Abstract
The aim of this study was to evaluate the effect and safety of N‐acetylcysteine (NAC) inhalation spray in the treatment of patients with coronavirus disease 2019 (COVID‐19). This randomized controlled clinical trial study was conducted on patients with COVID‐19. Eligible patients (n = 250) were randomly allocated into the intervention group (routine treatment + NAC inhaler spray one puff per 12 h, for 7 days) or the control group who received routine treatment alone. Clinical features, hemodynamic, hematological, biochemical parameters and patient outcomes were assessed and compared before and after treatment. The mortality rate was significantly higher in the control group than in the intervention group (39.2% vs. 3.2%, p < 0.001). Significant differences were found between the two groups (intervention and control, respectively) for white blood cell count (6.2 vs. 7.8, p < 0.001), hemoglobin (12.3 vs. 13.3, p = 0.002), C‐reactive protein (CRP: 6 vs. 11.5, p < 0.0001) and aspartate aminotransferase (AST: 32 vs. 25.5, p < 0.0001). No differences were seen for hospital length of stay (11.98 ± 3.61 vs. 11.81 ± 3.52, p = 0.814) or the requirement for intensive care unit (ICU) admission (7.2% vs. 11.2%, p = 0.274). NAC was beneficial in reducing the mortality rate in patients with COVID‐19 and inflammatory parameters, and a reduction in the development of severe respiratory failure; however, it did not affect the length of hospital stay or the need for ICU admission. Data on the effectiveness of NAC for Severe Acute Respiratory Syndrome Coronavirus‐2 is limited and further research is required.
Keywords: clinical trial, N‐acetylcysteine, Severe Acute Respiratory Syndrome Coronavirus‐2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a highly infectious respiratory disease caused by a new coronavirus, Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2). COVID‐19 disease emerged in Wuhan city, China in late 2019 and resulted in a global pandemic leading to more than 5.4 million deaths worldwide. 1 , 2 The rapid transmission of this highly pathogenic virus has necessitated the urgent need for global development and immediate application of therapeutic approaches and preventive measures against COVID‐19 disease. 3 Prevaccination, supportive care and oxygen therapy remain key management elements of COVID‐19 though there is no effective treatment for acute COVID‐19 disease. 4 , 5 , 6 The emergence of SARS‐CoV‐2 variants have increased the transmissibility and severity of infection and may also reduce the level of immunity of current vaccines. 7 , 8 , 9 In addition, the unequal distribution of vaccines worldwide has made it difficult to curtail the pandemic, 10 , 11 hence the need to accelerate the discovery and development of safe and effective agents as adjunctive therapies.
The pathogenetic mechanism of COVID‐19 is not fully understood 12 ; however, upon reaching the lower respiratory tract, it appears that the virus spike protein binds to the angiotensin‐converting enzyme 2 (ACE2), allowing it to enter the alveolar cells. 13 ACE2 is an enzyme that catalyzes the conversion of angiotensin II to angiotensin 1−7; therefore, elevated levels of serum angiotensin II in patients with COVID‐19 indicate the inactivation of this enzyme. 14 The increased levels of angiotensin II after inactivation of ACE2 by SARS‐CoV‐2 leads to a redox imbalance in the alveolar epithelium cells, causing apoptosis, breakdown of the alveolar‐capillary barrier resulting in impaired gas exchange and respiratory failure. 15 , 16 , 17 Evidence suggests that the excessive production of angiotensin II can be blocked by N‐acetylcysteine (NAC), preventing its cleavage to angiotensin 1–7 by ACE2, which may reduce pulmonary disease severity. 18 , 19 , 20
NAC has been used as a mucolytic in clinical practice for several decades to treat medical conditions including bronchitis, acute respiratory distress syndrome (ARDS), paracetamol intoxication, chemotherapy‐related toxicity, doxorubicin cardiotoxicity, heavy metal intoxication, ischemia‐reperfusion cardiac injury, human immunodeficiency virus infection or acquired immunodeficiency syndrome, and neuropsychiatric disorders. 21 , 22 , 23 , 24 NAC's antioxidant effect may attenuate the oxidative stress environment created by cytokine storm syndrome and the production of reactive oxygen species (ROS). 25 Given these properties, we hypothesized that NAC might be a potential adjuvant therapy against SARS‐CoV‐2. Therefore, we conducted this single‐center, prospective, randomized, open‐labeled, controlled clinical trial study to evaluate the therapeutic effect and safety of NAC inhalation spray in patients with COVID‐19.
2. MATERIALS AND METHODS
2.1. Trial design
This single‐center, prospective, randomized, open‐labeled, controlled clinical trial was conducted to investigate the therapeutic efficacy of NAC inhalation spray in the clinical management of patients with COVID‐19 admitted to Baqiyatallah hospital, Tehran, Iran, from May 2021 until August 2021. The protocol study was reviewed and approved by the Ethics Committees of Baqiyatallah University of Medical Sciences (IR.BMSU.REC.1399.123), following the Declaration of Helsinki of the World Medical Association. 26 This study was registered at the Iranian Registry of Clinical Trials (IRCT20080901001165N55) dated 23‐05‐2020. Written informed consent was obtained from all patients or their legal representatives if they could not provide consent. The study was conducted and reported following the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement. 27
2.2. Study population
Symptomatic adult patients with a positive COVID‐19 diagnosis were eligible for study participation if they met all the inclusion criteria. Inclusion criteria included (a) age of ≥18, (b) positive result on a reverse‐transcriptase–polymerase‐chain‐reaction assay of a specimen collected from a nasopharyngeal swab that confirmed COVID‐19 infection, (c) chest computed tomography (CT) results that confirmed COVID‐19 infection and (d) less than 7 days since the onset of symptoms. The exclusion criteria included (a) any allergy or hypersensitivity to NAC, (b) signs of the imminent need for intubation or the need for intensive care unit (ICU) admission due to increased respiratory effort, decreased level of consciousness, and oxygen saturation (SpO2) less than 90% with supplemental oxygen, (c) being a participant in another clinical trial at the same time, (d) pregnancy or breastfeeding and (e) unwilling to participate or unable to give informed consent.
