Abstract
Background
There are limited data regarding COVID‐19 vaccination during pregnancy.
Objectives
To evaluate the effects of COVID‐19 vaccination received during pregnancy on SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation, COVID‐19‐related intensive care unit (ICU) admission and maternal–fetal complications.
Search strategy
MEDLINE, CINHAL, Embase, Scopus and CENTRAL databases, as well as ClinicalTrials.gov, reference lists, related articles and grey literature sources.
Selection criteria
Randomised controlled trials, non‐randomised studies of interventions, pregnant women, COVID‐19 vaccination during pregnancy.
Data collection and analysis
Study selection, risk‐of‐bias assessment, data extraction and assessment of the certainty of evidence using the GRADE method were performed independently by two authors. Meta‐analyses were performed using Cochrane RevMan 5.4. PROSPERO registration number: CRD42022308849.
Main results
We included 14 observational studies (362 353 women). The administration of a COVID‐19 vaccine during pregnancy resulted in a statistically significant reduction in SARS‐CoV‐2 infection (OR 0.46, 95% CI 0.28–0.76) and COVID‐19‐related hospitalisation (OR 0.41, 95% CI 0.33–0.51). The effect appeared to be greater in fully vaccinated women, for both infection (OR 0.31, 95% CI 0.16–0.59) and hospitalisation (OR 0.15, 95% CI 0.10–0.21). However, the certainty of evidence was very low. The difference in COVID‐19‐related ICU admission between vaccinated and unvaccinated individuals did not reach statistical significance (OR 0.58, 95% CI 0.13–2.58). Finally, there were no statistically significant differences in any of the maternal–fetal complications considered in the included studies.
Conclusions
COVID‐19 vaccination administered during pregnancy seems to reduce SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation, with no significant effects on maternal–fetal complications.
Keywords: COVID‐19 vaccine, effectiveness, meta‐analysis, pregnancy, SARS‐CoV‐2, systematic review
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and there has been a rapid increase in COVID‐19 cases and related deaths since it was identified in early December 2019. 1 SARS‐CoV‐2 infection during pregnancy is associated with severe illness, with an increased risk of intensive care unit (ICU) admission, maternal death and adverse pregnancy outcomes. 2 , 3 , 4 , 5 COVID‐19 affects pregnancy in part because the immune system is directed towards fetal tolerance. 6 In addition, SARS‐CoV‐2 is targeted to the respiratory and cardiovascular systems, which are physiologically stressed during pregnancy. 7 Moreover, SARS‐CoV‐2 infection of the maternal placental surface may induce acute or chronic placental insufficiency, leading to pregnancy complications. 7 , 8
Studies have shown that COVID‐19 vaccination during pregnancy was associated with lower odds of severe or critical COVID‐19 during the pandemic, 9 , 10 , 11 and it has been reported that vaccinated individuals were less likely to experience adverse pregnancy outcomes. 12 , 13 , 14 , 15 As a result, the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynaecologists and the Society for Maternal–Fetal Medicine have each issued guidance supportive of offering COVID‐19 vaccines during pregnancy. 8 , 16 , 17 , 18 , 19
COVID‐19 vaccine hesitancy remains high, however, and concerns about safety and effectiveness are commonly cited barriers to vaccination among pregnant women. 20
As pregnant women were excluded from phase‐III trials, the effects of the vaccine on mother and child were not based on results obtained in the monitored setting of a clinical trial, but instead were estimated based on delayed reports of pregnancy outcomes from healthcare settings. 21 , 22 As a result, there are very limited data regarding the effectiveness of COVID‐19 vaccines in pregnant women. Thus, the aim of this systematic review (SR) was to assess the effects of COVID‐19 vaccination received during pregnancy on SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation, COVID‐19‐related ICU admission and maternal–fetal complications.
2. METHODS
We followed the Cochrane Handbook (v6.3) in conducting the study and the PRISMA Statement 2020 in reporting the results. 23 , 24 We registered the protocol in PROSPERO (www.crd.york.ac.uk/prospero) with registration number CRD42022308849.
We used the following inclusion criteria to select studies:
Study designs: randomised controlled trials (RCTs) and non‐randomised studies of interventions (NRSI), such as non‐randomised controlled trials, cohort studies and case–control studies.
Participants: pregnant women in all three trimesters.
Interventions: any type of COVID‐19 vaccination administered during pregnancy.
Comparators: absence of COVID‐19 vaccination (no intervention, placebo vaccine).
Outcomes: incidence of SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation, COVID‐19‐related ICU admission and maternal–fetal complications.
With the collaboration of a professional librarian, we searched the electronic bibliographic databases: MEDLINE (PubMed), CINHAL (EBSCOhost), EMBASE, Scopus (Ovid) and Cochrane Central Register of Controlled Trials (CENTRAL). In addition, to identify other relevant studies, we searched ClinicalTrials.gov, the reference lists of other SRs on the topic and the reference lists of the included articles, and grey literature sources such as databases of conference proceedings, theses and Google Scholar (scholar.google.com). The search strategies used for each database, following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses literature search extension (PRISMA‐S), are available in Appendix S1. 25 We limited the search to articles published in English, Italian, French and Spanish from 2019 to 4 February 2022, with no setting restrictions. We used Rayyan (www.rayyan.com) to eliminate duplicate records.
