Summary
In chronic lymphocytic leukaemia (CLL) the efficacy of SARS‐CoV‐2 vaccination remains unclear as most studies have focused on humoral responses. Here we comprehensively examined humoral and cellular responses to vaccine in CLL patients. Seroconversion was observed in 55.2% of CLL with lower rate and antibody titres in treated patients. T‐cell responses were detected in a significant fraction of patients. CD4+ and CD8+ frequencies were significantly increased independent of serology with higher levels of CD4+ cells in patients under a Bruton tyrosine kinase (BTK) or a B‐cell lymphoma 2 (BCL‐2) inhibitor. Vaccination skewed CD8+ cells towards a highly cytotoxic phenotype, more pronounced in seroconverted patients. A high proportion of patients showed spike‐specific CD4+ and CD8+ cells producing interferon gamma (IFNγ) and tumour necrosis factor alpha (TNFα). Patients under a BTK inhibitor showed increased production of IFNγ and TNFα by CD4+ cells. Vaccination induced a Th1 polarization reverting the Th2 CLL T‐cell profile in the majority of patients with lower IL‐4 production in untreated and BTK‐inhibitor‐treated patients. Such robust T‐cell responses may have contributed to remarkable protection against hospitalization and death in a cohort of 540 patients. Combining T‐cell metrics with seroprevalence may yield a more accurate measure of population immunity in CLL, providing consequential insights for public health.
Keywords: chronic lymphocytic leukaemia, COVID‐19, T‐cellular response, Th1 polarization, vaccine
Abbreviations
- CLL
chronic lymphocytic leukaemia
- CML
chronic myeloid leukaemia
- DMSO
dimethyl sulphoxide
- FACS
fluorescence‐activated cell sorting
- IFNγ
interferon gamma
- ITK
interleukin‐2‐inducible kinase
- PB
peripheral blood
- PBMC
PB mononuclear cell
- Th
T helper
- TNFα
tumour necrosis factor alpha
INTRODUCTION
Patients with chronic lymphocytic leukaemia (CLL) are at increased risk of poor COVID‐19 outcomes, compared with the general population. 1 , 2 Retrospective international multicentre cohorts of patients with CLL reported 30%–34% case fatality rates in severe COVID‐19. 1 , 2 , 3 , 4 , 5 Accordingly, experts have generally recommended that CLL patients should receive vaccines against SARS‐CoV‐2, the rapid development of which had a major role in decreasing COVID‐19‐related mortality and hospitalizations, both in clinical trials and in nationwide studies. 6 , 7
The BNT162b2 mRNA COVID‐19 vaccine that leads to transient expression of the SARS‐CoV‐2 spike protein was developed in 2020. 8 The phase 3 trial of the vaccine showed around 95% efficacy in preventing symptomatic COVID‐19 infection, 8 although data on CLL patients are limited as the registration trials excluded participants with haematological malignancies. Thus, the evaluation of vaccine effectiveness against severe forms of COVID‐19 in CLL remains an unmet need.
Patients with a history of CLL may have inherent immune defects due to the intrinsic immunosuppressive nature of the disease, prior treatments and comorbidities, which may hinder the efficacy of COVID‐19 vaccines. 9 Recent evidence indicates that antibody‐mediated response to COVID vaccination is impaired in patients with CLL, in both previously untreated patients and those undergoing therapy. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 To date, few studies describe cellular responses to determine immunogenicity of the SARS‐CoV‐2 vaccine. 15 , 16 , 17 , 18 This characterization remains mainly based on interferon gamma (IFNγ)‐mediated T‐cell responses, although the functional profile of T cells is certainly more diverse and the combination of functions that confer protection from infection is uncertain.
In this work we provide a comprehensive characterization of CD4+ and CD8+ T‐cell‐mediated responses to SARS‐CoV‐2 vaccine in CLL and correlate this information with serological findings and CLL‐directed treatments. We found that vaccination impacted specific T‐cell subsets, increased cytotoxic T‐cell activation and influenced cytokine production and polarization in CLL patients. T‐cellular immunity in CLL was present in seronegative patients and was affected by specific CLL‐directed treatments. The findings have significant implications for immunity against secondary COVID‐19 in a fragile setting.
METHODS
Sample collection
Peripheral blood (PB) samples were collected in heparin‐coated tubes (BD Vacutainer) before anti‐SARS‐CoV‐2 vaccine administration and 6–142 days after the second dose. Non‐heparinized tubes were used for collection of sera. PB mononuclear cells (PBMCs) were isolated from heparinized blood of CLL patients by Ficoll density gradient centrifugation (Nycomed). Isolated PBMCs were then cryopreserved in cell recovery medium [20% dimethyl sulphoxide (DMSO), 80% heat‐inactivated fetal bovine serum] and stored at −80°C until use. All the studies were performed on frozen samples.
Serological analysis
Blood serum samples were tested for the presence of anti‐spike IgG antibodies using the LIAISON® SARS‐CoV‐2 TrimericS IgG (DiaSorin) chemiluminescence immunoassay. The test was performed on the LIAISON XL analyser according to the manufacturer's instructions. The chemiluminescent signal measured by a photomultiplier in relative light units is proportional to the concentration of anti‐spike antibodies in the samples expressed in arbitrary units (AU/ml). The analyser automatically calculates the levels of anti‐spike IgG antibodies in binding antibody units per ml (BAU/ml). Samples with an antibody concentration greater than 33.8 BAU/ml were considered positive.
Flow cytometry analysis
Peripheral blood mononuclear cells from CLL patients were stained with the following antibodies: APC‐anti‐CD2, PacificBlue‐anti‐CD3, PC7‐anti‐CD4, FITC‐anti‐CD8, KromeOrange‐anti‐CD45 (Beckman Coulter). PB cells were also stained with Beckman Coulter antibody PC7‐anti‐CD27, FITC‐anti‐CD57 and PE‐TexasRed‐anti‐CD45RA (eBioscience). Multiparametric flow cytometry was used to define and analyse the CD4+ and CD8+ T‐cell compartments, including naïve (CD45RA+CD27+), central memory (CD27+CD45RA−), effector memory (CD27−CD45RA−), and effector memory T cells that re‐express CD45RA (EMRA) (CD27−CD45RA+) T cells. In addition, for SARS‐CoV‐2‐specific B cells, we stained FITC‐CD19+ B cells with recombinant SARS‐CoV‐2 spike‐prot (HEK)‐biotin plus anti‐biotin‐PECY7, according to the manufacturer's instructions. Cells were acquired with a Navios EX Flow Cytometer (Beckman Coulter) and analysed using Kaluza Analysis Software (Beckman Coulter). For IC‐FACS (fluorescence‐activated cell sorting) analysis (cytokines), cells were first stained with surface antibodies PC5.5‐anti‐CD4 (Beckman Coulter) and Alexa‐Fluor780‐anti‐CD8 (eBioscience) and then fixed/permeabilized for intracellular staining of cytokines using APC‐anti‐IFNγ, PECY7‐anti‐IL‐4, and FITC‐anti‐TNFα (eBioscience).
For CD4+ T‐cell polarization analysis, PBMCs from CLL patients were stained with the following antibodies: PerCP‐Cy5.5‐anti‐ CD3, APC/Cyanine7‐anti‐HLA‐DR, PE‐Cy7‐anti‐CD45RO (Biolegend), FITC‐anti‐CD8 (Miltenyi Biotec), BV650‐anti‐CD4, PE‐CF594‐anti‐CD25, BV786‐anti‐CD27, BV605‐anti‐CD38, Alexa Fluor® 647‐anti‐CD127, Alexa Fluor 700‐anti‐CD183, PE‐anti‐CD196 (CCR6), and BV421‐anti‐CD197 (CCR7) (BD Biosciences). Cell acquisition and analysis were performed using FACS CANTO (BD Biosciences).
