Abstract
Salmonella enterica serovar Typhimurium proliferates within cultured epithelial and macrophage cells. Intracellular bacterial proliferation is, however, restricted within normal fibroblast cells. To characterize this phenomenon in detail, we investigated the possibility that the pathogen itself might contribute to attenuating the intracellular growth rate. S. enterica serovar Typhimurium mutants were selected in normal rat kidney fibroblasts displaying an increased intracellular proliferation rate. These mutants harbored loss-of-function mutations in the virulence-related regulatory genes phoQ, rpoS, slyA, and spvR. Lack of a functional PhoP-PhoQ system caused the most dramatic change in the intracellular growth rate. phoP- and phoQ-null mutants exhibited an intracellular growth rate 20- to 30-fold higher than that of the wild-type strain. This result showed that the PhoP-PhoQ system exerts a master regulatory function for preventing bacterial overgrowth within fibroblasts. In addition, an overgrowing clone was isolated harboring a mutation in a previously unknown serovar Typhimurium open reading frame, named igaA for intracellular growth attenuator. Mutations in other serovar Typhimurium virulence genes, such as ompR, dam, crp, cya, mviA, spiR (ssrA), spiA, and rpoE, did not result in pathogen intracellular overgrowth. Nonetheless, lack of either SpiA or the alternate sigma factor RpoE led to a substantial decrease in intracellular bacterial viability. These results prove for the first time that specific serovar Typhimurium virulence regulators are involved in a response designed to attenuate the intracellular growth rate within a nonphagocytic host cell. This growth-attenuating response is accompanied by functions that ensure the viability of intracellular bacteria.
Salmonella enterica serovar Typhimurium causes food-borne diseases in humans and animals ranging from self-limited gastroenteritis to bacteremia and systemic infections (52). Major hallmarks of serovar Typhimurium pathogenesis include the exploitation of epithelial cell functions in inducing bacterial uptake and resistance to antimicrobial attack of phagocytic cells (reviewed in references 11, 20, and 73). These mechanisms enable serovar Typhimurium to efficiently penetrate the intestinal epithelial barrier and disseminate to deeper tissues (52, 72). In vitro and in vivo models have demonstrated that both responses are required for elicitation of disease (15, 19). Serovar Typhimurium also proliferates extensively within membrane-bound vacuoles of cultured epithelial and macrophage cells (16, 21, 25, 45). This process initiates 4 to 6 h after bacterial internalization (25) and ends at a stage at which the vacuole containing fast-growing bacilli entirely fills the preexisting host cell cytosol. In vitro models have shown that the average increase in viable intracellular bacteria ranges from 10- to 100-fold during the first 24 h of infection depending on the cell line used (45, 65). In contrast, the increase in the intracellular growth rate estimated in vivo is in the range of 5- to 10-fold every 24 h (2, 63, 70). The discrepancy in the growth rates between the two models raises the question whether serovar Typhimurium intracellular proliferation occurs in vivo to the same extent as in cultured cell lines. Likewise, there is not yet clear evidence that a process of massive intracellular proliferation is absolutely required for a fatal outcome of the infection. Indeed, several studies have reported that massive pathogen growth is not required to cause host death. Thus, an enhanced production of immune mediators, such as tumor necrosis factor alpha and interleukin-1, may lead to fatal consequences for the host even while the host harbors nonsaturating bacterial doses (39).
The extent to which Salmonella intracellular proliferation is required for virulence is at present unknown. An early report claimed that intraepithelial proliferation of serovars Typhimurium and Choleraesuis was essential for virulence, since in vitro-selected mutants displaying a lower intracellular growth rate behaved as attenuated in the murine typhoid model (17, 40). However, more recent studies have shown that intraepithelial proliferation might not be relevant in vivo. Thus, confocal microscopy analysis performed on liver and spleen tissue sections of infected mice has shown that the majority of proliferating bacteria reside and multiply almost exclusively in CD18-expressing phagocytes (8, 46, 58). Interestingly, these studies did not provide evidence for infected cells carrying as many intracellular bacteria as in vitro-infected cultured cells. Morbidity and mortality are severely compromised when the macrophage cell population is selectively immunodepleted in susceptible animals (31), suggesting that phagocytes are the main host cell types permissive for serovar Typhimurium intracellular growth. In addition, intracellular proliferation of S. enterica serovar Typhimurium seems to be significantly stimulated in tumor cells implanted in animals compared to other tissues or organs of the infected animal (44, 55). Altogether, these observations indicate that intraepithelial proliferation of serovar Typhimurium, if it exists, might be limited during the course of the infection.
The identity of the serovar Typhimurium functions required for intraepithelial proliferation is still a matter of debate. The functionality of the Salmonella pathogenicity island (SPI-2)-encoded type III secretion system (TTSS) was initially proposed as essential for intraepithelial growth (4). However, SPI-2 has been recently shown to be strictly required for intramacrophage survival and dispensable for intraepithelial growth (32). Other studies have reinforced the concept that SPI-2 is required to elicit enteritis and systemic disease as well as to promote intracellular bacterial growth within phagocytes located in target organs (3, 62).
A series of cell biology studies, based almost exclusively on in vitro models, have attempted to provide clues to how serovar Typhimurium initiates intracellular growth. It is known that bacterial proliferation within cultured epithelial cells correlates with alterations in the distribution of host lysosomal membrane glycoproteins (LGPs) and the formation of filamentous structures named Sifs (25, 32). Pathogen interference with the host vacuolar trafficking machinery has also been proposed as a major strategy promoting intracellular growth (22, 48, 64). Bacterial proteins required for alteration of the lysosomal glycoprotein distribution include SifA (65), the SPI-2-encoded proteins SseF and SseG (32), and the two-component system OmpR-EnvZ (49). SpiC, a SPI-2-encoded protein, has recently been shown to prevent endosome-lysosome fusion events in macrophages (69). Whether SpiC performs a similar function in epithelial cells is at present unknown. Interestingly, serovar Typhimurium mutants lacking proteins required for Sif formation retain the ability to grow intracellularly within epithelial cells (3, 32, 65), suggesting that Sif formation is dispensable for intraepithelial bacterial proliferation.
We have recently demonstrated that virulent serovar Typhimurium strains are unable to proliferate within certain nonphagocytic cell types such as normal fibroblasts (45). Intracellular bacteria are enclosed within membrane-bound vacuoles in which they are unable to initiate growth (45). Considering that the in vivo model has not provided any evidence for massive pathogen proliferation within nonphagocytic cells, we have employed the fibroblast infection model to characterize the mechanisms underlying the control of serovar Typhimurium intracellular growth within nonphagocytic cells.
In an attempt to define the factors involved in preventing intracellular growth of serovar Typhimurium within fibroblast cells, we raised the hypothesis that the absence of bacterial growth could also be the result, at least in part, of a response mounted by the pathogen itself. If this prediction is correct, bacterial mutants lacking functions related to this response should overgrow. A basic positive selection procedure allowed us to isolate serovar Typhimurium mutants with an enhanced intracellular growth rate. The study has led us to uncover a novel role for specific pathogen-encoded regulators, most of them previously characterized by their contribution to intramacrophage survival. To our knowledge, this is the first report that proves the existence of a growth self-attenuating response triggered by an intracellular bacterial pathogen within a eucaryotic cell.
MATERIALS AND METHODS
Bacterial strains.
S. enterica serovar Typhimurium strains used in this study are listed in Table 1. Unless otherwise indicated, the reference parental strain for the infection assays was the mouse virulent strain SL1344 (36). Mutant alleles obtained from other investigators in different genetic backgrounds were transduced into SL1344 before the phenotypic analysis of intracellular proliferation within eucaryotic cells. The only exception was the series of nonpolar in-frame deletion mutants in the spv genes, which, due to the lack of an appropriate selection marker, were tested in comparison with the parental wild-type (wt) strain SR11 (Table 1). The bank of MudJ transposon-generated mutants was constructed in SL1344 according to previously described methods (37). MudJ and flanking regions present in overgrowing clones were passaged by P22 HT-mediated transduction to a clean SL1344 genetic background. Upon confirmation of the linkage between the phenotype and the transposon insertion, the exact location of the insertion was mapped by direct chromosomal sequencing (see below). Plasmid pEG5433 containing the wt phoPQ locus (29) was used to complement phoP- and phoQ-null mutants.
TABLE 1.