The selection process consisted of two phases: an initial screening by title and abstract and a second selection step, in which the full texts were read. Both steps were carried out independently by two authors (MT and CT), and disagreements were resolved through discussion with a third reviewer (SS). We report the number of studies retrieved and the number of included and excluded articles at every step in the Results section using the PRISMA 2020 flow diagram (Figure 1). 24
FIGURE 1.
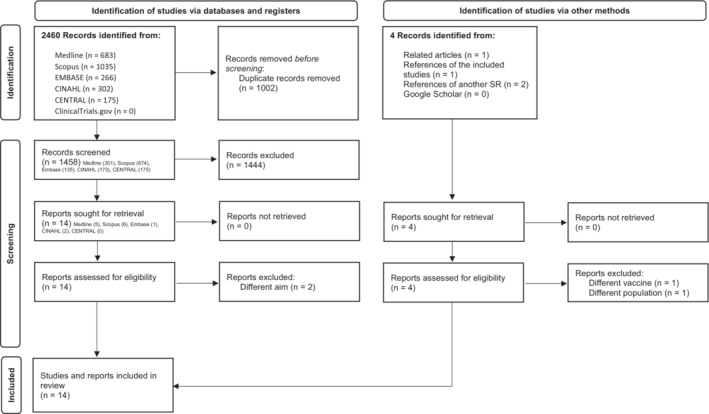
PRISMA 2020 flow diagram of the selection process
Data extraction was performed independently by two reviewers (MT and CT) using a data extraction sheet in Microsoft Excel (Microsoft, Redmond, WA, USA). Any discrepancies were resolved through discussion. In the case of uncertain or missing data, we contacted the study authors by email, with a maximum of two emails sent to each author if no response was received. To complete the information in Table 1 and Tables S1 and S2, we requested additional information from the authors of all included studies; however, authors from only six of the included studies replied to the email. 13 , 15 , 26 , 27 , 28 , 29 In cases in which information is missing because of the lack of response or provision of additional data, we have filled in the table with the inscription ‘ns’ (not specified).
TABLE 1.
Characteristics of the included studies
| Author, year | Study design | Sample size | Vaccinated, x (%) | Not vaccinated, x (%) | Vaccine type, x/n (%) | Vaccination period (trimester I, II, III), x/n (%) | Parity (N = nulliparous; P = parous), x/n (%) | Age (years) |
|---|---|---|---|---|---|---|---|---|
| Blakeway, 2022 | Retrospective cohort study | 1328 | 140 (10.5) | 1188 (89.5) |
Pfizer = 109/140 (77.8) Moderna = 18/140 (12.9) AstraZeneca = 13/140 (9.3) |
I = 0/140 (0.0) II = 20/140 (14.3) III = 120/140 (85.7) |
V: N = 78/140 (55.7) P = 62/140 (44.3) NV: N = 595/1188 (50.1) P = 593/1188 (49.9) |
V = 35.0 (31.7–37.0) NV = 33.0 (30.0–36.0) median (IQR) |
| Bleicher, 2021 | Prospective cohort study | 326 | 202 (62) | 124 (38) | Pfizer = 202/202 (100) | ns | ns |
V = 31.7 ± 3.9 NV = 30.2 ± 5.09 mean ± SD |
| Butt, 2021 | Prospective cohort study | 814 | 407 (50) | 407 (50) |
Pfizer = ns Moderna = ns |
I = 323/407 (79.4) II = 84/407 (20.6) |
ns |
V = 32 (29–36) NV = 32 (28–36) median (IQR) |
| Dagan, 2022 | Prospective cohort study | 21 722 | 10 861 (50) | 10 861 (50) | Pfizer = 10 861/10 861 (100) |
I = 2814/10 861 (26) II = 5242/10 861 (48) III = 2805/10 861 (26) |
ns |
V = 30 (26–33) NV = 30 (26–33) median (range) |
| Goldshtein, 2021 | Retrospective cohort study | 15 060 | 7530 (50) | 7530 (50) | Pfizer = 7530/7530 (100) |
I = 1581/7530 (21) II = 3464/7530 (46) III = 2485/7530 (33) |
V: N = 3447/7530 (45.8) P = 4083/7530 (54.2) NV: N = 3447/7530 (45.8) P = 4083/7530 (54.2) |
V = 31.1 ± 5.01 NV = 30.4 ± 5.53 mean ± SD |
| Kharbanda, 2021 | Case–control study | 105 446 | 15 079 (14.3) | 90 367 (85.7) |
Pfizer = 8218/15 079 (54.5) Moderna = 6333/15 079 (42.0) Janssen = 528/15 079 (3.5) |
ns | ns | ns |
| Lipkind, 2022 | Retrospective cohort study | 46 079 | 10 064 (21.8) | 36 015 (78.2) |
Pfizer = 5478/10 064 (54.4) Moderna = 4162/10 064 (41.4) Janssen = 424/10 064 (4.2) |
I = 172/10 064 (1.7) II = 3668/10 064 (36.5) III = 6224/10 064 (61.8) |
ns |
V = 32.3 ± 4.5 NV = 29.8 ± 5.3 mean ± SD |
| Magnus, 2021 | Case–control study | 18 477 | 1003 (5.4) | 17 474 (94.5) |
Pfizer = 790/1003 (78.7) Moderna = 137/1003 (13.7) AstraZeneca = 76/1003 (7.6) |
ns |
V: N = 643/1003 (64.1) P = 360/1003 (35.9) NV: N = 10 701/17 474 (61.2) P = 6773/17 474 (38.8) |
ns |
| Morgan, 2022 | Retrospective cohort study | 10 092 | 1332 (13.2) | 8760 (86.8) |
Pfizer = 883/1332 (66.3) Moderna = 382/1332 (28.7) Janssen = 67/1332 (5.0) |
ns | ns |
V = 32.1 ± 5.9 NV = 27.8 ± 4.9 mean ± SD |
| Rottenstreich, 2021 | Retrospective cohort study | 1775 | 712 (40.1) | 1063 (59.9) | Pfizer = 712/712 (100) | III = 712/712 (100) | ns |
