Abstract
Post‐exertional symptom exacerbation (PESE) is a characteristic symptom of post‐COVID syndrome (PCS). This prospective study investigated the effect of a 6‐week structured World Health Organization (WHO) Borg CR‐10 5‐phase pacing protocol on PESE episodes and quality of life in a cohort of individuals with long‐standing PCS (average duration of symptoms was 17 months). Participants received weekly telephone calls with a clinician to complete the Leeds PESE questionnaire (LPQ) and identify the appropriate phase of the pacing protocol. EQ‐5D 5L was completed at the intervention's beginning and end to measure overall health. Thirty‐one participants completed the 6‐week protocol, with a statistically and clinically significant reduction in the average number of PESE episodes (from 3.4 episodes in Week 1 to 1.1 in Week 6), with an average decrease of 16% (95% CI: 9%−24%; p < 0.001) each week, and reduction across all three exertional triggers (physical, cognitive, and emotional). Physical activity levels showed moderate improvements during the intervention period. Mean EQ‐5D 5L scores improved from 51.4 to 60.6 points (paired difference of 9.2 points, 95% CI: 3.2−15.2 points; p = 0.004). A structured pacing protocol significantly reduces PESE episodes and improves overall health in PCS.
Keywords: autonomic dysfunction, Borg CR‐10, C19‐YRS, dysautonomia, fatigue, long COVID, PACS, post‐exertional malaise (PEM), rate of perceived exertion (RPE), SARS‐CoV‐2, WHO
1. INTRODUCTION
The COVID‐19 pandemic has cost millions of lives and adversely affected most countries' health economies. Most survivors have mild symptoms from the severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) infection and recover within subsequent weeks. However, a significant proportion (up to 10%) of patients have persistent symptoms for more than 4 weeks beyond the acute phase (long COVID). 1 The ONS surveys suggest that almost 2 million report COVID‐19 symptoms persisting for more than 4 weeks; 807 000 (41% of all people with long COVID) for more than a year; and 403 000 (19% of those with long COVID) for more than 2 years. 2 Post‐COVID syndrome (PCS) is used for persistent symptoms beyond 12 weeks after the acute infection. 1
PCS is a multisystem condition with more than 200 symptoms across 10 organ systems that have been reported in the literature. 3 The common symptoms are breathlessness, fatigue, palpitations, dizziness, pain, brain fog (cognitive problems), anxiety, depression, posttraumatic stress, skin rash, and allergic reactions. PCS can be a remitting and relapsing condition with a protracted course causing significant distress and disability in many individuals. 4 , 5 Many of these symptoms overlap with dysautonomia, suggesting that autonomic impairment may play an important role in the underlying pathology of PCS. 6
Post‐exertional symptom exacerbation (PESE) or post‐exertional malaise (PEM), or a “crash” in layman's terms, has been reported as a characteristic symptom of PCS. 3 PESE is defined as worsening symptoms following physical or mental exertion, typically 12−48 h after activity and lasting days or (rarely) weeks. 7 It is thought to involve an abnormal response following physical, cognitive, emotional, or orthostatic exertion, that would not have caused a problem before illness. 8 With PESE, everyday activities that require mild effort can result in deteriorating physical and mental stamina, plus exacerbation of other symptoms. 9
A body of existing literature explores PESE within myalgic encephalomyelitis (ME) or chronic fatigue syndrome (CFS), 10 but little is known about PESE within PCS patients. Research exploring physical activity progression and PESE in people with PCS has not reported symptom exacerbation in response to exertion. 11 , 12 , 13 This may be because the incidence of PESE in PCS has not been identified using condition‐specific validated self‐report tools. Therefore, a systematic approach toward a detailed appraisal of PESE in PCS is warranted.
More detailed rehabilitation recommendations are needed to aid recovery for individuals living with PCS who report a high symptom burden associated with activity. This prospective study aimed to investigate the effect of pacing daily activities based on the World Health Organization (WHO) Borg CR‐10 rating of perceived exertion (RPE) pacing protocol 14 on reducing PESE episodes using a condition‐specific PESE questionnaire. In addition to physical exertion, we also aimed to evaluate the role of all types of exertion (physical, cognitive, and emotional) on PESE and the type of symptoms that constitute PESE in PCS.