2.3. Randomization and intervention
Eligible patients were randomly assigned (1:1) to the NAC or the control groups. The Block randomization method was used to randomize the patients. The randomization code was generated by computer in permuted blocks of 6. Block randomization was performed using the sealed envelope technique and computer‐generated random numbers by Random Allocation Software© (RAS; Informer Technologies, Inc.). All patients in the study groups received standard care regimens based on the latest version of the Ministry of Health of Iran protocols for COVID‐19. 28 In addition to routine treatment, the intervention group received NAC spray (@sinadarou.co) one puff (200 μg per puff) every 12 h for 7 days.
2.4. Data collection
Clinical data were collected on the specific case report forms for each patient that included: (a) demographics information: (age, gender, height, weight, and body mass index); (b) comorbidities: diabetes mellitus, hypertension, chronic kidney disease, malignancy, asthma and chronic obstructive pulmonary disease; (c) symptoms: fever, chills, dyspnea, chest pain, cough, sore throat, headache, myalgia, diarrhea, nausea, vomiting, olfactory, and generalized weakness; (d) vital signs or hemodynamic parameters: systolic blood pressure (SBP‐mmHg), diastolic blood pressure (DBP‐mmHg), heart rate (HR) beats per minute (BPM), respiratory rate (RR per minute) and oxygen saturation (SpO2‐%); (e) hematological data: white blood cell (WBC‐cell/mm3), neutrophil count (cells/µl), lymphocyte count (cell/mm3), platelet (Plt‐cell/mm3) and hemoglobin (Hb‐g/dl); (f) biochemical data: erythrocyte sedimentation rate (ESR‐mm/h), C‐reactive protein (CRP‐mg/L), creatinine (CR‐mg/dl), urea (mg/dl), aspartate aminotransferase (AST‐U/L) and alanine aminotransferase (ALT‐U/L); (g) CT score based on chest CT scan (noted below); (h) National Early Warning Score (NEWS‐noted below); (i) hospital length of stay (LOS‐day); (j) need for ICU admission and (k) mortality. All symptoms, hemodynamic, hematological and biochemical parameters were collected for each patient before and after the intervention.
2.5. Chest CT scan
All patients underwent chest CT examinations in a supine position before and after the intervention. CT scan examinations were performed with a 16‐row detector CT scanner (general electric GE; optima) and reviewed by two radiologists with 12 and 10 years of experience in thoracic imaging. To quantify the extent of lung lesions, a thin‐section CT involvement score was assigned based on all abnormal areas shown: each of the five lobes of the lung was assigned a visual score: score 0, no involvement; score 1, less than 5% involvement; score 2, 5%−25% involvement; score 3, 26%−49% involvement; score 4, 50%−75% involvement; and score 5, greater than 75% involvement. There was a score of 0−5 for each lobe, with a total possible score of 0−25. 29
2.6. Calculation of NEWS score
NEWS score included the following seven vital signs parameters: RR, oxygen saturation (SpO2) that was measured by pulse oximetry, supplementary oxygen requirement, systolic arterial blood pressure (SBP), pulse rate (PR), body temperature (T), and AVPU (Alert, responds to Voice, responds to Pain, Unresponsive) score based on the Glasgow Coma Scale (GCS) (The AVPU score was derived from the GCS as follows: A = 14−15, V = 9−13, P = 4−8, U = 3). 30 Patients with a score between 0 and 4 were considered to have low risk, those with a score of 5 or 6 were considered to have medium risk, and those with a score ≥ 7 were considered to have a high risk. 30
2.7. Statistical analysis
The Shapiro–Wilk test was used to test data normality. Normal distributed data were reported as mean and standard deviation for continuous variables and percentage (%) for categorical characteristics. Non‐normal distributed continuous data were reported as median and interquartile range. Comparison of demographic characteristics and clinical variables between two study groups were assessed using χ 2 or Fisher's exact tests for categorical variables and Mann–Whitney u test for non‐normally distributed continual variables. To compare symptoms as categorical variables, we used the χ 2 test or Fisher exact test between two groups and the McNemar test within groups pre and postintervention. Mann–Whitney u test and Wilcoxon Ranks test was used for comparing the non‐normal distributed continuous variables (hemodynamic, hematological, biochemical, CT score, and NEWS score) between groups and within groups pre and postintervention. All statistical analysis was carried out using SPSS software (ver.21) (SPSS Inc.). In all analyses, p Values less than 0.05 were considered significant.
3. RESULTS
3.1. Trial population
From May 2021 to August 2021, 250 out of 260 patients with COVID‐19 referred to Baqiyatallah Hospital in Tehran, Iran, met the inclusion criteria and gave informed written consent to participate in the study. Patients were randomly assigned through block randomization 1:1 into the intervention group (n = 125) or the control group (n = 125). No patient (in either study group) was eliminated during the follow‐up period, and 250 patients completed the study and were included in the analysis. Figure 1 shows the consolidated standards of reporting trials (CONSORT) flowchart of participants through each stage of a randomized trial.
Figure 1.
