Abstract
Primary gastric squamous cell carcinoma (GSCC) is an extremely rare malignancy with a poor prognosis. Despite the improved knowledge regarding its pathogenesis and biology, the treatment options remain limited. The present study reported on the unique case of a mismatch repair-deficient (dMMR) primary GSCC in a 79-year-old woman reporting fatigue and symptoms of upper gastrointestinal tract bleeding. Physical examination revealed abdominal pain at palpation. Gastroscopy revealed a large, exophytic, bleeding tumor. Medical imaging confirmed a mushroom-like polyp in the lumen of the stomach, with no signs of disease spread. Total gastrectomy and D2 lymphadenectomy were performed. Pathological examination of the post-operational material confirmed a well-differentiated SCC invading the mucosa, submucosa and muscle layer. There were no signs of dissemination observed in any of the 32 excised lymph nodes. Notably, according to the last follow-up, the patient remains well, supporting the 5-year GSCC survival rate statistics. To the best of our knowledge, this is the first such GSCC case reported in the Surgical Oncology Outpatient Clinic (Copernicus Memorial Hospital, Lodz, Poland) and these findings add to the limited data on GSCC. Although this is a very rare condition, it should always be considered during the process of diagnosis of gastric tumors.
Keywords: case report, diagnosis, gastric cancer; mismatch repair deficiency, primary gastric squamous cell carcinoma, surgical treatment, 5-year survival rate
Introduction
Cancer is a global health problem and the second leading cause of death in developed countries (1). A significant part of that problem is gastric cancer, being the fifth most common cancer worldwide (2). Despite numerous improvements in systemic treatment, patients diagnosed at an advanced stage have poor prognosis (3), and in such cases, medical procedures may be more of a palliative nature (4). For some patients with metastatic, unresectable, microsatellite instability-high (MSI-H), or mismatch repair-deficiency solid tumors, the US Food and Drug Administration (FDA) has approved treatment with immune checkpoint inhibitors such as pembrolizumab (5).
Primary squamous cell carcinoma of the stomach (GSCC) is an extremely rare condition, described for the first time in 1895 (6,7). The first guidelines for GSCC histopathological diagnosis were published in 1965 (8). The current incidence of GSCC is described as 0.04 to 0.07% of all gastric carcinomas (9–13). GSCC is more common in men, with a male-to-female ratio of 5 to 1 (14). GSCC patients are usually diagnosed in the sixth