This diagnostic study investigates if there is a reproducible and robust blood lipidome profile characteristic for schizophrenia, depression, and bipolar disorder.
Key Points
Question
Is there a robust and reproducible blood lipidome profile characteristic of severe mental disorders?
Findings
In this diagnostic study including 1552 individuals, significant blood plasma lipidome alterations were identified that reproducibly and accurately separated individuals with schizophrenia from controls in 3 population-distinct cohorts from China, Western Europe, and Russia. Parallel analysis of lipid alterations present in the blood plasma of individuals with major depressive disorder and bipolar disorder further revealed a significant transdiagnostic overlap.
Meaning
Study results suggest that a shared profile of quantitative plasma lipid alterations is characteristic of population-diverse individuals affected by severe mental disorders.
Abstract
Importance
No clinically applicable diagnostic test exists for severe mental disorders. Lipids harbor potential as disease markers.
Objective
To define a reproducible profile of lipid alterations in the blood plasma of patients with schizophrenia (SCZ) independent of demographic and environmental variables and to investigate its specificity in association with other psychiatric disorders, ie, major depressive disorder (MDD) and bipolar disorder (BPD).
Design, Setting, and Participants
This was a multicohort case-control diagnostic analysis involving plasma samples from psychiatric patients and control individuals collected between July 17, 2009, and May 18, 2018. Study participants were recruited as consecutive and volunteer samples at multiple inpatient and outpatient mental health hospitals in Western Europe (Germany and Austria [DE-AT]), China (CN), and Russia (RU). Individuals with DSM-IV or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnoses of SCZ, MDD, BPD, or a first psychotic episode, as well as age- and sex-matched healthy controls without a mental health–related diagnosis were included in the study. Samples and data were analyzed from January 2018 to September 2020.
Main Outcomes and Measures
Plasma lipidome composition was assessed using liquid chromatography coupled with untargeted mass spectrometry.
Results
Blood lipid levels were assessed in 980 individuals (mean [SD] age, 36 [13] years; 510 male individuals [52%]) diagnosed with SCZ, BPD, MDD, or those with a first psychotic episode and in 572 controls (mean [SD] age, 34 [13] years; 323 male individuals [56%]). A total of 77 lipids were found to be significantly altered between those with SCZ (n = 436) and controls (n = 478) in all 3 sample cohorts. Alterations were consistent between cohorts (CN and RU: [Pearson correlation] r = 0.75; DE-AT and CN: r = 0.78; DE-AT and RU: r = 0.82; P < 10−38). A lipid-based predictive model separated patients with SCZ from controls with high diagnostic ability (area under the receiver operating characteristic curve = 0.86-0.95). Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were found to be similar to those of SCZ (BPD: r = 0.89; MDD: r = 0.92; P < 10−79). Assessment of detected alterations in individuals with a first psychotic episode, as well as patients with SCZ not receiving medication, demonstrated only limited association with medication restricted to particular lipids.
Conclusions and Relevance
In this study, SCZ was accompanied by a reproducible profile of plasma lipidome alterations, not associated with symptom severity, medication, and demographic and environmental variables, and largely shared with BPD and MDD. This lipid alteration signature may represent a trait marker of severe psychiatric disorders, indicating its potential to be transformed into a clinically applicable testing procedure.
Introduction
There is an increasing interest in the use of lipids as potential biomarkers of pathophysiological processes, particularly psychiatric disorders, where currently, to our knowledge, no clinically useful diagnostic tests exist. Changes in lipid profiles have been repeatedly observed in serum and plasma across patients with psychiatric diagnoses, however, mostly in small-scale studies. Clinical lipid measurements revealed an increase in triglyceride levels in individuals with schizophrenia (SCZ) who were receiving medication, those who were drug naïve, those with a first episode of psychosis,1,2,3 and individuals with major depressive disorder (MDD) before treatment.4 Nonclinical lipidomics studies examining a broader scope of lipid classes yielded repeated observations of decreased ether phospholipids in patients with SCZ5,6,7,8,9,10 and less consistent alterations of acylcarnitines.11,12,13,14,15 MDD studies also reported decreased levels of ether phospholipids and decreased levels of acylcarnitines.16,17,18,19 Further, increased ceramide levels were reproducibly shown in both MDD and bipolar disorder (BPD),20 with specific ceramide species proposed as potential depression markers.21
Although these results demonstrate the potential of lipids as peripheral biological markers of psychiatric disorders,6,19 the existence of a reliable and specific signature of these disorders, sufficiently independent of demographic and environmental variables, remains unclear. Among examined studies, all but 3 were based on a single cohort, and the replication of obtained results in a second cohort was only successful in 2 of 3 instances.18,19,22 Similarly, only 3 publications included patients with 2 different psychiatric diagnoses,15,20,23 with none of them involving an untargeted, global lipidomics analysis.
Methods
Study Participants
Participants were recruited in 3 geographic locations (1) the Chongqing, China (CN) cohort, from July 17, 2009, to January 15, 2014; (2) the Germany and Austria (DE-AT) cohort, from February 6, 2012, to August 16, 2017; and (3) the Moscow, Russia (RU) cohort and the first psychotic episode in Russia (fepRU) cohort, from November 30, 2017, to May 18, 2018. A detailed description of recruitment procedures is available in the eMethods in Supplement 1. Diagnoses were established using either the DSM-IV (CN and DE-AT) or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) (RU) criteria, applied by experienced clinicians. Exclusion criteria varied slightly between cohorts but consistently included severe somatic illnesses potentially associated with psychiatric symptom manifestation, in line with ICD-10/DSM-IV diagnostic criteria. The protocols of this study were reviewed and approved by the Ethics Committee of Chongqing Medical University, the ethics committee of the Mental Health Research Centre, the Interdisciplinary Ethics Committee, Moscow, Russia, the ethics committees of the University of Munich, and the local ethics committees of the participating centers in Germany and Austria, as required. Written informed consent was obtained for all participants. Detailed materials and methods can be found in the eMethods in Supplement 1. This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guidelines.
Lipidome Measurements, Spectral Data Analysis, and Annotation
Blood collection, lipid extraction, chromatography, and mass spectrometry measurements, lipid data processing, and compound annotation were conducted as in Tkachev et al,24 with small modifications (eMethods and eAppendix in Supplement 1). Lipidome measurements were conducted in 4 temporally separate experimental runs, corresponding to the 4 cohorts (CN, DE-AT, RU, and fepRU). Within cohorts, samples were processed in a randomized order.
Confounder Assessment
Prior to the statistical testing between patient and control samples, we conducted assessment and correction of confounding variable associations with the lipid intensities data, including (1) fasting status, (2) smoking status, 3) body mass index equilibration, and (4) age and sex fold-change correction (eMethods and eTable 1 in Supplement 1). For the DE-AT cohort, a balanced subsample was used for statistical analysis).
Statistical Analysis
Definition of SCZ-Associated Features
To find statistical differences between SCZ and controls, we used Mann-Whitney U tests in the CN, DE-AT, and RU cohorts separately, with the P values adjusted using Benjamini-Hochberg multiple testing correction at a false discovery rate (FDR) of .10. Features passing this threshold in each of the 3 cohorts, resulting in the calculated FDR of .001, were defined as SCZ-associated features.
Correlations Between Cohorts and Disorders
To assess similarity of the lipid profiles between cohorts or disorders, the Pearson correlations between average log ratios between disease and controls (ie, the differences between median abundances in disease and median abundances in control individuals, after base-2 log transformation) were calculated.
Definition of SCZ-Associated Lipid Classes
To define lipid classes most affected by SCZ, the union of the following significant classes were taken: triacylglyceride (TAG), acylcarnitine (CAR), phosphatidylcholine (PC), phosphatidylcholine plasmalogen (PC-P), ceramide (Cer) (1-sided binomial test for SCZ-associated lipids), and TAG, CAR, PC-P, phosphatidylethanolamine (PE; Gene Set Enrichment Analysis, gsea R package [R Project for Statistical Computing],25 all annotated lipids). These lipid classes were considered in the lipid enzyme analysis.
