Abstract
Early intracellular development in vitro of the cyst-forming protozoon Sarcocystis singaporensis and the influence of a monoclonal antibody on invasion, intracellular localization, and development of sporozoites were studied. As revealed by immunofluorescence using parasite-specific antibodies which labeled the parasitophorous vacuole membrane (PVM) and by ultrastructural analysis, sporozoites invaded pneumonocytes of the rat via formation of a parasitophorous vacuole (PV). About half of the sporozoites left this compartment within the first 8 h postinfection to enter the host cell cytosol. By semiquantitative analysis of acetyl-histone H4 expression of sporozoites, a marker linked to early gene expression of eukaryotic cells, we show (supported by ultrastructural analysis) that escape from the PV appears to be necessary for early intracellular development. More than 90% of sporozoites located in the cytosol expressed high levels of acetylated histone H4 in the nucleus, whereas only a quarter of the intravacuolar sporozoites exhibited a similar signal. As revealed by ultrastructural analysis, young schizonts all resided in the cytosol. Specific binding of a monoclonal antibody (11D5/H3) to sporozoites before invasion significantly enhanced their escape from the PV, whereas cell invasion itself remained unaffected. The antibody actually increased proliferation of the parasites in vitro, providing a further link between residence in the cytosol and successful intracellular development. Monoclonal antibody 11D5/H3 precipitated a major 58-kDa antigen from oocyst-sporocyst extracts and reacted with the cytoplasm and the surface of sporozoites in immunofluorescence assays. Collectively, the observed antibody-parasite interaction suggests the existence of a signaling event that influences intracellular development of Sarcocystis.
Cyst-forming coccidia of the genus Sarcocystis are among the most prevalent parasites of livestock and are responsible for considerable economic losses (10, 15). Furthermore, recent outbreaks of Sarcocystis-induced disease among humans in tropical countries (3) underscore the increasing importance of these parasites for public health (31).
A peculiar aspect of Sarcocystis infection has been known for a long time but has received little attention: the possible escape of sporozoites from the parasitophorous vacuole (PV) after invasion of host cells. Early observations in vivo and ultrastructural studies of infected cell cultures of bovine pulmonary artery endothelial cells and bovine monocytes revealed that Sarcocystis cruzi sporozoites as well as the resulting schizonts were located free in the host cell cytoplasm, i.e., not surrounded by a PV (11, 34). We have extended this observation to Sarcocystis singaporensis, a species that infects rats as intermediate hosts and specifically develops inside endothelial cells and pneumonocytes, a characteristic that renders it a suitable model for Sarcocystis infections in the laboratory (21). To date, however, nothing is known about a possible escape from the PV and whether or not sporozoites of Sarcocystis spp. enter the host cell via formation of a PV in the first place. For instance, recent evidence regarding malaria parasites indicates that formation of a PV is not necessarily the only entry route of apicomplexans into a cell (29). Although circumstancial observations suggest that residence in the cytosol is necessary for Sarcocystis sporozoites to develop into schizonts, no investigations on possible metabolic changes that could indicate such a transition have been performed. The relatively long generation times during asexual development of Sarcocystis (21, 34) hamper, for instance, the measurement of proliferation based upon uptake of labeled DNA precursor molecules, which is quite a straightforward approach for Toxoplasma gondii (17, 28). To address the questions outlined above, we studied invasion of sporozoites of S. singaporensis into rat pneumonocytes at the light microscopical and ultrastructural level, and examined the level of acetyl-histone H4-mediated gene expression during early intracellular development in vitro. Acetylation-deacetylation of histones is thought to play a central role in transcriptional control in eukaryotic cells, and a link between signal-regulated acetylation of histone H4 and gene transcription has been established (1, 19). Acetylation of histones has been shown to play also a role in apicomplexan parasites (8, 18).
During experiments on host cell invasion of S. singaporensis, we made the intriguing observation that a parasite-specific monoclonal antibody (MAb) positively influenced intracellular development of sporozoites. Therefore, it was the aim of the second part of this work to investigate this phenomenon in more detail and to characterize the molecule recognized by the antibody. Although it is known that parasites capture antibodies through immunoglobulin binding proteins (Fc receptors) which can increase their infective capacity (33, 37), interaction of a parasite-specific antibody with its antigen on the protozoan's surface has not been observed to raise infectivity.
MATERIALS AND METHODS
Parasites.
The experiments were carried out with a strain of S. singaporensis (S5) characterized in detail previously (5, 22). This so-called wild type was passaged twice between snakes and laboratory rats after isolation from a wild-caught reticulated python in Thailand. Sporocysts were harvested from feces of infected pythons and purified on Percoll gradients. Sporozoites for infection of cultured cells were freshly excysted and purified as previously described (21). Before infection of cell cultures, they were stored for up to 2 h at 25°C in serum-free Ham's F12K medium (Life Technologies, Eggenstein, Germany).
Antibodies and other reagents.
For detection of sporozoites in cell cultures, a rabbit serum prepared against sporozoites of S. singaporensis (K3) was used (21, 22). The sporozoite-specific MAbs 11D5/H3 (immunoglobulin G2a [IgG2a]) and 2C6/E9 (IgG2b) were generated as described earlier (23). MAb 2C6/E9 reacted with the apical third of the sporozoite's cytoplasm and pellicle in indirect immunofluorescence and detected a high-molecular-weight antigen in Western blottings different from the antigen recognized by MAb 11D5/H3 (T. Jäkel, unpublished data). Two clones, G155-178 (anti-TNP antibody; PharMingen, San Diego, Calif.) and a MAb developed against the nematode Acanthocheilonema viteae (gift from Richard Lucius, Institute of Molecular Parasitology, Humboldt University, Berlin, Germany), served as isotype (IgG2a) controls. MAbs were used as hybridoma supernatants or purified by protein A affinity chromatography using protein A-Sepharose CL-4B according to the instructions of the manufacturer (Pharmacia Biotech). Rabbit polyclonal antibodies developed against a peptide (including four acetylation sites) corresponding to amino acids 2 to 19 of Tetrahymena histone H4 (1) were purchased from Upstate Biotechnology (Lake Placid, N.Y.). Second-step reagents included fluorescein isothiocyanate (FITC) and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG and goat anti-mouse IgG (Sigma-Aldrich, Deisenhofen, Germany). A fluorescent lipophilic marker, tracer F (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-5,5′-disulfonic acid), was purchased from Molecular Probes (Eugene, Oreg.). For staining of cell nuclei, 4′,6-diamidino-2-phenylindol (DAPI) was used (Sigma-Aldrich).
