Abstract
Background
Ischemia with Non-Obstructive Coronary Arteries (INOCA) is common clinically, particularly among women, but its prevalence among patients with at least moderate ischemia and the relationship between ischemia severity and non-obstructive atherosclerosis severity are unknown.
Objective
We investigated predictors of INOCA in enrolled, non-randomized ISCHEMIA trial participants, sex differences and the relationship between ischemia and atherosclerosis in patients with INOCA.
Methods
Core laboratories independently reviewed screening noninvasive stress tests (nuclear imaging, echocardiography, magnetic resonance imaging or non-imaging exercise tolerance testing), and CCTA, blinded to results of the screening test. INOCA was defined as all stenoses <50% on CCTA in a patient with moderate or severe ischemia on stress testing. INOCA patients, who were excluded from randomization, were compared to randomized participants with ≥50% stenosis in ≥1 vessel and moderate or severe ischemia.
Results
Among 3,612 participants with core laboratory-confirmed moderate or severe ischemia and interpretable CCTA, 476 (13%) had INOCA. Patients with INOCA were younger, predominantly female, and had fewer atherosclerosis risk factors. For each stress testing modality, extent of ischemia tended to be less among patients with INOCA, particularly with nuclear imaging. There was no significant relationship between severity of ischemia and extent or severity of non-obstructive atherosclerosis on CCTA. On multivariable analysis, women odds ratio 4.19 (95% confidence interval, 3.37–5.20) for INOCA compared with men.
Conclusions
Among participants enrolled in ISCHEMIA with core laboratory-confirmed moderate or severe ischemia, the prevalence of INOCA was 13%. Severity of ischemia was not associated with severity of non-obstructive atherosclerosis.
Keywords: ischemia, stress testing, coronary CT angiography, INOCA
Condensed Abstract:
We investigated predictors of ischemia with non-obstructive coronary arteries (INOCA) and the relationship between stress test findings and atherosclerosis in INOCA (defined as all stenoses <50%) in ISCHEMIA enrolled, non-randomized participants. Among 3,612 enrollees with core laboratory-confirmed moderate or severe ischemia and interpretable coronary computed tomography angiography (CCTA), 476 (13%) had INOCA. Ischemia tended to be less extensive with INOCA vs. obstructive coronary artery disease, particularly for nuclear imaging. On multivariable analysis, women had >4-fold odds of INOCA vs. men. INOCA was common with moderate or severe ischemia. There was no correlation between severity of ischemia and of non-obstructive atherosclerosis.
Introduction
Increasingly, clinicians encounter patients with demonstrable myocardial ischemia who have no obstructive coronary arteries as defined by the absence of any 50% or greater stenosis on noninvasive anatomic imaging or invasive coronary angiography (INOCA). These patients are at increased risk for major adverse cardiac events (1–3) and all-cause mortality as compared with people who have normal coronary arteries. (4,5) In addition, patients with INOCA suffer from impaired functional status and decreased quality of life. (6) Evidence to guide treatment in these patients is currently limited. (1,7) The American College of Cardiology-National Cardiovascular Data Registry and National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) study estimated that at least 3–4 million women and men with signs and symptoms suggestive of ischemia have INOCA in the United States. (8) The WISE demonstrated a 13% risk of 10-year all-cause mortality among patients with signs and symptoms of INOCA, higher than the 2.8% 10-year mortality in a nationally representative cohort of controls without symptoms who were approximately the same age during a similar time period.(4) The risks of death and of major adverse cardiovascular events were higher in those with angiographic evidence of non-obstructive atherosclerosis than with angiographically normal coronary arteries in multiple studies of symptomatic patients, but the relationship between the degree of atherosclerosis and ischemia in INOCA patients was not explored in these analyses.(9,10)
Despite the requirement for moderate or severe ischemia on a stress test as a prerequisite for entry into the ISCHEMIA trial, 21% of enrolled participants who underwent coronary computed tomography angiography (CCTA) were found to have trial-defined non-obstructive CAD, and were therefore not randomized.(11)
The ISCHEMIA trial provides a unique opportunity to better understand and clarify this common clinical entity and evaluate its prevalence in a large, multicenter, international study that included independent core lab assessment of both ischemia and anatomy. In this analysis, we characterized patients enrolled in ISCHEMIA with myocardial ischemia and no coronary stenosis ≥50% on CCTA, including sex differences, predictors of INOCA and the relationship between ischemia on stress testing and severity of atherosclerosis on CCTA.
Methods and patient population
The ISCHEMIA trial screened and enrolled 8518 patients who had moderate or severe ischemia on stress testing at baseline, as determined locally at 320 enrolling sites in 37 countries.(11) Blinded CCTA was performed in most enrolled participants (68%) and was reviewed by a core laboratory to exclude left main stenosis of at least 50% and to confirm obstructive CAD meeting trial criteria before randomization. CCTA was not usually performed if the estimated glomerular filtration rate (eGFR) was less than 60 mL/min or if invasive coronary angiography had been recently performed.(11) For the purposes of this analysis, we considered ≥50% stenosis in any vessel to indicate obstructive CAD, regardless of the type of stress test used to qualify the participant for the trial.
Stress testing was performed for clinical indications and included imaging modalities (nuclear myocardial perfusion imaging (MPI), either single-photon emission computed tomography [SPECT] or positron emission tomography [PET]), stress echocardiography and cardiac magnetic resonance [CMR]) or non-imaging exercise tolerance testing (ETT). Ischemia severity was interpreted in a blinded fashion by core laboratories, whether or not a participant was ultimately randomized, according to criteria in Supplemental Table 1. Only core laboratory determination of ischemia is included in these analyses. The imaging core laboratories also identified any infarcted segments. In supplemental analyses, we included participants who had mild or no ischemia based on core laboratory review.
CCTA scans were interpreted according to Society of Cardiovascular Computed Tomography (SCCT) guidelines for segmental stenosis of 0%, 1–24%, 25–49%, 50–69% or 70–100%.(12,13) In ISCHEMIA, CCTA anatomic eligibility criteria after positive imaging stress tests required at least 50% stenosis in at least 1 major coronary artery. The CCTA eligibility criteria for patients with positive non-imaging stress tests required at least 70% stenosis in a proximal or middle segment of a coronary artery. Non-imaging stress tests were used to qualify for ISCHEMIA in 23% of enrollees. However, in this study we used the 50% stenosis threshold to define INOCA regardless of stress test type.
The segment stenosis score was used as a measure of overall CAD plaque extent and the segment involvement score was used as a measure of overall coronary artery plaque distribution.(14) To calculate the segment stenosis score, each individual coronary segment was graded as having no to severe plaque (scores from 0 to 4) based on extent of obstruction of coronary luminal diameter. Then the extent scores of all 16 individual segments were summed to yield a total score ranging from 0 to 64. The segment involvement score was defined as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of luminal stenosis within each segment (minimum = 0; maximum = 16). Normal CCTA was defined as segment involvement score equal to zero (i.e., no plaque).
For this analysis, we excluded enrolled participants if they did not have a CCTA performed, if they had prior CABG or PCI or if CCTA was not evaluable for the presence of 50% stenosis. We also excluded those with no or mild ischemia based on stress core laboratory interpretation. The study cohort therefore consisted of 476 INOCA participants and 3,136 randomized ISCHEMIA trial participants who had at least 50% stenosis on CCTA according to the CT core laboratory and had moderate or severe ischemia according to the stress core laboratories.
Enrolled participants who were excluded from randomization were not followed up after exclusion. However, those who had stress echocardiography as the qualifying stress test were eligible for enrollment into the CIAO-ISCHEMIA ancillary study (NCT01471522)(15).
