Abstract
The global epidemic of coronavirus disease 2019 (COVID‐19) endangers more and more people. Many studies on cutaneous manifestations related to COVID‐19 have emerged, but their prevalence has varied widely. The objective of this study was to conduct a meta‐analysis estimating the prevalence of skin manifestations in COVID‐19. Four databases PubMed, Web of Science, CBM, and CNKI were searched, and the results were screened by two reviewers. A random‐effects model was used to evaluate the overall prevalence. Heterogeneity was assessed by I 2. Further subgroup analyses were conducted by region, sample size, sex, age, and severity of COVID‐19. A funnel plot and Egger's test were performed to assess publication bias. The pooled prevalence of cutaneous manifestation of 61 089 patients in 33 studies was 5.6% (95% confidence intervals [CI] = 0.040–0.076, I 2 = 98.3%). Severity of COVID‐19 was probably the source of heterogeneity. Studies with sample size <200 report higher prevalence estimates (10.2%). The prevalence of detailed types was as follows: maculopapular rash 2%, livedoid lesions 1.4%, petechial lesions 1.1%, urticaria 0.8%, pernio‐like lesions 0.5%, vesicular lesions 0.3%. Petechial lesions and livedoid lesions contain a higher proportion of severe patients than other skin manifestations. The prevalence rates of pernio‐like lesions, urticaria and petechial lesions vary greatly in different regions.
Keywords: COVID‐19, cutaneous manifestations, meta‐analysis, prevalence, systematic review
1. INTRODUCTION
In December 2019, a new type of virus named severe acute respiratory syndrome, coronavirus type 2, emerged and has spread to nearly every corner of the globe causing societal instability, and it has generated unprecedented global concern and responses. Globally, as of 3 June 2022, there have been 528 816 317 confirmed cases of COVID‐19, reported to the World Health Organization (WHO). 1
The most effective way to address the pandemic is the prevention of further infection. Thus, early diagnosis and subsequent quarantine is an effective strategy. The symptoms of COVID‐19 infection are various, it can be asymptomatic, 2 and it can span from a mild influenza‐like illness to life‐threatening complications. 3 While the lung is the primary viral target, a significant proportion of patients presented initially with atypical symptoms, such as diarrhea and nausea. In some severe cases, it may be associated with cellular immune deficiency, coagulation activation, myocardia injury, hepatic injury, and kidney injury. 4 More and more studies have reported that COVID‐19 is associated with dermatological manifestations. The skin, including mucous membranes, is an organ that frequently presents lesions caused by viral infections. 5 In a viral infection, exanthems usually occur as an immune hypersensitivity response to viral DNA or RNA. 6 Jamiolkowski et al. 7 and Guarneri C et al. 8 showed the significance of paying attention to skin manifestations in the diagnosis of COVID‐19. However, the prevalence of skin manifestations in COVID‐19 patients varies widely, from 0.2% to 20.4%. 9 , 10 Evidence is accumulating that cutaneous manifestations associated with COVID‐19 are extremely polymorphic. 11 A study in Spain has identified five common skin symptoms in 375 patients, containing pseudo‐chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis. 12 With increasing research, the main types of reported skin manifestations connected with COVID‐19 are also distinct. 13 , 14 , 15
Although there are two meta‐analyses 16 , 17 focusing on the prevalence of skin lesions in COVID‐19 patients, their prevalence rates are different, with 5.69% in Rajan et al. 16 study and 1.0% in Sameni et al. 17 study. Moreover, many of the included studies were case reports. Therefore, with the increasing number of studies, the data needs to be updated in time. In this study, we performed a meta‐analysis to estimate the prevalence of cutaneous manifestations and their main types related to COVID‐19.
2. MATERIALS AND METHODS
2.1. Search strategy
The literature search was performed using four databases (PubMed, Web of Science, CBM, and CNKI) from 1 December 2019 to 25 April 2022. The following keywords were used: ‘SARS‐CoV‐2’, ‘Coronavirus Disease 2019 Virus’, ‘2019 Novel Coronavirus’, ‘2019 Novel Coronaviruses’, ‘Coronavirus, 2019 Novel’, ‘Novel Coronavirus, 2019’, ‘Wuhan Seafood Market Pneumonia Virus’, ‘SARS‐CoV‐2 Virus’, ‘SARS CoV 2 Virus’, ‘SARS‐CoV‐2 Viruses’, ‘Virus, SARS‐CoV‐2’, ‘2019‐nCoV’, ‘COVID‐19 Virus’, ‘COVID 19 Virus’, ‘COVID‐19 Viruses’, ‘Virus, COVID‐19’, ‘Wuhan Coronavirus’, ‘Coronavirus, Wuhan’, ‘SARS Coronavirus 2’, ‘Coronavirus 2, SARS’ or ‘Severe Acute Respiratory Syndrome Coronavirus 2’ and ‘exanthema’, ‘skin manifestation’, ‘skin disease’, ‘exanthema’, ‘cutaneous’, ‘rash’, ‘chilblain‐like’, ‘vesicular’, ‘maculopapular’, ‘pernio’, ‘urticarial’, ‘livedo’, ‘vesicular’, or ‘petechial’.
2.2. Study setting and design
Our study is a systematic literature review conducted based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 18
2.3. Selection criteria
The inclusion criteria were as follows: (1) Study population: patients diagnosed with COVID‐19 irrespective of age and sex; (2) Study design: cross‐sectional studies, case–control studies, and retrospective/prospective cohort studies; (3) Outcome: prevalence rate of cutaneous manifestations related to COVID‐19 was the outcome measure, and (4) no language limit.
Studies with any of the following exclusion criteria were excluded from our study: (1) Studies exclusively reporting drug‐related skin manifestations or skin adverse events related to personal protective equipment related to COVID‐19; (2) Case reports.
2.4. Quality assessment
Quality assessment was performed by two researchers using Agency for Health Research and Quality (AHRQ) for the cross‐sectional study and the Newcastle–Ottawa scale for the cohort study.
2.5. Data extraction
Two independent researchers pulled out the data from the articles included. All disagreements were resolved by consensus with a third researcher. Information about the enrolled studies is listed in Table 1, including: (1) the first author's name, (2) publication time, (3) country, (4) number of total patients diagnosed with COVID‐19, (5) number of patients with cutaneous manifestations, and (6) type of cutaneous manifestations and their respective numbers.
TABLE 1.