Prediction Model Differentiating SCZ and Control Samples
To construct a model differentiating between SCZ and control samples based on their blood plasma lipid profiles, we used 365 annotated lipids (after exclusion of lipids affected by fasting status) and logistic regression with lasso regularization (inverse of regularization strength parameter C = 0.1 in sklearn logistic regression). Prior to model construction, we conducted cohort data normalization as described in the eMethods in Supplement 1. We used samples from the 3 cohorts to construct a training set and a test set of samples not included in the training set from one of the cohorts. We performed 100 such train/test splits to estimate the mean and the SD of area under the receiver operating characteristic curve (AUROC) values. To validate the model, the training set comprised samples from all CN, DE-AT, and RU cohorts, and the fepRU cohort was used as a test set. We further conducted analyses using other regularization parameters and feature selections as described in the eMethods in Supplement 1.
BPD- and MDD-Associated Lipids
To define BPD- and MDD-associated lipids, we used the same statistical procedure as in the SCZ analysis. Lipids significant in both cohorts were defined as BPD- and MDD-associated lipids.
We collected data from 16 publications6,7,8,9,10,11,13,14,17,18,19,20,22,26,27,28 (eTable 2 in Supplement 1 and eTable 3 in Supplement 2) reporting alterations in individual lipid species in patients with SCZ, BPD, and MDD both receiving and not receiving pharmacological treatment. We correlated average log ratios between SCZ and controls calculated for our data with reported disorder-control differences.
We defined enzymes directly linked to lipid classes using KEGG API and KEGG LinkDB,29 and genes associated with plasma lipid concentrations (lipid-associated genes) by merging the 2 gene summaries listed in Tabassum et al.30 Data were analyzed from March 2018 to September 2020, using Bioconductor software, version 3.6; R, version 3.4 (R Project for Statistical Computing); scikit-learn, version 0.19; and Python, version 3.6 (Python Software Foundation). All P values were 2-sided unless specified explicitly as 1-sided, and significance was set at P < .05 unless otherwise specified. Detailed statistical analysis is included in eMethods in Supplement 1.
Results
Study Setup
In this diagnostic study, we searched for a robust blood plasma lipidome signature of SCZ, assessed its specificity in comparison to BPD and MDD, and evaluated the possibility of transforming it into a clinically applicable testing procedure. To achieve this, we analyzed control (n = 478), SCZ (n = 436), BPD (n = 184), and MDD (n = 256) samples collected at 3 environmentally, culturally, and demographically diverse locations: CN, DE-AT, and RU (Figure 1, Table, and eTable 4 in Supplement 2). We further collected samples from 104 patients with a first psychotic episode who had no long-term psychopharmacology use in Moscow, Russia (fepRU data set). Blood lipid levels were assessed in 980 individuals (mean [SD] age, 36 [13] years; 510 male individuals [52%]; 470 female individuals [48%]) diagnosed with SCZ, BPD, MDD, or those with a first psychotic episode and in 572 controls (mean [SD] age, 34 [13] years; 323 male individuals [56%]; 249 female individuals [44%]).
Figure 1. Three Experimental Cohorts.
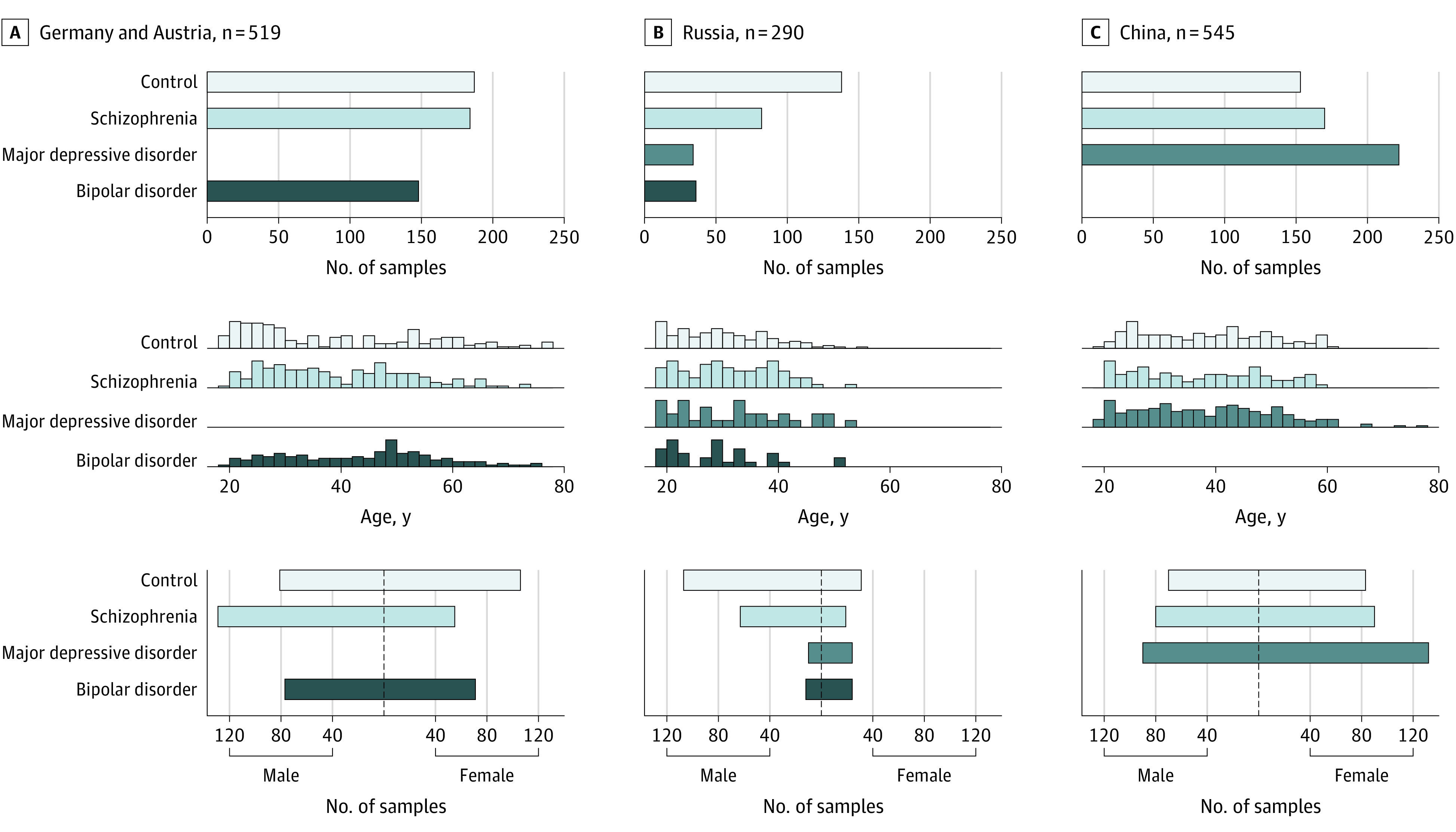
Schematic representation of the 3 sampling locations, Germany and Austria (A), Russia (B), and China (C). The top of each panel shows the number of individuals sampled at the location and the number of individuals sampled for each diagnosis group: control, schizophrenia, major depressive disorder, and bipolar disorder. The middle of each panel shows the distribution of ages for the diagnosis groups within each sample cohort. The bottom of each panel shows the distribution of sex for the diagnosis groups within each sample cohort.