Cell culture and infection.
For infection experiments, monolayers of L2 rat pneumonocytes (ATCC, CCL 149) were used. It has been shown that these cells support intracellular development of sporozoites into schizonts and merozoites (21). Cells were grown in Ham's F12K medium supplemented with 5% fetal bovine serum and infected cultures maintained as described earlier (21). For infection, cells grown on coverslips (1.3 cm2) were inoculated with either 105 (kinetic study and analysis of acetyl-histone H4 expression) or 1.5 × 105 to 2 × 105 (experiments with MAb 11D5/H3) sporozoites of S. singaporensis.
Immunoprecipitation.
For immunoprecipitation of the antigen recognized by MAb 11D5/H3, a cell extract of 2.5 × 107 to 4.0 × 107 sporocysts was obtained by suspending the parasites in 4 ml of cold (4°C) lysis buffer (150 mM NaCl, 50 mM sodium borate, 0.1% Nonidet P-40, and 0.5% sodium deoxycholate; pH 8.0) containing the protease inhibitors phenylmethylsulfonyl fluoride (100 μg/ml), aprotinin (1 μg/ml), and leupeptin (1 μg/ml) (all reagents were from Roche, Mannheim, Germany). Then, sporocysts were vortexed in the presence of glass beads (2-mm diameter) for 12 min and incubated on ice for 45 min. Afterwards, the suspension was spun at 11,500 × g for 10 min at 4°C and the supernatant was aspirated for further use. Approximately 50 μg of biotin-7–NHS (d-biotinoyl-ɛ-aminocapronic acid-N-hydroxysuccinimide ester) per 107 sporocysts was added to the protein solution and incubated for 15 min at 4°C. The biotinylation reaction was stopped by addition of 50 mM NH4Cl. Precipitation was initiated by suspending protein A-agarose beads in the cell extract for 90 min at 4°C on a roller-rocker according to the instructions of the manufacturer (Cellular Labeling and Immunoprecipitation Kit; Roche). For preabsorption, the sample was centrifuged at 12,000 × g for 20 s, the supernatant carefully removed and repeatedly mixed with protein A-agarose. One milliliter of supernatant of the hybridoma cell line 11D5/H3 was added to 4 ml of cell extract and incubated on ice for 2 h. As control, sporozoite extracts were treated with an irrelevant isotype-matched MAb and the K3 antibodies prepared against sporozoites. Antigen and stage specificity was controlled for by incubating MAb 11D5/H3 with bovine serum albumin (0.6 mg/ml) or extracts of bradyzoites (24), respectively. Protein A-agarose was added, and the mixture was incubated overnight on a roller-rocker at 4°C. The complexes were removed by centrifugation and repeatedly washed with washing buffers containing various concentrations of Tris base (50 to 10 mM) and NaCl (150 to 500 mM). Finally, complexes were solubilized in gel-loading buffer (pH 6.8) containing 0.125 M Tris base, 4% sodium dodecyl sulfate (SDS), 20% glycerol, and 2% or 4% 2-mercaptoethanol and were heated for 3 min in boiling water. Protein A-agarose was removed by centrifugation at 8,000 × g for 1 min. Five independent immunoprecipitation experiments were performed.
Gel electrophoresis and chemiluminescent protein detection.
Immunoprecipitated samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (25). Approximately 5 to 30 ng of target protein per lane was separated at 25 mA of constant current in 0.05 M Tris-buffer (pH 8.3) containing 0.38 M glycine and 0.1% SDS using 4% stacking gels and 12.5% resolving gels cast in a minigel chamber (Bio-Rad). Electrical transfer onto 0.45-μm-pore-size nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) was performed for 1.5 h at 250 mA and 4°C in 25 mM Tris buffer, pH 8.3, containing 192 mM glycine, 0.1% SDS, and 20% methanol. Afterwards, nitrocellulose membranes were washed twice with Tris-buffered saline (TBS; 50 mM Tris base, 150 mM NaCl, pH 7.5) and nonspecific binding was blocked overnight with 3% skimmed milk powder in phosphate-buffered saline (PBS) at 4°C. Membranes were incubated with streptavidin-peroxidase (20 mU/ml) for 30 min at 20°C and washed thoroughly with TBS containing 0.1% Tween 20. Finally, blots were incubated with a luminol solution at 20°C according to the instructions of the manufacturer (BM Chemiluminescence Blotting Kit; Roche) and after 1 min were exposed to X-ray film (Fuji Medical X-Ray RX) for various time intervals (1 s to 90 min).
Indirect immunofluorescence.
Detection of parasites in infected cell cultures and reaction of antibodies with acetone-fixed air-dried zoites were performed as described previously (20, 21). For detection of acetylated histone H4, cells were fixed onto coverslips for 1 min at 20°C using methanol supplemented with 5% acetic acid, followed by another fixation step for 20 min at −20°C with pure methanol. Cells were washed with PBS at 4°C, incubated with anti-acetyl histone H4 antibody diluted 1:200 in PBS for 2.5 h at 20°C, and washed again, and the primary antibodies were detected with FITC-conjugated goat anti-rabbit IgG diluted 1:200. In some experiments, three parallel stains were employed. To stain for acetylated histone H4 and sporozoite antigens, coverslips were first treated with anti-acetyl-histone H4 antibodies (and TRITC-conjugated goat anti-rabbit IgG diluted 1:200), followed by K3 serum (and anti-rabbit IgG FITC conjugate); lipophilic tracer F was added to enhance contrast of host cell structures. Although the former two antibodies derived from the same animal species, different target structures (nucleus versus cytoplasm) resulted in clearly distinguishable labels. As control, samples were treated with PBS instead of the primary antibody. To facilitate the counting of intracellular parasites and host cells in the invasion experiments, host cell nuclei were stained with DAPI before labeling of parasites. Therefore, cells on coverslips were fixed for 15 min at 37°C in methanol containing 0.1 μg of DAPI/ml. Afterwards, cells were washed with PBS and stained with K3 antibodies. All samples were examined with a Zeiss Axiophot microscope equipped with epifluorescence and phase-contrast optics.