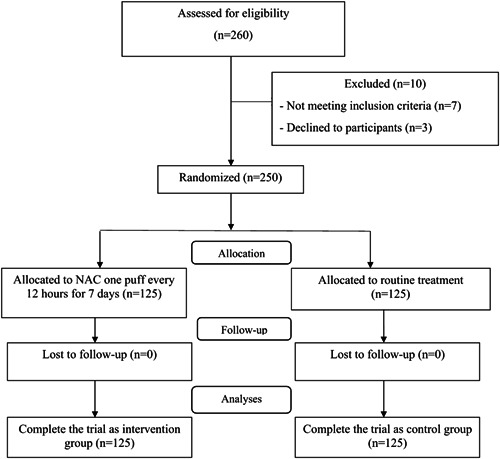
CONSORT flow diagram for this study. CONSORT, Consolidated Standards of Reporting Trials.
3.2. Demographic characteristics and clinical outcomes
A comparison of the demographic characteristics and clinical outcomes between the two groups is presented in Table 1. Two hundred and fifty patients with COVID‐19, including 138 (55.2%) men and 112 (44.8%) women, were enrolled in this study. Patients in the intervention and control groups showed similar demographic characteristics and clinical data. There was no difference between the two groups in terms of gender (p = 0.203), smoking status (p = 0.811), comorbidities (p = 0.899), height (0.548), weight (p = 0.112), and BMI (p = 0.052). However, the mean age of patients in the intervention group was significantly higher than in the control groups (57.25 ± 15.80 vs. 52.77 ± 16.21, p = 0.02). Regarding the clinical outcomes, there was no difference between the two groups for hospital LOS (p = 0.814) and the need for ICU admission (p = 0.274); however, nearly 40% of patients in the control group died, which was significantly higher than the intervention group (39.2% vs. 3.2%, p < 0.001).
Table 1.
Comparison of demographic characteristics and clinical data between two groups of study
| Variables | Total participants (n = 250) | Intervention group (n = 125) | Control group (n = 125) | p Value | |
|---|---|---|---|---|---|
| Gender | Male (%) | 138 (55.2) | 64 (51.2) | 74 (59.2) | 0.203a |
| Female (%) | 112 (44.8) | 61 (48.8) | 51 (40.8) | ||
| Age (year) | Mean ± SD (Range) | 55.01 ± 16.13 (18−90) | 57.25 ± 15.80 (18−90) | 52.77 ± 16.21 (24−89) | 0.022 * , b |
| Median (IQR) | 54 (41‐67) | 56 (45−71) | 53 (39−65) | ||
| Hight (cm) | Mean ± SD (Range) | 169.76 ± 10.24 (124−194) | 169.53 ± 10.62 (150−194) | 169.99 ± 9.88 (124−191) | 0.548b |
| Median (IQR) | 170 (161−178) | 168 (160−179) | 169.9 (162. 5−178) | ||
| Weight (kg) | Mean ± SD (Range) | 77.86 ± 17.08 (50–171) | 76.72 ± 18.87 (50−171) | 78.96 ± 15.08 (50−120) | 0.112b |
| Median (IQR) | 76 (67−86) | 75 (64−85) | 78 (67.5−87.5) | ||
| BMI | Mean ± SD (Range) | 27.07 ± 5.79 (16.79−60.55) | 26.77 ± 6.63 (16.79−60.55) | 27.37 ± 4.81 (19.53−43.57) | 0.052b |
| Median (IQR) | 26.19 (23.30−29.37) | 25.05 (22.8−28.7) | 26.66 (23.8−29.7) | ||
| Smoking status | Yes (%) | 19 (7.6) | 10 (8) | 9 (7.2) | 0.811a |
| No (%) | 231 (92.4) | 115 (92) | 116 (92.8) | ||
| Comorbidities | Yes (%) | 111 (44.4) | 56 (44.8) | 55 (44) | 0.899a |
| No (%) | 139 (55.6) | 69 (55.2) | 70 (56) | ||
| Comorbidity | DM (%) | 49 (19.6) | 23 (18.4) | 26 (20.8) | 0.633a |
| types | HTN (%) | 63 (25.2) | 29 (23.2) | 34 (27.2) | 0.466a |
| IHD (%) | 26 (10.4) | 14 (11.2) | 12 (9.6) | 0.679a | |
| CKD (%) | 20 (8.1) | 8 (6.4) | 12 (9.6) | 0.351a | |
| Cancer (%) | 6 (2.3) | 5 (4) | 1 (0.8) | 0.213c | |
| Asthma (%) | 8 (3.2) | 6 (4.8) | 2 (1.6) | 0.281c | |
| COPD (%) | 4 (1.6) | 3 (2.4) | 1 (0.8) | 0.622c | |
| Hospital LOS | Mean ± SD (Range) | 11.89 ± 3.56 (7−37) | 11.98 ± 3.61 (8−31) | 11.81 ± 3.52 (7–37) | 0.814b |
| Median (IQR) | 11 (10−13) | 11 (10−13) | 12 (10−13) | ||
| ICU needed | Yes (%) | 23 (9.2) | 9 (7.2) | 14 (11.2) | 0.274a |
| No (%) | 227 (90.8) | 116 (92.8) | 111 (88.8) | ||
| Mortality | Yes (%) | 53 (21.2) | 4 (3.2) | 49 (39.2) | <0.001 * , a |
| No (%) | 197 (78.8) | 121 (96.8) | 76 (60.8) | ||
Note: Bold values are statistically significant p values.
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; IHD, ischemic heart disease; HTN, hypertension.
p Value based on χ 2 test;
p Value based on Mann–Whitney u test;
p Value based on Fisher exact test;
p < 0.05 considered as significant.
3.3. Symptoms
A comparison of pre and postintervention symptoms between two study groups of study is shown in Table 2. Four common symptoms in both groups were cough (60.4%), myalgia (51.2%), dyspnea (50.4%), and fever (46.4%). There were no differences in the frequency of symptoms preintervention among patients in the NAC and control groups, indicating the homogeneity of the participants in the study (p > 0.05). In postintervention, dyspnea (7.2% vs. 19.2%, p = 0.005) and generalized weakness (3.2% vs. 9.6%, p = 0.039) were significantly reduced in the NAC group than the control group. In addition, all symptoms were significantly decreased within groups pre and postintervention (p < 0.001).