Table. Summary of Cohorts’ Demographic Variables.
| Cohort | Diagnosis | No. of participants | Age, mean (SD), y | Female sex, No. (%) | Duration of illness, median (IQR), y | Inpatient/outpatient | Symptom score, mean (SD) | Scale details |
|---|---|---|---|---|---|---|---|---|
| CN | SCZ | 170 | 36.9 (11.6) | 90 (53) | 2 (1-8) | Outpatient | 82 (28) | PANSS |
| DE-AT | SCZ | 184 | 39.2 (12.8) | 55 (30) | 11 (5-20) | 54% Inpatient | 56 (18) | PANSS |
| RU | SCZ | 82 | 31.2 (8.4) | 19 (23) | 8 (4-14) | Inpatient | 99 (16) | PANSS |
| DE-AT | BPD | 148 | 43.3 (13.4) | 71 (48) | 10 (4-18) | 32% Inpatient | 12 (11) | BDI |
| 4 (6) | YMRS | |||||||
| RU | BPD | 36 | 28.2 (8.9) | 24 (67) | 5 (4-9) | Inpatient | 20 (6) | HAMD |
| CN | MDD | 222 | 37.7 (12.3) | 132 (59) | 1 (0-5) | Outpatient | 23 (7) | HAMD |
| RU | MDD | 34 | 32.0 (10.3) | 24 (71) | 6 (2-10) | Inpatient | 18 (4) | HAMD |
| CN | Control | 153 | 37.8 (11.3) | 83 (54) | NA | NA | NA | NA |
| DE-AT | Control | 187 | 38.3 (16.2) | 106 (57) | NA | NA | NA | NA |
| RU | Control | 138 | 29.5 (8.3) | 31 (22) | NA | NA | NA | NA |
Abbreviations: BDI, Beck Depression Inventory; BPD, bipolar disorder; CN, China; DE-AT, Germany and Austria; HAMD, Hamilton Depression Rating Scale; MDD, major depressive disorder; NA, not applicable; PANSS, Positive and Negative Syndrome Scale; RU, Russia; SCZ, schizophrenia; YMRS, Young Mania Rating Scale.
We measured blood plasma lipid intensities using liquid chromatography coupled with untargeted mass spectrometry (LC-MS) yielding 1361 reproducibly detected features. Features analysis based on retention time, mass-to-charge, LC-MS2 fragmentation patterns resulted in 395 computationally annotated lipids (eTable 5 in Supplement 2) from 16 lipid classes characteristic for the blood plasma lipidome31 (eFigure 1 in Supplement 1). Lipids associated with fasting status (n = 30), of which 27 were free fatty acids, were discarded, leaving 365 annotated compounds and 1280 detected features (eTable 5 in Supplement 2 and eMethods in Supplement 1).
Plasma Lipidome Alterations Associated With SCZ
We identified statistically significant lipid intensities between SCZ and controls in each cohort (38%-61% per cohort; Wilcoxon rank-sum test, Benjamini-Hochberg–corrected FDR = 10%; permutation test, P < .001) (eTable 5 in Supplement 2 and eMethods in Supplement 1), after correction for confounders (DE-AT cohort subsample n = 122 for SCZ; n = 121 for control) (eTable 1 in Supplement 1). Among them, 213 lipids were significant in all 3 cohorts, CN, DE-AT, RU, an overlap exceeding chance expectation (SCZ-associated features) (permutation test, P < .001; calculated FDR = 0.1%) (eFigure 2 in Supplement 1). Furthermore, the direction of the intensity alterations correlated positively and significantly between the cohorts (Pearson correlation of the average lipid abundance log ratios between SCZ and controls, r ≥ 0.75; P ≤ .001) (eFigure 2 in Supplement 1; eTable 5 in Supplement 2). These results indicate that the multicohort approach to SCZ-associated alterations’ definition was effective at minimizing the impact of intrinsically diverse human demographic and environmental variables, such as ancestry, diet, lifestyle, and undetected health conditions and metabolic disorders.
The 213 SCZ-associated lipid features comprised 77 lipids from 14 classes annotated by their LC-MS2 fragmentation patterns (SCZ-associated lipids) (Figure 2A and B; eTable 6 in Supplement 2). The most prominent alterations at the lipid class level included increases in Cer, TAG, and PC and decreases in CAR and PC-P (1-sided binomial test, P < .05 for each cohort) (eTable 7 and eMethods in Supplement 1). Alterations in some of these classes, including TAGs, CARs, and PC-Ps could also be detected using all 365 putatively annotated lipids (GSEA, adjusted P < .05; significant alteration in lipid classes: TAG, CAR, PC-P, PE) (eTable 7 and eMethods in Supplement 1). Significant SCZ-associated alterations of TAG, CAR, plasmanyl-phosphatidylcholine (PC-O)/PC-P, and plasmanyl-phosphatidylethanolamine (PE-O)/phosphatidylethanolamine plasmalogen (PE-P) lipid classes coincided well with previous reports6,7,8,9,10,11,13,14,26 (eFigure 3 and eTable 2 in Supplement 1 and eTable 3 in Supplement 2), whereas there was no literature-based consensus for alterations of PCs and PEs, and the detected increase in Cer has not been previously reported, to our knowledge. Of note, the SCZ-associated lipid differences were similar between patients with high and low symptom severity, assessed using the Positive and Negative Syndrome Scale (PANSS)32 (CN: r = 0.91; DE-AT: r = 0.94; RU: r = 0.94 ; P < .001) (eFigure 4 in Supplement 1 and eTable 4 in Supplement 2). This result suggests that SCZ-associated lipid alterations might represent a trait rather than a state marker of the psychiatric disorder.
Figure 2. Schizophrenia (SCZ)-Associated Lipidome Alterations and Classification Modeling.
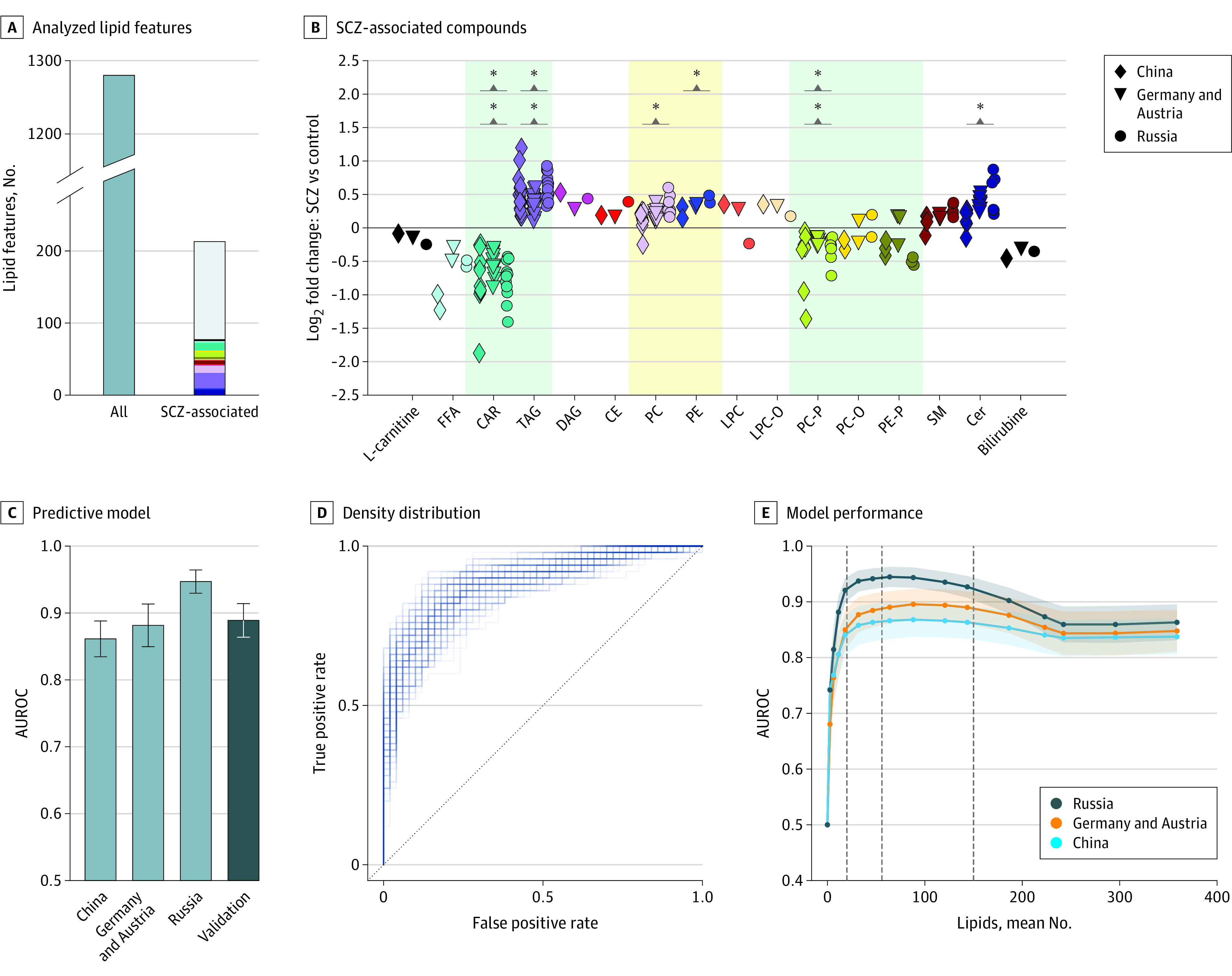
