Abstract
Aims
To assess weight change in the Healthier You: NHS Diabetes Prevention Programme (NHS DPP) delivered via video conferencing (remote) sessions or delivered via specific digital interventions through apps or websites, during the COVID‐19 pandemic compared to group‐based face‐to‐face interventions, pre‐pandemic.
Methods
Prospectively collected national service‐level data relating to individuals with non‐diabetic hyperglycaemia (HbA1c 42–47 mmol/mol (6.0%–6.4%) or fasting plasma glucose 5.5–6.9 mmol/L) referred to the NHS DPP from June 2016 to March 2022.
Results
Between March 2020 and March 2022, 335,961 people were referred to the programme and were offered a choice of remote or digital intervention. This was preceded by 556,793 people referred to the face‐to‐face programme between June 2016 and February 2022. Uptakes to intervention sessions were 47% for those offered a choice and 39% for face‐to‐face. Remote and digital participants were significantly younger (60 and 56 vs. 65 years) and heavier (86.1 kg and 91.0 kg vs. 84.1 kg) compared to face‐to‐face. Weight change was assessed for 42,407 remote, 7699 digital and 97,205 face‐to‐face participants with sufficient time to have finished the programme and no missing data. Mean weight losses for participants attending at least one intervention session were: 2.40 (2.36–2.44) kg, 2.59 (2.49–2.68) kg and 2.01 (1.98–2.04) kg for remote, digital and face‐to‐face participants respectively. Corresponding mean weight losses for those who completed the programme were: 3.24 (3.19–3.30) kg, 4.76 (4.60–4.92) kg and 3.04 (3.00–3.07) kg. There were no significant differences in weight change between interventions by ethnicity and deprivation.
Conclusions
Weight losses achieved through remote and digital interventions were greater than those previously achieved through face‐to‐face interventions, without evidence of exacerbation of health inequalities.
Keywords: Prevention of type 2 diabetes
What's new?
While several clinical trials have demonstrated the effectiveness of remotely administered lifestyle interventions to prevent type 2 diabetes in those at risk, there are few reports of these interventions implemented at scale in real‐world settings.
During the COVID‐19 pandemic, weight losses for remote and digital interventions were greater than previously achieved through group‐based face‐to‐face interventions and were greater for digital than for remote interventions. Remote and digital interventions attracted younger participants with higher baseline weights than face‐to‐face, with no exacerbation of health inequalities.
The effectiveness of remotely administered lifestyle interventions demonstrated in clinical trials can be realized through delivery in real‐world settings and at scale.
1. INTRODUCTION
In 2016, the National Health Service (NHS) in England established The Healthier You: NHS Diabetes Prevention Programme (NHS DPP), achieving universal population coverage 2 years later. 1 The NHS DPP was developed to prevent or delay the onset of type 2 diabetes in adults identified with non‐diabetic hyperglycaemia (NDH, also termed prediabetes) (HbA1c 42–47 mmol/mol (6.0%–6.4%) or fasting plasma glucose (FPG) 5.5–6.9 mmol/L), through group‐based face‐to‐face structured lifestyle interventions. Early analyses found that people completing the programme had a mean weight loss of 3.3 (95% CI: 3.2–3.4) kg. 1 Recently, an independent evaluation of the programme has suggested a 3.7% absolute risk reduction in type 2 diabetes incidence in those that completed the programme compared to those that attended the initial assessment but did not complete. 2
In 2017, the NHS in England commissioned a pilot digital diabetes prevention programme. The digital programme offered similar support, assistance and guidance as the face‐to‐face programme but through the use of digital platforms that include mobile apps which allow users to access health coaches, online peer support groups, wearable technologies that monitor levels of exercise, and the ability to set and monitor goals electronically. The pilot demonstrated that weight loss and reduction in HbA1c were comparable to those achieved through group‐based face‐to‐face programme delivery. 3
On the 16 March 2020, the UK prime minister announced that all non‐essential contact and travel must stop. This was followed by the announcement of the first UK national lockdown on the 23 March 2020. Discussions were immediately held with the providers and commitments were gained to move to remote delivery via video conferencing and digital interventions through apps or websites. Contract Variation Orders were issued to formally allow remote and digital delivery using the same service specification as in‐person delivery. While this change presented significant logistical and operational challenges for providers, remote and digital delivery was in place and being delivered within 3 weeks of the initial announcement.
Using data up to March 2022, we aimed to assess weight change and programme completion for participants who accessed remote or digital interventions in the NHS DPP following pandemic onset, compared to the same outcomes achieved by participants accessing face‐to‐face interventions in the NHS DPP prior to the pandemic.
2. METHODS
2.1. Study design and intervention
This was a service evaluation in England evaluating the effectiveness of remote and digital interventions in the NHS DPP following onset of the COVID‐19 pandemic in comparison to face‐to‐face delivery prior to pandemic onset, using prospectively collected national service‐level data relating to those referred from programme launch in June 2016 to March 2022.
Face‐to‐face intervention sessions were offered to adults with NDH between June 2016 and February 2020. The service was delivered according to a national service specification by one of five service providers selected through a national competitive process. Each provider followed the same broad structure of an initial assessment, core sessions and maintenance sessions, with a minimum total of 13 face‐to‐face group‐based sessions, over at least 9 months, constituting at least 16 h contact time. 1 , 4
From March 2020, in response to the social distancing requirements of the COVID‐19 pandemic, programme delivery across the whole country switched to participants being offered a choice between remote group‐based intervention sessions and digital intervention sessions. Remote intervention sessions mirrored the face‐to‐face group‐based dynamic and were delivered via video‐conferencing such as Zoom or Microsoft Teams, whereas digital interventions were delivered to an individual through apps or websites. Digital interventions consisted of nine engagement periods, each lasting 30 days (270 days in total), in addition to digital registration. Engagement was defined as a minimum of two episodes of active engagement within at least one of six categories of engagement in each 30‐day period; communication with a health coach, accessing educational content, logging information against goals, peer support forum, use of interactive tools and time spent in the app (Data S1). Weights were recorded at registration, at 90 days, 180 days and at the end of the digital intervention. Weights were assigned to subsequent sessions until another weight was available.