S. enterica serovar Typhimurium strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| 14028s | Wild type | 15 |
| MS5996s | 14028s phoQ5996::Tn10 | 14 |
| EG5799 | 14028s spiR(ssrA)5799::MudJ | 51 |
| SL2823 | 14028s soxRS::Ampr | 13 |
| SL2918 | 14028s fnr::Tn10 | Stephen J. Libby |
| SL2236 | 14028s slyA::pRR10ΔtrfA Penr | 42 |
| 12023spvA | 14028s spvA::Kmr | 35 |
| ST173 | LT2 lrp-1::Tn5 | Michael P. Spector |
| SFS78 | UK1 aldB2::MudJ mviA(rssB)::Camr | Michael P. Spector |
| SL1344 | hisG46 rpsL; virulent strain | 36 |
| SV4056 | SL1344 phoP7953::Tn10 | 23 |
| SV4365 | SL1344 phoQ5996::Tn10 | This work |
| EG5170 | SL1344 phoP5170::MudJ | E. A. Groisman |
| GVB311 | SL1344 rpoE::Kmr | 38 |
| SV4232 | SL1344 rpoE::KmrphoP7953::Tn10 | This work |
| SV4237 | SL1344 spiR(ssrA)5799::MudJ | This work |
| SV4241 | SL1344 spiR5799::MudJ phoP7953::Tn10 | This work |
| SV4386 | SL1344 spiA::KIXX | This work |
| SV4387 | SL1344 spiA::KIXX phoP7953::Tn10 | This work |
| CJD359 | SL1344 ompR1009::Tn10 | 6 |
| SV4235 | SL1344 ompR1009::Tn10 | This work |
| SV4236 | SL1344 ompR1009::Tn10 phoP5170::MudJ | This work |
| SV1610 | SL1344 dam228::MudJ | 23 |
| SV4089 | SL1344 dam228::MudJ phoP7953::Tn10 | 23 |
| SMS521 | SL1344 crp773::Tn10 | Michael Spector |
| SV4197 | SL1344 phoQ201 zzz::MudJ | This work |
| SV4198 | SL1344 slyA21::MudJ | This work |
| SV4210 | SL1344 rpoS1151::MudJ | This work |
| SV4215 | SL1344 igaA188 zzz::MudJ | This work |
| SV4431 | SL1344 zzz::MudJ | P22 (SV4215) × SL1344 |
| SV4221 | SL1344 spvA101::MudJ | This work |
| SV4222 | SL1344 rpoS1152::MudJ | This work |
| SV4224 | SL1344 phoQ201 | This work |
| SV4234 | SL1344 spvA101::MudJ | This work |
| SV4254 | SL1344 igaA188 zhf-6311::Tn10dTet | This work |
| SV4255 | SL1344 zhf-6311::Tn10dTet, nonmucoid | P22 (SV4254) × SL1344 |
| SV4312 | SL1344 spvA103::MudJ | This work |
| SV4313 | SL1344 spvA104::MudJ | This work |
| SV4314 | SL1344 spvR105::MudJ | This work |
| ST97 | SL1344 relA21::Tn10 | 60 |
| SV4285 | SL1344 relA21::Tn10 phoP5170::MudJ | This work |
| SV4253 | SL1344 lrp-1::Tn5 | This work |
| SV4286 | SL1344 lrp-1::Tn5 phoP7953::Tn10 | This work |
| SMS593 | SL1344 oxyR::Tn10 | 61 |
| SV4287 | SL1344 oxyR::Tn10 phoP5170::MudJ | This work |
| SV4233 | SL1344 mviA(rssB)::Camr | This work |
| SV4288 | SL1344 mviA(rssB)::CamrphoP7953::Tn10 | This work |
| SV4261 | SL1344 soxRS::Ampr | This work |
| SV4262 | SL1344 fnr::Tn10 | This work |
| SMS438 | SL1344 rpoS::Ω-Ampr | 53 |
| SV4240 | SL1344 rpoS::Ω-AmprphoP7953::Tn10 | This work |
| SV4250 | SL1344 slyA::pRR10ΔtrfA Penr | This work |
| SV4251 | SL1344 spvA::pRR10ΔtrfA PenrphoP7953::Tn10 | This work |
| SV4257 | SL1344 spvA::Kmr | This work |
| SV4258 | SL1344 spvA::KmrphoP7953::Tn10 | This work |
| χ3306 | SR11, wild type | 76 |
| UF082 | χ3306 ΔspvR | 76 |
| UF104 | χ3306 ΔspvA | 76 |
| UF051 | χ3306 ΔspvB | 76 |
Infection of eucaryotic cells.
HeLa cells (ATCC CCL2) and NRK-49F rat fibroblasts (ATCC no. CRL-1570) were grown and infected with the different serovar Typhimurium strains as described previously (45). Briefly, bacteria were grown overnight in Luria-Bertani (LB) medium at 37°C in static conditions, and 5 μl of the culture (approximately 106 CFU) was added to eucaryotic cells cultured to up to 60 to 70% confluency in 24-well plastic plates (approximately 105 eucaryotic cells/well). These conditions accounted for a multiplicity of infection of 10:1 (bacterium/eucaryotic cell ratio). Cells were infected for 20 to 30 min depending on the extent of bacterial invasion and monitored by microscopic observation and estimation of cultured cells displaying membrane ruffles. Infection was maintained until 50 to 60% of cultured cells showed signs of bacterial uptake. Noninternalized bacteria were further removed by three successive washes with phosphate-buffered saline (PBS), pH 7.4. Fresh growth medium containing 100 μg of gentamicin ml−1 was then added and maintained up to 2 h postinfection, the time at which the antibiotic concentration was lowered to 10 μg ml−1. At different postinfection times, infected cells were lysed with 1% Triton X-100–PBS (pH 7.4) (5 min, room temperature), and the number of viable intracellular bacteria was determined by plating of appropriate lysate dilutions in LB agar and colony counting. All samples were run at least in duplicate. The average invasion rate obtained in standard 30-min infection assays was 1 to 2% in HeLa cells and 0.2 to 0.5% in NRK-49F fibroblasts. The intracellular proliferation rate (Ipro) was calculated as the ratio of the number of viable intracellular bacteria present at 24 h to those present at 2 h postinfection.
Selection of serovar Typhimurium mutants with increased intracellular growth rate in fibroblasts.
Selection of overgrowing bacterial mutants was addressed with NRK-49F fibroblasts, since these cells, in contrast to HeLa epithelial cells, are nonpermissive for bacterial intracellular proliferation (45). These fibroblasts do not show signs of cytotoxicity at any time postinfection as tested by dye exclusion assays (data not shown). To select for serovar Typhimurium isolates with high Ipro values within NRK-49F fibroblasts, 10 pools, each representing 5,000 kanamycin-resistant clones of serovar Typhimurium, were first obtained by MudJ mutagenesis (37). These pools were used separately to infect NRK-49F fibroblast cultures in three to four successive infection cycles. Each of these cycles consisted of 30 min of incubation of NRK-49F fibroblasts with bacteria, 1.5 h in growth medium containing 100 μg of gentamicin ml−1, and up to 72 h of maintenance of the infection in growth medium containing 10 μg of gentamicin ml−1. The rationale for infecting up to 72 h was the fact that the viability of intracellular wt serovar Typhimurium within NRK-49F fibroblasts decreases more than 90% at this postinfection time (45), thus rendering the enrichment procedure more effective. In all cases, the inoculum for the intermediate infection cycles was prepared by direct inoculation of the 1% Triton X-100–PBS (pH 7.4) detergent lysate obtained at 72 h into fresh LB medium and further overnight incubation at 37°C. In parallel, the Ipro value of the pool was calculated in every infection cycle to validate the enrichment for overgrowing mutants within each pool. Upon the last infection cycle, the 1% Triton X-100–PBS (pH 7.4) lysate was plated, and mutants belonging to the pools that showed higher Ipro values were tested individually in NRK-49F fibroblasts. Analysis of individual clones was made only with pools 1, 2, 3, 4, and 6, in which the Ipro value after the last infection cycle was at least 2 orders of magnitude higher than that obtained in wt strain infections. Mapping of the MudJ insertions showed that mutations in genes such as spvA and rpoS were selected in different pools. Mutations in phoQ, slyA, spvR, and igaA genes were selected once, but in different pools.
Mapping of mutations linked to intracellular overgrowth phenotype.