2. METHODS
2.1. Study design and participants
This was a longitudinal prospective cohort of individuals with PCS assessed during a 6‐month period in a community COVID‐19 rehabilitation service. Before data collection, the Health Research Authority toolkit was completed. Ethical approval was given by Leeds Community Healthcare Trust Research and Development (SE/0131) and Leeds Beckett University (93585). Participants were individuals over 18 years old, with confirmed or suspected COVID‐19 infection and PCS symptoms for more than 12 weeks. Individuals lacking the capacity to consent, or those who could not commit to 6 weekly telephone calls, were excluded.
Suitable individuals were identified by email or by face‐to‐face clinic appointment. They were then provided with an information sheet, giving key information about the study. Patients provided written consent to confirm their participation in the 6‐week evaluation period, where they were to follow the WHO Borg CR‐10 pacing protocol for physical activity guidance.
2.2. WHO Borg CR‐10 pacing protocol
The guidance is presented as five incremental phases of activity, where a recommended RPE of 0−10 (0 being lowest and 10 being the maximal level of exertion) is increased through each phase (Table 1). It is recommended that individuals use the Borg CR‐10 scale as a subjective assessment of how hard they feel they are working. Suggested activities suitable for the RPE at each phase are provided. The phases begin with a “preparation for return to activity” phase, RPE 0−1, where breathing exercises and gentle stretches are suggested activities, and concludes with “return to baseline exercises,” RPE 8−10, where patients should be able to complete pre‐COVID‐19 activities. The guidance advises the individual to stay at each phase for a minimum of 7 days. The individual can use the example activities as a guide, but ultimately, could apply the guidance to any activity they deemed as exertional, for example, cleaning/housework, to manage the intensity of daily activities to reduce triggering PESE. Individuals are reminded within each phase that if their RPE score exceeds the upper limit suggested, to not continue with that activity within that phase.
Table 1.
WHO Borg CR‐10 pacing protocol
| Phase | RPE (0−10) | Example activities |
|---|---|---|
|
1 “Preparation for return to exercise” |
0−1 |
Controlled breathing exercises Gentle stretching & balance exercises Gentle walking |
|
2 “Low‐intensity activity” |
2−3 |
Walking Light household/gardening tasks |
|
3 “Moderate‐intensity activity” |
4−5 |
Brisk walking Introducing inclines Jogging Resistance exercises |
|
4 “High‐intensity exercises” |
5−7 |
Running Cycling Swimming Dancing |
|
5 “Return to baseline” |
8−10 | Regular exercise/sports/activities |
Abbreviation: RPE, rating of perceived exertion.
2.3. Data collection
Participants were provided with a copy of WHO pacing protocol guidance (Supporting Information: Material 1). 14 Participants received weekly telephone calls with a clinician, who administered a PESE questionnaire (Supporting Information: Material 2) to identify episodes of PESE that week and reviewed individuals' associated activity levels. This PESE questionnaire was adapted from the DSQ‐PEM 15 using items from the modified C19‐YRS questionnaire 16 to assess the number, severity, duration, and impact of PESE specific to PCS. Informed by the participant's answers, the clinician then discussed the target activity level for the following week, based on the WHO Borg CR‐10 pacing protocol. Participants also completed a copy of the EuroQol EQ‐5D‐5L 17 quality of life questionnaire at the beginning and the end of the study, rating their health in five dimensions and a visual analog scale (VAS) to rate overall health.
2.4. Statistical methods
All statistical analyses were conducted using Stata version 17 (StataCorp. Stata statistical software: Release 17.0; Stata Corporation:, 2021.) and the IBM Statistical Package for the Social Sciences (SPSS).
Basic demographic and clinical characteristics were tabulated to describe the cohort. Multilevel mixed‐effects Poisson regression was used to model counts of the number of PESE episodes experienced using the mepoisson command in Stata, allowing for the 6 weekly serial measures within each participant and with random slopes to allow individuals to have different symptom trajectories over time. The primary outcome was the total number of PESE episodes, but this was repeated for the number of episodes associated with physical, cognitive, and emotional exertions separately. Results are presented as the week‐on‐week percentage change in a number of episodes, with 95% confidence intervals (CI), adjusted for age and gender as covariates. p < 0.05 was taken to indicate statistical significance. We further explored some of the symptoms more commonly experienced within each episode, including fatigue, cognitive dysfunction (“brain fog”) or speech and language difficulty, breathlessness, headache, musculoskeletal aches, pains or weakness, and palpitations. These were modeled as random intercept models because of the smaller numbers presenting with each symptom.
