Abstract
Post‐acute sequelae of COVID‐19 (PASC) are long‐term consequences of SARS‐CoV‐2 infection that can substantially impair the quality of life. Underlying mechanisms ranging from persistent viruses to innate and adaptive immune dysregulation have been discussed. Here, we profiled the plasma of 181 individuals from the cohort study for digital health research in Germany (DigiHero), including individuals after mild to moderate COVID‐19 with or without PASC and uninfected controls. We focused on soluble factors related to monocyte/macrophage biology and on circulating SARS‐CoV‐2 spike (S1) protein as a potential biomarker for persistent viral reservoirs. At a median time of 8 months after infection, we found pronounced dysregulation in almost all tested soluble factors, including both pro‐inflammatory and pro‐fibrotic cytokines. These immunological perturbations were remarkably independent of ongoing PASC symptoms per se, but further correlation and regression analyses suggested PASC‐specific patterns involving CCL2/MCP‐1 and IL‐8 that either correlated with sCD162, sCD206/MMR, IFN‐α2, IL‐17A and IL‐33, or IL‐18 and IL‐23. None of the analyzed factors correlated with the detectability or levels of circulating S1, indicating that this represents an independent subset of patients with PASC. These data confirm prior evidence of immune dysregulation and persistence of viral protein in PASC and illustrate its biological heterogeneity that still awaits correlation with clinically defined PASC subtypes.
Keywords: cytokine/chemokine, COVID‐19, long COVID, macrophage, monocyte, SARS coronavirus
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) caused by the zoonotic severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a systemic multiorgan disease with a broad severity spectrum ranging from asymptomatic to fatal outcomes, especially in risk groups. 1 , 2 While most individuals mount lasting SARS‐CoV‐2‐directed immune responses 3 and the rapid development of effective and safe vaccines helped to prevent severe disease courses and mitigate the pandemic progression, it is now clear that a substantial proportion of SARS‐CoV‐2‐infected individuals do not fully recover but has persisting health impairments beyond 4 weeks of symptom onset that can last for months and significantly impact the quality of life. 4 , 5 These post‐acute sequelae of COVID‐19 (PASC), or post‐COVID‐19 conditions as suggested by the World Health Organization (WHO), are reported in 12.7%–87% of patients and encompass a wide range of systemic, respiratory, neuropsychiatric, and cardiac manifestations including fatigue, head and body aches, memory defects, dyspnea, palpitations as well as sleep and anxiety disorders. 4 , 5 , 6 , 7 , 8 , 9 Pre‐existing comorbidities like obesity and diabetes, as well as age and severity of acute disease, might represent risk factors, but lasting symptoms are also common among young individuals with mild disease courses and after vaccination. 10 , 11
While the epidemiological and clinical characterization of PASC is relatively advanced, mechanistic insights into the pathophysiological underpinnings of this condition are still limited. A potential trigger of ongoing sequelae is persistent immunogenic viral reservoirs. SARS‐CoV‐2 RNA and spike or other proteins that might fuel ongoing and generate de‐novo SARS‐CoV‐2‐specific or superantigenic T cell responses 10 , 12 , 13 , 14 have been detected in the respiratory tract, the gut, the brain, the kidney, and circulating in the blood months after acute disease. 1 , 15 , 16 , 17 These findings might also mirror unrepaired virus‐induced tissue damage that could account for some of the organ‐specific symptoms in PASC. 5 , 18 , 19 , 20 Autoimmunity represents another potential driver of PASC. Adaptive immune responses during acute COVID‐19 show imprints of autoreactivity and are characterized by the production of a variety of different autoantibodies that are also found in post‐acute phases and in the SARS‐CoV‐2‐induced postinfection multisystem inflammatory syndrome in children (MIS‐C). 6 , 21 , 22 , 23 , 24 In addition, dysbiosis of the microbiome that either result in the persisting production of inflammatory mediators like lipopolysaccharides that might promote inflammation or the long‐term depletion of anti‐inflammatory modulators is discussed. 25 , 26
We recently reported persisting elevation of a triad of monocyte/macrophage‐related cytokines—interleukin‐1β (IL‐1β), IL‐6, and tumor necrosis factor (TNF) 8–10 months after SARS‐CoV‐2 infection in patients with PASC. 6 We hypothesized that these cytokines are secreted by tissue‐resident macrophages that engage into a self‐sustaining pro‐inflammatory loop that may fuel PASC. Such macrophage imprinting has been previously reported to be potentially induced through engulfment of spike protein by tissue‐resident macrophage in early disease phases. 27 , 28
Since these data corroborate the role of classical pro‐inflammatory monocytes/macrophages in PASC, we designed a refined liquid biomarker panel with more focus on nonclassical pro‐fibrotic monocytes/macrophages as well as monocyte/macrophage–T cell interactions to be run on biosamples from the DigiHero study cohort. These analyses also addressed the relation of these markers to persisting viral antigens as a potential trigger for systemic dysregulation. Our data illustrate the pronounced dysregulation of monocyte/macrophage‐related Type 1 and/or Type 2 cytokines in some individuals with PASC and the long‐term circulation of spike protein in others. Together, these data further refine the molecular underpinnings of PASC and suggest the existence of different PASC subtypes.