Direct chromosomal sequencing was used to determine the exact location of MudJ insertions in serovar Typhimurium isolates displaying an increased Ipro. Chromosomal DNA was prepared as follows: bacteria grown overnight in 1.5 ml of LB medium were spun down and suspended in the same volume of 10 mM Tris-HCl (pH 8.0)–25 nM EDTA (pH 8.0). An 0.55-ml volume of lysozyme solution (10 mg ml−1 in 10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0]) was added, and the mix was incubated for 20 min at 37°C. Proteinase K (100 μg ml−1) was added, and the mix was incubated for 1 h at 55°C. After three to four phenol and chloroform-isoamyl alcohol (24:1) extractions, DNA was precipitated with ammonium acetate and absolute ethanol and finally suspended in 10 mM Tris-HCl (pH 8.0)–1 mM EDTA (pH 8.0). DNA was further sheared with a 23-gauge needle and cleaned in a Sephadex G-50 column. The oligonucleotide used as a primer specific for the left arm of the MudJ transposon was 5′ CGAATAATCCAATGTCCTCC 3′. Conditions for chromosomal sequencing were (i) incubation of 2 μg of chromosomal DNA at 58°C for 30 min; (ii) addition of 13 pmol of primer oligonucleotide, 16 μl of BigDye Terminator mix, 1 μl of ThermoFidelase I (Fidelity Systems, Gaithersburg, Md.), and H2O up to 40 μl; (iii) PCR (95°C) for 5 min (95°C for 30 s, 53°C for 20 s, and 60°C for 4 min) (55 cycles), 60°C for 5 min, and holding at 4°C; and (iv) DNA precipitation with Centrisep spin columns (catalog no. 401763; Applied Biosystems–Perkin-Elmer, Madrid, Spain). To define the spontaneous mutation present in strains SV4197-SV4224 (phoQ) and SV4215-SV4254 (igaA), the phoPQ and igaA genes were PCR amplified from chromosomal DNA extracted from wt (SL1344) and mutant serovar Typhimurium strains. The pairs of primers used to amplify the phoPQ genes were pho-1D (5′ GAGTTGACCCGTGGCAAGCG 3′) and pho-1R (5′ GCGTTCAAGAAAGTCGGGCC 3′). To amplify the igaA gene, the oligonucleotides yF-1Dbis (5′ CATTGTGCGACATACCG 3′) and yF-1Rbis (5′ TATGCATGGGGAACTCC 3′) were used. The PCR products were sequenced over the full length of the genes using oligonucleotides corresponding to internal sequences as primers. All the partial sequences were confirmed from products obtained in two independent PCR amplifications. The point mutation in the overgrowing SV4197-SV4224 (phoQ201) strains consisted of a C→T nucleotide change that caused an R (arginine)→W (tryptophan) amino acid change at position 313 of the PhoQ protein. In the SV4215-SV4254 (igaA188) strains, the point mutation consisted of a G→A nucleotide change that caused an R (arginine)→H (histidine) amino acid change at position 188 of the IgaA protein.
Immunofluorescence.
Serovar Typhimurium-infected cells were fixed with 3% paraformaldehyde for 10 min at room temperature. Labeling for immunofluorescence was performed with the following primary antibodies: monoclonal mouse immunoglobulin G (IgG) antibody MLK33 (gift of J. M. Slauch, University of Illinois), which recognizes serovar Typhimurium lipopolysaccharide (LPS), and polyclonal rabbit antibody C38 (gift of I. Sandoval, Centro de Biología Molecular “Severo Ochoa,” Madrid, Spain), which recognizes rat LGPs. The secondary antibodies included goat anti-mouse IgG conjugated to Texas Red, goat anti-mouse IgG conjugated to fluorescein isothiocyanate, and goat anti-rabbit IgG conjugated to Texas Red. DAPI (4,6-diamidino-2-phenylindole; 1 μg ml−1) was added for 1 min to stain nuclei. The staining was performed as described previously (45).
Nucleotide sequence accession number.
The phoPQ nucleotide sequence of serovar Typhimurium strain SL1344 was deposited in the EMBL database under the accession no. AJ272210/STY272210. The igaA gene is under the accession no. AJ301649/SEN301649.
RESULTS
Isolation of S. enterica serovar Typhimurium mutants with increased ability to proliferate within fibroblasts.
S. enterica serovar Typhimurium persists in a resting or slow-growing state within NRK-49F fibroblasts (45). To investigate whether this reduced proliferation was a consequence of a bacterial self-attenuation growth response, we undertook a genetic strategy to identify the hypothetical functions involved. The rationale was that mutations causing loss of function in hypothetical growth-attenuating genes should result in bacterial strains with increased proliferation. A bank of at least 50,000 independent transposon MudJ-generated mutants derived from the virulent serovar Typhimurium strain SL1344 was used (36, 37). Upon three to four cycles of infection of NRK-49F cells, several clones showing enhanced Ipro rates were chosen for further characterization. When tested individually, all these isolates displayed invasion rates similar to that of the wt strain (0.2 to 0.5% [data not shown]). However, the ratio of viable intracellular bacteria at 24 h to that at 2 h (Ipro value) obtained for these clones was substantially higher than the ratio obtained for the wt strain (Table 2). The phenotypic analysis allowed us to differentiate two major groups of overgrowing bacterial mutants. One class was represented by isolate SV4197, which showed an Ipro value up to 24-fold higher than that exhibited by the wt strain (Table 2). The second class included nine isolates (SV4198, SV4210, SV4215, SV4221, SV4222, SV4234, SV4312, SV4313, and SV4314) with Ipro values 6- to 13-fold higher than that of the wt (Table 2). Linkage of the MudJ transposon insertion to the overgrowth phenotype was demonstrated in all isolates, except SV4197 and SV4215 (see below). Because insertion mutations usually cause loss of function, the success of these hunts for mutants indicated that specific serovar Typhimurium factors exerting negative control on bacterial intracellular growth do exist.
TABLE 2.
S. enterica serovar Typhimurium mutants selected for high Ipro in NRK-49F cells
| Strain | Selected mutationa | Iprob |
|---|---|---|
| SL1344 | None, wild type | 1.7 ± 0.4 |
| SV4197c | Point mutation in phoQ (R313W); zzz::MudJ | 41.0 ± 6.0 |
| SV4198 | slyA21::MudJ | 12.0 ± 0.7 |
| SV4210 | rpoS1151::MudJ | 11.5 ± 4.3 |
| SV4215c | Point mutation in igaA (R188H); zzz::MudJ | 15.2 ± 3.2 |
| SV4431d | igaA+ zzz::MudJ (insertion from SV4215) | 3.7 ± 1.1 |
| SV4221 | spvA101::MudJ | 14.5 ± 2.8 |
| SV4222 | rpoS1152::MudJ | 10.2 ± 2.7 |
| SV4224c | phoQ201 (R313W) | 32.5 ± 7.5 |
| SV4234 | spvA102::MudJ | 22.0 ± 2.3 |
| SV4254c | igaA (R188H) zhf-6311::Tn10dTet | 17.7 ± 4.2 |
| SV4255d | igaA+zhf-6311::Tn10dTet (nonmucoid derivative of SV4254) | 3.1 ± 0.4 |
| SV4312 | spvA103::MudJ | 18.4 ± 5.1 |
| SV4313 | spvA104::MudJ | 11.4 ± 1.2 |
| SV4314 | spvR105::MudJ | 20.6 ± 4.7 |
MudJ insertions were mapped by direct chromosomal sequencing.
Means ± standard deviations of triplicate samples from representative experiments. The assay was repeated a minimum of three times for each strain.
Mutation identified in PCR-amplified phoPQ or igaA loci.
Control strains that link igaA to negative control of intracellular growth (see text).
The PhoP-PhoQ two-component system is a negative regulator of bacterial growth within fibroblasts.
Standard genetic analysis showed that the mutation harbored by the isolate SV4197, which displayed the highest Ipro value, was not linked to the MudJ insertion. However, this strain had distinct phenotypic traits: inability to grow on N-minimal plates containing low levels of Mg2+ (8 μM) and formation of dark colonies on green plates. These phenotypes are characteristic of phoP or phoQ mutants (26; our unpublished observations). Moreover, the high-Ipro phenotype of SV4197 was 90% cotransducible with purB, which is closely linked to the phoPQ locus (29). To avoid any undesirable effect of the MudJ insertion present in SV4197 and not linked to control of intracellular growth rate, the spontaneous mutation was passaged using the purB marker to a wt genetic background to construct strain SV4224. This strain retained all phenotypes related to lack of a functional PhoP-PhoQ system, including the inability to grow on N-minimal plates with low magnesium and to induce an mgtC::MudJ transcriptional fusion in micromolar concentrations of Mg2+ (data not shown). To ascertain the type of mutation harbored by strains SV4197 and SV4224, the promoter and entire coding region of the phoPQ genes from SL1344 (wt), SV4197, and SV4224 were PCR amplified and sequenced (see Materials and Methods). As expected, the sequence analysis showed that SV4197 and its derivative SV4224 harbored the same mutation consisting of a spontaneous nucleotide change in the phoQ gene that resulted in a substitution of an R (arginine) for a W (tryptophan) at position 313 of the PhoQ protein. The two-component regulatory system PhoP-PhoQ has been shown elsewhere to play a central regulatory function in Salmonella virulence, mediating, among other processes, the survival of intracellular bacteria within phagocytic cells (28, 52). Our results unveil a novel, unexpected function for the PhoP-PhoQ system: the attenuation of serovar Typhimurium proliferation within normal cultured fibroblasts.