The potential for different trajectories in specified subgroups was formally explored by including an interaction term in the models. Symptom trajectories were compared by gender, age, and the initial agreed activity level. The number of PESE episodes each week is presented graphically alongside average activity level targeted, severity of episodes experience, and duration of episodes experienced.
The five dimensions from the EQ‐5D‐5L were reported separately and combined into a single index through mapping onto the EQ‐5D‐3L value sets. 18 Paired t‐tests were used to compare the overall health rating before and after the study, and to compare the EQ‐5D index before and after the study.
3. RESULTS
3.1. Descriptive statistics
In total, 34 participants consented to this evaluation, but 3 (9%) were excluded from data analysis because they did not complete the full 6‐weeks follow up period, leaving 31 participants for data analysis. Table 2 shows participant characteristics at recruitment. Participants had a mean age of 47 years (standard deviation: 9 years), were predominantly employed (90%), White British, the majority women, with most attempting earlier phases 1−3 of the WHO Borg protocol. The median self‐reported duration of PCS symptoms for this cohort was 17 months (interquartile range [IQR]: 12−27 months). Duration of symptoms was determined from the initial C19‐YRS questionnaire, where patients report the data of a positive COVID‐19 test or the onset of suspected COVID‐19 infection. Patients had been under the care of the Leeds Community Long COVID Rehabilitation clinic for a median of 12 months (IQR: 9−15 months).
Table 2.
Participant characteristics
| Characteristic | n | % |
|---|---|---|
| Age (years) | ||
| <40 | 6 | 19 |
| 40−49 | 12 | 39 |
| 50−59 | 10 | 32 |
| 60+ | 3 | 10 |
| Gender | ||
| Female | 22 | 71 |
| Male | 9 | 29 |
| Ethnic group | ||
| White British | 28 | 90 |
| South Asian | 2 | 6 |
| Black | 1 | 3 |
| Employment status | ||
| Employed | 28 | 90 |
| Unemployed | 2 | 6 |
| Retired | 1 | 3 |
| Other coexisting medical conditions | ||
| Hypertension | 5 | 16 |
| Diabetes | 4 | 13 |
| Respiratory conditions | 3 | 13 |
| Anxiety | 5 | 16 |
| Depression | 4 | 13 |
| Cardiovascular conditions | 4 | 13 |
| Duration of PCS symptoms (months) | ||
| <12 | 5 | 16 |
| 12−23 | 14 | 45 |
| 24+ | 12 | 39 |
| Attending rehabilitation service (months) | ||
| <10 | 8 | 26 |
| 10−15 | 17 | 55 |
| 16+ | 6 | 19 |
| Initial activity level (Week 1) | ||
| Phase 1 | 5 | 16 |
| Phase 2 | 14 | 45 |
| Phase 3 | 11 | 35 |
| Phase 4 | 1 | 3 |
| Phase 5 | 0 | 0 |
| Initial number of PESE episodes (Week 1) | ||
| 0 | 6 | 19 |
| 1 or 2 | 11 | 35 |
| 3 or more | 14 | 45 |
| Initial duration of PESE episodes (Week 1) (h) | ||
| 0 | 6 | 19 |
| 1−3 | 10 | 32 |
| 4−10 | 5 | 16 |
| 11−23 | 2 | 6 |
| 24+ | 8 | 26 |
Abbreviations: PCS, post‐COVID syndrome; PESE,post‐exertional symptom exacerbation.
3.2. Episodes of PESE
Patients who followed the WHO Borg pacing protocol for 6 weeks reported a reduction in the number of PESE episodes experienced, from a mean of 3.4 episodes in Week 1 down to 1.1 in Week 6. Multilevel mixed‐effects Poisson regression showed the number of episodes of PESE experienced decreased on average by 16% (95% CI: 9%−24%; p < 0.001) each week following the WHO Borg pacing protocol (Table 2). The marked decrease in the number of episodes of PESE was achieved at the same time as moderate improvements in targeted physical activity levels, as indicated by the phased return to previous activity levels based on the WHO Borg pacing protocol (Figure 1).