Characteristics of all studies describing cutaneous manifestations related to COVID‐19 in the meta‐analysis
| Author | Published time | Country | Total patients | Patients with cutaneous manifestations | Types of cutaneous manifestations | Quality evaluation score |
|---|---|---|---|---|---|---|
| Mendez 57 | September 2020 | Spain | 75 | 14 | Pernio‐like lesions (6), maculopapular rash (4), urticaria (2), livedo reticularis‐like lesions (1), vesicular eruption (1) | 9 |
| Askin 58 | July 2020 | Turkey | 210 | 34 (17 cases of erythema caused by hand washing and 1 case of itching related to vancomycin were excluded) | Maculopapular rash (12), urticaria (7), purpuric rash (4), necrosis (4), enanthema and apthous stomatitis (3), vesicular eruption (3), pernio (1) | 6 |
| Fernandez 59 | December 2020 | Spain | 144 | 5 | Livedo reticularis‐like lesions (4), maculopapular rash (1) | 7 |
| Dalal 60 | June 2020 | India | 102 | 13 | Pruritus (8), maculopapular rash (3), urticaria (2) | 6 |
| De Giorgi 11 | May 2020 | Italy and China | 678 | 53 | Erythematous rash (37), urticaria (14), vesiculation (2) | 7 |
| Jimenez 61 | July 2020 | America | 21 | 6 | Erythema multiforme‐like (3), purpuric (2), papulovesicular (1) | 7 |
| Rerknimitr 20 | September 2020 | Thailand | 153 | 12 (5 cases were not sure whether related to COVID‐19, and 6 cases caused by complications or treatment or equipment were excluded) | Urticaria (5), maculopapular rash (4), vesicular (2), necrosis (1) | 7 |
| Recalcati 9 | March 2020 | Italy | 88 | 18 | Erythematous rash (14), urticaria (3), chickenpox‐like vesicles (1) | 6 |
| Guan 10 | April 2020 | China | 1099 | 2 | – | 9 |
| Andina 62 | April 2021 | Spain | 50 | 18 | Erythematous macules and papules (17), acral ischemic lesion (1) | 8 |
| Pangti 63 | August 2021 | India | 138 | 10 | Weals (3), purpura and petechiae (3), Chilblain‐like (1), macular erythematous rash (1), desquamation (1), aphthous ulcers (1) | 6 |
| Thuangtong 15 | June 2021 | Thailand | 93 | 7 (1 case of psoriasis was excluded as the basic disease) | Maculopapular rash (3), urticaria (2), petechiae (1), eczema (1) | 8 |
| Rekhtman 24 | April 2021 | America | 296 | 35 | Ulcer (13), purpura (9), necrosis (5), nonspecific erythema (4), morbilliform eruption (4), pernio‐like lesions (4), vesicles (1) | 8 |
| Yildiray 64 | June 2021 | Turkey | 266 | 5 | Urticaria (3), vesicular (2) | 7 |
| Al Ali 13 | July 2021 | Oman | 374 | 5 (6 cases related to drugs and 1 case of acute attack of atopic dermatitis were excluded) | Maculopapular rash (2), transient pruritic erythema (1), palmoplantar erythema (1), urticaria (1) | 10 |
| G Brancacci 65 | March 2021 | Italy | 417 | 7 | Chilblain‐like (4), purpuric (2), papular and pustular rash (1) | 6 |
| Unterluggauer 14 | July 2021 | Austria | 102 | 16 (1 case caused by drugs was excluded) | Livedo reticularis (6), splinter hemorrhage‐like lesions (4), subcutaneous nodules (2), others (4) | 7 |
| Parcha 25 | May 2021 | America | 1230 | 887 | – | 8 |
| Sun 66 | August 2020 | China | 3128 | 52 | Urticaria (27), erythema and papules (15), scratch (5), rhagades (3), chilblains (2) | 7 |
| Gaspari 27 | April 2021 | Italy | 1409 | 21 | – | 6 |
| Jakhar 67 | December 2021 | India | 71 | 13 | Urticarial lesions (4), macular erythema (2), morbilliform rash (2), mucosal manifestations (5) | 6 |
| Bryan 68 | 2022 | America | 1086 | 10 | Erythematous papules (4), blisters (3), pruritic rash (1), urticarial rash (1), diffuse vitiligo‐like rash (1) | 6 |
| Tan 23 | June 2022 | Philippines | 507 | 39 | Morbilliform rash (17), livedo reticularis (7), petechial rash (6), vesicular rash (4), pernio or chilblains (2), urticaria (3) | 6 |
| Zengarini 69 | January 2022 | Italy | 1053 | 0 | – | 9 |
| Vera 22 | March 2022 | Spain | 2929 | 96 | – | 8 |
| Murugan 26 | March 2022 | India | 988 | 23 | – | 8 |
| Sugai 70 | March 2022 | Japan | 1245 | 7 | – | 6 |
| Rekhtman 71 | February 2021 | America | 12 | 4 | Nonspecific erythema (3), morbilliform (1) | 7 |
| Hedou 72 | April 2020 | France | 103 | 5 | Erythematous rash (2), urticaria (2), other (1) | 6 |
| Mascitti 73 | October 2021 | Thailand | 28 957 | 2756 | – | 7 |
| Giavedoni 74 | October 2020 | Spain | 2761 | 58 | Maculopapular rash (12), urticaria (4), chilblain‐like lesions (17), papulo‐vesicular eruptions (8), livedo reticularis (4), other (13) | 6 |
| Joob 75 | May 2020 | Thailand | 48 | 1 | Maculopapular rash (1) | 5 |
| Garg 76 | April 2020 | America | 180 | 2 | – | 8 |
2.6. Statistical analyses
The pooled prevalence of cutaneous manifestations related to COVID‐19 was calculated using a random‐effects model. Statistical heterogeneity was assessed by I 2 and p‐value. I 2: 0%–25% indicates no heterogeneity; 25%–50%, modest heterogeneity; 50%, high heterogeneity. When the heterogeneity is higher than 50%, random‐effects models are used to obtain estimates, while the heterogeneity is lower than 50%, we use fixed‐effects models. We conducted a sensitivity analysis to evaluate the stability of the results and investigate each study's influence by excluding a single study sequentially. A funnel plot and Egger's test were performed to assess publication bias. All statistical analyses were carried out by STATA 14.0. A p‐value <0.05 was considered as a statistical difference.
3. RESULTS
3.1. Description of eligible studies
The initial search yielded 6237articles. We removed 1868 duplicates and assessed 4369 articles for title/abstract screening, among them 101 articles were selected for full‐text reading, and seven articles were added by manual searching. After screening the literature based on the inclusion criteria, 33 articles were identified for analysis (Figure 1). Among them, 24 articles described specific rash types. Ten studies focused on adults and five were related to children. The countries of origin were Spain, Turkey, India, China, Italy, America, Thailand, Oman, Philippines, Austria, Japan, and France. Most of the research was in Asia containing the largest patient population (n = 37 379). In addition, 33 participants with skin manifestations were excluded due to excessive hand washing, drug allergy, and recurrence of original skin disease in five articles. 13 , 14 , 15 , 19 , 20 In total, 33 studies including 61 089 patients met the inclusion criteria and were selected for meta‐analysis. The characteristics of the selected studies were summarized in Table 1.
FIGURE 1.
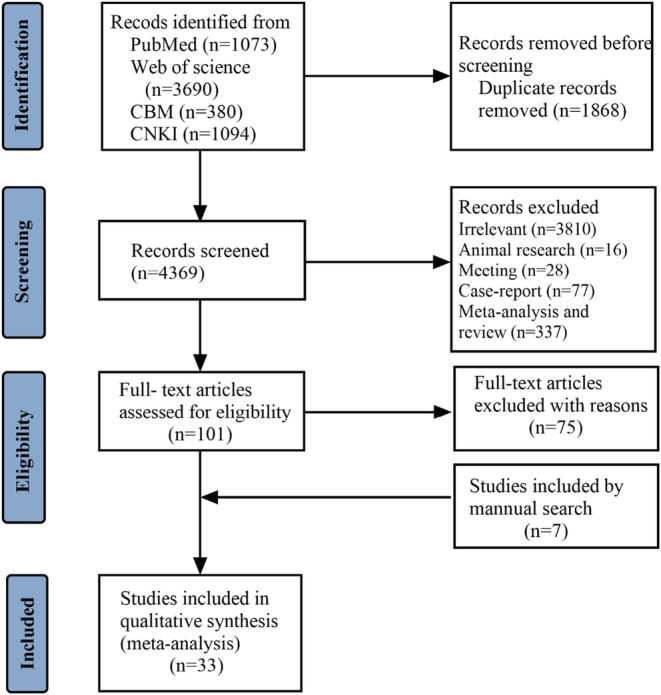
Flow diagram of the study selection process
3.2. Results of the meta‐analysis
The severity and impacts of COVID‐19 around the world have not been uniformly distributed across populations. 21 A total of 16 among 33 studies describe the detailed number of severe and critical COVID‐19 patients, with the pooled prevalence of 15.7% (1597/24 951). There were 10 studies in Asia, occupying the highest prevalence of 21.5% (1306/5006). The lowest prevalence estimates of 1.0% (118/12 306) was conducted by America, which was contained in one study. The pooled prevalence of Europe in five studies lies between Asia and America, accounting for 11.9% (173/7639).
There were six countries in seven studies describing the mortality of COVID‐19 patients, with the total mortality rate of 1.96% (442/22 535). The lowest mortality rate 0.02% (1/5933) was reported by Spain. 22 The mortality rate of 21.89% (111/507) reported by Philippines ranked first. 23 Furthermore, the mortality rates in the other five studies from low to high were as follows: less than 0.43% (54/12 599) in America, 24 , 25 1.01% (1/988) in India, 26 1.36% (15/1099) in China, 10 17.81% (251/1409) in Italy, 27 respectively.
3.3. Meta‐analysis of the prevalence of cutaneous manifestations in COVID‐19 patients
The overall pooled prevalence of cutaneous manifestations in COVID‐19 patients was 5.6%, which was shown in the forest plot in Figure 2, with significant heterogeneity stated among studies (95% confidence interval [CI] = 0.040–0.076, n = 61 089, I 2 = 98.3%). Sensitivity analysis excluding one study, did not significantly affect the overall prevalence of COVID‐19 patients with skin lesions, which indicated that our analysis was stable (Figure 3). There were signs of publication bias when the funnel plot of overall skin manifestations was examined visually (Figure 4), however, Egger's test did not show publication bias (p = 0.274).
FIGURE 2.