Table 2.
Comparison of symptoms on pre‐ and post‐intervention between two groups of study
| Symptoms | Intervention group (n = 125) | Control group (n = 125) | p Valuea | |
|---|---|---|---|---|
| Fever | Before (yes, %) | 63 (50.4) | 53 (42.4) | 0.205 |
| After (yes, %) | 4 (3.2) | 1 (0.8) | 0.175 | |
| p Value b | <0.001 * | <0.001 * | ||
| Chills | Before (yes, %) | 58 (46.4) | 56 (44.8) | 0.800 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Cough | Before (yes, %) | 73 (58.4) | 78 (62.4) | 0.518 |
| After (yes, %) | 12 (9.6) | 18 (14.4) | 0.243 | |
| p Value b | <0.001 * | <0.001 * | ||
| Dyspnea | Before (yes, %) | 60 (48.4) | 66 (53.2) | 0.446 |
| After (yes, %) | 9 (7.2) | 24 (19.2) | 0.005 * | |
| p Value b | <0.001 * | <0.001 * | ||
| Chest pain | Before (yes, %) | 33 (26.4) | 33 (26.4) | 0.970 |
| After (yes, %) | 8 (6.4) | 5 (4) | 0.393 | |
| p Value b | <0.001 * | <0.001 * | ||
| Sore throat | Before (yes, %) | 25 (20) | 27 (21.6) | 0.755 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Headache | Before (yes, %) | 30 (24) | 38 (30.4) | 0.256 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Myalgia | Before (yes, %) | 58 (46.4) | 70 (56) | 0.129 |
| After (yes, %) | 5 (4) | 5 (4) | 0.999 | |
| p Value b | <0.001 * | <0.001 * | ||
| Diarrhea | Before (yes, %) | 19 (15.2) | 28 (22.4) | 0.145 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Nausea | Before (yes, %) | 40 (32) | 35 (28) | 0.490 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Vomiting | Before (yes, %) | 10 (8) | 14 (11.2) | 0.390 |
| After (yes, %) | 0 | 0 | ||
| p Value b | <0.001 * | <0.001 * | ||
| Olfactory | Before (yes, %) | 34 (27.2) | 24 (19.2) | 0.057 |
| After (yes, %) | 11 (8.8) | 10 (8) | 0.668 | |
| p Value b | <0.001 * | <0.001 * | ||
| Weakness | Before (yes, %) | 43 (34.4) | 54 (43.2) | 0.153 |
| After (yes, %) | 4 (3.2) | 12 (9.6) | 0.039 * | |
| p Value b | <0.001 * | <0.001 * | ||
Note: Bold values are statistically significant p values.
p Value based on χ2 test or Fisher exact test;
p Value based on McNemar test;
p < 0.05 considered as significant.
3.4. Vital signs, hematological, and biochemical parameters findings
Comparison of vital signs and hematological parameters pre and postintervention between two groups of study are shown in Table 3. There were no differences between the two groups in terms of vital signs and hematological parameters, except (intervention vs. control) RR (18 vs. 17, p = 0.036), SpO2 (93% vs. 92%, p = 0.014) and lymphocytes (6.8 vs. 11.0, p < 0.001). The results for postintervention (intervention vs. control) showed significant differences in the median of WBC (6.2 vs. 7.8, p < 0.001) and Hb (12.3 vs. 13.3, p = 0.002) between groups. Significant differences were seen for pre and postintervention for vital signs and hematological parameters (p < 0.001), except for DBP and WBC.
Table 3.
Comparison of vital signs and hematological parameters on pre and postintervention between two groups of study
| Parameters | Intervention group (n = 125) | Control group (n = 125) | p Valuea | |
|---|---|---|---|---|
| SBP (mmHg) | Before | 130 (110−135) | 130 (120−139.5) | 0.194 |
| After | 125 (120−130) | 125 (120–130) | 0.332 | |
| p Value b | 0.043 * | 0.009 * | ||
| DBP (mmHg) | Before | 80 (73−89) | 80 (75−90) | 0.533 |
| After | 80 (75−85) | 80 (75−85) | 0.816 | |
| p Value b | 0.582 | 0.223 | ||
| HR (BPM) | Before | 96 (87−103) | 95 (87−100) | 0.402 |
| After | 83 (78−90) | 83 (78−91) | 0.427 | |
| p Value b | <0.0001 * | <0.001 * | ||
| RR (RPM) | Before | 18 (16−19) | 17 (16–18) | 0.036 * |
| After | 14 (14−15) | 15 (14–16) | 0.091 | |
| p Value b | <0.0001 * | <0.0001 * | ||
| SpO2 (%) | Before | 93 (93−96) | 92 (89−94) | 0.014 * |
| After | 94 (93−96) | 94 (93−95) | 0.581 | |
| p Value b | <0.0001 * | <0.0001 * | ||
| WBC | Before | 6.97 (5.08−9.505) | 6.86 (5.20−9.35) | 0.981 |
| (×103 cell/mm3) | After | 6.2 (5.06−8.050) | 7.80 (5.89−10.17) | <0.001 * |
| p Value b | 0.070 | 0.088 | ||
| Neutrophils | Before | 82 (75.05−87.95) | 78.2 (71.2–86) | 0.011 * |
| (cells/µL) | After | 69 (58–78) | 67.9 (59−78) | 0.851 |
| p Value b | <0.0001 * | <0.0001 * | ||
| Lymphocyte | Before | 6.8 (4.15−10.75) | 11 (6.800−15.1) | <0.001 * |
| (×103 cell/mm3) | After | 24.4 (17.0−32.7) | 23 (13.85−33.0) | 0.205 |
| p Value b | <0.0001 * | <0.0001 * | ||
| Hb (g/dl) | Before | 13.5 (11.6−15) | 14 (12.5–15.4) | 0.078 |
| After | 12.3 (10.35−13.9) | 13.3 (11.35−14.6) | 0.002 * | |
| p Value b | <0.0001 * | <0.0001 * | ||
| Platelet | Before | 195 (150.5−251.5) | 200 (154.5–240.5) | 0.955 |
| (×103 cell/mm3) | After | 260 (205.5–317.5) | 251 (195−327.5) | 0.764 |
| p Value b | <0.0001 * | <0.0001 * | ||
Note: Bold values are statistically significant p values.