SARS‐CoV‐2 spike stimulation
Peripheral blood mononuclear cells (5 × 106) isolated from CLL patients were stimulated to evaluate the presence of specific SARS‐CoV‐2 T cells. For each patient we performed SARS‐CoV‐2‐specific T stimulation (PepTivator SARS‐CoV‐2 Prot_S; Miltenyi Biotec 0.6 nmol/ml), DMSO (negative control) and PMA/ionomycin (eBioscience™ Cell Stimulation Cocktail) (positive control) for 6 h. We added Brefeldin A (1 μg/ml) after 2 h and then incubated for a further 4 h. Then, cells were collected and stained for cytokine production or T‐cell polarization. Only patients with a positive response to PMA/ionomycin stimulation were included in the analysis. The values obtained in the DMSO well were subtracted from those of the SARS‐CoV‐2‐specific‐stimulated well to obtain the values reported in the graphs.
Statistical analysis
Statistical analyses were performed with the GraphPad Prism software (GraphPad Software, Inc.). The non‐parametric Mann–Whitney U‐test was used for comparisons between two independent groups, for example in serological studies, T‐cell and T‐cell subset studies. The Wilcoxon signed‐rank test was used for pairwise group comparisons such as in studies regarding cytokines production and T‐cell polarization. Correlation analysis was performed by using the Spearman's correlation coefficient (ρ). Results were considered statistically significant when p was less than 0.05, and otherwise were indicated as not significant (N.S.).
RESULTS
Impaired SARS‐CoV‐2 antibody responses to vaccination in CLL patients
A total of 134 CLL patients were enrolled in the study. The median age was 70 years (range 30–93) and 89 (66.4%) were male (patient baseline demographic and disease characteristics are summarized in Table S1). Most of the participants received an mRNA‐based COVID‐19 vaccine (94.8%) with the remainder (5.2%) receiving an adenoviral vaccine. The efficacy of vaccination in terms of seroconversion was assessed in infection‐naïve patients by measuring responses after a median of 20 days (range 6–142 days) from the second vaccine dose.
Serological analysis revealed detectable levels of SARS‐CoV‐2 antibodies in 55.2% of CLL patients with a median anti‐spike IgG response of 396 BAU/ml (range 36.4–14 500) (Figure S1, left panel). The percentage of seroconversion was 57.8% when restricting the analysis to 109 patients analysed in the window period of 14–30 days and 47.8% in the remaining CLL analysed at longer time‐points. As a comparator, we analysed a cohort of 100 chronic myeloid leukaemia (CML) patients, a chronic haematological malignancy previously described to produce a humoral response in a high proportion of patients. 13 , 19 , 20 SARS‐CoV‐2 antibodies were found in 90.0% of CML patients with a median anti‐spike IgG response of 1350 BAU/ml (range 37.4–17 100) (Figure S1, right panel). Our data showed that in CLL compared to CML anti‐SARS‐CoV‐2 vaccine response is significantly lower (mean 1191 ± 297.2 BAU/ml vs. 2027 ± 282.9 BAU/ml; p < 0.0001) (Figure 1A, left panel).
FIGURE 1.
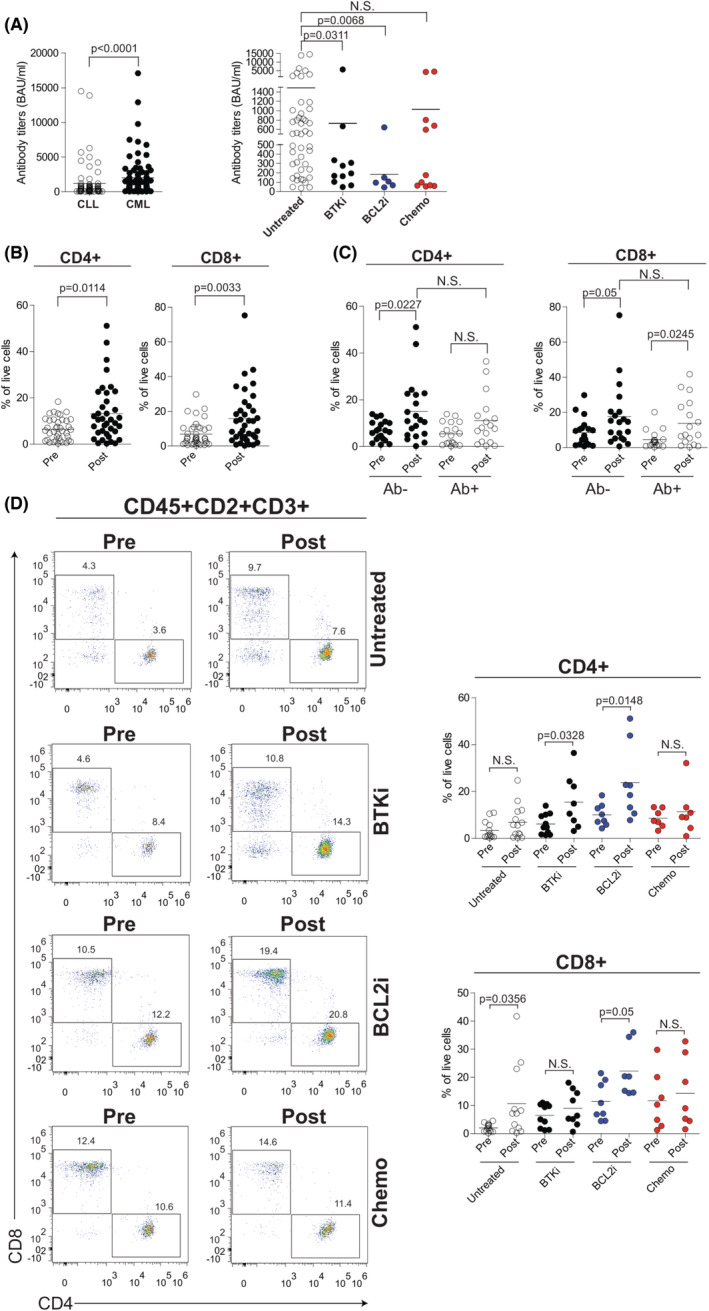
Humoral response and T‐cell levels after SARS‐CoV‐2 vaccination in CLL. (A) Left panel, anti‐SARS‐CoV‐2 antibody levels in seroconverted patients with CLL (n = 74) or chronic myeloid leukaemia (CML) (n = 90). Right panel, anti‐SARS‐CoV‐2 antibody titres in CLL seroconverted patients from untreated (N = 46), Bruton tyrosine kinase inhibitor (BTKi) (N = 11), B‐cell lymphoma 2 inhibitor BCL2i (N = 6), and chemo (n = 11) subgroups. (B) Frequency of CD4+ (left) and CD8+ (right) T cells within the CD2+CD3+ T‐cell compartment in CLL patients before (pre) and after (post) vaccine administration. (C) Frequency of CD4+ (left) and CD8+ (right) T cells within the CD2+CD3+ T‐cell compartment, in seronegative (Ab−) and seropositive (Ab+) CLL patients before (pre) and after (post) vaccine administration. (D) Flow‐cytometric analysis of CD4+ and CD8+ T cells within the CD2+CD3+ T‐cell compartment in CLL patients per treatment status before (pre) and after (post) vaccine administration. Representative plots are shown in the left panel. Right panel, frequency of CD4+ (upper) and CD8+ (bottom) in each treatment subgroup of CLL patients. Data are presented as scatter dot plots and the mean values (horizontal lines) are shown. p value (Mann–Whitney test) is reported above each comparison. CLL, chronic lymphocytic leukaemia; N.S., not significant
CLL patient responsiveness to vaccination was further validated by a bead‐based flow cytometric immunoassay evaluating the percentage of SARS‐CoV‐2‐specific B cells in 14 serologically positive and 16 serologically negative selected patients. SARS‐CoV‐2 spike protein S1 subunit staining of CD19+ cells was significantly higher in seroconverted patients compared to serologically negative CLL (1.72 ± 0.55% vs. 0.12 ± 0.05%; p < 0.0001) (Figure S2A). No correlation was observed between the levels of anti‐spike IgG and SARS‐CoV‐2 specific B‐cell percentages in serologically positive CLL (Figure S2B).