Electron microscopy.
Infected cell monolayers were fixed with 2.5% glutaraldehyde (Serva) in 0.1 M cacodylate buffer (pH 7.4) at 4°C for 1 h, washed with buffer several times, and gently scraped off the substrate. Fixed cells were pelleted by centrifugation in 1% low-melting-point agar (Sigma-Aldrich) diluted in 0.1 M cacodylate buffer at 37°C. After the polymerization of agar, cells were postfixed with 1% OsO4 in cacodylate buffer for 2 h at 4°C. Samples were dehydrated, embedded in Araldite (Serva), and stained as described previously (21).
Image analysis.
For documentation of electrophoretic separations and calculation of molecular weight, a video camera and software package were used (BIO-CAPT, BIO-GENE, Vilber Lourmat, France).
Statistical analyses.
Data were analyzed by nonparametric (Kruskal-Wallis analysis of variance [ANOVA] on ranks followed by multiple comparison procedures according to Student-Newman-Keuls) or parametric (Student's t test, ANOVA) methods, using Sigma Stat version 2.0 (Jandel Scientific, San Rafael, Calif.).
RESULTS
Escape of sporozoites of S. singaporensis from PV precedes schizogonic development and is linked to enhanced levels of acetyl-histone H4-mediated gene expression.
Sporozoites of S. singaporensis enter their host cells within the first 2 h after inoculation of cell cultures (21). In a first set of experiments, we investigated the type of intracellular localization of sporozoites in L2 pneumonocytes at various time intervals after infection (1, 2, 4, 8, and 16 h) by indirect immunofluorescence using rabbit antibodies (K3) prepared against sporozoites and by ultrastructural analysis. At the light microscopical level, the K3 antibodies reacted with parasite antigens incorporated into the PVM, which allowed directly visualization of the membrane. Within the first 16 h postinfection, almost all of the intravacuolar sporozoites were entirely surrounded by a fluorescent signal delimiting the PV, whereas such a label was absent in intracytosolic stages (Fig. 1A and E). In some cases, sporozoites were apparently caught in the moment of entering the cytosol, indicated by a discontinuous label of the PVM (Fig. 1B and C). Intravacuolar and intracytosolic stages were easily distinguishable in phase-contrast images, as only intravacuolar sporozoites were surrounded by a distinct halo representing the PV (Fig. 1B). Intravacuolar sporozoites, which appeared as slender and dark forms, assumed a more stumpy appearance and showed a lighter cytoplasm once located in the cytoplasm of the host cell (Fig. 1D and E).
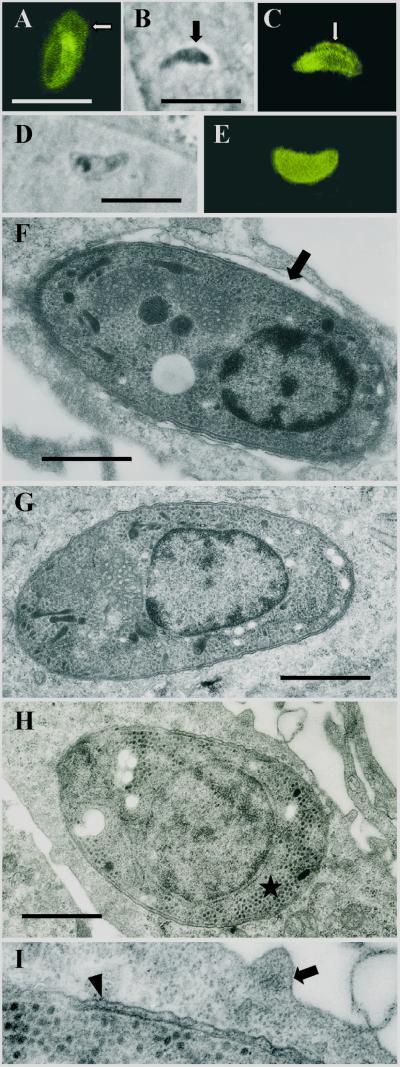
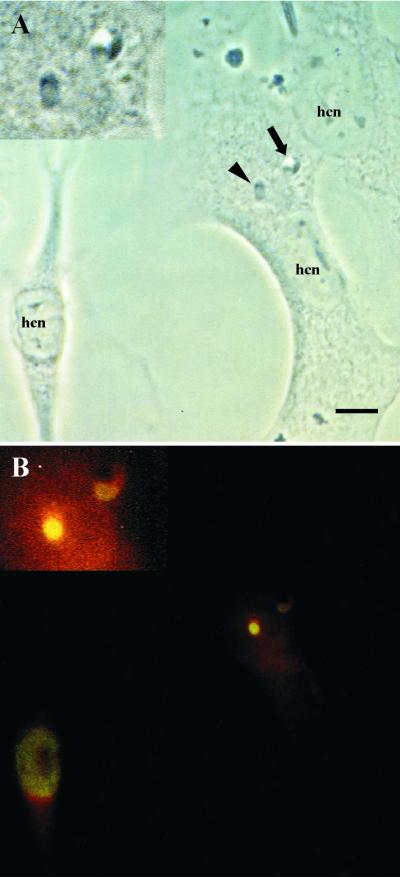
FIG. 1.