Abbreviations: DBP, diastolic blood pressure; Hb, hemoglobin; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; SpO2,Saturated pressure of oxygen; WBC, white blood cell.
p Value based on Mann–Whitney u test;
p Value based on Wilcoxon Ranks test;
p < 0.05 considered as significant.
Comparison of biochemical parameters pre and postintervention between the two groups of study are presented in Table 4. There were no differences between the two groups, except for urea (27 vs. 17, p < 0.0001) and ALT (31 vs. 37, p = 0.009), which were significantly higher and lower in the NAC group than in the control group, respectively. The results postintervention (intervention vs. control) showed significant differences in median CRP (6.0 vs. 11.5, p < 0.0001) and AST (33 vs. 25.5, p < 0.0001). Significant differences pre and postintervention were seen for biochemical parameters (p < 0.001), except ALT within the control group (p = 0.315).
Table 4.
Comparison of biochemical parameters on pre and postintervention between two groups of study
| Biochemical parameters | Intervention group (n = 125) | Control group (n = 125) | p Valuea | |
|---|---|---|---|---|
| ESR (mm/h) | Before | 32 (13−50.5) | 29 (14.5−54) | 0.878 |
| After | 12 (7−19) | 13 (9−29) | 0.057 | |
| p Value b | <0.0001 * | <0.0001 * | ||
| CRP (mg/L) | Before | 19.7 (10.8−41.65) | 18.10 (6.55−62.70) | 0.423 |
| After | 6 (2−11.80) | 11.5 (6.55–25.1) | <0.0001 * | |
| p Value b | <0.0001 * | <0.0001 * | ||
| CR (mg/dl) | Before | 1.1 (0.930−1.33) | 1.1 (1−1.3) | 0.600 |
| After | 1 (0.875−1.23) | 1.01 (0.90−1.200) | 0.546 | |
| p Value b | <0.003 * | 0.003 * | ||
| Urea (mg/dl) | Before | 27 (22−39) | 17 (13−31) | <0.0001 * |
| After | 19 (14−31) | 19 (13−34) | 0.764 | |
| p Value b | <0.0001 * | 0.1780 | ||
| AST (U/L) | Before | 33 (27−43) | 32 (20–41.75) | 0.087 |
| After | 32 (26–51) | 25.5 (19–41.75) | <0.0001 * | |
| p Value b | 0.039 * | 0.031 * | ||
| ALT (U/L) | Before | 31 (24–45) | 37 (28–51.5) | 0.009 * |
| After | 37 (28.5−51.5) | 37 (23–53) | 0.385 | |
| p Value b | 0.001 * | 0.315 | ||
Note: Bold values are statistically significant p values.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, creatinine; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate.
p Value based on Mann–Whitney u test;
p Value based on Wilcoxon Ranks test;
p < 0.05 considered as significant.
3.5. CT scores and NEWS scores
Comparison of CT score and NEWS score pre and postintervention between the intervention and control groups are shown in Table 5. There were no differences in the median CT and NEWS scores between NAC and control groups preintervention. The median score of CT was significantly lower in the intervention group than in the control group post‐intervention (6 vs. 7, p < 0.0001); however, the median score of NEWS was not different between the two groups postintervention (2 vs. 1, p = 0.184). CT scores differed significantly between groups (p < 0.0001), and both CT and NEWS scores were significantly decreased with groups for both the intervention and control groups (p < 0.0001).
Table 5.
Comparison of CT score and NEWS score on pre‐ and post‐intervention between two groups of study
| Biochemical parameters | Intervention group (n = 125) | Control group (n = 125) | p Valuea | |
|---|---|---|---|---|
| CT score | Before | 9 (7−13) | 10 (7−14) | 0.375 |
| After | 6 (4−8) | 7 (5−12) | <0.0001 * | |
| p Value b | <0.0001 * | <0.0001 * | ||
| NEWS score | Before | 6 (3−7) | 5 (3−6) | 0.148 |
| After | 2 (1−3) | 1 (1−2) | 0.184 | |
| p Value b | <0.0001 * | <0.0001 * | ||
Note: Bold values are statistically significant p values.
p Value based on Mann–Whitney u test;
p Value based on Wilcoxon Ranks test;
p < 0.05 considered as significant.
4. DISCUSSION
In this single‐center, prospective, randomized, controlled trial, and 250 patients with COVID‐19 infection were randomly divided into two equal groups. Patients who received standard of care were compared with patients who additionally received a single puff (200 μg/puff) of inhalation spray of NAC every 12 h for 7 days. Symptoms in both study groups were significantly improved on the seventh day compared to the first day of hospitalization. Treatment with NAC led to significantly lower frequencies of dyspnea and weakness compared to the control group. There were significant differences between the two groups in terms of WBC, Hb, CRP and AST postintervention. Furthermore, both CT score and NEWS score were significantly reduced within both groups; however, NAC treatment significantly reduced the CT score compared to the control group. The mortality rate was significantly lower in the intervention group than in the control group despite the increased age of the subjects randomized to the intervention arm. No differences were observed in vital signs, the requirement for ICU admission and hospital LOS between the intervention and control groups. No adverse drug reaction occurred in any of the study subjects treated with NAC; thus, NAC can be considered a safe adjunct treatment in coronavirus disease.