decade of life (13,15), in an advanced stage of the disease. The tumor stage is recognized to be the most important prognostic factor (15). Most commonly, a GSCC tumor is located in the upper third of the stomach (16). Although the chemotherapeutic options continue to develop, surgery remains the most effective therapeutic option (12,13). Even so, the prognosis for GSCC patients, especially those in advanced stages, is poor (17).
Case report
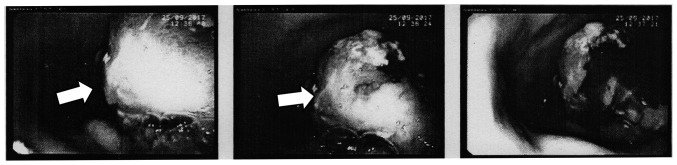
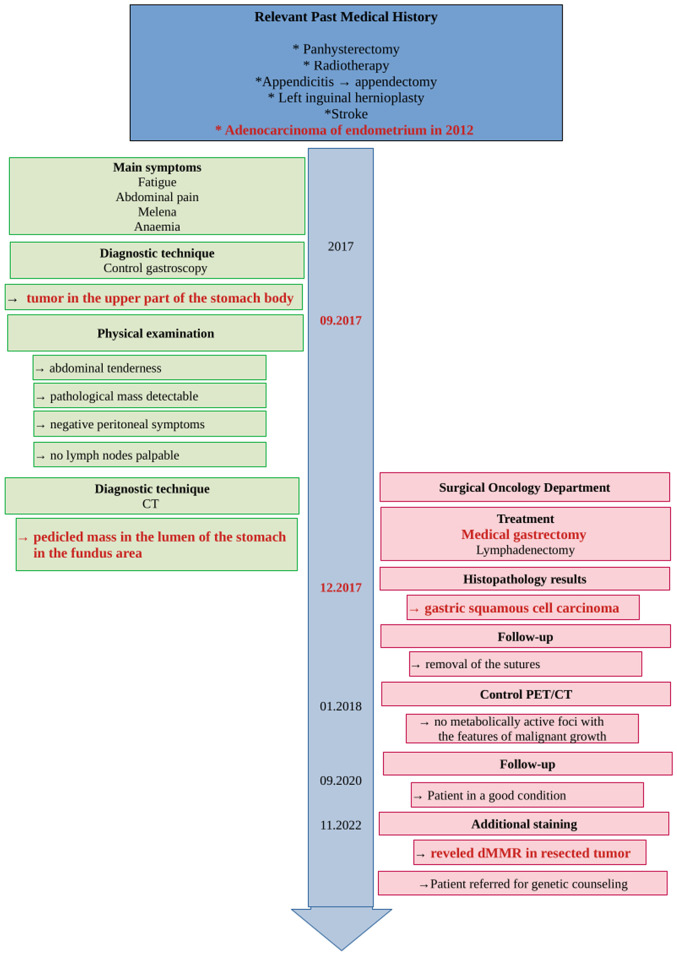
In December 2017, a 79-year-old Polish woman suffering from fatigue, abdominal pain, melena, and anemia was admitted to Surgical Oncology Outpatient Clinic (Medical University of Lodz, Copernicus Memorial Hospital, Lodz, Poland). She denied chronic diseases and ongoing pharmacological treatment. Her past medical history included adenocarcinoma of the endometrium (treated with panhysterectomy and radiotherapy in 2012), appendectomy due to appendicitis, left inguinal hernioplasty, and stroke. Physical examination revealed abdominal tenderness without any pathological mass detectable, with negative peritoneal symptoms and no lymph nodes palpable. A diagnostic gastroscopy (Fig. 1) was performed. A large, exophytic, bleeding tumor was found in the upper part of the stomach body.
Figure 1.
Diagnostic gastroscopy. The arrow indicates a large tumor that is visible in the upper part of the stomach body, in the greater curvature of the stomach.
Abdominal ultrasonography did not reveal any pathology. An abdominal Computer Tomography (CT) (Fig. 2) scan showed a pedicled mass (49×40×42 mm) in the lumen of the stomach in the fundus area, without features of surrounding fatty tissue invasion, and no pathological abdominal lymph nodes. There were no signs of disease spread in medical imaging.
Figure 2.
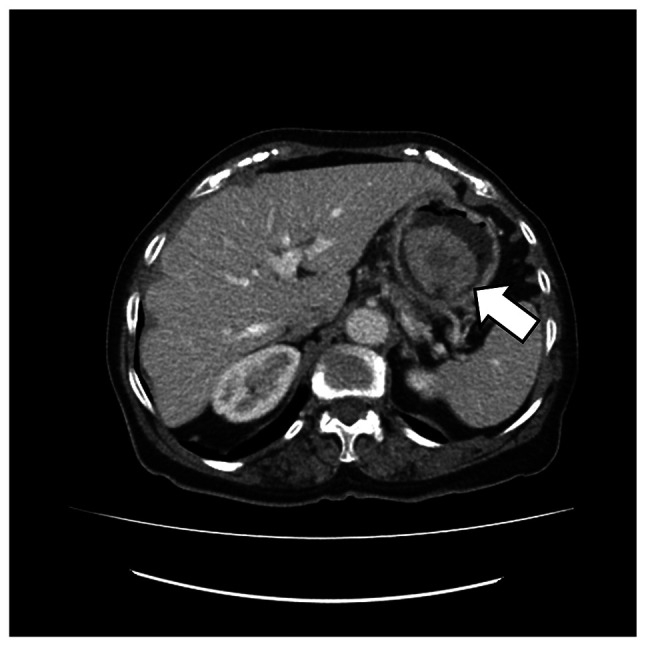
The abdominal CT before the operation. The arrow indicates the massive tumor in the lumen of the stomach, in the fundus area.
Taking into consideration the patient's condition and test results, particularly the signs of active gastrointestinal bleeding, a strong suspicion of gastric cancer arose. The patient was informed about the clinical diagnosis and surgical treatment was proposed. The patient initially declined, but changed her mind after one month and was immediately admitted to the Surgical Oncology Department. Radical gastrectomy with Roux-en-Y loop and D2 lymphadenectomy was performed without complications. The patient was discharged home in good general condition 10 days later, without any clinically-significant blood morphology deviations.