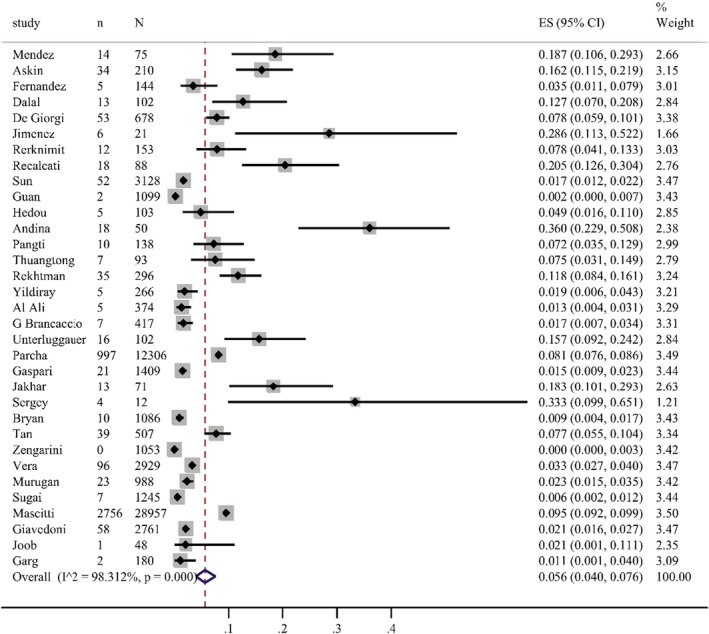
Forest plot showing the prevalence rate of overall cutaneous manifestations in COVID‐19 patients, with 95% CI
FIGURE 3.
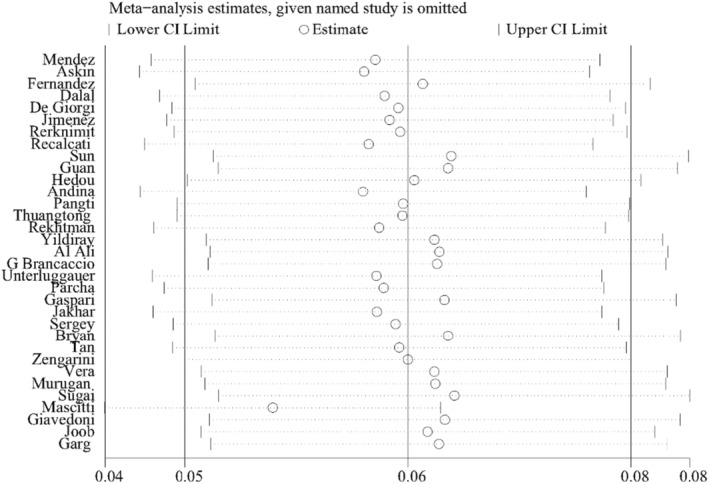
Sensitivity analysis of overall cutaneous manifestations in COVID‐19 patients
FIGURE 4.
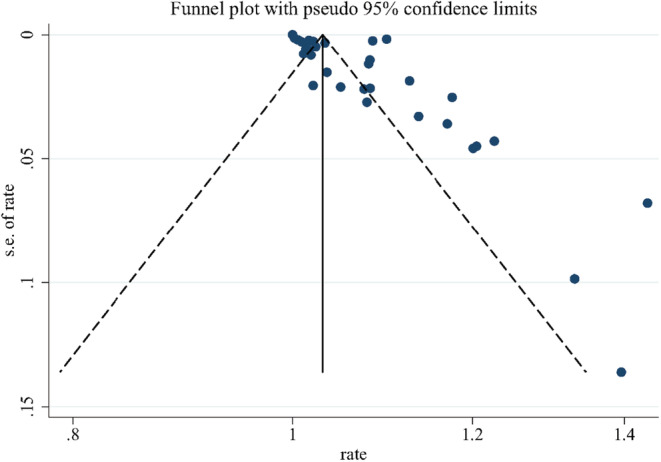
Funnel plot of overall cutaneous manifestations in COVID‐19 patients
3.4. Subgroup analysis
To explore the potential factors of heterogeneity, we carried out subgroup analyses according to region, sample size, sex, age and severity of COVID‐19. The results are summarized in Table 2.
TABLE 2.
Subgroup analyses and meta‐regression of studies based on region, sample size, sex, age, and severity of COVID‐19
| Groups | Included studies | Sample size | Prevalence (95% CI) | p | ph | I 2(%) | Meta‐regression | |
|---|---|---|---|---|---|---|---|---|
| (95% CI) | p′ | |||||||
| Total | 0.056 (0.040–0.076) | 0.000 | 0.000 | 98.3% | ||||
| Region | ||||||||
| Asian | 15 studies | 37 379 | 0.057 (0.032–0.081) | 0.000 | 0.000 | 99.4% | 0.975–1.053 | 0.479 |
| Europe | 10 studies | 8078 | 0.043 (0.028–0.058) | 0.000 | 0.000 | 89.8% | ||
| America | 6 studies | 13 901 | 0.072 (0.025–0.119) | 0.003 | 0.000 | 98.8% | ||
| Sample size | ||||||||
| >200 | 17 studies | 58 656 | 0.043 (0.024–0.062) | 0.000 | 0.000 | 99.4% | 0.900–0.991 | 0.023 |
| <200 | 16 studies | 1524 | 0.102 (0.070–0.134) | 0.000 | 0.000 | 86.0% | ||
| Sex | ||||||||
| Male | 9 studies | 2308 | 0.046 (0.026–0.067) | 0.000 | 0.000 | 89.2% | 0.924–1.053 | 0.671 |
| Female | 9 studies | 1865 | 0.027 (0.011–0.042) | 0.001 | 0.000 | 81.2% | ||
| Age | ||||||||
| Adults | 10 studies | 31 172 | 0.069 (0.039–0.099) | 0.000 | 0.000 | 96.2% | 0.932–1.132 | 0.563 |
| Children | 5 studies | 16 285 | 0.072 (0.034–0.110) | 0.000 | 0.000 | 98.2% | ||
| Severity | ||||||||
| Non‐severe | 6 studies | 982 | 0.081 (0.040–0.122) | 0.000 | 0.000 | 86.0% | 0.911–1.200 | 0.466 |
| Severe | 3 studies | 283 | 0.121 (0.083–0.159) | 0.000 | o.441 | 0.0% | ||
Abbreviations: CI, confidence interval; I 2, 0–25, no heterogeneity; 25–50, modest heterogeneity; 50, high heterogeneity; ph, p‐value of heterogeneity, p‐value of Q‐test for the heterogeneity test.
3.4.1. Stratification by region
We found 15 studies conducted in Asia, with a pooled prevalence estimate of 5.7% (95% CI 0.032–0.081), I 2 = 99.4%. In Europe, there were 10 studies, the pooled prevalence was 4.3% (95% CI 0.028–0.058), I 2 = 89.8%. The pooled prevalence estimates of six studies conducted in America was 7.2% (95% CI 0.025–0.119), I 2 = 98.8%. The results of the subgroup analysis related to region demonstrated that the heterogeneity also remained high. We found no study conducted in Africa reporting the prevalence of skin manifestations in COVID‐19.
3.4.2. Stratification by sample size
A pooled prevalence of 10.2% (95% CI 0.070–0.134), I 2 = 86.0% was estimated from 16 studies with a sample size <200. There were 17 studies presenting prevalence estimates with a sample size >200, and the pooled prevalence was 4.3% (95% CI 0.024–0.062), I 2 = 99.4%. The results grouped by sample size still showed high heterogeneity.
3.4.3. Stratification by sex
There were nine studies conducting prevalence estimates stratified by sex. The pooled prevalence was 4.6% in men (95% CI 0.026–0.067), I 2 = 89.2% and 2.7% in women (95% CI 0.011–0.042), I 2 = 81.2%, respectively. Which also displayed high heterogeneity.
3.4.4. Stratification by age
There were 10 studies involving adults, with pooled prevalence of 6.9% (95% CI 0.039–0.099), I 2 = 96.2%. Children were included in five studies, the pooled prevalence was 7.2% (95% CI 0.034–0.110), I 2 = 98.2%. The high heterogeneity noticed remained in both subgroups.
3.4.5. Stratification by severity of COVID‐19
We found six studies involving non‐severe (asymptomatic, mild to moderate symptoms) COVID‐19 patients, the pooled prevalence was 8.1% (95% CI 0.040–0.122), I 2 = 86.0%. Severe COVID‐19 patients were included in three studies, with the pooled prevalence of 12.1% (95% CI 0.083–0.159), I 2 = 0.0%, which indicated the severity of COVID‐19 was probably the source of high heterogeneity.