To date, few clinical studies have examined the therapeutic effect of NAC on COVID‐19 disease. 31 However, the rationale for using NAC in the prevention or adjunct treatment of COVID‐19 has been largely inferred from previous research on other viruses, such as influenza and respiratory syncytial virus. 32 , 33 , 34 SARS‐CoV‐2, by binding to the ACE2 receptors in the host's body, can infect the cells of many structures related to the respiratory, cardiac, neurological, hematological, renal and gastrointestinal systems. COVID‐19 induces a severe cytokine storm syndrome in pulmonary structures, leading to oxidative stress, inflammation and alveolar damage. 35 , 36 A high ratio of ROS to glutathione (GSH) appears to reflect the severity of symptoms and prolonged hospitalization of COVID‐19 patients. 37 NAC, which acts as a precursor of GSH inside cells, has been used in many conditions to restore or protect against GSH depletion and has a wide safety margin. Promising results have been reported in the ability of NAC to synthesize glutathione, improve the T lymphocyte proliferative response and modulate the inflammatory pathway. Glutathione precursors such as NAC have been suggested as a potential therapeutic approach to block nuclear factor kappa‐light‐chain‐enhancer of activated B cells activation and address cytokine storm syndrome and respiratory distress in patients with COVID‐19 pneumonia. 38 , 39 , 40 , 41 During the period of our study, little information was available on the administration of NAC. It was derived mainly from scattered case reports of intravenous or oral administration of NAC, and no information was available on its inhalation. Until now, inhalation administration of NAC has generally been associated with mucolytic activity, in contrast to oral administration of NAC, which is mainly associated with antioxidant activity. This molecule, as well as other thiol derivatives, acts primarily on the lower respiratory tract to loosen mucus, as the main target of the drug is mucin. However, some studies have shown that inhaled NAC is also effective on oxidative stress and that patients with higher oxidative stress may be good responders to inhaled NAC therapy, as the glutathione replenished by inhaled NAC can reverse the oxidant‐antioxidant imbalance. 37 , 42
In accord with the current study, a multi‐center, prospective cohort study by Assimakopoulos et al. 43 in 2021 was conducted on hospitalized patients with moderate or severe COVID‐19: patients who received standard of care were compared with patients who additionally received oral NAC (600 mg) for 14 days. The results showed that oral NAC administration (1200 mg/day) in patients with COVID‐19 pneumonia reduces the risk for mechanical ventilation (MV) and 14‐day and 28‐day mortality. In addition, NAC improved the PO2/FiO2 ratio over time and decreased WBC, CRP, d‐dimer and lactic acid dehydrogenase (LDH) levels. A randomized study by Gaynitdinova et al. 44 in 2021 evaluated NAC efficacy in the treatment of moderate COVID‐19‐associated pneumonia in 22 patients who received standard therapy (hydroxychloroquine, azithromycin, enoxaparin, dexamethasone, and tocilizumab) compared with 24 patients who received NAC (1200–1500 mg/day) intravenously. A significant increase in SpO2 and ventilatory function and a more rapid reduction in the degree of lung damage occurred in the patients receiving NAC compared to the control group. The rate of CRP reduction and the decrease of hospitalization duration in the NAC group was significantly higher compared to the standard treatment group. Moreover, a single‐center, randomized, double‐blind, and placebo‐controlled trial by Altay et al. 45 in 2021 evaluated a combined metabolic cofactor supplementation (CMCS) consisting of l‐serine, NAC, nicotinamide riboside, and l‐carnitine tartrate in 309 adult patients with laboratory‐confirmed COVID‐19 infection, reported reductions in recovery time and liver enzymes associated with hepatic function in CMCS compared to placebo.
Contrary to these positive findings, in a pilot randomized clinical trial, 92 patients with mild‐moderate COVID‐19 associated ARDS were treated with standard‐of‐care treatment and either placebo (n = 45) or intravenous NAC 40 mg/kg/day for three consecutive days besides standard‐of‐care treatment (n = 47). No differences were observed in rates of 28‐day mortality, ICU and hospital stay and invasive MV use between the intervention and control groups. 46 In addition, a double‐blind, placebo‐controlled, randomized, single‐center trial conducted at the Emergency Department of a hospital in Brazil, where 135 patients diagnosed with severe COVID‐19 were assigned 1:1 to either 21 000 mg (approximately 300 mg/kg) of intervenous NAC or placebo. 47 This study found no difference in the progression to severe respiratory failure requiring invasive or noninvasive MV, hospital LOS, admission to ICU and mortality. 47 These conflicting results may be related to differences in the study population (moderate or severe COVID‐19 patients), research environment (ICU or general wards), long‐term or short‐term treatment with NAC, or different doses of NAC that were used. Therefore, well‐designed prospective clinical trials are essential to confirm that NAC can be considered adjunctive therapy with the standard COVID‐19‐based protocol.
To the best of our knowledge, this is the first study to use the NAC inhaler spray. The main strength of our study was that it was randomized and well‐designed, with accurate follow‐up and observation of all subjects. All patients were admitted under the same conditions and received standard treatment protocols based on WHO and Ministry of Health of Iran guidelines for COVID‐19 alongside NAC for the intervention group. However, the results of the current study should be interpreted in light of the study's limitations. A drug synergism effect with other components of the standardized protocol‐driven care cannot be ruled out and therapeutic dose monitoring was not performed in patients. Due to the unique circumstances of the pandemic, repeating, and conducting some important laboratory indices were not possible for all patients.