Early intracellular development (1 h to 2 days postinfection) of sporozoites of S. singaporensis in L2 rat pneumonocytes in vitro. Immunofluorescence images, corresponding phase-contrast images, and ultrastructural morphology are shown. Bars, 1 μm (F to I) or 10 μm (A to E). (A) Immunofluorescent staining of a sporozoite 2 h postinfection using rabbit anti-sporozoite antibodies (K3). The banana-shaped sporozoite is located inside a PV, and the PVM (arrow) is labeled due to incorporated parasite molecules. The label of the PVM persisted up to 18 h postinfection but was reduced or absent at later intervals. (B) Phase-contrast image of a sporozoite which appears to leave the PV and enter the cytoplasm of the host cell (2 h postinfection). The arrow indicates the PV, which is clearly visible as a halo surrounding the parasite. Note the slender appearance and dark cytoplasm of the zoite. (C) Corresponding immunofluorescent staining with K3 antibodies. The arrow points at the PVM, which seems to be absent at the left (apical) end of the sporozoite (the nucleus is located closer to the posterior end; see panels F and G), possibly a sign of a disrupted PVM. Note that the K3 antibodies preferentially label the apical portion of this stage. (D) Phase-contrast image of a sporozoite located inside the cytosol; these stages assumed a stumpy appearance and the parasite's cytoplasm became lucent. (E) Corresponding immunofluorescence showing that the K3 label is evenly distributed throughout the cytoplasm. (F) Ultrastructure of a sporozoite 1 h postinfection. The zoite resides in a PV (arrow). Note the parasite's electron-dense cytoplasm and remnants of membranes adhering to the pellicle, indicating recent invasion of the cell. (G) Ultrastructure of a sporozoite residing free in the cytoplasm 2 h postinfection. (H) Late sporozoite/young schizont 2 days postinfection. Note the absence of rhoptries and micronemes, the enlarged nucleus, and formation of islets of granules of the crystalloid body (asterisk). (I) Enlarged view of the host-parasite interface of the previous specimen. The schizont resides inside the cytosol; the arrowhead indicates the pellicle, and the arrow indicates the plasmalemma of the host cell.
Ultrastructural analysis of samples collected 1 h postinfection of L2 cells with sporozoites confirmed that invasion into host cells was accompanied by formation of a PV because most of the intracellular sporozoites rested within a membrane-bound vacuole (Fig. 1F). However, sporozoites located free in the cytosol had already formed at that time and were observed with increasing frequency at later intervals (Fig. 1G). To determine the type of intracellular localization of developing sporozoites (early schizonts), we prepared ultrathin sections of L2 cells 2 and 3 days postinfection, a time when the first schizonts usually appear in cultured cells (21). Developing sporozoites were recognized by an enlarged nucleus, the absence of apical organelles such as rhoptries and micronemes, and, particularly, the condensation of granules of the crystalloid body to electron-dense islets of variable size (Fig. 1H). All of these stages found in ultrathin sections of infected L2 cells (n = 46) were located inside the cytosol of the host cell (Fig. 1H and I), not in a PV, indicating that only intracytosolic sporozoites transformed to schizonts. Intravacuolar sporozoites had largely disappeared by day 2 postinfection. Most of them appeared to be degraded inside the PV of their host cell because these stages showed signs of cellular disintegration, such as a patchy K3 label of the pellicle and cytoplasm in immunofluorescence or the appearance of cell fragments at the ultrastructural level.
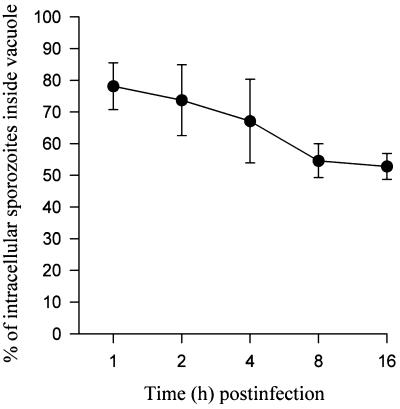
When we quantified changes in intracellular localization of sporozoites at various time intervals (on the basis of the light microscopical criteria described above), we observed that about 20% of the intracellular zoites had left the vacuole within the first hour (Fig. 2), which was consistent with the ultrastructural findings. Escape from the PV appeared to be terminated 8 h postinfection because proportions were similar to those at 16 h, when about half of the intracellular sporozoites resided inside the cytosol (Fig. 2).
FIG. 2.
Kinetics of the escape of sporozoites of S. singaporensis from the PV of L2 pneumonocytes. Shown is the mean (± standard deviation) percentage of intravacuolar sporozoites out of all that were in intracellular stages at various time intervals after infection. At 2 h postinfection, all extracellular sporozoites were removed from the cell cultures by washing with medium. The graph summarizes three independent experiments, whereby two coverslips were fixed and analyzed in each experiment and 200 intracellular sporozoites were checked on each coverslip.
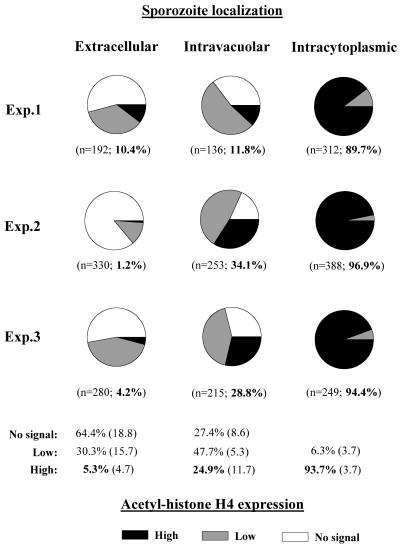
Although the ultrastructural results suggested that only intracytosolic sporozoites transformed to schizonts, we wanted to investigate whether residence in the cytosol was actually paralleled by early developmental activity. Therefore, we examined an early indicator of cellular activity, acetylation of histone H4, which is linked to early gene expression in the cell. As revealed by single-cell analysis of parasites labeled with anti-acetyl-histone H4 antibody in immunofluorescence, intracytosolic sporozoites showed a strong nuclear label whereas the reaction was weak or completely absent in most of the intravacuolar sporozoites (Fig. 3). This was observed 12, 18, and 24 h postinfection. Shortly after host cell invasion (6 h), a similar trend was visible; however, histone H4 acetylation of intracytosolic sporozoites was relatively low. Semiquantitative analysis of acetyl-histone H4 expression at 18 h postinfection showed that more than 90% of the intracytosolic sporozoites showed a high degree of acetyl-histone H4 expression, whereas only about a quarter of the intravacuolar sporozoites exhibited a comparable activity (Fig. 4). This indicated that escape from the PV was linked to an enhanced state of developmental activity of sporozoites of S. singaporensis.
FIG. 3.