A, Numbers of all analyzed lipid features for all studied mental disorders and SCZ-associated lipids. The colors correspond to the lipid classes displayed in panel B. The y axis was broken to increase lipid class color visibility. B, The average lipid abundance log ratios between individuals with SCZ and control individuals for the 77 SCZ-associated compounds, sorted according to lipid classes. Asterisks denote significance at the lipid class level (eTable 7 in Supplement 1). Shading marks reported lipid class alterations in SCZ (eTable 2 in Supplement 1): green (good) and yellow (mixed) agreement without our results. C, Area under the receiver operating characteristic curve (AUROC) estimates for predictive model separating individuals with SCZ and control individuals. The model was trained on samples merged from China, Germany and Austria, and Russia, and tested separately on samples from China, Germany and Austria, Russia, and the cohort of patients with a first psychotic episode (fepRU; indicated as “Validation”) subsets. D, The density distribution formed by the individual AUROC curves of the models trained on different random subsets from the 3 primary cohorts and tested using fepRU samples. E, Model performance depending on the number of lipids used in the model. Lines correspond to mean performance in each cohort over 100 train/test splits. Shaded areas cover the estimated SDs. First dashed gray line denotes 20 features. Dashed black line indicates the mean number of features for the parameter C = 0.1. Second dashed gray line denotes 150 features. CAR indicates acylcarnitine; CE, cholesteryl ester; Cer, ceramide; DAG, diacylglycerol; FFA, free fatty acid; LPC, lysophosphatidylcholine; LPC-0, lysoplasmanyl-phosphatidylcholine; PC, phosphatidylcholine; PC-0, plasmanyl-phosphatidylcholine; PC-P, phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE-P, phosphatidylethanolamine plasmalogen; SM, sphingomyelin; TAG, triacylglyceride.
Predictive Classification and Intercohort Reproducibility Assessment
Although most of the lipid abundance differences between SCZ and controls had moderate amplitudes, they were sufficient to separate individuals with SCZ from controls with good accuracy using a predictive model. Specifically, a lasso logistic regression model trained on the 3 merged cohorts and all 365 annotated lipids accurately separated individuals with SCZ and control individuals (train/test shuffle split cross validation, performance on test samples, mean [SD] AUROC: RU cohort, 0.95 [0.02]; DE-AT cohort, 0.88 [0.03], and CN cohort, 0.86 [0.03]) (Figure 2C; eMethods in Supplement 1). Further, the model performed just as accurately in classifying first psychotic episode and control samples in the fepRU data set (AUROC mean [SD], 0.89 [0.025]) (Figure 2C and D; eMethods in Supplement 1) and did not depend on symptom severity (RU cohort: r = −0.15; P = .17; DE-AT cohort: r = 0.06; P = .47; CN cohort: r = 0.19; P = .07) (eMethods in Supplement 1). This result demonstrated good generalizability of the model, particularly because no first psychotic episode samples were used for model training. With the chosen regularization parameter (C = 0.1), 64 lipids were used in the model (eTable 5 in Supplement 2), but models based on 20 to 150 features performed similarly well (Figure 2E; eMethods in Supplement 1). Although this result highlights the performance stability, it precludes an optimal model definition. For instance, although the model trained using 20 best performing features (eMethods in Supplement 1) yielded a mean (SD) AUROC of 0.89 (0.025) on the fepRU data set not used in this feature selection, a model based on the remaining 345 features performed just as well (mean [SD] AUROC, 0.91 [0.025] on fepRU data set).
Models trained on a single cohort retained a good intracohort performance but displayed reduced intercohort generalizability (eFigure 5, eTable 8, and eMethods in Supplement 1). Likewise, the results from 1-dimensional statistics showed, on average, a 25% disagreement rate between the cohorts instead of the statistically expected 5% (eFigure 6 and eMethods in Supplement 1). Accordingly, minimization of the cohort-specific associations by considering overlapping statistical differences resulted in more than 90% concordance with the fourth (fepRU) data set, further supporting the applied multicohort approach.
Lipid Alterations in SCZ Associate With Other Psychiatric Disorders
We next examined lipid intensity alterations associated with MDD and BPD, each represented in our data set by 1 larger and 1 smaller cohort (Figure 1, Table, and eTable 4 in Supplement 2). Comparison of significant differences identified independently in each cohort after the confounder correction (eTable 1 in Supplement 1) yielded 97 lipids altered in both MDD cohorts (MDD-associated lipids) and 47 lipids altered in both BPD cohorts (BPD-associated lipids) (Wilcoxon test, BH-corrected FDR = 10% in each cohort independently; permutation test, P < .01) (eTable 5 in Supplement 2 and eMethods in Supplement 1). As for SCZ-associated differences, the MDD- and BPD-associated alterations detected in each cohort correlated significantly and positively between the cohorts, supporting their reliability (Pearson correlation of the average lipid abundance log ratios between BPD and controls, r = 0.9, P < .001 or MDD and controls, r = 0.27, P = .007) (eFigure 7 in Supplement 1).
Of the 97 MDD-associated and 47 BPD-associated lipid features, 30 and 28 overlapped with the 213 SCZ-associated features, and 7 among all 3 disorders, significantly more than expected by chance (permutation test, P < .001) (eFigure 8 in Supplement 1). Further, lipid intensity alterations detected in MDD and BPD correlated strongly with those observed in SCZ (Pearson correlation for average lipid abundance log ratios between SCZ/MDD/BPD and controls; for MDD-SCZ comparison, r = 0.92, P < .001; for BPD-SCZ comparison, r = 0.89, P < .001) (Figure 3A; eFigure 9 in Supplement 1). The comparison of SCZ-associated differences found in our study to published disorder-associated blood plasma lipidome alterations similarly showed significant correlation not only with SCZ studies, but also with studies of MDD and BPD6,7,8,9,10,11,13,14,17,18,19,20,22,26,27,28 (Spearman correlation, ρ = 0.58 / 0.78, P < .001) (eFigures 10 and 11, eTable 2, and eMethods in Supplement 1, and eTable 3 in Supplement 2). Although not excluding the existence of disorder-specific lipidome differences, these results suggest a largely similar association of SCZ, BPD, and MDD with blood plasma lipidome composition.
Figure 3. Comparison of the Blood Plasma Lipidome Alterations Among 3 Psychiatric Disorders.