Statistical analysis
Baseline characteristics, including patient demographics, medical history, and ischemia severity by stress test modality are presented for enrolled participants with vs. without obstructive CAD on CCTA and by sex among those with no obstructive CAD (“INOCA”). Categorical variables are presented as counts (percentages), and differences between groups are assessed using the Chi-Square test or Fisher’s exact test. For categorical variables with ordered levels, the Cochran-Armitage test for trend was applied. Continuous variables are presented as the number of non-missing values and median (Q1, Q3); differences between groups are assessed using the Wilcoxon rank-sum test.
CCTA findings including segment stenosis score, segment involvement score, number of vessels with >0% stenosis, and percentage of participants with entirely normal CCTA (i.e., all visualized segments had 0% stenosis) were compared between women and men who had no obstructive CAD using the Wilcoxon rank-sum for continuous measures or a Chi-square test or Fisher’s exact test for categorical measures. Similar analysis methods were used when comparing ischemia severity between patients with vs. without obstructive CAD.
A multivariable logistic regression model was fit to assess the association between demographics and clinical characteristics with the presence of INOCA. Odds ratios (95% CI) and associated p-values were reported for each covariate. To account for possible nonlinear relationships, continuous covariates were modeled as restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles of each covariate’s empirical distribution. Missing data were accounted for using multiple imputation (100 imputations). When analyzing stress-imaging tests, we did not incorporate type of stress or exercise testing parameters for exercise stress tests in multivariable models, due to frequently missing data in many participants who were excluded from randomization. Discrimination ability of the model was assessed by reporting the C-index, which was calculated over all 100 “imputed” models. All analyses were performed using SAS version 9.4 (Cary, NC).
Results
Patient population
There were 8,518 enrolled patients in the ISCHEMIA trial. After initial exclusion of 2,760 persons who had no study CCTA performed and 455 patients not evaluable for obstructive CAD ≥ 50%, 147 with a prior CABG, and 945 with prior PCI, there were 4,211 participants, of whom 688 (16.3%) had no CCTA-defined coronary stenosis ≥50% (Figure 1). We then excluded 212 participants with mild or no ischemia according to the stress core laboratories, yielding a final cohort of 476 patients with INOCA for this analysis. We excluded 387 participants with no or mild ischemia from the comparator CAD cohort, resulting in a total of 3411 participants with obstructive CAD. Baseline characteristics of patients with no obstructive CAD who did or did not have moderate or severe ischemia according to the stress core laboratories appear in Supplemental Table 2. Overall, 27.3% of INOCA patients had entirely normal coronary arteries, and the remainder had some degree of atherosclerosis.
Figure 1. Study Flow Diagram.
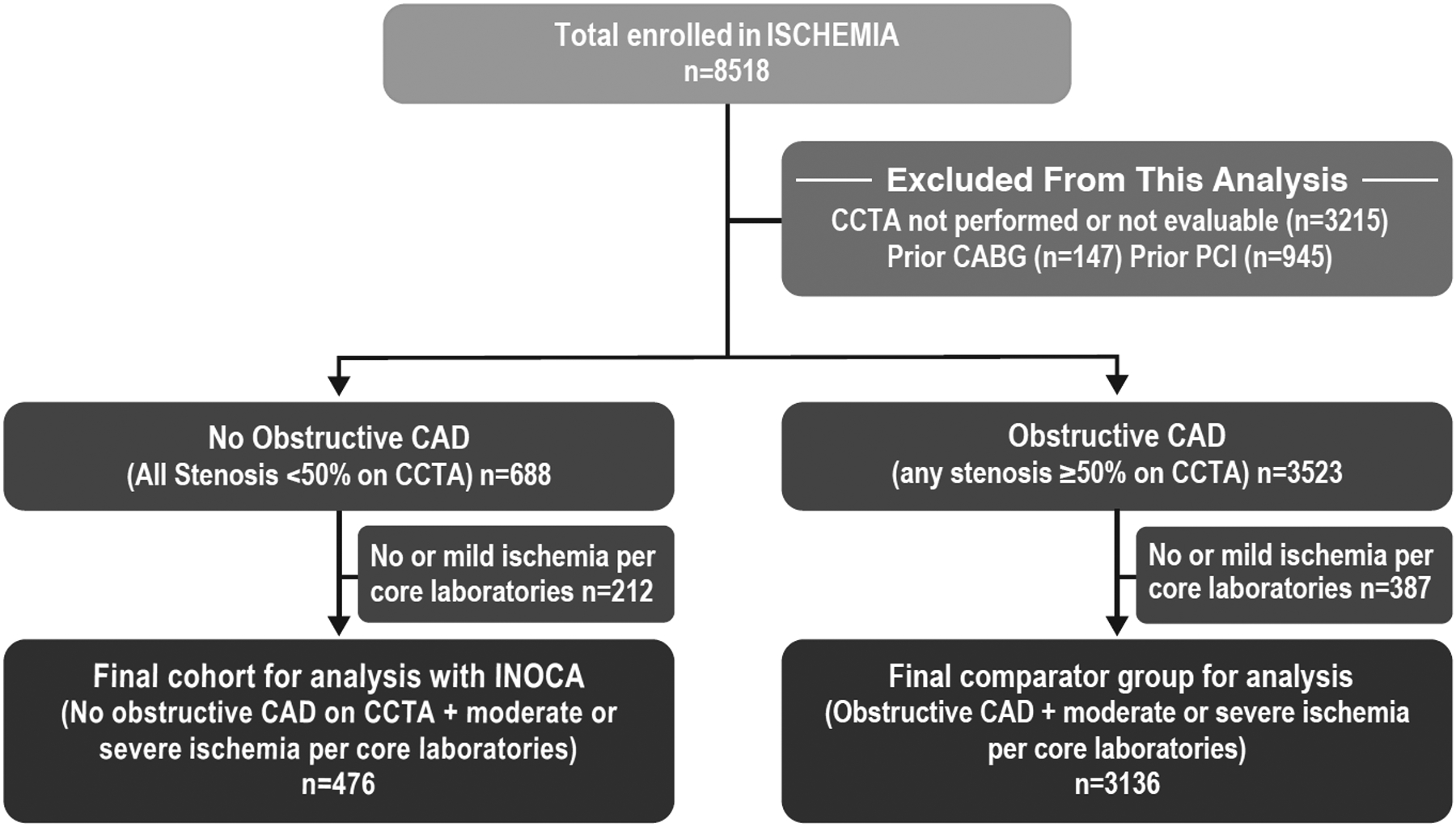
This analysis included participants enrolled in the ISCHEMIA trial. Those without a coronary CT angiogram (CCTA) were excluded. We excluded participants with prior coronary artery bypass grafting (CABG) or prior percutaneous coronary intervention (PCI), because they had obstructive coronary artery disease (CAD) at one time. We compared participants with no obstructive CAD, defined as all stenoses on CCTA <50%, who were excluded from randomization in the trial, to randomized trial participants with at least one stenosis of ≥50% on CCTA, termed obstructive CAD. The final cohort for analysis consisted only of participants with moderate or severe ischemia as determined by core laboratories.
Enrolled participants with INOCA and moderate or severe ischemia (n=476, 13%) were younger than the 3,136 participants with obstructive CAD and moderate or severe ischemia (median age 60 vs. 63 years, p<.001) (Central Illustration). As compared with participants with obstructive CAD, participants with INOCA were more likely to be female (53.6% vs. 20.6%, p<.001) and less likely to have history of hypertension (59.4% vs. 66.5%, p=.002) or diabetes (25.4% vs. 39.7%, p<.001) (Table 1).
Central Illustration.