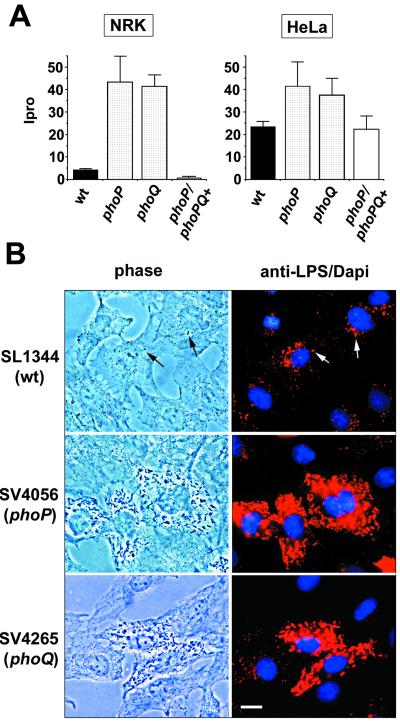
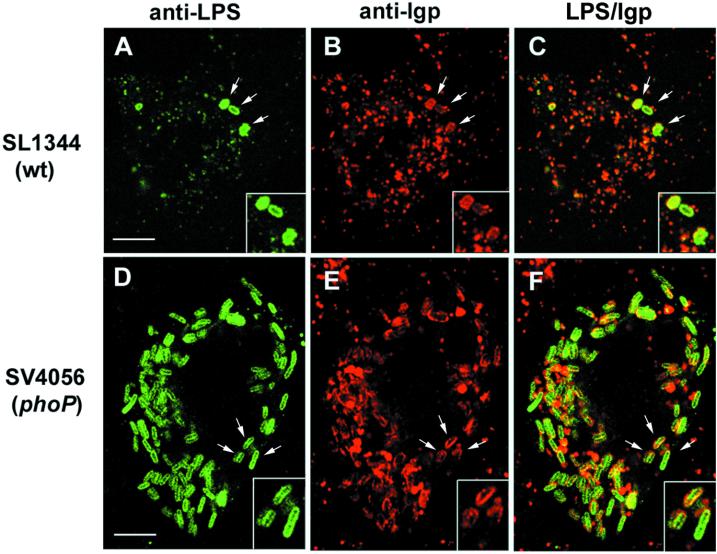
To confirm the involvement of the PhoP-PhoQ system in this novel pathogen intracellular response, previously reported phoP- and phoQ-null mutant alleles were transduced into the SL1344 background (strains SV4056 [phoP7956::Tn10] and SV4365 [phoQ5996::Tn10], respectively), and the resulting strains were tested for proliferation within fibroblasts. The absence of either PhoP or PhoQ protein caused a dramatic increase in the ability of intracellular bacteria to initiate growth (Fig. 1A). Increased proliferation was also observed in other nonpermissive cell lines, such as 3T3 mouse fibroblasts (data not shown), but not in HeLa cells (Fig. 1A). These results suggest that the self-attenuating growth response triggered by intracellular bacteria might occur only in specific nonphagocytic cell lines. Two additional observations confirmed that the PhoP-PhoQ system exerts negative control on bacterial growth within NRK-49F fibroblasts. First, the high intracellular growth rate shown by the phoP mutant in NRK-49F fibroblasts was abolished by complementation with plasmid pEG5433 containing the wt phoPQ genes (29) (Fig. 1A). Second, high intracellular bacterial growth rates were obtained with distinct phoP mutant alleles irrespective of the genetic background (data not shown). Microscopic analysis of infected NRK-49F fibroblasts confirmed the kinetics data and showed that the absence of either PhoP or PhoQ regulatory protein caused exacerbated intracellular bacterial proliferation (Fig. 1B). Despite the marked difference in the intracellular growth rate between HeLa epithelial cells and NRK-49F fibroblasts (45), the phenomenon of massive release of vesicles containing LPS occurs to the same extent in the two cell types (Fig. 1B) (24). Nonetheless, the vast amount of LPS observed in the cytosol of wt-infected NRK-49F fibroblasts might also derive in part from bacteria that are degraded by the fibroblast. Indeed, our previous electron microscopy analysis demonstrated the occurrence of bacterial degradation within NRK-49F fibroblasts (45). We then reasoned whether the capacity of phoP or phoQ mutants to proliferate extensively within NRK-49F fibroblasts was associated with avoidance of bacterial degradation by altering the intracellular trafficking route. Confocal immunofluorescence microscopy demonstrated that intracellular wt and phoP bacteria are enclosed in compartments containing LGPs irrespective of the extent of bacterial growth (Fig. 2). Moreover, the accessibility of fluid endocytic tracers as dextran sulfate to the phagosome remains blocked in wt- or phoP or phoQ mutant-infected fibroblasts (data not shown). These observations agree essentially with the data previously obtained in the epithelial cell infection model (22). Altogether, these results prove that the PhoP-PhoQ two-component regulatory system is required to attenuate intracellular bacterial growth within fibroblast cells. However, this role is apparently not exerted by modulating processes such as the secretion of LPS vesicles (24), the targeting of bacteria to LGP-containing compartments (22, 25), or the disconnection of the phagosome with the endocytic route (22).
FIG. 1.
The PhoP-PhoQ two-component regulatory system is required to attenuate intracellular growth within fibroblasts. (A) Intracellular growth rates (Ipro values) of wt (SL1344), phoP7953::Tn10 (SV4056), and phoQ5996::Tn10 (SV4365) serovar Typhimurium strains in NRK-49F fibroblasts and HeLa epithelial cells. Strains harboring the pEG5433 plasmid containing the phoPQ locus are marked as phoP/phoPQ+. Data are representative of a minimum of five experiments. (B) Absence of attenuating growth response in intracellular phoP and phoQ mutants. NRK-49F fibroblast cells were fixed at 24 h postinfection and processed for immunofluorescence microscopy as described elsewhere (45). Primary antibody was monoclonal mouse IgG antibody MLK33 anti-serovar Typhimurium LPS. Secondary antibody was goat anti-mouse IgG conjugated to Texas Red. DAPI (1 μg ml−1) was added to stain nuclei. Arrows indicate two intracellular wt bacteria that do not exhibit massive proliferation. Bar, 10 μm.
FIG. 2.
wt and phoP bacteria are equally targeted to LGP-containing vacuoles of NRK-49F fibroblasts. Infected NRK-49F cells were fixed at 24 h postinfection and processed for confocal immunofluorescence microscopy as described elsewhere (45). Primary antibodies were mouse monoclonal antibody MLK33 anti-serovar Typhimurium LPS and rabbit polyclonal C38 antibody anti-rat LGP. Secondary antibodies were anti-mouse IgG conjugated to fluorescein isothiocyanate and anti-rabbit IgG conjugated to Texas Red. Arrows show examples of colocalization of bacteria with the late endosome-lysosomal marker. Bar, 5 μm.
The virulence transcriptional regulators RpoS, SlyA, and SpvR mediate attenuation of intracellular bacterial growth in fibroblasts.
