages in patients. Among these compounds, four compounds including hanfangchin A demonstrated appreciable synergistic effects with remdesivir.
4.2.2. Flavonoids
Su, Yao, Zhao, Li, Liu, et al. (2020) reported the antiviral effects of a patented Chinese traditional medicine for treating respiratory tract infection, Shuanghuanglian preparation, against SARS‐CoV‐2. It was shown that the oral liquid and lyophilized injection of Shuanghuanglian, and their bioactive components inhibited 3CLpro of SARS‐CoV‐2 and SARS‐CoV‐2 replication in Vero E6 cells in a dose dependent manner. The two bioactive constituents of the preparation contributing to the antiviral activity were flavonoids identified as baicalin and baicalein. X‐Ray protein crystallography showed that baicalein binding with 3CLpro of the virus was markedly dissimilar from the other known 3CLpro inhibitors. Baicalein was shown to position itself productively in the core of the substrate‐binding region by interacting with the crucial oxyanion loop and S1/S2 subsites and effectively preventing access of substrate to the catalytic dyad within the active site. The results verified the traditional use of a patented Chinese traditional medicine through the exploration of its in vitro potency against SARS‐CoV‐2 the and identification of its bioactive constituents (Su, Yao, Zhao, Li, J Liu, et al., 2020). In another in vitro study on baicalein and baicalin, isolated from Scutellaria baicalensis, both compounds showed marked antiviral activity by inhibiting the RdRp of the virus. Baicalein was shown to be more potent and it has the potential to be further developed into a selective non‐nucleoside polymerase inhibitor of the virus (Zandi et al., 2021).
It was recently reported that myricetin, a common flavonoid found in food, covalently suppressed the 3CLpro of SARS‐CoV‐2. Crystal structure analysis of myricetin and its derivatives bound to protease revealed that the pyrogallol group of the compounds acted as an electrophile to covalently modify the catalytic cysteine. Structure–activity analysis revealed that some of the myricetin derivatives possessed strong antiviral activity and were stable for oral administration. The results provide underlying mechanisms of covalent binding of the myricetin derivatives as non‐peptidomimetic covalent inhibitors against 3CLpro. The results highlighted pyrogallol as a potential candidate for designing targeted covalent ligands (Su et al., 2021). In vitro study of five flavonoid aglycones isolated from K. aegyptiaca, A. hierochuntica, and C. reticulata against SARS‐CoV‐2 in Vero E6 cells demonstrated that pectolinarigenin and tangeretin showed the highest inhibition against the virus with IC50 values of 12.4 and 2.5 μg/ml, respectively. Their IC50 values indicate that they could be potential candidates for in vivo studies in SARS‐CoV‐2 infected animal models (Al‐Karmalawy et al., 2021).
4.2.3. Terpenoids
Several studies have revealed that several triterpenoids and steroidal agents were able to inhibit the ACE2 carboxypeptidase domain and S protein RBD interaction. The S protein‐ACE2 binding was significantly inhibited by the triterpenoids such as oleanolic acids, glycyrrhetinic acid, and betulinic acid (Carino et al., 2020). High throughput screening of 91 natural compounds using AlphaScreen to identify SARS‐CoV‐2 3CLpro inhibitors revealed that an FDA‐approved drug, all‐trans retinoic acid (ATRA) (tretinoin) showed anti‐3CLpro activity. AlphaScreen and immunoblotting assay were performed to confirm the suppressive effect of ATRA against the virus, showing an IC50 value of 24.7 μM. The compound also inhibited SARS‐CoV‐2 replication in Vero E6/TMPRSS2 and Calu‐3 cells with IC50 values of 2.69 and 0.82 μM, respectively. ATRA also demonstrated an inhibitory effect against the replication of SARS‐CoV‐2 VOC. These findings indicate that ATRA has the potential to be developed into an antiviral agent against SARS‐CoV‐2 (Morita et al., 2021). Glycyrrhizin, a triterpene glycoside, is the major bioactive constituent of G. glabra root, exhibited potent inhibition against SARS‐CoV‐2 in Vero E6 cells by blocking viral replication through inhibition of the viral Mpro. Glycyrrhizin completely inhibited replication of SARS‐CoV‐2 at 1 mg/ml (postentry conditions) and 0.5 mg/ml (combined pre and postentry conditions). It was suggested that health products that contained an appreciable amount of glycyrrhizin may provide great benefit for patients battling SARS‐CoV‐2. Glycyrrhizin has good potential to be further investigated as as antiviral agent for clinical use to heal COVID‐19 patients (van de Sand et al., 2021).
Artemisinins, and sesquiterpene lactones from Artemisia annua, are known for their anti‐malarial properties and they have also been documented to possess various pharmacological activities, including antiviral, anticancer, and immunomodulation. Nine artemisinin‐related compounds were identified systematically for their in vitro antiviral effect against SARS‐CoV‐2 in Vero E6 cells. Their mechanism of antiviral action was determined by using a time‐of‐drug‐addition assay. The therapeutic potential of certain bioactive metabolites against COVID‐19 was predicted by a pharmacokinetic prediction model. Among the tested compounds, arteannuin B showed the highest anti‐SARS‐CoV‐2 potential with an EC50 of 10.28 μM, while lumefantrine showed therapeutic promise due to high plasma and lung drug concentrations after multiple dosing. It was revealed by a mechanistic analysis that both active compounds, lumefantrine and arteannuin B, inhibited the virus at the postentry step of virus infection. The results of this study indicated that artemisinins possessed significant in vitro antiviral activity and have a huge prospect to be leading candidates for the development of anti‐SARS‐CoV‐2 drugs (Cao et al., 2020). In vitro assay has been performed to evaluate the effect of the diterpenoid, andrographolide, and its fluorescence derivative nitrobenzoxadiazole‐conjugated andrographolide (Andro‐NBD) on purified SARS‐CoV‐2 Mpro. Both compounds inhibited the SARS‐CoV‐2 and SARS‐CoV Mpro, with Andro‐NBD shown to covalently bonded its fluorescence to these proteases. Based on mass spectrometry analysis, andrographolide was suggested to form a covalent bond with the active site Cys145 of either SARS‐CoV‐2 Mpro or SARS‐CoV Mpro (Shi et al., 2020). The anti‐SARS‐CoV‐2 activity of A. paniculata extract and andrographolide in Calu‐3 cells by the plaque assay showed that the plant samples significantly inhibited the infectious virions production with IC50 values of 0.036 μg/ml and 0.034 μM, respectively (Sa‐Ngiamsuntorn et al., 2021). Based on this IC50 value, andrographolide has so far been the most potent phytochemical identified for its antiviral activity against the COVID‐19 virus. Angrographolide has a high potential to be developed into an antiviral candidate against the virus.