2.2. Data source
The NHS DPP minimum dataset was used to identify all those with NDH referred to the NHS DPP between June 2016 and March 2022. Age, sex and postcode are recorded at the point of referral. Ethnicity, weight and height are recorded at initial assessment. Between June 2016 and February 2020 for those on face‐to‐face interventions, body weight and height were independently recorded by coaches employed by the provider in light indoor clothing at each intervention session using class 3 scales, while from March 2020, for those on digital and remote interventions, they were self‐reported. All remote and digital participants were given guidance by providers on correct self‐measurement of weight. This included using the same scales for each measurement taken, taking measurements at the same time of day, and taking care to be consistent with clothing. Weight measurements between 35 kg and 300 kg were considered valid.
2.3. Participants
We analysed data from three groups of participants based on starting date in the programme: participants who attended at least one remote intervention session between March 2020 and March 2022 (remote); participants who engaged with at least one digital intervention session between March 2020 and March 2022 (digital) and participants who attended their first face‐to‐face intervention session between June 2016 and February 2019 (F2F). Participants who attended a first face‐to‐face intervention session between March 2019 and February 2020, with insufficient time to finish the face‐to‐face intervention prior to pandemic onset, were excluded from the F2F group. These people were offered the opportunity to complete the programme remotely but were also excluded from the remote group analysed here. Due to data quality issues identified, data from one provider were not included in these analyses.
2.4. Outcomes
The primary outcome was change in weight for all participants who attended at least one intervention session, who had sufficient time to finish the programme and for whom there were no missing data for age, sex, ethnicity, deprivation, height and weight. Secondary outcomes were “completion” of the programme, defined as attendance or engagement of at least 60% of sessions, and completer weight change in this sub‐group. Completion rates were calculated with the number of people who had attended at least one intervention session as the denominator. For both primary and secondary outcomes, weight change was calculated as the difference in weight between the first and last sessions attended for all participants who by 31st March 2022 had had time to finish the 9‐month programme. The baseline measurement was defined as the weight measured at the first intervention session attended. Weight change greater than five standard deviations from the mean was deemed erroneous and recorded as missing.
2.5. Covariates
Individual factors (age, sex, ethnicity, socioeconomic status, baseline BMI, weight and number of sessions attended) and programme factors (provider) were identified as potential outcome moderators. Sex was recorded as male, female or indeterminate. Participants were grouped into 10‐year age‐bands and self‐reported ethnicity as white, Asian, black, mixed or other. Socioeconomic status was measured using quintiles of Index of Multiple Deprivation associated with the Lower Super Output Area derived from participant postcode. 5 All variables also include an unknown category where either the participant declined to give the relevant information, or a value was not recorded. BMI was calculated and participants were classified as healthy‐weight/underweight, overweight or obese, defined according to their reported ethnicity, or if their ethnicity was not known or not recorded, according to the white ethnicity group in‐line with the National Institute for Heath and Care Excellence guidelines. 6
2.6. Statistical analyses
Linear regression models were used to identify factors associated with change in weight with adjustment made for age, sex, ethnicity, baseline BMI grouping, quintile of deprivation, Provider and baseline weight measurements. Logistic regression models were used to identify characteristics associated with programme completion with adjustment made for age, sex, ethnicity, baseline BMI grouping, quintile of deprivation and Provider. Separate linear and logistic regression models were run by intervention type (F2F, remote and digital). Differences between these models were assessed using non‐overlapping confidence intervals.
Statistical significance was defined as p‐value <0.05 and confidence intervals (CI) were set at 95%. All data were analysed using Stata version 16.
2.7. Data access
This service evaluation involves assessment of anonymized data collected during routine service delivery; NHS England has published an information governance framework setting out the legal basis for data collection and data flows, ensuring that the service and its evaluation are delivered in compliance with data protection legislation. 7
3. RESULTS
Between June 2016 and March 2022, 892,754 people with NDH were referred into the programme and of those, 473,396 (53%) attended an initial assessment and 373,898 (42%) attended at least one intervention session. With programme duration at least 9 months, there were 297,684 participants who had attended at least one intervention session and who also had sufficient time to finish the programme before data was extracted. Among these participants, 156,902 (53%) attended at least 60% of sessions. Data S2 outlines the number of participants at each stage in the programme. Characteristics of participants at each stage in the programme are shown in Table 1. At each stage in the programme, there was a greater loss of people aged under 60 years and from the most deprived quintile (Table 1).
TABLE 1.