2. MATERIALS AND METHODS
2.1. The population‐based cohort study for digital health research in Germany (DigiHero)
The here analyzed individuals essentially reflect the discovery cohort of the COVID‐19 module of the DigiHero study. 6 This subcohort encompasses 181 participants from the DigiHero discovery cohort who were interviewed with an online questionnaire on the clinical course of their SARS‐CoV‐2 infection, its postinfection sequelae, and SARS‐CoV‐2 vaccination status. Interviews and blood sampling were performed until 9th of October 2021. The study was approved by the institutional review board (approval numbers 2020‐076) and was conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed written consent was obtained from all participants or legal representatives. Plasma samples were isolated by centrifugation of whole blood for 15 min at 2000g, followed by centrifugation at 12,000g for 10 min. Samples were stored at −80°C until further use.
2.2. Biological samples and data from the biobank of the Halle COVID cohort (HACO)
Plasma samples from acute nonhospitalized COVID‐19 (n = 15 mild to moderate severity) were used as a control group. Included patients were 18 years or older and had self‐reported symptoms for maximum of 4 weeks and a positive antigen or antibody test. Samples were collected between April and December 2020, where the ancestral SARS‐CoV‐2 strain was the dominant variant. Informed written consent was obtained and the study was approved by the institutional review board (approval number 2020‐039) and conducted in accordance with the ethical principles stated by the Declaration of Helsinki. The collected plasma samples were isolated as described above.
2.3. Profiling of human plasma for monocyte/macrophage‐related soluble factors, anti‐SARS‐CoV‐2 antibodies, and circulating SARS‐CoV‐2 spike protein
Plasma levels of IL‐5, IL‐9, IL‐17F, IL‐18, IL‐22, IL‐23, IL‐33, and CCL2/MCP‐1 were measured using the respective LEGENDplex capture beads and corresponding detection antibodies from the LEGENDplex Human Inflammation Panel (Cat. No. 740809) and Human Th Panel (Cat. No. 741027) (BioLegend). For quantification of soluble CD206 (MMR), the Human MMR ELISA (RayBiotech, Cat. No. ELH‐MMR) was used, for quantification of soluble CD163, the Human CD163 Quantikine ELISA Kit (R&D Systems, Cat. No. DC1630). Profiling of antibodies directed against the spike (S1) protein and the nucleocapsid protein (NCP) of SARS‐CoV‐2 were performed using the anti‐SARS‐CoV‐2‐ELISA IgG (Cat. No. EI 2606‐9601G) and anti‐SARS‐CoV‐2‐NCP (Cat. No. EI 2606‐9601‐2G) ELISA kits from Euroimmun (Lübeck, Germany). Circulating S1 protein was measured using the RayBio COVID‐19 S‐Protein (S1RBD) ELISA kit (RayBiotech, Cat. No. ELV‐COVID19S1). All kits were used according to the manufacturer's instructions. Read‐out of the LEGENDplex system was performed on a BD FACSCelesta; enzyme‐linked immunosorbent assays (ELISAs) were read on a Tecan Spark Microplate reader.
2.4. Statistical analysis
All bar/dot plots, as well as logistic regression and Spearman rank‐order correlation analysis for plasma levels over time, were generated using GraphPad PRISM 8.3.1 (GraphPad Software). Differences in plasma cytokine levels were studied by unpaired t‐test with Welch's correction and Welch´s ANOVA. Correlations were calculated using the R package corrplot. Ranges of p values are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
3. RESULTS
3.1. Characteristics of post‐acute COVID‐19 cohort
To study post‐COVID‐19 perturbations in monocyte/macrophage‐related soluble factors, we randomly selected 181 individuals from the discovery cohort of DigiHero 6 for profiling. The subcohort consisted of 91 individuals with ongoing PASC at the time of blood sampling (65 females, 26 males), 62 individuals who never reported PASC (26 females, 36 males), and 28 individuals without prior COVID‐19 (17 females, 11 males) (Figure 1A). All participants were recruited until October 2021. Median time from infection to sampling was 8 months for individuals with ongoing PASC (range 1–17 months) and 7.5 months for individuals without PASC (range 4–17 months) (Figure 1B), the median age was 51 for both post‐COVID‐19 groups and 50 for the never COVID‐19 group (Figure 1C). Most individuals in the analyzed groups had received at least one vaccination (73% of individuals without PASC, 77% of individuals with ongoing PASC, and 83% of individuals without prior COVID‐19). All vaccinations were performed post‐COVID‐19 (median 6 months, range 4–12). Notably, postinfection vaccination in this cohort was not associated with the resolution of PASC symptoms, 6 which is in line with recent data. 1 , 11 , 29 , 30
Figure 1.