V = 30.6 ± 5.8 NV = 29.5 ± 6 mean ± SD |
| Shimabukuro, 2021 | Retrospective cohort study | 3958 | 3958 (100) | 0 (0.0) |
Pfizer = 2136/3958 (54.0) Moderna = 1822/3958 (46.0) |
Periconception = 92/3958 (2.3) I = 1132/3958 (28.6) II = 1714/3958 (43.3) III = 1019/3958 (25.7) |
ns | ns |
| Stock, 2022 | Prospective cohort study | 130 875 |
18 399 (14.0) 25 917 vaccinations |
112 476 (86.0) |
Pfizer = 20 572/25 917 (79.4) Moderna = 3224/25 917 (12.4) AstraZeneca = 2121/25 917 (8.2) |
I = 9905/25 917 (38.2) II = 9317/25 917 (35.9) III = 6695/25 917 (25.8) |
ns | ns |
| Theiler, 2021 | Retrospective cohort study | 2002 | 140 (7.0) | 1862 (93) |
Pfizer = 127 (90.7) Moderna = 12 (8.6) Janssen = 1 (0.7) |
ns |
V: N = 56 (40.0) P = 84 (60) NV: N = 546 (29.3) P = 1316 (70.7) |
V = 31.8 ± 3.7 NV = 30.5 ± 5.2 mean ± SD |
| Wainstock, 2021 | Retrospective cohort study | 4399 |
Total = 913 (20.8) 1 dose = 155 (17.0) 2 doses = 758 (83.0) |
3486 (79.2) | Pfizer‐BioNTech = 913 (100) | ns | ns |
V = 30.6 ± 5.3 NV = 28.2 ± 5.7 mean ± SD |
Abbreviations: IQR, interquartile range; ns, not specified; NV, not vaccinated; SD, standard deviation; V, vaccinated.
We collected data on reports (first author, publication year, study design), participants (sample size, sample characteristics), intervention (vaccine type received, vaccination strategy) and outcomes (incidence of SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation, COVID‐19‐related ICU admission and maternal–fetal complications).
Two authors (MT and IP) independently assessed the risk of bias (RoB) of the included studies using the Risk of Bias in Non‐randomised Studies of Interventions (ROBINS‐I) tool, 30 and resolved disagreements by discussion with a third author (SS). We used robvis (visualisation tool) to produce the RoB summary and RoB graph. 31
We reported the results as follows:
Primary outcomes: SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation and COVID‐19‐related ICU admission.
Secondary outcomes: maternal–fetal complications.
We conducted a meta‐analysis of data for both primary and secondary outcomes reported by at least two included studies, using odds ratio (ORs) as a measure of effect size. We judged the effectiveness based on statistical significance (i.e. the 95% CI of the effect between groups did not include the null value).
We used the DerSimonian–Laird random‐effects model as a conservative approach to account for different sources of heterogeneity among studies. Statistical heterogeneity of the studies was evaluated using the I 2 test. We conducted a sensitivity analysis for primary outcomes in which we considered only studies and results from fully vaccinated women (i.e. at least 14 days after receiving the necessary vaccine doses, to define the vaccination as complete, which was either one or two doses depending on the vaccine type). We planned another sensitivity analysis to compare the effects of different COVID‐19 vaccination strategies (different types, doses or timing), but we did not retrieve enough data to perform this analysis.
We examined publication bias using funnel plots. The Egger test for funnel plot asymmetry was not performed because no meta‐analysis included at least ten studies. 23
We performed statistical analyses using Cochrane RevMan 5.4 (www.cochrane.org). We assessed the certainty of the body of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. 32 Two authors (MT and SS) independently assessed the quality of evidence as high, moderate, low or very low by considering five domains that can reduce the quality of evidence (study design and RoB, inconsistency, indirectness, imprecision, publication bias) and three that can increase the quality of evidence (large magnitude of an effect, dose–response gradient, effect of plausible residual confounding). 32 Following the Cochrane Handbook (v6.3), 33 we used GRADEpro GDT (www.gradepro.org) to elaborate a summary of findings table for the outcomes investigated.
No patients were involved in this research.