Characteristics of participants at each stage in the programme between June 2016 and March 2022
| Referrals | Attended initial assessment | Attended at least one intervention session | Attended at least one intervention session and had time to have finished the programme | Completed the programme | |
|---|---|---|---|---|---|
| Overall | 892,754 (100%) | 473,396 (100%) | 373,898 (100%) | 297,684 (100%) | 156,902 (100%) |
| 18–29 | 10,973 (1%) | 4524 (1%) | ** | 2297 (1%) | 601 (0%) |
| 30–39 | 46,329 (5%) | 21,250 (4%) | 15,921 (4%) | 11,704 (4%) | 3945 (3%) |
| 40–49 | 112,562 (13%) | 52,768 (11%) | 40,292 (11%) | 30,431 (10%) | 12,182 (8%) |
| 50–59 | 201,809 (23%) | 102,361 (22%) | 80,091 (21%) | 62,206 (21%) | 30,321 (19%) |
| 60–69 | 233,429 (26%) | 133,343 (28%) | 108,320 (29%) | 87,490 (29%) | 50,891 (32%) |
| 70–79 | 211,556 (24%) | 122,780 (26%) | 98,947 (26%) | 81,017 (27%) | 48,030 (31%) |
| 80+ | 75,892 (9%) | 36,347 (8%) | 27,048 (7%) | 22,539 (8%) | 10,932 (7%) |
| Unknown | 204 (0%) | 23 (0%) | ** | 0 (0%) | 0 (0%) |
| Male | 406,414 (46%) | 211,915 (45%) | 166,250 (44%) | 132,710 (45%) | 69,988 (45%) |
| Female | 478,282 (54%) | 260,404 (55%) | 206,960 (55%) | 164,336 (55%) | 86,544 (55%) |
| Unknown/indeterminate | 8058 (1%) | 1077 (0%) | 688 (0%) | 638 (0%) | 370 (0%) |
| Asian | N/a | 59,189 (13%) | 45,748 (12%) | 35,640 (12%) | 15,538 (10%) |
| Black | N/a | 34,778 (7%) | 28,054 (8%) | 21,132 (7%) | 10,944 (7%) |
| Mixed | N/a | 8656 (2%) | 6907 (2%) | 5353 (2%) | 2588 (2%) |
| Other | N/a | 7453 (2%) | 5508 (1%) | 4504 (2%) | 2016 (1%) |
| White | N/a | 334,613 (71%) | 267,392 (72%) | 214,043 (72%) | 118,471 (76%) |
| Unknown | N/a | 28,707 (6%) | 20,289 (5%) | 17,012 (6%) | 7345 (5%) |
| IMD 1 (most deprived) | 182,410 (20%) | 85,391 (18%) | 62,458 (17%) | 49,309 (17%) | 20,658 (13%) |
| IMD 2 | 186,879 (21%) | 93,415 (20%) | 71,263 (19%) | 56,297 (19%) | 27,890 (18%) |
| IMD 3 | 179,764 (20%) | 96,610 (20%) | 76,692 (21%) | 60,911 (20%) | 33,005 (21%) |
| IMD 4 | 172,190 (19%) | 97,673 (21%) | 79,631 (21%) | 63,474 (21%) | 35,836 (23%) |
| IMD 5 (least deprived) | 169,230 (19%) | 99,545 (21%) | 83,466 (22%) | 67,339 (23%) | 39,311 (25%) |
| Unknown | 2281 (0%) | 762 (0%) | 388 (0%) | 354 (0%) | 202 (0%) |
**Suppressed due to small numbers
Of those referred, 335,961 (38%) were offered a choice of a remote or digital intervention between March 2020 and March 2022 and 556,793 (62%) were offered a F2F intervention between June 2016 and February 2020, with characteristics of people referred similar between referral offers (Data S3). Uptakes to the intervention sessions were 47% for those offered a choice of a remote or digital intervention and 39% for F2F. For people offered the remote or digital intervention, uptake was higher in those aged 30–69 years, while uptake for people offered the F2F intervention was higher in older people (Data S4).
Of those that attended at least one intervention session: 131,100 (35%) attended remote sessions, 26,169 (7%) accessed digital sessions and 119,367 (32%) attended F2F sessions. The remaining 97,262 (26%) participants attended a first F2F intervention session between March 2019 and February 2020 with insufficient time to finish the F2F intervention prior to pandemic onset. These participants were excluded from further analyses. Characteristics of participants who accessed remote, digital and F2F are shown in Table 2: the remote participants and the digital participants were younger than those in the F2F group with mean ages (SD) of 60 (13), 56 (13) and 65 (12) years respectively. Valid weights at baseline were recorded for 71% of remote participants, 89% of digital participants and 96% of F2F participants. The mean baseline weight for remote participants was 86.1 kg, and for digital participants was 91.0 kg, both significantly heavier than the mean baseline weight for F2F participants which was 84.1 kg. After adjustment for differences in age, sex, ethnicity and deprivation, the mean baseline weight for remote participants was 0.32 (0.15–0.49) kg, p = 0.0002 greater than the mean baseline weight for F2F participants while the mean baseline weight for digital participants was 2.76 (2.49–3.01) kg, p < 0.0001 greater.
TABLE 2.