Acetyl-histone H4 expression of sporozoites is dependent upon the type of intracellular localization in L2 pneumonocytes 18 h postinfection. Shown are a phase-contrast image (A) and the corresponding indirect immunofluorescence image (B) produced by using rabbit anti-acetyl-histone H4 antibodies and FITC-conjugated goat anti-rabbit IgG; a lipophilic stain (red) was used to visualize host cell structures. Insets show a magnification of the parasites. Bar, 10 μm. (A) Two sporozoites, one inside a PV (arrow), the other inside the cytosol (arrowhead) of the same host cell (hcn, host cell nucleus). Note the clearly visible halo surrounding the intravacuolar sporozoite. (B) High level of nuclear expression of acetyl-histone H4 by the intracytosolic sporozoite, whereas the intravacuolar stage shows a low signal. Because the epitope recognized by the antibody is highly conserved among species, the antibody reacts with the nuclei of L2 pneumonocytes. Note that acetyl-histone H4 expression is restricted to one of the three host cells; this one had just started to contract and was partially detached from the monolayer, indicating the onset of mitosis.
FIG. 4.
Semiquantitative analysis of acetyl-histone H4 expression in nuclei of intracellular and extracellular sporozoites of S. singaporensis at 18 h postinfection of pneumonocytes based on the staining characteristics shown in the previous figure. Accordingly, nuclear labeling was ranked as high, low, or no signal, and numbers in each category are expressed as proportions. Shown are the results of three independent experiments whereby three coverslips were examined in each experiment. Figures in parentheses immediately below the pie charts indicate the total number of sporozoites evaluated in each compartment (extracellular, intravacuolar, and intracytosolic), followed by the percentage of high-level expression. The lower part of the graph shows mean percentages (standard deviation in parentheses) for each compartment and level of signal. Values of high expression are in bold.
MAb 11D5/H3 does not inhibit cell invasion of sporozoites but enhances escape from PV and their subsequent proliferation.
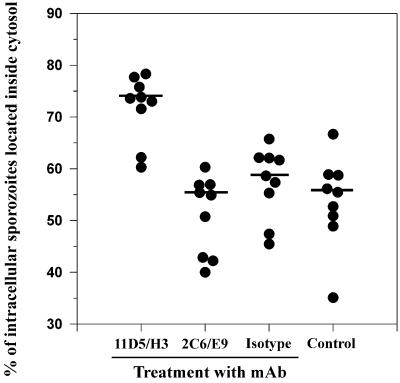
We tested the effect of the species and stage-specific MAb 11D5/H3 (22, 23) on host cell invasion of sporozoites. Therefore, sporozoites were incubated for 60 min at 37°C with increasing concentrations (10, 50, or 100 μg/ml) of the antibody diluted in Ham's F12K medium, washed, inoculated onto cultures of L2 cells, and allowed to invade for 12 h. Preincubation of sporozoites with MAb 11D5/H3 did not alter the invasive behavior of sporozoites in three independent experiments, whereby about 1,800 host cells were evaluated in each experiment. Mean numbers of intracellular sporozoites per 100 host cells (20.3 ± 8.7, 15.8 ± 6.8, and 18.1 ± 8.5 [mean ± standard deviation] at 10, 50, or 100 μg/ml, respectively; n = 60) were not significantly different from invasion rates of isotype-treated (21.2 ± 10.5; n = 60; 100 μg of antibody/ml) or untreated (19.9 ± 9.3; n = 60) sporozoites (one-way ANOVA; d.f. = 4; F = 1.66; P = 0.16). However, MAb 11D5/H3 (50 μg/ml) significantly influenced intracellular behavior of sporozoites in L2 pneumonocytes. Treatment increased the median proportion of intracytosolic sporozoites by almost 40% compared with an isotype control or a parasite-reactive MAb directed against a different antigen (Fig. 5). The same effect was observed when treating sporozoites with hybridoma supernatants of 11D5/H3 which usually contained lower concentrations of antibody (data not shown). We also checked whether the reaction of MAb 11D5/H3 with live sporozoites influenced acetyl-histone H4-mediated gene expression of the parasites. Staining of the same cell cultures for acetyl histone H4 revealed that 11D5/H3 did not alter acetyl-histone H4 expression of sporozoites as values for intracytosolic stages were higher than 93% (93.3 to 98.7%; n = 4 coverslips) compared to 15.2 to 26.9% for intravacuolar stages, similar to the results reported earlier (Fig. 4).
FIG. 5.
Influence of preincubation of sporozoites with MAb 11D5/H3 on their subsequent intracellular localization. Sporozoites were treated with 50 μg of MAb 11D5/H3 per ml before inoculation onto cell cultures. An isotype-matched irrelevant MAb and the sporozoite-reactive MAb 2C6/E9 were used, both at 50 μg/ml, and medium alone served as controls. The number of intracytosolic stages was determined as described before and expressed as a percent of all intracellular parasites. The graph summarizes three independent experiments whereby three coverslips were examined for each treatment and 200 to 300 sporozoites were evaluated on each coverslip. Data are shown as dot plots, and bars indicate the median. Kruskal-Wallis ANOVA and pairwise multiple comparison procedures (Student-Newman-Keuls method) revealed that the median for 11D5/H3 treatment was significantly higher than those of all other treatments (d.f. = 3; H = 19.096; P < 0.001); no statistically significant differences occurred between the other groups.
To compare the proliferative potential of 11D5/H3-treated sporozoites with isotype-treated stages, merozoites were harvested from sporozoite-infected cultures and their cumulative numbers were determined between 5 to 12 days postinfection. Significantly higher numbers of merozoites were collected from 11D5/H3-treated samples. In three independent experiments, a mean total of 3,605 ± 716 (± standard deviation; n = 9) merozoites were grown per coverslip compared to 2,733 ± 391 (n = 9) after isotype treatment. This demonstrated that binding of MAb 11D5/H3 to sporozoites significantly increased proliferation of the parasite (Student's t test; P = 0.044).
MAb 11D5/H3 recognizes antigen on surface and in cytoplasm of sporozoites and precipitates a 58-kDa molecule.
As revealed by indirect immunofluorescence using air-dried acetone-fixed stages, MAb 11D5/H3 stained the pellicle as well as the cytoplasm of sporozoites (Fig. 6A). To test whether the antibody actually reacted with the surface of live sporozoites, some of the coverslips used for the invasion experiments were treated with FITC-conjugated anti-mouse IgG, as they contained numerous extracellular as well as intracellular sporozoites which had been preincubated with the mouse MAb 11D5/H3. Extracellular sporozoites exhibited a typical surface label (Fig. 6B and C). Intracellular sporozoites were not stained by anti-mouse IgG, indicating that sporozoites had shed the antibody or antigen-antibody complex once penetrating the host cell.