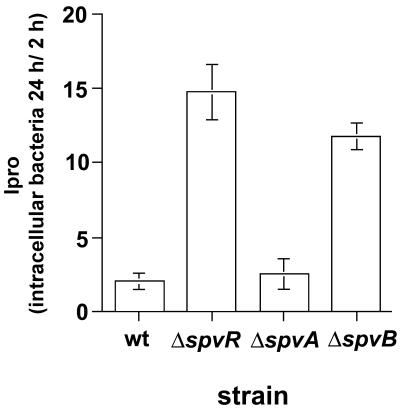
Transductional analysis showed that mutants belonging to the phenotypic class II (Ipro values 6- to 13-fold above those of the wt [Table 2]), except strain SV4215, harbored independent MudJ insertions 100% linked to overgrowth phenotype. The only exception was represented by strain SV4215 (Table 2; also see below). Sequencing the chromosomal regions flanking the MudJ in these mutants showed that the MudJ was inserted in the slyA, rpoS, spvA, and spvR genes (Table 2). Four distinct insertions were mapped in the spvA gene (strains SV4221, SV4234, SV4312, and SV4313), two were mapped in the rpoS gene (strains SV4210 and SV4220), and one was mapped in the slyA and spvR genes (strains SV4198 and SV4314, respectively) (Table 2). All these genes, except spvA, have been shown previously to be essential for Salmonella responses linked to resistance to macrophage attack (slyA and rpoS) and intracellular proliferation in vivo (spvR) (5, 12, 50, 74). SlyA, the alternative sigma factor RpoS, and SpvR are all transcriptional regulators (9). To confirm the involvement of these factors in attenuation of bacterial growth rate in fibroblasts, the Ipro value in fibroblasts was calculated for SL1344 derivatives carrying previously characterized slyA or rpoS mutant alleles (35, 42, 53). All mutant strains behaved like the isolates selected for overgrowth in NRK-49F fibroblasts (Table 3). Moreover, a series of in-frame nonpolar deletion mutations in the spvR, spvA, and spvB genes was used to define in more detail the involvement of the proteins encoded by these genes in the growth-attenuating response. The ΔspvR and ΔspvB mutants, but not the ΔspvA mutant, displayed a higher Ipro than did the wt (Fig. 3). These results are consistent with the role in virulence previously assigned to SpvB. Furthermore, they indicate that SpvB is essential to repress bacterial growth within fibroblasts and suggest that the phenotype of the spvA::MudJ mutations selected in the overgrowing mutants SV4221, SV4234, SV4312, and SV4313 is likely due to polar effects on the expression of downstream genes. Altogether, these experiments validate our selection method and demonstrate that the virulence-related regulators SlyA, RpoS, and SpvR, as well as the SpvB protein, play a role in the attenuation of bacterial growth within fibroblasts. It is relevant that, unlike the epithelial or macrophage infection models, the fibroblast infection system provides a discernible phenotype for serovar Typhimurium spv mutants.
TABLE 3.
Intracellular growth rates of S. enterica serovar Typhimurium mutants harboring defined slyA, rpoS, and spvA mutations
| Strain | Genotypea | Iprob
|
|
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| SL1344 | Wild type | 1.0 ± 0.05 | 3.4 ± 0.6 |
| SV4056 | phoP7953::Tn10 | 26.0 ± 2.4 | 32.0 ± 4.9 |
| SMS438 | rpoS::Ω-Ampr | 11.2 ± 1.9 | |
| SV4240 | rpoS::Ω-AmprphoP7953::Tn10 | 23.0 ± 1.1 | |
| SV4250 | slyA::pRR10ΔtrfA Penr | 12.0 ± 2.3 | |
| SV4251 | slyA::pRR10ΔtrfA PenrphoP7953::Tn10 | 26.0 ± 5.5 | |
| SV4257 | spvA::Kmr | 25.5 ± 1.7 | |
| SV4258 | spvA::KmrphoP7953::Tn10 | 33.0 ± 3.6 | |
All mutant strains are isogenic with strain SL1344.
Shown are means ± standard deviations of two representative experiments. The assay was repeated a minimum of three times for each strain.
FIG. 3.
SpvR and SpvB proteins are involved in attenuation of intracellular growth within NRK-49F fibroblasts. Shown are the Ipro values at 24 h postinfection for wt (SR11 and χ3306) and the in-frame nonpolar deletion mutant derivatives UF082 (ΔspvR), UF104 (ΔspvA), and UF051 (ΔspvB). Bars represent means ± standard deviations of a representative experiment from a total of three repetitions.
As the Ipro values of slyA, rpoS, and spvR regulatory mutants were lower than those of phoP or phoQ mutants, we investigated whether the function of these proteins as negative regulators of bacterial intracellular growth was dependent on the PhoP-PhoQ system. If such an association existed, double mutants carrying a nonfunctional PhoP-PhoQ system and a deficiency in any of the other regulators would display the same intracellular growth phenotype, e.g., similar Ipro values. The intracellular growth rates of phoP slyA, phoP rpoS, and phoP spvR double mutants were then determined (Table 3). No additive effect on Ipro value was observed for any of the double mutants. These results suggest that the role of SlyA, RpoS, and SpvR regulators related to intracellular growth attenuation might be under the control of the PhoP-PhoQ two-component system.
A novel gene, igaA, participates in the growth attenuation response within fibroblasts.
Strain SV4215, which belongs to class II of the overgrowth mutants with intermediate Ipro values in NRK-49F fibroblasts, failed to show genetic linkage between the overgrowth phenotype and the MudJ insertion: transduction of the MudJ insertion to the wt resulted in transductants with wt Ipro values (strain SV4431 [Table 2]). Interestingly, the SV4215 mutant was mucoid on plates. To determine whether the mucoidy phenotype was linked to the ability to overgrow in fibroblasts, strain SV4215 was mutagenized with Tn10dTet. Six pools, each of 5,000 Tetr colonies, were then prepared and lysed with P22. These lysates were used to transduce the wt, selecting Tetr resistance. Mucoid colonies were then sought among the Tetr transductants. One Tetr mucoid transductant, propagated as strain SV4254, proved to retain the high Ipro value of the parental strain SV4215 (Table 2), indicating that mucoidy and loss of attenuation of intracellular growth were linked. To rule out the possibility that the cotransducible Tn10dTet insertion (hereafter zhf-6311::Tn10dTet) had an effect on intracellular growth, a nonmucoid strain carrying the zhf-6311::Tn10dTet insertion (SV4255) was also used to infect NRK-49F fibroblasts. This strain showed an Ipro value similar to that of the wt (Table 2), confirming that the zhf-6311::Tn10dTet insertion had no effect on intracellular proliferation. The cotransduction frequency between mucoidy and the zhf-6311::Tn10dTet insertion was around 0.35, suggesting that the locus controlling capsule production and the zhf-6311::Tn10dTet insertion were 12 to 14 kb apart. Mapping with locked-in P22 prophages (1) indicated that zhf-6311::Tn10dTet was located at centisome 75 on the chromosome of serovar Typhimurium, near cysG. Three-factor crosses indicated that the locus under study, named igaA for intracellular growth attenuator, mapped near mrcA. The gene order was aroB-mcrA-igaA. An open reading frame homologous to yrfF of Escherichia coli (80% identity, 89% homology) and to umoB of Proteus mirabilis (39% identity, 57% homology) exists at this position. While the biological function of YrfF has not been investigated in E. coli, the UmoB protein has been shown previously to modulate expression of flhDC, which controls the swarming phenotype in P. mirabilis (7). Sequencing of PCR products of the igaA region from SL1344 (wt), SV4215, and SV4254 strains showed that the YrfF-UmoB-related open reading frame of strains SV4215 and SV4254 harbored a point mutation causing an amino acid substitution of R (arginine) for H (histidine) at position 188 of the putative IgaA protein. Attempts to construct igaA-null mutants were unsuccessful (data not shown), suggesting that igaA may be an essential gene. If such is the case, the igaA188 mutation must not be null. As in the tests performed with SlyA, RpoS, and SpvR regulators, the possibility of a functional relationship between IgaA and PhoP-PhoQ was tested. A phoP igaA double mutant displayed an Ipro value similar to that of a phoP mutant (data not shown), suggesting that the IgaA function might belong to the same proliferation control pathway as the PhoP-PhoQ system. This view is supported by the observation that, unlike SV4255 (zhf-6311::Tn10dTet), the strain SV4254 (igaA188 zhf-6311::Tn10dTet) is attenuated for virulence in the murine typhoid model (unpublished observations). Altogether, these observations provide evidence that the IgaA function is involved in negative control of bacterial proliferation within NRK-49F fibroblasts.
Specificity of the response involved in attenuating pathogen growth rate.
Although 40 to 50 genes have been shown elsewhere to be required for Salmonella pathogenesis (30), our selection procedure produced only a very small subset of virulence proteins. To determine whether additional regulators were involved in the attenuation of the bacterial growth rate within fibroblasts, we tested mutants affected in the following regulatory proteins: (i) RpoE, an alternative sigma factor (38); (ii) SpiR (SsrA) and SpiA, a regulator and a type III structural protein, respectively, both encoded in SPI-2 (34, 51); (iii) OmpR, a transcriptional regulator controlling outer membrane protein content (43); (iv) Dam, a DNA-adenine methylase required for systemic disease (23, 33); (v) the global regulators Crp, RelA, and Lrp (18); (vi) OxyR and SoxRS, related to oxidative stress (13, 67); (vii) MviA, a regulator required for eliciting systemic disease in mice (66); and (viii) Fnr, which controls switching to anaerobic metabolism. Unlike the mutations in the phoP, phoQ, slyA, rpoS, spvR, and igaA genes, none of the other virulence-related mutations resulted in an Ipro value higher than that of the wt (Table 4). Moreover, when combined with phoP-null mutant alleles, no alteration in the capacity of intracellular bacteria to attenuate growth was detected (Table 4). These results suggest that the growth-attenuating response is under the control of a specific subset of virulence-related regulatory proteins. Even though none of these mutants displayed an overgrowth phenotype, two of them, the spiA and rpoE mutants, were defective for maintenance of viability in the intracellular environment of fibroblasts, with Ipro values in the range of 30% of that of the wt (Table 4). The combination of the mutations producing survival defects (spiA and rpoE) with phoP mutant alleles also caused a marked decrease in the intracellular growth rate. Nonetheless, the Ipro value of the spiA phoP or rpoE phoP double mutants was still 10-fold higher than that showed by single spiA or rpoE mutants (Table 4). These results may indicate that not all the population of intracellular bacteria is affected to the same extent by the fibroblast defense mechanisms. Interestingly, the low Ipro value of the spiA mutant was not reproduced by the spiR mutant, which suggests that the spiR mutant might retain a residual activity of the SPI-2-encoded TTSS in the intracellular environment of fibroblast cells.