Characteristics and mean weight at baseline for those who have attended or remotely accessed at least one intervention session by March 2022, by intervention type
| N | Percentage | % weight recorded at start | Mean weight at baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Face‐to‐face | Remote | Digital | Face‐to‐face | Remote | Digital | Face‐to‐face | Remote | Digital | Face‐to‐face | Remote | Digital | |
| Overall | 119,367 | 131,100 | 26,169 | 100% | 100% | 100% | 96% | 71% | 89% | 84.1 | 86.1 | 91.0 |
| 18–29 | 466 | ** | 597 | 0% | ** | 2% | 92% | 65% | 89% | 95.9 | 97.1 | 100.7 |
| 30–39 | 3136 | 7442 | 2226 | 3% | 6% | 9% | 96% | 68% | 88% | 89.7 | 93.6 | 97.7 |
| 40–49 | 9430 | 17,320 | 4625 | 8% | 13% | 18% | 96% | 69% | 88% | 89.9 | 91.4 | 95.3 |
| 50–59 | 21,590 | 31,309 | 8066 | 18% | 24% | 31% | 96% | 70% | 90% | 89.0 | 90.3 | 92.9 |
| 60–69 | 37,160 | 35,777 | 6681 | 31% | 27% | 26% | 96% | 71% | 90% | 85.0 | 85.7 | 88.1 |
| 70–79 | 36,724 | 30,068 | 3432 | 31% | 23% | 13% | 96% | 72% | 87% | 80.7 | 80.0 | 82.4 |
| 80+ | 10,861 | 7584 | 542 | 9% | 6% | 2% | 95% | 70% | 86% | 75.4 | 74.0 | 75.9 |
| Unknown | 0 | ** | 0 | 0% | ** | 0% | 0% | ** | 0% | n/a | ** | n/a |
| Male | 54,094 | 57,092 | 11,436 | 45% | 44% | 44% | 96% | 72% | 89% | 90.7 | 91.6 | 96.3 |
| Female | 64,989 | 73,819 | 14,722 | 54% | 56% | 56% | 96% | 69% | 89% | 78.6 | 81.6 | 86.8 |
| Unknown/indeterminate | 284 | 189 | 11 | 0% | 0% | 0% | 96% | 80% | 82% | 81.6 | 86.0 | 102.1 |
| Asian | 12,809 | 17,982 | 3812 | 11% | 14% | 15% | 97% | 69% | 85% | 76.0 | 77.5 | 79.1 |
| Black | 6771 | 13,002 | 2213 | 6% | 10% | 8% | 96% | 62% | 84% | 86.4 | 88.7 | 91.5 |
| Mixed | 1991 | 2607 | 838 | 2% | 2% | 3% | 97% | 65% | 85% | 84.8 | 88.4 | 89.3 |
| Other | 1880 | 1742 | 549 | 2% | 1% | 2% | 96% | 65% | 80% | 80.5 | 81.5 | 81.8 |
| White | 86,647 | 90,714 | 18,103 | 73% | 69% | 69% | 96% | 73% | 91% | 85.2 | 87.4 | 93.7 |
| Unknown | 9269 | 5053 | 654 | 8% | 4% | 2% | 94% | 62% | 82% | 84.0 | 85.5 | 88.6 |
| IMD 1 (most deprived) | 19,634 | 22,007 | 4253 | 16% | 17% | 16% | 96% | 71% | 89% | 85.7 | 88.3 | 94.9 |
| IMD 2 | 22,351 | 26,089 | 5243 | 19% | 20% | 20% | 96% | 69% | 87% | 84.6 | 87.4 | 91.9 |
| IMD 3 | 24,007 | 27,465 | 5570 | 20% | 21% | 21% | 96% | 70% | 89% | 84.0 | 85.9 | 90.6 |
| IMD 4 | 25,331 | 27,647 | 5639 | 21% | 21% | 22% | 96% | 71% | 90% | 83.7 | 85.3 | 90.1 |
| IMD 5 (least deprived) | 27,806 | 27,845 | 5449 | 23% | 21% | 21% | 96% | 72% | 90% | 82.9 | 84.2 | 88.5 |
| Underweight/healthy | 17,134 | 15,106 | 3265 | 14% | 12% | 12% | 100% | 100% | 100% | 62.8 | 63.8 | 64.0 |
| Overweight | 39,754 | 28,555 | 6843 | 33% | 22% | 26% | 100% | 100% | 100% | 76.5 | 77.6 | 78.2 |
| Obese | 55,348 | 44,285 | 12,911 | 46% | 34% | 49% | 100% | 100% | 100% | 96.1 | 99.3 | 103.8 |
| Unknown | 7131 | 43,154 | 3150 | 6% | 33% | 12% | 30% | 10% | 8% | 84.0 | 85.5 | 88.6 |
**Suppressed due to small numbers
Completion and weight change were assessed for participants with sufficient time to have finished the programme, with no missing or unknown data for age, sex, ethnicity, deprivation, height and weight, of which there were 42,407 (52%) remote participants, 7699 (55%) digital participants and 97,205 (81%) F2F participants. Full details of missing data can be found in Data S5. Data on weight were missing at the first or last session attended for 43% of remote participants, 43% of digital participants and 10% of F2F participants. F2F and remote participants with higher baseline weights were less likely to record an end weight at their last session attended (Data S6).
Of those with no missing data, 62% of remote participants and 48% of digital participants completed the programme compared to 53% of F2F participants. The number of sessions attended is shown in Data S7. Univariate analyses of completion of the programme are shown in Data S8. Logistic regression analysis showed that after adjustment, remote participants had 1.47 (95% CI:1.43–1.51, p < 0.001) odds of completing the programme and digital participants had 0.81 (0.77–0.85, p < 0.001) odds of completing the programme compared to F2F participants (Data S9). For remote participants, odds ratios (OR) for completing the programme for Asian, black, mixed and other ethnicities compared to white ethnicity, were higher than the corresponding ORs for F2F. ORs for remote participants aged 80 years and over compared to those aged 60–69 years and for remote participants from the most deprived quintile (IMD1) and from IMD 3 compared to IMD 5 (least deprived) quintile, were lower than the corresponding ORs for F2F (Data S10). For digital participants, ORs for those of Asian and black ethnicity compared to white ethnicity, were higher than the corresponding ORs for F2F while ORs for men compared to women were lower than the corresponding ORs for F2F participants (Data S10).