3. RESULTS
3.1. Selection process
We found 2460 records during the database search, and after the removal of duplicates, we screened 1458 records and evaluated 14 full‐text reports for inclusion in the review. In addition, we identified four reports from other sources: one in the related articles in MEDLINE (PubMed), another in the references of the included studies and two in the references of another SR. Ultimately, we excluded a total of four studies, 34 , 35 , 36 , 37 and included 14 reports describing 14 studies. The flow diagram of the selection process can be found in Figure 1, the reasons for exclusion are listed in Table S3 and the citations of the included studies are listed in Table S4.
3.2. Characteristics of the individual studies
No RCTs were found. All the included studies were observational: four prospective cohort studies, 15 , 26 , 38 , 39 eight retrospective cohort studies, 10 , 11 , 13 , 14 , 27 , 40 , 41 , 42 and two case–control studies. 28 , 29 The entire population consisted of 362 353 women, of whom 70 740 received at least one dose of a COVID‐19 vaccine during pregnancy and 291 613 were not vaccinated against COVID‐19 during pregnancy. The characteristics of the included studies are reported in Table 1.
3.3. Effect of vaccination on SARS‐CoV‐2 infection, COVID‐19‐related hospitalisation and COVID‐19‐related ICU admission
Meta‐analysis of eight studies showed a significant reduction in the probability of SARS‐CoV‐2 infection in vaccinated women (OR 0.46, 95% CI 0.28–0.76, p = 0.002), with a high heterogeneity (I 2 = 94%) (Figure 2A). 10 , 11 , 13 , 15 , 26 , 38 , 39 , 40 The sensitivity analysis considering only fully vaccinated women showed a stronger effect (OR 0.31, 95% CI 0.16–0.59, p = 0.0004), but the heterogeneity remained substantial (I 2 = 75%) (Appendix S2). 10 , 11 , 15 , 26
FIGURE 2.
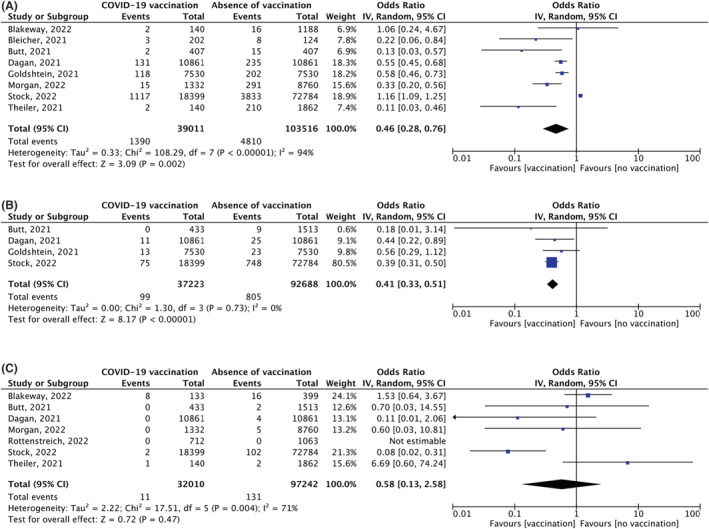
Forest plots of meta‐analysis for primary outcomes (A) Outcome: incidence of SARS‐CoV‐2 infection. Comparison: vaccinated vs non‐vaccinated pregnant women. (B) Outcome: COVID‐19‐related hospitalisation. Comparison: vaccinated vs non‐vaccinated pregnant women. (C) Outcome: COVID‐19‐related ICU admission. Comparison: vaccinated vs non‐vaccinated pregnant women.
Meta‐analysis of four studies identified a significant reduction of COVID‐19‐related hospitalisation in vaccinated women (OR 0.41, 95% CI 0.33–0.51, p < 0.00001), with no heterogeneity (I 2 = 0%) (Figure 2B). 13 , 15 , 26 , 39 The sensitivity analysis considering only fully vaccinated women showed a stronger effect (OR 0.15, 95% CI 0.10–0.21, p < 0.00001), with no heterogeneity (I 2 = 0%) (Appendix S2). 15 , 26
Meta‐analysis of seven studies did not identify a significant reduction in COVID‐19‐related ICU admissions in vaccinated women (OR 0.58, 95% CI 0.13–2.58, p = 0.47), with a high heterogeneity (I 2 = 71%) (Figure 2C). 10 , 11 , 15 , 26 , 39 , 40 , 41 The effect size was not substantially modified in the sensitivity analysis considering only fully vaccinated women (OR 0.53, 95% CI 0.05–5.95, p = 0.61) (Appendix S2).
We report all primary outcomes data in Table S1.
3.4. Effect of vaccination on maternal‐fetal complications
Eight studies evaluated maternal complications occurring during pregnancy in vaccinated versus unvaccinated women (Table S2). 10 , 11 , 13 , 38 , 39 , 40 , 41 , 42 We performed a meta‐analysis for the following outcomes: composite pregnancy complications (OR 0.99, 95% CI 0.81–1.21, p = 0.93), hypertensive disorders and pre‐eclampsia (OR 1.11, 95% CI 0.86–1.42, p = 0.42), placental abruption (OR 0.60, 95% CI 0.29–1.21, p = 0.15), thromboembolism (OR 2.44, 95% CI 0.12–51.05, p = 0.57), postpartum haemorrhage (OR 0.89, 95% CI 0.62–1.29, p = 0.54), puerperal fever (OR 0.91, 95% CI 0.55–1.50, p = 0.71) and maternal death (OR 2.19, 95% CI 0.09–53.82, p = 0.63). No significant differences between vaccinated and unvaccinated women were observed for these outcomes (Appendix S3).