4.2.4. Other phytochemicals
Curcumin has been revealed in previous studies to show potent antiviral against several viruses, including SARS‐CoV, HIV, influenza A, and HCV (Praditya et al., 2019). The antiviral effects of an extract of turmeric root, the content of turmeric capsule, and curcumin at subtoxic concentrations against SARS‐CoV‐2 in Vero E6 and human Calu‐3 cells were determined by Bormann et al. (2021). The study revealed that all samples particularly curcumin exhibited potent antiviral activity against SARS‐CoV‐2 in cell culture supernatants by reducing the RNA level. Curcumin as a major ingredient in turmeric root or capsules contributed mainly to the antiviral activity. In both cell‐free and cell‐based antiviral assays on 56 polyphenols, curcumin (above 10 μg/ml) was among the compounds exhibiting strong inhibition against S glycoprotein binding to the ACE2 receptor and thus cellular entry of pseudo‐typed SARS‐CoV‐2 virions (Goc et al., 2021). In another in vitro assay on the antiviral activity of curcumin against SARS‐CoV‐2 in Vero E6 cells, the compound significantly inhibited virus replication with an IC50 value of 0.44 μM which was much lower than that of hydrochloroquine (1.72 μM) (Kandeil et al., 2021). The results of the above studies provide further proof that curcumin is a promising candidate for further development into an antiviral agent to combat SARS‐CoV‐2.
Carolacton, a macrolide keto‐carboxylic acid with antibacterial potency, is produced by the myxobacterium Sorangium cellulosum. Carolacton exhibited potent inhibition at a sub‐micromolar concentration (IC50 of 0.14 μM) against SARS‐CoV‐2 infection in Vero E6 cells. It was shown that carolacton was a methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) inhibitor which could potently inhibit a variety of viruses, including SARS‐CoV‐2. Thus, it is a potential target for developing COVID‐19 drugs (Anderson et al., 2020). Yang et al. (2021) highlighted the high potency of resveratrol in inhibiting SARS‐CoV‐2 replication in cultured Vero E6 cells, showing an EC50 of 4.48 μM. Resveratrol is a stilbenoid, a polyphenolic phytoalexin. Interestingly, when 50 μM of resveratrol was added after virus infection the inhibitory rate for viral replication in cells was greater than 98% which was similar to the full‐time treatment, however, the inhibitory rate for cells pre‐treated with resveratrol for 2 h was less than 20%. The results suggest that the suppressive effects of the compound on virus replication have a strong presence after the viruses are inoculated into the Vero cells. The in vitro antiviral effect of resveratrol was also reported by Pasquereau et al. (2021). There was a reduction of the viral titer on SARS‐CoV‐2 when treated with resveratrol in Vero E6 cells as measured by qRT‐PCR.
The discovery of a potent agent with dual targeting ability, that is, downregulation of SARS‐CoV‐2 replication and host dysregulated inflammatory pathways, is a novel discovery that may lead to the design and development of a promising agent with antiviral and antiinflammatory effects for prevention and treatment of COVID‐19. Recently, there are a few reports on the ability of phytochemicals to exhibit dual targeting ability. For example, phillyrin, a lignan, isolated from Forsythia suspensa showed potent antiinflammatory and antiviral properties against the SARS‐CoV‐2 and HCoV‐229 E in vitro. The antiviral effect was determined in Vero E6 cells, whilst measurement of levels of expression of proinflammatory cytokines (TNF‐α, IL‐6, IL‐1β, MCP‐1, and IP‐10) was performed in Huh‐7 cells. Phyllyrin demonstrated strong inhibition of the proliferation of the virus in Vero E6 cells, with an IC50 value of 63.90 μg/ml and a significant reduction in the proinflammatory cytokine expression in Huh‐7 cells at the protein and mRNA levels. Phyllyrin inhibited the proinflammatory cytokines expression by modulating the NF‐кB signaling pathway (Ma, Li, et al., 2020). Bioinformatics analysis revealed that phillyrin interacted with 192 common core targets and 25 biological pathways to treat the co‐infection of SARS‐COV‐2 and influenza virus. It was suggested that the compound activates the PI3K‐AKT, RAS, and HIF‐1 signaling pathways to regulate the body's immune and antiinflammatory effects, thereby reducing the severity of diseases caused by SARS‐COV‐2 and influenza virus infections (Lai et al., 2021).
Another example of the dual targeting ability of phytochemicals is demonstrated by a study by Runfeng et al. (2020). They reported the antiinflammatoryand antiviral effects of Chinese traditional medicine, Lianhuaqingwen, against SARS‐CoV‐2. The formulation noticeably downregulated replication of the virus in Vero E6 cells, affected virus morphology and significantly blocked viral replication in Vero E6 cells, and markedly suppressed pro‐inflammatory cytokines production in virus‐infected Huh‐7 cells at the mRNA levels. Another traditional Chinese medicine (TCM), the Liu Shen capsule, was shown to possess dual targeting ability. The preparation could markedly suppress replication of SARS‐CoV‐2 in Vero E6 cells, and significantly attenuate pro‐inflammatory cytokines production (IL‐1β, IL‐6, IL‐8, TNF‐α, CXCL‐10/IP‐10, and CCL‐2/MCP‐1) at the mRNA levels in Huh‐7 cells. Liu Sen capsule also suppressed the key proteins in the NF‐κB/MAPK signaling pathway via inhibition of the expression of p‐p38 MAPK, p‐NF‐κB p‐IκBα, and p65, while IκBα expression was increased. The results suggest that this traditional medicine has the potential to be used as an alternative candidate to treat COVID‐19 (Ma, Pan, et al., 2020). However, the effective constituents of both Chinese traditional formulations contributing to the antiviral and antiinflammatory effects were not reported. Figure 2 shows the chemical structures of some compounds with antiviral effects against SARS‐CoV‐2 as identified by in vitro studies.
FIGURE 2.