For all participants who attended at least one intervention session with no missing data and sufficient time to finish the programme, the mean baseline weight was 85.5 kg for remote participants, 91.9 kg for digital participants and 83.9 kg for F2F participants with mean weight changes of −2.40 (−2.44 to −2.36) kg for remote participants, −2.59 (−2.68 to −2.49) kg for digital participants and −2.01 (−2.04 to −1.98) kg for F2F participants (Table 3). Those who attended more sessions lost more weight (Figure 1). Linear regression analysis showed that after adjustment, remote participants lost 0.32 (0.28–0.36) kg, p < 0.001 more weight and digital participants lost 1.19 (1.11–1.28) kg more weight than F2F participants (Data S11). For remote participants, the difference in weight loss for those aged 18–29 years compared to those aged 60–69 years and the differences in weight loss for those who were overweight or obese compared to those of normal weight, were greater than the corresponding differences for F2F participants. The difference in weight loss between men and women was smaller than for F2F (Data S12). For digital participants, the difference in weight loss between men and women and the differences in weight loss for those who were overweight or obese compared to those of normal weight were greater than for F2F (Data S12).
TABLE 3.
Mean weight change for all participants who attended at least one intervention session with no missing data and who have had time to finish the programme by March 2022, by intervention type
| N | Mean weight at baseline | Mean weight change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F2F | Remote | Digital Choice | F2F | Remote | Digital Choice | F2F | Remote | Digital Choice | |
| Overall | 97,205 | 42,407 | 7699 | 83.9 | 85.5 | 91.9 | −2.01 (−2.04 to −1.98) | −2.40 (−2.44 to −2.36) | −2.59 (−2.68 to −2.49) |
| 18–29 | 337 | 432 | 174 | 95.8 | 97.1 | 101.0 | −0.30 (−0.71 to 0.11) | −1.82 (−2. 18 to −1.45) | −0.68 (−1.25 to −0.11) |
| 30–39 | 2415 | 2204 | 583 | 89.6 | 92.4 | 98.8 | −0.97 (−1.12 to −0.83) | −1.82 (−1.97 to −1.66) | −1.45 (−1.75 to −1.15) |
| 40–49 | 7449 | 5060 | 1264 | 90.1 | 90.9 | 97.7 | −1.21 (−1.30 to −1.12) | −1.95 (−2.07 to −1.84) | −1.92 (−2.14 to −1.69) |
| 50–59 | 17,354 | 9833 | 2410 | 88.8 | 90.1 | 94.1 | −1.64 (−1.70 to −1.57) | −2.33 (−2.42 to −2.24) | −2.62 (−2.80 to −2.44) |
| 60–69 | 30,489 | 11,987 | 2074 | 84.9 | 85.2 | 89.0 | −2.24 (−2.29 to −2.19) | −2.69 (−2.78 to −2.61) | −3.31 (−3.51 to −3.11) |
| 70–79 | 30,262 | 10,348 | 1024 | 80.6 | 79.8 | 82.6 | −2.31 (−2.36 to −2.27) | −2.62 (−2.70 to −2.54) | −2.94 (−3.20 to −2.69) |
| 80+ | 8899 | 2543 | 170 | 75.3 | 73.7 | 75.7 | −1.92 (−1.98 to −1.86) | −1.92 (−2.04 to −1.81) | −2.00 (−2.45 to −1.55) |
| Male | 44,183 | 19,036 | 3522 | 90.5 | 91.1 | 97.1 | −2.23 (−2.27 to −2.19) | −2.54 (−2.61 to −2.48) | −2.72 (−2.87 to −2.57) |
| Female | 53,022 | 23,371 | 4177 | 78.5 | 81.0 | 87.6 | −1.82 (−1.86 to −1.79) | −2.29 (−2.34 to −2.23) | −2.48 (−2.60 to −2.35) |
| Asian | 11,236 | 5684 | 917 | 76.0 | 77.3 | 79.2 | −0.99 (−1.05 to −0.93) | −1.41 (−1.50 to −1.32) | −1.59 (−1.81 to −1.37) |
| Black | 5885 | 3499 | 488 | 86.5 | 88.5 | 91.2 | −1.55 (−1.66 to −1.44) | −2.14 (−2.28 to −1.99) | −2.39 (−2.78 to −2.00) |
| Mixed | 1776 | 730 | 180 | 84.8 | 88.2 | 87.8 | −1.69 (−1.87 to −1.51) | −1.79 (−2.07 to −1.51) | −2.00 (−2.57 to −1.43) |
| Other | 1666 | 556 | 108 | 80.5 | 81.4 | 83.3 | −1.39 (−1.58 to −1.21) | −1.90 (−2.22 to −1.57) | −2.41 (−3.16 to −1.66) |
| White | 76,642 | 31,938 | 6006 | 85.0 | 86.7 | 94.2 | −2.21 (−2.25 to −2.18) | −2.63 (−2.68 to −2.58) | −2.78 (−2.89 to −2.66) |
| IMD 1 (most deprived) | 15,762 | 7004 | 1333 | 85.7 | 87.9 | 96.2 | −1.59 (−1.66 to −1.53) | −1.94 (−2.04 to −1.84) | −2.06 (−2.29 to −1.84) |
| IMD 2 | 18,212 | 7915 | 1428 | 84.4 | 87.0 | 93.2 | −1.83 (−1.90 to −1.77) | −2.28 (−2.38 to −2.18) | −2.30 (−2.53 to −2.07) |
| IMD 3 | 19,831 | 8742 | 1621 | 83.8 | 85.3 | 91.2 | −2.05 (−2.11 to −1.99) | −2.42 (−2.51 to −2.33) | −2.62 (−2.83 to −2.41) |
| IMD 4 | 20,805 | 9200 | 1677 | 83.5 | 84.7 | 91.0 | −2.21 (−2.27 to −2.15) | −2.63 (−2.72 to −2.54) | −2.81 (−3.02 to −2.60) |
| IMD 5 (least deprived) | 22,595 | 9546 | 1640 | 82.9 | 83.6 | 89.0 | −2.22 (−2.28 to −2.16) | −2.60 (−2.69 to −2.51) | −3.00 (−3.21 to −2.78) |
| Underweight/healthy | 14,699 | 7607 | 1083 | 62.7 | 64.1 | 64.6 | −1.29 (−1.34 to −1.24) | −1.24 (−1.31 to −1.17) | −1.51 (−1.69 to −1.33) |
| Overweight | 34,396 | 14,011 | 2293 | 76.4 | 77.7 | 78.7 | −1.98 (−2.02 to −1.94) | −2.25 (−2.31 to −2.18) | −2.57 (−2.73 to −2.41) |
| Obese | 48,110 | 20,789 | 4323 | 95.8 | 98.7 | 105.8 | −2.25 (−2.29 to −2.20) | −2.93 (−2.99 to −2.86) | −2.86 (−3.01 to −2.72) |
FIGURE 1.
