Abstract
One of the more recently identified bacterial exportation systems is the type IV secretion mechanism, which is characterized by a multiprotein complex that spans the inner and outer bacterial membranes and contains a pilin component. The most thoroughly studied type IV secretion system is encoded by the virB operon of Agrobacterium tumefaciens. In Bartonella henselae, 8 of the 10 virB operon genes share extensive homology and arrangement with the virB operon of A. tumefaciens. Sequencing of the region upstream of the B. henselae virB2 gene revealed a region with sequence homology to the vir box of A. tumefaciens. This possible promoter region was cloned upstream of the green fluorescent protein reporter gene in the promoterless vector pANT3 and used to transform B. henselae. Minimal reporter gene expression was seen in the transformed bacteria cultivated in the absence of host cells, but expression was strongly induced in intracellular bacteria cultivated with human microvascular endothelial cells. Deletion of an 87-bp fragment, which contained the putative vir box from the 5′ end of the promoter region, diminished intracellular induction of the reporter gene. Host cell induction of the 17-kDa antigen gene, which replaces virB5 in B. henselae, was also demonstrated at the protein level using specific antiserum. Thus, expression of the virB genes of B. henselae is induced in bacteria, which have invaded host cells, through a mechanism that may be similar to the environment-sensing mechanism found in the virB operon of A. tumefaciens.
Bartonella henselae is a fastidious gram-negative bacillus that is capable of causing a wide variety of disease syndromes. The most common B. henselae-associated diseases (BAD) are cat-scratch disease, which is most often seen in immunocompetent children, and bacillary angiomatosis (BA), which is common in AIDS patients and other immunosuppressed individuals. BA is a proliferative disorder of the vascular endothelial cells resulting in the development of tumor-like lesions on the skin and internal organs (36). BA is caused by both B. henselae and Bartonella quintana (17, 32, 38, 39). Bartonella-induced vascular proliferation was first attributed to infection with Bartonella bacilliformis (14). This proliferation is manifested as the eruptive phase (verruga peruana) of Carrion's disease, which is endemic to Peru (26). Although the epidemiology of diseases caused by different Bartonella spp. differs, the ability of Bartonella spp. to induce angiogenic lesions in infected patients represents a common mechanism of pathogenesis that is unique to this genus.
B. henselae is a facultative intracellular bacterium which has been shown to attach and invade human endothelial cells (12). Surface pili are thought to play an important role in the initial attachment to host cells, since nonpiliated strains are less invasive (5). In general, primary isolates of B. henselae are thought to be more heavily piliated than isolates that have been extensively cultivated on laboratory media (5). Invasion of human endothelial cells has been shown to occur with large aggregates of bacteria as well as with individual organisms (12). However, some reports indicate that B. henselae may induce endothelial cell proliferation independent of bacterial invasion (8, 21). The factor from B. henselae that is responsible for causing endothelial cell proliferation and ultimately angiogenesis has not yet been identified. In addition, the mechanism by which the B. henselae factor is delivered to endothelial cells to mediate proliferation has not been elucidated.
Recently an operon has been identified in B. henselae that is homologous and colinear to the virB operon of Agrobacterium tumefaciens (22, 29). The B. henselae virB genes share sequence homology to the virB genes of A. tumefaciens; however, in B. henselae the virB5 gene is replaced by the gene encoding the immunodominant 17-kDa antigen found only in Bartonella spp. (22). Despite the presence of high levels of antibody to the 17-kDa antigen in sera from most patients with Bartonella infections, minimal reactivity with this protein was observed on Western blots using B. henselae cultivated on cell-free laboratory medium (2). This suggests that the 17-kDa protein may not be expressed at high levels when grown on routine culture medium. Because of the importance of the virB operon in (i) intracellular survival for other bacteria, (ii) delivery of effector molecules to host cells mediating pathogenesis in other bacteria, and (iii) apparent low-level expression of the 17-kDa antigen in B. henselae bacteria cultivated on laboratory medium, the induction of this operon in the intracellular environment of host cells was examined.
MATERIALS AND METHODS
Growth conditions for cell lines and bacterial strains.
Human microvascular endothelial cells (HMEC-1) (1) were cultured in MCBD131 cell culture media (Gibco/BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum, 10 ng of epidermal growth factor/ml, and 1 μg of hydrocortisone/ml. Escherichia coli strains were grown using Luria-Bertani broth or agar (Difco, Detroit, Mich.) with appropriate antibiotics (ampicillin, 100 μg/ml; kanamycin, 50 μg/ml). B. henselae strains were cultivated on heart infusion agar (Difco) supplemented with 5% rabbit blood or 1% bovine hemoglobin (chocolate agar) at 37°C in 5% CO2. For some experiments, B. henselae strains were incubated with supplemented MCBD131 at 37°C in 5% CO2. Antibiotic concentrations for cultivation of B. henselae strains were 200 μg/ml for streptomycin and 50 μg/ml for kanamycin where indicated. Plasmids and bacterial strains are described in detail in Table 1.