5. CONCLUSION
The present study provides evidence that the use of 400 μg/day of NAC inhaler spray for 7 days in patients with COVID‐19 pneumonia prevents the development of severe respiratory failure. Despite admitted COVID‐19 patients treated with NAC being older and therefore at greater risk of serious COVID‐19 disease, the results showed that NAC administered by inhaled spray was associated with better survival when added to standard treatment in hospitalized patients with COVID 19 pneumonia. However, NAC therapy had no effect on hospital LOS and the requirement for ICU admission. These findings need to be confirmed by future prospective clinical trials; however, until then, considering the excellent safety profile and low cost of NAC, its use as adjunct therapy in COVID‐19 should be considered.
AUTHOR CONTRIBUTIONS
All authors contributed to the study design. Testing and data collection were performed by Yunes Panahi, Mostafa Ghanei, and Morteza Rahimi. The data analysis and interpretation was performed by Abbas Samim, Amirhossein Sahebkar and Amir Vahedian‐Azimi. Draft of the manuscript was written by Yunes Panahi, Amir Vahedian‐Azimi, and Amirhossein Sahebkar. Mostafa Ghanei and Stephen L. Atkin provided critical revisions. All authors approved the final version of the manuscript for submission.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The protocol study was reviewed and approved by the Ethics Committees of Baqiyatallah University of Medical Sciences (IR.BMSU. REC.1399.123), in accordance with the Declaration of Helsinki of the World Medical Association. This study Registered at Iranian Registry of Clinical Trials (IRCT20080901001165N55) dated 23‐05‐2020. Written informed consent was obtained from all patients or their legal representatives if they were unable to provide consent. In addition, the study was conducted and reported in accordance with the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement.
ACKNOWLEDGMENTS
Thanks to guidance and advice from the “Clinical Research Development Unit of Baqiyatallah Hospital.”
Panahi Y, Ghanei M, Rahimi M, et al. Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: an open‐label randomized controlled clinical trial. J Med Virol. 2022;95:e28393. 10.1002/jmv.28393
Contributor Information
Amir Vahedian‐Azimi, Email: amirvahedian63@gmail.com.
Amirhossein Sahebkar, Email: amir_saheb2000@yahoo.com.
DATA AVAILABILITY STATEMENT
All data collected and analyzed during the current study are available from the authors on a reasonable request.
REFERENCES
- 1. Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID‐19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS‐CoV‐2. Biochimie. 2020;179:85‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baloch S, Baloch MA, Zheng T, Pei X. The coronavirus disease 2019 (COVID‐19) pandemic. Tohoku J Exp Med. 2020;250(4):271‐278. [DOI] [PubMed] [Google Scholar]
- 3. Mourmouris P, Tzelves L, Roidi C, Fotsali A. COVID‐19 transmission: a rapid systematic review of current knowledge. Osong Public Health Res Perspect. 2021;12(2):54‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu R, Wang L, Kuo HCD, et al. An update on current therapeutic drugs treating COVID‐19. Curr Pharmacol Rep. 2020;6(3):56‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaur SP, Gupta V. COVID‐19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhama K, Sharun K, Tiwari R, et al. COVID‐19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16(6):1232‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh DD, Parveen A, Yadav DK. SARS‐CoV‐2: emergence of new variants and effectiveness of vaccines. Front Cell Infect Microbiol. 2021;11:777212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aleem A, Akbar Samad AB, Slenker AK. Emerging Variants of SARS‐CoV‐2 And Novel Therapeutics Against Coronavirus (COVID‐19). StatPearls. StatPearls Publishing, Copyright©; 2022. [Google Scholar]
- 9. Ramos AM, Vela‐Pérez M, Ferrández MR, Kubik AB, Ivorra B. Modeling the impact of SARS‐CoV‐2 variants and vaccines on the spread of COVID‐19. Commun Nonlinear Sci Numer Simul. 2021;102:105937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyder AA, Hyder MA, Nasir K, Ndebele P. Inequitable COVID‐19 vaccine distribution and its effects. Bull World Health Organ. 2021;99(6):406‐406A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sawal I, Ahmad S, Tariq W, Tahir MJ, Essar MY, Ahmed A. Unequal distribution of COVID‐19 vaccine: a looming crisis. J Med Virol. 2021;93(9):5228‐5230. [DOI] [PubMed] [Google Scholar]
- 12. Harrison AG, Lin T, Wang P. Mechanisms of SARS‐CoV‐2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care. 2020;24(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beyerstedt S, Casaro EB, Rangel ÉB. COVID‐19: angiotensin‐converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS‐CoV‐2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandes IG, de Brito CA, Dos Reis VMS, Sato MN, Pereira NZ. SARS‐CoV‐2 other respiratory viruses: what does oxidative stress have to do with it? Oxid Med Cell Longev. 2020;2020:8844280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saponaro F, Rutigliano G, Sestito S, et al. ACE2 in the era of SARS‐CoV‐2: controversies and novel perspectives. Front Mol Biosci. 2020;7:588618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐Converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the Renin‐Angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ullian ME, Gelasco AK, Fitzgibbon WR, Beck CN, Morinelli TA. N‐acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J Am Soc Nephrol. 2005;16(8):2346‐2353. [DOI] [PubMed] [Google Scholar]
- 19. Boesgaard S, Aldershvile J, Poulsen HE, Christensen S, Dige‐Petersen H, Giese J. N‐acetylcysteine inhibits angiotensin converting enzyme in vivo. J Pharmacol Exp Ther. 1993;265(3):1239‐1244. [PubMed] [Google Scholar]