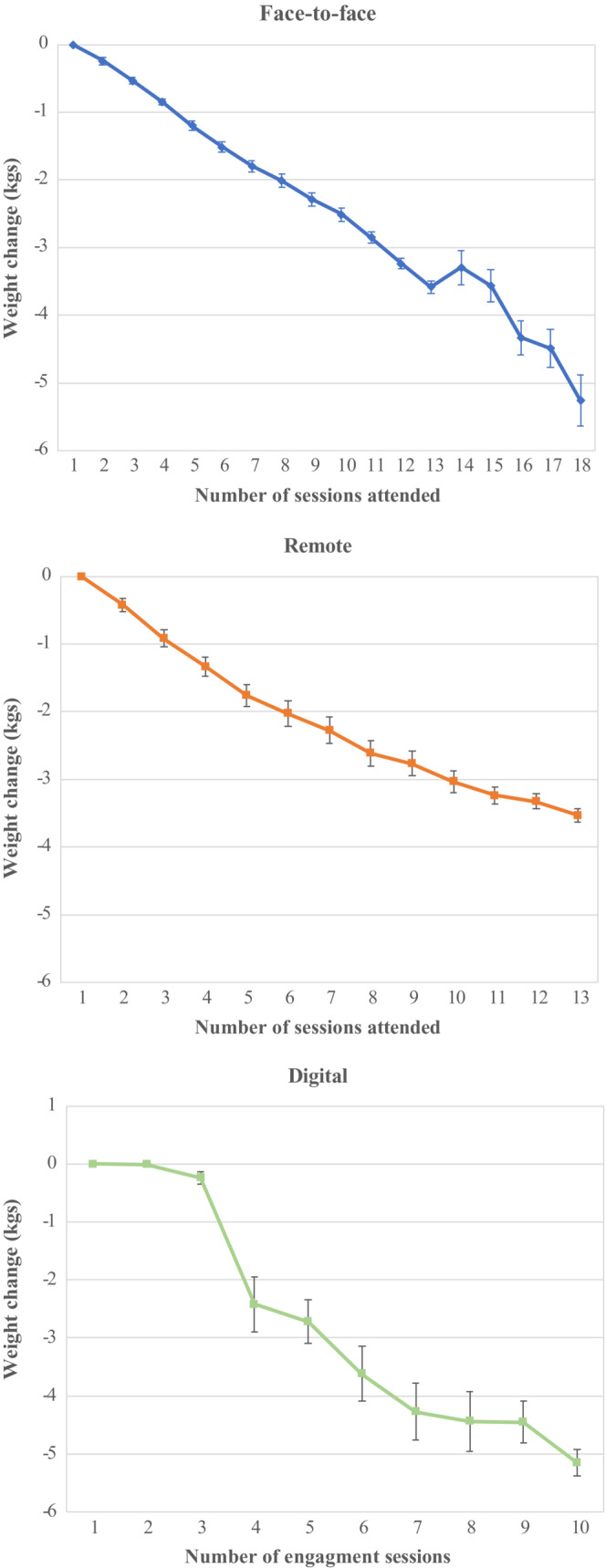
Mean weight change by number of sessions attended (face‐to‐face) or remotely accessed (remote and digital) for those with no missing data and who had time to finish the programme by March 2022
The mean baseline weights for those who completed the programme and with no missing data were 84.6 kg for remote participants, 86.8 kg for digital participants and 82.8 kg for F2F participants, significantly lower than the mean baseline weights for all participants who had attended at least one intervention session, with no missing weight data who had time to finish the programme. This difference was particularly marked for digital participants (−5.1 kg). Mean weight changes were −3.24 (−3.30 to −3.19) kg for remote participants, −4.76 (−4.92 to −4.60) kg for digital participants and −3.04 (−3.07 to −3.00) kg for F2F participants. Linear regression analysis showed that after adjustment, remote participants lost 0.31 (0.25–0.37) kg, p < 0.001 more weight, and digital participants lost 2.26 (2.11–2.41) kg more weight than F2F participants (Data S13). For remote participants, the difference in weight loss between those aged 18–29 years compared to those aged 60–69 years and the differences in weight loss for those who were overweight or obese compared to those of normal weight was greater than the corresponding differences for F2F participants. The difference in weight loss between men and women was smaller than for F2F (Data S14). For digital participants, the difference in weight loss between those who were obese and those who were of normal weight was greater than for F2F (Data S14).
4. DISCUSSION
To our knowledge, this is the largest cohort reported internationally of participants accessing interventions remotely or digitally following the onset of the COVID‐19 pandemic that aim to prevent the onset of type 2 diabetes in those with NDH. Compared to the weight loss achieved through accessing group‐based face‐to‐face interventions in the NHS DPP, the current evaluation has demonstrated greater weight loss for those accessing remote and digital interventions following pandemic onset. Furthermore, weight loss was greater for those accessing digital interventions than those accessing remote interventions following pandemic onset.