TABLE 1.
Description of plasmids and bacterial strains
| Plasmid or strain | Application or genotype | Reference |
|---|---|---|
| Plasmids | ||
| pUC19 | Ampr:lacZα | 42 |
| pICB.4D7 | pUC19 with 4.0-kb EcoRI B. henselae Houston-1 genomic DNA fragment isolated by virB2 hybridization | This work |
| pCB182 | Promoterless lacZ galK:Ampr | 30 |
| pCBlacZ | pCB182 with B. henselae htr P1 promoter | |
| pSIR4 | pCB182 with E. coli lacZ promoter in forward orientation | |
| pANT3 | Promoterless gfpmut3:Kanr | 19 |
| pVirBF | pCB182 with B. henselae virB promoter region in the forward orientation | This work |
| pVirBR | pCB182 with B. henselae virB promoter region in the reverse orientation | This work |
| pVBGFPF | pANT3 with B. henselae virB promoter region in the forward orientation | This work |
| pVBGFPR | pANT3 with B. henselae virB promoter region in the reverse orientation | This work |
| pVBDEL | pANT3 with 87-bp 5′ deletion of the B. henselae virB promoter region | This work |
| Strains | ||
| B. henselae | ||
| Houston-1 | ATCC 49882 | 24 |
| 882Str | Streptomycin-resistant laboratory-adapted strain of ATCC 49882 | 19 |
| E. coli | ||
| DH5α | endA1 hsdR17 (rK−mK+) glnV44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacIZYA-argF)U169 deoR (φ80dlacΔ[lacZ]M15) | |
| JM109 | F′ traD36 lacIq Δ(lacZ)M15 proA+B+/e14−(McrA−) Δ(lac-proAB) thi gyrA96 (Nalr) endA1 hsdR17 (rK−mK+) relA1 supE44 recA1 | |
| CB454 | F− ΔlacZ−lacY+galK rpsL thi recA56 | 30 |
Cloning of the virB promoter region.
To define potential promoter sequences of the B. henselae virB operon, a 422-bp virB2 DNA probe (derived from the 5′-most end of the virB operon) was constructed by PCR amplification of B. henselae Houston-1 genomic DNA using the primers VB2F and VB2R (29). The probe was digoxigenin-dUTP labeled for use in Southern blotting as described in the Genius System protocol (Boehringer Mannheim, Indianapolis, Ind.). Hybridized probe was detected using the Phototope-Star chemiluminescence detection kit (New England Biolabs, Beverly, Mass.). A 4.0-kb EcoRI band of B. henselae was identified by Southern blotting and targeted for cloning into pUC19. Potential E. coli clones of the 4.0-kb EcoRI fragment were screened by colony blotting using the virB2-digoxigenin probe under the same conditions as described above.
DNA sequencing.
To find the nucleotide sequence of the region upstream of the virB operon, internal and M13 universal primers were used to sequence the insert of pICB.4D7 (Table 1). DNA sequencing was performed by 35S-dATP labeling with the dideoxynucleotide termination system using Sequenase T7 DNA polymerase (Amersham Life Science, Cleveland, Ohio). DNA sequences were recorded and analyzed using DNasis software, version 2.5 (Hitachi, San Bruno, Calif.). The putative promoter inserts of the lacZ (pCB182) and green fluorescent protein (GFP) (pANT3) reporter constructs (described below) were determined by the same method using vector-specific primers flanking the inserted promoter region.
Promoter constructs.
To determine if the region upstream of the virB operon has promoter activity, a 362-bp fragment of DNA amplified by PCR using primers PVirF and PVirR (Fig. 1) constructed with XbaI sites was first cloned upstream of the lacZ reporter gene in pCB182 (30). Promoter orientations were confirmed by sequencing, and both the forward (pVirBF) and reverse (pVirBR) directions were obtained after transformation of E. coli CB454 (Table 1).
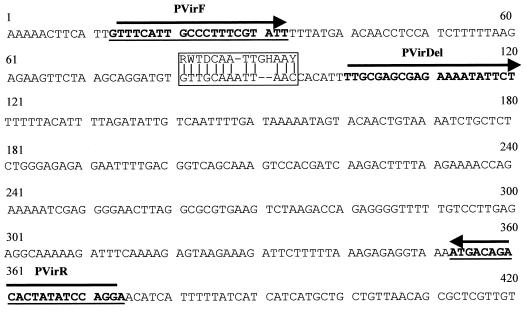
FIG. 1.