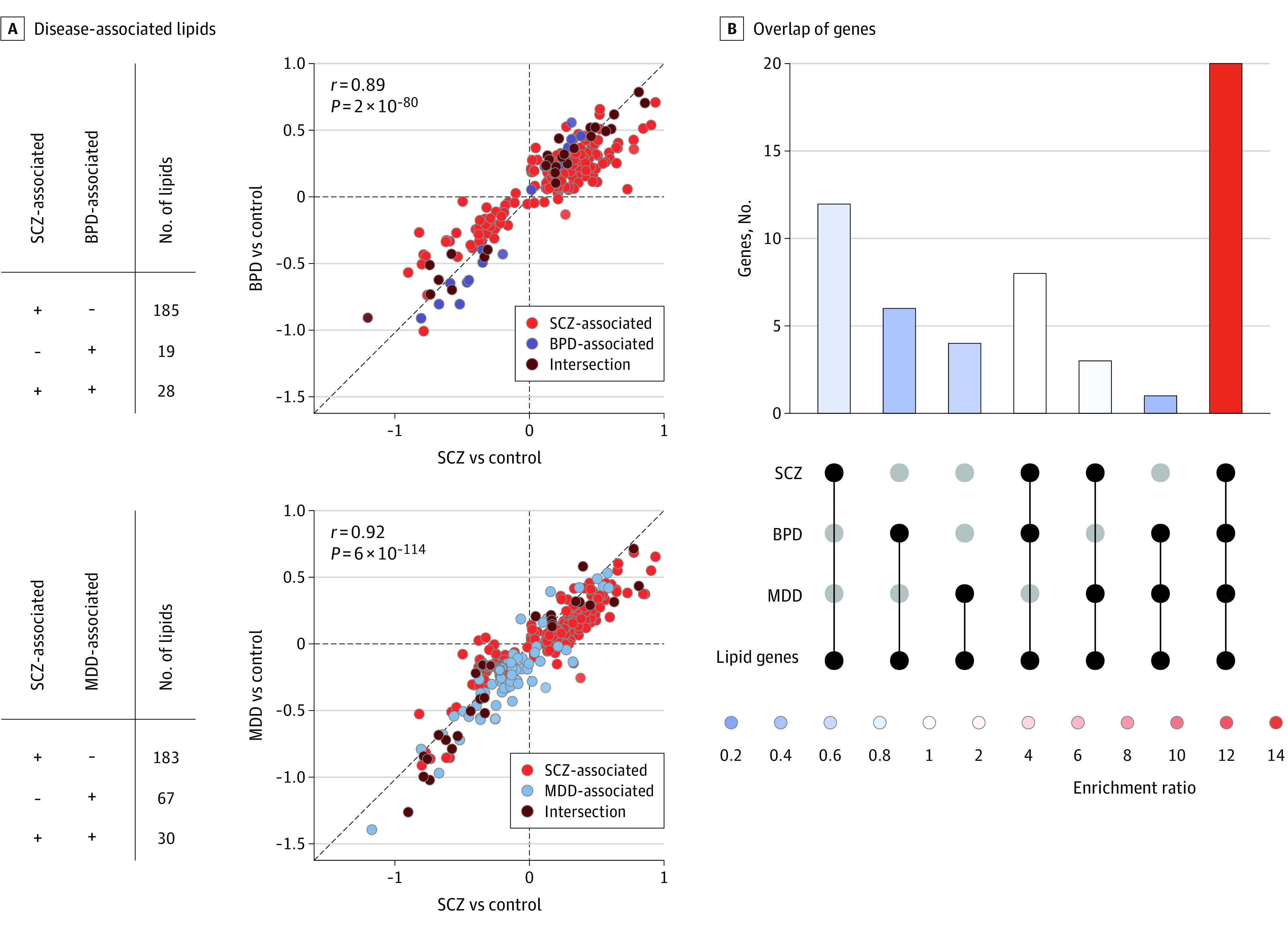
A, Number of disease-associated lipids that are common (+ +) or different (− +, + −) for the disorders and pairwise comparisons of average lipid abundance log ratios (log2 fold-change) between the different disorders and controls. Log2 fold-changes were averaged for 3 (for schizophrenia [SCZ]) or 2 (for bipolar disorder [BPD] and major depressive disorder [MDD]) cohorts. Circles represent the SCZ-, BPD-, and MDD-associated lipid features, as well as their intersection. The diagonal line indicates y = x. B, Intersection of genes associated with SCZ, BPD, and MDD, considering only the subset of 84 genes linked to the blood plasma lipid level variation. Colors correspond to the fold of enrichment, calculated for each group by subsampling random groups of genes of the same size 1000 times.
Association Between Lipidome Alterations and Disorder-Linked Genes
We next compared identified lipidome alterations with reported genetic associations, defined using GRASP.33 For 3 of the 6 lipid classes most affected by SCZ (eTable 7 in Supplement 1), the corresponding sets of lipid enzymes were enriched with SCZ-associated and MDD-associated genes (for SCZ and PC, PE, Cer: permutation enrichment test, P = .03, P = .02, and P = .09, respectively; for MDD and PC, PE, Cer: permutation enrichment test, P = .046, P = .02, and P = .03, respectively; for BPD and PC, PE, Cer: permutation enrichment test, nonsignificant) (eTable 9 and eMethods in Supplement 1). At the same time, only 2 of the 10 nonaffected lipid classes displayed such enrichment for at least 1 disorder (DAG and sphingomyelin [SM]) (eTable 9 in Supplement 1).
A recent genome-wide association study identified 84 genes harboring single-nucleotide variants associated with individual lipid levels in the human blood plasma.30 Although the well-established overlap of genes linked to SCZ, BPD, and MDD was reproduced in our analysis (permutation test, P < .001) (eFigure 12 in Supplement 1), this overlap was far greater when only lipid-associated genes were assessed. Specifically, 20 of the 84 lipid-level–associated genes were linked to all 3 disorders simultaneously, 15-fold more than expected by chance (permutation test, P < .001) (Figure 3C and eFigure 12 and eMethods in Supplement 1). This result could provide a partial explanation for the observed overlap of lipidome alterations between the disorders.
Association Between SCZ-Associated Lipidome Alterations and Medication
Most of the patients assessed in our study received long-term antipsychotic medication, shown to affect some of the plasma lipid compounds.6,7,8,11,12,13,15,34,35,36 To assess the association of medication, we evaluated 13 patients with SCZ in the DE-AT cohort who were not medicated for at least 6 months prior to blood sample collection (eTable 4 in Supplement 2), and the first psychotic episode cohort with patients medicated for less than 1 week. Comparison of the lipid intensity differences between controls and either patients receiving medication or 13 patients with SCZ who were not receiving medication revealed highly correlated alterations in both patient groups (Pearson correlation, r = 0.75, P < .001) (Figure 4A). This similarity was well above the chance expectation calculated by the random subsampling procedure (empirical P = .02) (eFigure 13 and eMethods in Supplement 1) and was further improved by exclusion of triglycerides, a lipid class with the most extensively demonstrated association with treatment with antipsychotic drugs1,2,3 (empirical P = .001) (eFigure 13 in Supplement 1). Accordingly, direct comparison of the profiles of patients who were receiving medication and those who were not showed that long-term antipsychotic withdrawal tended to attenuate SCZ-associated differences for TAGs, SMs, and some PCs and ceramides, whereas enhancing them for CARs (Figure 4A; eFigure 14 in Supplement 1).
Figure 4. The Cohort of Patients With a First Psychotic Episode in Russia (fepRU) and Association With Medication.
