Abstract
Background and purpose
There have been over 500 million confirmed cases of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), also known as coronavirus disease 2019 (COVID‐19), across the globe. To date, a broad spectrum of neurological manifestations following acute infections as well as COVID‐19 vaccines have been reported. The aim of this study was to describe the spectrum of neurological manifestations seen in the ‘COVID‐19 clinic’ established in a tertiary Movement Disorders clinic.
Methods
In this consecutive case‐series study over the period March 2020–January 2022, clinical information regarding demographic data, clinical history and examination findings, investigation results and video recordings of outpatients with motor manifestations associated with COVID‐19 infection or vaccination were reviewed.
Results
Twenty‐one adult patients were reviewed in this ad hoc clinic at Toronto Western Hospital. The majority of the patients were female (76%) and the mean (range) age was 50.7 ± 17.2 (21–80) years. Nine patients (43%) presented with motor manifestations following COVID‐19 infection. Twelve patients (57%) developed neurological symptoms following at least one dose of the mRNA or viral vector‐based COVID‐19 vaccine. The most common manifestation observed was a functional movement disorder (43%). The vaccine group demonstrated a higher number of functional disorders compared to the infection group (58% vs. 22%; p = 0.08).
Conclusion
Functional motor manifestations can be associated with COVID‐19 and are likely to be under‐reported. In view of the co‐existence of functional symptoms, movement disorders and mental health conditions observed in this study, we would advocate the use of dedicated COVID‐19 Neurology clinics with full access to an experienced multidisciplinary team.
Keywords: COVID‐19, functional, movement disorders, SARS‐CoV‐2, vaccine
INTRODUCTION
There have been over 500 million confirmed cases of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), also known as coronavirus disease 2019 (COVID‐19), across the globe [1]. Since the first reported cases of COVID‐19, a broad spectrum of neurological manifestations following acute infections have been described, ranging from mild and non‐specific symptoms, such as headaches, brain fog and olfactory dysfunction, through to cases of Guillain–Barré syndrome (GBS), encephalitis and cerebrovascular events. Regardless of the infection severity, COVID‐19 patients are at risk of developing neurological problems in both inpatient and outpatient settings [2, 3, 4, 5, 6].
The underlying pathophysiology of neurological complications associated with COVID‐19 is complex, and in some cases, multifactorial in origin. Proposed mechanisms include: systemic disease resulting in hyperinflammation, multi‐organ failure or a hypercoagulable state; direct viral invasion resulting in encephalitis, vasculitis or muscular damage; and post‐infectious immune‐mediated complications, which can result in conditions such as GBS in the peripheral nervous system or acute disseminated encephalomyelitis (ADEM) in the central nervous system [7].
Movement disorders related to COVID‐19 infection are increasingly being described, although they remain relatively uncommon. Myoclonus has been most commonly reported, however, other movements disorders include ataxia, postural tremor, parkinsonism, eye movement abnormalities, choreiform movements and functional neurological disorders (FNDs) [8, 9, 10]. Finally, neurological manifestations associated with COVID‐19 have also extended to involve patients receiving the COVID‐19 vaccine, although a causal link with the vaccine is yet to be clearly determined [11].
In July 2020, the Canadian Institutes of Health Research funded the Canadian COVID‐19 Prospective Cohort Study (CANCOV) [12] to collect data on 2000 COVID‐19 patients across the spectrum of severity of illness in five provinces. Following comprehensive assessments carried out by the General Internal Medicine team, hospitalized non‐intensive care unit (ICU) patients and non‐hospitalized patients who required further neurological work‐up for neurocognitive issues, peripheral neuropathy symptoms and movement disorders were referred to the respective subspeciality clinics in the neurology service.
The aim of this study was to describe and summarize a consecutive cohort of patients encountered in the outpatient setting at the Toronto Western Hospital presenting with motor neurological disorders associated with COVID‐19 infection or following vaccination.
METHODS
In this consecutive case‐series study over the period March 2020–January 2022, patients with a motor neurological manifestation associated with COVID‐19 infection (or the vaccine) reviewed in the ‘COVID‐19 clinic’ at the Movement Disorders Centre in Toronto Western Hospital were included. The COVID‐19 clinic was not part of the University Health Network's FND program. Patients were directly referred from the aforementioned CANCOV study (n = 10) as well as from general practitioners (n = 9) or General Neurology (n = 1). Separate to the CANCOV study, three additional patients with vaccination‐related side effects were referred by General Neurology to the COVID‐19 clinic due to a clinical demand (Figure 1).
FIGURE 1.
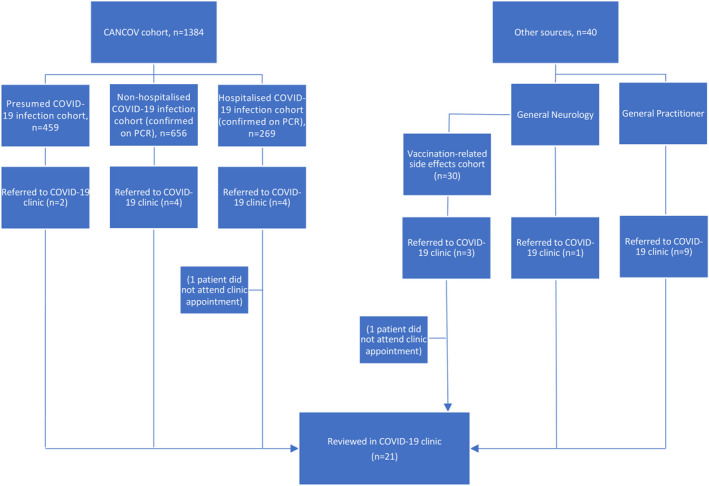
Flow chart of patients referred to the COVID‐19 clinic
Clinical information regarding demographic data, clinical history and examination findings, investigation results, including nerve conduction studies (NCS), electromyogram (EMG) studies and magnetic resonance imaging (MRI), as well as video recordings, were reviewed and summarized. All patients underwent a full neurological examination with a focused assessment for a movement disorder.