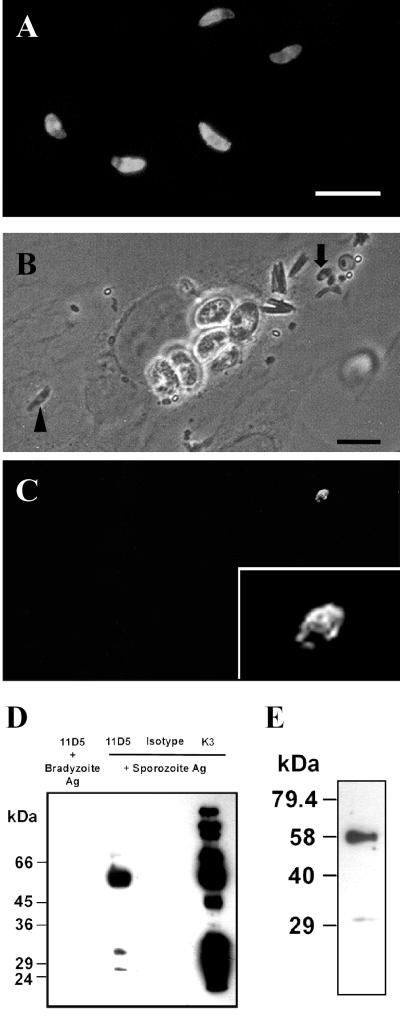
FIG. 6.
Characterization of MAb 11D5/H3 by indirect immunofluorescence and immunoprecipitation of the target antigen. Bars, 10 μm. (A) Staining pattern of air-dried sporozoites when the antibody was applied after the permeabilization of parasites with acetone. (B and C) Phase-contrast image and corresponding immunofluorescence of L2 pneumonocytes ethanol-acetone-fixed 1 h postinfection with sporozoites. Sporozoites were preincubated with mouse MAb 11D5/H3 before inoculation onto cells. After fixation and permeabilization of cells, 11D5/H3 was visualized with FITC-conjugated anti-mouse IgG. An extracellular sporozoite (arrow; above the focus level of host cell cy- toplasm) is labeled on the surface (inset shows a magnification) as indicated by the patchiness of membrane staining, whereas the label is absent on an intracellular sporozoite (arrowhead). The latter resides inside a PV which is visible by the surrounding halo. (D) Electrophoretic separation of the antigen precipitated by MAb 11D5/H3 from sporocyst-sporozoite extracts in gel-loading buffer containing 4% 2-mercaptoethanol (lane 2). A major band at 58 kDa was detected, whereby two minor bands appeared at 27 and 31 kDa. MAb 11D5/H3 did not react with bradyzoite extracts (lane 1), indicating that the antigen was not expressed in bradyzoites. An irrelevant isotype-matched MAb (IgG2a) and a sporozoite-specific rabbit serum (K3) served as negative and positive controls, respectively. (E) Electrophoretic separation of the antigen precipitated by 11D5/H3 from sporocyst-sporozoite extracts as performed before, except that reducing conditions were lowered to 2% 2-mercaptoethanol.
In Western blottings, MAb 11D5/H3 did not react with Nonidet-P40 (24) or detergent-free extracts of sporozoites separated by electrophoresis under reducing or nonreducing conditions. Additionally, reactivity in immunofluorescence was abrogated by short fixation of sporozoites with 1% formaldehyde (or glutaraldehyde), suggesting that the epitope recognized by the antibody was sensitive to conformational changes (T. Jäkel, unpublished data). Therefore, we immunoprecipitated the antigen from biotinylated extracts of sporocysts and sporozoites before electrophoretic separation. This revealed that MAb 11D5/H3 reacted with a major band at 58 kDa whereby additional reactions were seen with antigens at 31 and 27 kDa (Fig. 6D). The two bands at low molecular masses largely disappeared when the concentration of mercaptoethanol was reduced by half (Fig. 6E). No resolution was achieved under nonreducing conditions. Figure 6D further shows that MAb 11D5/H3 was stage specific, i.e., it did not react with bradyzoites, and that the K3 antibodies, among others, also precipitated a 58-kDa molecule.
DISCUSSION
In the present investigation, we provide evidence that a MAb binding to the surface of sporozoites of S. singaporensis enhances their escape from the PV after invasion of host cells in vitro. We further show that escape from the PV is associated with higher metabolic activity (as indicated by increased acetyl-histone H4 expression) of sporozoites compared to stages that remain in this compartment and that the antibody actually increases proliferation. Collectively, these data indicate the antibody's positive influence on the parasite's development, suggesting the existence of a signaling mechanism that could link a receptor on the surface of the parasite with intrinsic components. Increased infectivity was observed for Trypanosoma cruzi when monoclonal IgG1 antibodies, specific or nonspecific for the parasite, bound to Fc receptors on the surface of the trypanosomes (33). However, the involvement of Fc receptors on the observed escape process of Sarcocystis sporozoites seems rather unlikely. An isotype-matched irrelevant antibody and an antibody directed against a different antigen of the parasite did not enhance escape, suggesting that modulation of infective behavior was mediated by specific binding of MAb 11D5/H3 to its antigen.
As demonstrated for the first time for a Sarcocystis species, sporozoites reside in a PV after invasion and before they enter the cytosol. Given its close relationship to other apicomplexan parasites (30), an active penetration of the cell and formation of a PV could be expected (9, 27, 35). However, the latter has not been demonstrated before. Here, visualization of the PV at the light microscopical level was facilitated by antibodies that reacted with parasite-derived molecules incorporated into, or attached to, the PV membrane (PVM). Such antigens have been identified as rhoptry and dense-granule proteins in T. gondii and other apicomplexans (4, 13, 26, 32). The dense-granule protein GRA5 of T. gondii was constantly detected between 2 and 24 h postinfection in the PVM of sporozoite-infected host cells (36). In contrast to sporozoites, merozoites (reference 22 and unpublished data by T. Jäkel) as well as bradyzoites (14) of Sarcocystis develop inside a PV. Thus, escape of the PV by sporozoites of Sarcocystis is a unique feature that is unparalleled among the coccidia but shared with piroplasms, T. cruzi, and various intracellular bacteria (2). Recently, it was observed that Plasmodium sporozoites can disrupt the hepatocyte plasma membrane to move freely in the cytosol (29), indicating that entry into the host cell is not necessarily associated with formation of a PV. According to the results presented here, Sarcocystis sporozoites use the “classical” entry mechanism. Because all extracellular sporozoites were removed from the cell cultures after 2 h, the increased frequency of intracytosolic stages at later intervals suggests that sporozoites entered the cytosol via disruption of the PVM, not by penetrating the plasmalemma.