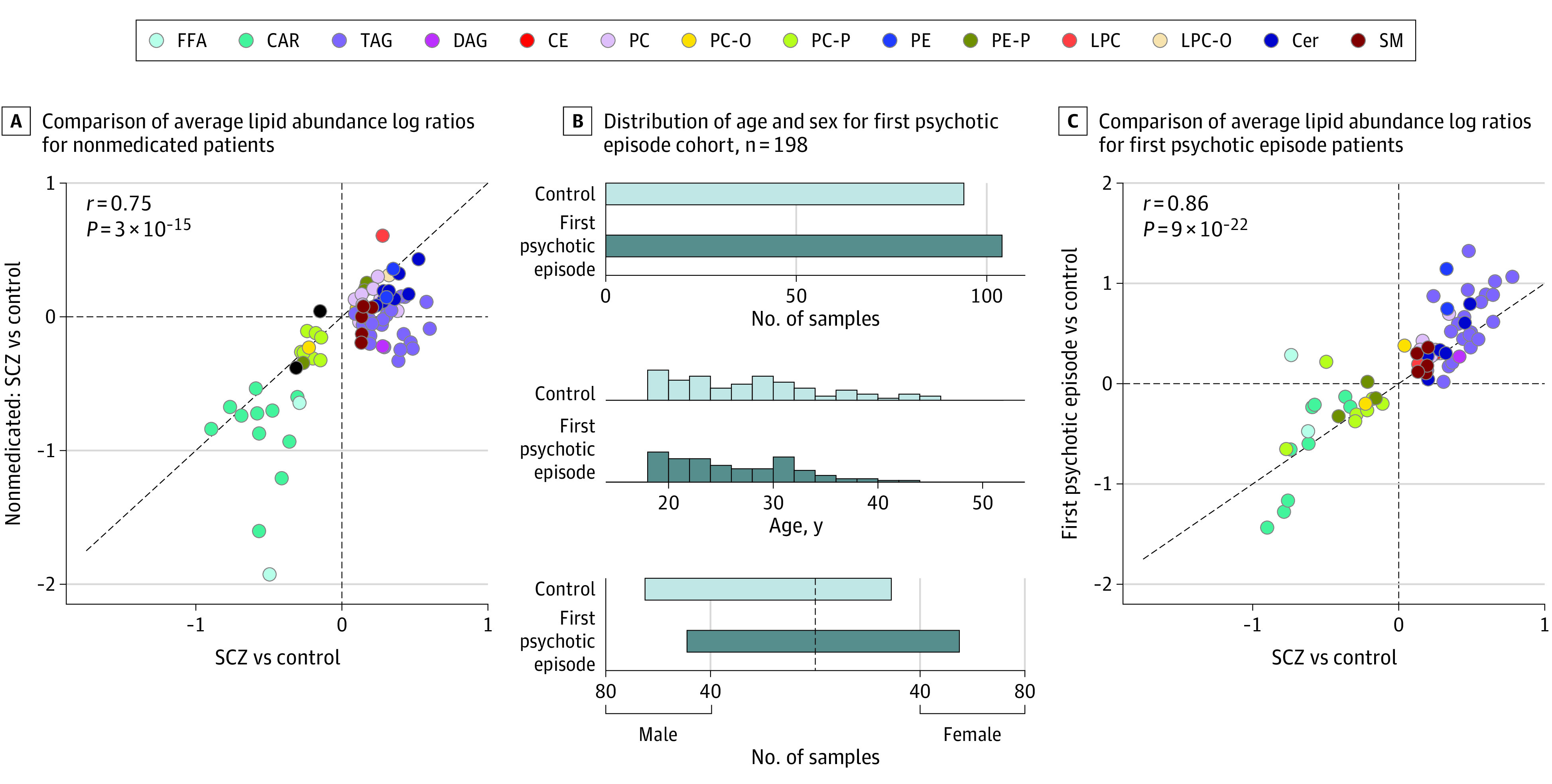
A, For the 77 schizophrenia (SCZ)-associated lipids, comparison of average lipid abundance log ratios (log2 fold-change) between individuals with SCZ and control individuals assessed for all patients with SCZ (n = 122, Germany and Austria cohort) and those who were not medicated (n = 13, Germany and Austria cohort). The diagonal line indicates y = x. Colors correspond to lipid classes. B, Sample information for the fepRU cohort. Top: number of individuals sampled for each diagnoses group: control and first psychotic episode. Middle: distribution of ages for the diagnosis groups. Bottom: distribution of sex for the diagnosis groups. C, Comparison of the SCZ-associated lipid intensity differences (log2 fold-change) measured in patients with SCZ from the 3 primary cohorts (n = 436, averaged for the 3 cohorts) and in patients with a first episode of psychosis (n = 104, fepRU cohort). Circles represent 70 SCZ-associated lipids detected in the fepRU cohort. The diagonal line indicates y = x. CAR indicates acylcarnitine; CE, cholesteryl ester; Cer, ceramide; DAG, diacylglycerol; FFA, free fatty acid; LPC, lysophosphatidylcholine; LPC-0, lysoplasmanyl-phosphatidylcholine; PC, phosphatidylcholine; PC-0, plasmanyl-phosphatidylcholine; PC-P, phosphatidylcholine plasmalogen; PE, phosphatidylethanolamine; PE-P, phosphatidylethanolamine plasmalogen; SM, sphingomyelin; TAG, triacylglyceride.
In addition to this analysis, assessment of patients with a first psychotic episode (fepRU cohort; n = 104 for patients with a first psychotic episode; n = 94 for controls) (Figure 4B; eTable 10 in Supplement 2) treated with antipsychotics for less than 1 week similarly reproduced SCZ-associated differences identified in long-term patients (70 of the 77 annotated SCZ-associated lipids detected in fepRU data; Pearson correlation for average log ratios between SCZ and controls, and first psychotic episode and controls, r = 0.86, P < .001) (Figure 4C; eTable 11 in Supplement 2). Taken together, these results indicate that the identified SCZ-associated alterations cannot be attributed to medication effects. A detailed description of all supporting statistical analysis, including literature comparison (eTable 12 in Supplement 2), lipid class enzyme enrichment (eTable 13 in Supplement 2), and confounding factors assessment (eFigures 15, 16, 17 in Supplement 1), can be found in eMethods in Supplement 1.
Discussion
By leveraging the multicohort design of this diagnostic study, we searched for reproducible lipid alterations in the blood plasma of patients with SCZ and aimed to assess their specificity with respect to MDD and BPD, paving the way toward a lipid-based clinically applicable test for psychiatric disorders. We identified a set of 77 lipids significantly and reproducibly associated with SCZ in the 3 culturally and demographically diverse cohorts. Consolidating published results, we reproduced repeatedly reported alterations (eg, decrease of ether phospholipids, PC-P, PC-O, PE-P), resolved contradictory accounts (eg, decrease in acylcarnitines), and identified novel alterations (eg, increase in ceramides).
We further showed that a multivariate model based on subsets of annotated lipids separated SCZ from control individuals with the predictive performance (AUROC) greater than 0.9. The model performance did not drop when applied to an independent first psychotic episode cohort, demonstrating its good generalizability. Further, the model demonstrated an AUROC greater than 0.9 using from 20 to approximately 150 lipids as an input, allowing flexible feature selection for future clinical applications.
Our investigation of MDD and BPD, conducted in parallel with the SCZ measurements, revealed substantial similarities of lipid alterations among the 3 disorders. This result aligns with lipid alteration similarities between disorders reported for particular lipid classes and individual disorders: Cer alterations in both MDD and BPD,20 and ether phospholipid (PC-P, PC-O, PE-P) alterations in SCZ5,6,7,8,9,10 and MDD.16,17,18,19 Further, the SCZ-associated alterations identified in our study correlated positively and significantly with lipid alterations reported not only for SCZ but also for patients with MDD and BPD.6,7,8,9,10,11,13,14,17,18,19,20,22,26,27,28 In addition to the lipidome effect similarities, the 3 disorders display a well-established overlap of clinical manifestations and underlying genetic factors.37,38,39,40,41,42,43 Our reanalysis of SCZ, MDD, and BPD genomic associations reproduced this overlap and revealed a significant enrichment of enzymes corresponding to the disorder-associated lipid classes, in line with recent findings.44 Additionally, genes associated with plasma lipidome variation30 overlapped strongly with the genetic markers shared by SCZ, MDD, and BPD, suggesting a link between the disorders’ commonalties in both genetics and lipid phenotypes.
Limitations
Our study has several limitations. Our focus on the lipidome alterations shared by demographically distinct patient groups excludes population-specific disorder effects. Further, although we demonstrated that most of the SCZ-associated alterations were not associated with medications, the sample size of patients not receiving medication was not sufficient to exclude treatment associations with the individual lipids. Finally, although a multicohort design minimizes the outcomes of unaccounted confounding demographic and genetic variables, it would not exclude any systematic biases, such as ubiquitous disorder-linked lifestyle alterations and somatic comorbidities.45,46,47,48 This uncertainty is exemplified by the fact that 3 emerging biomarkers of cardiovascular disease (Cer d18:1/16:0, Cer d18:1/18:0, Cer d18:1/24:1),49 a common SCZ comorbidity, were among the 77 SCZ-associated lipids. Further studies, including better structured and more deeply phenotyped independent cohorts, are necessary to address these issues.