RESULTS
Twenty‐one adult patients were included in this case series, two of whom were reviewed entirely virtually. The majority of the patients were female (76%) and the mean (range) age was 50.7 ± 17.2 (21–80) years. Almost half of the patients (48%) had a pre‐existing neurological disorder and over two‐thirds of the cohort had at least one medical comorbidity (Tables 1 and 2). Most patients developed symptoms within 7 days and weeks‐months in the vaccine‐related (10/12 patients, 83%) and infection groups (6/9 patients, 67%), respectively (Table 1). No abnormal investigation results were identified, which included seven NCS/EMG studies, three electroencephalogram studies and 14 brain MRI scans.
TABLE 1.
Summary of clinical characteristics
| Infection‐related | Vaccine‐related | Total (%) | |
|---|---|---|---|
| Mean (range) age, years | 49.9 years (21–75) | 51.3 years (33–80) | 50.7 years (21–80) |
| Sex, n (%) | |||
| Male | 4 (19) | 1 (5) | 5 (24) |
| Female | 5 (24) | 11 (52) | 16 (76) |
| Pre‐existing neurological condition(s), n (%) | |||
| Absent | 4 (19) | 7 (33) | 11 (52) |
| Present | 5 (24) | 5 (24) | 10 (48) |
| B12 deficiency | 1 | — | |
| Tremor syndrome | — | 1 | |
| PLMD | 1 | — | |
| Previous cerebrovascular event and mild parkinsonism | — | 1 | |
| Pituitary adenoma (trans‐sphenoidal surgery) | — | 1 | |
| Retinitis pigmentosa | 1 | ||
| Scoliosis/low back pain | — | 1 | |
| Fatigue following COVID‐19 infection (post‐covid syndrome) | — | 1 | |
| Ulnar nerve neuropathy | 1 | — | |
| Carpal tunnel syndrome | 1 | — | |
| Medical comorbidities, n (%) | |||
| Absent | 3 (14) | 3 (14) | 6 (29) |
| Present | 6 (29) | 9 (43) | 15 (71) |
| Hypothyroidism | 1 | 3 | |
| Respiratory disease (asthma) | 1 | — | |
| Cardiovascular disease (hypertension, hypercholesterolemia) | 1 | 3 | |
| Adrenal insufficiency | — | 1 | |
| OSA | 1 | — | |
| Type 2 diabetes | 1 | — | |
| IBS | — | 1 | |
| Osteoarthritis | — | 1 | |
| Other: lymphoma | 1 | ||
| Pre‐existing mental health illness, n (%) | |||
| Absent | 9 (43) | 4 (19) | 13 (62) |
| Present | — | 8 (33) | 8 (38) |
| Anxiety | — | 6 | |
| Previous history of psychological trauma | — | 2 | |
| Time of onset from insult to neurological manifestations, n (%) | |||
| <24 h (same day) | 1 | 6 | 7 (33) |
| <1 week | — | 4 | 4 (19) |
| <1 month | 4 | — | 4 (19) |
| 5–8 months | 2 | 1 | 3 (14) |
| Unclear | 2 | 1 | 3 (14) |
| Neurological diagnoses, n (%) | |||
| Myoclonus | 3 | — | |
| Eyelid myokymia | 1 | — | |
| De novo tremor syndrome | 1 | — | |
| Unmasking of tremor syndrome | 2 | — | |
| Functional | 2 | 7 | |
| Seizure | — | 2 | |
| Other | — | 3 | |
| Clinical outcomes, n (%) | |||
| Overall improvement/full recovery | 5 (24) | 9 (43) | 14 (67) |
| Ongoing follow‐up/therapies | 4 (19) | — | 4 (19) |
| Referred to other disciplines (epilepsy, physiotherapy, etc.) | — | 3 (14) | 3 (14) |
| Investigation a , n (%) | |||
| Normal | 8 | 10 | 18 (86) |
| Abnormal | — | — | — |
| N/A | 1 | 2 | 3 (14) |
Abbreviations: CSF, cerebral spinal fluid; CT, computed tomography; IBS, irritable bowel syndrome; MRI, magnetic resonance imaging; N/A, not available; OSA, obstructive sleep apnea; PLMD, periodic limb movement disorder.
Blood work, CSF, neurophysiological studies, imaging (CT, MRI).
TABLE 2.
Demographic and clinical features of the patients included in this case series.