Antibody titres were further assessed in relation to CLL treatment status dividing patients into four groups including untreated (N = 60), BTK inhibitor (BTKi, N = 31), BCL‐2 inhibitor (BCL2i, N = 20), and chemoimmunotherapy (Chemo, N = 23) treated patients. The vast majority of patients in BTKi (N = 29) and BCL2i (N = 16) subgroups were under active treatment while only eight out of 23 patients were on active chemoimmunotherapeutic treatment. This analysis demonstrated that the humoral response was more frequent in untreated CLL (76.7%) than in patients exposed to a BTKi (35.5%), BCL2i (30.0%) and chemoimmunotherapy (47.8%) (Figure S3). Seroconversion rate was reduced to 37.5% when considering patients under active chemoimmunotherapy. Moreover, means of antibody titres in seropositive patients were higher in untreated patients (1488 ± 443.8 BAU/ml), compared to CLL on BTKi (728.5 ± 498.8 BAU/ml; p = 0.0311), BCL2i (184.3 ± 93.08 BAU/ml; p = 0.0068) and chemoimmunotherapy (1027 ± 503.3 BAU/ml; p = 0.2307) (Figure 1A, right panel). Previous treatment with anti‐CD20 therapies reduced the chances of developing a humoral response to COVID‐19 vaccine (22/55 seropositive patients, 40%), particularly in patients exposed to anti‐CD20 within less than 12 months before vaccination (2/8 seropositive patients, 25%) (Figure S4). These data indicate that antibody responses were lower during active treatment and after exposure to anti‐CD20 therapy.
We did not find statistically significant associations between antibody response and sex, Binet stage (for untreated patients), cytogenetics, NOTCH1 and TP53 mutational status (Table S2). A statistically significant association was observed with age. Patients aged <70 years showed antibody titres significantly higher (1747 ± 530.8 BAU/ml vs. 603.3 ± 212.4 BAU/ml; p = 0.012) compared with older patients (≥70 years) (Table S2). Moreover, the percentage and antibody titres of responders were higher in the IGHV‐mutated compared to IGHV‐unmutated patients (62.9% vs. 44.4%; 1478 ± 518.7 BAU/ml vs. 654 ± 226.7 BAU/ml; p = 0.4313) (Table S2) although this finding did not reach statistical significance.
SARS‐CoV‐2 vaccination induced alterations of CD4 + and CD8 + T‐cell frequencies and subset composition in CLL
To assess the cellular immunogenicity of the SARS‐CoV‐2 vaccine in CLL, we evaluated the percentages of different CD4+ and CD8+ T‐cell subsets in PB samples from 62 patients collected before vaccine and after administration of the second dose. Not all experiments were performed on each patient's serial sample; therefore, the actual N for each experiment was less than 40.
As shown in Figure 1B, we observed a significant increase in total CD4+ and CD8+ frequencies following vaccination (from 6.44 ± 0.76% to 13.25 ± 2.02% and from 6.85 ± 1.10% to 15.80 ± 2.59%; p = 0.0114 and p = 0.0033 respectively). These T‐cell changes were independent of the serological status as CD4+ and CD8+ populations were increased either in patients developing or not developing an antibody (Ab) response to SARS‐CoV‐2 (Ab+ and Ab− patients respectively) (Figure 1C). Conversely, variation of T cells in response to vaccine was influenced by CLL‐directed treatments. The increase in CD4+ cells was significantly pronounced in patients under targeted therapy with either a BTK or a BCL‐2 inhibitor (from 6.08 ± 1.31% to 15.47 ± 4.03% and from 9.97 ± 1.68% to 23.78 ± 5.54% respectively; p = 0.0328 and p = 0.0148 respectively) while untreated patients and those exposed to chemoimmunotherapy showed a not significant increase tendency (from 3.42 ± 1.06% to 6.90 ± 1.90% and from 8.59 ± 1.48% to 11.36 ± 3.79% respectively) (Figure 1D). After vaccination, CD8+ cells were 3.3‐log increased in untreated CLL (from 2.05 ± 0.37% to 10.70 ± 3.69%, p = 0.0356) and 1.27‐log increased in patients under BCL‐2 inhibitor‐based therapy (from 11.46 ± 2.43% to 22.19 ± 3.50%, p = 0.05), while BTK inhibition and chemoimmunotherapy exposure did not significantly affect this T‐cell population (Figure 1D).
Both CD4+ and CD8+ T cells were categorized into naïve (Naïve; CD45RA+CD27+), central memory (CM; CD45RA−CD27+), effector memory (EM; CD45RA−CD27−) and CD45RA+ effector memory T (EMRA; CD45RA+CD27−) cells. 21 Although the percentage of the investigated CD4+ and CD8+ T‐cell subsets did not significantly change from pre‐ to postvaccination in the full cohort of 62 CLL patients (Figure S5), we found differences when patients were stratified for the humoral response or CLL‐directed treatments. Specifically, after vaccination, Ab− patients displayed a reduction of CD4+ Naïve T cells (from 23.65 ± 4.17% to 12.33 ± 2.20%) that results significantly lower when compared to the same population of Ab+ patients (12.33 ± 2.20% vs. 25.64 ± 3.43, p = 0.029; Figure S6A). Moreover, CD8+ Naïve T cells were significantly 1.22‐log increased (from 12.33 ± 2.23% to 21.45 ± 4.08%; p = 0.036; Figure S6B). Vaccination did not induce any significant effect in the other examined T‐cell subset either in Ab− or Ab+ patients (Figure S6A,B). Furthermore, in patients stratified for CLL‐directed treatments, we documented a significant 1.31‐log expansion of the CD8+ Naïve T‐cell compartment between pre‐ and postvaccine samples in patients under a BTK inhibitor (from 12.4 ± 4.1% to 26.8 ± 5.1%, p = 0.0356) (Figure S7B).
Interestingly, alterations in some of the investigated T‐cell subsets were already present before vaccine administration in CLL patients under a BCL‐2 inhibitor, displaying a significant reduction of CD4+ Naïve cells and the expansion of EM cells compared to untreated CLL (31.38 ± 4% vs. 14.97 ± 3.97% and 22.34 ± 4.09% vs. 33.44 ± 8.34%; p = 0.0116 and p = 0.0485 respectively) (Figure S8A). When comparing postvaccination samples, we confirmed expanded CD4+ EM cells in BCL‐2‐inhibitor‐treated versus untreated CLL while both CD4+ and CD8+ CM were higher in all CLL‐directed treatment groups compared to untreated patients (Figure S7A,B).