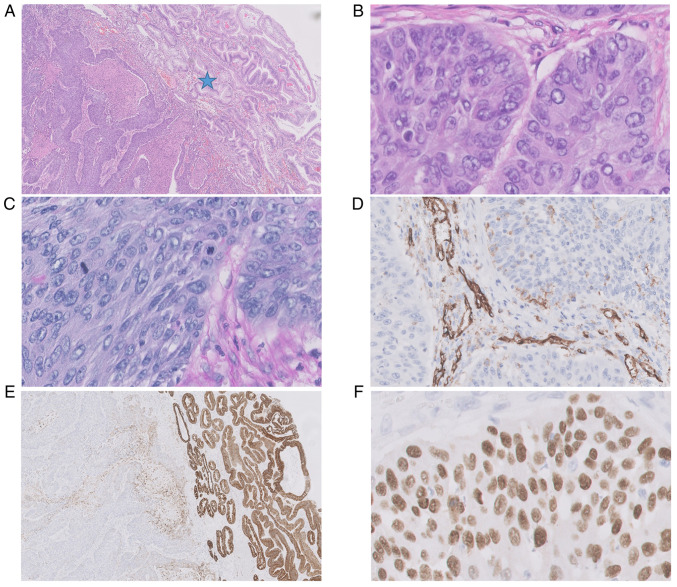
The tumor specimen used for histopathological analysis were taken by surgery. The histopathological analysis of post-operational material (Fig. 3) revealed a large tumor (5×6×4 cm) with central ulceration and bleeding signs in the lumen of the stomach, located 10 cm from the proximal end of the specimen. The tumor had invaded the mucosa, submucosa and muscle layer. The radial margin was assessed as 0.2 cm. Immunostaining was positive for p63 (Fig. 3F), and negative for CK7 (Fig. 3E), CK19, CK20, and CDX2. Histochemical staining with AB/PAS was negative (Fig. 3C). Additionally, no vascular invasion was apparent in the tumor (anti-CD31 staining) (Fig. 3D). This all favored a diagnosis of squamous cell carcinoma G2. All 32 removed regional lymph nodes were free from cancer cells.
Figure 3.
Histopathological picture of primary gastric squamous cell carcinoma in the antrum. (A and B) Tumor cells showed only squamous cell differentiation without any formation of glands with central necrosis (HE 20×, 400×); (C) AB/PAS histochemical staining was completely negative in tumor cells (AB/PAS, 400×); (D) no vascular invasion was seen in the tumor, CD31 was positive in the endothelial cells of stromal vessels [IHC reaction with anti-CD31 antibody (DAKO), 400×]; the pre-existent prepyloric mucosa was intact, (A) with no infiltration by carcinoma (asterisk) and was (E) positive in anti-CK7 staining (IHC reaction with anti-CK7 antibody (DAKO), 20×); (F) p63 IHC positive expression in squamous cell carcinoma (IHC reaction with anti-p63 antibody (DAKO), 400×). AB/PAS, alcian blue/periodic acid-Schiff; HE, hematoxylin and eosin; IHC, immunohistochemical.
A selected representative block of tumor tissue was stained immunohistochemically (IHC) with MSH2 (Monoclonal Mouse Anti-Human antibody, Clone FE11), MSH6 (Monoclonal Rabbit Anti-Human antibody, Clone EP49), MLH1 (Monoclonal Mouse Anti-Human antibody, Clone ES05), and PMS2 (Monoclonal Rabbit Anti-Human antibody, Clone EP51) (Agilent Dako, Glostrup, Denmark) to characterize DNA-mismatch repair (MMR) protein expression patterns. Immunohistochemistry (IHC) was performed in tissue sections of formalin-fixed, paraffin-embedded (FFPE). We used EnVision FLEX+, Mouse, High pH (Link) (Dako) in Autostainer Link 48 with the HIER (heat-induced epitope retrieval) method using the panel of following antibodies: CK-7 (Clone OV-TL 12/30, Mouse monoclonal, Catalog No. IR619), CK19 (Clone RCK108, Mouse monoclonal, Catalog No. IR615), CK20 (Clone Ks20.8, Mouse monoclonal, Catalog No. IR777), CDX2 (Clone DAK-CDX2, Mouse monoclonal, Catalog No. IR080), CD31 (Clone JC70A, Mouse monoclonal, Catalog No. IR610), p63 (Clone DAK-P63, Mouse monoclonal, Catalog No. IR662), MLH1 (Clone ES05, Mouse monoclonal, Catalog No. IR076), PSM2 (Clone EP51, Rabbit monoclonal, Catalog No. IR087), MSH2 (Clone FE11, Mouse monoclonal, Catalog No. IR085), MSH6 (Clone EP49, Rabbit monoclonal, Catalog No. IR086), all provided by DAKO, Agilent. All primary antibodies were ready-to-use (RTU), and the incubation time was 20 min at room temperature. To distinguish mucins in tissue sections, we used Alcian Blue/PAS Stain Kit, Artisan, Ready-to-use (Catalog No. AR178, DAKO, Agilent), which is intended to identify acidic and neutral mucins in tissue sections on the Artisan Link Staining Systems.