The effectiveness of remote and digital lifestyle interventions to prevent type 2 diabetes in those at risk has now been demonstrated by several clinical trials, including comparisons to usual care and to in‐person delivery. 8 , 9 , 10 , 11 A recent systematic review of eight clinical trials found that six of these reported significant reductions in weight and/or glycaemic parameters in comparison to control groups, with results comparable to, or in some cases more effective than, in‐person delivery. 11
By necessity, many routine healthcare interactions following onset of the COVID‐19 pandemic relied on remote delivery methods to replace in‐person delivery. However, there have been few evaluations assessing the relative clinical effectiveness and associated outcomes of remote delivery, compared to the historical effectiveness and outcomes of in‐person delivery. The evaluation presented here has identified five important patterns. Firstly, baseline weights were significantly greater following, compared to prior to, pandemic onset, confirming the results we demonstrated previously. 12 However, it is not clear whether the increase in baseline weight reflects an increase in weight in the general population, or a change in the population entering the programme. Others have also examined the impact of the pandemic on weight. 13 , 14 , 15 , 16 In a study of 11,534 attendees at a US medical facility in Massachusetts, there was an increase in weight among women (+0∙51 kg), but a decrease in men (−0∙81 kg), with the highest risk of weight gain among people under 40 years. 14 A study of 2447 individuals in Lithuania found that nearly a third (32%) gained weight during lockdown with nearly half (49%) eating more than usual and two thirds (61%) reporting a decrease in physical activity. 16
Secondly, remote delivery following pandemic onset was associated with greater completion rates, whereas digital delivery was associated with lower completion rates. However, differences in completion rates may relate to factors other than mode of intervention delivery, including changes in working patterns and lifestyle behaviours that were disrupted during the pandemic.
Thirdly, the mean weight of people choosing digital interventions was higher than those in the other two groups. We speculate that this may be a reflection of the weight stigma associated with obesity, which may lead people with more severe obesity to avoid group‐based environments or that people with more severe obesity may perceive an individual digital offer as more personalised to their needs than a group programme. 17 The potential increase in body weight during the pandemic, may have led to an increase in people choosing digital. However, heavier participants were also less likely to complete the programme.
Fourthly, digital delivery attracted younger participants than the traditional group‐based face‐to‐face approach, as we have previously observed in the pilot digital stream of the NHS DPP. 18 We are now also able to report that group‐based remote delivery is also more attractive to younger participants compared to a traditional group‐based face‐to‐face approach, though the differences were smaller. This finding is important to help boost uptake of interventions among working age people particularly in view of the burgeoning numbers in England with young‐onset Type 2 diabetes. 19 , 20
Fifthly, despite concerns of a digital divide, there was little evidence that remote and digital delivery were associated with exacerbation of health inequalities compared to the traditional group‐based face‐to‐face approach. 21 , 22 While those of greater socioeconomic deprivation and black, Asian, mixed and other ethnicities are appropriately represented in those referred, 19 we have previously described progressively greater attrition through the face‐to‐face programme of these groups. 1 This disparity was also apparent in this study where the OR for completion for Asian groups was 24% lower than white groups, and where Asian and black groups lost less weight than those of white ethnicity. Remote delivery, however, was associated with improved retention of those of Asian, Black, mixed and other ethnicities. In addition, there has been a new round of provider procurement with a new specification requiring providers to offer a tailored service for all individuals from an Asian background who require specific cultural/language tailoring.
A major strength of this study is that it represents data from a large national population cohort of people at risk of type 2 diabetes. However, there are also some limitations. Real‐world data have been used to compare modes of interventional delivery. As such, there was no randomisation in intervention group allocation. Furthermore, interventional delivery for the comparator group was not contemporaneous and had preceded pandemic onset; there was a reliance on self‐reported weights following pandemic onset and there is a tendency to under‐report weight with self‐reported measurements. 23 In addition, there were substantial missing weight data following pandemic onset, which might have had an effect on the estimation of weight difference, and participants with higher baseline weights were less likely to record an end weight at their last session attended, so that the pattern of missing weight data may have led to an underestimation of weight loss following pandemic onset. Future analyses, however, will assess weight, and HbA1c, longitudinally using data from the National Diabetes Audit.
The findings of this service evaluation provide reassurance that the clinical effectiveness of remote and digital interventions in the NHS DPP offered during the pandemic has maintained, or perhaps enhanced, the weight loss observed in face‐to‐face interventions before the pandemic, with no evidence of adverse impacts on health inequalities.
AUTHOR CONTRIBUTIONS
Emma Barron and Jonathan Valabhji conceived the study. Emma Barron and Dominque Bradley managed the data and carried out the statistical analysis. All the authors collaborated in interpretation of the results and drafting of the manuscript. Emma Barron and Jonathan Valabhji had the final responsibility for the decision to submit for publication.
FUNDING INFORMATION
No funding.
CONFLICTS OF INTEREST
JV is the national clinical director for diabetes and obesity at NHS England. CB is an adviser to the NHS Diabetes Programme. BY is clinical lead for the National Diabetes Audit and a trustee of Diabetes UK. KK has been a consultant and speaker for Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly and Merck Sharp & Dohme; has received grants in support of investigator‐initiated trials from Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Pfizer and Boehringer Ingelheim; has served on advisory boards for Novo Nordisk, Sanofi Aventis, Lilly and Merck Sharp & Dohme; and is supported by the UK National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. EM is managing director of a not‐for‐profit Community Interest Company, HeLP‐Digital, which exists to disseminate a digital diabetes self‐management programme, HeLP‐Diabetes, across the NHS. NW is supported by the Medical Research Council (grant MC_UU_00006/1). SJ is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and Oxford NIHR Collaboration and Leadership in Applied Health Research. All other authors declare no competing interests.