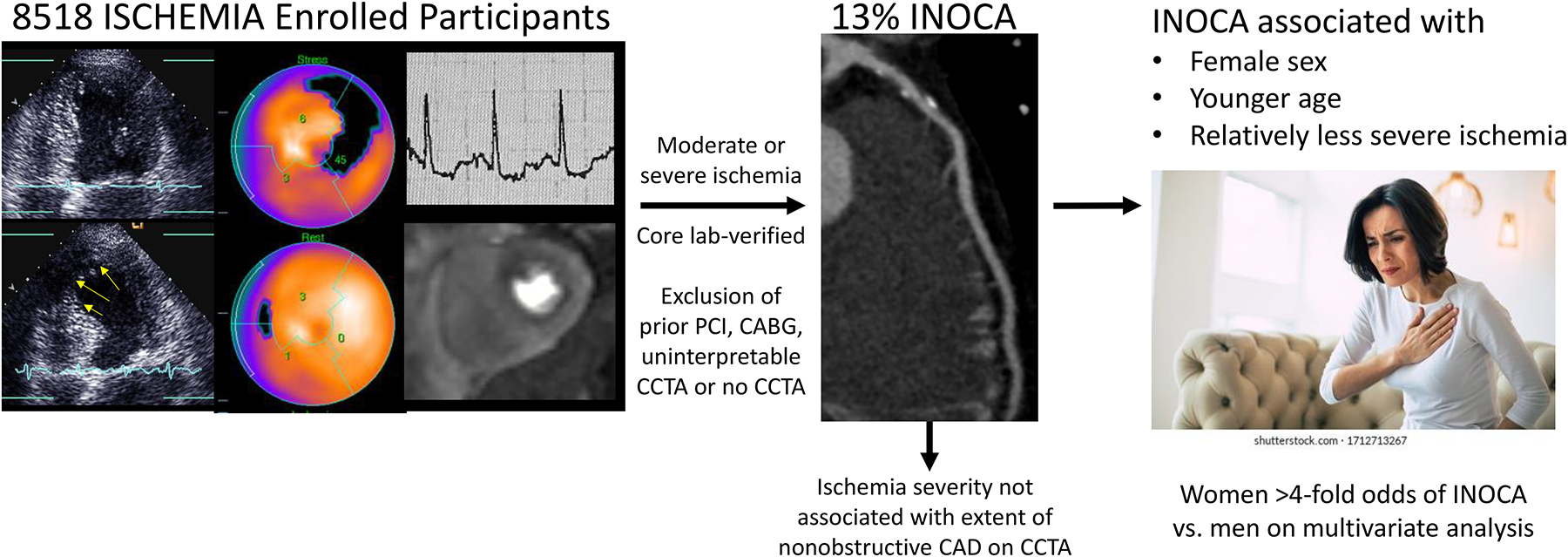
We analyzed the prevalence of INOCA, defined as coronary CT angiogram (CCTA) showing < 50% diameter stenosis in all coronary arteries, among participants enrolled in ISCHEMIA. Participants with INOCA identified on CCTA were excluded from randomization in the trial. Clinical and stress test variables associated with INOCA are shown. Female sex was strongly associated with INOCA on multivariable analysis. Ischemia severity and extent of non-obstructive coronary artery disease (CAD) on CCTA were not correlated, whether CAD was assessed based on the number of segments with plaque, or incorporated the severity of plaque and the number of segments affected, in the segment stenosis score.
Table 1.
Enrolled Participant Baseline Characteristics by Presence of Obstructive Coronary Artery Disease on CCTA.
| Demographics | |||
| Age at Enrollment (yrs.) | <.001 | ||
| Median (25th, 75th) | 60 (53, 68) | 63 (56, 69) | |
| Female Sex | 255/476 (53.6%) | 646/3,136 (20.6%) | <.001 |
| Race | 0.107 | ||
| American Indian or Alaskan Native | 2/467 (0.4%) | 7/3,112 (0.2%) | |
| Asian | 155/467 (33.2%) | 1,103/3,112 (35.4%) | |
| Native Hawaiian or Other Pacific Islander | 2/467 (0.4%) | 9/3,112 (0.3%) | |
| Black or African American | 27/467 (5.8%) | 108/3,112 (3.5%) | |
| White | 281/467 (60.2%) | 1,873/3,112 (60.2%) | |
| Multiple Races Reported | 0/467 (0.0%) | 12/3,112 (0.4%) | |
| Ethnicity | 0.381 | ||
| Hispanic or Latino | 78/451 (17.3%) | 456/2,909 (15.7%) | |
| Not Hispanic or Latino | 373/451 (82.7%) | 2,453/2,909 (84.3%) | |
| Clinical History | |||
| Hypertension | 281/473 (59.4%) | 2,081/3,127 (66.5%) | 0.002 |
| Diabetes | 121/476 (25.4%) | 1,244/3,136 (39.7%) | <.001 |
| Prior Myocardial Infarction | 16/474 (3.4%) | 245/3,109 (7.9%) | <.001 |
| Cigarette Smoking | <.001 | ||
| Never Smoked | 278/452 (61.5%) | 1,452/3,087 (47.0%) | |
| Former Smoker | 131/452 (29.0%) | 1,265/3,087 (41.0%) | |
| Current Smoker | 43/452 (9.5%) | 370/3,087 (12.0%) | |
| Qualifying Stress Test Modality | |||
| Nuclear | 110/476 (23.1%) | 1,281/3,136 (40.8%) | <.001 |
| Echocardiogram | 174/476 (36.6%) | 677/3,136 (21.6%) | <.001 |
| CMR | 9/476 (1.9%) | 103/3,136 (3.3%) | 0.102 |
| ETT | 183/476 (38.4%) | 1,075/3,136 (34.3%) | 0.075 |
The proportion of participants with INOCA was 110/1381 (7.9%) among those who had stress nuclear imaging as the qualifying stress test, 174/851 (20.4%) for stress echocardiography, 183/1258 (14.5%) for ETT and 9/112 (8.0%) for CMR. There was no significant difference in the proportion of participants for whom ETT was the qualifying stress test modality between those with INOCA vs. obstructive CAD. However, overall there were fewer stress nuclear tests and more stress echocardiograms among INOCA participants.
The proportion of participants with INOCA who had at least one infarcted segment on stress imaging was 16.4% (18/110) for nuclear imaging and 8.0% (14/174) for stress echocardiography.
Stress test results in participants with INOCA vs. obstructive CAD
For each of the stress test modalities, the proportion of patients with no obstructive CAD was higher when there was moderate rather than severe ischemia, and this relationship was strongest for stress nuclear, the largest subset (Figure 2 and, excluding those with normal coronary arteries on CCTA, in Supplemental Figure 1; including patients rated by core laboratories as having mild or no ischemia, in Figure 3). For both stress echo and stress nuclear testing, there was a lower likelihood of INOCA with increasing ischemia severity using modality-specific parameters (Supplemental Figure 2). This relationship was not evaluated for ETT, which does not provide a quantitative measure of ischemia, or for CMR, due to low sample size.
Figure 2. Likelihood of INOCA based on ischemia severity and stress imaging modality.