| Case | Sex | Age | Clinical summary | Pre‐existing neurological condition | Medical comorbidities | Examination findings | Investigations | Diagnosis | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|
| COVID‐19 infection‐related cases | |||||||||
| 1 | F | 75 | 6–12 months’ history of isolated head tremor following a presumed COVID‐19 infection several months prior (anosmia, breathlessness, cough). Family history positive for tremor. Double vaccinated with AstraZeneca with no reported side effects | Periodic limb movement disorder (confirmed on sleep study in 2019) | Obstructive sleep apnea, fibromyalgia, osteoarthritis | ‘No‐no’ head tremor, with additional features including hand tremor, dystonic posturing | Brain MRI demonstrating small vessel disease | Underlying tremor syndrome unmasked by COVID‐19 infection | Ongoing follow‐up in Movement Disorders clinic |
| 2 | F | 21 | New‐onset tremor and pain in left hand following COVID‐19 infection (confirmed on RT‐PCR). Double vaccinated with Moderna with no reported side effects. No previous history of mental health illness, however, subsequently developed anxiety | Carpal tunnel syndrome | Nil | Abnormal finger movements akin to minipolymyoclonus (Video 1 Segment 1) | Normal EMG/NCS | De novo COVID‐19 infection‐related movement disorder | Spontaneous clinical recovery of movement disorder. Anxiety improved with mental health input |
| 3 |
M |
34 | Muscle twitching, heaviness in limbs, extreme fatigue, headaches following a presumed COVID‐19 infection (anosmia, breathlessness, cough). Double vaccinated with Pfizer‐BioNTech with no reported side effects. No previous history of mental health illness, however, subsequently developed anxiety | Nil | Nil | Functional slowness. Sensory symptoms suggestible (applying a tuning fork resolved them) | Normal EMG/NCS, CK, autoimmune panel, brain MRI | FND following COVID‐19 infection | Improved without medical intervention (including anxiety) |
| 4 |
M |
46 | New‐onset jerky finger movements interfering with daily activities following COVID‐19 infection (confirmed on RT‐PCR). Double vaccinated with Pfizer‐BioNTech with no reported side effects | Ulnar neuropathy | Hypercholesterolemia | Jerky finger movements akin to minipolymyoclonus (Video 1 Segment 2) | Normal EMG/NCS | De novo COVID‐19 Infection‐related movement disorder | Improved without medical intervention |
| 5 | F | 39 | Sudden and persistent twitching of left eyelid following presumed COVID‐19 infection (history of anosmia, new cough and headaches). No previous history of mental health illness, however, subsequently developed anxiety. Double vaccinated with Pfizer‐BioNTech with no reported side effects | Nil | Nil | Left upper and lower lid myokymia, worse when looking to the left (Video 4) | Normal brain MRI and MG screening | Eyelid/facial myokymia following COVID‐19 infection | Improved without medical intervention (including anxiety) |
| 6 | M | 59 | New onset of postural hand tremor 2–3 weeks following confirmed COVID‐19 infection (RT‐PCR) requiring hospital admission but no ICU admission. Family history positive for tremor. Double vaccinated with Moderna with no reported side effects | Nil | Type 2 diabetes mellitus | Postural hand tremor (R > L), possible myoclonic component (Video 3) | NA | De novo tremor following COVID‐19 infection | Ongoing follow‐up in Movement Disorders clinic |
| 7 | M | 74 | New‐onset bilateral hand shaking, fatigue and weakness following confirmed COVID‐19 infection (RT‐PCR) requiring hospital admission but no ICU admission. Received Pfizer‐BioNTech vaccination without side effects | Retinitis pigmentosa (legally blind) | Recent investigation for lymphoma | Action‐induced myoclonic movements in hands (Video 2) | Normal brain MRI and EMG/NCS | De novo action‐induced myoclonus following COVID‐19 infection | Clinical improvement following commencement of Clonazepam |
| 8 | F | 68 | Acute exacerbation of hand tremor (present since childhood, intermittent and mild) following confirmed COVID‐19 infection (RT‐PCR) requiring hospital admission but no ICU admission. No side effects from COVID‐19 vaccination (AstraZeneca). No previous history of mental health illness, however, subsequently developed anxiety | Nil | Asthma (mild) | Bilateral hand action tremor and ‘no‐no’ head tremor | Normal brain MRI | ET unmasked by COVID‐19 infection | Ongoing follow‐up in Movement Disorders clinic |
| 9 | F | 33 | Development of left hemi‐body tic‐like movements and involuntary vocalizations following COVID‐19 infection (confirmed on RT‐PCR). History of transient tics during childhood. No side effects following two doses of Moderna vaccine. No previous history of mental health illness, however, subsequently developed anxiety | B12 replacement | Hypothyroidism | Irregular small amplitude involuntary movements of the left foot, mild premonitory symptoms. Completely suppressible and distractible. | Normal brain MRI | FND (atypical tic‐like movements) following COVID‐19 infection | Ongoing follow‐up in Movement Disorders clinic |
| Vaccine‐related cases | |||||||||
| 10 | F | 54 | Brief episode of euphoria following the first dose of vaccination (Pfizer‐BioNTech), followed by a sensation of internal tremors, intolerance to heat, brain fog, formication (scalp), change in food tolerance and extreme tiredness. History of anxiety | Nil | Hypothyroidism | No focal neurological deficit elicited | CT head normal | FND following COVID‐19 vaccination | Improved after diagnosis and without other medical intervention (including anxiety) |
| 11 | F | 80 | Proximal weakness, myalgia, fatigue and brain fog following first and second doses of COVID‐19 vaccination (Pfizer‐BioNTech and Moderna) | Pituitary adenoma (trans‐sphenoidal surgery) | Adrenal and thyroid insufficiency following transsphenoidal surgery; asthma; hypertension; hypercholesterolemia | Proximal weakness in the upper and lower limbs, with no associated neuropathy or focal neurological deficit | NA | Unmasking of polymyalgia rheumatica following COVID‐19 vaccination vs. decompensation of adrenal insufficiency | Resolution of symptoms following a temporary increase in steroid dose |
| 12 | F | 71 | Acute on chronic worsening of leg weakness following first dose of vaccination (Pfizer‐BioNTech). No previous history of mental health illness, however, subsequently developed anxiety | Scoliosis (previous spinal surgery), falls | Arthritis | Hyperalgesia in lower extremities on sensory examination. Left plantar reflex upgoing | Normal EMG, no acute change on MRI brain and spine (multilevel degenerative changes particularly at L4‐5 and L5‐S1) | Acute on chronic exacerbation of lower limb symptoms following COVID‐19 vaccination | Referred to physiotherapy for strength conditioning |