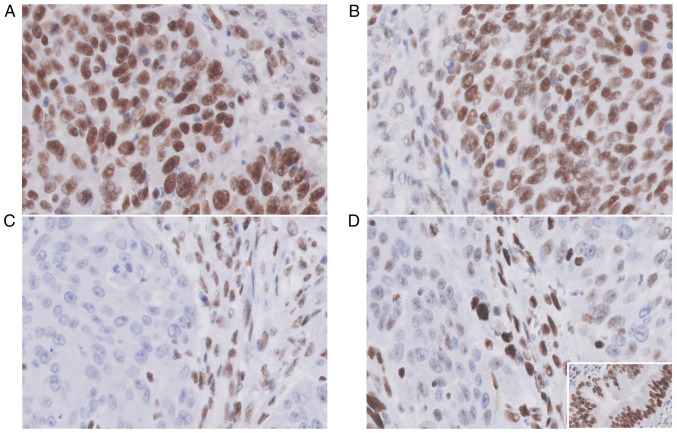
A positive external control was performed on normal gastric tissue (Fig. 4D, inset). Positive nuclear staining was performed for each MMR protein in lymphocytes, stroma and endothelial cells as a positive internal control (Fig. 4A-D) (18).
Figure 4.
MMR protein expression patterns showed positive nuclear staining (MMR-proficient) of (A) MLH1 protein (anti-MLH1 ES05, 400×) and (B) PSM2 protein (anti-PSM2 EP51); (C) MSH2 nuclear staining was totally negative (MMR-deficient) (anti-MSH2 FE11, 400×); (D) The staining pattern for MSH6 immunoexpression was heterogeneous (anti-MSH6 EP49, 400×) with only a partial loss of nuclear staining; (D, inset) The external control was positive in the benign glandular epithelium; (A-D) The internal control was positive in lymphocytes, stroma and endothelial cells for each MMR protein staining.
A lack of nuclear reactivity was considered as loss of expression (negative in 100% of tumor cells) (MMR-deficient). At least 1% nuclear staining of each antibody in tumor cells was considered as retained expression (positive in tumor cells) (MMR-proficient) (19). As the IHC indicated MSH2 loss of expression, the tumor was diagnosed as mismatch-repair deficient.
The existence of metabolically-active malignant-type lesions was excluded by whole-body PET-CT scan (Fig. 5), and the diagnosis of GSCC was maintained.
Figure 5.
Whole body PET-CT scan: no metabolically-active foci are present.
In 2018, a routine CT scan failed to find any sign of disease recurrence. Interestingly, five years after surgery (in 2022), the patient is in good health condition and remains under the supervision of the Outpatient Clinic. Due to a past history of endometrial cancer (in 2012) and diagnosed dMMR in GSCC tissue, the patient was referred for genetic counseling (Fig. 6).
Figure 6.
Case report timeline.
Discussion
Almost 100 cases of primary gastric squamous cell carcinoma had been published by the year 2016 (20). The most frequent localization of GSCC is the proximal 1/3 of the gastric wall (11,17). Diagnosis of GSCC can be excluded by esophagus invasion and localization in gastric cardia (21). Pathogenetic differences between GSCC and gastric adenocarcinoma are still unclear. One metastatic theory suggests that basal cells in the gastric mucosa can transform into squamous cells, then into squamous cell carcinoma cells (7). One study proposes that squamous differentiation may occur in previously-existing adenocarcinoma (9), while Takita et al suggest that the Epstein-Barr virus may play a part in this process: they confirmed the presence of the Epstein-Barr in surgically removed GSCC (22). However, there is no information about previous stomach malignancies or Epstein-Barr infection in records of the currently-described patient.