SARS‐CoV‐2 vaccination skewed CD8 + T‐cell response towards a terminally differentiated CD8 + CD27−CD57 + high cytotoxic phenotype
CD8+ cells have been characterized for the expression of CD57 and CD27 markers, in order to further separate these T cells into functionally distinct subsets. Concomitant loss of CD27 and expression of CD57 on CD8+ T cells is observed during prolonged stimulation and is associated with a fully differentiated effector phenotype. 22 This allowed classifying CD8+ T cells into early differentiated (CD27+ CD57+) cells that can proliferate and differentiate into terminally differentiated (CD27− CD57+) highly cytotoxic T cells with high expression of perforin and granzyme B, generated during chronic immune activation. 22
SARS‐CoV‐2 vaccination induced a significant decrease of early differentiated CD8+CD27+CD57+ T cells (from 11.28 ± 1.59% to 4.43 ± 0.63%, p < 0.0001) and a reduction of terminally differentiated CD8+CD27−CD57+ T cells (from 48.32 ± 2.97% to 40.35 ± 3.03%, p = 0.0913) (Figure 2A). These changes determined a significant increase of the ratio between terminally and early differentiated CD8+ cells (from 7.71 ± 1.14 to 19.39 ± 3.23, p = 0.0023) (Figure 2B), indicating that SARS‐CoV‐2 vaccination skewed CD8+ cells towards a highly cytotoxic phenotype.
FIGURE 2.
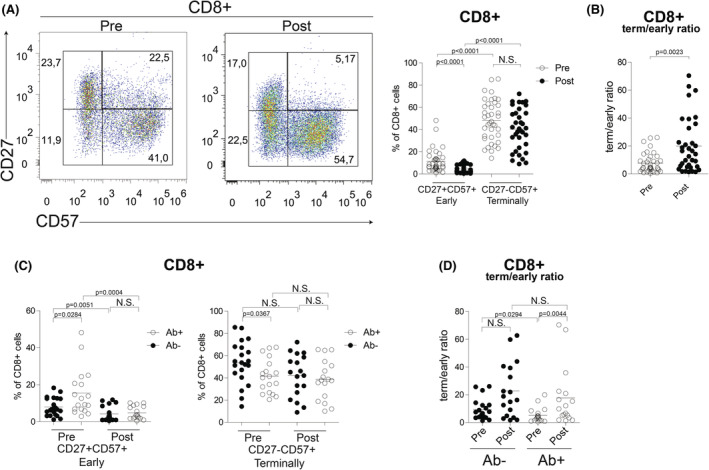
CD8+ T‐cell skewing after SARS‐CoV‐2 vaccination in CLL. (A) Flow cytometric analysis of CD27+CD57+ (early differentiated) and CD27−CD57+ (terminally differentiated) T cells within the CD8+ T‐cell compartment before (pre) and after (post) SARS‐CoV‐2 vaccination in CLL patients. Representative plots are shown in the left panel. Right panel, frequency of CD27+CD57+ (early differentiated) and CD27−CD57+ (terminally differentiated) T cells within the CD8+ T‐cell compartment before (pre, white dots) and after (post, black dots) SARS‐CoV‐2 vaccination in CLL patients. (B) Ratio of terminally/early differentiated CD8+ T cells, before (pre, white dots) and after (post, black dots) SARS‐CoV‐2 vaccine administration. (C) Frequency of CD27+CD57+ (early differentiated) and CD27−CD57+ (terminally differentiated) T cells within the CD8+ T‐cell compartment before (pre) and after (post) SARS‐CoV‐2 vaccination in seronegative (Ab−, black dots) and seropositive (Ab+, white dots) CLL patients. (D) Ratio of terminally/early differentiated CD8+ T cells, before (pre) and after (post) SARS‐CoV‐2 vaccine administration in seronegative (Ab−, black dots) and seropositve (Ab+, white dots) CLL patients. Data are presented as scatter dot plots and the mean values (horizontal lines) are shown. p value (Mann–Whitney test) is reported above each comparison. CLL, chronic lymphocytic leukaemia; N.S., not significant
Interestingly, patients who developed antibodies after SARS‐CoV‐2 vaccine showed a significantly higher frequency of early differentiated T cells (Ab− 8.01 ± 1.08% vs. Ab+ 15.53 ± 2.95%; p = 0.0284) and a low frequency of terminally differentiated T cells (Ab− 53.65 ± 4.19% vs. Ab+ 41.59 ± 3.64%; p = 0.0367) even before vaccination compared to seronegative patients (Figure 2C). As expected, after vaccination a significant reduction of early differentiated T cells was observed either in Ab− and Ab+ patients (Figure 2C). Upon vaccination, the ratio between terminally and early differentiated CD8+ cells was significantly increased in both Ab− and Ab+ patients, the latter displaying the higher magnitude (from 9.396 ± 1.71 to 22.8 ± 4.602 and from 5.2 ± 1.250 to 18.7 ± 5.4 respectively) (Figure 2D) suggesting a potential correlation between T‐cell differentiation capacity and antibodies responses.
SARS‐CoV‐2 spike peptide stimulation induced changes in cytokine production by CLL T cells
To better characterize T‐cell immune responses induced by vaccination, we analysed cytokine‐producing T‐cell populations, after a median of 30 days (range 10–142) following the second dose of vaccine. For this purpose, we used multiparameter intracellular FACS analysis to determine the phenotypic characteristics of the cytokine‐producing T‐cell populations.
Cryopreserved PBMCs from 39 CLL patients were stimulated with a panel of SARS‐CoV‐2 spike‐spanning peptide‐pools and analysed for intracellular cytokines IFNγ, TNFα, to determine the functional activity of CD4+ and CD8+ T‐cell responses. PMA/ionomycin stimulation was used as a positive control for T‐cell activation and DMSO to define the baseline level of cytokine production. One patient with no PMA/ionomycin response was excluded from the analysis.
After vaccination, median frequencies of spike‐specific CD4+ and CD8+ T cells producing IFNγ were 0.46% (range 0%–7.86%) and 0.70% (range 0%–43.99%) respectively (Figure 3A). Median percentages of TNFα produced by CD4+ and CD8+ T cells were 0.47% (range 0%–15.27%) and 0.12% (range 0%–52.4%) respectively (Figure 3A). Increase of this cytokine production was significantly higher than in DMSO control.
FIGURE 3.
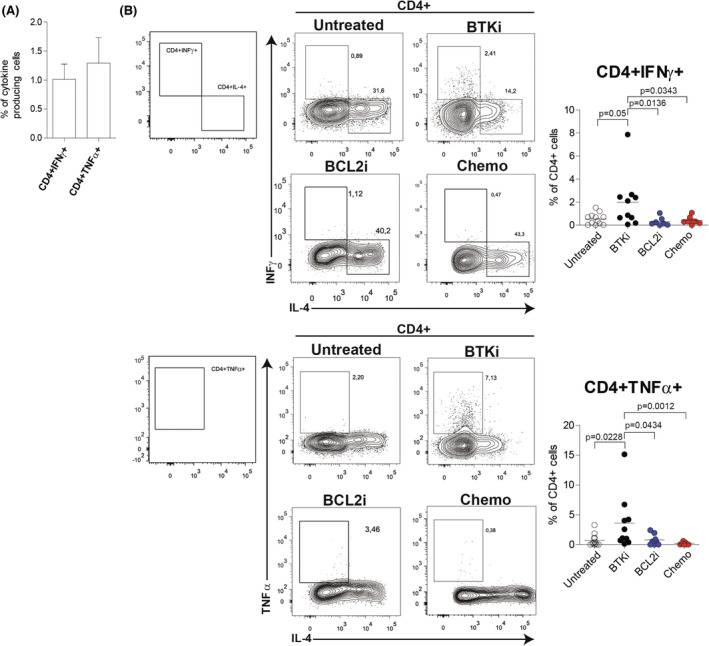
Cytokine production induced by SARS‐CoV‐2 antigenic stimulation in CD4+ T cells from vaccinated CLL patients. (A) Frequency of CD4+ T cells producing IFNγ or TNFα after SARS‐CoV‐2 antigenic peptide stimulation (N = 38) represented as mean ± standard error of the mean. (B) Flow cytometric analysis of CD4+IFNγ+ (upper panel) and CD4+TNFα+ (bottom panel) cells in CLL patients per treatment subgroup. Gating strategy (left panel) and representative plots (middle panel) are shown. Right panel, data are presented as scatter dot plots and the mean values (horizontal lines) are shown. p value (Mann–Whitney test) is reported above each comparison. CLL, chronic lymphocytic leukaemia; IFNγ, interferon gamma; N.S., not significant; TNFα, tumour necrosis factor alpha
Based on the background level of cytokines in DMSO controls, we set 0.2% as the cut‐off level for identifying positive T‐cell immunity against SARS‐CoV‐2. We found that either CD4+ or CD8+ T cells from 26 out of 38 (68.4%) vaccinated patients produced IFNγ after stimulation with spike SARS‐CoV‐2 peptide‐pools (Table S3). CD4+ TNFα positivity was observed in 23/38 (60.5%) patients whereas only 15/38 (39.5%) patients showed CD8+TNFα‐positive cells over the cut‐off level of 0.2%. These data revealed spike‐specific T‐cell responses to vaccine in a significant fraction of CLL patients.