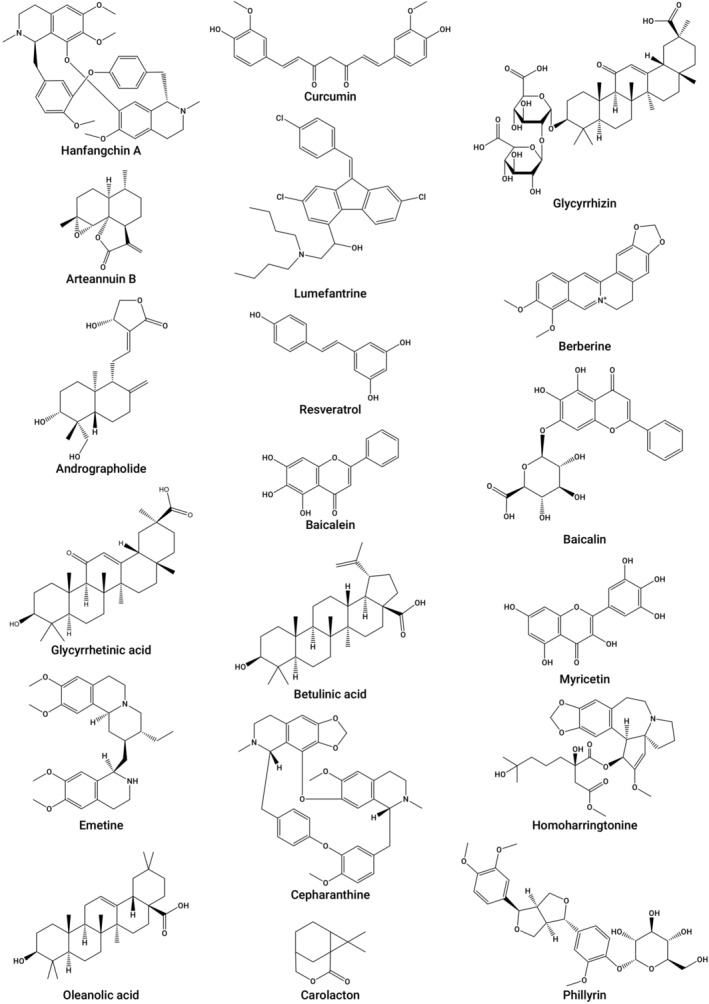
The chemical structures of compounds with antiviral effects against SARS‐CoV‐2 as identified by in vitro studies. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
The inhibitory effects of the compounds against SARS‐CoV‐2 occurred at different steps of viral replication, ranging from binding with ACE2 receptor, entry, and fusion to viral replication. Their mechanisms of antiviral actions involved targeting several molecular targets which include SARS‐CoV‐2 S glycoprotein, 3CLpro, PLpro, RdRp, and N proteins. The chemical structures of the phytochemicals active against SARS‐CoV‐2 were diversified and there was no study yet to relate their chemical structures to antiviral activity. None of the bioactive compounds which exhibited strong antiviral effects against SARS‐CoV‐2 has been subjected to a structure–activity relationships (SAR) study as they were not structurally related. To evaluate SAR, a series of analogs of the hit compounds have to be synthesized and their anti‐SARS‐CoV‐2 activities have to be tested. The most potent compound in a series of analogs will then be selected as a drug candidate for further experimental and preclinical studies on its effects in SARS‐CoV‐2‐infected animal models.
4.3. In vivo antiviral effects of phytochemicals against SARS‐CoV‐2
Initially, the emergency use of antimalarial drugs and antiviral agents to treat COVID‐19 was under compassionate use protocols and clinical trials, based on in vitro activity against SARS‐CoV‐2 and limited clinical experience. Although some of these repurposed drugs such as chloroquine and remdesivir exhibited strong in vitro inhibition of SARS‐CoV‐2 replication, only very few of them have been evaluated for their effects in SARS‐CoV‐2‐infected animal models. Recently, Qiao et al. (2021) reported the in vitro and in vivo inhibitory activities of 32 new bicycloproline‐containing compounds synthesized from either telaprevir or boceprevir against Mpro of SARS‐CoV‐2 in Vero E6 cells and a transgenic mouse model of SARS‐CoV‐2 infection. The IC50 values of the compounds in the in vitro study ranged from 7.6 to 748.5 nM. Among the compounds, MI‐30 and MI‐09 demonstrated strong antiviral activity in both in vitro and in vivo assays. It was observed that intraperitoneal oral administration with MI‐30 or MI‐09 markedly reduced lung lesions and viral loads. Interestingly, in a recent study, PIKfyve inhibitors including apilimod which have shown strong antiviral activity in vitro against many coronaviruses including SARS‐CoV‐2, id not inhibit the virus replication in a murine model of COVID‐19. The results indicated that the in vitro antiviral potency of the compounds did not translate to in vivo efficacy in an animal model (Logue et al., 2022).
Until now, there have been few in vivo studies to evaluate the antiviral activity of natural products or phytochemicals in SARS‐CoV‐2‐infected animal models. A TCM, Pudilan Xiaoyan oral liquid (PDL), containing four herbs (Corydalis bungeana, I. indigotica, S. baicalensis, and Taraxacum mongolicum) (PDL) and more than 181 ingredients, showed potent antiviral effects against SARS‐CoV‐2 in Vero E6 cells and SARS‐CoV‐2‐infected hACE2 transgenic mice (Deng et al., 2020). PDL demonstrated the ability to effectively suppress SARS‐CoV‐2 replication in vitro with an EC50 value of 1.078 mg/ml, half‐cytotoxic concentration (CC50) value of 8.914 mg/ml, and selectivity index of 8.27°C. Also, PDL showed a potent in vivo inhibitory effect against SARS‐CoV‐2. When PDL at 4 ml/kg was administered by intragastric route from 1‐h postvirus inoculation, then once daily for 5 days to SARS‐CoV‐2‐infected hACE2 transgenic mice, the animals showed an improved weight loss and significantly reduced the viral RNA copies of the lung. Histopathological analysis of lung tissues of treated mice and control mice indicated that the former had mild interstitial pneumonia along with small amounts of inflammatory cells infiltrated, albeit the alveolar interstitium expanded. The results also showed that in PDL‐treated mice, the degree of pulmonary inflammation was markedly attenuated, suggesting the pneumonia in virus‐infected mice could be relieved by treatment with PDL.
Up to now, only crude extracts have been used to evaluate the in vivo antiviral effects of plants in SARS‐CoV‐2‐infected animal models. The bioactive phytochemicals responsible for the antiviral activity have to be isolated and identified, and the extracts used in the studies have to be standardized using the bioactive compounds as the chemical markers. The antiviral effects and mechanisms of action of the bioactive phytochemicals have to be evaluated in SARS‐CoV‐2‐infected animal models. More elaborate experimental and preclinical investigations including pharmacokinetics and pharmacodynamics studies together with bioavailability and toxicological studies in animal models are required before the compounds are ready for human studies to develop them into antiviral drugs to treat COVID‐19.
4.4. Clinical trials
Initially, some antimalarial drugs and antiviral agents have been allowed emergency use by FDA and WHO to combat the COVID‐19 pandemic, under compassionate use protocols based on in vitro studies against the virus and limited clinical studies. However, human studies on the use of these investigational and repurposed drugs have now been discontinued as they showed little benefit in treating the disease and some showed adverse effects (Oren et al., 2020). At present, clinical trials on phytochemicals for their antiviral properties against COVID‐19 viruses have been sparse, mainly due to insufficient data generated from preclinical studies which include toxicological, pharmacokinetic, and pharmacodynamic studies. Many randomized controlled trials (RCTs) on TCM have been carried out and some have shown that TCM plus routine treatment was significantly better than routine treatment alone in improving the clinical symptoms. In these RCTs, TCM treatment plus routine treatment were reported to improve chest images and reduce clinical deterioration, development of ARDS, use of mechanical ventilation, and overall mortality rates in patients with COVID‐19. It also improved the negativity rate SARS‐CoV‐2 nucleic acid test as compared to routine treatment alone (Qiu et al., 2020; Wang et al., 2021; Xing & Liu, 2021). However, the level of evidence on the effectiveness of TCM to treat COVID‐19 needs further improvement.