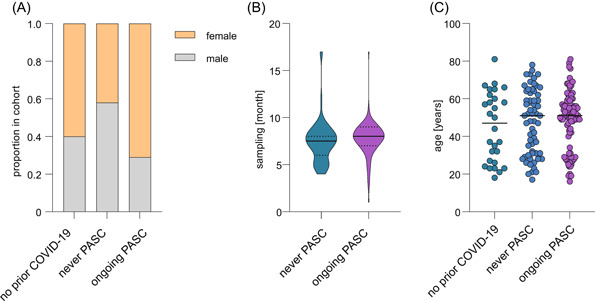
Clinical and epidemiological characteristics of the post‐COVID‐19 cohort. (A) Sex distribution in the analyzed cohort comprised individuals with ongoing PASC (n = 91), individuals with prior COVID‐19 who never reported PASC (n = 62), and individuals without prior COVID‐19 (n = 28). (B) Violin plot of median blood sampling time point (continuous line) relative to positive polymerase chain reaction or antigen test for the post‐COVID‐19 groups. Dotted lines separate quartiles. (C) Median age of indicated groups. PASC, post‐acute sequelae of COVID‐19.
3.2. Plasma soluble factors associated with pro‐inflammatory and pro‐fibrotic macrophages are increased in post‐acute COVID‐19
We selected soluble plasma factors for profiling that are associated with distinct activation states or phenotypes of monocytes/macrophages in COVID‐19. Our panel included both pro‐inflammatory cytokines (e.g., IL‐17, IL‐18, IL‐23) and more pro‐fibrotic cytokines (e.g., IL‐5, IL‐9). 31 , 32 , 33 We also included the shedded forms of two characteristic monocyte/macrophage surface molecules, namely the soluble mannose receptor (sMMR/sCD206/sMRC1) and the soluble haptoglobin‐hemoglobin receptor (sCD163), 34 which are rather associated to macrophages responding to Type 2 cytokines.
We observed markedly increased plasma levels of IL‐5, IL‐9, IL‐17F, IL‐18, IL‐22, IL‐23, IL‐33, CCL2/MCP‐1, and sCD163 but only marginally increased levels for sCD206/sMMR in post‐COVID‐19 disease phases as compared to individuals who never had COVID‐19 (Figure 2). The mean levels of IL‐5, IL‐9, IL‐17F, IL‐22, IL‐23, and IL‐33 tended towards higher values in individuals with ongoing PASC as compared to individuals who never reported PASC, while this trend was reversed for IL‐18 and CCL2/MCP‐1 (Figure 2). Notably, we did not observe sex‐specific patterns.
Figure 2.
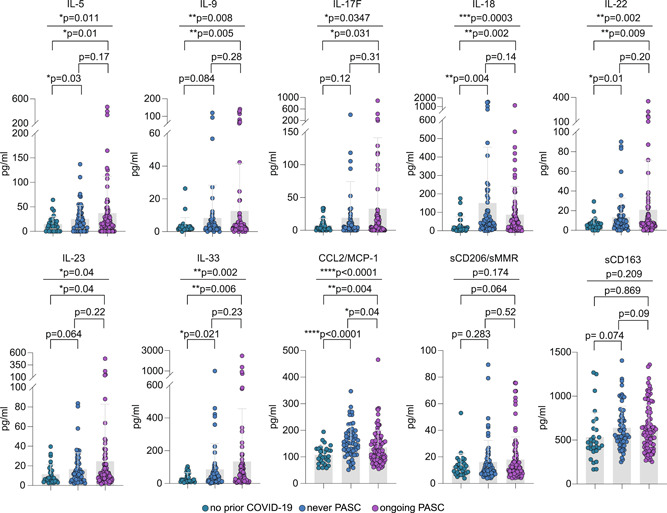
Profiling of plasma monocyte/macrophage‐related soluble factors from individuals with ongoing PASC, without PASC, and without SARS‐COV‐2 infection. Mean plasma cytokine/chemokine/soluble factor levels of individuals with no prior COVID‐19 (n = 28), individuals who never reported PASC postinfection (n = 62), or with ongoing PASC (n = 91). Error bars indicate ±SD. Statistical analysis: Welch's analysis of variance for comparison of all three groups and two‐sided Welch corrected t‐test for comparison of the no prior COVID‐19 versus never PASC, no prior COVID‐19 versus ongoing PASC, and never PASC versus ongoing PASC groups. PASC, post‐acute sequelae of COVID.
To explore potential clusters of dysregulated soluble factors, we performed a large correlation analysis also, including IL‐1β, IL‐4, IL‐6, IL‐8, IL‐13, IL‐17A, TNF, LTA (TNF‐β), and IFN‐α2 from our previously published report since these factors have been found increased in postinfection biosamples. 6 This analysis revealed a characteristic pattern of correlating cytokines in post‐COVID‐19 samples relatively independent of PASC (Figure 3A,B), but not in uninfected individuals (Figure 3C). There were only very few significant correlations that were evident in the PASC setting, but not in post‐COVID‐19 patients without PASC. Two factors that were remarkable in this respect were CCL2/MCP‐1 and IL‐8. Both showed no specific correlations in individuals without prior COVID‐19 or without PASC, but a clear positive correlation with each other and additional factors in individuals with PASC (Figure 3A–C). CCL2/MCP‐1 was strongly correlated with IL‐18 and IL‐23; IL‐8 was correlated with the shedded macrophage molecules sCD206/MMR and sCD163, as well as IL‐17A, IFN‐α2, and IL‐33. This pointed to potential PASC subgroups.
Figure 3.