| 13 | F | 44 | Sudden change in gait (bouncing gait) following second dose of vaccination (Moderna). No side effects following first dose (Pfizer‐BioNTech). History of traumatic childhood | Presumed COVID‐19 infection earlier in the year with post‐covid syndrome (fatigue and weakness) | Nil | Bouncing gait, huff and puff sign positive | Brain MRI and cervical spine normal | FND following COVID‐19 vaccination | Improved after receiving diagnosis of FND and without other medical intervention |
| 14 | F | 33 | Acute onset of generalized muscle twitches fleeting between limbs, trunk and scalp (described as a “pin‐ball machine”) following COVID‐19 vaccination (Pfizer‐BioNTech). History of anxiety | Nil | Nil | Normal | Normal blood work (including CK) | FND following COVID‐19 vaccination | Improved after diagnosis and without other medical intervention (including anxiety) |
| 15 | F | 52 | Isolated episode of altered awareness (associated with tongue bite and prolonged post‐ictal phase) within 6 h of receiving the first dose of vaccination (Pfizer‐BioNTech) | Nil | Previous thyroidectomy | Normal | Normal brain MRI and EEG | First seizure following COVID‐19 vaccination | No further events. Referred to Epilepsy service. |
| 16 | F | 43 | Slowing of movements, shaking in limbs, pain, whole body spasms, excessive fatigue immediately following first dose of vaccination, presenting to ER on a number of occasions. History of traumatic childhood | Nil | IBS | Jerky, tremulous, and writhing limb movements, highly variable, entrainable and distractable (Video 5) | Normal brain MRI, CSF analysis, and EMG/NCS | FND following COVID‐19 vaccination | Clinical improvement after diagnosis. Anxiety improved with mental health input |
| 17 | F | 39 | Two episodes of loss of awareness associated with tongue bite and post‐ictal phase. First episode occurred 2 days following the second vaccination (Pfizer‐BioNTech). Second episode took place 4.5 months later in the context of sleep deprivation. No history of mental health illness, however, subsequently developed anxiety | Nil | Previous thyroidectomy | Normal a | EEG (2) | Two seizures following vaccination—possible underlying epilepsy | Commenced on Levetiracetam. No further events. Referred to Epilepsy service |
| 18 | F | 34 | Increased perception of the colour red, hyperalgesia in the limbs, pre‐syncope, lethargy, effortful movements, subjective heaviness in limbs, mental fatigue and tremor following the first dose of COVID‐19 vaccination (Pfizer‐BioNTech). History of anxiety | Nil | Nil | Normal except for slightly diminished ankle jerks | Normal brain MRI and EMG | FND following COVID‐19 vaccination | Improved after diagnosis and without other medical intervention (including anxiety) |
| 19 | F | 42 | Acute pain syndrome following vaccination into ipsilateral arm (Pfizer‐BioNTech), followed by loss of range of movement in the shoulder and development of right‐hand tremor 4 months later. History of anxiety | Nil | Hypertension | Variable hand tremor variable, entrainable and distractible (Video 6) a | NA | FND following COVID‐19 vaccination | Improved after diagnosis and without other medical intervention (including anxiety). |
| 20 |
M |
49 | Sudden worsening of pre‐existing tremor syndrome and bilateral forearm pain (dorsal aspects) following the second dose of COVID‐19 vaccine (Pfizer‐BioNTech). History of anxiety | ET (family history positive), previous C3/4 laminectomy | Hypertension, hypercholesterolemia | Variable and distractible finger flexion‐extension tremor (L > R). Looping appearance of Archimedes spirals (Video 7) | Normal brain MRI and spine | FND following COVID‐19 vaccination | Improved after diagnosis and without other medical intervention (including anxiety) |
| 21 | F | 75 | Recurrent brief episodes of stabbing‐like pain near the left pre‐auricular and temporal region within a few days of receiving first dose of COVID‐19 vaccine (Pfizer‐BioNTech). Symptoms returned following second dose but not with third dose. History of anxiety | Mild parkinsonism (not on L‐dopa), previous cerebrovascular event | Hypertension, obstructive sleep apnea | Normal | Normal serum inflammatory markers and brain MRI | Cranial neuralgia following COVID‐19 vaccination(s) | Spontaneous clinical recovery without medical intervention. |
Abbreviations: CK, creatinine kinase; CSF, cerebrospinal fluid; EEG, electroencephalogram; EMG, electromyogram; ET, essential tremor; FND, functional neurological disorder; IBS, irritable bowel syndrome; MG, myasthenia gravis; MRI, magnetic resonance imaging; NA, not available; NCS, nerve conduction studies.
Virtual assessment.
All patients received at least one dose of the vaccination: 12 patients received the Pfizer‐BioNTech vaccine, four received the Moderna vaccine, three patients received the AstraZeneca vaccine, and two patients received either a mixture of Pfizer‐BioNTech/Moderna vaccines or AstraZeneca/Moderna vaccines. Twelve and nine patients were double and partially vaccinated, respectively. All cases of COVID‐19 infections predated the first dose of the vaccine except for one patient who contracted COVID‐19 within 11 days of receiving the first vaccination dose.
COVID‐19 infection
Nine patients (43%) presented with neurological manifestations following COVID‐19 infection (confirmed by RT‐PCR in five cases or based on clinical features as PCR testing was not readily available in the remaining four cases). Three patients (14%) developed myoclonus following COVID‐19 infection. Small‐amplitude jerky finger movements with a clinical phenotype similar to minipolymyoclonus, with no accompanying pathological neurological features on examination and neurophysiological testing, were observed in two patients (Table 2). In both cases, this was on a background of a fully recovered ulnar or median neuropathy (Video 1 Segments 1 and 2). The third patient developed action‐induced myoclonus following an acute illness with COVID‐19 infection (Video 2). An underlying tremor syndrome was unmasked in three patients (14%). The first case involved a patient with a history of undiagnosed essential tremor, who became more symptomatic following an acute illness with COVID‐19 that required hospitalization (ward‐level care). The second case was a patient with a strong family history of tremor, who developed an isolated (likely dystonic) head tremor after contracting COVID‐19. The third case involved a patient with a strong family history of tremor, who developed a postural hand tremor several weeks after an acute COVID‐19 infection that required hospitalization (ward‐level care; Video 3). One patient developed isolated eyelid myokymia following infection (Video 4), and two further patients were diagnosed with FND (functional slowness and atypical tic‐like movements).