There were already a few case studies carried out to evaluate the efficacy of phytochemicals as anti‐SARS‐CoV‐2 in humans. In a case study, compassionate use of glycyrrhizin at 150 mg (3 times per day for 8 days) among other potential antivirals by a COVID‐19 patient showed improvement after 12 h of treatment and later recovered from the disease. However, the effectiveness of glycyrrhizin to treat COVID‐19 has to be proven by further systematic and controlled clinical studies (Ding et al., 2020). Glycyrrhizin has been shown to target the S‐RBD‐ACE2 complex, reducing the buildup of intracellular ROS, managing thrombi, preventing the rapid formation of airway exudates, and encouraging endogenous interferon against the virus (Luo et al., 2020). In a preliminary clinical study on 18 hospitalized patients with severe COVID‐19, berberine was found to be able to reduce circulating inflammatory mediators. However, whether berberine has any antiviral effect against the virus was unclear and further experiments are needed to clarify this (Zhang et al., 2020).
5. CONCLUSIONS
For the past 2 years since the emergence of SARS‐CoV‐2 in 2019, intensive research on the antiviral effects of natural products particularly phytochemicals against SARS‐CoV‐2 has been focusing on in silico and in vitro antiviral studies of natural products which have previously shown promising results against the earlier coronaviruses, SARS‐CoV and MERS‐CoV. However, the in silico and in vitro screening methods used to test the efficacy of the phytochemicals against the human viral disease were mostly not validated. In vivo studies on the antiviral effects of phytochemicals against SARS‐CoV‐2‐infected animal models are still lacking. More than a hundred phytochemicals have been identified with potential antiviral activity against SARS‐CoV‐2 protein targets through in silico studies. Until now there are several phytochemicals such as hanfangchin A, curcumin, glycyrrhizin arteannuin B, lumefantrine, berberine, betulinic acid, oleanolic acid, baicalin, baicalein, carolacton, homoharringtonine, emetine, cepharanthine, phillyrin, andrographolide, resveratrol, and myricetin that have been reported to exhibit promising results in vitro studies as potent inhibitors of SARS‐CoV‐2. Among these active compounds, andrographolide showed the highest inhibition against the virus with an IC50 value of 0.034 μM. The diterpenoid inhibited SARS‐CoV‐2 by forming a covalent bond with the active site Cys145 of Mpro in Vero E6 cells. It also suppressed virus replication in Calu‐3 cells. Other compounds with strong antiviral effects against the virus, arranged based on IC50 values (ranging from 0.1 to 0.63 μM) are as follows: Betulinic acid, carolacton, cepharanthine, glycyrrhizin, emetine, and myricetin. However, no SAR studies have been reported on bioactive natural products due to their chemical diversity.
Further experimental and preclinical studies on the effects of these compounds in SARS‐CoV‐2‐infected animal models have to be performed. Pharmacokinetics and pharmacodynamics studies together with bioavailability, metabolism, and toxicological studies in animal models have to be carried out to provide sufficient efficacy and safety data before clinical studies can be performed to develop them into antiviral drugs to battle COVID‐19. However, the in vivo antiviral effects of plants in SARS‐CoV‐2‐infected animal models have so far been carried out using crude extracts, and the phytochemicals responsible for the antiviral effects have not been identified. Although a few case studies have been reported to evaluate the effects of phytochemicals as anti‐SARS‐CoV‐2 in human, no systematic clinical trials against the COVID‐19 viruses has been carried out. This is mainly due to insufficient data generated from preclinical studies which include toxicological, pharmacokinetic, and pharmacodynamic studies. The search for natural products of plant origin as new chemical leads for the development of safe and potent anti‐SARS‐CoV‐2 agents will continue to gain much major research interest.
FUNDING INFORMATION
No grant from funding agencies in the public, commercial, or not‐for‐profit sectors has been received to write this review.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
Jantan, I. , Arshad, L. , Septama, A. W. , Haque, M. A. , Mohamed‐Hussein, Z.‐A. , & Govender, N. T. (2022). Antiviral effects of phytochemicals against severe acute respiratory syndrome coronavirus 2 and their mechanisms of action: A review. Phytotherapy Research, 1–21. 10.1002/ptr.7671
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- Agrawal, N. , & Goyal, A. (2022). Potential candidates against COVID‐19 targeting RNA‐dependent RNA polymerase: A comprehensive review. Current Pharmaceutical Biotechnology, 23(3), 396–419. [DOI] [PubMed] [Google Scholar]
- Ahidjo, B. A. , Loe, M. W. C. , Ng, Y. L. , Mok, C. K. , & Chu, J. J. H. (2020). Current perspective of antiviral strategies against COVID‐19. ACS Infectious Diseases, 6(7), 1624–1634. [DOI] [PubMed] [Google Scholar]
- Al‐Karmalawy, A. A. , Farid, M. M. , Mostafa, A. , Ragheb, A. Y. , H. Mahmoud, S. , Shehata, M. , … Marzouk, M. M. (2021). Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS‐CoV‐2. Molecules, 26(21), 6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, D. E. , Cui, J. , Ye, Q. , Huang, B. Y. , Zu, W. H. , Gong, J. , … Tan, X. (2020). Orthogonal genome‐wide screenings in bat cells identify MTHFD1 as a target of broad antiviral therapy. Proceedings of the National Academy of Sciences, 118(39), e2104759118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A. , Sarkar, A. , & Maulik, U. (2020). Molecular docking study of potential phytochemicals and their effects on the complex of SARS‐CoV2 spike protein and human ACE2. Scientific Reports, 10(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman, Z. R. , Usher, M. , Olson, A. , Chipman, J. G. , Brunsvold, M. E. , Beilman, G. , … Lusczek, E. R. (2022). Comparison of outcomes and process of care for patients treated at hospitals dedicated for COVID‐19 care vs other hospitals. JAMA Network Open, 5(3), e220873. 10.1001/jamanetworkopen.2022.0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozari, M. , & Hosseinzadeh, H. (2021). Natural products for COVID‐19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytotherapy Research, 35(2), 864–876. [DOI] [PubMed] [Google Scholar]