Generally, the established risk factors for GSCC include male sex, with a male-to-female incidence ratio ranging from 5: 1 (11,15,23) to 2.3: 1 (17), African/Caribbean ethnicity (17), presence of squamous cell metaplasia and tobacco use (24,25). In addition, five-year survival depends on the stage at which the treatment was started. At stages I and IV, survival rates do not differ significantly between GSCC and gastric adenocarcinoma (I-80% vs. 79%, II-67.5% vs. 43.2%, III-39.7% vs. 28.7%, IV-6% vs. 5%); however, GSCC patients are rarely diagnosed in early stage (stage I-7.4% vs. 22.8% in gastric adenocarcinoma). Results of the treatment depend strongly on initial qualification for surgical treatment (yes-5-year OS 59.2%, no-17.4%) (17). The patient described herein was diagnosed in stage IB (pT2pN0cM0), which gives her an 80% possibility of 5-year survival according to Dong et al (17).
Surprisingly, the pathological report revealed a deficiency in mismatch repair mechanisms, with negative staining for MSH2. Given her past medical history of endometrial cancer, the patient was referred to genetic counseling to revise the hereditary dMMR background and perform differentiation diagnostics of Lynch Syndrome type II OMIM#120435 and Muir-Torre Syndrome OMIM#158320. A literature search using EMBASE, MEDLINE, PubMed and Cochrane databases with the terms gastric cancer, squamous cell carcinoma and mismatch repair deficiency did not reveal any other case of mismatch repair deficient, primary gastric squamous cell carcinoma ever published.
Although chemotherapy and radiotherapy have well-established places in the algorithms of gastric adenocarcinoma treatment (26,27), no indications exist for GSCC, since the outcome of chemotherapy in these patients is unknown. Data on GSCC treatment is only available from case reports or series with the largest cohorts reaching 56 patients. Based on existing literature, GSCC treatment in Europe is based around cisplatin, 5-fluorouracil, oxaliplatin, capecitabine, docetaxel, gemcitabine and S1; however, a Japanese publication (11) notes that mitomycin, epirubicin and bleomycin can also be applied in both systemic and intrahepatic infusions.
Although the majority of publications describe chemotherapy in a palliative setting (28), a few radical resections with adjuvant chemotherapy (29) and radiation were noted (11,25,30). Only one patient was reported to undergo neoadjuvant treatment, which resulted in partial response to 5-FU/cisplatin systemic treatment (11); nevertheless, 5-FU/cisplatin seems to be chosen most often in the reviewed publications. Unfortunately, as no randomized clinical trials have been conducted on GSCC patients, decisions on chemotherapy should be made individually by the multidisciplinary team. In the present case, adjuvant chemotherapy was not advised by the medical oncologist, based on the presence of upper gastrointestinal bleeding prior to the surgery and the poor performance status of the patient post gastrectomy (according to Eastern Cooperative Oncology Group-ECOG 2). The patient's treatment was standard and included only surgical intervention.
In our case, negative IHC nuclear staining of MSH2 indicates possible microsatellite instability (MSI). Furthermore, based on recent reports, weak, heterogeneous nuclear expression of MSH2 protein requires testing for MSI mutations; in our case, the MSH6 protein showed only focally weak nuclear expression in a few cells. It should be emphasized that molecular testing is mandatory in cases with loss/patchy MMR-IHC in daily clinical practice, to avoid misdiagnosis and consequently inadequate therapeutic choices (31).
Immune checkpoint inhibitors have been approved by the FDA and they have demonstrated survival benefits among patients with MSI-H/dMMR diagnosis (3). Hence, pembrolizumab appears an attractive potential treatment option in the case of GSCC relapse or dissemination.