DNA sequence of the promoter region of the B. henselae virB operon. The vir box sequence of A. tumefaciens (above) and the homologous region in B. henselae are boxed (where R = A or G; W = A or T; D = A, G, or T; H = A, C, or T; and Y = C or T). The primers used to generate the reporter constructs are in bold. The virB2 start codon is located at position 352. The B. henselae nucleotide sequence is numbered as in GenBank.
A second set of reporter constructs was created using the same primers as above (PVirF and PVirR) and inserted into the XbaI site of pANT3, which contains a promoterless GFP gene (gfpmut3) which has been optimized for analysis by flow cytometry. Plasmid constructs ligated in both the forward (pVBGFPF) and reverse (pVBGFPR) orientations (Table 1) were obtained and confirmed by PCR and sequencing after transforming E. coli JM109. An additional clone was constructed using the same downstream primer (PVirR) in conjunction with a new upstream primer (PVirDel) to produce a PCR product lacking 87 bp of the 5′ region (Fig. 1). This amplicon was also inserted upstream of the gfpmut3 reporter gene in pANT3 (pVBDEL).
Transformation of B. henselae 882Str.
Transformations of B. henselae were performed as previously described (25). Briefly, 3-day-old B. henselae 882Str grown on streptomycin chocolate agar was harvested and washed three times in ice-cold 10% glycerol and resuspended in a 100-μl total volume at approximately 109 CFU/ml. To 40 μl of the B. henselae 882Str suspension 1.0 μg of plasmid DNA, derived from E. coli JM109 harboring GFP reporter constructs, was added, and the mixture was transferred to precooled 0.1-cm-gap-width cuvettes (Bio-Rad, Hercules Calif.). Cells were electroporated using a Bio-Rad Pulse Controller II for 4.6 ms with field strength equivalents of 12.5 kV/cm and a constant capacitance of 25 μF. Samples were placed in 1 ml of recovery broth (RPMI 1640 with glutamine, 1% HEPES buffer, 1% sodium pyruvate, 5% fetal calf serum [heat inactivated], 5% rabbit blood lysate) and incubated at 37°C with 5% CO2 for 7 h. Transformants were selected by growth on heart infusion agar–5% rabbit blood with kanamycin. Confirmation of the transformants as B. henselae 882Str was accomplished by extracting total DNA from each of the putative clones followed by PCR amplification using the B. henselae htrA gene primers CAT1 and CAT2 as previously described (3). Amplification of the kanamycin gene of pANT3 was also performed to confirm the presence of the plasmid using the primers TN903KN1 (5′-CCGATGCGCCAGAGTTTCTGAA-3′) and TN903KN2 (5′-ACCTATTAATTTCCCCTCGTCAAAA-3′) (25).
Flow cytometry.
All samples were run on a FACScan flow cytometer (Becton Dickinson) and analyzed with Cell Quest software (Becton Dickinson) to examine both immunostained bacteria and bacteria expressing GFP. For GFP production, B. henselae 882Str reporter constructs were cultured with HMEC-1 or in MCDB131 alone for 24 h at 37°C. Bacteria cultivated with HMEC-1 were treated with gentamicin (200 μg/ml) for 2 h to kill extracellular bacteria, and cells were harvested using 2 mM EDTA. Titration experiments followed by plating showed that this treatment was sufficient to kill all cell-free B. henselae. Infected HMEC-1 cells were lysed in 0.1% saponin, cell debris was collected by low-speed centrifugation, and the bacteria in the supernatant were subjected to flow cytometry. Suspensions of both cocultivated and cell-free bacteria were first differentiated using forward and side scatter so that gating and analysis would not include any remaining HMEC-1 cells or debris. The gated, viable bacteria were observed on a histogram plot for the presence of GFP (FL1) versus the bacterial cell counts. The detection settings for the FL1 channel were set with respect to the control samples. In the final analysis, histogram plots for the samples were overlaid for GFP expression.
Western blotting.
B. henselae Houston-1 was incubated in cell culture media alone or with HMEC-1 for 24 or 48 h at 37°C with 5% CO2. HMEC-1 cocultures were treated with gentamicin, as described for flow cytometry, and infected cells were harvested in 2 mM EDTA and lysed in 0.1% saponin. Cell debris was collected by low-speed centrifugation. Bacteria remaining in the supernatant from the HMEC-1 lysate and cell-free bacteria were lysed in 1× sample buffer (Novex, San Diego, Calif.). Samples were used for Western blotting by standard methods (40). Rabbit polyclonal serum to the 17-kDa antigen (diluted 1:200) was used and has previously been shown to recognize the 17-kDa antigen of B. henselae, Bartonella clarridgeiae, B. quintana, and Bartonella elizabethae (37). Immunoreactive bands were detected using goat anti-rabbit antibody conjugated with horseradish peroxidase (KPL, Gaithersburg, Md.) diluted 1:5,000 and reacted with the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (KPL).