- Bormann, M. , Alt, M. , Schipper, L. , van de Sand, L. , Le‐Trilling, V. T. K. , Rink, L. , … Krawczyk, A. (2021). Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS‐CoV‐2 in vitro. Viruses, 13(10), 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borse, S. , Joshi, M. , Saggam, A. , Bhat, V. , Walia, S. , Marathe, A. , … Tillu, G. (2021). Ayurveda botanicals in COVID‐19 management: An in silico multi‐target approach. PLoS One, 16(6), e0248479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly, L. , Druce, J. D. , Catton, M. G. , Jans, D. A. , & Wagstaff, K. M. (2020). The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Research, 178, 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, R. , Hu, H. , Li, Y. , Wang, X. , Xu, M. , Liu, J. , … Zhong, W. (2020). Anti‐SARS‐CoV‐2 potential of artemisinins in vitro. ACS Infectious Diseases, 6(9), 2524–2531. [DOI] [PubMed] [Google Scholar]
- Carino, A. , Moraca, F. , Fiorillo, B. , Marchianò, S. , Sepe, V. , Biagioli, M. , … Fiorucci, S. (2020). Hijacking SARS‐CoV‐2/ACE2 receptor interaction by natural and semi‐synthetic steroidal agents acting on functional pockets on the receptor binding domain. Frontiers in Chemistry, 8, 572885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan, M. , Bhardwaj, V. K. , Kumar, A. , Kumar, V. , Kumar, P. , Enayathullah, M. G. , … Kumar, S. (2022). Theaflavin 3‐gallate inhibits the main protease (Mpro) of SARS‐CoV‐2 and reduces its count in vitro. Scientific Reports, 12, 13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Li, J. , Luo, C. , Liu, H. , Xu, W. , Chen, G. , … Jiang, H. (2006). Binding interaction of quercetin‐3‐β‐galactoside and its synthetic derivatives with SARS‐CoV 3CLpro: Structure–activity relationship studies reveal salient pharmacophore features. Bioorganic & Medicinal Chemistry, 14(24), 8295–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka, K. , Witek‐Krowiak, A. , Skrzypczak, D. , Mikula, K. , & Młynarz, P. (2020). Phytochemicals containing biologically active polyphenols as an effective agent against Covid‐19‐inducing coronavirus. Journal of Functional Foods, 73, 104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy, K. T. , Wong, A. Y. L. , Kaewpreedee, P. , Sia, S. F. , Chen, D. , Hui, K. P. Y. , … Yen, H. L. (2020). Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS‐CoV‐2 replication in vitro. Antiviral Research, 178, 104786. 10.1016/j.antiviral.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, E. C. , Nofchissey, R. A. , Ye, C. , & Bradfute, S. B. (2021). The iminosugars celgosivir, castanospermine and UV‐4 inhibit SARS‐CoV‐2 replication. Glycobiology, 31(4), 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong, Y. , Kriegenburg, F. , De Haan, C. A. , & Reggiori, F. (2017). Coronavirus nucleocapsid proteins assemble constitutively in high molecular oligomers. Scientific Reports, 7(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W. , Xu, Y. , Kong, Q. , Xue, J. , Yu, P. , Liu, J. , … Bao, L. (2020). Therapeutic efficacy of Pudilan Xiaoyan Oral liquid (PDL) for COVID‐19 in vitro and in vivo . Signal Transduction and Targeted Therapy, 5(1), 1–3. 10.1038/s41392-020-0176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, H. , Deng, W. , Ding, L. , Ye, X. , Yin, S. , & Huang, W. (2020). Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID‐19 patients. Journal of Medical Virology, 92(10), 2200–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai, P. , Batool, M. , Shah, M. , & Choi, S. (2015). Middle East respiratory syndrome coronavirus: Transmission, virology and therapeutic targeting to aid in outbreak control. Experimental & Molecular Medicine, 47(8), e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evary, Y. M. , Masyita, A. , Kurnianto, A. A. , Meidianto, A. R. , & Yusnita, R. (2022). Molecular docking of phytochemical compounds of Momordica charantia as potential inhibitors against SARS‐CoV‐2. Current Drug Targets, 22(3), 57–63. [DOI] [PubMed] [Google Scholar]
- Ganeshpurkar, A. , Gutti, G. , & Singh, S. K. (2019). RNA‐dependent RNA polymerases and their emerging roles in antiviral therapy. Viral Polymerases, Structures, Functions and Roles as Antiviral Drug Targets, 1–42. 10.1016/B978-0-12-815422-9.00001-2 [DOI] [Google Scholar]
- Gentile, D. , Patamia, V. , Scala, A. , Sciortino, M. T. , Piperno, A. , & Rescifina, A. (2020). Putative inhibitors of SARS‐CoV‐2 main protease from a library of marine natural products: A virtual screening and molecular modeling study. Marine Drugs, 18(4), 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, R. , Chakraborty, A. , Biswas, A. , & Chodhary, S. (2020). Evaluation of green tea polyphenols as novel corona virus (SARSCoV‐2) main protease (Mpro) inhibitors‐an in silico docking and molecular dynamics simulation study. Journal of Biomolecular Structure and Dynamics, 39(12), 4362–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, R. , Palbag, S. , & Ghosh, D. (2020). In‐silico screening of active molecules from medicinal plant resources for controlling SARS‐CoV‐2 infection. Journal of Ayurvedic and Herbal Medicine, 6(3), 149–153. [Google Scholar]
- Giofrè, S. V. , Napoli, E. , Iraci, N. , Speciale, A. , Cimino, F. , Muscarà, C. , … Saija, A. (2021). Interaction of selected terpenoids with two SARS‐CoV‐2 key therapeutic targets: An in silico study through molecular docking and dynamics simulations. Computers in Biology and Medicine, 134, 104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc, A. , Sumera, W. , Rath, M. , & Niedzwiecki, A. (2021). Phenolic compounds disrupt spike‐mediated receptor‐binding and entry of SARS‐CoV‐2 pseudo‐virions. PLoS One, 16(6), e0253489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, P. Y. (2020). Exploring active compounds of Jinhua Qinggan granules for prevention of COVID‐19 based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs, 24, 1685–1693. [Google Scholar]
- Goyzueta‐Mamani, L. D. , Barazorda‐Ccahuana, H. L. , Mena‐Ulecia, K. , & Chávez‐Fumagalli, M. A. (2021). Antiviral activity of metabolites from Peruvian plants against SARS‐CoV‐2: An in silico approach. Molecules, 26(13), 3882. 10.3390/molecules26133882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Große, M. , Ruetalo, N. , Layer, M. , Hu, D. , Businger, R. , Rheber, S. , … Schubert, U. (2021). Quinine inhibits infection of human cell lines with SARS‐CoV‐2. Viruses, 13(4), 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. , Singh, A. K. , Kushwaha, P. P. , Prajapati, K. S. , Shuaib, M. , Senapati, S. , & Kumar, S. (2021). Identification of potential natural inhibitors of SARS‐CoV2 main protease by molecular docking and simulation studies. Journal of Biomolecular Structure and Dynamics, 39(12), 4334–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, T. Y. , Wu, S. L. , Chen, J. C. , Li, C. C. , & Hsiang, C. Y. (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin‐converting enzyme 2 interaction. Antiviral Research, 74(2), 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne, J. R. , & Vohl, M. C. (2020). Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS‐CoV illness severity. American Journal of Physiology‐Endocrinology and Metabolism, 318(5), E830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D. , Yu, H. , Wang, T. , Yang, H. , Yao, R. , & Liang, Z. (2021). Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Journal of Medical Virology, 93(1), 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L. , Yuen, T. T. T. , Ye, Z. , Liu, S. , Zhang, G. , Chu, H. , & Yue, J. (2021). Berbamine inhibits SARS‐CoV‐2 infection by compromising TRPMLs‐mediated endolysosomal trafficking of ACE2. Signal Transduction and Targeted Therapy, 6(1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34(10), 2471–2492. [DOI] [PubMed] [Google Scholar]