It is important to emphasize the major limitations of our case report. Firstly, diagnostic MMR analysis was not initially performed, as the patient did not have metastatic cancer, which would have required other treatment options and the aforementioned MMR testing. Upon additional diagnostics, we detected IHC MSH2 negative nuclear staining, indicating possible microsatellite instability (MSI). Secondly, the limitation of our study is also the lack of assessment of the microvessel density with CD31 expression, which may be a potential prognostic biomarker. CD31 immunohistochemical staining was performed to assess tumor angioinvasion.
As a particularly rare malignancy, GSCC presents a challenging problem in setting a correct diagnosis and in planning optimal treatment. Nevertheless, despite its generally serious prognosis and poor treatment outcomes, early diagnosis and effective treatment are possible. There is a need for further research in the field of GSCC, especially for developing diagnostic methods and new systemic treatment options.
The presented case report adds to the limited amount of data available on GSCC. More importantly, it serves as an important reminder that despite being a very rare condition, it should still be a consideration when diagnosing patients with gastric tumors.
This is the first reported case of dMMR primary gastric squamous cell cancer. Based on the reviewed literature, the MSI/MMR status of previously published GSCC cases remains unknown. Therefore, in the event of a diagnosis of GSCC, we encourage clinicians to consider MSI/MMR testing. This could directly benefit the patients by indicating the need for immune checkpoint inhibition implementation in cases with disseminated disease.
Acknowledgements
The Department of Surgical Oncology is the scientific, structural part of the Medical University of Lodz, located in the Copernicus Memorial Hospital in Lodz. The Surgical Oncology Outpatient Clinic is the medical, structural part of the Copernicus Memorial Hospital in Lodz.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JarJ, AM, JagJ and KLG conceptualized the study; JarJ, DJK and AM performed an operation or performed histopathological examination of the patient's cancer tissue; JagJ, AM, EW and KLG wrote and prepared the original draft; EW reviewed the literature and treatment methods; JagJ, AM, EW, JarJ, AKS and KLG analyzed the data; JagJ, AM, EW, JarJ, KLG, DJK and AKS reviewed and edited the manuscript. JagJ, KLG and JarJ confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Xu L, Yin S, Jiang J, Hong C, He Y, Zhang C. Risk factors and prognostic impact of postoperative complications in patients with advanced gastric cancer receiving neoadjuvant chemotherapy. Curr Oncol. 2022;29:6496–6507. doi: 10.3390/curroncol29090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: Novel translational implications. Int J Mol Sci. 2021;22:3805. doi: 10.3390/ijms22083805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci AD, Rizzo A, Rojas Llimpe FL, Di Fabio F, De Biase D, Rihawi K. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER paradigm shift? Cancers (Basel) 2021;13:1664. doi: 10.3390/cancers13071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricci AD, Rizzo A, Brandi G. DNA damage response alterations in gastric cancer: Knocking down a new wall. Future Oncol. 2021;17:865–868. doi: 10.2217/fon-2020-0989. [DOI] [PubMed] [Google Scholar]
- 6.Segura S, Pender J, Dodge J, Brandwein SL, El-Fanek H. Primary squamous cell carcinoma of the stomach: A case report and review of the literature. Conn Med. 2016;80:209–212. [PubMed] [Google Scholar]
- 7.Altshuler JH, Shaka JA. Squamous cell carcinoma of the stomach. Review of the literature and report of a case. Cancer. 1966;19:831–838. doi: 10.1002/1097-0142(196606)19:6<831::AID-CNCR2820190613>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::AID-CNCR2820180209>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::AID-CNCR2820240518>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Muto M, Hasebe T, Muro K, Boku N, Ohtsu A, Fujii T, Ono M, Taijiri H, Mukai K, Yoshida S. Primary squamous cell carcinoma of the stomach: A case report with a review of Japanese and Western literature. Hepatogastroenterology. 1999;46:3015–3018. [PubMed] [Google Scholar]
- 11.Amuluru K, Gupta H. Primary squamous cell carcinoma of the stomach: A case report. J Gastrointest Cancer. 2010;41:24–26. doi: 10.1007/s12029-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 12.Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91–94. [PubMed] [Google Scholar]