3.5. Meta‐regression
Results from the meta‐regression of prevalence on sample size indicate that studies with sample size <200 report higher prevalence estimates (p′ = 0.023). The results of meta‐regression are summarized in Table 2.
3.6. Specific dermatological manifestations
According to the results of these studies, 12 countries reported 20 types of skin manifestations among patients with COVID‐19. Among them, maculopapular rash/morbilliform exanthem was the most common, with a pooled prevalence of 2.0% (95% CI = 0.014–0.026, n = 10 069, I 2 = 85.4%) in 19 studies, with a forest plot in supplementary. The prevalence of maculopapular rash/ morbilliform exanthem ranged from 0.4% to 34%. Livedoid lesions were displayed in five studies, with pooled prevalence 1.4% (95% CI = 0.001–0.027, n = 3589, I 2 = 74.9%), the range was 0.1%–5.9%. Eight studies covered petechial lesions. The prevalence ranged from 0.5% to 10.8%, and the pooled prevalence was 1.1% (95% CI = 0.006–0.015, n = 1784, I 2 = 44.1%). Sixteen studies displayed urticaria. The prevalence ranged from 0.1% to 5.6%, and the pooled prevalence of it was 0.8% (95% CI = 0.005–0.012, n = 9833, I 2 = 74.4%). Pernio‐like lesions were shown in eight studies, the prevalence varied from 0.1% to 8%, and the pooled prevalence was 0.5% (95% CI = 0.001–0.009, n = 7532, I 2 = 74.6%). There were 12 studies describing vesicular lesions, the prevalence ranged from 0.3% to 4.8%, the pooled prevalence was 0.3% (95% CI = 0.002–0.005, n = 6558, I 2 = 0.0%). The forest plots are displayed in Figures 5, 6, 7, 8, 9, 10. The estimates of the six types of skin manifestations are shown in Table 3.
FIGURE 5.
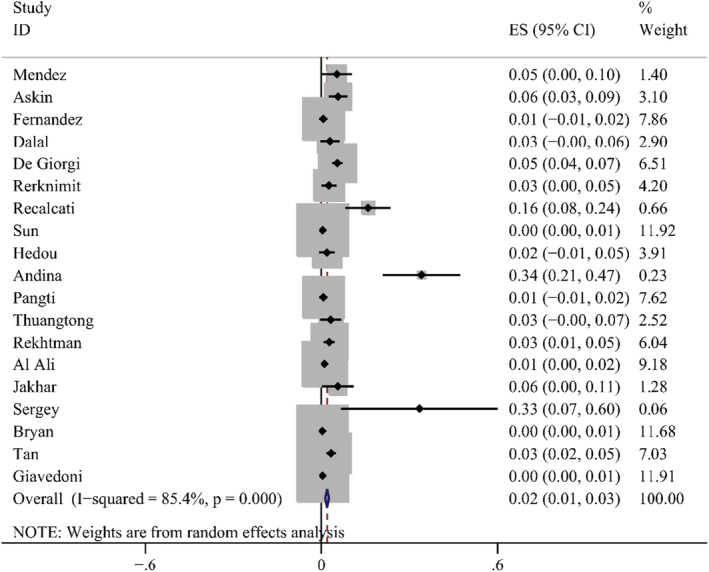
Forest plot showing prevalence rate of maculopapular rash in COVID‐19 patients, with 95% CI
FIGURE 6.
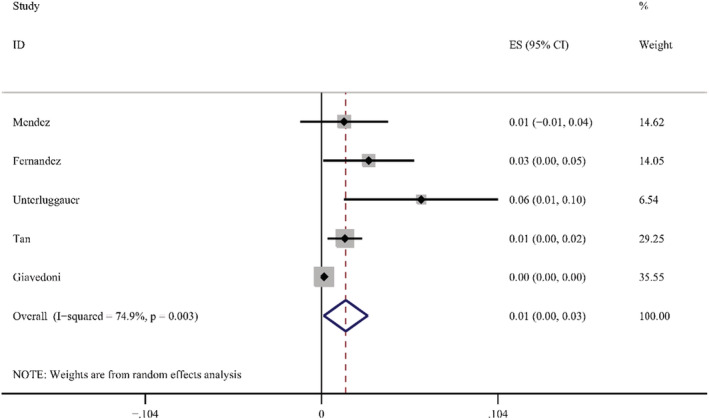
Forest plot showing prevalence rate of livedoid lesions in COVID‐19 patients, with 95% CI
FIGURE 7.
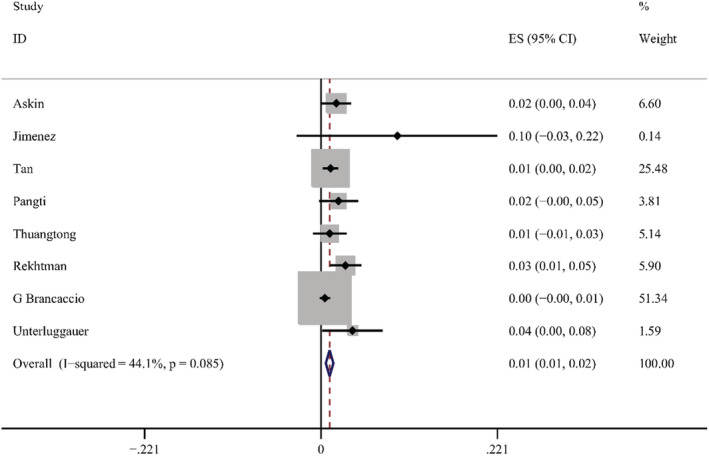
Forest plot showing prevalence rate of petechial lesions in COVID‐19 patients, with 95% CI
FIGURE 8.
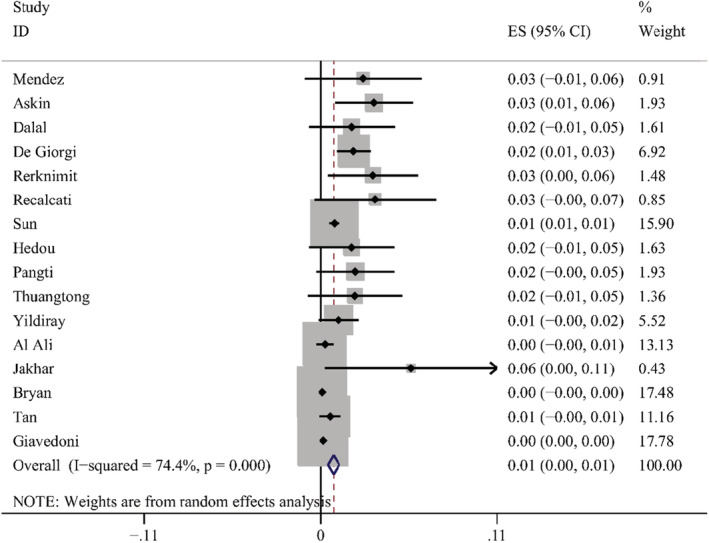
Forest plot showing prevalence rate of urticaria in COVID‐19 patients, with 95% CI
FIGURE 9.
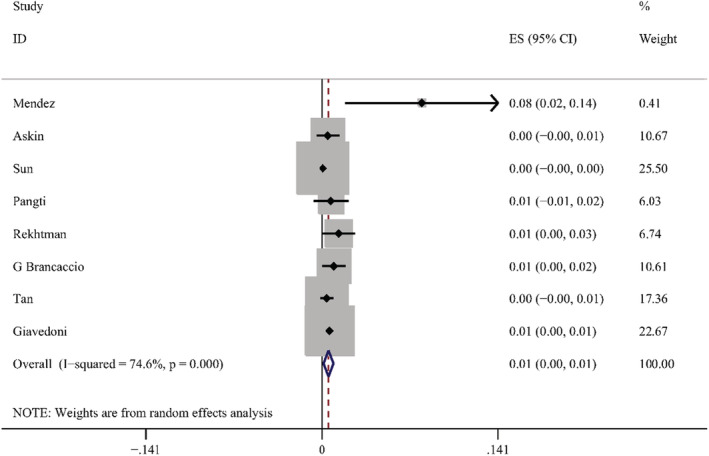
Forest plot showing prevalence rate of pernio‐like lesions in COVID‐19 patients, with 95% CI
FIGURE 10.