- Jackson, C. B. , Farzan, M. , Chen, B. , & Choe, H. (2022). Mechanisms of SARS‐CoV‐2 entry into cells. Nature Reviews Molecular Cell Biology, 23(1), 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaz, Z. , Al‐Jassani, M. J. , & Haruna, U. (2020). Screening of common herbal medicines as promising direct inhibitors of Sars‐Cov‐2 in silico. Annual Research & Review in Biology, 35(8), 53–67. 10.9734/arrb/2020/v35i830260 [DOI] [Google Scholar]
- Kandeil, A. , Mostafa, A. , Kutkat, O. , Moatasim, Y. , Al‐Karmalawy, A. A. , Rashad, A. A. , … Ali, M. A. (2021). Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2. Pathogens, 10(6), 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. M. , Chung, Y. S. , Jo, H. J. , Lee, N. J. , Kim, M. S. , Woo, S. H. , … Han, M. G. (2020). Identification of coronavirus isolated from a patient in Korea with COVID‐19. Osong Public Health and Research Perspectives, 11(1), 3–7. 10.24171/j.phrp.2020.11.1.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, J. , Arai, H. , Iida, H. , Mukai, J. , Furukawa, K. , Ohtsu, S. , … Uemura, N. (2021). A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID‐19. Clinical and Translational Science, 14(5), 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, K. B. , Shin, H. J. , Kim, H. S. , Kim, B. T. , Kim, S. J. , & Kim, C. (2020). Repurposing screens of FDA‐approved drugs identify 29 inhibitors of SARS‐CoV‐2. Journal of Microbiology and Biotechnology, 30, 1843–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Choudhir, G. , Shukla, S. K. , Sharma, M. , Tyagi, P. , Bhushan, A. , & Rathore, M. (2021). Identification of phytochemical inhibitors against main protease of COVID‐19 using molecular modeling approaches. Journal of Biomolecular Structure and Dynamics, 39(10), 3760–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Y. , Han, T. , Lao, Z. , Li, G. , Xiao, J. , & Liu, X. (2021). Phillyrin for COVID‐19 and influenza Co‐infection: A potential therapeutic strategy targeting host based on bioinformatics analysis. Frontiers in Pharmacology, 12, 754241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. W. , Tsai, F. J. , Tsai, C. H. , Lai, C. C. , Wan, L. , Ho, T. Y. , … Chao, P. D. L. (2005). Anti‐SARS coronavirus 3C‐like protease effects of Isatis indigotica root and plant‐derived phenolic compounds. Antiviral Research, 68(1), 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. C. , Ho, C. T. , Chuo, W. H. , Li, S. , Wang, T. T. , & Lin, C. C. (2017). Effective inhibition of MERS‐CoV infection by resveratrol. BMC Infectious Diseases, 17(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue, J. , Chakraborty, A. R. , Johnson, R. , Goyal, G. , Rodas, M. , Taylor, L. J. , … Frieman, M. B. (2022). PIKfyve‐specific inhibitors restrict replication of multiple coronaviruses in vitro but not in a murine model of COVID‐19. Communications Biology, 5(1), 1–9. 10.1038/s42003-022-03766-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung, J. , Lin, Y. S. , Yang, Y. H. , Chou, Y. L. , Shu, L. H. , Cheng, Y. C. , … Wu, C. Y. (2020). The potential chemical structure of anti‐SARS‐CoV‐2 RNA‐dependent RNA polymerase. Journal of Medical Virology, 92(6), 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, P. , Liu, D. , & Li, J. (2020). Pharmacological perspective: Glycyrrhizin may be an efficacious therapeutic agent for COVID‐19. International Journal of Antimicrobial Agents, 55(6), 105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. , Li, R. , Pan, W. , Huang, W. , Liu, B. , Xie, Y. , … Yang, Z. (2020). Phillyrin (KD‐1) exerts anti‐viral and anti‐inflammatory activities against novel coronavirus (SARS‐CoV‐2) and human coronavirus 229 E (HCoV‐229 E) by suppressing the nuclear factor kappa B (NF‐κB) signaling pathway. Phytomedicine, 78, 153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. , Pan, W. , Li, R. , Liu, B. , Li, C. , Xie, Y. , … Yang, Z. (2020). Liu Shen capsule shows antiviral and anti‐inflammatory abilities against novel coronavirus SARS‐CoV‐2 via suppression of NF‐κB signaling pathway. Pharmacological Research, 158, 104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, I. S. , Jarrar, Y. B. , Alshaer, W. , & Ismail, S. (2020). SARS‐CoV‐2 entry in host cells‐multiple targets for treatment and prevention. Biochimie, 175, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, J. S. , Johnson, J. B. , Steel, J. C. , Broszczak, D. A. , Neilsen, P. M. , Walsh, K. B. , & Naiker, M. (2020). Natural product‐derived phytochemicals as potential agents against coronaviruses: A review. Virus Research, 284, 197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, P. S. (2006). The molecular biology of coronaviruses. Advances in Virus Research, 66, 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehany, T. , Khalifa, I. , Barakat, H. , Althwab, S. A. , Alharbi, Y. M. , & El‐Sohaimy, S. (2021). Polyphenols as promising biologically active substances for preventing SARS‐CoV‐2: A review with research evidence and underlying mechanisms. Food Bioscience, 40, 100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merarchi, M. , Dudha, N. , Das, B. C. , & Garg, M. (2021). Natural products and phytochemicals as potential anti‐SARS‐CoV‐2 drugs. Phytotherapy Research, 35(10), 5384–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre, S. , Srivastava, T. , Naik, S. , & Patravale, V. (2021). Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID‐19: A review. Phytomedicine, 85, 153286. 10.1016/j.phymed.2020.153286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, T. , Miyakawa, K. , Jeremiah, S. S. , Yamaoka, Y. , Sada, M. , Kuniyoshi, T. , … Ryo, A. (2021). All‐trans retinoic acid exhibits antiviral effect against SARS‐CoV‐2 by inhibiting 3CLpro activity. Viruses, 13(8), 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogidigo, J. O. , Iwuchukwu, E. A. , Ibeji, C. U. , Okpalefe, O. , & Soliman, M. E. (2022). Natural phyto, compounds as possible noncovalent inhibitors against SARS‐CoV2 protease: Computational approach. Journal of Biomolecular Structure and Dynamics, 40(5), 2284–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, H. , Watashi, K. , Saso, W. , Shionoya, K. , Iwanami, S. , Hirokawa, T. , … Nishioka, K. (2020). Multidrug treatment with nelfinavir and cepharanthine against COVID‐19. bioRxiv. 10.1101/2020.04.14.039925 [DOI]