Figure 1.
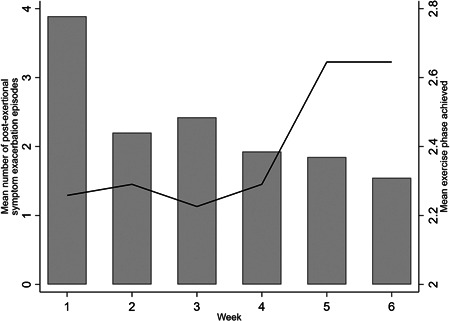
Mean number of episodes of post‐exertional symptom exacerbation and mean exercise phase by week
The frequency of PESE episodes from each trigger based on the weekly results, provides additional insight, as shown in Figure 2. We have used the frequency of episodes with each type of trigger instead of the % because some episodes were reported to be potentially associated with more than one type of trigger. The improvement was seen across all three types of exertion (physical, cognitive, and emotional) causing PESE, but particularly those related to physical exertion (Table 3 and Figure 2).
Figure 2.
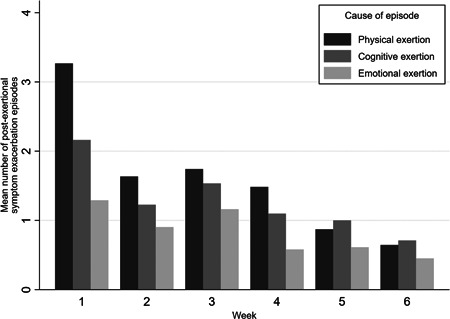
Mean number of episodes of post‐exertional symptom exacerbation by reported physical, cognitive, and emotional exertion associated with the episode
Table 3.
Symptom trajectories over time
| Symptom | % decrease in number of episodes per weeka | 95% CI | p Value |
|---|---|---|---|
| All episodes of PESE | 16 | 9%−24% | p < 0.001 |
| PESE related to | |||
| Physical exertion | 29 | 20%−37% | p < 0.001 |
| Cognitive exertion | 20 | 12%−29% | p < 0.001 |
| Emotional exertion | 27 | 14%−39% | p < 0.001 |
| PESE symptoms | |||
| Fatigue | 10 | 0%−19% | p = 0.05 |
|
Cognitive dysfunction or speech & language difficulty |
2 | −12% to 16% | p = 0.8 |
| Breathlessness | 20 | 0%−39% | p = 0.05 |
| Headache or migraine | 21 | 3%−39% | p = 0.02 |
| Musculoskeletal aches, pains, or weakness | 9 | −6% to 23% | p = 0.2 |
| Palpitations | 29 | −5% to 63% | p = 0.1 |
| Number of PESE symptoms | 13 | 5%−21% | p = 0.002 |
| Duration of PESE symptoms | −2 | −14% to 10% | p = 0.7 |
Abbreviations: CI, confidence interval; PESE, post‐exertional symptom exacerbation.
Adjusted for age and gender.
The most common individual symptoms experienced during an episode were fatigue, cognitive dysfunction (“brain fog”) or speech and language difficulty, breathlessness, headache or migraine, musculoskeletal aches, pains or weakness, and palpitations. The most substantial improvements were seen in fatigue, breathlessness and headache, and possibly palpitations, but less so in cognitive dysfunction, though small numbers with individual symptoms resulted in wide CIs (Table 3 and Figure 3). Another improvement observed was in the average number of symptoms within each PESE episode reported across the 6‐weeks. At Week 1, participants reported an average of 2.68 symptoms exacerbated by activity, compared to 1.68 reported at Week 6.
Figure 3.
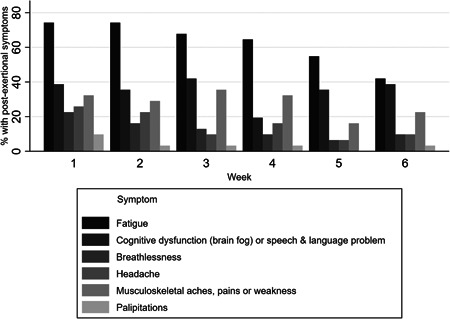
Proportion reporting individual symptoms of post‐exertional symptom exacerbation episodes
There was no evidence of any differences in improvement rates between different subgroups based on sex, age, or starting phase of the pacing protocol (Table 4). However, numbers in each subgroup were small and power to detect differences between subgroups was low, as indicated by wide overlapping CIs.