Nine studies evaluated fetal complications occurring during pregnancy in vaccinated versus unvaccinated women (Table S2). 10 , 11 , 13 , 14 , 15 , 38 , 40 , 41 , 42 We performed a meta‐analysis for the following outcomes: pregnancy loss (OR 1.04, 95% CI 0.96–1.13, p = 0.36), fetal abnormalities (OR 0.91, 95% CI 0.40–2.07, p = 0.82), small for gestational age (OR 1.01, 95% CI 0.87–1.17, p = 0.88), intrauterine growth restriction (OR 0.97, 95% CI 0.62–1.52, p = 0.90), preterm birth (OR 0.82, 95% CI 0.64–1.06, p = 0.12), stillbirth (OR 0.73, 95% CI 0.28–1.87, p = 0.51), meconium‐stained amniotic fluid (OR 0.78, 95% CI 0.58–1.05, p = 0.10), neonatal ICU admission (OR 0.91, 95% CI 0.58–1.44, p = 0.69) and hypoxic ischaemic encephalopathy (OR 4.42, 95% CI 0.18–108.91, p = 0.36). No significant differences were observed for these outcomes between vaccinated and unvaccinated women (Appendix S3).
3.5. Risk of bias within studies
The overall RoB was serious for 12, 10 , 11 , 14 , 15 , 26 , 27 , 28 , 29 , 38 , 40 , 41 , 42 and moderate for two, 13 , 39 of the included studies. This judgement was primarily influenced by the confounding bias domain, in which most of the studies had a serious RoB. 10 , 11 , 14 , 15 , 26 , 27 , 28 , 29 , 38 , 40 , 41 , 42 In the domains of the selection of participants and the selection of reported results, all studies had a moderate RoB. 10 , 11 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 38 , 39 , 40 , 41 , 42 Finally, in the biases resulting from the classification of the intervention, 10 , 11 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 39 , 40 , 42 deviations from intended interventions, 10 , 11 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 38 , 39 , 40 , 41 , 42 missing data, 10 , 11 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 39 , 40 , 41 , 42 and the measurement of outcome domains, 10 , 11 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 38 , 39 , 40 , 41 , 42 most of the studies obtained a low RoB. The attrition rate of the included studies can be found in Table S5, the RoB assessment is presented in Figure S1 and the justifications for each judgement are listed in Table S6.
3.6. Risk of publication bias
The funnel plots showed gaps and asymmetries for both primary and secondary outcomes, which could suggest the presence of publication bias (Appendix S4). However, we must consider that the asymmetry could result from several factors, such as non‐reporting biases, poor methodological quality, leading to spuriously inflated effects in smaller studies, true heterogeneity, artefacts and chance. 23 Furthermore, none of the outcomes were addressed in at least ten studies, so one must be cautious in making a visual interpretation, and performing a statistical test for asymmetry (i.e. Egger test) is not appropriate. 23 In any case, the probable presence of publication bias was considered relevant in the GRADE approach.
3.7. Certainty in the body of evidence
All considered outcomes started the GRADE process with a low certainty of evidence because the data were obtained from observational studies. 32 All of the outcomes were downgraded to having a very low certainty of evidence because of the high RoB in most of the included studies and the probable presence of publication bias. In addition, some outcomes also exhibited unexplained high (I 2 > 60%) or very high (I 2 > 90%) heterogeneity and wide (with a range greater than 0.5 OR points) or very wide (with a range greater than 1.0 OR points) confidence intervals. For these reasons, the evidence is very uncertain for all outcomes considered. For more information, see the summary of findings tables with footnotes explaining judgements for primary (Figure 3) and secondary outcomes (Appendix S5).
FIGURE 3.
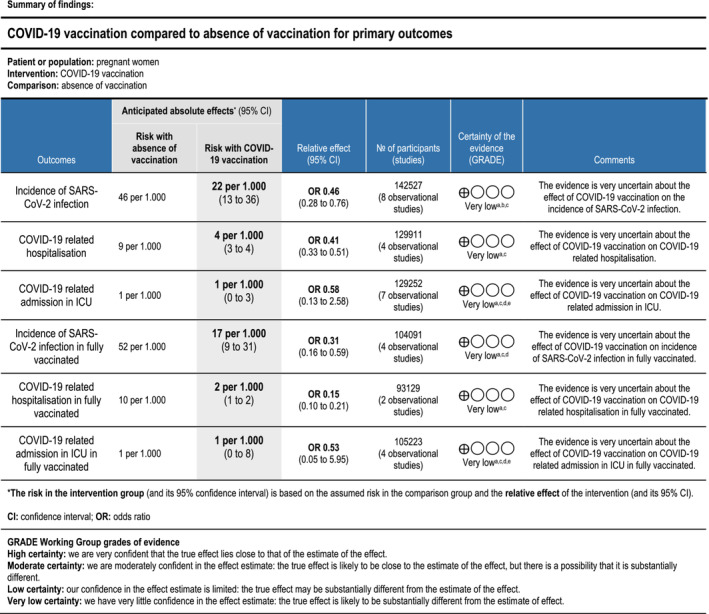
GRADE summary of findings table for primary outcomes. aDowngrade by one level due to a high risk of bias in most of the included studies for this outcome (see the ROB assessment results with ROBINS I). bDowngrade by two levels due to unexplained high heterogeneity, I2 > 90% (see the forest plots and the results section). cDowngrade by one level due to probable publication bias (see the funnel plots and the results section). dDowngrade by one level due to unexplained heterogeneity, I2 > 60% (see the forest plots and the results section). eDowngrade by two levels due to very wide confidence intervals. Confidence interval range greater than 1.0 OR points.