VIDEO 1.
(Segments 1 and 2) – minipolymyoclonus
VIDEO 2.
Action‐induced myoclonus
VIDEO 3.
Tremor
VIDEO 4.
Eyelid myokymia
COVID‐19 vaccination
Twelve patients (57%) developed neurological symptoms following at least one dose of the COVID‐19 vaccine. There were two cases of a first seizure (10%) following vaccination. Other cases included one patient who developed increased leg weakness on a background of longstanding back issues; a second patient developed increased proximal weakness resulting from a decompensation of adrenal insufficiency and was also subsequently diagnosed with polymyalgia rheumatica by a rheumatologist; and a third patient developed a brief episode of auriculotemporal neuralgia.
The most common neurological manifestation observed, however, was FND (n = 9, 43%). Six patients demonstrated motor FND, such as functional slowness (n = 1), functional gait (n = 1), choreiform‐like (n = 1; Video 5), tic‐like (n = 1) and tremor (n = 2; Videos 6 and 7). The remaining three patients complained of internal tremors, generalized muscle twitching or weakness in spite of having an unremarkable neurological examination. These patients variably reported other functional symptoms, such as extremely effortful voluntary movements, fleeting sensory symptoms, and increased sensitivity to external stimuli (Table 2). Overall, the vaccine group demonstrated a higher number of FNDs compared to the infection group: 58% (n = 7) versus 22% (n = 2; p = 0.08 [Figure 2]). A significant proportion of the FND patients (n = 7, 78%) had a pre‐existing history of anxiety or a history of traumatic childhood, especially in the vaccine group. No patients in the infection group had a known history of a mental health illness.
VIDEO 5.
Functional movement disorder
VIDEO 6.
Functional tremor (virtual consult)
VIDEO 7.
Functional tremor
FIGURE 2.
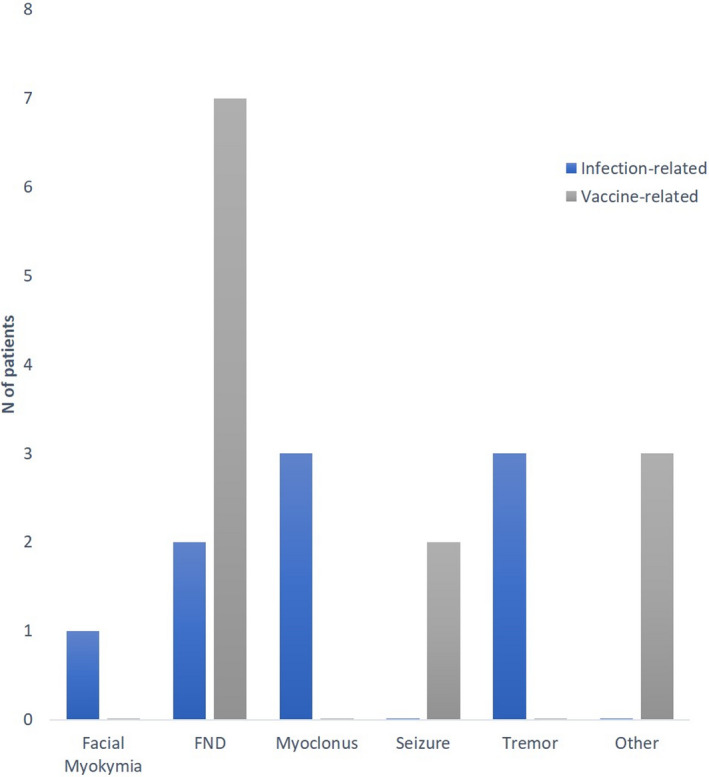
Summary of diagnosis made in the COVID‐19 clinic at the Movement Disorders Centre, Toronto Western Hospital. Abbreviation: FND, functional neurological disorder.
DISCUSSION
In this study, we describe a case series of 21 adult patients presenting with a motor neurological manifestation following COVID‐19 infection or the vaccination. Two distinctive patient groups were referred to the COVID‐19 clinic; the first cohort involved patients who had developed motor neurological manifestations following an acute illness with COVID‐19 infection or vaccination. Several non‐mutually exclusive pathophysiological mechanisms have been proposed, which will be discussed in the following paragraphs.
COVID‐19 infection
Two patients developed small‐amplitude jerky finger movements with a phenomenology similar to minipolymyoclonus following COVID‐19 infection, which was on a background of a fully recovered neuropathy. The clinical significance of these movements is unclear and, to the best of our knowledge, this type of movement disorder might be the expression of a very mild myoclonus [13] or the result of a subtle denervation following nerve damage, although electrophysiology was not suggestive of residual or chronic denervation. This cohort also included patients who had developed neurological disorders that were ‘unmasked’ by COVID‐19 infection. In particular, three cases of an underlying tremor syndrome were diagnosed, which included a case of essential tremor (undiagnosed tremor with transient aggravation following infection, which led to referral to the COVID‐19 clinic), the second case was an isolated de novo (likely dystonic) head tremor and the third a case was a patient who developed a de novo postural‐action tremor.