Table 4.
Symptom trajectories over time by age, gender, and initial level of activity
| Subgroup | % decrease in number of episodes per weeka | 95% CI | p Value for interaction |
|---|---|---|---|
| All episodes of PESE by gender | |||
| Females | 14 | 5%−22% | |
| Males | 23 | 11%−36% | p = 0.2 |
| All episodes of PESE by age (years) | |||
| <50 | 12 | 2%−22% | |
| 50+ | 23 | 13%−34% | p = 0.1 |
| All episodes of PESE by initial exercise | |||
| Phase 1 or 2 | 18 | 9%−28% | |
| Phase 3 or 4 | 14 | 3%−25% | p = 0.5 |
Abbreviations: CI, confidence interval; PESE, post‐exertional symptom exacerbation.
Adjusted for age and gender.
Whilst the number of PESE episodes and number of symptoms per PESE episode reduced markedly over 6 weeks, when an episode did occur, the severity of those symptoms, and the duration of the episode, remained similar over the 6 weeks. The number of participants who did not experience PESE over a 7‐day period increased over the 6‐weeks from 6 to 13 (Figure 4).
Figure 4.
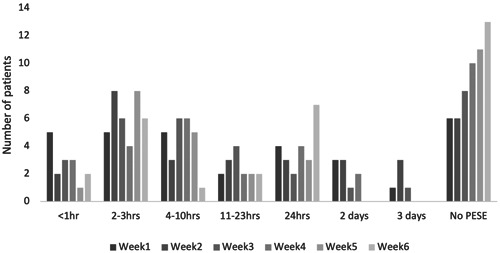
Duration of PESE episodes over the 6‐week intervention period. PESE, post‐exertional symptom exacerbation.
The participants overall health, as rated by the EQ‐5D VAS, improved from a mean of 51.4 points to a mean of 60.6 points (paired difference of 9.2 points, 95% CI: 3.2−15.2 points; p = 0.004). The EQ‐5D index also improved from a mean of 0.49−0.63 (paired difference 0.14, 95% CI: 0.08−0.20; p < 0.001). This improvement was seen across all domains (Figures 5, 6, 7).
Figure 5.
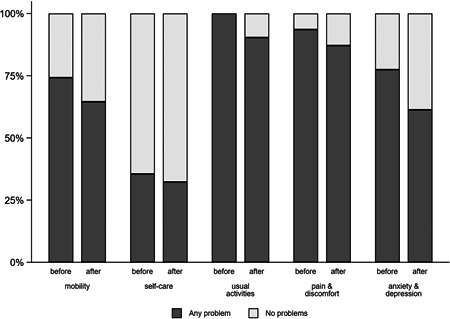
Any problems across five dimensions of the EQ‐5D‐5L before and after the study
Figure 6.
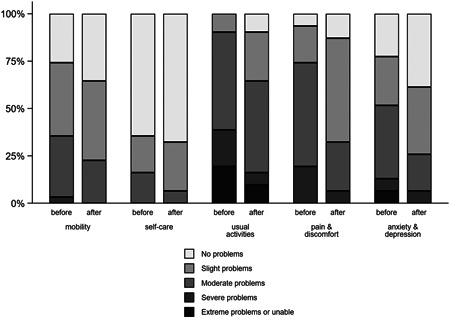
Health ratings across five dimensions of the EQ‐5D‐5L before and after the study
Figure 7.
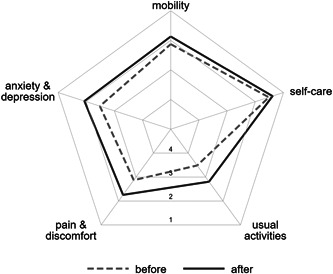
Radar plot of health ratings across five dimensions of the EQ‐5D‐5L before and after the study
3.3. Adverse effects
Four participants could not comply with the protocol guidance, due to being unwell with an acute viral infection separate from COVID, a second infection with COVID‐19, or an exacerbation of PEM, which significantly limited capabilities.