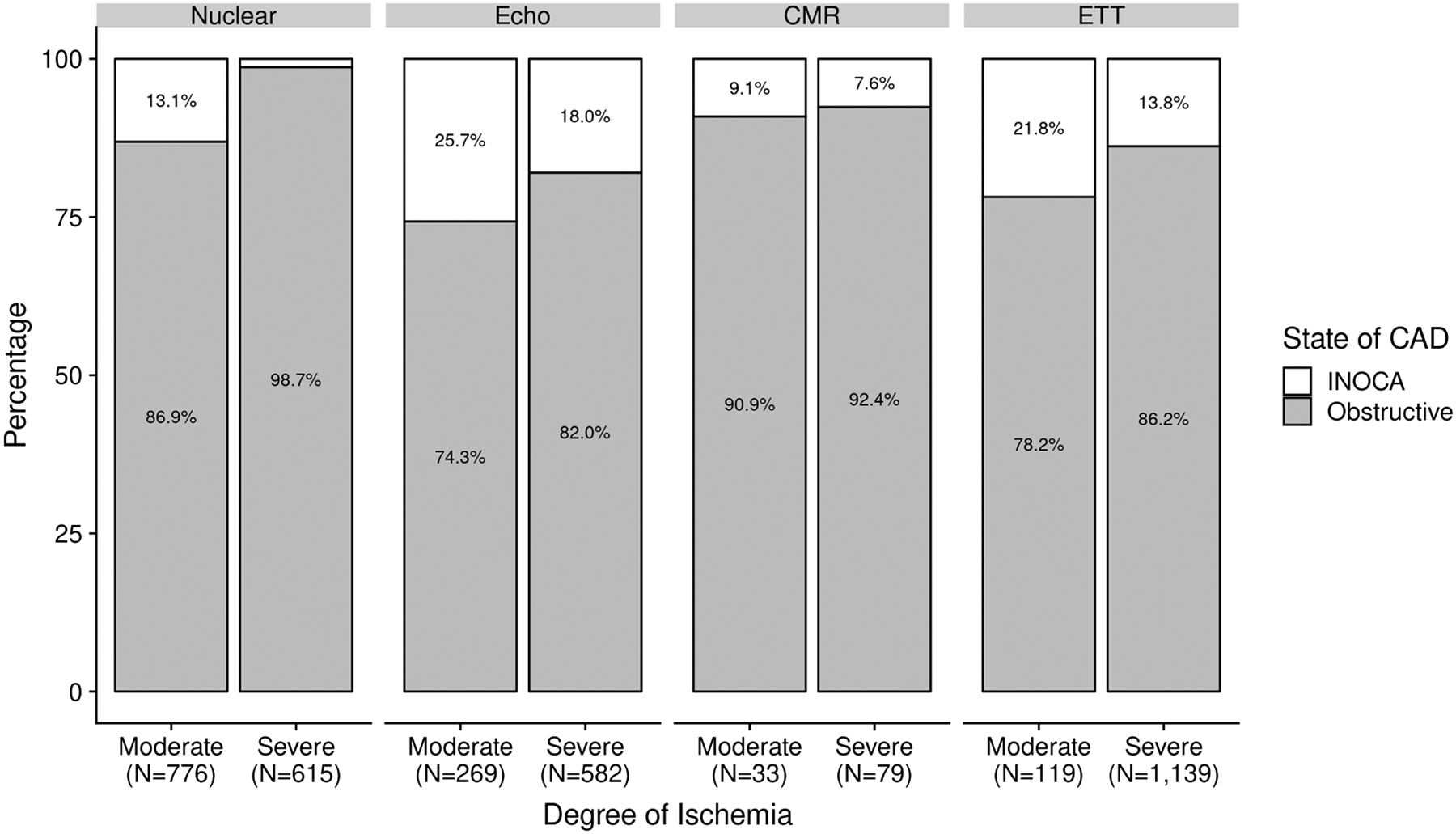
See Supplemental Table 1 for definitions of moderate and severe ischemia.
Figure 3. Ischemia severity in patients with no obstructive CAD vs. obstructive CAD, including those rated by core laboratories as showing no or mild ischemia.
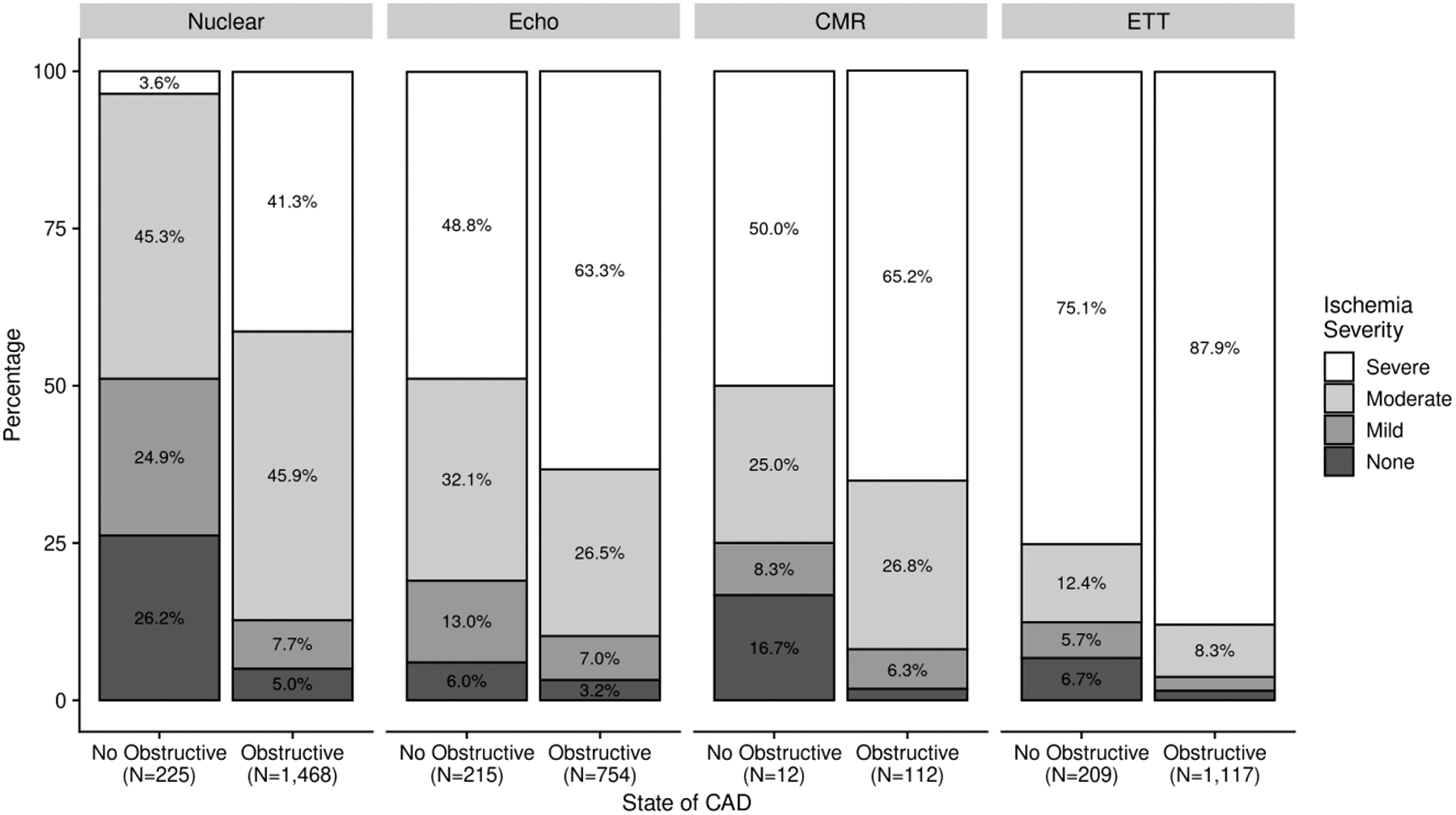
See Supplemental Table 1 for definitions of moderate and severe ischemia. Note that in the remainder of analyses in this article, INOCA is defined as moderate or severe ischemia with <50% stenosis in all major epicardial vessels.
On multivariable analysis, in participants with core laboratory-determined moderate or severe ischemia, the finding of no obstructive CAD on CCTA was associated with younger age, female sex, absence of diabetes, absence of prior MI, lower ischemia severity and stress test modality (Table 2). In particular, the odds ratio for diagnosing INOCA among women vs. men was 4.19 (95% CI: 3.37, 5.20, p<.001). The C-statistic for this model was 0.763 (95% CI: 0.761, 0.765).
Table 2.