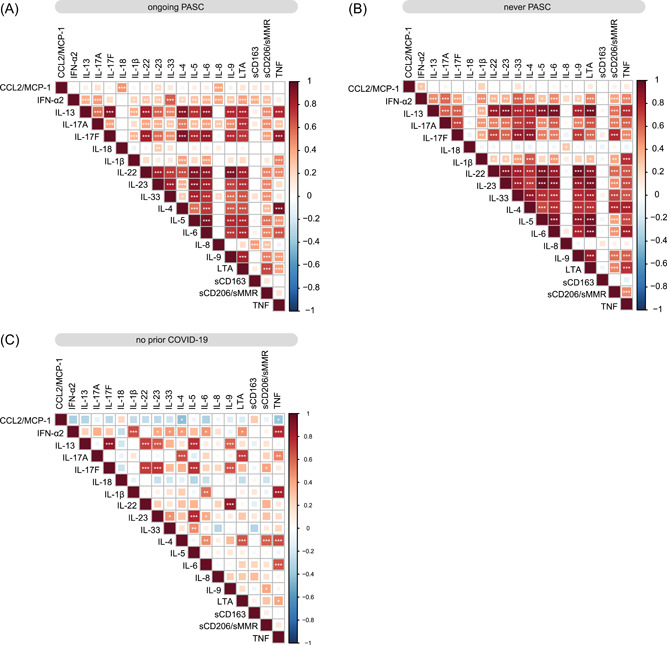
Correlation analysis of plasma soluble factors. (A–C) Correlation matrix of all analyzed soluble factors for individuals with ongoing PASC (n = 91) (A), individuals who never reported PASC postinfection (n = 62) (B), or individuals with no prior COVID‐19 (n = 28) (C). PASC, postacute sequelae of COVID.
3.3. Some perturbations persist longer in patients with PASC compared to individuals without PASC
Recent data suggest that clinical and biological markers decline in most COVID‐19 survivors over time, although with different kinetics. 10 , 14 , 35 , 36 To address this question in our cohort, we asked if individuals with PASC showed slower normalization of the strong perturbations in monocyte/macrophage‐related factors than individuals without PASC. Since no repetitive sampling was performed in the DigiHero cohort, this analysis was restricted to interpatient group comparisons (Figure 1B). To close the gap of early postinfection samples that were unavailable in the DigiHero cohort, we quantified the set of soluble factors in additional plasma samples from individuals with mild to moderate acute COVID‐19 (nine females, six males; median age 68 [range 23–85]; median sampling on Day 15 after symptom onset [range 1–23]) collected as part of the independent Halle COVID‐19 (HACO) cohort. 37 Despite the limitation of this cross‐sectional approach using two cohorts, the selection of these two cohorts from the same pandemic wave, including individuals with similar acute COVID‐19 courses in an outpatient setting and identical sample preparation and quantification, ensured correction for technical confounders as well as selection and survivorship biases. Linear regression and Spearman rank‐order correlation analysis revealed a relatively clear negative correlation between sampling time point and plasma levels for many of the dysregulated soluble factors in individuals without PASC (Figure 4A). In patients with PASC, such over‐time normalization was less evident for some of the measured factors (Figure 4B). While Spearman correlation analysis showed a quite clear negative time correlation for IL‐5 and IL‐17F in individuals without PASC, this was not observed in individuals with PASC (Figure 4B). This suggested that individuals with PASC might show prolonged perturbations of the analyzed soluble factors.
Figure 4.
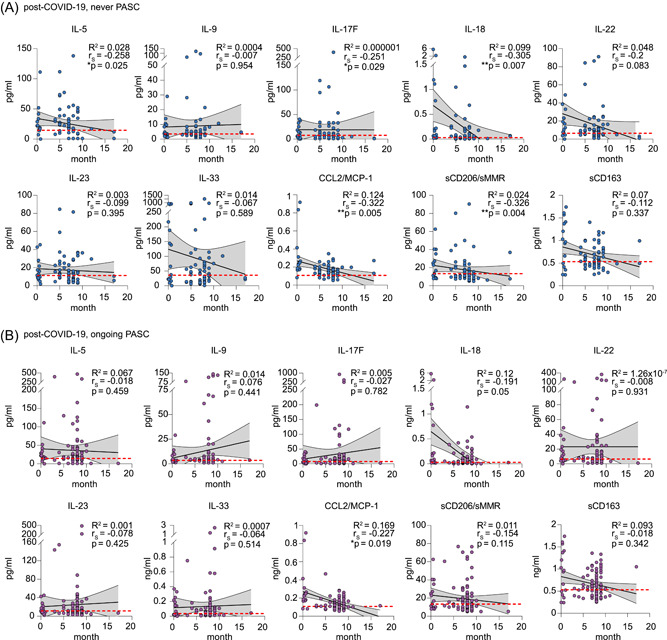
Association of plasma soluble factors with sampling time point postinfection. (A, B) Linear regression of plasma cytokine levels and sampling time point postinfection in individuals without PASC (n = 62) (A) and with ongoing PASC (n = 91) (B). Both cohorts also comprise cytokine data from individuals with mild/moderate acute COVID‐19 (n = 15). The dotted red lines indicate the mean plasma level determined in individuals without prior COVID‐19 (n = 28). Correlation coefficient R 2, Spearman correlation coefficients (r S), and p values are indicated. PASC, post‐acute sequelae of COVID.