4. DISCUSSION
Our evaluation has found that participants who were managed using the WHO Borg CR‐10 pacing protocol 14 showed a significant reduction in episodes of PESE coupled with improvements in activity levels over 6 weeks. PESE comprises a variety of symptoms triggered not only by physical exertion but also by cognitive and emotional exertion. This study adds to the current understanding by demonstrating the potential of a structured pacing protocol to gradually improve activity levels while substantially decreasing the number of PESE episodes or “crashes” in PCS.
For individuals with long COVID, exertion intolerance has been identified as a severe symptom. 19 Yet, current advice on safely returning to PA without worsening their symptoms is unclear, with patients reporting receiving differing advice from healthcare professionals. 20 Pacing was identified as only the fourth recommended strategy, 20 despite NHS “Your COVID Recovery” guidance stating that pacing is the standard recommendation to manage daily activities. 21 Salman et al. 22 state there is no straightforward evidence‐based approach to guide a patient back to PA following COVID‐19 infection, but an individualized, gradual approach should be taken. Recommendations in international guidelines are currently being revised, namely about pacing. 19
Depending on patient rehabilitation goals, a phased approach can be used to increase the physical activity threshold to baseline, guideline levels or beyond. 23 Individual differences need to be heavily considered, as a patient who was not physically active before COVID‐19 will likely have a lower baseline than a regularly active individual. Hartle et al. 24 reported that the onset of PESE occurred within minutes following physical exertion compared to within hours after cognitive effort. Our findings highlight that PESE was triggered by physical, cognitive, and emotional factors. This is where the Borg CR‐10 PRE scale within the protocol can be used as a specific tool to assist with managing activity intensity to allow patients to identify their perceived exertion level and functional activities, without triggering symptom exacerbation and potentially hindering progress in their rehabilitation. The WHO protocol can be applied to any activity the patient deems suitable to engage in.
Advice surrounding “paced” or “gradual” approaches to manage symptoms have been cited amongst the existing literature exploring physical activity within ME and CFS, 25 , 26 with a particular interest in graded exercise therapy and cognitive behavioral therapy, compared to self‐pacing approaches. 27 Studies have struggled to identify a single, objective biomarker to track PESE or PEM following physical exertion. However, research conducted by Nelson et al. 26 assessed the use of heart rate (HR) parameters to detect differences in autonomic balance in ME/CFS patients in an attempt to detect PESE. It was identified that ME/CFS patients displayed HR alterations, including increases in HR during a 24 h time frame. Monitoring HR parameters is a relatively complex task for a patient to undertake independently. Consequently, utilizing the Borg CR‐10 scale to simplify activity level management has been shown as an effective measure to reduce PESE in PCS patients.
Moving forward, there may be something to learn from pacing strategies employed within PCS management to implement within ME/CFS as continuing research emerges. Sukocheva et al. 28 summarize that extensive data collection and registration of symptoms are needed to determine whether long‐term PCS symptoms are due to a postviral syndrome such as ME/CFS. Post‐COVID‐19 studies have been initiated in the United Kingdom, such as the posthospitalisation COVID‐19 study (PHOSP‐COVID) which intends to prospectively follow 10 000 patients for 1 year 29 in addition to the National Institute for Health Research funding 15 longitudinal COVID‐19 focused research programs to improve our understanding of PCS and potential treatment options, such as LOCOMOTION. 30
This study has a few limitations. The small sample size raises the uncertainty about the potential size of improvement. In particular, we could not undertake any subgroup analysis to identify which individuals are most likely to benefit from this approach or for whom it may be contraindicated. Larger numbers would also be required to identify trends in individual symptoms where the most improvement is seen. However, the overall pattern is one of substantial improvement, particularly in those relating to physical exertion. This is consistent with the WHO protocol being based on phased increases in physical activity rather than cognitive or emotional activity, which would be harder to quantify.
The cohort does not entirely represent the PSC population in terms of an ethnic group or general population in the local area. In particular, the cohort is relatively young and White British. Notably, no concurrent control group was used to compare and demonstrate a causal effect of the WHO Borg protocol based on physical activity. A randomized controlled trial, with a more significant number of participants representative of the broader population of PCS, would be required to confirm this rehabilitation intervention.