Factors Associated with INOCA on Multivariable Analysis
| Characteristic | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age at Enrollment (per 10-year change) | 0.72 (0.64, 0.81) | <.001 |
| Female Sex | 4.19 (3.37, 5.20) | <.001 |
| Race (White vs. Non-white) | 1.27 (0.96, 1.68) | 0.089 |
| Hypertension | 0.84 (0.67, 1.06) | 0.146 |
| Diabetes | 0.56 (0.44, 0.71) | <.001 |
| Prior MI | 0.57 (0.33, 0.98) | 0.042 |
| Cigarette Smoking1 | 0.097 | |
| Former Smoker vs. Never Smoker | 0.85 (0.66, 1.09) | |
| Current Smoker vs. Never Smoker | 0.68 (0.47, 0.99) | |
| Estimated GFR from Enrollment (per 30-mL/min higher) | 1.09 (0.93, 1.27) | 0.292 |
| Severe vs. Moderate Ischemia | 0.45 (0.35, 0.58) | <.001 |
| Imaging Modality1 | <.001 | |
| Echocardiogram vs. Nuclear | 2.97 (2.23, 3.94) | |
| CMR vs. Nuclear | 1.11 (0.53, 2.34) | |
| ETT vs. Nuclear | 2.59 (1.85, 3.61) |
P-value reported is for the overall effect of the variable
C-statistic for this model = 0.763 (95% CI: 0.761, 0.765)
Relationship Between Ischemia Severity and Extent of Non-Obstructive Atherosclerosis on CCTA
There was no significant correlation between ischemia severity and the severity of non-obstructive atherosclerosis as measured by either the segment involvement score or the segment stenosis score, which includes the number of segments affected and severity of stenosis (Figure 4). The median segment involvement score was 3 (IQR, 0–8).
Figure 4. Relationship between severity of ischemia and extent and severity of non-obstructive stenosis on CCTA.
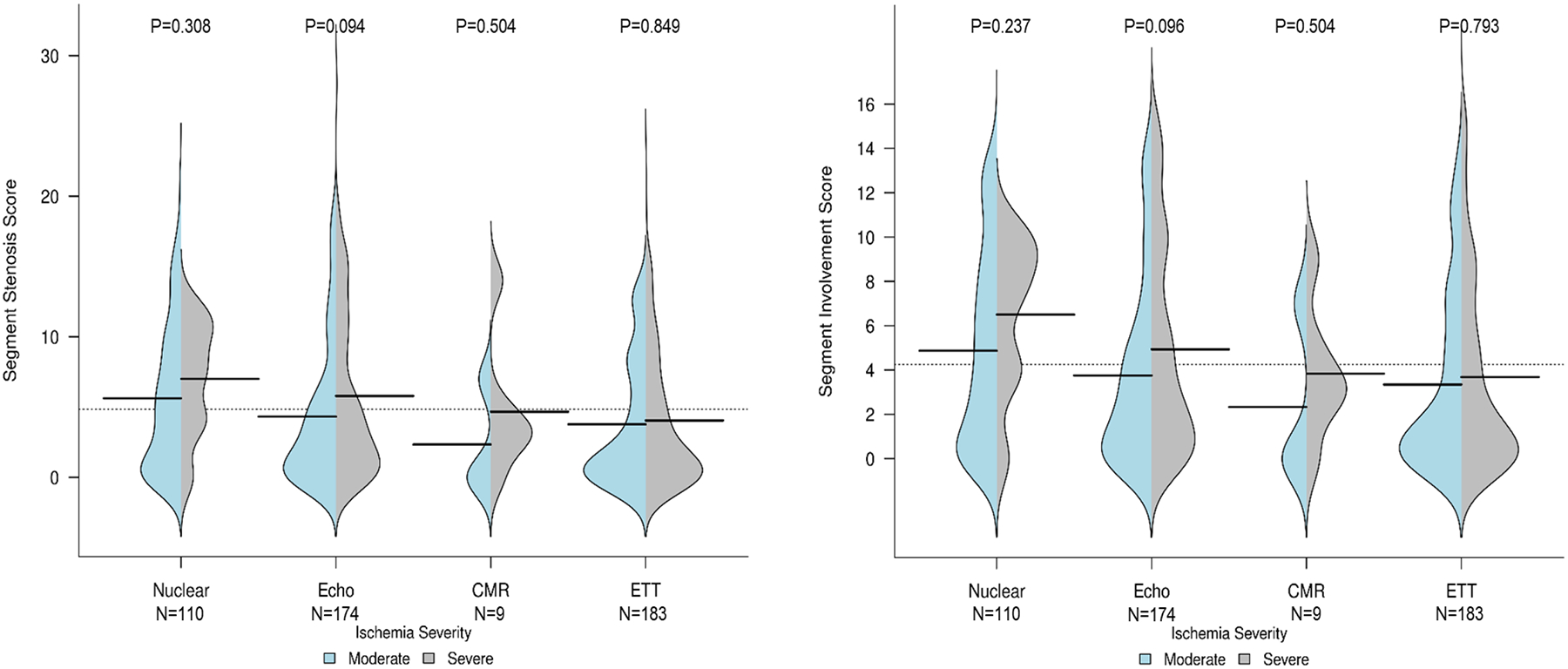
The bean plots show the severity of non-obstructive atherosclerosis as measured by the segment stenosis score (left) and the segment involvement score (right). For each bean, patients with moderate ischemia as determined by core laboratories are shown on the left in blue and those with severe ischemia in gray on the right. The median value is shown with a horizontal black line, and the width illustrates the frequency of each value on the y-axis. To calculate the segment stenosis score, each individual coronary segment was graded as having no to severe plaque (i.e., scores from 0–4) based on extent of obstruction of coronary luminal diameter. Then the extent scores of all 16 individual segments were summed to yield a total score ranging from 0–64. The segment involvement score was defined as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of luminal stenosis within each segment (minimum=0; maximum=16). Data are presented for each stress testing modality.
P-values comparing segment stenosis score between participants with moderate vs. severe ischemia overall for each modality were all >0.05. The same was true for segment involvement score.
Sex Differences in Clinical Characteristics and Imaging Findings Related to INOCA
Women with INOCA were older than men with INOCA (median age 61 years vs 59, p=0.026), and more women than men with INOCA had never smoked (71.9% vs. 49.5%; p <.001). Among the 476 participants with INOCA, a greater proportion of men had a nuclear (SPECT/PET) qualifying stress test compared to women (29.0% vs. 18.0%, p=0.005, Table 3) and a lower proportion had stress echocardiography (25.8% vs. 45.9%, p<0.001), similar to usage of the various stress testing modalities in the trial overall.(16) Women were more likely than men to have no obstructive CAD regardless of the stress test modality: INOCA was present in 15.1% of women vs. 5.9% of men undergoing stress nuclear testing, p<0.001; 41.2% of women vs. 10.1% of men undergoing stress echocardiography p<0.001; and 30.3% of women vs. 10.1% of men undergoing ETT, p<0.001).
Table 3.