It is not clear why a large portion of sporozoites of S. singaporensis remains in the PV after invasion and finally becomes degraded. One simple explanation could be that in vitro conditions were not optimal for the parasites. However, the observed behavior appears to be characteristic of the wild-type sporozoites used here, because sporozoites of the frequently passaged strain S1 (24) showed an escape rate of more than 90% in the same cell type (T. Jäkel, unpublished data). We used wild-type parasites because they were subjected to few (two) passages in the laboratory, and, therefore, presumably reflected the natural situation. We have provided evidence that frequent passages in the laboratory from the intermediate to the definitive host reduce the fitness of Sarcocystis in terms of a reduction of transmission stages (24). Because wild-type sporozoites of S. singaporensis reacted to binding of an antibody with enhanced escape from the PV and increased proliferation, this indicates that they are capable of adjusting replication upon certain stimuli. Hence, laboratory-passaged parasites may have lost this ability. The potential for variation of replication could represent a selective advantage for successful transmission in the wild.
To date, it is not known why sporozoites of Sarcocystis enter the host cell cytosol. It has been speculated (34) that it may represent a means of escaping a potentially hostile host cell environment (such as phagosomes, for instance); however, no evidence exists to support this notion. Here we show that localization in the cytosol is associated with enhanced levels of acetyl-histone H4-linked gene expression in sporozoites. The induction of immediate-early (IE) genes, such as c-fos or c-jun, correlates well with a nucleosomal response, the acetylation and phosphorylation of histones via extracellular signal-regulated kinase cascades (1, 7), whereby alterations in chromatin and nucleosome structure seem to be a cause and not an effect of IE gene induction (7). Because IE genes are directly involved in growth control of the cell and, hence, early indicators of replication (6), we interpret detection of high levels of acetyl-histone H4 in the nucleus of sporozoites as an early sign of their forthcoming proliferation. Therefore, essential stimuli for growth of sporozoites could be largely restricted to the cytosol and our data suggest that entering this compartment is necessary for sporozoites to develop further. However, about a quarter of the intravacuolar sporozoites also showed high levels of gene expression (as well as some extracellular stages). It is possible that these sporozoites started development in the cytosol of a neighboring cell before they left the host to reinvade a new cell. Sporozoites of S. singaporensis remain capable for extended periods to leave a host cell upon a certain stimulus (21). Additionally, our ultrastructural data support the view that only intracytosolic sporozoites undergo development into schizonts because late sporozoites/early schizonts were all located free inside the host cell cytoplasm. Because binding of MAb 11D5/H3 to sporozoites actually increased numbers of merozoites, the next generation of the parasite, these results strengthen the link between residence in the cytosol and successful proliferation.
Evidence that the molecule recognized by MAb 11D5/H3 is located on the surface of sporozoites stemmed from the observation that the antibody reacted with the surface of fixed and live zoites and that the fluorescent label was lost after invasion of host cells. This shedding phenomenon has been studied in detail for T. gondii (12), and results of a recent study suggest that antigen-antibody binding is not ruptured but that surface protein-antibody complexes and zoite membrane are shed (16). Because MAb 11D5/H3 also reacted with the cytoplasm of sporozoites as revealed by indirect immunofluorescence, it is likely that the protein is synthesized there and transported to the surface. As whole sporozoite extracts were prepared for immunoprecipitation in the presence of detergent, we have no information on the exact localization of the 58-kDa molecule; this has to be further evaluated by cell fractionation and immuno-electron microscopy. Two additional bands with masses of 27 and 31 kDa appeared after electrophoretic separation if the concentration of reducing agent was increased, suggesting a contribution of disulfide bonds to the structure of the protein. As the combined molecular mass of the two molecules is 58 kDa, they might constitute subunits. Interestingly, parasite-specific IgG2b antibodies of infected rats detected molecules at 58 and 27 kDa in sporozoite extracts of S. singaporensis in a previous study (24). Therefore, whether the IgG2b response of infected rats targets the same antigen and exerts a similar influence on parasite development like MAb 11D5/H3 should be tested.