Since the emergence of the very first cases of COVID‐19 approximately 2 years ago, there has been an increasing number of studies describing neurological disorders associated with COVID‐19 infection. One particular study, which involved a very large cohort of patients, found that neurological manifestations were prevalent in up to 80% of hospitalized patients with COVID‐19, mainly encephalopathy, headaches, anosmia, coma and stroke [2]. The study also found that patients with pre‐existing neurological conditions were more likely to develop neurological signs and/or syndromes with COVID‐19. Movement disorders related to COVID‐19 infection are increasingly being described, with myoclonus as one of the most common abnormal movements reported in hospitalized COVID‐19 patients [14, 15]. Notably, neuropathy has also been described after COVID‐19 [16]. Proposed mechanisms include a combination of neurological injury from systemic dysfunction due to hypoxaemia [17, 18], and immunological dysfunction, which can lead to either a hyperinflammatory state (cytokine storm and microglia activation), a post‐infectious immune‐mediated phenomenon resulting in cases of GBS or ADEM, and/or an increase in autoantibody reactivity against a number of immune‐related proteins, including cytokines and type I interferons [19, 20, 21, 22, 23]. Other proposed mechanisms described in the literature include dysfunction of the renin‐angiotensin system since SARS‐CoV‐2 binds to angiotensin‐converting enzyme 2 (ACE2) receptors in order to enter cells. Downregulation of ACE2 can lead to inhibition of mitochondrial function and damage to vascular endothelial cells [24]. Although rare, another mechanism described involves direct viral invasion of the nervous system, such as via the olfactory system causing hyposmia [25]. One study, however, found that COVID‐19 brains demonstrated very low levels of detectable virus and did not correlate with histopathological changes observed on autopsy [26]. Neurological disorders unmasked by COVID‐19 infection, or the vaccination (see below), are possibly caused by the loss of compensation due to the stress sustained by the body. This hypothesis has previously been suggested for the rare case of parkinsonism seen after COVID‐19 [27].
COVID‐19 vaccination
With the advent of effective COVID‐19 vaccines, the frequency and severity of COVID‐19 infections have fortunately decreased. However, the vaccination has introduced new challenges in terms of neurological side effects. Problems unmasked by COVID‐19 vaccination included adrenal insufficiency followed by polymyalgia rheumatica, as well as a case of increased leg weakness due to a worsening of chronic low back pain. Seizures are an uncommon neurological manifestation following COVID‐19 vaccination and only three cases have been reported to date [28, 29]. An additional two cases were identified in our cohort; both patients developed a seizure (first episode) within 6 and 48 hours of receiving the first dose of vaccination, respectively. The second patient went on to develop another seizure 4 months later. This observation has previously been reported in some children after vaccination [30]. An extensive neurological work‐up was normal in both cases.
The second group of patients reviewed in the COVID‐19 clinic experienced neurological symptoms that were unlikely to be directly related to COVID‐19 infection or the vaccine. Frequently, the connection was made by the patient retrospectively based on the timing of events, followed by hypervigilance of non‐specific symptoms, such as feeling a muscle twitch during an acute COVID‐19 illness or developing increased pain following vaccination, which was often fueled by a heightened state of anxiety. In fact, the anxiety generated by becoming infected or receiving the vaccine [31] could direct the patient's attention towards the body, a mechanism also possible to explain some of the patients referred to the COVID‐19 clinic. Accordingly, a significant proportion of our patients developed de novo anxiety following recovery from COVID‐19 infection (n = 5) or vaccination (n = 2); however, a deterioration in mental health following atypical changes in societal norms, including repeated lockdowns and social isolation cannot be excluded. Thus, the psychiatric consequence of the pandemic and the vaccination campaigns as well as the increased attention towards the body can likely explain the common occurrence of FNDs, the most common neurological manifestation observed in this study (43%). Two and four patients, following COVID‐19 infection and following vaccination, respectively, were diagnosed with a functional movement disorder (clinically established based on the Fahn and Williams criteria [32]). Our study demonstrated that the vast majority of FND patients achieved a full clinical recovery, or had markedly improved, especially after receiving a prompt diagnosis (Table 2). Here, we were also able to diagnose a patient with a functional movement disorder (Patient 19) using telemedicine, which has become an invaluable clinical tool during the pandemic [33].
The development of functional symptoms following COVID‐19 vaccination has recently been described [34] and is overall related to a well‐known ‘immunization stress‐related response’ that has already been reported with other vaccination campaigns [35]. Neuropsychiatric disorders following COVID‐19 have also been found to be relatively common; a systematic review by Groff et al. discovered that COVID‐19 survivors were at risk of developing neurological and mental health disorders, such as difficulty concentrating (23.8%), memory deficits (18.6%), cognitive impairment (17.1%) and generalized anxiety disorder (29.6%) [36]. Moreover, a nationwide study in the United Kingdom demonstrated that a significant proportion of COVID‐19 patients (43%) developed neuropsychiatric disorders, including affective disorders, which often involved younger patients [37]. Another study involving the follow‐up of 100 COVID‐19 survivors after discharge from hospital found that post‐traumatic stress disorder symptoms were more often seen in female patients, which were more severe compared to male patients and were associated with moderate or severe levels of fatigue [38]. More recently, a study reported that up to 78.9% of patients with a functional movement disorder were acutely admitted to hospital due to the development of severe symptoms during the pandemic [39]. Additionally, functional tic‐like behaviors in children and young adults have also generated great interest recently, which is thought to be associated with social media use during the pandemic; the phenomenology is distinct however from classic tics, specifically involving more complex movements and a sudden worsening when in the company of others [40].
How common are these problems?