3.4. Plasma levels of circulating spike protein are detectable in a substantial proportion of patients after COVID‐19, especially in those with PASC
Persistent immunogenic viral antigens like the SARS‐CoV‐2 S1 spike protein are potential drivers of PASC that might also fuel systemic cytokine and chemokine perturbations. To study this hypothesis, we profiled our cohort for levels of circulating S1. Since the S1 antigen has been detected in plasma after vaccination, 38 , 39 we restricted this analysis to individuals without prior vaccination. Around 35% of individuals with prior COVID‐19 but no PASC showed measurable levels of circulating S1 protein (Figure 5A). In the ongoing PASC group, circulating S1 was detected in around 64% of individuals (Figure 5A). This group also showed numerically higher circulating S1 levels as compared to individuals without PASC (Figure 5B). However, the detectability or level of circulating S1 did not show a clear correlation with any of the soluble factors dysregulated in individuals with ongoing PASC (Figure 5C). Nevertheless, it should be noted that for three individuals with detectable plasma S1, the levels of TNF, IL‐1β, IL‐6, and/or IL‐8 were in the upper range of values detected in the ongoing PASC group. Of note, levels of circulating S1 showed a trend toward a positive correlation with S1 and NCP antibody levels, suggesting that persistent viral proteins may sustain the immune response (Figure 5C).
Figure 5.
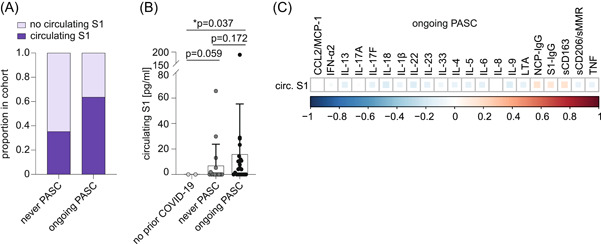
Persistence of circulating S1 protein and correlation with monocyte/macrophage‐related soluble factors and SARS‐CoV‐2‐directed antibodies in unvaccinated individuals with ongoing PASC. (A) Proportion of individuals with detectable levels of circulating S1 (cS1) protein in unvaccinated individuals with prior COVID‐19 who never experienced PASC (n = 17) and with ongoing PASC (n = 22). (B) Mean plasma levels of circulating SARS‐CoV‐2 spike (S1) protein in unvaccinated individuals with ongoing PASC (n = 22), individuals with prior COVID‐19 who never reported PASC (n = 17), and individuals without prior COVID‐19 (n = 2). Error bars indicate ±SD. Statistical analysis: one‐sided Welch corrected t‐test. (C) Correlation matrix of indicated soluble factors with levels of circulating S1 and S1/NCP antibodies in unvaccinated individuals with ongoing PASC. NCP, nucleocapsid protein; PASC, post‐acute sequelae of COVID.
4. DISCUSSION
In this work, we provide evidence for the long‐term and surprisingly strong dysregulation of monocyte/macrophage‐related cytokines, chemokines, and other soluble factors in individuals with a history of COVID‐19. While individuals with PASC tended to show more dysregulation, the correlation patterns of these factors were remarkably independent of ongoing symptoms with a few exceptions. We also observed circulating SARS‐CoV‐2 S1 spike protein in a substantial proportion of individuals with a history of COVID‐19 even many months after infection—especially in the subset of individuals with PASC. While the soluble “immune” factors showed strong correlations with each other, we did not find a strong correlation with the detectability or level of circulating S1. This was a relevant finding that we interpreted as indicative of distinct subgroups of PASC that may result from divergent underlying mechanisms. Unfortunately, the presumable PASC subsets suggested by our analyses—individuals with predominant macrophage dysregulation versus individuals with persistent viral proteins or reservoirs—were rather small subgroups. Therefore, no reliable correlation analysis of these molecular patterns with the clinical characteristics registered in the context of the DigiHero trial could be performed.