4. DISCUSSION
4.1. Main findings
Our SR demonstrated that the administration of a COVID‐19 vaccine during pregnancy resulted in a statistically significant reduction in SARS‐CoV‐2 infection (OR 0.46, 95% CI 0.28–0.76) and COVID‐19‐related hospitalisation (OR 0.41, 95% CI 0.33–0.51), but the certainty of evidence was very low. The effect appeared to be greater for both infection (OR 0.31, 95% CI 0.16–0.59) and hospitalisation (OR 0.15, 95% CI 0.10–0.21) when considering only fully vaccinated women, although the level of certainty was still very low. Conversely, the difference in ICU admissions related to COVID‐19 did not reach statistical significance (OR 0.58, 95% CI 0.13–2.58), probably because of the small number of total cases among both vaccinated and unvaccinated women.
Finally, there was no significant difference between vaccinated and unvaccinated women in any of the maternal–fetal complications considered in the included studies.
4.2. Strengths and limitations
Our SR is subject to several limitations. Of the few studies that addressed the questions of our SR, all were observational and most presented a serious RoB. Moreover, five of the 14 included studies did not recruit women with a history of SARS‐CoV‐2 infection, 13 , 26 , 39 , 41 , 42 whereas the other studies did not provide information about SARS‐CoV‐2 infection history. 10 , 11 , 14 , 15 , 27 , 28 , 29 , 38 , 40 This could indicate selection bias. Moreover, the included studies did not report data stratified by trimester of pregnancy; as a result, we were unable to study the outcomes in each trimester. Additionally, we must consider that time‐varying exposure outcomes (i.e. pregnancy loss, preterm birth, stillbirth, placental abruption and maternal death) can occur at any time during pregnancy, and if they occur early, participants have a lower likelihood of getting the vaccine. This may affect the results by creating a bias suggesting a protective effect of the vaccine on such outcomes. 43 In our case, it may have particularly influenced the outcome of the meta‐analysis for preterm birth (OR 0.82, 95% CI 0.64–1.06) (Appendix S3). Finally, we have restricted the eligibility based on the language of publication, and thus otherwise eligible studies could have been excluded.
On the other hand, our SR also had several strengths, including the completion of a sensitive search in multiple databases, high methodological quality, according to the standards, and the use of the GRADE approach. 32
4.3. Interpretation
The results of our SR should be interpreted with caution because of the very low level of certainty of the evidence. Nevertheless, COVID‐19 vaccination administered during pregnancy seems to reduce the incidence of SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation, with no significant effects on maternal–fetal complications. These findings should be considered by both clinicians and pregnant women and could help to overcome vaccine hesitancy. Reducing the number of infections or hospitalisations is an important goal that would limit the risk of pregnancy and perinatal complications associated with symptomatic or severe COVID‐19, 3 , 4 , 5 , 44 , 45 prevent hospital‐related adverse events, 46 , 47 , 48 and reduce the economic burden on healthcare facilities.
A recent SR and meta‐analysis published by Prasad et al. addressed a similar question, but there are a few differences between their review and ours. 49 Some of the studies in their review included women vaccinated before pregnancy or non‐pregnant individuals. In addition, they did not consider COVID‐19‐related hospitalisation and many maternal–fetal complications (i.e. composite pregnancy complications, puerperal fever, small for gestational age, intrauterine growth restriction, meconium‐stained amniotic fluid and hypoxic ischaemic encephalopathy). Nonetheless, their results are similar to our results; however, they found a significant reduction in stillbirth in the vaccinated cohort (OR 0.85, 95% CI 0.73–0.99, p < 0.01, I 2 = 93.9%) and we did not. This discrepancy may result from the fact that they included data from two study registers, which increased the sample size.
Another SR and meta‐analysis studied the effect of vaccination on SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation during pregnancy. 50 It included only six observational studies published up to September 2021. Its results are consistent with our findings.
Additional SRs found that COVID‐19 vaccination does not appear to be associated with maternal–fetal complications; rather, it was associated only with common adverse reactions, such as transient headache, pain at the injection site and fatigue. 50 , 51 , 52 , 53 , 54
Our study contributed to the knowledge on the topic by including new studies in the SR and providing additional data related to the certainty of evidence using the GRADE method. 32
5. CONCLUSION
COVID‐19 vaccination administered during pregnancy seems to reduce the incidence of SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation, with no significant effects on maternal–fetal complications. However, the certainty of evidence is very low. For future research, we recommend high‐quality RCTs to increase the level of the certainty of evidence, performing studies or generating data comparing different vaccination strategies with each other (e.g. different types, doses or timing) and further data stratification according to the trimester of pregnancy to enable subgroup analysis and meta‐regression.