In our study, 10 patients from the CANCOV study (n = 1384) were referred to the COVID‐19 clinic during 2018–2022 (0.65% of the cohort; Figure 1). Of the nine patients reviewed who had presented with a movement disorder, one patient from the non‐hospitalized COVID‐19 infection (confirmed on PCR) cohort and one patient from the presumed infection cohort were diagnosed with FND (i.e., two patients out of 1115 [0.18%]). Furthermore, three out of 30 patients with vaccination‐related symptoms were referred separately from the CANCOV study by general neurologists; of these three patients, one was diagnosed with FND (3.3%). Regarding the neuromuscular clinic, the experience at our centre is that approximately 5% of the patients referred for further evaluation during the pandemic (5 out of 100 patients) were thought to be functional in origin. This percentage is very similar in comparison to a study that was recently carried out in our deep brain stimulation clinic, which demonstrated that 4.5% of the total referrals (excluding Parkinson's disease) were eventually diagnosed with FND [41]. Furthermore, a study from another tertiary care movement disorders clinic reported that 8.2% (45 out of 550 patients) of new referrals (children and adults) were eventually diagnosed with FND during the pandemic, which was a 60.1% increase in incidence compared to the year prior (5.1%) [42].
On the whole, the exact prevalence of neurological manifestations associated with COVID‐19 infection or vaccination is not entirely clear at present due to publication bias. For example, a number of case reports have emphasized the occurrence of parkinsonism following COVID‐19; however, this is most likely a very rare occurrence [27]. Our study aimed to address this gap by describing a consecutive series of patients with motor neurological manifestations related to COVID‐19 infection or the vaccine. However, given the small sample size of this study and the selected cohort of patients who were specifically referred to this clinic, this may not be wholly representative of the type of patients reviewed in other clinics or centres.
CONCLUSIONS
Overall, our study shows that in a consecutive cohort of outpatients referred to a movement disorder clinic, a functional etiology is common, especially following vaccination. The underlying pathophysiology of neurological complications associated with COVID‐19 or the vaccine is complex and multiple mechanisms/etiologies are possible. Herein, we propose a practical approach to subcategorizing neurological manifestations into three groups: (i) manifestations following acute infection or vaccination; (ii) neurological disorders unmasked by COVID‐19 infection or the vaccination and; (iii) functional neurological disorders following infection or vaccination.
With the ongoing COVID‐19 pandemic, neurologists are likely to encounter increasing numbers of patients with neurological manifestations associated with COVID‐19 infection and vaccinations. We would therefore advocate establishing dedicated COVID‐19 clinics along with a multidisciplinary approach in view of the co‐existence of functional symptoms, movement disorders and mental health illnesses as highlighted in this study.
AUTHOR CONTRIBUTIONS
Wilson K. W. Fung: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing—original draft, Writing—review and editing. Qais Sa'di: Data curation, Investigation, Methodology, Resources, Writing—review and editing. Hans Katzberg: Data curation, Methodology, Resources, Validation, Writing—review and editing. Robert Chen: Data curation, Formal Analysis, Resources, Validation, Writing—review and editing. Anthony E. Lang: Methodology, Validation, Resources, Writing—review and editing. Angela M. Cheung: Data curation, Formal Analysis, Investigation, Methodology, Resources, Validation, Writing—review and editing. Alfonso Fasano: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing
FUNDING INFORMATION
This study was partly funded by the University of Toronto and University Health Network Chair in Neuromodulation to AF.
CONFLICT OF INTEREST
None relevant to this work.
APPROVAL STATEMENT
AF has IRB approval for retrospective chart reviews of patients under his care.
CONSENT STATEMENT
Informed consent was not required as this was a retrospective case series. All patients had provided written consent for videotaping.
Fung WKW, Sa’di Q, Katzberg H, et al. Functional disorders as a common motor manifestation of COVID‐19 infection or vaccination. Eur J Neurol. 2022;00:1‐14. doi: 10.1111/ene.15630
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organisation . WHO coronavirus (COVID‐19) dashboard; 2022. Accessed June 16, 2022. https://covid19.who.int/
- 2. Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID‐19—a report for the GCS‐NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4(5):e2112131. doi: 10.1001/jamanetworkopen.2021.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boesl F, Audebert H, Endres M, Pruss H, Franke C. A neurological outpatient Clinic for Patients with Post‐COVID‐19 syndrome—a report on the clinical presentations of the first 100 patients. Front Neurol. 2021;12:738405. doi: 10.3389/fneur.2021.738405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pilotto A, Cristillo V, Cotti Piccinelli S, et al. Long‐term neurological manifestations of COVID‐19: prevalence and predictive factors. Neurol Sci. 2021;42:4903‐4907. doi: 10.1007/s10072-021-05586-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I, CoviCare Study Team . Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID‐19 in an outpatient setting. Ann Intern Med. 2021;174(9):1252‐1260. doi: 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized Covid‐19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073‐1085. doi: 10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID‐19. Nat Rev Neurol. 2020. Nov;16(11):636‐644. doi: 10.1038/s41582-020-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sulzer D, Antonini A, Leta V, et al. COVID‐19 and possible links with Parkinson's disease and parkinsonism: from bench to bedside. Npj Parkinsons Dis. 2020;6:18. doi: 10.1038/s41531-020-00123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yüksel MF, Yildirim M, Bektas O, Sahin S, Teber D. A sydenham chorea attack associated with COVID‐19 infection. Brain Behav Immunity ‐ Health. 2021;13:100222. doi: 10.1016/j.bbih.2021.100222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandão PRP, Grippe TC, Pereira DA, Munhoz RP, Cardoso F. New‐onset movement disorders associated with COVID‐19. Tremor Other Hyperkinet Mov (NY). 2021;8(11):26. doi: 10.5334/tohm.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID‐19 vaccines and SARS‐CoV‐2 infection. Nat Med. 2021;27:2144‐2153. doi: 10.1038/s41591-021-01556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Canadian COVID‐19 prospective cohort study; 2020. Accessed February 7, 2022. https://cancov.net/