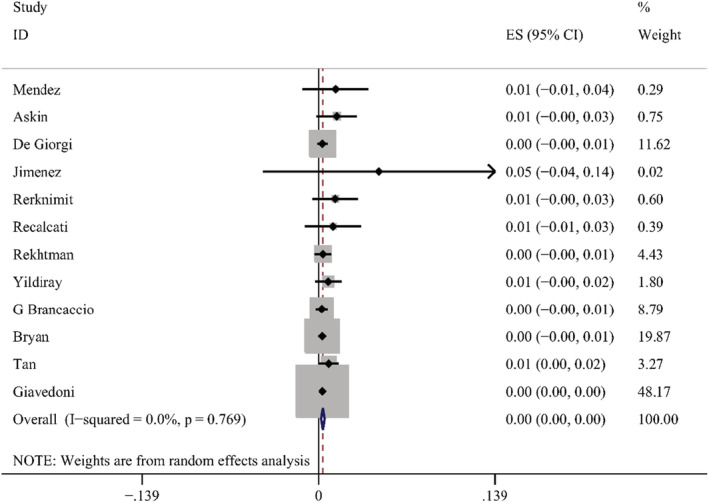
Forest plot showing prevalence rate of vesicular lesions in COVID‐19 patients, with 95% CI
TABLE 3.
Prevalence of six types of skin manifestations
| Type | Included studies | Sample size | Prevalence (95% CI) | p | ph | I 2(%) |
|---|---|---|---|---|---|---|
| Maculopapular rash/morbilliform exanthem | 19 studies | 10 069 | 2% (0.014–0.026) | 0.000 | 0.000 | 85.4% |
| Livedoid lesions | 5 studies | 3589 | 1.4% (0.001–0.027) | 0.031 | 0.003 | 74.9% |
| Urticaria | 16 studies | 9833 | 0.8% (0.005–0.012) | 0.000 | 0.000 | 74.4% |
| Petechial lesions | 8 studies | 1784 | 1.1% (0.006–0.015) | 0.000 | 0.085 | 44.1% |
| Pernio‐like lesions | 8 studies | 7532 | 0.5% (0.001–0.009) | 0.007 | 0.000 | 74.6% |
| Vesicular lesions | 12 studies | 6558 | 0.3% (0.002–0.005) | 0.000 | 0.769 | 0.0% |
Abbreviations: CI, confidence interval; I 2, 0–25, no heterogeneity; ph, p‐value of heterogeneity, p‐value of Q‐test for the heterogeneity test.
We also estimated the proportion of severe/critical patients in each skin type. Among these six skin types, the largest proportion of severe/critical patients was petechial lesions, with a percentage of 59.5% (10/17). The second was livedoid lesions, the proportion was 35.4% (2/5). Then followed by vesicular lesions and urticaria, the percentages of them were 18.2% (3/11) and 17.8% (4/15), respectively. Pernio‐like lesions contained the least severe/critical patients, there was only one in 32. The prevalence of severe/critical patients in each skin type are summarized in Table 4.
TABLE 4.
The prevalence of severe/critical patients in each skin type
| Type | Included studies | Severe or critical patients/patients of this type | Percentage (95% CI) | p | ph | I 2(%) |
|---|---|---|---|---|---|---|
| Pernio‐like lesions | 5 | 1/32 | 0.000 (0.000–0.085) | 1.000 | 0.989 | 0.000% |
| Maculopapular rash/morbilliform exanthem | 7 | 6/36 | 0.089 (0.000–0.264) | 0.049 | 0.374 | 7.089% |
| Urticaria | 5 | 4/15 | 0.178 (0.000–0.538) | 0.084 | 0.204 | 32.605% |
| Vesicular lesions | 4 | 3/11 | 0.182 (0.000–0.599) | 0.123 | 0.820 | 0.000% |
| Petechial lesions | 5 | 10/17 | 0.595 (0.288–0.875) | 0.000 | 0.791 | 0.000% |
| Livedoid lesions | 2 | 2/5 | 0.354 (0.000–0.908) | 0.084 | – | – |
Abbreviations: CI, confidence interval; I 2, 0–25, no heterogeneity; ph, p‐value of heterogeneity, p‐value of Q‐test for the heterogeneity test.
3.7. Subgroup analysis of each skin type according to region
3.7.1. Maculopapular rash/morbilliform exanthem
We found nine studies in Asia, with a pooled prevalence of 2.2% (95% CI = 0.011–0.032, n = 4776). Six studies were conducted in Europe, their pooled prevalence was 4.1% (95% CI = 0.014–0.069, n = 3221). There were three studies in America, occupying the lowest pooled prevalence of 1.8% (95% CI = −0.012–0.049, n = 1394).
3.7.2. Livedoid lesions
There was one study in Asia, the prevalence of livedoid lesions was 1.4% (n = 507). Europe had 4 studies, with a pooled prevalence of 1.8% (95% CI = −0.003–0.039, n = 3082). There was no report about livedoid lesions in America.
3.7.3. Petechial lesions
Four studies were conducted by Asia, and the pooled prevalence was 1.4% (95% CI = 0.006–0.021, n = 948). There were two studies in Europe, with the pooled prevalence of 0.6% (95% CI = −0.001–0.012, n = 519). America had two studies either, with a higher prevalence of 3.2% (95% CI =0.013–0.051, n = 317).
3.7.4. Urticaria
We found 10 studies in Asia, with a pooled prevalence of 1.0% (95% CI = 0.005–0.015, n = 5042). Four studies were conducted by Europe, their pooled prevalence was 1.4% (95% CI = −0.003–0.031, n = 3027). One study was reported in America, with the smallest prevalence rate of 0.1% (−0.001–0.003, n = 1086).
3.7.5. Pernio‐like lesions
There were four studies in Asia, their pooled prevalence was 0.1% (95% CI = 0.000–0.002, n = 3983). Three studies were contained in Europe, with a pooled prevalence of 1.0% (95% CI = −0.001–0.020, n = 3253). America had one study, the prevalence was 1.4% (95% CI = 0.000–0.027, n = 296), which was the highest.
3.7.6. Vesicular lesions
Four studies were contained in Asia, with a pooled prevalence of 0.9% (95% CI = 0.004–0.015, n = 1136). The pooled prevalence rate of four studies (95% CI = 0.001–0.005, n = 3341) in Europe was the same as that of three studies (95% CI = 0.000–0.006, n = 1403) in America, both of them were 0.3%.
The data of subgroup analysis of each skin type according to region was summarized in Table 5.
TABLE 5.
Subgroup analysis of each skin type according to region
| Type | Region | Included studies | Sample size | Prevalence (95% CI) | p | ph | I 2(%) |
|---|---|---|---|---|---|---|---|
| Pernio‐like lesions | Asian | 4 studies | 3983 | 0.001 (0.000–0.002) | 0.077 | 0.408 | 0.0% |
| Europe | 3 studies | 3253 | 0.010 (−0.001–0.020) | 0.073 | 0.051 | 66.5% | |
| America | 1 study | 296 | 0.014 (0.000–0.027) | 0.044 | – | – | |
| Maculopapular rash/morbilliform exanthem | Asian | 9 studies | 4776 | 0.022 (0.011–0.032) | 0.000 | 0.000 | 76.1% |
| Europe | 6 studies | 3221 | 0.041 (0.014–0.069) | 0.003 | 0.000 | 89.0% | |
| America | 3 studies | 1394 | 0.018 (−0.012–0.049) | 0.236 | 0.003 | 83.0% | |
| Urticaria | Asian | 10 studies | 5042 | 0.010 (0.005–0.015) | 0.000 | 0.038 | 49.3% |
| Europe | 4 studies | 3027 | 0.014 (−0.003–0.031) | 0.110 | 0.094 | 53.1% | |
| America | 1 study | 1086 | 0.001 (−0.001–0.003) | 0.317 | – | – | |
| Vesicular lesions | Asian | 4 studies | 1136 | 0.009 (0.004–0.015) | 0.001 | 0.856 | 0.0% |
| Europe | 4 studies | 3341 | 0.003 (0.001–0.005) | 0.002 | 0.747 | 0.0% | |
| America | 3 studies | 1403 | 0.003 (0.000–0.006) | 0.043 | 0.621 | 0.0% | |
| Petechial lesions | Asian | 4 studies | 948 | 0.014 (0.006–0.021) | 0.000 | 0.809 | 0.0% |
| Europe | 2 studies | 519 | 0.006 (−0.001–0.012) | 0.080 | 0.078 | 67.9% | |
| America | 2 studies | 317 | 0.032 (0.013–0.051) | 0.001 | 0.317 | 0.0% | |
| Livedoid lesions | Asian | 1 study | 507 | 0.014 (0.004–0.024) | 0.008 | – | – |
| Europe | 4 studies | 3082 | 0.018 (−0.003–0.039) | 0.085 | 0.015 | 71.5% | |
| America | – | – | – | – | – | – |
Abbreviations: CI, confidence interval; I 2, 0–25, no heterogeneity; ph, p‐value of heterogeneity, p‐value of Q‐test for the heterogeneity test.