Baseline Characteristics and Stress Test Findings of Women and Men with No Obstructive Coronary Artery Disease on CCTA.
| Demographics | |||
| Age at Enrollment (years, median, 25th, 75th) | 61 (53, 68) | 59 (52, 66) | 0.026 |
| Race | 0.014 | ||
| American Indian or Alaskan Native | 2/249 (0.8%) | 0/218 (0.0%) | |
| Asian | 68/249 (27.3%) | 87/218 (39.9%) | |
| Native Hawaiian or Other Pacific Islander | 0/249 (0.0%) | 2/218 (0.9%) | |
| Black or African American | 16/249 (6.4%) | 11/218 (5.0%) | |
| White | 163/249 (65.5%) | 118/218 (54.1%) | |
| Multiple Races Reported | 2/249 (0.8%) | 0/218 (0.0%) | |
| Hispanic or Latino Ethnicity | 49/241 (20.3%) | 29/210 (13.8%) | 0.068 |
| Clinical History | |||
| Hypertension | 161/253 (63.6%) | 120/220 (54.5%) | 0.045 |
| Diabetes | 64/255 (25.1%) | 57/221 (25.8%) | 0.862 |
| Prior Myocardial Infarction | 7/253 (2.8%) | 9/221 (4.1%) | 0.432 |
| Cigarette Smoking | <.001 | ||
| Never Smoked | 174/242 (71.9%) | 104/210 (49.5%) | |
| Former Smoker | 53/242 (21.9%) | 78/210 (37.1%) | |
| Current Smoker | 15/242 (6.2%) | 28/210 (13.3%) | |
| Qualifying Stress Test Modality | |||
| Nuclear | 46/255 (18.0%) | 64/221 (29.0%) | 0.005 |
| Echocardiogram | 117/255 (45.9%) | 57/221 (25.8%) | <.001 |
| CMR | 8/255 (3.1%) | 1/221 (0.5%) | 0.042 |
| ETT | 84/255 (32.9%) | 99/221 (44.8%) | 0.008 |
| Left Ventricular Ejection Fraction, Median (25th, 75th) | N=150 62 (57, 67) |
N=100 60 (55, 64) |
0.007 |
| Exercise Stress Test Details * | |||
| Peak METs Achieved | N=94 | N=67 | <.001 |
| Median (25th, 75th) | 7.0 (5.9, 8.7) | 8.4 (7.0, 10.2) | |
| Heart Rate at Peak Stress (bpm) | N=107 | N=76 | 0.341 |
| Median (25th, 75th) | 149 (137, 160) | 148 (142, 162) | |
| Systolic Blood Pressure at Peak Stress (mmHg) | N=100 | N=71 | 0.314 |
| Median (25th, 75th) | 161 (150, 186) | 170 (150, 192) | |
| Diastolic Blood Pressure at Peak Stress (mmHg) | N=83 | N=67 | 0.729 |
| Median (25th, 75th) | 81 (77, 90) | 80 (79, 90) |
includes ETT and exercise stress imaging
Sex Differences in the Extent of Non-Obstructive Atherosclerosis on CCTA
The segment stenosis score and segment involvement score were significantly higher in men compared to women (all p<0.001) (Table 4). There was no interaction between sex and ischemia severity with respect to segment stenosis score or segment involvement score.
Table 4.
Extent and severity of coronary atherosclerosis among enrolled participants with INOCA by sex, and among randomized women and men who underwent CCTA by sex*
| CCTA Findings | |||||||
| Segment Stenosis Score (SSS) Median (25th, 75th) | 3 (0, 8) | 2 (0, 6) | 4 (1, 10) | <.001 | 24 (19, 30) | 22 (16, 27) | 25 (19, 31) |
| Segment Involvement Score (SIS) Median (25th, 75th) | 3 (0, 7) | 2 (0, 5) | 4 (1, 9) | <.001 | 12 (10, 13) | 11 (9, 13) | 12 (10, 13) |
| Participants with Entirely Normal CCTAa | 130/476 (27.3%) | 78/255 (30.6%) | 52/221 (23.5%) | 0.085 | NA | NA |
p<0.001 for comparisons between sexes and with INOCA participants. Randomized participants were included in this table only if they underwent CCTA that was interpretable for SSS and SIS.
All visualized segments have 0% stenosis.
Discussion
In this analysis of ISCHEMIA trial enrollees, we observed that INOCA was present in 13%, defined here as no coronary stenosis ≥50% in a participant with moderate or severe ischemia and a fully evaluable CCTA with no prior PCI or CABG. Thus, mechanisms other than advanced atherosclerosis may be responsible for ischemia, even when a high degree of ischemia is present. For each stress test modality, extent of ischemia tended to be less among patients with INOCA than in patients with obstructive CAD, particularly for participants undergoing stress nuclear imaging. There was no significant correlation between severity of ischemia and severity of non-obstructive atherosclerosis on CCTA. On multivariable analysis, females had 4.2-fold odds of INOCA compared with men and were younger. INOCA patients were less likely to have cardiac risk factors including hypertension, diabetes, or cigarette smoking.
In this study the proportion of patients with no obstructive CAD was far lower than in series of patients undergoing clinically indicated CCTA or invasive angiography, due to the requirement for core laboratory confirmed moderate or severe ischemia.(10,17–19) It was lower than the overall proportion excluded from randomization due to lack of trial-defined obstructive CAD (21%) because we restricted this analysis to participants with moderate or severe ischemia according to core laboratories and with interpretable CCTA. In addition, the trial definition required ≥70% stenosis for participants enrolled after ETT. (20)
One proposed mechanism for stress-induced ischemia in the absence of epicardial coronary obstruction is coronary microvascular dysfunction.(3) Reduced coronary flow reserve (CFR) has been observed across the entire spectrum of CAD, from severe atherosclerosis to those with normal appearing angiograms. (21) Reduced coronary flow reserve is also found in patients with systemic inflammation and endothelial dysfunction. (22) Many pro-inflammatory conditions have been associated with INOCA such as chronic pulmonary disease, heart failure with preserved ejection fraction, systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease. Coronary artery spasm is another mechanism of INOCA and may coexist with coronary microvascular dysfunction. (23) Given that coronary artery spasm is often not identified on non-invasive testing and that both endothelial dysfunction and microvascular coronary disease may be associated with mild ischemia, or no ischemia, on stress testing, the prevalence of INOCA in our study is likely an underestimate of the true prevalence in the general population.
Female sex and lower degrees of ischemia severity were the strongest independent predictors of INOCA on multivariate analysis. Women were approximately 4-fold more likely than men to have INOCA and were more frequently excluded from trial randomization due to INOCA.(24) However, it should be noted that INOCA is not exclusively a disease of females, and 46% of patients with INOCA in this analysis were male. This represents a reason that equal representation of women in trials of revascularization for CAD is challenging, and cannot be remedied by equitable screening. However, it is consistent with data from over 6,000 patients in the Advanced Cardiovascular Imaging Consortium registry without known CAD, in which female sex was independently associated with the absence of obstructive CAD on CCTA performed within 3 months of a positive stress test.(25) In that study, stress test results were not predictive of obstructive CAD, in contrast to the current analysis.
The likelihood of INOCA was highest for participants enrolled after stress echocardiography, followed by ETT and then nuclear or CMR perfusion imaging. For both stress nuclear and stress echocardiography, the likelihood of INOCA was lower with increasing ischemia severity, but this relationship was more striking for nuclear than echocardiography. When considering ETT, it should be noted that trial eligibility required severe ischemia. The differences between modalities in the relationship between ischemia severity and no obstructive CAD were most apparent in supplemental analyses that considered the entire enrolled cohort, without restricting to tests verified to show moderate or severe ischemia by the core laboratories. This may relate to variability in interpretation between sites and core laboratories, inherent differences between stress test modalities, or both.