Our screening effort in this well‐characterized cohort of patients focused strongly on the monocyte/macrophage compartment and its network of soluble factors. Monocytes and macrophages represent one of the most important cellular immune subsets that are associated with the heterogeneous courses and severity of acute COVID‐19 40 , 41 and are discussed as central for PASC. 14 , 27 , 28 , 42 , 43 , 44 , 45 The here reported cytokine and chemokine data not only corroborate this hypothesis and the importance of pro‐inflammatory and pro‐fibrotic monocytes and macrophages, 6 but also suggest a complex role of monocyte/macrophage‐centered factors known to regulate the TH1/TH2 balance in PASC. This is in line with the reported differential activation of classical and nonclassical monocytes in PASC. 14 , 46 One of the most emblematic cytokines in this respect is IL‐33, which has been originally described as a pro‐inflammatory member of the IL‐1 family but can also induce TH2 responses and act as damage‐associated molecular pattern (DAMP). IL‐33 was suggested to drive the acute severity of COVID‐19 in concert with granulocyte‐macrophage colony‐stimulating factor, to mediate TH2 polarization, and induce chronic pulmonary fibrosis. In addition, it may also mediate the differentiation of monocytes to alternatively activated macrophages that may regenerate damaged bronchial epithelial tissue. 47 , 48
An evolving body of evidence suggests that the wide spectrum of PASC symptoms mirrors the existence of different pathological subgroups. 35 , 46 , 49 In line with this notion, we identified two PASC‐specific correlation patterns consisting of IL‐8 and CCL2/MCP‐1 with either sCD162, sCD206/MMR, IFN‐α2, IL‐17A, and IL‐33, or IL‐18 and IL‐23, which might hint towards distinct disease mechanisms. The correlation of IL‐17A with IFN‐α2 and IL‐8 is notable given the importance of type I interferons for SARS‐CoV‐2 clearance and the pathogenic role of tissue‐resident TH17 cells that interact with pro‐inflammatory and pro‐fibrotic macrophages in the lung of SARS‐CoV‐2‐infected individuals leading to IL‐8 secretion. 50 In contrast, the correlation of CCL2/MCP‐1, IL‐8, IL‐18, and IL‐23 in a subset of participants might be interpreted as a transition from pro‐inflammatory TH1‐like responses in the acute phase towards a more pronounced TH2 response in PASC that is associated with macrophage‐dependent lung fibrosis potentially driven by an exacerbated reaction to Type 2 cytokines. This is also in line with the lower levels of CCL2/MCP‐1 and IL‐8 in individuals with ongoing PASC as compared to individuals without PASC after SARS‐CoV‐2 infection that was also observed by others. 44 CCL2/MCP‐1, IL‐8, IL‐18, and IL‐23 have all been described as pro‐fibrotic in lung, liver, and/or heart 51 , 52 , 53 , 54 and might indicate ongoing tissue damage in PASC. 55 , 56 Interestingly, IL‐8 was found in all PASC‐associated cytokine signatures underscoring its importance for long‐lasting sequelae.
Persistent viral antigens, especially the S1 spike and NCP proteins, have been detected in multiple tissues postinfection and might provide a reservoir sustaining immune responses. 1 , 15 , 16 , 17 We also observed persisting circulating S1 in postinfection samples with a higher frequency of detection and increased levels in individuals with ongoing PASC. Notably, S1 levels did numerically correlate with SARS‐CoV‐2 antibody titers but not with any of the analyzed soluble immune factors. Nevertheless, a few individuals with circulating S1 had relatively high plasma levels of TNF, IL‐1β, IL‐6, and/or IL‐8, supporting the superantigenic features of the spike protein. 13 In line with a recent publication analyzing 31 PASC patients, 17 these data suggest that individuals with PASC that have circulating S1 represent a different disease subset independent of monocyte/macrophage reprogramming. In addition, there are individuals with circulating S1 postinfection who do not develop PASC. Given the small number of involved samples in both data sets, the pathological relevance of circulating S1 needs further validation in larger cohorts.
Overall, these data are indicative of a variety of molecular subtypes in PASC that need to be dissected in future studies with a clear theragnostic aim.
AUTHOR CONTRIBUTIONS
Mascha Binder, Rafael Mikolajczyk, Michael Gekle, Christoph Schultheiß, Lisa Paschold, Cornelia Gottschick, Bianca Klee, Matthias Girndt, Thomas Frese, Daniel Sedding, and Jessica I. Höll designed the COVID‐19 module of the DigiHero cohort study. Daniel Sedding and Jochen Dutzmann provided the HACO patient cohort. Christoph Schultheiß and Lisa Paschold conducted experiments. Mascha Binder, Christoph Schultheiß, Lisa Paschold, Edith Willscher, and Lidia Bosurgi analyzed and interpreted the data. Mascha Binder, Christoph Schultheiß, Edith Willscher, and Lisa Paschold drafted the manuscript. All authors critically revised and approved the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The DigiHero study is conducted by a consortium of the Medical Faculty of the Martin‐Luther‐University Halle‐Wittenberg, including the following PIs: Mascha Binder, Thomas Frese, Michael Gekle, Matthias Girndt, Jessica I. Höll, Patrick Michl, Rafael Mikolajczyk, Matthias Richter, and Daniel Sedding. We thank Aline Patzschke, Christoph Wosiek, Katrin Nerger, and Bianca Gebhardt for excellent technical assistance. We sincerely thank healthy donors, patients, and their household members for participating in this study. Flow cytometry was performed at the UKH FACS sorting core facility. This project was partially funded by the CRC 841 of the German Research Foundation (to Mascha Binder) as well as by the Medical Faculty of the Martin Luther University Halle (Saale). Open Access funding enabled and organized by Projekt DEAL.
Schultheiß C, Willscher E, Paschold L, et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post‐acute sequelae of COVID‐19. J Med Virol. 2022;95:e28364. 10.1002/jmv.28364
DATA AVAILABILITY STATEMENT
The authors confirm that the primary data of this study will be available upon request.