Conclusions
In this blood plasma lipidome diagnostic analysis conducted in 3 independent cohorts of patients with SCZ, results suggest significant and reproducible abundance alterations of 77 lipids. Further analysis suggested that these alterations are present in individuals with a first psychotic episode, not associated with medication and symptom severity, and largely shared by patients with MDD and BPD. Accordingly, we showed that predictive models using anywhere from 20 to 150 of assessed lipids reliably separated individuals with SCZ and controls, laying a foundation for the development of lipid-based clinical evaluation of the psychiatric disorder risks.
eMethods
eFigure 1. Detected Features and Annotated Compounds
eFigure 2. SCZ-Associated Features and the Agreement of Their Abundance Alterations Between Cohorts
eFigure 3. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL
eFigure 4. Comparison of SCZ-Associated Lipid Differences Between Patients With High and Low PANSS Scores
eFigure 5. Area Under the Receiver Operating Characteristic Curve (AUROC) Values for Lasso Logistic Regression Model Delineating SCZ and CTL Individuals
eFigure 6. Reproducibility of the Lipid Intensity Difference Direction Between SCZ and CTL Between and Within the Cohorts
eFigure 7. Comparison of Statistically Significant BPD- and MDD-Associated Lipid Intensity Differences Between 2 Independent Cohorts
eFigure 8. Intersection of MDD-Associated, BPD-Associated, and SCZ-Associated Lipid Features
eFigure 9. Comparison Blood Plasma Lipidome Alterations Between Disorders Within Each of the Cohorts
eFigure 10. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL
eFigure 11. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL by Individual Study
eFigure 12. Overlap of Genetic Variants Linked to Psychiatric Disorders and Blood Plasma Lipid Levels
eFigure 13. Correlation Between Lipid Profiles of Nonmedicated Individuals and the SCZ Profile, Compared to the Correlation Between Random Lipid Profiles and the SCZ Profile
eFigure 14. Comparison of the Lipid Intensity Distributions in Medicated and Nonmedicated SCZ Samples
eFigure 15. Dependence of the Lipid Intensity Values on the Fasting Period Prior to Sample Collection
eFigure 16. The Distribution of the Body Mass Index (BMI) for Subsets of CTL and SCZ Samples Used in Analysis of DE-AT Cohort. The P-Value of the Mann–Whitney U Test is Marked Within the Plot
eFigure 17. Effect of Smoking on SCZ-Associated Lipid Intensity Differences
eTable 1. Confounding Factors Assessment
eTable 2. Summary of Studies Reporting Lipid Alterations in Psychiatric Disorders at the Level of Individual Lipid Species
eTable 7. SCZ-Associated Lipid Classes Analysis
eTable 8. Intra- and Inter- Cohort Performance of the SCZ vs CTL Classifier
eTable 9. Enrichment of Disease-Associated Genes Among Lipid Enzymes
eAppendix
eReferences
eTable 3. Alterations for SCZ-Associated Lipids Reported in the Literature
eTable 4. Sample Information for DE-AT, CN, and RU Cohorts
eTable 5. Quantified Lipid Features and Related Information
eTable 6. SCZ-Associated Lipids and Related Information
eTable 10. Sample Information for fepRU Data set
eTable 11. SCZ-Associated Lipids Information for fepRU Data set
eTable 12. Previously Reported Alterations for Various Lipid Species
eTable 13. Linking of Lipid Maps to KEGG
Data Sharing Statement
References
- 1.Vancampfort D, Wampers M, Mitchell AJ, et al. A meta-analysis of cardiometabolic abnormalities in drug naïve, first-episode and multiepisode patients with schizophrenia vs general population controls. World Psychiatry. 2013;12(3):240-250. doi: 10.1002/wps.20069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry. 2017;211(6):339-349. doi: 10.1192/bjp.bp.117.200907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode nonaffective psychosis: a systematic review and meta-analysis. Schizophr Res. 2017;190:18-27. doi: 10.1016/j.schres.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 4.Wei YG, Cai DB, Liu J, et al. Cholesterol and triglyceride levels in first-episode patients with major depressive disorder: a meta-analysis of case-control studies. J Affect Disord. 2020;266:465-472. doi: 10.1016/j.jad.2020.01.114 [DOI] [PubMed] [Google Scholar]
- 5.Kaddurah-Daouk R, McEvoy J, Baillie R, et al. Impaired plasmalogens in patients with schizophrenia. Psychiatry Res. 2012;198(3):347-352. doi: 10.1016/j.psychres.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Cheng SL, Fei Q, et al. Metabolic profiling identifies phospholipids as potential serum biomarkers for schizophrenia. Psychiatry Res. 2019;272:18-29. doi: 10.1016/j.psychres.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Yan L, Zhou J, Wang D, et al. Unbiased lipidomic profiling reveals metabolomic changes during the onset and antipsychotics treatment of schizophrenia disease. Metabolomics. 2018;14(6):80. doi: 10.1007/s11306-018-1375-3 [DOI] [PubMed] [Google Scholar]
- 8.Leppik L, Parksepp M, Janno S, et al. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2020;270(1):59-70. doi: 10.1007/s00406-018-0971-6 [DOI] [PubMed] [Google Scholar]
- 9.Wood PL, Unfried G, Whitehead W, Phillipps A, Wood JA. Dysfunctional plasmalogen dynamics in the plasma and platelets of patients with schizophrenia. Schizophr Res. 2015;161(2-3):506-510. doi: 10.1016/j.schres.2014.11.032 [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Sun X, Maziade M, et al. Characterising phospholipids and free fatty acids in patients with schizophrenia: a case-control study. World J Biol Psychiatry. 2021;22(3):161-174. doi: 10.1080/15622975.2020.1769188 [DOI] [PubMed] [Google Scholar]
- 11.Cao B, Wang D, Pan Z, et al. Characterizing acyl-carnitine biosignatures for schizophrenia: a longitudinal pre- and posttreatment study. Transl Psychiatry. 2019;9(1):19. doi: 10.1038/s41398-018-0353-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao B, Jin M, Brietzke E, et al. Serum metabolic profiling using small molecular water-soluble metabolites in individuals with schizophrenia: a longitudinal study using a pre-posttreatment design. Psychiatry Clin Neurosci. 2019;73(3):100-108. doi: 10.1111/pcn.12779 [DOI] [PubMed] [Google Scholar]
- 13.Kriisa K, Leppik L, Balõtšev R, et al. Profiling of acylcarnitines in first episode psychosis before and after antipsychotic treatment. J Proteome Res. 2017;16(10):3558-3566. doi: 10.1021/acs.jproteome.7b00279 [DOI] [PubMed] [Google Scholar]
- 14.Cao B, Wang D, Pan Z, et al. Metabolic profiling for water-soluble metabolites in patients with schizophrenia and healthy controls in a Chinese population: a case-control study. World J Biol Psychiatry. 2020;21(5):357-367. doi: 10.1080/15622975.2019.1615639 [DOI] [PubMed] [Google Scholar]
- 15.Cuturic M, Abramson RK, Breen RJ, Edwards AC, Levy EE. Comparison of serum carnitine levels and clinical correlates between outpatients and acutely hospitalised individuals with bipolar disorder and schizophrenia: a cross-sectional study. World J Biol Psychiatry. 2016;17(6):475-479. doi: 10.1080/15622975.2016.1178803 [DOI] [PubMed] [Google Scholar]
- 16.Knowles EEM, Huynh K, Meikle PJ, et al. The lipidome in major depressive disorder: shared genetic influence for ether-phosphatidylcholines, a plasma-based phenotype related to inflammation, and disease risk. Eur Psychiatry. 2017;43:44-50. doi: 10.1016/j.eurpsy.2017.02.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirkan A, Isaacs A, Ugocsai P, et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family–based lipidomics study. J Psychiatr Res. 2013;47(3):357-362. doi: 10.1016/j.jpsychires.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Li J, Zheng P, et al. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal Bioanal Chem. 2016;408(23):6497-6507. doi: 10.1007/s00216-016-9768-5 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Zheng P, Zhao X, et al. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J Proteome Res. 2015;14(5):2322-2330. doi: 10.1021/acs.jproteome.5b00144 [DOI] [PubMed] [Google Scholar]