Patients with no obstructive CAD had lower degrees of ischemia on average than patients with obstructive CAD on CCTA in ISCHEMIA for every stress testing modality, most notably for nuclear imaging. Only 1.3% of patients with severe ischemia on stress nuclear imaging had INOCA. Perfusion imaging appears to have better specificity for obstructive CAD at the upper range of ischemia severity than wall motion imaging, though we cannot formally test this due to the selected nature of our trial-eligible population. For example, an unacceptable level of angina was an exclusion criterion. Prior studies have shown that stress echocardiography had a mean sensitivity of 84% and mean specificity of 87% for detecting obstructive CAD.(26) Because stress echocardiography has been documented to have higher specificity and lower sensitivity than stress nuclear imaging, a referral pattern may exist such that lower-risk patients were sent more frequently for stress echocardiography.(27,28) In addition, stress echocardiography may be preferred in women, particularly younger women, to avoid radiation exposure (29). Women enrolled in ISCHEMIA were less likely to have had nuclear imaging performed, more likely to have had stress echocardiography and overall in the trial, women had less severe ischemia as compared with men.(24) Women are well known to have higher prevalence of INOCA. Women also develop atherosclerotic disease later than men in general.(30) Alternatively, these findings may represent true differences in the diagnostic accuracy of the various stress test modalities for obstructive CAD, and/or the severity of inducible wall motion abnormalities that aligns with a particular severity of perfusion defect may be different than that reflected by the inclusion criteria for the ISCHEMIA trial. The accuracy of stress echocardiography may be reduced in cases with exercise-induced hypertension, and hemodynamic data were not incorporated into the interpretation at the stress echocardiography core laboratory in ISCHEMIA. There may be greater variability in interpretation of stress echocardiography than perfusion imaging between sites and core laboratories. It is also possible that stress echocardiography and/or exercise tolerance testing have greater sensitivity for microvascular ischemia than stress perfusion imaging. Studies describing the sequence of events in the ischemic cascade (namely, perfusion abnormality followed by abnormal wall motion, ECG changes and then symptoms) were performed in the setting of epicardial stenosis.(31,32) Epicardial stenosis by its nature creates regional abnormalities in perfusion, while microvascular ischemia may be more widespread across the myocardium and yet patchy in its effects on a particular segment.(33) These inherent differences in the mechanism of ischemia have the potential to lead to different patterns of stress testing abnormalities, an area for further study. Finally, it may be the case that at least some of these stress tests were falsely positive for ischemia, such as due to artifacts. Furthermore, given these observations, it would be important to determine in future studies the potential differences in association with independent markers of ischemia such as myocardial flow reserve by PET or invasive CFR measurements. as well as prognosis based upon the modality used to identify patients felt to have INOCA.
On CCTA, there was a median of three segments affected by atherosclerosis in our patients with INOCA. In line with prior studies, most INOCA patients did have some demonstrable atherosclerosis.(34,35) However, neither the number of involved segments (segment involvement score) nor the segment stenosis score, which takes into account the severity of stenosis by segment, was associated with ischemia severity for any stress testing modality. This suggests that atherosclerosis may not be the main contributor to ischemia in these patients. However, our analysis is limited by the multiple methods of ischemia testing employed and the limited range of ischemia severity based on trial entry criteria. More importantly, atherosclerotic plaque features other than stenosis severity (e.g., low attenuation, positive remodeling, napkin ring sign, spotty calcification), and lumen volume may be more closely associated with ischemia than stenosis severity.(36) We did not have access to these parameters for the present analysis. In addition, atherosclerosis is common at sites of coronary spasm.(37)
The CorMicA study investigated underlying mechanisms of ischemia in patients with INOCA. In CorMicA, coronary microvascular disease, coronary artery spasm or both was identified in 89% of consecutive male and female patients who were referred for coronary angiography for evaluation of typical or atypical chest pain and found to have no obstructive CAD.(23) This was even higher than a prior study at the Mayo Clinic, in which 63% of patients had abnormalities on invasive testing; noninvasive stress tests had limited accuracy for identifying invasive testing abnormalities(38). Using invasive and noninvasive methods, the WISE program demonstrated that many patients with INOCA have ischemia on gold-standard testing, and that abnormal coronary flow reserve is associated with adverse cardiovascular outcomes(39,40). Based on this prior literature and because independent core laboratories confirmed moderate or severe ischemia without knowledge of CAD status, it is likely that most patients with INOCA enrolled in the ISCHEMIA trial had true ischemia.
Our study results reinforce the need to address gaps in knowledge about INOCA diagnosis and management, including the diagnostic accuracy of the various non-invasive modalities as compared with the reference standards of invasive coronary reactivity testing or positron emission tomography, how non-invasive test findings relate to future risk, and the best medical therapy and lifestyle measures to improve symptoms and reduce risk of cardiovascular events, among others.
Limitations
CCTA was not performed in all enrolled patients, which could bias toward a higher prevalence of INOCA in patients with moderate or severe ischemia, since lower eGFR is associated with increased risk of CAD (41). Stress testing methods are subject to artifacts that have the potential to mimic ischemia; thus, some patients may have had false positive stress tests. CCTA and invasive angiography do not always agree on stenosis severity. We have not yet assessed FFR by CCTA, or quantified plaque volume or plaque length as a measure of plaque diffuseness, or examined lumen volume. However, we found no relationship between segment involvement score, a marker of the diffuseness of plaque and ischemia severity. We did not perform invasive testing to investigate mechanisms of INOCA, which was outside the scope of the trial. Angina and clinical outcomes were not recorded in patients excluded from trial randomization in ISCHEMIA, but were reported separately in the Changes in Ischemia and Angina over One year among ISCHEMIA trial (CIAO-ISCHEMIA) ancillary study(15). Many patients with INOCA as proven by invasive testing in clinical practice do not have abnormal stress imaging, and may have mild ischemia, exercise ECG abnormalities alone or normal stress test results. In some cases, calcification could have resulted in overestimation of stenosis severity, so some patients included in the obstructive CAD group may in fact have had INOCA. Breast attenuation on perfusion imaging, and alteration in lead placement to avoid breast tissue on ETT, may contribute to false positive stress test results in women. Sample size was limited. Other determinants of myocardial ischemia such as valvular heart disease, left ventricular outflow tract obstruction, left ventricular hypertrophy, or diastolic function were not recorded. Therefore, our population may possibly include some patients with different cardiac and systemic conditions associated with ischemia other than intrinsic coronary dysfunction.
Conclusions
Among patients with core lab determined moderate or severe ischemia at baseline who were enrolled but not randomized in ISCHEMIA, the prevalence of INOCA was 13%. INOCA was more common among women, younger patients and patients with relatively less severe ischemia. Women had less extensive non-obstructive atherosclerosis than men. There was no relationship between extent of non-obstructive atherosclerosis and severity of ischemia in patients with INOCA.
Supplementary Material
Core Clinical Competencies and Translational Outlook implications.
Competency in Medical Knowledge 1:
Ischemia with no obstructed coronary arteries (INOCA) is observed in both men and women, including with all degrees of ischemia on stress testing.
Competency in Medical Knowledge 2:
INOCA is more common among women than men, 4-fold in this study. Still, 46% of INOCA patients in this study were male.
Competency in Interpersonal & Communication Skills:
It is important to explain to patients that a CT showing non-obstructive atherosclerosis does not equate to the absence of heart disease.
Translational Outlook 1:
Severity of ischemia in INOCA patients was not correlated with severity of non-obstructive atherosclerosis in this study. Future studies will investigate whether atherosclerotic plaque features other than stenosis severity, and lumen volume, may be more closely associated with ischemia than stenosis severity or the number of arterial segments with plaque.
Sources of Funding:
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565
Other Support:
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Amgen Inc; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck, Sharp & Dohme Corp. and Sunovion Pharmaceuticals
Disclosure Statements:
Dr. Harmony Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work;
Dr. Ariel Diaz reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Derek Cyr reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Leslee J. Shaw reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. G.B. John Mancini reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Jonathon Leipsic reports Consultant and stock options- circle cvi and heartflow Research grant - GE Healthcare and Edwards, Speakers bureau- philips and ge healthcare.
Dr. Matthew J. Budoff reports grants from National Heart, Lung, and Blood Institute during the conduct of the study and grant support from General Electric
Dr. James K. Min reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. He serves in the Medical Advisory Board for Arineta. He receives salary and has ownership interest for Cleerly, Inc.
Dr. Cameron Hague reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Daniel S. Berman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study and receives software royalties from Cedars-Sinai Medical Center.
Dr. Bernard R. Chaitman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Michael H. Picard reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Sean W. Hayes reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Marielle Scherrer-Crosbie reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Raymond Y. Kwong reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Renato D. Lopes reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work.