AUTHOR CONTRIBUTIONS
MT, PG, and RC proposed the review project and identified the framework. MT, IP, CT, SS, GS, PG and RC defined the protocol, developed the search strategy, extracted the data and assessed the RoB. SS provided methodological support and performed statistical analyses. SS and MT applied the GRADE method. All authors approved the final version of the article and agreed to be accountable for all aspects of it.
ACKNOWLEDGEMENTS
None.
FUNDING INFORMATION
None.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interests form available to view online as supporting information.
ETHICS APPROVAL
None.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Tormen M, Taliento C, Salvioli S, Piccolotti I, Scutiero G, Cappadona R, et al. Effectiveness and safety of COVID‐19 vaccine in pregnant women: A systematic review with meta‐analysis. BJOG. 2022;00:1–10. 10.1111/1471-0528.17354
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yanes‐Lane M, Winters N, Fregonese F, Bastos M, Perlman‐Arrow S, Campbell JR, et al. Proportion of asymptomatic infection among COVID‐19 positive persons and their transmission potential: a systematic review and meta‐analysis. PLoS One. 2020;15(11):1–21. 10.1371/journal.pone.0241536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS‐CoV‐2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327(8):748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez‐Portilla RJ, Sotiriadis A, Chatzakis C, Torres‐Torres J, Espino y Sosa S, Sandoval‐Mandujano K, et al. Pregnant women with SARS‐CoV‐2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet Gynecol. 2021;57(2):224–31. [DOI] [PubMed] [Google Scholar]
- 5. Wang H, Li N, Sun C, Guo X, Su W, Song Q, et al. The association between pregnancy and COVID‐19: a systematic review and meta‐analysis. Am J Emerg Med. 2022;56:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arthurs AL, Jankovic‐Karasoulos T, Roberts CT. COVID‐19 in pregnancy: what we know from the first year of the pandemic. Biochim Biophys Acta Mol Basis Dis. 2021;1867(12):166248. 10.1016/j.bbadis.2021.166248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan DSA, Hamid LR, Ali A, Salam RA, Zuberi N, Lassi ZS, et al. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID‐19‐infected pregnant women: a systematic review and meta‐analysis. BMC Pregnancy Childbirth. 2021;21(1):1–14. 10.1186/s12884-021-04250-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status – United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021;138(2):281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan JA, Biggio JR, Martin JK, Mussarat N, Chawla HK, Puri P, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139(1):107–9. [DOI] [PubMed] [Google Scholar]
- 11. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS‐CoV‐2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rottenstreich A, Zarbiv G, Oiknine‐Djian E, Vorontsov O, Zigron R, Kleinstern G, et al. The effect of gestational age at BNT162b2 mRNA vaccination on maternal and neonatal SARS‐CoV‐2 antibody levels. Clin Infect Dis. 2022;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS‐CoV‐2 infection in pregnant women. JAMA. 2021;326(8):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipkind HS, Vazquez‐Benitez G, DeSilva M, Vesco KK, Ackerman‐Banks C, Zhu J, et al. Receipt of COVID‐19 vaccine during pregnancy and preterm or small‐for‐gestational‐age at birth – Eight Integrated Health Care Organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, et al. SARS‐CoV‐2 infection and COVID‐19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28(3):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The American College of Obstetricians and Gynecologists (ACOG) . ACOG and SMFM recommend COVID‐19 vaccination for pregnant individuals. 2021. [cited 2022 Apr 18]. Available from: https://www.acog.org/news/news‐releases/2021/07/acog‐smfm‐recommend‐covid‐19‐vaccination‐for‐pregnant‐individuals
- 17. Shimabukuro T. COVID‐19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP). 2021. [cited 2022 Apr 18]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2021‐02/28‐03‐01/05‐covid‐Shimabukuro.pdf
- 18. Centers for Disease Control and Prevention (CDC) . COVID‐19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID‐19. [cited 2022 Apr 18]. Available from: https://emergency.cdc.gov/han/2021/han00453.asp
- 19. Chinn J, Sedighim S, Kirby KA, Hohmann S, Hameed AB, Jolley J, et al. Characteristics and outcomes of women with COVID‐19 giving birth at US academic centers during the COVID‐19 pandemic. JAMA Netw Open. 2021;4(8):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sallam M. COVID‐19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 2021;9(2):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Spall HGC. Exclusion of pregnant and lactating women from COVID‐19 vaccine trials: a missed opportunity. Eur Heart J. 2021;42(28):2724–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor MM, Kobeissi L, Kim C, Amin A, Thorson AE, Bellare NB, et al. Inclusion of pregnant women in COVID‐19 treatment trials: a review and global call to action. Lancet Glob Health. 2021;9(3):e366–71. 10.1016/S2214-109X(20)30484-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane; 2022. [cited 2022 Apr 18]. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rethlefsen M, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA‐S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butt AA, Chemaitelly H, Al Khal A, Coyle PV, Saleh H, Kaleeckal AH, et al. SARS‐CoV‐2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Investig. 2021;131(23):e153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kharbanda EO, Haapala J, DeSilva M, Vazquez‐Benitez G, Vesco KK, Naleway AL, et al. Spontaneous abortion following COVID‐19 vaccination during pregnancy. JAMA. 2021;326(16):1629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid‐19 vaccination during pregnancy and first‐trimester miscarriage. N Engl J Med. 2021;385(21):2008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and Shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods. 2021;12(1):55–61. [DOI] [PubMed] [Google Scholar]