- 13.Schmidt C, Schmid A, Lüttges JE, Kremer B, Henne-Bruns D. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology. 2001;48:1033–1036. [PubMed] [Google Scholar]
- 14.González-Sánchez JA, Vitón R, Collantes E, Rodríguez-Montes JA. Primary squamous cell carcinoma of the stomach. Clin Med Insights Oncol. 2017;11:1179554916686076. doi: 10.1177/1179554916686076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SH, Lee JH, Kim K, Shin DH, Kim JY, Sol MY, Choi KU. Primary squamous cell carcinoma of the stomach: A case report. Oncol Lett. 2014;8:2122–2124. doi: 10.3892/ol.2014.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi H, Matsutani T, Fujita I, Kanazawa Y, Nomura T, Hagiwara N, Hosone M, Katayama H, Uchida E. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58–62. doi: 10.5230/jgc.2014.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong C, Jiang M, Tan Y, Kong Y, Yang Z, Zhong C, Li D, Yuan Y. The clinicopathological features and prognostic factors of gastric squamous cell carcinoma. Medicine (Baltimore) 2016;95:e4720. doi: 10.1097/MD.0000000000004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato A, Sato N, Sugawara T, Takahashi K, Kito M, Makino K, Sato T, Shimizu D, Shirasawa H, Miura H, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770–776. doi: 10.1097/PAS.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma M, Yang Z, Miyamoto H. Loss of DNA mismatch repair proteins in prostate cancer. Medicine (Baltimore) 2020;99:e20124. doi: 10.1097/MD.0000000000020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis: A case report and review of literature. Hum Pathol. 2009;40:1494–1498. doi: 10.1016/j.humpath.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol Radium Ther Nucl Med. 1967;101:447–449. doi: 10.2214/ajr.101.2.447. [DOI] [PubMed] [Google Scholar]
- 22.Takita J, Kato H, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Kuwano H. Primary squamous cell carcinoma of the stomach: A case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology. 2005;52:969–974. [PubMed] [Google Scholar]
- 23.Volpe CM, Hameer HR, Masetti P, Pell M, Shaposhnikov YD, Doerr RJ. Squamous cell carcinoma of the stomach. Am Surg. 1995;61:1076–1078. [PubMed] [Google Scholar]
- 24.Chen Y, Zhu H, Xu F, Cao Y, Gu X, Wan Y, Gou H. Clinicopathological characteristics, treatment, and prognosis of 21 patients with primary gastric squamous cell carcinoma. Gastroenterol Res Pract. 2016;2016:306254. doi: 10.1155/2016/3062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Waagner W, Wang Z, Picon AI. A rare case of a primary squamous cell carcinoma of the stomach presenting as a submucosal mas. Case Rep Surg. 2015;2015:482342. doi: 10.1155/2015/482342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karaca M, Tural D, Kocoglu H, Selcukbiricik F, Bilgetekin I, Özet A. Adjuvant chemotherapy for gastric cancer in elderly patients has same benefits as in younger patients. J Cancer Res Ther. 2018;14:593–596. doi: 10.4103/0973-1482.172588. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Liu J, Gao S, Yang Y, Kong W, Ren W, Zhu L, Yang M, Wei J, Zou Z, et al. Use of simultaneous radiation boost achieves high treatment response rate in patients with metastatic gastric cancer. J Cancer Res Ther. 2018;14:36–39. doi: 10.4103/jcrt.JCRT_387_17. [DOI] [PubMed] [Google Scholar]
- 28.Meng Y, Zhang J, Wang H, Zhang Y, Sun R, Zhang Z, Gao F, Huang C, Zhang S. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach: Case report. Medicine (Baltimore) 2017;96:e9224. doi: 10.1097/MD.0000000000009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman Rojas P, Parikh J, Vishnubhotla P, Oharriz JJ. Primary gastric squamous cell carcinoma. Cureus. 2018;10:e2389. doi: 10.7759/cureus.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gülçiçek OB, Solmaz A, Özdoğan K, Erçetin C, Yavuz E, Yiğitbaş H, Çelebi F, Altınay S. Primary squamous cell carcinoma of the stomach. Ulus Cerrahi Derg. 2015;32:221–223. doi: 10.5152/UCD.2015.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zito Marino F, Amato M, Ronchi A, Panarese I, Ferraraccio F, De Vita F, Tirino G, Martinelli E, Troiani T, Facchini G, et al. Microsatellite status detection in gastrointestinal cancers: PCR/NGS is mandatory in negative/patchy MMR immunohistochemistry. Cancers (Basel) 2022;14:2204. doi: 10.3390/cancers14092204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.