Analysis of CLL treatment subgroups revealed a CD4+IFNγ positivity in 8/10 (80%) patients who were on a BTK inhibitor, whereas only 3/7 (42.9%) patients on BCL‐2 inhibitor developed T‐cell immunity. CD4+IFNγ positivity was found in 9/13 (69.2%) untreated patients and 6/8 (75.0%) patients treated with chemoimmunotherapy (Table S3). Positive SARS‐CoV‐2‐specific CD4+TNFα+ cell responses were detected in 9/10 (90.0%) patients treated with a BTK inhibitor, in 5/7 (71.4%) patients treated with a BCL‐2 inhibitor, in 2/8 (25.0%) patients treated with chemotherapy and in 7/13 (53.8%) untreated patients (Table S3).
Notably, we found a significantly higher production of IFNγ and TNFα by CD4+ cells in patients under BTK inhibitor compared to other treatments (Figure 3B). Cytokine levels in CD8+ cells were not influenced by CLL treatment (Figure S9).
Moreover, we detected positivity for at least one cytokine in either CD4+ or CD8+ in 20/21 (95.2%) Ab− and in 15/17 (88.2%) Ab+ patients, indicating a high response rate even in the absence of seroconversion (no cytokine production in 1/21 and 2/17 Ab− and Ab+ patients respectively, Table S3). IFNγ and TNFα positivity in both CD4+ and CD8+ was detected in 6/38 (15.8%) patients, four of whom were on a BTK inhibitor. Overall, 40% of patients treated with a BTK inhibitor had a complete cytokine response to SARS‐CoV‐2 peptides. Only three individuals (7.9%) showed no T‐cellular response and one was defined ‘double negative’ with neither a cellular nor a humoral response after vaccination.
SARS‐Cov‐2 vaccination induced polarization of spike‐specific CD4 + T cells towards a Th1 profile
Given the role of CD4+ T helper (Th) cell subsets in immune response to viral infections, we next explored the functional polarization, induced by vaccination, of SARS‐CoV‐2‐specific CD4+ T cells into Th1 or Th2 subsets, by first analysing the expression of CCR6 and CD183 markers. 23
Of 29 analysed patients, 24 showed a significant 1.28‐log increase of CD4+CCR6−CD183+ Th1 cells after SARS‐CoV‐2 spike peptide stimulation compared to DMSO (Figure 4A). In keeping with these data, we also found an increased production of IFNγ and TNFα by CD4+ cells, further supporting a Th1 polarized response induced by vaccine (Figure 4B). Consistently, we identified a significant impairment of the Th2 profile in 20/28 patients showing a reduction of CD4+CCR6−CD183− and a decrease of IL‐4‐producing CD4+ cells percentages compared to DMSO (Figure 4C; Table S4). Altered Th1/Th2 polarization was not influenced by either the serological status or CLL treatments (Table S4). Nevertheless, we detected a decreased IL‐4 production in untreated and BTK‐inhibitor‐treated patients after SARS‐CoV‐2 spike peptide stimulation, indicating a more pronounced Th1 response in these subgroups (Figure 4D). We also analysed Th17 and Treg subsets and did not find differences between SARS‐CoV‐2‐specific stimulation and DMSO (Figure S10; Table S4).
FIGURE 4.
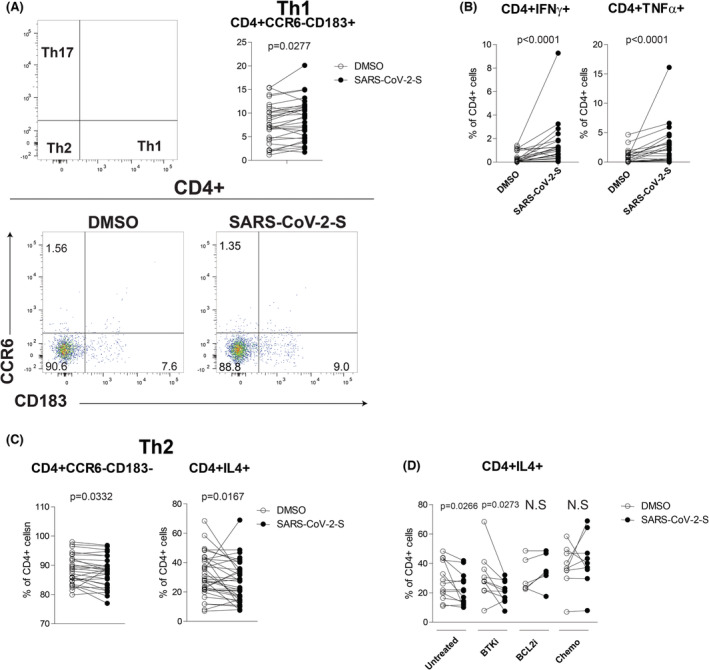
CD4+ T‐cell polarization following SARS‐CoV‐2 antigenic stimulation in vaccinated CLL. (A) Flow cytometric analysis of CD4+ T‐cell polarization towards Th2 (CCR6−CD183−), Th1 (CCR6−CD183+) and Th17 (CCR6+CD183−) profile after SARS‐CoV‐2 antigenic peptide stimulation in CLL patients, DMSO served as control. Gating strategy (upper left) and representative plots (bottom) are shown. Upper right panel, paired frequencies of CD4+CCR6−CD183+ Th1 T cells after SARS‐CoV‐2 spike peptide stimulation compared to DMSO in stimulated CLL T cells (N = 29). (B) Paired frequencies of CD4+IFNγ+ (left) and CD4+TNFα+ after SARS‐CoV‐2 spike peptide stimulation compared to DMSO in stimulated CLL T cells (N = 29). (C) Left panel, paired frequencies of CD4+CCR6−CD183− Th2 T cells after SARS‐CoV‐2 spike peptide stimulation compared to DMSO in stimulated CLL T cells (N = 29). Right panel, paired frequencies of CD4+IL4+ after SARS‐CoV‐2 spike peptide stimulation compared to DMSO in stimulated CLL T cells (N = 29). (D) Paired frequencies of CD4+ IL4+ after SARS‐CoV‐2 spike peptide stimulation compared to DMSO in stimulated CLL T cells in each different group of CLL treatment. Data are presented as dot and line diagrams. p value (Wilcoxon paired test) is reported above each comparison. CLL, chronic lymphocytic leukaemia; DMSO, dimethyl sulphoxide; IFNγ, interferon gamma; N.S., not significant; TNFα, tumour necrosis factor alpha
Altogether these data demonstrated that the vaccine modulated the Th1/Th2 balance towards the development of a Th1 phenotype, 24 thus allowing an antiviral response by T‐cell compartment.