- 32. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations (updated October 2013). 2013. [cited 2022 Apr 19]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
- 33. Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 63 (updated February 2022). Cochrane; 2022. [cited 2022 Apr 18]. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 34. Aslam J, Masroor M, Mehmood QUA, Arshad M, Jabeen S, Mushtaq MA. Maternal mortality with SARS‐COV‐2 during its 4th wave in Pakistan: the vaccine paradox and pregnancy. J Coll Physicians Surg Pak. 2022;32(1):119–21. 10.29271/jcpsp.2022.01.119 [DOI] [PubMed] [Google Scholar]
- 35. Beharier O, Plitman Mayo R, Raz T, Sacks KN, Schreiber L, Suissa‐Cohen Y, et al. Efficient maternal to neonatal transfer of antibodies against SARS‐CoV‐2 and BNT162b2 mRNA COVID‐19 vaccine. J Clin Invest. 2021;131(13):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de la Cruz Conty ML, Encinas Pardilla MB, Garcia Sanchez M, Gonzalez Rodriguez L, Muner‐Hernando ML, Royuela Vicente A, et al. Impact of recommended maternal vaccination programs on the clinical presentation of SARS‐COV‐2 infection: a prospective observational study. Vaccines (Basel). 2021;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kadali RAK, Janagama R, Peruru SR, Racherla S, Tirumala R, Madathala RR, et al. Adverse effects of COVID‐19 messenger RNA vaccines among pregnant women: a cross‐sectional study on healthcare workers with detailed self‐reported symptoms. Am J Obstet Gynecol. 2021;225:458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bleicher I, Kadour‐Peero E, Sagi‐Dain L, Sagi S. Early exploration of COVID‐19 vaccination safety and effectiveness during pregnancy: interim descriptive data from a prospective observational study. Vaccine. 2021;39(44):6535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dagan N, Barda N, Biron‐Shental T, Makov‐Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID‐19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–5. [DOI] [PubMed] [Google Scholar]
- 40. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, le Doare K, et al. COVID‐19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener‐Well Y, Grisaru‐Granovsky S. Covid‐19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID‐19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fell DB, Dimitris MC, Hutcheon JA, Ortiz JR, Platt RW, Regan AK, et al. Guidance for design and analysis of observational studies of fetal and newborn outcomes following COVID‐19 vaccination during pregnancy. Vaccine. 2021;39(14):1882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei SQ, Bilodeau‐Bertrand M, Liu S, Auger N. The impact of COVID‐19 on pregnancy outcomes: a systematic review and meta‐analysis. CMAJ. 2021;193(16):E540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lassi ZS, Ana A, Das JK, Salam RA, Padhani ZA, Irfan O, et al. A systematic review and meta‐analysis of data on pregnant women with confirmed COVID‐19: clinical presentation, and pregnancy and perinatal outcomes based on COVID‐19 severity. J Glob Health. 2021;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sikora A, Zahra F. Nosocomial infections. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2022. [cited 2022 Apr 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559312/ [Google Scholar]
- 47. Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health‐care‐associated infection in developing countries: systematic review and meta‐analysis. Lancet. 2011;377(9761):228–41. [DOI] [PubMed] [Google Scholar]
- 48. Duke GJ, Moran JL, Bersten AD, Bihari S, Roodenburg O, Karnon J, et al. Hospital‐acquired complications: the relative importance of hospital‐ and patient‐related factors. Med J Aust. 2022;216(5):242–7. [DOI] [PubMed] [Google Scholar]
- 49. Prasad S, Kalafat E, Blakeway H, Townsend R, O'Brien P, Morris E, et al. Systematic review and meta‐analysis of the effectiveness and perinatal outcomes of COVID‐19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Effectiveness and safety of COVID‐19 vaccine among pregnant women in real‐world studies: a systematic review and meta‐analysis. Vaccines (Basel). 2022;10(2):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pratama NR, Wafa IA, Budi DS, Putra M, Wardhana MP, Wungu CDK. mRNA Covid‐19 vaccines in pregnancy: a systematic review. PLoS One. 2022;17(2):1–21. 10.1371/journal.pone.0261350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Falsaperla R, Leone G, Familiari M, Ruggieri M. COVID‐19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. 2021;20(12):1619–28. 10.1080/14760584.2021.1986390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu W, Sivajohan B, McClymont E, Albert A, Elwood C, Ogilvie G, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals and their infants. Int J Gynecol Obstet. 2022;156(3):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rawal S, Tackett RL, Stone RH, Young HN. COVID‐19 vaccination among pregnant people in the United States: a systematic review. Am J Obstet Gynecol MFM. 2022;4(4):100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6
Data S7
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