Dr. Roxy Senior reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Sudhanshu K. Dwivedi reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Todd D. Miller reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Benjamin J.W. Chow reports grants from National Heart, Lung, and Blood Institute during the conduct of the study and holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research. He receives research support from TD Bank, CV Diagnostix and AusculSciences, and Siemens Healthineers. He has equity interest in General Electric.
Dr. Ramesh de Silva reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Gregg W. Stone reports grants and personal fees from National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Terumo, personal fees from Amaranth, personal fees from Shockwave, personal fees and other from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Abiomed, personal fees from Claret, personal fees from Sirtex, personal fees and other from Ancora, personal fees and other from Qool Therapeutics, other from Cagent, other from Applied Therapeutics, other from Biostar family of funds, other from MedFocus family of funds, personal fees and other from SpectraWave, personal fees from MAIA Pharmaceuticals, personal fees and other from Orchestra Biomed, other from Aria, personal fees from Vectorious, other from Cardiac Success, outside the submitted work.
Dr. William E. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work; Dr. Sripal Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, personal fees from Pfizer, personal fees from Amgen, personal fees from Reata, outside the submitted work.
Dr. Sean M. O’Brien reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. David J. Maron reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; St. Jude Medical, Inc.; Volcano Corporation; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc.; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Abbreviations:
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CIAO-ISCHEMIA
Changes in Ischemia and Angina over One year among ISCHEMIA trial CMR – cardiac magnetic resonance imaging
- eGFR
estimated glomerular filtration rate
- ETT
exercise tolerance testing
- INOCA
ischemia with non-obstructive coronary arteries
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- PCI
percutaneous coronary intervention
- SIHD
stable ischemic heart disease
- WISE
Women’s Ischemia Syndrome Evaluation
Footnotes
Disclaimer: The manuscript contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
References
- 1.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and No Obstructive Coronary Artery Disease (INOCA): What Is the Risk? Journal of the American Heart Association 2018;7:e008868–e008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang FY, Huang BT, Lv WY et al. The Prognosis of Patients With Nonobstructive Coronary Artery Disease Versus Normal Arteries Determined by Invasive Coronary Angiography or Computed Tomography Coronary Angiography: A Systematic Review. Medicine (Baltimore) 2016;95:e3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunadian V, Chieffo A, Camici PG et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. European Heart Journal 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenkre TS, Malhotra P, Johnson BD et al. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagidipati NJ, Mudrick DW, Chiswell K, Brucker A, Peterson ED, Douglas PS. Sex differences in long-term outcomes of patients across the spectrum of coronary artery disease. Am Heart J 2018;206:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw LJ, Merz CNB, Pepine CJ et al. The Economic Burden of Angina in Women With Suspected Ischemic Heart Disease. Circulation 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 7.Turgeon RD, Pearson GJ, Graham MM. Pharmacologic Treatment of Patients With Myocardial Ischemia With No Obstructive Coronary Artery Disease. The American Journal of Cardiology 2018;121:888–895. [DOI] [PubMed] [Google Scholar]
- 8.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati M, Cooper-DeHoff RM, McClure C et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen L, Hvelplund A, Abildstrøm SZ et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European Heart Journal 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS, Reynolds HR, Bangalore S et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiology 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leipsic J, Abbara S, Achenbach S et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 13.Mancini GBJ, Leipsic J, Budoff MJ et al. Coronary CT Angiography Followed by Invasive Angiography in Patients With Moderate or Severe Ischemia-Insights From the ISCHEMIA Trial. JACC: Cardiovascular Imaging 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min JK, Shaw LJ, Devereux RB et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds HR PM, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El-Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, Kunichoff DF, Anthopolos R, Alfakih K, Abdul-Nour K, Khouri M, Bershtein L,. Natural History of Patients with Ischemia and No Obstructive Coronary Artery Disease: the CIAO-ISCHEMIA Study. Circulation Journal 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds HR, Shaw LJ, Min JK et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw LJ, Shaw RE, Merz CN et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787–801. [DOI] [PubMed] [Google Scholar]
- 18.Maddox TM, Stanislawski MA, Grunwald GK et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min JK, Dunning A, Lin FY et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. Journal of the American College of Cardiology 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 20.Hochman JS, Reynolds HR, Bangalore S et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 22.Sakr SA, Abbas TM, Amer MZ et al. Microvascular angina. The possible role of inflammation, uric acid, and endothelial dysfunction. Int Heart J 2009;50:407–19. [DOI] [PubMed] [Google Scholar]
- 23.Ford TJ, Berry C. How to Diagnose and Manage Angina Without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial. Interv Cardiol 2019;14:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds HR, Shaw LJ, Min JK et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinnaiyan KM, Raff GL, Goraya T et al. Coronary Computed Tomography Angiography After Stress Testing: Results From a Multicenter, Statewide Registry, ACIC (Advanced Cardiovascular Imaging Consortium). Journal of the American College of Cardiology 2012;59:688–695. [DOI] [PubMed] [Google Scholar]
- 26.Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress Echocardiography. Part I. Exercise Echocardiography: Techniques, Implementation, Clinical Applications, and Correlations. Mayo Clinic Proceedings 1995;70:5–15. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. Jama 1998;280:913–20. [DOI] [PubMed] [Google Scholar]
- 28.Neglia D, Rovai D, Caselli C et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8. [DOI] [PubMed] [Google Scholar]
- 29.Mieres JH, Shaw LJ, Arai A et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005;111:682–96. [DOI] [PubMed] [Google Scholar]
- 30.Chaitman BR, Bourassa MG, Davis K et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981;64:360–367. [DOI] [PubMed] [Google Scholar]
- 31.Hauser AM, Gangadharan V, Ramos RG, Gordon S, Timmis GC, Dudlets P. Sequence of mechanical, electrocardiographic and clinical effects of repeated coronary artery occlusion in human beings: Echocardiographic observations during coronary angioplasty. Journal of the American College of Cardiology 1985;5:193–197. [DOI] [PubMed] [Google Scholar]
- 32.Leong-Poi H, Rim S-J, Le DE, Fisher NG, Wei K, Kaul S. Perfusion Versus Function: The Ischemic Cascade in Demand Ischemia. Circulation 2002;105:987–992. [DOI] [PubMed] [Google Scholar]
- 33.Lanza GA, Crea F. Primary Coronary Microvascular Dysfunction. Circulation 2010;121:2317–2325. [DOI] [PubMed] [Google Scholar]
- 34.Khuddus MA, Pepine CJ, Handberg EM et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenstrom I, Maaniitty T, Uusitalo V et al. Frequency and angiographic characteristics of coronary microvascular dysfunction in stable angina: a hybrid imaging study. Eur Heart J Cardiovasc Imaging 2017;18:1206–1213. [DOI] [PubMed] [Google Scholar]
- 36.Stuijfzand WJ, van Rosendael AR, Lin FY et al. Stress Myocardial Perfusion Imaging vs Coronary Computed Tomographic Angiography for Diagnosis of Invasive Vessel-Specific Coronary Physiology: Predictive Modeling Results From the Computed Tomographic Evaluation of Atherosclerotic Determinants of Myocardial Ischemia (CREDENCE) Trial. JAMA Cardiol 2020;5:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin ES, Ann SH, Singh GB et al. OCT-Defined Morphological Characteristics of Coronary Artery Spasm Sites in Vasospastic Angina. JACC Cardiovascular imaging 2015;8:1059–1067. [DOI] [PubMed] [Google Scholar]
- 38.Cassar A, Chareonthaitawee P, Rihal CS et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv 2009;2:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.AlBadri A, Bairey Merz CN, Johnson BD et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson BD, Shaw LJ, Buchthal SD et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–9. [DOI] [PubMed] [Google Scholar]
- 41.Joosen IA, Schiphof F, Versteylen MO et al. Relation between mild to moderate chronic kidney disease and coronary artery disease determined with coronary CT angiography. PLoS One 2012;7:e47267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