ETHICS STATEMENT
This service evaluation involves assessment of anonymized data collected during routine service delivery; NHS England has published an information governance framework setting out the legal basis for data collection and data flows, ensuring that the service and its evaluation are delivered in compliance with data protection legislation.
Supporting information
Data S1:
Barron E, Bradley D, Safazadeh S, et al. Effectiveness of digital and remote provision of the Healthier You: NHS Diabetes Prevention Programme during the COVID‐19 pandemic. Diabet Med. 2023;00:e15028. doi: 10.1111/dme.15028
DATA AVAILABILITY STATEMENT
I confirm that my Data Availability Statement (pasted below) complies with the Expects Data Policy. Research data are not shared.
REFERENCES
- 1. Valabhji J, Barron E, Bradley D, et al. Early outcomes from the English National Health Service Diabetes Prevention Programme. Diabetes Care. 2020;43:152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parkinson B, MCManus E, Meacock R; M Sutton Health Organisation, Politics and Economics (HOPE) research group . The effectiveness of the NHS Diabetes Prevention Programme at reducing type 2 diabetes incidence amongst people with non‐diabetic hyperglycaemia. 10.1111/dme.14809 [DOI]
- 3. Ross J, Barron E, McGough B, et al. Uptake and impact of the English National Health Service digital diabetes prevention programme: observational study. BMJ Open Diabetes Res Care. 2022;10(3):e002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatterton H, Younger T, Fischer A, Khunti K. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ. 2012;345:e4624. [DOI] [PubMed] [Google Scholar]
- 5. English Indices of deprivation . 2019. Accessed June 22, 2022. https://www.gov.uk/government/statistics/english‐indices‐of‐deprivation‐2019
- 6. National Institute for Health and Care Excellence . BMI: preventing ill health and premature death in black, Asian and other minority ethnic groups. Accessed May 8, 2022. https://www.nice.org.uk/guidance/ph46 [PubMed]
- 7. NHS England and NHS Improvement . Diabetes Prevention Programme Information Governance and Data Flows Framework. 2019. Accessed May 8, 2022. https://www.england.nhs.uk/wp‐content/uploads/2019/09/diabetes‐prevention‐programme‐information‐governance‐and‐data‐flows‐framework.pdf
- 8. Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta‐analysis. Prev Med. 2017;100:194‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Rhoon L, Byrne M, Morrissey E, et al. A systematic review of the behaviour change techniques and digital features in technology‐driven type 2 diabetes prevention interventions. Digit Health. 2020;6:205520762091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bian RR, Piatt GA, Sen A, et al. The effect of technology‐mediated diabetes prevention interventions on weight: a meta‐analysis. J Med Internet Res. 2017;19:e4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villegas V, Shah A, Manson JE, Tobis DK. Prevention of type 2 diabetes through remotely‐administered lifestyle programs: a systematic review. Contemp Clin Trials. 2022;119:106817. [DOI] [PubMed] [Google Scholar]
- 12. Valabhji J, Barron E, Bradley D, Bakhai C, Khunti K, Jebb S. Effect of the COVID‐19 pandemic on body weight in people at high risk of type 2 diabetes referred to the English NHS diabetes prevention Programme. Lancet Diabetes Endocrinol. 2021;9(10):649‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhutani S, van Dellen MR, Cooper JA. Longitudinal weight gain and related risk behaviors during the COVID‐19 pandemic in adults in the US. Nutrients. 2021;13:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulugeta W, Desalegn H, Solomon S. Impact of the COVID‐19 pandemic lockdown on weight status and factors associated with weight gain among adults in Massachusetts. Clin Obes. 2021;11:e12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chew HSJ, Lopez V. Global impact of COVID‐19 on weight and weight‐related behaviours in the adult population: a scoping review. Int J Environ Res Public Health. 2021;18:1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kriaucioniene V, Bagdonaviciene L, Rodríguez‐Pérez C, Petkeviciene J. Associations between changes in health behaviours and body weight during the COVID‐19 quarantine in Lithuania: the Lithuanian COVIDiet study. Nutrients. 2020;12(10):3119. doi: 10.3390/nu12103119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albury C, Strain WD, Le Brocq S, et al. The importance of language in engagement between health‐care professionals and people living with obesity: a joint consensus statement. Lancet Diabetes Endocrinol. 2020;8(5):447‐455. [DOI] [PubMed] [Google Scholar]
- 18. McGough B, Murray E, Brownlee E, et al. The healthier you: NHS Diabetes Prevention Programme: digital mods of delivery engage younger people. Diabet Med. 2019;36(11):1510‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barron E, Clark R, Hewings R, Smith J, Valabhji J. Progress of the Healthier You: NHS Diabetes Prevention Programme: referrals, uptake and participant characteristics. Diabet Med. 2018;35(4):513‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NHS Digital . National Diabetes Audit: Young people with Type 2 diabetes, 2019–20. Available at: Young People with Type 2 Diabetes, 2019‐20‐NHS Digital.
- 21. Estacio EV, Whittle R, Protheroe J. The digital divide: examining socio‐demographic factors associated with health literacy, access and use of internet to seek health information. J Health Psychol. 2019;24(12):1668‐1675. [DOI] [PubMed] [Google Scholar]
- 22. Van Deursen AJ, Van Dijk JA. The first‐level digital divide shifts from inequalities in physical access to inequalities in material access. New Media Soc. 2019;21(2):354‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self‐reported height and weight in a large, nationwide cohort of U.S. adults. PLoS One. 2020;15:e0231229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
I confirm that my Data Availability Statement (pasted below) complies with the Expects Data Policy. Research data are not shared.