REFERENCES
- 1.Alberts A S, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N W, Webster P. Phagolysosomal escape by intracellular pathogens. Parasitol Today. 1991;7:335–340. doi: 10.1016/0169-4758(91)90212-7. [DOI] [PubMed] [Google Scholar]
- 3.Arness M K, Brown J D, Dubey J P, Neafie R C, Granstrom D E. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am J Trop Med Hyg. 1999;61:548–553. doi: 10.4269/ajtmh.1999.61.548. [DOI] [PubMed] [Google Scholar]
- 4.Beckers C J M, Dubremetz J F, Mercereau-Puijalon O, Joiner K A. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonsong P, Hongnark S, Suasa-ard K, Khoprasert Y, Promkerd P, Hamarit G, Nookarn P, Jäkel T. Rodent management in Thailand. In: Singleton G, Hinds L, Leirs H, Zhang Z, editors. Ecologically-based rodent management. Canberra, Australia: ACIAR; 1999. pp. 338–357. [Google Scholar]
- 6.Chen T A, Allfrey V G. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc Natl Acad Sci USA. 1987;84:5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton A L, Rose S, Barratt M J, Mahadevan L C. Phosphoacetylation of histone H3 on c-fos and c-jun-associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darkin-Rattray S J, Gurnett A M, Myers R W, Dulski P M, Crumley T M, Allocco J J, Cannova C, Meinke P T, Colletti S L, Bednarek M A, Singh S B, Goetz M A, Dombrowski A W, Polishook J D, Schmatz D M. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci USA. 1996;93:13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrowolski J M, Sibley L D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 10.Dubey J P. Toxoplasma, Neospora, Sarcocystis, and other tissue cyst-forming coccidia of humans and animals. In: Kreier J P, editor. Parasitic protozoa. Vol. 6. San Diego, Calif: Academic Press; 1993. pp. 1–157. [Google Scholar]
- 11.Dubey J P, Speer C A, Fayer R. Sarcocystosis of animals and man. Boca Raton, Fla: CRC Press; 1989. [Google Scholar]
- 12.Dubremetz J F, Rodriguez C, Ferreira E. Toxoplasma gondii: redistribution of monoclonal antibodies on tachyzoites during host cell invasion. Exp Parasitol. 1985;59:24–32. doi: 10.1016/0014-4894(85)90053-0. [DOI] [PubMed] [Google Scholar]
- 13.Dubremetz J F, Garcia-Réguet N, Conseil V, Fourmaux M N. Apical organelles and host-cell invasion by Apicomplexa. Int J Parasitol. 1998;28:1007–1013. doi: 10.1016/s0020-7519(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 14.Entzeroth R. Invasion and early development of Sarcocystis muris (Apicomplexa, Sarcocystidae) in tissue cultures. J Protozool. 1985;32:446–453. doi: 10.1111/j.1550-7408.1985.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 15.Fayer R, Elsasser T H. Bovine sarcocystosis: how parasites negatively affect growth. Parasitol Today. 1991;7:250–255. doi: 10.1016/0169-4758(91)90242-g. [DOI] [PubMed] [Google Scholar]
- 16.Hakansson S, Morisaki H, Heuser J, Sibley L D. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999;10:3539–3547. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halonen S K, Chiu F-C, Weiss L M. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun. 1998;66:4989–4993. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hettmann C, Soldati D. Cloning and analysis of a Toxoplasma gondii histone acetyltransferase: a novel chromatin remodelling factor in apicomplexan parasites. Nucleic Acids Res. 1999;27:4344–4352. doi: 10.1093/nar/27.22.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson R H, Ladurner A G, King D S, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 20.Jäkel T, Burgstaller H, Frank W. Sarcocystis singaporensis: studies on host specificity, pathogenicity, and potential use as a biocontrol agent of wild rats. J Parasitol. 1996;82:280–287. [PubMed] [Google Scholar]
- 21.Jäkel T, Henke M, Weingarten B, Kliemt D, Seidinger S. In vitro cultivation of the vascular phase of Sarcocystis singaporensis. J Euk Microbiol. 1997;44:293–299. doi: 10.1111/j.1550-7408.1997.tb05669.x. [DOI] [PubMed] [Google Scholar]
- 22.Jäkel T, Archer-Baumann C, Boehmler A M, Sorger I, Henke M, Kliemt D, Mackenstedt U. Identification of a subpopulation of merozoites of Sarcocystis singaporensis that invades and partially develops inside muscle cells in vitro. Parasitology. 1999;118:235–244. doi: 10.1017/s0031182098003734. [DOI] [PubMed] [Google Scholar]
- 23.Jäkel T, Khoprasert Y, Endepols S, Archer-Baumann C, Suasa-ard K, Promkerd P, Kliemt D, Boonsong P, Hongnark S. Biological control of rodents using Sarcocystis singaporensis. Int J Parasitol. 1999;29:1321–1330. doi: 10.1016/s0020-7519(99)00081-8. [DOI] [PubMed] [Google Scholar]
- 24.Jäkel T, Khoprasert Y, Kliemt D, Mackenstedt U. Immunoglobulin subclass responses of wild brown rats to Sarcocystis singaporensis. Int J Parasitol. 2001;31:273–283. doi: 10.1016/s0020-7519(00)00172-7. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Lecordier L, Mercier C, Sibley L D, Cesbron-Delauw M-F. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol Biol Cell. 1999;10:1277–1287. doi: 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingelbach K, Joiner K A. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci. 1998;111:1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- 28.Mineo J R, McLeod R, Mack D, Smith J, Khan I A, Ely K H, Kasper L H. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;9:3951–3964. [PubMed] [Google Scholar]
- 29.Mota M M, Pradel G, Vanderberg J P, Hafalla J C R, Frevert U, Nussenzweig R S, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 30.Mugridge N B, Morrison D A, Jäkel T, Heckenroth A R, Tenter A M, Johnson A M. Effects of sequence alignment and structural domains of ribosomal DNA on phylogeny reconstruction for the protozoan family Sarcocystidae. Mol Biol Evol. 2000;17:1842–1853. doi: 10.1093/oxfordjournals.molbev.a026285. [DOI] [PubMed] [Google Scholar]
- 31.Nichols G L. Food-borne protozoa. Br Med Bull. 2000;56:209–235. doi: 10.1258/0007142001902905. [DOI] [PubMed] [Google Scholar]
- 32.Ossorio P N, Dubremetz J F, Joiner K A. A soluble secretory protein of the intracellular parasite Toxoplasma gondii associates with the parasitophorous vacuole membrane through hydrophobic interactions. J Biol Chem. 1994;269:15350–15357. [PubMed] [Google Scholar]
- 33.Rodriguez De Cuna C, Kierszenbaum F, Wirth J J. Binding of the specific ligand to Fc receptors on Trypanosoma cruzi increases the infective capacity of the parasite. Immunology. 1991;72:114–120. [PMC free article] [PubMed] [Google Scholar]
- 34.Speer C A, Burgess D E. In vitro development and antigen analysis of Sarcocystis. Parasitol Today. 1988;4:46–49. doi: 10.1016/0169-4758(88)90064-6. [DOI] [PubMed] [Google Scholar]
- 35.Suss-Toby E, Zimmerberg J, Ward G E. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc Natl Acad Sci USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilley M, Fichera M E, Jerome M E, Roos D S, White M W. Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains a subset of dense-granule proteins. Infect Immun. 1997;65:4598–4605. doi: 10.1128/iai.65.11.4598-4605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vercammen M, El Bouhdidi A, Ben Messaoud A, De Meuter F, Bazin H, Dubremetz J-F, Carlier Y. Identification and characterization of a Fc receptor activity on the Toxoplasma gondii tachyzoite. Parasite Immunol. 1998;20:37–47. doi: 10.1046/j.1365-3024.1998.t01-1-00124.x. [DOI] [PubMed] [Google Scholar]