3.8. Sensitivity analysis and publication bias
Sensitivity analysis excluding one study did not significantly affect the prevalence of these cutaneous manifestations. The funnel plots and Egger's tests of maculopapular rash/morbilliform exanthem, urticaria, vesicular lesions, pernio‐like lesions, and petechial lesions indicated publication bias (p < 0.05).
4. DISCUSSION
To our knowledge, two meta‐analyses have reported the prevalence of skin manifestations in COVID‐19. 16 , 17 Most of the included articles in the previous meta‐analysis 17 are case reports, and the involved countries and sample size are few. Furthermore, all articles are reported in 2020, which do not cover the newly published research in the last 2 years. Meta‐analyses and reviews in the study of Lee DS et al. 28 and Zhao et al. 29 only emphasized the proportion of each skin manifestation type other than the prevalence of skin manifestations in COVID‐19 patients. The chief strength of our study is that we evaluate newer and more large‐scale studies and present a more accurate meta‐analysis.
In our meta‐analysis, we evaluated the data from 33 studies covering 61 089 patients. The pooled prevalence of severe and critical COVID‐19 patients in 16 studies is 15.7% (1597/24951), and the total mortality rate of six countries in seven studies is 2.0% (442/22 535), which are higher than 0.3% (38 640/14 149 190) and 1.0% (6 565 854/628 404 291) respectively reported by the WHO. 30 This is largely due to the fact that most of our data come from inpatients, and the proportion of outpatients is very small. Besides, one study has shown that among hospitalized COVID‐19 patients, the risk of mortality is substantially higher, ranging from 1.4% to 28%. 31 Moreover, we have found that the highest proportion of severe and critical patients is in Asia, followed by Europe, and the lowest is in America. Many factors could influence the severity of the disease such as country average age and weather temperatures, and administration of vaccine. 32 The lowest mortality rate is reported by Spain, and the report of Philippines ranks first. Up to now, the risk factors related to the mortality of COVID‐19 patients in low and middle‐income countries have not been well studied. 33
The pooled prevalence of overall cutaneous manifestations associated with COVID‐19 in our study is 5.6% (95% CI = 0.040–0.076, I 2 = 98.3%). We conducted subgroup analysis due to the high level of heterogeneity in the present study. Our subgroup analysis revealed that I 2 value of studies including severe COVID‐19 patients was 0.0%, indicating that the severity of COVID‐19 was probably the source of heterogeneity in our study. Some skin manifestations are more common in severe patients, urticarial eruptions are usually an indicator of severe disease. 34 A study in Spain indicates that 19% of COVID‐19 patients had urticarial lesions, which is a bad prognosis, as the mortality rate is 2%. 12 COVID‐19 adults with livedoid lesions tend to need intensive care support. 35 Mohammed et al. study 36 showed that vascular rashes in the spectrum of livedo/purpura/necrosis were significantly related to severe forms of COVID‐19. The skin manifestations related to COVID‐19 deserve physicians' continuous attention, especially in patients with severe COVID‐19 infection. Results from the meta‐regression of prevalence on sample size indicate that studies with sample size <200 report higher prevalence estimates. Subgroup analysis by age shows that the prevalence of children (7.2%, 95% CI 0.034–0.110) is comparable to that in adults (6.9%, 95% CI 0.039–0.099). In children, cutaneous signs of COVID‐19 may be the predominant or only clue of infection. 37 Multisystem inflammatory syndrome in children (MIS‐C) is considered a rare but serious complication of SARS‐CoV‐2 infection. The clinical symptoms usually include continuous fever, gastrointestinal symptoms (abdominal pain, vomiting, diarrhea), skin rashes, and conjunctivitis, 38 and this syndrome is currently being seen worldwide, especially among children 0–5 years of age, the prevalence of dermatologic symptoms was highest. 39 We found no study conducted in Africa reporting the prevalence of skin manifestations in COVID‐19; subgroup analyses on Asia, Europe, and America shows that there is no significant difference of pooled prevalence among them. Sex was not a factor affecting the prevalence of skin manifestations in COVID‐19 either.
There are six types of skin manifestations which are the most common among all skin manifestations. The prevalence of skin manifestation in COVID‐19 patients is as follows: maculopapular rash/ morbilliform exanthem 2%, livedoid lesions 1.4%, petechial lesions 1.1%, urticaria 0.8%, pernio‐like lesions 0.5%, and vesicular lesions 0.3%. Which is different from some studies showing that pernio‐like lesions may represent the most common and characteristic skin manifestations of COVID‐19. 40 , 41 One possible reason is that most of the patients screened in our study are inpatients, and the moderate severity was correlated with maculopapular rash, 42 and many of the pernio‐like lesions appeared either in asymptomatic individuals or in patients who endorsed mild symptoms. 43 , 44
Maculopapular rash/ morbilliform exanthem occupies the highest prevalence (2.0%) among all cutaneous manifestations, and accounts for 41% (166/405) in COVID‐19 patients with skin manifestations, which is consistent with the study of Matteo Bassetti et al. 45 These lesions mainly occur either because of viral infections or as side effects of the administered drugs, 46 and the side effects of drugs are also an influence factor that is difficult to eliminate completely. These may be the possible reasons for the highest prevalence of maculopapular rash in our study. Moreover, we estimated the proportion of severe and critical patients with maculopapular rash, and found it was relatively low with a rate of 8.9%. There is no distinct difference in the prevalence of maculopapular rash among three regions.
The pooled prevalence of livedoid lesions is the second among these skin rashes, and they account for the second highest proportion of severe and critical patients (35.4%). The major reason is that livedoid lesions mainly appeared in severe patients. Harjas et al. 47 study also indicates that livedoid lesions primarily appeared in elderly patients with more severe infections. In addition, livedoid lesions are considered as the pattern most associated with mortality. 48 The prevalence rates of livedoid lesions have no significant difference among these three regions.
Petechial lesions have the highest proportion of severe and critical patients, with a rate of 59.5%. That's mainly because petechial lesions usually appear in the most severe cases of SARS‐CoV‐2 infection, 49 which is further confirmed by Rabia Ghafoor et al. 42 study. Genovese et al. 50 study indicated that purpuric lesions have the highest rate of COVID‐19 related mortality. The prevalence of petechial lesions in America occupies the highest position, Europe has the lowest rate, and the possible reason is not yet quite clear.
For urticaria, the proportion of severe/critical patients in urticaria is at a relatively low level among the six skin manifestations. Allegra demonstrates that it may present with a severe clinical course of COVID‐19. 51 Thus, Seque suggests that it is associated with mild systemic conditions with low mortality. 52 Moreover, Pathania has reported that emotional stress related to COVID‐19, rather than the infection itself, may trigger the urticaria. 53 The subgroup analysis of urticaria displayed that the prevalence of urticaria in America was obviously lower than in Asia and Europe, and the possible reason deserves further discussion.
The prevalence of pernio‐like lesions is low. However, pernio‐like lesions and vesicular lesions may represent the most common and characteristic skin manifestations of COVID‐19, and the pernio‐like lesions are very useful as epidemiological markers. 40 In addition, the proportion of severe and critical patients with pernio‐like lesions is the lowest (0.00%) among the six types of skin manifestations, which is further explained that pernio‐like lesions are more common in asymptomatic or mild patients. In the subgroup analysis of pernio‐like lesions according to region, the prevalence of them in Asia is significantly lower than in Europe and America, which is consistent with Tan et al. 54 study. One explanation of this phenomenon is that compared with Chinese and African populations, the frequency of minor allele that is related to antiviral cascade reaction is more common in white populations. 55
In our study, the prevalence of vesicular lesions is the lowest among the six skin manifestations. Vesicular lesions are considered a specific pattern of skin lesion related to COVID‐19, as they are not commonly seen in drug reactions. 52 Mohammed et al. 36 study indicates that vesicular lesions are associated with moderate COVID‐19, which may be due to a direct cytopathic effect. Similarly, we did not observe the obvious distinction of prevalence among three regions. The skin manifestations in COVID‐19 patients differ by race and country remains controversial, one most likely reason is racial differences or other geopolitical factors, 56 and the possible reasons deserve further discussion.