TABLE 4.
Role of S. enterica serovar Typhimurium virulence regulators in attenuation of intracellular growth within NRK-49F cells
| Strain | Mutated gene(s)a | Iprob
|
|
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| SL1344 | None (wild type) | 1.0 ± 0.05 | 4.4 ± 0.35 |
| SV4056 | phoP | 26.0 ± 2.4 | 45.0 ± 3.5 |
| GVB311 | rpoE | 0.3 ± 0.1 | |
| SV4232 | rpoE phoP | 2.8 ± 1.1 | |
| SV4237 | spiR | 1.6 ± 0.25 | |
| SV4241 | spiR phoP | 25.9 ± 0.9 | |
| SV4386 | spiA | 0.38 ± 0.13 | |
| SV4387 | spiA phoP | 3.5 ± 0.8 | |
| SV4235 | ompR | 4.8 ± 0.9 | |
| SV4236 | ompR phoP | 35.0 ± 3.5 | |
| SV1610 | dam | 4.5 ± 0.9 | |
| SV4089 | dam phoP | 36.2 ± 5.0 | |
| SMS521 | crp | 1.05 ± 0.2 | |
| SV4284 | crp phoP | 15.9 ± 2.3 | |
| ST97 | relA | 1.1 ± 0.3 | |
| SV4285 | relA phoP | 34.6 ± 2.5 | |
| SV4253 | lrp | 1.2 ± 0.2 | |
| SV4286 | lrp phoP | 26.6 ± 0.4 | |
| SMS593 | oxyR | 2.3 ± 0.4 | |
| SV4287 | oxyR phoP | 32.8 ± 8.3 | |
| SV4233 | mviA | 2.6 ± 0.4 | |
| SV4288 | mviA phoP | 26.5 ± 10 | |
| SV4261 | soxS | 4.5 ± 1.3 | |
| SV4262 | fnr | 4.4 ± 0.3 | |
All mutant strains are isogenic with strain SL1344.
Shown are means ± standard deviations of two representative experiments. The assay was repeated a minimum of three times for each strain.
DISCUSSION
In this study, we present evidence that a specific subset of S. enterica serovar Typhimurium virulence determinants prevent pathogen overgrowth with cultured normal fibroblasts. This was unexpected, considering that virulence functions are generally associated with promotion of survival and proliferation in the intracellular environment of the host cell. Interestingly, recent data obtained with animal models support the idea that massive intracellular proliferation of serovar Typhimurium occurs predominantly within CD18-expressing phagocytes and not in epithelial cells (31, 46, 58). These reports raise the interesting question of whether, during the infection, the invasion of nonphagocytic cells by S. enterica might be followed by adaptation of the pathogen to a slow-growth or resting state. In this respect, a very recent report by Hernández-Pando et al. has provided evidence by in situ PCR that nonphagocytic cells carry Mycobacterium tuberculosis that remains latent in the lungs of asymptomatic individuals (35a). In this study, it was shown that fibroblasts are the host cell type that exhibits a stronger signal for mycobacterial DNA (35a).
To evaluate the significance of the lack of S. enterica growth within nonphagocytic cells, we reasoned whether the lifestyle of this pathogen within cultured fibroblasts could somehow reflect the growth kinetics of S. enterica in animal tissues. In addition, fibroblasts must be considered a potential host cell type for S. enterica since they are efficiently invaded in vitro by this pathogen and located in anatomical sites close to the infection loci. Though no study has yet proved that S. enterica infects fibroblasts upon penetration of the intestinal epithelium, it is known that fibroblasts exist in the lamina propria (56, 57). These so-called intestinal subepithelial myofibroblasts modulate the mobility, differentiation, and proliferation of epithelial and parenchymal cells by secreting a large number of cytokines, chemokines, growth factors, and other immunomodulatory molecules (56, 57). A nonproliferative intracellular infection of these subepithelial fibroblasts by S. enterica would provide the bacteria with the opportunity to orchestrate the physiology of the intestinal epithelium while remaining masked from recognition by immune cells. As in the case of M. tuberculosis (35a), a latent S. enterica infection of cells with minimal antigen presentation capacity, such as fibroblasts, would perhaps enable the pathogen to persist for a long time. In accord with these postulates, we have obtained preliminary data with the calf ligated loop assay that suggest that the PhoP-PhoQ system might mediate intracellular growth attenuation at the level of the lamina propria (P. Watson and F. Garcia-del Portillo, unpublished observations).
In principle, two non-mutually exclusive processes could explain the attenuation of serovar Typhimurium growth within fibroblasts. On one side, normal fibroblasts, though not considered professional phagocytes, could mount an effective antibacterial response. In this context, Eriksson et al. have proposed that S. enterica triggers nitric oxide (NO) release by macrophages and that mutants which do not stimulate host NO release display an intracellular growth advantage (10). We have observed that NO levels of NRK-49F fibroblast cells increase two- to threefold in response to serovar Typhimurium infection. However, blockage of NO production does not result in an increase of the intracellular growth rate (data not shown). Therefore, unlike the macrophage model, attenuation of intracellular bacterial growth in fibroblasts does not seem to rely, at least entirely, on host NO production.
An alternative hypothesis to explain the absence of massive intracellular proliferation of serovar Typhimurium within fibroblasts is that intracellular bacteria mount a specific response to restrain themselves from massive growth. Regardless of the relative contribution of the fibroblast to attenuation of bacterial growth, a bacterial product(s) can arrest intracellular proliferation. A simple positive selection procedure provided us with overgrowing serovar Typhimurium mutants. The genetic characterization of these isolates revealed that functions previously known to be involved in intramacrophage survival and in vivo intracellular proliferation, such as PhoP-PhoQ, SlyA, SpvR, and RpoS, are essential to down-regulating the Ipro in cultured fibroblasts. To our knowledge, these data represent the first example of attenuation of bacterial overgrowth in the intracellular environment of a nonphagocytic eucaryotic cell that is directed by pathogen factors.
The detailed characterization of the phenotype displayed by individual overgrowing clones permitted us to differentiate two major classes. In the first class, exemplified by phoP and phoQ mutants, a substantial derepression of the intracellular growth rate occurred at 24 h, reaching values up to 20- to 30-fold higher than those for the wt virulent strain. This observation was unexpected, considering that the lack of the PhoP-PhoQ two-component system had been always linked to major intramacrophage survival defects and marked attenuation in the murine typhoid model (30, 52). Our data demonstrate that the absence of either of these two regulatory proteins results in enhanced serovar Typhimurium intracellular proliferation within normal fibroblast cells. The point mutant allele phoQ201 (R313W) isolated in our screening was associated with loss of function for the PhoQ protein, since the overgrowing clone carrying this mutant allele was defective for growth in low-Mg2+ minimal medium and activation of different pag genes (unpublished observations). The phoQ201 mutation maps in the cytosolic transmitter domain of the PhoQ sensor protein. Interestingly, this point mutation does not affect synthesis or stability of the protein, as indicated by Western blotting analysis performed with phoQ-null mutants carrying the phoQ201 allele in a plasmid (data not shown). This result suggests that the PhoQ (R313W) protein, though probably able to receive the extracellular signal, is unable to transmit it to the PhoP transcriptional regulator. How the intracellular growth-attenuating function of the PhoP-PhoQ system is related to Salmonella pathogenesis is at present unknown. One could speculate that, besides its central role in promoting intramacrophage survival, the PhoP-PhoQ system might also prolong residence of intracellular bacteria that have invaded nonprofessional phagocytic cells. Indeed, it has been known for decades that a small percentage of Salmonella infections occurring in livestock and humans end with persistence of bacteria in host tissues, a phenomenon poorly characterized at a cellular or molecular level. Monitoring the phoP or phoQ mutants in intestinal and systemic sites upon oral administration could provide insights into this putative relationship.