Characteristics of pre‐ and postvaccination illness in CLL patients
We examined frequency, hospitalization and mortality rates of COVID‐19 infection during two distinct coronavirus waves comparing data for patients from October 2020 through May 2021 (prevaccination wave) to those from August 2021 through March 2022 (postvaccination wave). Patients were considered fully vaccinated after having received two doses of mRNA vaccine.
In a cohort of 540 CLL patients followed at our institution, the frequency of COVID‐19 before the introduction of vaccines was similar to that of the postvaccination phase (2.6% vs. 2.4% respectively). Hospitalization due to severe COVID‐19 in the second time period was reduced to 38.4% (5/13 positive patients) compared to 78.5% (11/14 positive patients) in the first time period, when the vaccine was not available. In the second observation period, three patients received monoclonal antibodies and one antiviral therapy with molnupiravir. Corticosteroids were widely applied in both waves. Information on the COVID variant was not available, although it is known that delta prevailed in the prevaccine phase while omicron in the postvaccine phase. The rate of deaths attributed to COVID‐19 infection was significantly reduced from 57.1% (8/14) to 7.7% (1/13), providing evidence in favour of vaccination for these patients.
DISCUSSION
The adaptive immune system comprises two major branches that respond to infection and vaccine administration through distinct but overlapping mechanisms. 25 , 26 , 27 The induction of appropriate immune responses by a vaccination is crucial for an effective protection against pathogen invasion.
In this study, we assessed responses of different branches of adaptive immunity (humoral immunity and CD4+ and CD8+ T cells) in a cohort of CLL patients who received the SARS‐CoV‐2 vaccine at a single academic medical centre.
Altered responses to COVID‐19 vaccines have been described already in the CLL population, although this characterization was mainly based on humoral responses after mRNA vaccines. 10 , 11 , 12 B‐cell defects and frequent hypogammaglobulinaemia have been considered leading causes of impaired serological responses to antibacterial 28 or antiviral 29 , 30 vaccines in CLL. Our results indicate that almost half (44.8%) of patients in our cohort were seronegative after vaccination, confirming low overall serological responses seen in previously published reports of 40%–64%. 11 , 12 , 13 In a systematic review and meta‐analysis of 12 studies including around 1500 CLL patients, pooled seropositivity rates were 51% following two doses of COVID‐19 vaccine. 15
In our cohort, the levels of SARS‐CoV‐2 antibodies were relatively low, compared to those in participants of a prospective observational study conducted among vaccinated healthcare workers used as reference. 31 , 32 Here, we also provided data showing that COVID‐19 vaccination generated robust humoral immunity in 90% of CML patients with higher antibody titres compared to CLL patients. These findings support previous observation that patients with CLL have the lowest rate of seropositivity among leukaemia patients. 15
Our analysis demonstrated that untreated CLL patients were more likely to test SARS‐CoV‐2 seropositive than patients exposed to therapy, indicating that a poor humoral response was compounded by immunosuppression related to chemotherapy, targeted agents and B‐cell‐depleting treatments such as CD‐20 monoclonal antibodies. This negative impact of CLL treatment on COVID‐19 vaccine responses was noted in subgroup analyses of published studies. 11 , 16 , 17 Shen et al. reported a strong association with vaccination failure in 74.4% of patients treated within the last 12 months. 17 Rates of seropositivity were lower in the setting of active treatment with either a BTK or a BCL‐2 inhibitor 11 while the lowest response rate was reported in the setting of anti‐CD20 monoclonal antibody therapy. 16 Indeed, CD20‐targeting agents, by producing a decrease in B lymphocytes, represent a risk factor for severe COVID‐19 infections.
Besides antibody production, many studies have shown that T‐cell responses are associated with reduced disease in COVID‐19 patients, suggesting the importance of SARS‐CoV‐2‐specific CD4+ and CD8+ T‐cell responses for effective protective immunity. 33 , 34 , 35 The spike protein is the SARS‐CoV‐2 component of both mRNA and adenovirus‐based vaccines and is a good target for CD4+ and CD8+ T‐cell responses. 34 , 36 , 37 To date, there are limited data on T‐cell immunity following SARS‐CoV‐2 vaccination of many immuno‐compromised patient groups including CLL. 18 , 38 , 39 Here, we collected PB samples from individuals prior to and after vaccination to ascertain how T‐cellular immune response emerged after SARS‐CoV‐2 vaccine. We identified significant increases in both CD4+ and CD8+ total T‐cell frequencies following vaccine administration in CLL patients independently of the serological status. As shown in previous studies, the magnitude of CD4+ and CD8+ T‐cell expansion depends on the amount of antigen available, as happens in the vaccine‐induced response. 34 , 40 , 41 , 42 , 43 In our study, the expanded T cells found in vaccinated CLL patients represent evidence of immune response to the vaccine, suggesting that patients who suffer from insufficient humoral response might still benefit from vaccination due to the cellular immune responses.
This expansion of total CD4+ and CD8+ T cells was influenced by CLL‐targeted treatments. In BTK‐inhibitor‐treated patients, we detected a strong increase of CD4+ cells compared to other groups after vaccine administration. This is in line with previous data showing that ibrutinib treatment increases numbers of both CD4+ and CD8+ T cells that lack the typical immunophenotypic features of CLL‐exhausted T cells. 44 Thus, despite the impaired humoral response, ibrutinib may contribute to preserve a certain degree of immunization through T‐cell activity. We also found that BCL‐2 inhibitor treatment induced a strong CD4+ T‐cell expansion and the highest increase of CD8+ T‐cell frequency after vaccination. In contrast to our findings, Ludwig et al. reported that venetoclax treatment reshapes the immune system leading to a decrease in circulating T‐cell numbers in mice. 45 Additionally, ex vivo and in vivo BCL‐2 inhibition led to shifting of global T‐cell populations towards a more memory T‐cell state with increased expression of BCL‐2, BCL‐XL, and MCL‐1. 45
Consistently, our in‐depth characterization of T‐cell subpopulations showed that before vaccination there was a lower frequency of CD4+ Naïve T cells and an expansion of EM T cells in BCL‐2‐inhibitor‐treated compared to untreated patients. Similar changes in these cell subsets were also observed after vaccination, suggesting that the poor response to vaccine in venetoclax‐treated patients was associated not only with a poor humoral response, but even with alterations in some specific T‐cell compartments prior to vaccination.
In CLL patients, the T‐cell compartment is dysfunctional as CLL cells determine a chronic stimulation of T cells that leads to a terminally differentiated and an exhausted T‐cell phenotype. 44 , 46 , 47 Under persistent antigenic stimulation such as vaccine, early differentiated CD8+CD27+CD57+ T cells are able to proliferate and differentiate into the most potent effector terminally differentiated CD8+CD27−CD57+ T cells. 22 Our data showed that CD8+ T cells were skewed towards a terminally differentiated phenotype that further expanded after the second dose of vaccination, compatible with the antigen stimulation.
Notably the ratio between terminally and early differentiated CD8+ T cells was increased both in patients developing and not developing antibodies after vaccination. These results suggest that CLL patients showing both humoral and T‐cell responses could be those who will better control a potential COVID‐19 infection. However, even in seronegative patients, the induction of a T cell‐mediated cytotoxicity by vaccination could be important, given the crucial role of cellular immunity for the viral clearance.