5. IMPLICATIONS
Our study provides a more comprehensive, up‐to‐date assessment of currently available evidence. Specifically, this is an attempt to report distinct estimates of the prevalence of various skin manifestations in COVID‐19 patients by meta‐analysis.
6. LIMITATIONS
Some limitations should be taken into consideration in our meta‐analysis. First, most of the studies are aimed at hospitalized patients, excluding isolated outpatients, which may affect the evaluated prevalence of cutaneous manifestations. Second, some factors are as follows: cutaneous adverse drug reaction, other cutaneous diseases, and chance occurrence, which may have an impact on the evaluated prevalence and cannot be eliminated from our analysis. Besides, large heterogeneity was observed among studies as indicated by high I 2 values. Finally, the funnel plots for each type of skin manifestation meta‐analysis indicate publication bias, backed up by significant results on Egger's test, suggesting that there are small studies with negative results that have not been published.
7. CONCLUSION
In conclusion, this meta‐analysis provides pooled estimates of the prevalence in COVID‐19 patients with skin manifestations. We conducted subgroup analysis due to the high level of heterogeneity, and found the severity of COVID‐19 was probably the source of heterogeneity in our study. Meta‐regression indicates that studies with a sample size <200 report higher prevalence estimates. There are six types of skin manifestations which are the most common among all skin manifestations. Prevalence of these skin manifestations from high to low is: maculopapular rash/morbilliform exanthem, livedoid lesions, petechial lesions, urticaria, pernio‐like lesions, vesicular lesions. Different skin manifestations are related to the severity of the patient's condition. Among the six skin manifestations, petechial lesions and livedoid lesions are the most frequent in severe and critical COVID‐19 patients. Furthermore, the prevalence rates of pernio‐like lesions, urticaria and petechial lesions vary greatly in different regions. The highest prevalence of pernio‐like lesions is in America, the lowest in Asia; Europe reported the highest prevalence of urticaria, America showed the smallest; petechial lesions' prevalence locates the first in America, Europe has the least of them. And the possible reasons need to be further explored.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
The author would like to thank all the researchers involved, especially Miss. Chunying Cui for her help in this study.
Li J, Wen W, Mu Z, Du X, Han X. Prevalence of cutaneous manifestations in COVID‐19: A meta‐analysis. J Dermatol. 2022;00:1–15. 10.1111/1346-8138.16672
REFERENCES
- 1. World Health Organization . WHO Coronavirus (COVID‐19) dashboard. 2021. [cited 2022 June 3]. Available from: https://covid19.who.int/info
- 2. Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)/COVID‐19 detection. Clin Microbiol Rev. 2021;34:e00228–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drago F, Ciccarese G, Merlo G, Trave I, Javor S, Rebora A, et al. Oral and cutaneous manifestations of viral and bacterial infections: not only COVID‐19 disease. Clin Dermatol. 2021;39:384–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korman AM, Alikhan A, Kaffenberger BH. Viral exanthems: an update on laboratory testing of the adult patient. J Am Acad Dermatol. 2017;76:538–50. [DOI] [PubMed] [Google Scholar]
- 7. Jamiolkowski D, Mühleisen B, Müller S, Navarini AA, Tzankov A, Roider E. SARS‐CoV‐2 PCR testing of skin for COVID‐19 diagnostics: a case report. Lancet. 2020;396:598–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guarneri C, Rullo EV, Pavone P, Berretta M, Ceccarelli M, Natale A, et al. Silent COVID‐19: what your skin can reveal. Lancet Infect Dis. 2021;21:24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–3. [DOI] [PubMed] [Google Scholar]
- 10. Guan W‐J, Ni Z‐Y, Hu Y, Liang W‐H, Ou C‐Q, He J‐X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Giorgi V, Recalcati S, Jia Z, Chong W, Ding R, Deng Y, et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID‐19): a prospective study from China and Italy. J Am Acad Dermatol. 2020;83:674–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galván Casas C, Català A, Carretero Hernández G, Rodríguez‐Jiménez P, Fernández‐Nieto D, Rodríguez‐Villa Lario A, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al Ali A, Al‐Shidhani S, Al‐Balushi F, Alhinai M, Al‐Azri AR, Al Lawati SAL, et al. Cutaneous manifestations of COVID‐19: an experience from Oman. Cureus. 2021;13:e16667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unterluggauer L, Pospischil I, Krall C, Saluzzo S, Kimeswenger S, Karolyi M, et al. Cutaneous manifestations of SARS‐CoV‐2 ‐ a two‐center, prospective, case‐controlled study. J Am Acad Dermatol. 2021;85:202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rattapon T, Nasikarn A, Charussri L, Daranporn T. Patient recovery from COVID‐19 infections: follow‐up of hair, nail, and cutaneous manifestations. Biomed Res Int. 2021;2021:5595016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bandhala Rajan M, Kumar‐M P, Bhardwaj A. The trend of cutaneous lesions during COVID‐19 pandemic: lessons from a meta‐analysis and systematic review. Int J Dermatol. 2020;59:1358–70. [DOI] [PubMed] [Google Scholar]
- 17. Fatemeh S, Bahareh H, Somayeh Y, Mehdi G, Parviz O, Javad NM, et al. COVID‐19 and skin manifestations: an overview of case reports/case series and meta‐analysis of prevalence studies. Front Med. 2020;7:573188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 19. Ozge A, Neval AR, Dorukhan AD, Kevser UT, Umit T, Zekayi K. Cutaneous manifestations in hospitalized patients diagnosed as COVID‐19. Dermatol Ther. 2020;33:e13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pawinee R, Chinathip T, Pattamon L, Watsamon J, Opass P, Thanyawee P, et al. Skin manifestations in COVID‐19: the tropics experience. J Dermatol. 2020;47:e444–6. [DOI] [PubMed] [Google Scholar]
- 21. Mendenhall E, Kohrt BA, Logie CH, Tsai AC. Syndemics and clinical science. Nat Med. 2022;28:1359–62. [DOI] [PubMed] [Google Scholar]
- 22. Garcia‐Vera C, Castejon‐Ramirez S, Miranda EL, Abadia RH, Ventura MG, Navarro EB, et al. COVID‐19 in children: clinical and epidemiological spectrum in the community. Eur J Pediatr. 2022;181:1243–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan CC, Dofitas BL, Frez MLF, Yap CDD, Uy JKK, Ciriaco‐Tan CP. Cutaneous manifestations of COVID‐19 IN a tertiary COVID referral hospital IN The Philippines. JAAD Int. 2022;7:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Grbic N, et al. Eruptions and related clinical course among 296 hospitalized adults with confirmed COVID‐19. J Am Acad Dermatol. 2021;84:946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parcha V, Booker KS, Kalra R, Kuranz S, Berra L, Arora G, et al. A retrospective cohort study of 12,306 pediatric COVID‐19 patients in the United States. Sci Rep. 2021;11:10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murugan TP, Ghosh U, Rajan RJ, Punnen A, Chandran J, Das Adhikari D, et al. Spectrum of COVID‐19 disease in children: a retrospective analysis comparing wave 1 and wave 2 from a tertiary Hospital in South India. Indian J Pediatr. 2022;89:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaspari V, Orioni G, Misciali C, Viviani F, Zengarini C. COVID‐19 skin eruptions: incidence in hospitalized patients in Bologna. Int J Dermatol. 2021;60:512–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DS, Mirmirani P, McCleskey PE, Mehrpouya M, Gorouhi F. Cutaneous manifestations of COVID‐19: a systematic review and analysis of individual patient‐level data. Dermatol Online J. 2020;26:13030/qt7s34p8rw. [PubMed] [Google Scholar]
- 29. Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID‐19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:2505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. COVID‐19 CORONAVIRUS PANDEMIC. [cited 2022 October 13]. Available from: https://www.worldometers.infocoronavirus
- 31. Spiegelhalter D. Use of “normal” risk to improve understanding of dangers of covid‐19. BMJ. 2020;370:m3259. [DOI] [PubMed] [Google Scholar]