- 20.Brunkhorst-Kanaan N, Klatt-Schreiner K, Hackel J, et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism. 2019;95:65-76. doi: 10.1016/j.metabol.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 21.Dinoff A, Herrmann N, Lanctôt KL. Ceramides and depression: a systematic review. J Affect Disord. 2017;213:35-43. doi: 10.1016/j.jad.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Kim EY, Lee JW, Lee MY, et al. Serum lipidomic analysis for the discovery of biomarkers for major depressive disorder in drug-free patients. Psychiatry Res. 2018;265:174-182. doi: 10.1016/j.psychres.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 23.Scola G, Versace A, Metherel AH, et al. Alterations in peripheral fatty acid composition in bipolar and unipolar depression. J Affect Disord. 2018;233:86-91. doi: 10.1016/j.jad.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 24.Tkachev A, Stekolshchikova E, Anikanov N, et al. Shorter chain triglycerides are negatively associated with symptom improvement in schizophrenia. Biomolecules. 2021;11(5):720. doi: 10.3390/biom11050720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, Sergushichev A. Fast gene set enrichment analysis. BioRxiv. Preprint posted online February 1, 2021. doi: 10.1101/060012 [DOI]
- 26.He Y, Yu Z, Giegling I, et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl Psychiatry. 2012;2:e149. doi: 10.1038/tp.2012.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracia-Garcia P, Rao V, Haughey NJ, et al. Elevated plasma ceramides in depression. J Neuropsychiatry Clin Neurosci. 2011;23(2):215-218. doi: 10.1176/jnp.23.2.jnp215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai H, Cao T, Li N, et al. Quantitative monitoring of a panel of stress-induced biomarkers in human plasma by liquid chromatography-tandem mass spectrometry: an application in a comparative study between depressive patients and healthy subjects. Anal Bioanal Chem. 2019;411(22):5765-5777. doi: 10.1007/s00216-019-01956-2 [DOI] [PubMed] [Google Scholar]
- 29.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27(1):29-34. doi: 10.1093/nar/27.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabassum R, Rämö JT, Ripatti P, et al. ; FinnGen Project . Genetic architecture of human plasma lipidome and its link to cardiovascular disease. Nat Commun. 2019;10(1):4329. doi: 10.1038/s41467-019-11954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burla B, Arita M, Arita M, et al. MS-based lipidomics of human blood plasma: a community-initiated position paper to develop accepted guidelines. J Lipid Res. 2018;59(10):2001-2017. doi: 10.1194/jlr.S087163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 33.Eicher JD, Landowski C, Stackhouse B, et al. GRASP v2.0: an update on the Genome-Wide Repository of Associations between SNPs and phenotypes. Nucleic Acids Res. 2015;43(database issue):D799-D804. doi: 10.1093/nar/gku1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aquino A, Alexandrino GL, Guest PC, et al. Blood-based lipidomics approach to evaluate biomarkers associated with response to olanzapine, risperidone, and quetiapine treatment in schizophrenia patients. Front Psychiatry. 2018;9:209. doi: 10.3389/fpsyt.2018.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suvitaival T, Mantere O, Kieseppä T, et al. Serum metabolite profile associates with the development of metabolic comorbidities in first-episode psychosis. Transl Psychiatry. 2016;6(11):e951. doi: 10.1038/tp.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEvoy J, Baillie RA, Zhu H, et al. Lipidomics reveals early metabolic changes in subjects with schizophrenia: effects of atypical antipsychotics. PLoS One. 2013;8(7):e68717. doi: 10.1371/journal.pone.0068717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross-Disorder Group of the Psychiatric Genomics Consortium . Genomic relationships, novel loci, and pleiotropic mechanisms across 8 psychiatric disorders. Cell. 2019;179(7):1469-1482.e11. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anttila V, Bulik-Sullivan B, Finucane HK, et al. ; Brainstorm Consortium . Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988-989. doi: 10.1016/j.biopsych.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium . Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705-1715.e16. doi: 10.1016/j.cell.2018.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krynicki CR, Upthegrove R, Deakin JFW, Barnes TRE. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr Scand. 2018;137(5):380-390. doi: 10.1111/acps.12873 [DOI] [PubMed] [Google Scholar]
- 43.Adler CM, Strakowski SM. Boundaries of schizophrenia. Psychiatr Clin North Am. 2003;26(1):1-23. doi: 10.1016/S0193-953X(02)00085-0 [DOI] [PubMed] [Google Scholar]
- 44.Maas DA, Martens MB, Priovoulos N, et al. Key role for lipids in cognitive symptoms of schizophrenia. Transl Psychiatry. 2020;10(1):399. doi: 10.1038/s41398-020-01084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders: a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318. doi: 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penninx BWJH, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20(1):63-73. doi: 10.31887/DCNS.2018.20.1/bpenninx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24(6):412-424. doi: 10.1016/j.eurpsy.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 48.Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20(1):47-52. doi: 10.31887/DCNS.2018.20.1/nsartorius [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani A, Dugo C. Ceramides and risk of major adverse cardiovascular events: a meta-analysis of longitudinal studies. J Clin Lipidol. 2020;14(2):176-185. doi: 10.1016/j.jacl.2020.01.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Detected Features and Annotated Compounds
eFigure 2. SCZ-Associated Features and the Agreement of Their Abundance Alterations Between Cohorts
eFigure 3. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL
eFigure 4. Comparison of SCZ-Associated Lipid Differences Between Patients With High and Low PANSS Scores
eFigure 5. Area Under the Receiver Operating Characteristic Curve (AUROC) Values for Lasso Logistic Regression Model Delineating SCZ and CTL Individuals
eFigure 6. Reproducibility of the Lipid Intensity Difference Direction Between SCZ and CTL Between and Within the Cohorts
eFigure 7. Comparison of Statistically Significant BPD- and MDD-Associated Lipid Intensity Differences Between 2 Independent Cohorts
eFigure 8. Intersection of MDD-Associated, BPD-Associated, and SCZ-Associated Lipid Features
eFigure 9. Comparison Blood Plasma Lipidome Alterations Between Disorders Within Each of the Cohorts
eFigure 10. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL
eFigure 11. Comparison of Detected and Published Average Lipid Abundance Log Ratios Between SCZ and CTL by Individual Study
eFigure 12. Overlap of Genetic Variants Linked to Psychiatric Disorders and Blood Plasma Lipid Levels
eFigure 13. Correlation Between Lipid Profiles of Nonmedicated Individuals and the SCZ Profile, Compared to the Correlation Between Random Lipid Profiles and the SCZ Profile
eFigure 14. Comparison of the Lipid Intensity Distributions in Medicated and Nonmedicated SCZ Samples
eFigure 15. Dependence of the Lipid Intensity Values on the Fasting Period Prior to Sample Collection
eFigure 16. The Distribution of the Body Mass Index (BMI) for Subsets of CTL and SCZ Samples Used in Analysis of DE-AT Cohort. The P-Value of the Mann–Whitney U Test is Marked Within the Plot
eFigure 17. Effect of Smoking on SCZ-Associated Lipid Intensity Differences
eTable 1. Confounding Factors Assessment
eTable 2. Summary of Studies Reporting Lipid Alterations in Psychiatric Disorders at the Level of Individual Lipid Species
eTable 7. SCZ-Associated Lipid Classes Analysis
eTable 8. Intra- and Inter- Cohort Performance of the SCZ vs CTL Classifier
eTable 9. Enrichment of Disease-Associated Genes Among Lipid Enzymes
eAppendix
eReferences
eTable 3. Alterations for SCZ-Associated Lipids Reported in the Literature
eTable 4. Sample Information for DE-AT, CN, and RU Cohorts
eTable 5. Quantified Lipid Features and Related Information
eTable 6. SCZ-Associated Lipids and Related Information
eTable 10. Sample Information for fepRU Data set
eTable 11. SCZ-Associated Lipids Information for fepRU Data set
eTable 12. Previously Reported Alterations for Various Lipid Species
eTable 13. Linking of Lipid Maps to KEGG
Data Sharing Statement