Our data also demonstrated the presence of functional spike‐specific T‐cell responses to vaccine, due to an increase of CD4+ T cells producing either IFNγ or TNFα, in a significant fraction of CLL patients. These findings are in line with previous data indicating that the presence of anti‐spike antibodies depends on spike‐specific CD4+ T cells. 34 , 48 Additionally, we found a more prominent CD4+ T‐cell response to SARS‐CoV‐2 vaccination than CD8+ T‐cell responses, confirming previous findings. 34 This cellular response was independent of the serological status as T‐cell responses were elicited in the absence or presence of circulating antibodies, consistent with a non‐redundant role as key determinants of immune protection against COVID‐19. 26 This contribution of a T‐cellular response to vaccination might implement the compromised humoral immunity protection against various current and emerging SARS‐CoV‐2 variants. 49
This study showed that CLL‐directed treatments influenced the magnitude of spike‐specific T‐cell responses to vaccine. Analysis on CLL treatment subgroups revealed a significantly higher production of IFNγ and TNFα by CD4+ T cells in patients under a BTK inhibitor compared to other treatments, while cytokine levels in CD8+ T cells were not influenced by CLL treatment. Published reports suggested a potential benefit from ibrutinib and acalabrutinib treatment in people with severe COVID‐19 infection. 2 In fact, ibrutinib treatment overcomes the negative impact of CLL on the cellular immunity 44 and could play an anti‐COVID‐19 effect by modulating cytokines and T cells. 50 In our study, the relatively low numbers of patients in each treatment subgroup do not allow to draw definite conclusions in favour of a specific CLL therapeutic choice during the pandemic.
Recent evidence demonstrated that ibrutinib restores T‐cell number and function in CLL patients by binding interleukin‐2‐inducible kinase (ITK) and by reducing the expression of exhaustion and inhibitory markers such as PD‐1 and CTLA‐4. 44 The ITK binding subverts Th2 immunity thereby potentiating Th1‐based immune responses. Generally, CLL cells produce IL‐6 and stimulate IL‐4 production by T cells, skewing the immune system towards a Th2 phenotype. 24 , 51 , 52 Less severe cases of SARS were associated with accelerated induction of a Th1 cell response, 46 , 53 whereas Th2 cell responses have been associated with enhancement of lung disease following infection in hosts parenterally vaccinated with inactivated SARS‐CoV‐2 viral vaccines. 54 , 55 Thus, an ideal COVID‐19 vaccine should be able to induce a Th1 cell‐like phenotype. Here, we demonstrated a significant expansion of Th1 cells after SARS‐CoV‐2 spike peptide stimulation supported by an increased production of IFNγ and TNFα cytokines by CD4+ cells. In addition, vaccinated CLL patients showed a significant impairment of the Th2 profile documented by a decrease of IL‐4‐producing CD4+ T cells. Interestingly, untreated and ibrutinib‐treated CLL showed the most relevant reduction of antigen‐specific IL‐4 producing Th2 cells and higher Th1 cytokines than other treatment groups, demonstrating that ibrutinib treatment may potentiate Th1‐based immune responses to SARS‐CoV‐2 vaccination. Further studies in larger cohorts of vaccinated CLL are needed to confirm this Th1 subset skewing in BTK‐inhibitor‐treated patients.
Overall, our study showed that although CLL patients did not develop a complete serological response to SARS‐CoV‐2 vaccination, a significant fraction of patients showed a T‐cellular response mediated by both terminally differentiated CD8+ T cells and functional Th1 cells. T‐cell responses were influenced by different CLL treatments with patients under a BTK inhibitor displaying higher IFNγ and TNFα production in CD4+ cells than in others. However, more evidence is needed to support potential implications for the therapeutic choice for CLL patients during the COVID‐19 vaccination era, given the small number of patients analysed in each treatment subgroup. Combining T‐cell metrics with seroprevalence may yield a more accurate measure of protective immunity in CLL patients providing consequential insights for public health.
AUTHOR CONTRIBUTIONS
Daniele Sorcini was responsible for data collection, statistical analysis and interpretation, and manuscript preparation; Filomena De Falco performed research, analysed results and contributed to manuscript preparation; Marco Gargaro performed T‐cell polarization experiments, Silvia Bozza and Alessandro Graziani performed serological analysis, Valerio Guarente, Valeria Cardinali, Alessandra Cipiciani provided clinical data, Arianna Stella, Francesco Maria Adamo, Estevao Carlos Silva Barcelos, Chiara Rompietti, Erica Dorillo, Clelia Geraci, Angela Esposito, Roberta Arcaleni, Silvia Capoccia, Maria Grazia Mameli, Lorenzo Moretti, contributed to performing experiments, Carlo Riccardi, Antonella Mencacci, Francesca Fallarino, Emanuela Rosati reviewed the manuscript and provided critical suggestions, Paolo Sportoletti designed, conceived and coordinated the study, analysed the statistics and wrote and revised the manuscript.
FUNDING INFORMATION
This work was partially funded by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG 2018‐ID. 21352 to Paolo Sportoletti); FIRC‐AIRC (3‐years fellowship ‘Filomena Todini’ ID. 23928 to Chiara Rompietti); 3‐years fellowship ‘Leonino Fontana and Maria Lionello’ ID. 26617 to Francesco Maria Adamo.
CONFLICT OF INTEREST
The authors declare no competing interests.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Figure S7.
Figure S8.
Figure S9.
Figure S10.
Table S1.
Table S2.
Table S3.
Table S4.
ACKNOWLEDGEMENTS
We are grateful to the patients who consented to the anonymous use of their clinical data for this study. This work was partially funded by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG 2018‐ID. 21352 to Paolo Sportoletti); FIRC‐AIRC (3‐years fellowship “Filomena Todini” ID. 23928 to Chiara Rompietti; 3‐years fellowship “Leonino Fontana and Maria Lionello” ID. 26617 to Francesco Maria Adamo).
Sorcini D, De Falco F, Gargaro M, Bozza S, Guarente V, Cardinali V, et al. Immune correlates of protection by vaccine against SARS‐CoV‐2 in patients with chronic lymphocytic leukaemia. Br J Haematol. 2022;00:1–13. 10.1111/bjh.18602
Daniele Sorcini and Filomena De Falco contributed equally.
Contributor Information
Daniele Sorcini, Email: daniele.sorcini@unipg.it.
Paolo Sportoletti, Email: paolo.sportoletti@unipg.it.