REFERENCES
- 1. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID‐19. Science. 2022;375(6585):1122‐1127. 10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- 2. Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID‐19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622‐642. 10.1016/S2213-2600(21)00218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehandru S, Merad M. Pathological sequelae of long‐haul COVID. Nat Immunol. 2022;23(2):194‐202. 10.1038/s41590-021-01104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultheiß C, Willscher E, Paschold L, et al. The IL‐1β, IL‐6, and TNF cytokine triad is associated with post‐acute sequelae of COVID‐19. Cell Rep Med. 2022;3(6):100663. 10.1016/j.xcrm.2022.100663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID‐19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452‐461. 10.1016/S0140-6736(22)01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie Y, Xu E, Bowe B, Al‐Aly Z. Long‐term cardiovascular outcomes of COVID‐19. Nat Med. 2022;28(3):583‐590. 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen EL, Goßling A, Adam G, et al. Multi‐organ assessment in mainly non‐hospitalized individuals after SARS‐CoV‐2 infection: The Hamburg City Health Study COVID Programme. Eur Heart J. 2022;43(11):1124‐1137. 10.1093/eurheartj/ehab914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post‐acute COVID‐19 sequelae. Cell. 2022;185(5):881‐895. 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS‐CoV‐2 infection. Nat Med. 2022;28(7):1461‐1467. 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS‐CoV‐2 persistence is associated with antigen‐specific CD8 T‐cell responses. EBioMedicine. 2021;64:103230. 10.1016/j.ebiom.2021.103230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noval Rivas M, Porritt RA, Cheng MH, Bahar I, Arditi M. Multisystem inflammatory syndrome in children and long COVID: the SARS‐CoV‐2 viral superantigen hypothesis. Front Immunol. 2022;13:941009. 10.3389/fimmu.2022.941009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild‐to‐moderate SARS‐CoV‐2 infection. Nat Immunol. 2022;23(2):210‐216. 10.1038/s41590-021-01113-x [DOI] [PubMed] [Google Scholar]
- 15. Zollner A, Koch R, Jukic A, et al. Postacute COVID‐19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;163(2):495‐506. 10.1053/j.gastro.2022.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun J, Xiao J, Sun R, et al. Prolonged persistence of SARS‐CoV‐2 RNA in body fluids. Emerging Infect Dis. 2020;26(8):1834‐1838. 10.3201/eid2608.201097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swank Z, Senussi Y, Manickas‐Hill Z, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post‐acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2022. 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guler SA, Ebner L, Aubry‐Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID‐19: first results from the national prospective observational Swiss COVID‐19 lung study. Eur Respir J. 2021;57(4):2003690. 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toraldo DM, Satriano F, Rollo R, et al. COVID‐19 IgG/IgM patterns, early IL‐6 elevation and long‐term radiological sequelae in 75 patients hospitalized due to interstitial pneumonia followed up from 3 to 12 months. PLoS One. 2022;17(2):e0262911. 10.1371/journal.pone.0262911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin Y, Wu J, Chen T, et al. Long‐term microstructure and cerebral blood flow changes in patients recovered from COVID‐19 without neurological manifestations. J Clin Invest. 2021;131(8):e147329. 10.1172/JCI147329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595(7866):283‐288. 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 22. Schultheiß C, Paschold L, Willscher E, et al. Maturation trajectories and transcriptional landscape of plasmablasts and autoreactive B cells in COVID‐19. iScience. 2021;24:103325. 10.1016/j.isci.2021.103325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porritt RA, Binek A, Paschold L, et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J Clin Invest. 2021;131(20):e151520. 10.1172/JCI151520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Q, Bastard P, Effort CHG, Cobat A, Casanova JL. Human genetic and immunological determinants of critical COVID19 pneumonia. Nature 2022;603:587‐598. 10.1038/s41586-022-04447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haran JP, Bradley E, Zeamer AL, et al. Inflammation‐type dysbiosis of the oral microbiome associates with the duration of COVID‐19 symptoms and long COVID. JCI Insight. 2021;6(20):e152346. 10.1172/jci.insight.152346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post‐acute COVID‐19 syndrome. Gut. 2022;71(3):544‐552. 10.1136/gutjnl-2021-325989 [DOI] [PubMed] [Google Scholar]
- 27. Theobald SJ, Simonis A, Georgomanolis T, et al. Long‐lived macrophage reprogramming drives spike protein‐mediated inflammasome activation in COVID‐19. EMBO Mol Med. 2021;13(8):e14150. 10.15252/emmm.202114150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohnacker S, Hartung F, Henkel F, et al. Mild COVID‐19 imprints a long‐term inflammatory eicosanoid‐ and chemokine memory in monocyte‐derived macrophages. Mucosal Immunol. 2022;15:515‐524. 10.1038/s41385-021-00482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID‐19 vaccination on the risk of developing long‐COVID and on existing long‐COVID symptoms: a systematic review. EClinicalMedicine. 2022;53:101624. 10.1016/j.eclinm.2022.101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuchida T, Hirose M, Inoue Y, Kunishima H, Otsubo T, Matsuda T. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with long COVID. J Med Virol. 2022;94(7):3416‐3420. 10.1002/jmv.27689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID‐19. Nature. 