Our study also identified other virulence-related regulatory proteins of serovar Typhimurium involved in the negative regulation of intracellular growth rate. Thus, knockout mutants defective in the transcriptional regulator SlyA, RpoS, or SpvR displayed 6- to 13-fold-higher intracellular growth rates than those of the wt strain. These transcriptional regulators have been shown to regulate Salmonella virulence. SlyA, like the PhoP-PhoQ system, is essential for Salmonella to withstand the macrophage antimicrobial attack. RpoS has been shown previously to be required for systemic disease (5). SpvR, which positively regulates the plasmid-encoded spvABCD operon (27), is also needed for systemic disease (41). Interestingly, neither rpoS nor spvR mutants display any intracellular survival defect in cultured macrophages. This lack of an in vitro phenotype agrees with the fact that RpoS positively regulates spvR expression (59). The interconnection between the PhoP-PhoQ system and the SlyA, RpoS, and SpvR regulators is at present undefined, although it has been proposed previously that the PhoP-PhoQ system may control in a positive manner the expression of the spvABCD operon (47). Whether this control is exerted via the SpvR regulator is unknown. Regulation of expression of the spvR gene is very complex, and besides RpoS, other proteins that exert control over spvR include H-NS, Crp, and Lrp as negative regulators and integration host factor (IHF) as a positive regulator. No overgrowing mutant was selected with a defect in the positive regulator IHF, which could indicate that loss of RpoS, but not of IHF, ensures a significant repression of the spvR gene in the intracellular environment of fibroblasts. Deletion of the spvR or spvB genes enhances the Ipro, which suggests that SpvB plays an important role in attenuating growth rate. Though the biological function of Spv proteins remains undefined, the SpvB protein exhibits mono(ADP-ribosyl)transferase activity toward host actin (54, 68). Although further work is required to determine the exact biological function of Spv proteins, our fibroblast model offers an opportunity to address this question. The fact that double mutants lacking phoP and rpoS or slyA or spvR did not display higher proliferation rates than did the single mutants suggests that SlyA, RpoS, and SpvR might be under the control of the PhoP-PhoQ system to attenuate growth within fibroblasts.
An additional mutant having a defect in a previously unknown protein of S. enterica was also selected as an overgrowing clone. This protein, which we named IgaA for intracellular growth attenuator, is homologous to the E. coli YrfF and P. mirabilis UmoB proteins. Whereas the biological function of YrfF is currently unknown, UmoB has been shown elsewhere to act as a positive regulator of FlhDC, the master regulator of flagella and swarming (7). More recently, FlhDC has been shown to repress cell division during P. mirabilis swarming, suggesting that UmoB could repress cell division via FlhDC. This biological function, if maintained in S. enterica, could sustain a putative negative control of cell division and growth exerted by IgaA in intracellular bacteria. Unlike the phoQ201 (R313W) point mutant allele, the igaA188 (R188H) mutant allele must confer a partial loss of function, since IgaA is an essential protein in a wt genetic background in laboratory conditions. The analysis of IgaA secondary structure predicts that it might be a membrane protein with at least four transmembrane domains. As the igaA188 point mutant overexpresses capsule material, there is likely an interconnection between IgaA function and structural (cps) or regulatory (rcsA and rcsCB) genes involved in capsule synthesis. It would be also interesting to link IgaA function with the PhoP-PhoQ two-component regulatory system.
Unlike the strains defective in the other regulators, the rpoE and spiA mutants exhibited a survival defect within fibroblasts, retaining only 30% viability as shown in comparison with the wt strain. Our data are consistent with the survival defect previously reported for rpoE mutants in epithelial cells and macrophages (38) and the loss of viability of spiA in macrophages. Likewise, our observations imply that fibroblasts might harbor antimicrobial activities analogous to some extent to the variety of macrophage defenses. Interestingly, in contrast to the spiA mutant, which does not have a functional SPI-2 TTSS, the spiR SPI-2 regulatory mutant showed the same survival rate as did the wt strain. This difference suggests that this spiR mutant may have residual activity of the SPI-2 TTSS, which might be sufficient to maintain viability. Similar differences in intracellular viability rates have been found in cultured macrophages (E. A. Groisman, unpublished observations).
An interesting characteristic of the response described in this study is that it relies on the function of a specific subset of virulence regulators. Thus, S. enterica virulence regulators, such as the OmpR-EnvZ two-component system, the Dam methylase, Crp, Cya, and MviA, among others, are dispensable for attenuation of the intracellular growth rate within fibroblasts. These differences illustrate the tremendous variety of intracellular responses that pathogens can trigger depending on distinct environmental cues present in specific host cell types.
A final question that arises from our observations is how to explain the lack of virulence shown in animal models by the class I and class II overgrowing mutants (phoQ, slyA, spvR, rpoS, and igaA). Whereas decreased bacterial survival and/or proliferation in cultured macrophages is primarily associated with attenuation (e.g., phoPQ and slyA mutants [71, 75]), massive proliferation within nonphagocytic cells could also result in a suicide strategy for these bacteria. Thus, lysis of an infected nonphagocytic cell due to intracellular bacterial overgrowth may lead to bacterial release into extracellular locations. These extracellular bacteria can be subsequently ingested by nearby activated phagocytic cells, in which these bacterial mutants rapidly lose viability. Thus, a combination of growth attenuation (via PhoP-PhoQ, SlyA, RpoS, and SpvR) and viability maintenance (via RpoE and SPI-2 TTSS) might provide the pathogen with the wherewithal to adapt to a long-persistence state within the infected nonphagocytic cell.
ACKNOWLEDGMENTS
We greatly appreciate the generosity of Paul Gulig, Stephen J. Libby, Ferric C. Fang, Mark Roberts, Gordon Dougan, Michael P. Spector, and David Holden for sending strains. We also thank James M. Slauch and I. Sandoval for providing antibodies; Nuria Gómez-López for her assistance in the PCR, phoPQ and igaA locus sequencing, and direct chromosomal sequencing of MudJ insertion sites; Amparo Haro for the confocal microscopy analysis; and Felix Solomon for strain construction.
This work was supported by grants from the Ministerio de Educación y Cultura of Spain (PM97-0148-C02), Comunidad de Madrid (08.2/0045.1/2000), European Union (QLK2-1999-00310), and North Atlantic Treaty Organization (NATO) (CRG971613). E.A.G. is an Associate Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Benson N R, Goldman B S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowe F, Lipps C J, Tsolis R M, Groisman E, Heffron F, Kusters J G. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect Immun. 1998;66:3372–3377. doi: 10.1128/iai.66.7.3372-3377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumell J H, Rosenberger C M, Gotto G T, Marcus S L, Finlay B B. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 2001;3:75–84. doi: 10.1046/j.1462-5822.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 5.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufour A, Furness R B, Hughes C. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol Microbiol. 1998;29:741–751. doi: 10.1046/j.1365-2958.1998.00967.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap N E, Benjamin W H, Berry A K, Eldridge J H, Briles D E. A ‘safe-site’ for Salmonella typhimurium is within splenic polymorphonuclear cells. Microb Pathog. 1992;13:181–190. doi: 10.1016/0882-4010(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 9.El-Gedaily A, Paesold G, Krause M. Expression profile and subcellular location of the plasmid-encoded virulence (Spv) proteins in wild-type Salmonella dublin. Infect Immun. 1997;65:3406–3411. doi: 10.1128/iai.65.8.3406-3411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson S, Bjorkman J, Borg S, Syk A, Pettersson S, Andersson D I, Rhen M. Salmonella typhimurium mutants that downregulate phagocyte nitric oxide production. Cell Microbiol. 2000;2:239–250. doi: 10.1046/j.1462-5822.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- 11.Ernst R K, Guina T, Miller S I. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J Infect Dis. 1999;179:S326–S330. doi: 10.1086/513850. [DOI] [PubMed] [Google Scholar]
- 12.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang F C, Vazquez-Torres A, Xu Y. The transcriptional regulator SoxS is required for resistance of Salmonella typhimurium to paraquat but not for virulence in mice. Infect Immun. 1997;65:5371–5375. doi: 10.1128/iai.65.12.5371-5375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 15.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay B B, Brumell J H. Salmonella interactions with host cells: in vitro to in vivo. Philos Trans R Soc Lond B Biol Sci. 2000;355:623–631. doi: 10.1098/rstb.2000.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay B B, Chatfield S, Leung K Y, Dougan G, Falkow S. Characterization of a Salmonella choleraesuis mutant that cannot multiply within epithelial cells. Can J Microbiol. 1991;37:568–572. doi: 10.1139/m91-095. [DOI] [PubMed] [Google Scholar]