DATA AVAILABILITY STATEMENT
All data and information concerning this study will be made available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–43. 10.1182/blood.2020006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, et al. COVID‐19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European research initiative on CLL, and CLL campus. Leukemia. 2020;34(9):2354–63. 10.1038/s41375-020-0959-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fürstenau M, Langerbeins P, De Silva N, Fink AM, Robrecht S, von Tresckow J, et al. COVID‐19 among fit patients with CLL treated with venetoclax‐based combinations. Leukemia. 2020;34(8):2225–9. 10.1038/s41375-020-0941-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuneo A, Scarfò L, Reda G, Varettoni M, Quaglia FM, Marchetti M, et al. Chronic lymphocytic leukemia management in Italy during the COVID‐19 pandemic: a campus CLL report. Blood. 2020;136(6):763–6. 10.1182/blood.2020006854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatzikonstantinou T, Kapetanakis A, Scarfò L, Karakatsoulis G, Allsup D, Cabrero AA, et al. COVID‐19 severity and mortality in patients with CLL: an update of the international ERIC and campus CLL study. Leukemia. 2021;35(12):3444–54. 10.1038/s41375-021-01450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuneo A, Rigolin GM, Coscia M, Quaresmini G, Scarfò L, Mauro FR, et al. Management of chronic lymphocytic leukemia in Italy during a one year of the COVID‐19 pandemic and at the start of the vaccination program. A Campus CLL report. Hematol Oncol. 2021;39(4):570–4. 10.1002/hon.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desai A, Gainor JF, Hegde A, Schram AM, Curigliano G, Pal S, et al. COVID‐19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18(5):313–9. 10.1038/s41571-021-00487-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018;11(1):57–70. 10.1080/17474086.2018.1407645 [DOI] [PubMed] [Google Scholar]
- 10. Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid‐19 vaccination in patients with chronic lymphocytic leukemia. Blood Cancer J. 2021;11(7):136. 10.1038/s41408-021-00528-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. 10.1182/blood.2021011568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roeker LE, Knorr DA, Pessin MS, Ramanathan LV, Thompson MC, Leslie LA, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047–9. 10.1038/s41375-020-01030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS‐CoV‐2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–3. 10.1016/j.ccell.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N, et al. Safety and efficacy of the BNT162b mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107(3):625–34. 10.3324/haematol.2021.279196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teh JSK, Coussement J, Neoh ZCF, Spelman T, Lazarakis S, Slavin MA, et al. Immunogenicity of COVID‐19 vaccines in patients with hematologic malignancies: a systematic review and meta‐analysis. Blood Adv. 2022;6(7):2014–34. 10.1182/bloodadvances.2021006333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liebers N, Speer C, Benning L, Bruch PM, Kraemer I, Meissner J, et al. Humoral and cellular responses after COVID‐19 vaccination in anti‐CD20‐treated lymphoma patients. Blood. 2022;139(1):142–7. 10.1182/blood.2021013445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen Y, Freeman JA, Holland J, Solterbeck A, Naidu K, Soosapilla A, et al. COVID‐19 vaccine failure in chronic lymphocytic leukemia and monoclonal B‐lymphocytosis; humoural and cellular immunity. Br J Haematol. 2022;197(1):41–51. 10.1111/bjh.18014 [DOI] [PubMed] [Google Scholar]
- 18. Mellinghoff SC, Robrecht S, Mayer L, Weskamm LM, Dahlke C, Gruell H, et al. SARS‐CoV‐2 specific cellular response following COVID‐19 vaccination in patients with chronic lymphocytic leukemia. Leukemia. 2022;36(2):562–5. 10.1038/s41375-021-01500-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrington P, Doores KJ, Radia D, O'Reilly A, Lam HPJ, Seow J, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) induces neutralising antibody and polyfunctional T‐cell responses in patients with chronic myeloid leukemia. Br J Haematol. 2021;194(6):999–1006. 10.1111/bjh.17568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breccia M, Abruzzese E, Accurso V, Attolico I, Barulli S, Bergamaschi M, et al. COVID‐19 infection in chronic myeloid leukemia after one year of the pandemic in Italy. A campus CML report. Br J Haematol. 2022;196(3):559–65. 10.1111/bjh.17890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- 22. Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. 10.1111/j.1365-2567.2011.03470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wingender G, Kronenberg M. OMIP‐030: characterization of human T cell subsets via surface markers. Cytometry A. 2015;87(12):1067–9. 10.1002/cyto.a.22788 [DOI] [PubMed] [Google Scholar]
- 24. Puzzolo MC, Del Giudice I, Peragine N, Mariglia P, De Propris MS, Cappelli LV, et al. TH2/TH1 shift under ibrutinib treatment in chronic lymphocytic leukemia. Front Oncol. 2021;11:637186. 10.3389/fonc.2021.637186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vardhana S, Baldo L, Morice WG 2nd, Wherry EJ. Understanding T cell responses to COVID‐19 is essential for informing public health strategies. Sci Immunol. 2022;7(71):eabo1303. 10.1126/sciimmunol.abo1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158–168.e14. 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rijkers G, Murk JL, Wintermans B, van Looy B, van den Berge M, Veenemans J, et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis. 2020;222(8):1265–9. 10.1093/infdis/jiaa463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mauro FR, Giannarelli D, Galluzzo CM, Vitale C, Visentin A, Riemma C, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021;35(3):737–46. 10.1038/s41375-020-0884-z [DOI] [PubMed] [Google Scholar]
- 29. Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IE, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185–9. 10.1182/blood.2020008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397–e399. 10.3324/haematol.2017.164285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bradley BT, Bryan A, Fink SL, Goecker EA, Roychoudhury P, Huang ML, et al. Anti‐SARS‐CoV‐2 antibody levels measured by the AdviseDx SARS‐CoV‐2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J Clin Microbiol. 2021;59(9):e0098921. 10.1128/JCM.00989-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS‐CoV‐2 antibody response following vaccination with BNT162b2 and mRNA‐1273. JAMA. 2021;326(15):1533–5. 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X, et al. Acute SARS‐CoV‐2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–877.e5. 10.1016/j.immuni.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861–80. 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan AT, Linster M, Tan CW, le Bert N, Chia WN, Kunasegaran K, et al. Early induction of functional SARS‐CoV‐2‐specific T cells associates with rapid viral clearance and mild disease in COVID‐19 patients. Cell Rep. 2021;34(6):108728. 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koch T, Mellinghoff SC, Shamsrizi P, Addo MM, Dahlke C. Correlates of vaccine‐induced protection against SARS‐CoV‐2. Vaccines (Basel). 2021;9(3):238. 10.3390/vaccines9030238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature. 2020;587(7833):270–4. 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 40. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T‐cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. 10.1038/nri778 [DOI] [PubMed] [Google Scholar]
- 41. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–9. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 42. Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8+ T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27(7):1280–9. 10.1038/s41591-021-01386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS‐CoV‐2 in UK convalescent individuals following COVID‐19. Nat Immunol. 2020;21(11):1336–45. 10.1038/s41590-020-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–64. 10.1172/JCI89756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ludwig LM, Hawley KM, Banks DB, Thomas‐Toth AT, Blazar BR, McNerney ME, et al. Venetoclax imparts distinct cell death sensitivity and adaptivity patterns in T cells. Cell Death Dis. 2021;12(11):1005. 10.1038/s41419-021-04285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–32. 10.1038/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T cells from CLL patients exhibit features of T‐cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–21. 10.1182/blood-2012-09-457531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blixt L, Wullimann D, Aleman S, Lundin J, Chen P, Gao Y, et al. T‐cell immune responses following vaccination with mRNA BNT162b2 against SARS‐CoV‐2 in patients with chronic lymphocytic leukemia: results from a prospective open‐label clinical trial. Haematologica. 2022;107(4):1000–3. 10.3324/haematol.2021.280300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li L, Muftuoglu M, Liang S, Basyal M, Lv J, Akdogan ME, et al. In‐depth analysis of SARS‐CoV‐2‐specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight. 2022;7(7):e156559. 10.1172/jci.insight.156559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGee MC, August A, Huang W. BTK/ITK dual inhibitors: modulating immunopathology and lymphopenia for COVID‐19 therapy. J Leukoc Biol. 2021;109(1):49–53. 10.1002/JLB.5COVR0620-306R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–83. 10.1016/s1074-7613(00)80533-6 [DOI] [PubMed] [Google Scholar]
- 52. Vazquez MI, Catalan‐Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74(2):318–26. 10.1016/j.cyto.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janice Oh HL, Ken‐En Gan S, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg Microbes Infect. 2012;1(9):e23. 10.1038/emi.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. A double‐inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–15. 10.1128/JVI.06048-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tseng CT, Sbrana E, Iwata‐Yoshikawa N, Newman PC, Garron T, Atmar RL, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. 10.1371/journal.pone.0035421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Figure S7.
Figure S8.
Figure S9.
Figure S10.
Table S1.
Table S2.
Table S3.
Table S4.
Data Availability Statement
All data and information concerning this study will be made available from the corresponding authors upon reasonable request.