- 32. Zawbaa HA‐O, Osama H, El‐Gendy AA‐O, Saeed HA‐O, Harb HA‐O, Madney YA‐O, et al. Effect of mutation and vaccination on spread, severity, and mortality of COVID‐19 disease. J Med Virol. 2022;94:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abebe HA‐OX, Mulugeta A, Berhe Y, Berhane K, Siraj A, Siraj D, et al. Risk factors for mortality among hospitalized COVID‐19 patients in Northern Ethiopia: a retrospective analysis. PLoS One. 2022;17:e0271124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huynh T, Sanchez‐Flores X, Yau J, Huang JT. Cutaneous manifestations of SARS‐CoV‐2 infection. Am J Clin Dermatol. 2022;23:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strom MA, Trager MH, Timerman D, Coromilas AJ, Burris K, Belsito DV, et al. Cutaneous findings in hospitalized and critically ill patients with COVID‐19: a case series of 15 patients. J Am Acad Dermatol. 2021;84:510–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohammed GF, Al‐Dhubaibi MS, Atef L. Cutaneous manifestations of coronavirus disease 2019: skin narratives and dialogues. J Clin Aesthet Dermatol. 2022;15:E77–81. [PMC free article] [PubMed] [Google Scholar]
- 37. Agnihothri R, Fox LP. Clinical patterns and morphology of COVID‐19 dermatology. Dermatol Clin. 2021;39:487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aldawas A, Ishfaq M. COVID‐19: multisystem inflammatory syndrome in children (MIS‐C). Cureus. 2022;14:e21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gisondi P, Piaserico S, Bordin C, Alaibac M, Girolomoni GA‐O, Naldi LA‐O. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34:2499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jia JL, Kamceva M, Rao SA, Linos E. Cutaneous manifestations of COVID‐19: a preliminary review. J Am Acad Dermatol. 2020;83:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghafoor RA‐O, Ali SA‐O, Goldust M. Cutaneous manifestations of coronavirus disease 2019. J Cosmet Dermatol. 2022;21:3667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmed H, Yusuf N. The cutaneous manifestations associated with COVID‐19: a review. Am J Dermatol Res Rev. 2020;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gisondi P, Piaserico S, Bordin C, Alaibac M, Girolomoni G, Naldi L. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34:2499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bassetti M, Massone C, Vena A, Dettori S, Conforti C, Giacobbe DR, et al. Skin manifestations in patients with coronavirus disease 2019. Curr Opin Infect Dis. 2022;35:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arefinia NA‐O, Ghoreshi ZA, Alipour AH, Iranmanesh B, Mehrolhasani N, Shamsi‐Meymandi S, et al. A comprehensive narrative review of the cutaneous manifestations associated with COVID‐19. Int Wound J. 2022. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh H, Kaur H, Singh K, Sen CK. Cutaneous manifestations of COVID‐19: a systematic review. Adv Wound Care. 2021;10:51–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Potekaev NN, Zhukova OV, Protsenko DN, Demina OM, Khlystova EA, Bogin VA‐O. Clinical characteristics of dermatologic manifestations of COVID‐19 infection: case series of 15 patients, review of literature, and proposed etiological classification. Int J Dermatol. 2020;59:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fernandez‐Lazaro D, Garrosa M. Identification, mechanism, and treatment of skin lesions in COVID‐19: a review. Viruses. 2021;13:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID‐19: current knowledge and future perspectives. Dermatology. 2021;237:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Allegra A, Asero R, Giovannetti A, Isola S, Gangemi S. Urticaria and coronavirus infection: a lesson from SARS‐CoV‐2 pandemic. Eur Ann Allergy Clin Immunol. 2021;53:51–4. [DOI] [PubMed] [Google Scholar]
- 52. Arai SC, Silva EMMSE, Maria PA, Jane T. Skin manifestations associated with COVID‐19. An Bras Dermatol. 2022;97:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID‐19: a worldwide review. JAAD Int. 2021;2:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maiti AA‐O. The African‐American population with a low allele frequency of SNP rs1990760 (T allele) in IFIH1 predicts less IFN‐beta expression and potential vulnerability to COVID‐19 infection. Immunogenetics. 2020;72:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tamai MA‐O, Sakamoto R, Goto N, Morimura O, Nishida T, Iwahashi H, et al. Cutaneous manifestations of coronavirus disease 2019 patients in Japan. J Dermatol. 2022;49:872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mendez Maestro I, Pena Merino L, Gonzalez U, del Tanago B, Aramburu Gonzalez A, Orbea Sopena A, et al. Skin manifestations in patients hospitalized with confirmed COVID‐19 disease: a cross‐sectional study in a tertiary hospital. Int J Dermatol. 2020;59:1353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Askin O, Altunkalem RN, Altinisik DD, Uzuncakmak TK, Tursen U, Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID‐19. Dermatol Ther. 2020;33:e13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fernandez‐Nieto D, Ortega‐Quijano D, Suarez‐Valle A, Jimenez‐Cauhe J, Jaen‐Olasolo P, Fernandez‐Guarino M. Lack of skin manifestations in COVID‐19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS‐CoV‐2 variant. A cross‐sectional study. J Eur Acad Dermatol Venereol. 2020;35:e183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ashish D, Deepak J, Vishal A, Ravi B. Dermatological findings in SARS‐CoV‐2 positive patients: an observational study from North India. Dermatol Ther. 2020;33:e13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jimenez‐Cauhe J, Ortega‐Quijano D, de Perosanz‐Lobo D, Burgos‐Blasco P, Vano‐Galvan S, Fernandez‐Guarino M, et al. Enanthem in patients with COVID‐19 and skin rash. JAMA Dermatol. 2020;156:1134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andina‐Martinez D, Nieto‐Moro M, Antonio Alonso‐Cadenas J, Anon‐Hidalgo J, Hernandez‐Martin A, Perez‐Suarez E, et al. Mucocutaneous manifestations in children hospitalized with COVID‐19. J Am Acad Dermatol. 2021;85:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pangti R, Gupta S, Nischal N, Trikha A. Recognizable vascular skin manifestations of SARS‐CoV‐2 (COVID‐19) infection are uncommon in patients with darker skin phototypes. Clin Exp Dermatol. 2020;46:180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yildiray Y, Ayse PS. Cutaneous manifestations of coronavirus disease in Turkey: a prospective study. Dermatol Sin. 2021;39:74–8. [Google Scholar]
- 65. Brancaccio G, Gussetti N, Sasset L, Alaibac M, Tarantello M, Salmaso R, et al. Cutaneous manifestations in a series of 417 patients with SARS‐CoV‐2 infection: epidemiological and clinical correlates of chilblain like lesions. Pathog Glob Health. 2021;115:483–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun Y, Zhou R, Zhang H, Rong L, Zhou W, Liang Y, et al. Skin is a potential host of SARS‐CoV‐2: a clinical, single‐cell transcriptome‐profiling and histologic study. J Am Acad Dermatol. 2020;83:1755–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jakhar D, Das A, Kaul S, Kaur I, Madke B, Dalal A. Prevalence and characteristics of dermatological manifestations in COVID‐19 positive dermatologists: report from a web‐based survey in India. J Eur Acad Dermatol Venereol. 2021;35:E832–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bryan A, Samant H, Asarkar A, Nathan C‐AO, Khandelwal A. Cutaneous manifestations in COVID‐19‐positive African American patients. Ochsner J. 2022;22:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Corrado Z, Alba G, Matteo G, Cecilia P, Emi D, Maria PB, et al. Estimating the incidence of Covid‐19 skin manifestations on the general population in a territorial setting. J Eur Acad Dermatol Venereol. 2022;36:e415–7. [DOI] [PubMed] [Google Scholar]
- 70. Sugai T, Fujita Y, Inamura E, Maya Y, Shimizu S. Prevalence and patterns of cutaneous manifestations in 1,245 COVID‐19 patients in Japan: a single‐Centre study. J Eur Acad Dermatol Venereol. 2022;36:e522–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Garg A. Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID‐19 and multisystem inflammatory syndrome in children. J Am Acad Dermatol. 2021;84:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson RM. Comment on ‘Cutaneous manifestations in COVID‐19: a first perspective’ by Recalcati S. J Eur Acad Dermatol Venereol. 2020;34:E299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hélène M, Patrick J, Alexandre B, Luc J, Agnès D, Xavier L, et al. Prognosis of rash and chilblain‐like lesions among outpatients with COVID‐19: a large cohort study. Eur J Clin Microbiol Infect Dis. 2021;40:2243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giavedoni P, Podlipnik S, Pericas JM, Fuertes de Vega I, Garcia‐Herrera A, Alos L, et al. Skin manifestations in COVID‐19: prevalence and relationship with disease severity. J Clin Med. 2020;9:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82:E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 ‐ COVID‐NET, 14 states, march 1‐30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