- 18.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 19.Galan J E, Curtiss R. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galan J E, Zhou D. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc Natl Acad Sci USA. 2000;97:8754–8761. doi: 10.1073/pnas.97.16.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-del Portillo F, Finlay B B. Invasion and intracellular proliferation of Salmonella within non-phagocytic cells. Microbiologia. 1994;10:229–238. [PubMed] [Google Scholar]
- 22.Garcia-del Portillo F, Finlay B B. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J Cell Biol. 1995;129:81–97. doi: 10.1083/jcb.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Del Portillo F, Pucciarelli M G, Casadesus J. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci USA. 1999;96:11578–11583. doi: 10.1073/pnas.96.20.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-del Portillo F, Stein M A, Finlay B B. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect Immun. 1997;65:24–34. doi: 10.1128/iai.65.1.24-34.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-del Portillo F, Zwick M B, Leung K Y, Finlay B B. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci USA. 1993;90:10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Vescovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 27.Grob P, Kahn D, Guiney D G. Mutational characterization of promoter regions recognized by the Salmonella dublin virulence plasmid regulatory protein SpvR. J Bacteriol. 1997;179:5398–5406. doi: 10.1128/jb.179.17.5398-5406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groisman E A. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groisman E A, Chiao E, Lipps C J, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groisman E A, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5:343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 31.Gulig P A, Doyle T J, Hughes J A, Matsui H. Analysis of host cells associated with the Spv-mediated increased intracellular growth rate of Salmonella typhimurium in mice. Infect Immun. 1998;66:2471–2485. doi: 10.1128/iai.66.6.2471-2485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy R L, Gonias L A, Stein M A. Aggregation of host endosomes by Salmonella requires SPI2 translocation of SseFG and involves SpvR and the fms-aroE intragenic region. Mol Microbiol. 2000;37:1417–1435. doi: 10.1046/j.1365-2958.2000.02092.x. [DOI] [PubMed] [Google Scholar]
- 33.Heithoff D M, Sinsheimer R L, Low D A, Mahan M J. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 34.Hensel M, Nikolaus T, Egelseer C. Molecular and functional analysis indicates a mosaic structure of Salmonella pathogenicity island 2. Mol Microbiol. 1999;31:489–498. doi: 10.1046/j.1365-2958.1999.01190.x. [DOI] [PubMed] [Google Scholar]
- 35.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 35a.Hernández-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, Rook G A W, Bjune G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–2137. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 36.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 37.Hughes K T, Roth J R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988;119:9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphreys S, Stevenson A, Bacon A, Weinhardt A B, Roberts M. The alternative sigma factor, ςE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan S A, Everest P, Servos S, Foxwell N, Zahringer U, Brade H, Rietschel E T, Dougan G, Charles I G, Maskell D J. A lethal role for lipid A in Salmonella infections. Mol Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 40.Leung K Y, Finlay B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby S J, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libby S J, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang F C, Guiney D G, Songer J G, Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci USA. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low K B, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin S L, Luo X, Miller S I, Zheng L, King I, Pawelek J M, Bermudes D. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Moya M, de Pedro M A, Schwarz H, Garcia-del Portillo F. Inhibition of Salmonella intracellular proliferation by non-phagocytic eucaryotic cells. Res Microbiol. 1998;149:309–318. doi: 10.1016/s0923-2508(98)80436-1. [DOI] [PubMed] [Google Scholar]
- 46.Matsui H, Eguchi M, Kikuchi Y. Use of confocal microscopy to detect Salmonella typhimurium within host cells associated with Spv-mediated intracellular proliferation. Microb Pathog. 2000;29:53–59. doi: 10.1006/mpat.2000.0370. [DOI] [PubMed] [Google Scholar]
- 47.Matsui H, Kawakami T, Ishikawa S, Danbara H, Gulig P A. Constitutively expressed phoP inhibits mouse-virulence of Salmonella typhimurium in an Spv-dependent manner. Microbiol Immunol. 2000;44:447–454. doi: 10.1111/j.1348-0421.2000.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 48.Meresse S, Steele-Mortimer O, Finlay B B, Gorvel J P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills S D, Ruschkowski S R, Stein M A, Finlay B B. Trafficking of porin-deficient Salmonella typhimurium mutants inside HeLa cells: ompR and envZ mutants are defective for the formation of Salmonella-induced filaments. Infect Immun. 1998;66:1806–1811. doi: 10.1128/iai.66.4.1806-1811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohl M E, Miller S I. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- 53.O'Neal C R, Gabriel W M, Turk A K, Libby S J, Fang F C, Spector M P. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J Bacteriol. 1994;176:4610–4616. doi: 10.1128/jb.176.15.4610-4616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otto H, Tezcan-Merdol D, Girisch R, Haag F, Rhen M, Koch-Nolte F. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol Microbiol. 2000;37:1106–1115. doi: 10.1046/j.1365-2958.2000.02064.x. [DOI] [PubMed] [Google Scholar]
- 55.Pawelek J M, Low K B, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 56.Powell D W, Mifflin R C, Valentich J D, Crowe S E, Saada J I, West A B. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 57.Powell D W, Mifflin R C, Valentich J D, Crowe S E, Saada J I, West A B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 58.Richter-Dahlfors A, Buchan A M, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbe-Saule V, Schaeffer F, Kowarz L, Norel F. Relationships between H-NS, sigma S, SpvR and growth phase in the control of spvR, the regulatory gene of the Salmonella plasmid virulence operon. Mol Gen Genet. 1997;256:333–347. doi: 10.1007/s004380050577. [DOI] [PubMed] [Google Scholar]
- 60.Rudd K E, Bochner B R, Cashel M, Roth J R. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J Bacteriol. 1985;163:534–542. doi: 10.1128/jb.163.2.534-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seymour R L, Mishra P V, Khan M A, Spector M P. Essential roles of core starvation-stress response loci in carbon-starvation-inducible cross-resistance and hydrogen peroxide-inducible adaptive resistance to oxidative challenge in Salmonella typhimurium. Mol Microbiol. 1996;20:497–505. doi: 10.1046/j.1365-2958.1996.5451068.x. [DOI] [PubMed] [Google Scholar]
- 62.Shea J E, Beuzon C R, Gleeson C, Mundy R, Holden D W. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanley T L, Ellermeier C D, Slauch J M. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J Bacteriol. 2000;182:4406–4413. doi: 10.1128/jb.182.16.4406-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steele-Mortimer O, Meresse S, Gorvel J P, Toh B H, Finlay B B. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 65.Stein M A, Leung K Y, Zwick M, Garcia-del Portillo F, Finlay B B. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol Microbiol. 1996;20:151–164. doi: 10.1111/j.1365-2958.1996.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 66.Swords W E, Giddings A, Benjamin W H. Bacterial phenotypes mediated by mviA and their relationship to the mouse virulence of Salmonella typhimurium. Microb Pathog. 1997;22:353–362. doi: 10.1006/mpat.1996.0117. [DOI] [PubMed] [Google Scholar]
- 67.Taylor P D, Inchley C J, Gallagher M P. The Salmonella typhimurium AhpC polypeptide is not essential for virulence in BALB/c mice but is recognized as an antigen during infection. Infect Immun. 1998;66:3208–3217. doi: 10.1128/iai.66.7.3208-3217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tezcan-Merdol D, Nyman T, Lindberg U, Haag F, Koch-Nolte F, Rhen M. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol Microbiol. 2001;39:606–619. doi: 10.1046/j.1365-2958.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- 69.Uchiya K, Barbieri M A, Funato K, Shah A H, Stahl P D, Groisman E A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valentine P J, Devore B P, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun. 1998;66:3378–3383. doi: 10.1128/iai.66.7.3378-3383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.VanCott J L, Chatfield S N, Roberts M, Hone D M, Hohmann E L, Pascual D W, Yamamoto M, Kiyono H, McGhee J R. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 72.Vazquez-Torres A, Fang F C. Cellular routes of invasion by enteropathogens. Curr Opin Microbiol. 2000;3:54–59. doi: 10.1016/s1369-5274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- 73.Vazquez-Torres A, Fang F C. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 2001;9:29–33. doi: 10.1016/s0966-842x(00)01897-7. [DOI] [PubMed] [Google Scholar]
- 74.Watson P R, Paulin S M, Bland A P, Libby S J, Jones P W, Wallis T S. Differential regulation of enteric and systemic salmonellosis by slyA. Infect Immun. 1999;67:4950–4954. doi: 10.1128/iai.67.9.4950-4954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wick M J, Harding C V, Twesten N J, Normark S J, Pfeifer J D. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol Microbiol. 1995;16:465–476. doi: 10.1111/j.1365-2958.1995.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 76.Wilson J A, Gulig P A. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential-phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology. 1998;144:1823–1833. doi: 10.1099/00221287-144-7-1823. [DOI] [PubMed] [Google Scholar]