2020;584(7821):463‐469. 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Idiz UO, Yurttas TT, Degirmencioglu S, et al. Immunophenotyping of lymphocytes and monocytes and the status of cytokines in the clinical course of Covid‐19 patients. J Med Virol. 2022;94:4744‐4753. 10.1002/jmv.27917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ling L, Chen Z, Lui G, et al. Longitudinal cytokine profile in patients with mild to critical COVID‐19. Front Immunol. 2021;12:763292. 10.3389/fimmu.2021.763292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gantzel RH, Kjær MB, Laursen TL, et al. Macrophage activation markers, soluble CD163 and mannose receptor, in liver fibrosis. Front Med. 2021;7:615599. 10.3389/fmed.2020.615599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Talla A, Vasaikar SV, Lemos MP, et al. Longitudinal immune dynamics of mild COVID‐19 define signatures of recovery and persistence. bioRxiv. 2021. 10.1101/2021.05.26.442666 [DOI] [Google Scholar]
- 36. Arish M, Qian W, Narasimhan H, Sun J. COVID‐19 immunopathology: from acute diseases to chronic sequelae. J Med Virol. 2022. 10.1002/jmv.28122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultheiß C, Paschold L, Simnica D, et al. Next‐generation sequencing of T and B cell receptor repertoires from COVID‐19 patients showed signatures associated with severity of disease. Immunity. 2020;53(2):442‐455. 10.1016/j.immuni.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogata AF, Cheng CA, Desjardins M, et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccine antigen detected in the plasma of mRNA‐1273 vaccine recipients. Clin Infect Dis. 2022;74(4):715‐718. 10.1093/cid/ciab465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bansal S, Perincheri S, Fleming T, et al. Cutting edge: circulating exosomes with COVID spike protein are induced by BNT162b2 (Pfizer‐BioNTech) vaccination prior to development of antibodies: a novel mechanism for immune activation by mRNA vaccines. J Immunol. 2021;207(10):2405‐2410. 10.4049/jimmunol.2100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knoll R, Schultze JL, Schulte‐Schrepping J. Monocytes and macrophages in COVID‐19. Front Immunol. 2021;12:720109. 10.3389/fimmu.2021.720109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li H, Li X, Wu Q, et al. Plasma proteomic and metabolomic characterization of COVID‐19 survivors 6 months after discharge. Cell Death Dis. 2022;13(3):235. 10.1038/s41419-022-04674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patterson BK, Francisco EB, Yogendra R, et al. Persistence of SARS CoV‐2 S1 protein in CD16+ monocytes in post‐acute sequelae of COVID‐19 (PASC) up to 15 months post‐infection. Front Immunol. 2022;12:746021. 10.3389/fimmu.2021.746021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kovarik JJ, Bileck A, Hagn G, et al. Multi‐omics provide evidence for an anti‐inflammatory immune signature and metabolic alterations in patients with long COVID syndrome – an exploratory study. medRxiv. 2022. [Google Scholar]
- 45. Brauns E, Azouz A, Grimaldi D, et al. Functional reprogramming of monocytes in patients with acute and convalescent severe COVID‐19. JCI Insight. 2022;7(9):e154183. 10.1172/jci.insight.154183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv. 2022. 10.1101/2022.08.09.22278592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dagher R, Copenhaver AM, Besnard V, et al. IL‐33‐ST2 axis regulates myeloid cell differentiation and activation enabling effective club cell regeneration. Nat Commun. 2020;11(1):4786. 10.1038/s41467-020-18466-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zizzo G, Cohen PL. Imperfect storm: is interleukin‐33 the Achilles heel of COVID‐19? Lancet Rheumatol. 2020;2(12):e779‐e790. 10.1016/S2665-9913(20)30340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID‐19 after hospitalisation (PHOSP‐COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275‐1287. 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao Y, Kilian C, Turner JE, et al. Clonal expansion and activation of tissue‐resident memory‐like TH17 cells expressing GM‐CSF in the lungs of patients with severe COVID‐19. Science Immunology. 2021;6(56):eabf6692. 10.1126/sciimmunol.abf6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee J, Choi JA, Ju H, Kim JE, Paik SY, Rao PV. Role of MCP‐1 and IL‐8 in viral anterior uveitis, and contractility and fibrogenic activity of trabecular meshwork cells. Sci Rep. 2021;11(1):14950. 10.1038/s41598-021-94391-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Senoo S, Taniguchi A, Itano J, et al. Essential role of IL‐23 in the development of acute exacerbation of pulmonary fibrosis. Am J Physiol. 2021;321(5):L925‐L940. 10.1152/ajplung.00582.2020 [DOI] [PubMed] [Google Scholar]
- 53. Xiao H, Li H, Wang JJ, et al. IL‐18 cleavage triggers cardiac inflammation and fibrosis upon β‐adrenergic insult. Eur Heart J. 2018;39(1):60‐69. 10.1093/eurheartj/ehx261 [DOI] [PubMed] [Google Scholar]
- 54. Zhang LM, Zhang J, Zhang Y, et al. Interleukin‐18 promotes fibroblast senescence in pulmonary fibrosis through down‐regulating Klotho expression. Biomed Pharmacother. 2019;113:108756. 10.1016/j.biopha.2019.108756 [DOI] [PubMed] [Google Scholar]
- 55. Dinnon KH 3rd, Leist SR, Okuda K, et al. SARS‐CoV‐2 infection produces chronic pulmonary epithelial and immune cell dysfunction with fibrosis in mice. Sci Transl Med. 2022;14:eabo5070. 10.1126/scitranslmed.abo5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McGroder CF, Zhang D, Choudhury MA, et al. Pulmonary fibrosis 4 months after COVID‐19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021;76(12):1242‐1245. 10.1136/thoraxjnl-2021-217031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the primary data of this study will be available upon request.