The small sample size restricted our ability to explore potentially different outcomes in subgroups of patients defined by comorbidities such as hypertension, type 2 diabetes, respiratory conditions, or psychological conditions as the numbers are simply too small to undertake statistical interactions on these characteristics. We have, however, explored the potential impact of gender, age, duration of PCS, and the initially agreed activity level. Also, the improvement in PESE seen was large enough to be detected, in part because of the efficient repeated measures design.
The results are impressive for many reasons: duration of symptoms (17 months), the substantial size of the improvement with a short 6‐week intervention, the longitudinal nature of the cohort, and the temporal dose–response trend. The findings of this study lend some weight to the evidence for potential benefit and that the significant reduction in the number of episodes of PESE is less likely to be explained by potential biases.
It is also essential to recognize the regular contact during the 6‐week follow‐up period, and whether the weekly telephone calls with a clinician contributed to accountability and encouraged patients to continue following the WHO Borg protocol. Further qualitative research would be needed to clarify patient motivations.
5. CONCLUSIONS
The WHO Borg CR‐10 is a structured pacing protocol shown for the first time in the current literature to substantially reduce PESE episodes whilst increasing activity levels even in a cohort of individuals with long‐standing PCS symptoms. This approach to pacing has the potential to be an effective rehabilitation intervention in PCS. The study also demonstrated that PESE episodes could be triggered by physical, cognitive, or emotional exertion and each episode comprises several symptoms with variable duration of each episode. Future research should investigate a longer pacing program in a clinical trial setting for both post‐acute and chronic stages of PCS and must explore the potential of this intervention to prevent the development of a chronic PCS state in these individuals.
AUTHOR CONTRIBUTIONS
Megan Parker and Manoj Sivan conceptualized the study. Megan Parker, Hannah Brady Sawant, Thuvia Flannery, Rachel Tarrant, Jenna Shardha, Denise Ross, Stephen Halpin, and Manoj Sivan (as the service evaluation and research lead) were involved in obtaining NHS service approvals, iterations to develop the PESE questionnaire, and regular supervision of the work within the Covid rehabilitation service. Megan Parker and Rebecca Banister were involved in obtaining approvals from Leeds Beckett University and MSc project registration and supervision. Data collection was mainly performed by Megan Parker with help from Hannah Brady Sawant, Thuvia Flannery. Darren C. Greenwood is the senior biostatistician in the team and helped perform all the statistical analysis presented in the draft. Megan Parker wrote the initial manuscript draft, which was edited by all authors and the final manuscript was approved by all authors.
CONFLICTS OF INTEREST
Manoj Sivan is the advisor to the WHO on Covid rehabilitation policy in Europe. Manoj Sivan, Denise Ross, Rachel Tarrant, and Jenna Shardha were involved in writing the WHO self‐management booklet which proposed the Borg CR‐10 pacing protocol that has been investigated in this study. 14
Supporting information
Supplementary file 1.
Supplementary file 2.
ACKNOWLEDGMENTS
The authors would like to thank individuals with PCS and healthcare professionals who provided valuable suggestions and feedback during the study and the iterative process of PESE questionnaire development.
Parker M, Sawant HB, Flannery T, et al. Effect of using a structured pacing protocol on post‐exertional symptom exacerbation and health status in a longitudinal cohort with the post‐COVID‐19 syndrome. J Med Virol. 2022;95:e28373. 10.1002/jmv.28373
DATA AVAILABILITY STATEMENT
The data sets used and analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. NICE . COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19. London: National Institute for Health and Care Excellence (NICE) Scottish Intercollegiate Guidelines Network (SIGN) and Royal College of General Practitioners (RCGP). 2022.
- 2. ONS . Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK: 7 July 2022. Office of National Statistics; 2022.
- 3. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DA, O'Brien KK. Conceptualising long COVID as an episodic health condition. BMJ Glob Health. 2021;6(9):e007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sivan M, Rayner C, Delaney B. Fresh evidence of the scale and scope of long covid. BMJ. 2021;373:n853. [DOI] [PubMed] [Google Scholar]
- 6. Larsen NW, Stiles LE, Miglis MG. Preparing for the long‐haul: autonomic complications of COVID‐19. Auton Neurosci. 2021;235:102841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC . Preventing worsening of symptoms. Centres for Disease Control and Prevention; 2022.