- 20. Jorge‐Aarón RM, Rosa‐Ester MP. N‐acetylcysteine as a potential treatment for COVID‐19. Future Microbiol. 2020;15:959‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landini G, Di Maggio T, Sergio F, Docquier JD, Rossolini GM, Pallecchi L. Effect of high N‐acetylcysteine concentrations on antibiotic activity against a large collection of respiratory pathogens. Antimicrob Agents Chemother. 2016;60(12):7513‐7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwalfenberg GK. N‐acetylcysteine: a review of clinical usefulness (an old drug with new tricks. J Nutr Metab. 2021;2021:9949453. 10.1155/2021/9949453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panahi Y, Ghanei M, Hashjin MM, Rezaee R, Sahebkar A. Potential utility of N‐acetylcysteine for treating mustard lung. Crit Rev Eukaryot Gene Expr. 2017;27(3):247‐266. 10.1615/CritRevEukaryotGeneExpr.2017019740 [DOI] [PubMed] [Google Scholar]
- 24. Panahi Y, Ostadmohammadi V, Raygan F, Sharif MR, Sahebkar A. The effects of N‐acetylcysteine administration on metabolic status and serum adiponectin levels in patients with metabolic syndrome: a randomized, double‐blind, placebo‐controlled trial. J Funct. Foods. 2022;99:105299. [Google Scholar]
- 25. McCarty MF, DiNicolantonio JJ. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. 2020;63(3):383‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Medical Association . World medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 27. Jayaraman J. Guidelines for reporting randomized controlled trials in paediatric dentistry based on the CONSORT statement. Int J Paediat Dent. 2020;31(suppl 1):38‐55. [DOI] [PubMed] [Google Scholar]
- 28. Rahmanzade R, Rahmanzadeh R, Hashemian SM, Tabarsi P. Iran's approach to COVID‐19: evolving treatment protocols and ongoing clinical trials. Front Public Health. 2020;8:551‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin‐section CT. Radiology. 2005;236(3):1067‐1075. [DOI] [PubMed] [Google Scholar]
- 30. Jones M. NEWSDIG: the National early warning score development and implementation group. Clin Med. 2012;12(6):501‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izquierdo JL, Soriano JB, González Y, et al. Use of N‐Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID‐19. Sci Prog. 2022;105(1):003685042210745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Flora S, Grassi C, Carati L. Attenuation of influenza‐like symptomatology and improvement of cell‐mediated immunity with long‐term N‐acetylcysteine treatment. Eur Respir J. 1997;10(7):1535‐1541. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Q, Ju Y, Ma Y, Wang T. N‐acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial. Medicine. 2018;97(45):e13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharafkhah M, Abdolrazaghnejad A, Zarinfar N, Mohammadbeigi A, Massoudifar A, Abaszadeh S. Safety and efficacy of N‐acetyl‐cysteine for prophylaxis of ventilator‐associated pneumonia: a randomized, double blind, placebo‐controlled clinical trial. Med Gas Res. 2018;8(1):19‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beltrán‐García J, Osca‐Verdegal R, Pallardó FV, et al. Oxidative stress and inflammation in COVID‐19‐associated sepsis: the potential role of anti‐oxidant therapy in avoiding disease progression. Antioxidants (Basel). 2020;9(10):936. 10.3390/antiox9100936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lo Presti E, Nuzzo D, Al Mahmeed W, et al. Molecular and pro‐inflammatory aspects of COVID‐19: the impact on cardiometabolic health. Biochim Biophys Acta Mol Basis Dis. 2022;1868(12):166559. 10.1016/j.bbadis.2022.166559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lana JFSD, Lana AVSD, Rodrigues QS, et al. Nebulization of glutathione and N‐Acetylcysteine as an adjuvant therapy for COVID‐19 onset. Adv Redox Res. 2021;3:100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID‐19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30:101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong KK, Lee SWH, Kua KP. N‐Acetylcysteine as adjuvant therapy for COVID‐19—a perspective on the current state of the evidence. J Inflamm Res. 2021;14:2993‐3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dominari A, Hathaway Iii D, Kapasi A, et al. Bottom‐up analysis of emergent properties of N‐acetylcysteine as an adjuvant therapy for COVID‐19. World J Virol. 2021;10(2):34‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Marco F, Foti G, Corsico AG. Where are we with the use of N‐acetylcysteine as a preventive and adjuvant treatment for COVID‐19? Eur Rev Med Pharmacol Sci. 2022;26(2):715‐721. [DOI] [PubMed] [Google Scholar]
- 42. Calverley P, Rogliani P, Papi A. Safety of N‐acetylcysteine at high doses in chronic respiratory diseases: a review. Drug Saf. 2021;44(3):273‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Assimakopoulos SF, Aretha D, Komninos D, et al. N‐acetyl‐cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID‐19 pneumonia: a two‐center retrospective cohort study. Infect Dis. 2021;53(11):847‐854. [DOI] [PubMed] [Google Scholar]
- 44. Gaynitdinova VV, Avdeev SN, Merzhoeva ZM, Berikkhanov ZG‐M, Medvedeva IV, N‐acetylcysteine TLG. As a part of complex treatment of moderately severe COVID‐associated pneumonia. Pul'monologiya. 2021;31(1):21‐29. [Google Scholar]
- 45. Altay O, Arif M, Li X, et al. Combined metabolic activators accelerates recovery in mild‐to‐moderate COVID‐19. Adv Sci (Weinh). 2021;8(17):e2101222. 10.1002/advs.202101222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taher A, Lashgari M, Sedighi L, Rahimi‐Bashar F, Poorolajal J, Mehrpooya M. A pilot study on intravenous N‐Acetylcysteine treatment in patients with mild‐to‐moderate COVID19‐associated acute respiratory distress syndrome. Pharmacol Rep. 2021;73(6):1650‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Alencar JCG, Moreira CL, Müller AD, et al. Double‐blind, randomized, placebo‐controlled trial with N‐acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2021;72(11):e736‐e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected and analyzed during the current study are available from the authors on a reasonable request.