- Oren, O. , Yang, E. H. , Gluckman, T. J. , Michos, E. D. , Blumenthal, R. S. , & Gersh, B. J. (2020). Use of chloroquine and hydroxychloroquine in COVID‐19 and cardiovascular implications: Understanding safety discrepancies to improve interpretation and design of clinical trials. Circulation. Arrhythmia and Electrophysiology, 13(6), e008688. 10.1161/CIRCEP.120.008688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, X. , Liu, Y. , Lei, X. , Li, P. , Mi, D. , Ren, L. , … Qian, Z. (2020). Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nature Communications, 11(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, P. , Rane, J. S. , Chatterjee, A. , Kumar, A. , Khan, R. , Prakash, A. , & Ray, S. (2021). Targeting SARS‐CoV‐2 spike protein of COVID‐19 with naturally occurring phytochemicals: An in silico study for drug development. Journal of Biomolecular Structure and Dynamics, 39(16), 6306–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso, I. L. , Revel, J. S. , & Stevens, J. F. (2020). Potential use of polyphenols in the battle against COVID‐19. Current Opinion in Food Science, 32, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. Y. , Jeong, H. J. , Kim, J. H. , Kim, Y. M. , Park, S. J. , Kim, D. , … Ryu, Y. B. (2012). Diarylheptanoids from Alnus japonica inhibit papain‐like protease of severe acute respiratory syndrome coronavirus. Biological and Pharmaceutical Bulletin, 35, 2036–2042. [DOI] [PubMed] [Google Scholar]
- Park, J. Y. , Yuk, H. J. , Ryu, H. W. , Lim, S. H. , Kim, K. S. , Park, K. H. , … Lee, W. S. (2017). Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 32(1), 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau, S. , Nehme, Z. , Haidar Ahmad, S. , Daouad, F. , Van Assche, J. , Wallet, C. , … Herbein, G. (2021). Resveratrol inhibits HCoV‐229 E and SARS‐CoV‐2 coronavirus replication in vitro. Viruses, 13(2), 354. 10.3390/v13020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala, B. , Patras, A. , & Dash, C. (2021). Phycobilins as potent food bioactive broad‐spectrum inhibitors against proteases of SARS‐CoV‐2 and other coronaviruses: A preliminary study. Frontiers in Microbiology, 12, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, L. , Vernès, L. , & Cadoret, J. P. (2021). Docking and in silico toxicity assessment of Arthrospira compounds as potential antiviral agents against SARS‐CoV‐2. Journal of Applied Phycology, 33(3), 1579–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno, A. , Padey, B. , Dubois, J. , Julien, T. , Traversier, A. , Dulière, V. , … Terrier, O. (2020). In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS‐CoV‐2. Antiviral Research, 181, 104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praditya, D. , Kirchhoff, L. , Brüning, J. , Rachmawati, H. , Steinmann, J. , & Steinmann, E. (2019). Anti‐infective properties of the golden spice curcumin. Frontiers in Microbiology, 10, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth, D. S. N. B. K. , Murahari, M. , Chandramohan, V. , Panda, S. P. , Atmakuri, L. R. , & Guntupalli, C. (2021). In silico identification of potential inhibitors from cinnamon against main protease and spike glycoprotein of SARS CoV‐2. Journal of Biomolecular Structure and Dynamics, 39(13), 4618–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, J. , Li, Y. S. , Zeng, R. , Liu, F. L. , Luo, R. H. , Huang, C. , … Yang, S. (2021). SARS‐CoV‐2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science, 371(6536), 1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Q. , Huang, Y. , Liu, X. , Huang, F. , Li, X. , Cui, L. , … Luo, L. (2020). Potential therapeutic effect of traditional Chinese medicine on coronavirus disease 2019: A review. Frontiers in Pharmacology, 11, 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia, K. , Kilianski, A. , Baez‐Santos, Y. M. , Baker, S. C. , & Mesecar, A. (2014). Structural basis for the ubiquitin‐linkage specificity and deISGylating activity of SARS‐CoV papain‐like protease. PLoS Pathogens, 10, e1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remali, J. , & Aizat, W. M. (2021). A review on plant bioactive compounds and their modes of action against coronavirus infection. Frontiers in Pharmacology, 11, 589044. 10.3389/fphar.2020.589044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva, L. , Yuan, S. , Yin, X. , Martin‐Sancho, L. , Matsunaga, N. , Pache, L. , … Chanda, S. K. (2020). Discovery of SARS‐CoV‐2 antiviral drugs through large‐scale compound repurposing. Nature, 586(7827), 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk, J. G. , Forthal, D. N. , Kalantar‐Zadeh, K. , Mehra, M. R. , Lavie, C. J. , Rizk, Y. , … Lewin, J. C. (2021). Expanded access programs, compassionate drug use, and emergency use authorizations during the COVID‐19 pandemic. Drug Discovery Today, 26(2), 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng, L. , Yunlong, H. , Jicheng, H. , & Weiqi, P. (2020). Lianhuaqingwen exerts anti‐viral and anti‐inflammatory activity against novel coronavirus (SARS‐CoV‐2). Pharmacological Research, 156, 104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa‐Ngiamsuntorn, K., Suksatu, A., Pewkliang, Y., Thongsri, P., Kanjanasirirat, P., Manopwisedjaroen, S., … Kongsomros, S. (2021). Anti‐SARS‐CoV‐2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. Journal of Natural Products, 8494, 1261–1270. [DOI] [PubMed] [Google Scholar]
- Salas Rojas, M. , Silva Garcia, R. , Bini, E. , Pérez de la Cruz, V. , León Contreras, J. C. , Hernández Pando, R. , … Pineda, B. (2021). Quinacrine, an antimalarial drug with strong activity inhibiting SARS‐CoV‐2 viral replication in vitro. Viruses, 13(1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki, S. G. , & Sawicki, D. L. (2005). Coronavirus transcription: A perspective. In Enjuanes L. (Ed.), Coronavirus replication and reverse genetics Current topics in microbiology and immunology, Vol. 287. Berlin, Heidelberg: Springer. 10.1007/3-540-26765-4_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehailia, M. , & Chemat, S. (2021). Antimalarial‐agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31‐binding hotspots of SARS‐CoV‐2 spike protein than hydroxychloroquine: Potential repurposing of artenimol for COVID‐19. Journal of Biomolecular Structure and Dynamics, 39(16), 6184–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaldam, M. A. , Yahya, G. , Mohamed, N. H. , Abdel‐Daim, M. M. , & Al Naggar, Y. (2021). In silico screening of potent bioactive compounds from honeybee products against COVID‐19 target enzymes. Environmental Science and Pollution Research, 28(30), 40507–40514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J. , Wan, Y. , Luo, C. , Ye, G. , Geng, Q. , Auerbach, A. , & Li, F. (2020). Cell entry mechanisms of SARS‐CoV‐2. Proceedings of the National Academy of Sciences, 117(21), 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, T. H. , Huang, Y. L. , Chen, C. C. , Pi, W. C. , Hsu, Y. L. , Lo, L. C. , … Lin, C. H. (2020). Andrographolide and its fluorescent derivative inhibit the main proteases of 2019‐nCoV and SARS‐CoV through covalent linkage. Biochemical and Biophysical Research Communications, 533(3), 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, S. K. , Shakya, A. , Prasad, S. K. , Singh, S. , Gurav, N. S. , Prasad, R. S. , & Gurav, S. S. (2021). An in‐silico evaluation of different Saikosaponins for their potency against SARS‐CoV‐2 using NSP15 and fusion spike glycoprotein as targets. Journal of Biomolecular Structure and Dynamics, 39(9), 3244–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, V. , Yadav, A. , & Sarkar, P. (2022). Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS‐CoV2. Materials Today: Proceedings, 49, 2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H. , Yao, S. , Zhao, W. , Li, M. , J Liu, J. , Shang, W.J. , Xie, H. et al. (2020). Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS‐CoV‐2 3CL protease in vitro. BioRxiv. 10.1101/2020.04.13.038687 [DOI]
- Su, H. , Yao, S. , Zhao, W. , Zhang, Y. , Liu, J. , Shao, Q. , … Xu, Y. (2021). Identification of pyrogallol as a warhead in design of covalent inhibitors for the SARS‐CoV‐2 3CL protease. Nature Communications, 12(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H. X. , Yao, S. , Zhao, W. F. , Li, M. J. , Liu, J. , Shang, W. J. , … Xu, Y. C. (2020). Anti‐SARS‐CoV‐2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacologica Sinica, 41(9), 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swargiary, A. , Mahmud, S. , & Saleh, M. A. (2022). Screening of phytochemicals as potent inhibitor of 3‐chymotrypsin and papain‐like proteases of SARS‐CoV2: An in silico approach to combat COVID‐19. Journal of Biomolecular Structure and Dynamics, 40(5), 2067–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, K. (2020). In vitro and animal models for SARS‐CoV‐2 research. Trends in Pharmacological Sciences, 41(8), 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand, L. , Bormann, M. , Alt, M. , Schipper, L. , Heilingloh, C. S. , Steinmann, E. , … Krawczyk, A. (2021). Glycyrrhizin effectively inhibits SARS‐CoV‐2 replication by inhibiting the viral main protease. Viruses, 13(4), 609. 10.3390/v13040609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S. , Twilley, D. , Esmear, T. , Oosthuizen, C. B. , Reid, A. M. , Nel, M. , & Lall, N. (2020). Anti‐SARS‐CoV natural products with the potential to inhibit SARS‐CoV‐2 (COVID‐19). Frontiers in Pharmacology, 11, 561334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, S. , Arokiyaraj, S. , Saravanan, M. , & Dhanraj, M. (2020). Molecular docking studies on the anti‐viral effects of compounds from Kabasura kudineer on SARS‐CoV‐2 3CLpro. Frontiers in Molecular Biosciences, 7, 613401. 10.3389/fmolb.2020.613401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahedi, H. M. , Ahmad, S. , & Abbasi, S. W. (2021). Stilbene‐based natural compounds as promising drug candidates against COVID‐19. Journal of Biomolecular Structure and Dynamics, 39(9), 3225–3234. [DOI] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Xu, B. , Zhang, Y. , Duan, Y. , Gao, R. , He, H. , … Li, J. (2021). Efficacy and safety of traditional Chinese medicine in coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Frontiers in Pharmacology, 12, 609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhang, X. , Omarini, A. B. , & Li, B. (2020). Virtual screening for functional foods against the main protease of SARS‐CoV‐2. Journal of Food Biochemistry, 44(11), e13481. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , … Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Research, 30(3), 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , & Yang, L. (2020). Turning the tide: Natural products and natural‐product‐inspired chemicals as potential counters to SARS‐CoV‐2 infection. Frontiers in Pharmacology, 11, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warowicka, A. , Nawrot, R. , & Goździcka‐Józefiak, A. (2020). Antiviral activity of berberine. Archives of Virology, 165(9), 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayasinghe, Y. S. , Bhansali, P. , Viola, R. E. , Kamal, M. A. , & Poddar, N. K. (2021). Natural products: A rich source of antiviral drug lead candidates for the management of COVID‐19. Current Pharmaceutical Design, 27(33), 3526–3550. [DOI] [PubMed] [Google Scholar]
- Wu, C. Y. , Jan, J. T. , Ma, S. H. , Kuo, C. J. , Juan, H. F. , Cheng, Y. S. E. , … Wong, C. H. (2004). Small molecules targeting severe acute respiratory syndrome human coronavirus. Proceedings of the National Academy of Sciences, 101(27), 10012–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, B. , Shen, X. , He, Y. , Pan, X. , Liu, F. L. , Wang, Y. , … Gao, Z. (2021). SARS‐CoV‐2 envelope protein causes acute respiratory distress syndrome (ARDS)‐like pathological damages and constitutes an antiviral target. Cell Research, 31(8), 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian, Y. , Zhang, J. , Bian, Z. , Zhou, H. , Zhang, Z. , Lin, Z. , & Xu, H. (2020). Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharmaceutica Sinica B, 10(7), 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D. , & Liu, Z. (2021). Effectiveness and safety of traditional Chinese medicine in treating COVID‐19: Clinical evidence from China. Aging and Disease, 12(8), 1850–1856. 10.14336/AD.2021.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Wei, J. , Huang, T. , Lei, L. , Shen, C. , Lai, J. , … Liu, Y. (2021). Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in cultured Vero cells. Phytotherapy Research, 35, 1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, B. , Yang, R. , Ma, Y. , Zhou, S. , Zhang, X. , & Liu, Y. (2017). A systematic review of the active saikosaponins and extracts isolated from radix Bupleuri and their applications. Pharmaceutical Biology, 55(1), 620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi, K. , Musall, K. , Oo, A. , Cao, D. , Liang, B. , Hassandarvish, P. , … Schinazi, R. F. (2021). Baicalein and baicalin inhibit SARS‐CoV‐2 RNA‐dependent‐RNA polymerase. Microorganisms, 9(5), 893. 10.3390/microorganisms9050893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. H. , Wu, K. L. , Zhang, X. , Deng, S. Q. , & Peng, B. (2020). In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. Journal of Integrative Medicine, 18(2), 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrieq, R. , Ahmad, I. , Snoussi, M. , Noumi, E. , Iriti, M. , Algahtani, F. D. , … Kadri, A. (2021). Tomatidine and patchouli alcohol as inhibitors of SARS‐CoV‐2 enzymes (3CLpro, PLpro and NSP15) by molecular docking and molecular dynamics simulations. International Journal of Molecular Sciences, 22(19), 10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