- 8. Twomey R, DeMars J, Franklin K, et al. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. 2022;102(4):pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ausubel B, Brown‐Clark J. When to talk to your provider about the relationship between long COVID and ME/CFS. Women's Health Activist. 2021;46(2):9‐11. [Google Scholar]
- 10. White AT, Light AR, Hughen RW, et al. Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology. 2010;47(4):615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halle M, Bloch W, Niess AM, et al. Exercise and sports after COVID‐19‐Guidance from a clinical perspective. Translational Sports Med. 2021;4(3):310‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post‐COVID to improve fatigue, breathlessness exercise capacity and cognition—a cohort study. Chron Respir Dis. 2021;18: 14799731211015691. doi:10.1177/14799731211015691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putrino D, Tabacof L, Tosto‐Mancuso J, et al. Autonomic conditioning therapy reduces fatigue and improves global impression of change in individuals with post‐acute COVID‐19 syndrome. Research Square. 2021. https://www.researchsquare.com/article/rs-440909/v1 [Google Scholar]
- 14. WHO . Support for rehabilitation: self‐management after COVID‐19‐related illness. Copenhagen: WHO Regional Office for Europe; 2021. https://apps.who.int/iris/handle/10665/344472
- 15. Cotler J, Holtzman C, Dudun C, Jason L. A brief questionnaire to assess post‐exertional malaise. Diagnostics. 2018;8(3):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sivan M, Preston N, Parkin A, et al. The modified COVID‐19 Yorkshire rehabilitation scale (C19‐YRSm) patient‐reported outcome measure for long Covid or post‐COVID‐19 syndrome. J Med Virol. 2022;94(9):4253‐4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20(10):1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health. 2012;15(5):708‐715. [DOI] [PubMed] [Google Scholar]
- 19. Buchberger B, Zwierlein R, Rohde V. Post‐corona‐fatigue—das bekannte bild in neuem Gewand? Der Onkologe. 2022;28(4):340‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright J, Astill S, Sivan M. The relationship between physical activity and long COVID: a cross‐sectional study. Int J Environ Res Public Health. 2022;19(9):5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NHSE . National commissioning guidance for post COVID services (July 2022). NHS England; 2022.
- 22. Salman D, Vishnubala D, Le Feuvre P, et al. Returning to physical activity after covid‐19. BMJ. 2021;372:m4721. [DOI] [PubMed] [Google Scholar]
- 23. Barker‐Davies RM, O'Sullivan O, Senaratne KPP, et al. The Stanford hall consensus statement for post‐COVID‐19 rehabilitation. Br J Sports Med. 2020;54(16):949‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartle M, Bateman L, Vernon SD. Dissecting the nature of post‐exertional malaise fatigue. Fatigue. 2021;9:33‐34. [Google Scholar]
- 25. Geraghty K, Hann M, Kurtev S. Myalgic encephalomyelitis/chronic fatigue syndrome patients' reports of symptom changes following cognitive behavioural therapy, graded exercise therapy and pacing treatments: analysis of a primary survey compared with secondary surveys. J Health Psychol. 2019;24(10):1318‐1333. [DOI] [PubMed] [Google Scholar]
- 26. Nelson MJ, Bahl JS, Buckley JD, Thomson RL, Davison K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review and meta‐analysis. Medicine. 2019;98(43):e17600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White P, Goldsmith K, Johnson A, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sukocheva OA, Maksoud R, Beeraka NM, et al. Analysis of post COVID‐19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J Adv Res. 2022;40:179‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans RA, Leavy OC, Richardson M, et al. Clinical characteristics with inflammation profiling of long COVID and association with 1‐year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respiratory Med. 2022;10(8):761‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sivan M, Greenhalgh T, Darbyshire JL, et al. LOng COvid multidisciplinary consortium optimising treatments and services across the NHS (LOCOMOTION): protocol for a mixed‐methods study in the UK. BMJ Open. 2022;12(5):e063505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1.
Supplementary file 2.
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author upon reasonable request.