- 13. Ganguly J, Chai JR, Jog M. Minipolymyoclonus: a critical appraisal. J Mov Disord. 2021;14(2):114‐118. doi: 10.14802/jmd.20166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark JR, Liotta EM, Reish NJ, et al. Abnormal movements in hospitalized COVID‐19 patients: a case series. J Neurol Sci. 2021;423:117377. doi: 10.1016/j.jns.2021.117377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following Coronavirus Disease 2019 (COVID‐19). Mov Disord Clin Pract. 2020;7(8):974‐976. doi: 10.1002/mdc3.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raahimi MM, Kane A, Moore CE, Alareed AW. Late onset of Guillain‐Barré syndrome following SARS‐CoV‐2 infection: part of ‘long COVID‐19 syndrome’? BMJ Case Rep. 2021;14(1):e240178. doi: 10.1136/bcr-2020-240178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid‐19. N Engl J Med. 2020;383(10):989‐992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radmanesh A, Derman A, Lui YW, et al. COVID‐19‐associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223‐E227. doi: 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellul MA, Benjamin L, Singh B, Lant S, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020. Sep;19(9):767‐783. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koralnik IJ, Tyler KL. COVID‐19: a global threat to the nervous system. Ann Neurol. 2020. Jul;88(1):1‐11. doi: 10.1002/ana.25807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020. May;19(5):383‐384. doi: 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boldrini M, Canoll PD, Klein RS. How COVID‐19 affects the brain. JAMA Psychiat. 2021;78(6):682‐683. doi: 10.1001/jamapsychiatry.2021.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595:283‐288. doi: 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 24. Lei Y, Zhang J, Schiavon CR, et al. SARS‐CoV‐2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323‐1326. doi: 10.1161/CIRCRESAHA.121.318902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meinhardt J, Radke J, Dittmayer C, Franz J, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci. 2021;24(2):168‐175. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- 26. Thakur KT, Miller EH, Glendinning MD, Al‐Dalahmah O, et al. COVID‐19 neuropathology at Columbia University Irving medical center/New York Presbyterian hospital. Brain. 2021;144(9):2696‐2708. doi: 10.1093/brain/awab148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez‐Latapi P, Fearon C, Fasano A, Lang AE. Parkinson's disease and COVID‐19: do we need to Be more patient? Mov Disord. 2021;36(2):277. doi: 10.1002/mds.28469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghosh R, Dubey S, Roy D, Mandal A, Naga D, Benito‐León J. Focal onset non‐motor seizure following COVID‐19 vaccination: a mere coincidence? Diabetes Metab Syndr. 2021;15(3):1023‐1024. doi: 10.1016/j.dsx.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu BD, Ugolini C, Jha P. Two cases of post‐Moderna COVID‐19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021;13(7):e16172. doi: 10.7759/cureus.16172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verbeek NE, Jansen FE, Vermeer‐de Bondt PE, et al. Etiologies for seizures around the time of vaccination. Pediatrics. 2014. Oct;134(4):658‐666. doi: 10.1542/peds.2014-0690 [DOI] [PubMed] [Google Scholar]
- 31. Hause AM, Gee J, Johnson T, et al. Anxiety‐related adverse event clusters after Janssen COVID‐19 vaccination—five U.S. mass vaccination sites. Morb Mortal Wkly Rep. 2021;70:685‐688. doi: 10.15585/mmwr.mm7018e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231‐257. [PubMed] [Google Scholar]
- 33. Zulman DM, Verghese A. Virtual care, telemedicine visits, and real connection in the era of COVID‐19: unforeseen opportunity in the face of adversity. JAMA. 2021;325(5):437‐438. doi: 10.1001/jama.2020.27304 [DOI] [PubMed] [Google Scholar]
- 34. Fasano A, Daniele A. Functional disorders after COVID‐19 vaccine fuel vaccination hesitancy. J Neurol Neurosurg Psychiatry. 2021;93:339‐340. doi: 10.1136/jnnp-2021-327000 [DOI] [PubMed] [Google Scholar]
- 35. Taylor S, Asmundson GJG. Immunization stress‐related responses: implications for vaccination hesitancy and vaccination processes during the COVID‐19 pandemic. J Anxiety Disord. 2021;84:102489. doi: 10.1016/j.janxdis.2021.102489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Groff D, Sun A, Ssentongo AE, et al. Short‐term and long‐term rates of Postacute sequelae of SARS‐CoV‐2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7(10):875‐882. doi: 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halpin SJ, McIvor C, Whyatt G, Adams A, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013‐1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 39. Machado M, Tarrano C, Mesrati F, Roze E, Vidailhet M, Aubignat M. Functional movement disorders during the COVID‐19 pandemic: Back to Charcot's era at the Salpêtrière. Mov Disord. 2022;37:432‐434. doi: 10.1002/mds.28875 [DOI] [PubMed] [Google Scholar]
- 40. Hull M, Parnes M. Tics and TikTok: functional tics spread through social media. Mov Disord Clin Pract. 2021;8(8):1248‐1252. doi: 10.1002/mdc3.13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gorodetsky C, Azevedo P, Fasano A. Functional patients referred for deep brain stimulation: how common is it? Mov Disord Clin Pract. 2022;9:841‐842. doi: 10.1002/mdc3.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hull M, Parnes M, Jankovic J. Increased incidence of functional (psychogenic) movement disorders in children and adults amid the COVID‐19 pandemic: a cross‐sectional study. Neurol Clin Pract. 2021. Oct;11(5):e686‐e690. doi: 10.1212/CPJ.0000000000001082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