Labeling of B. henselae Houston-1 with FITC.
B. henselae Houston-1 was cultured with HMEC-1 or in cell culture media alone for 4 days at 37°C. Cultures to be analyzed by flow cytometry were gentamicin treated, harvested, and lysed as described for the GFP samples. Both cell-associated and cell-free bacteria were incubated with either normal rabbit sera (NRS) or rabbit anti-17-kDa-antigen serum diluted 1:50 in PBST-BSA (136 mM NaCl, 2.7 mM KCl, 1.8 mM KH2PO4, 10.1 mM Na2HPO4, 0.5% bovine serum albumin [BSA], 0.1% Tween 20) for 1 h at 37°C. Samples were washed four times in PBST (without BSA). Bacteria were collected after each wash by centrifugation at 3,000 × g. Samples were incubated with goat anti-rabbit fluorescein isothiocyanate (FITC) conjugate (KPL) diluted 1:10 in PBST-BSA for 1 h at 37°C. Samples were washed four times in PBST with centrifugation and analyzed by flow cytometry.
Digital imaging of B. henselae 882Str GFP reporter constructs and FITC-labeled B. henselae Houston-1.
B. henselae bacteria expressing GFP or bacteria labeled with specific rabbit antiserum were examined by fluorescence microscopy and analyzed by digital imaging. B. henselae 882Str GFP reporter constructs or Houston-1 bacteria were incubated with HMEC-1 or in media alone on eight-well cell culture slides (Nunc, Inc., Naperville, Ill.). Extracellular bacteria were killed with gentamicin as for flow cytometry. Samples were fixed with 2% paraformaldehyde and either reacted with rabbit sera (described above) or analyzed directly for GFP. DNA was stained with antifade–4,6 diaminido-2 phenylindole (DAPI) (Vector, Burlingame, Calif.). Images of B. henselae 882Str GFP reporter constructs and B. henselae Houston-1 bacteria labeled with rabbit immune sera and FITC were captured using a Leitz Orthoplan 2 microscope with a charge-coupled capture device and the Smart Capture program (Vysis, Downer's Grove, Ill.) as previously described (25). GFP induction or FITC labeling was measured by pixel densities and reported as a ratio of GFP/DNA or FITC/DNA.
Nucleotide sequence accession number.
The DNA sequence of the promoter region of the B. henselae virB operon was appended to the existing B. henselae virB sequence deposited in GenBank under accession number U23447.
RESULTS
Identification of the putative virB promoter sequence.
Eight clones were identified that hybridized with the 422-bp virB2 probe. All eight clones contained an insert of approximately 4.0 kb that hybridized to the virB2 probe by Southern blotting (data not shown). Nucleotide sequencing upstream of the virB2 gene was performed, and analysis of the region revealed a 13-bp sequence located 259 nucleotides upstream of the start codon of virB2 that has homology to the vir box of A. tumefaciens (Fig. 1). The vir box sequence of A. tumefaciens has been shown to act as the −35 region and is necessary for transcription of the virB operon (9, 15). The B. henselae sequence aligns and matches 12 of the 14 residues (Fig. 1) found in the A. tumefaciens vir box consensus sequence, RWTDCAATTGHAAY (where R = A or G; W = A or T; D = A, G, or T; H = A, C, or T; and Y = C or T) (9). This putative promoter region was further analyzed in both E. coli and B. henselae using reporter gene constructs.
Induction of the GFP reporter gene by the B. henselae virB promoter region.
To determine if the region upstream of the virB operon has promoter activity, the 362-bp region defined by PvirF and PvirR (Fig. 1) was cloned upstream of the lacZ reporter gene of pCB182 in both the forward and reverse orientations. The new constructs (pVirBF or pVirBR) were used to transform E. coli CB454, and β-galactosidase assays were performed (43). The B. henselae htrA promoter and the E. coli lacZ promoter were used as positive controls, and pCB182 without an insert served as the negative control. Neither pVirBF or pVirBR directed transcription initiation of the β-galactosidase reporter gene to significant levels in E. coli CB454 compared to htrA or lacZ promoters (data not shown). This was not surprising, because the vir promoters of A. tumefaciens require the presence of an activator protein (VirG) for induction of the promoter (20). Transcription initiation from the A. tumefaciens virB promoter in E. coli has been reported to be inefficient in the absence of this transcription factor (20). Since similar transcription factors may be required for the B. henselae virB expression, the promoter assays were performed directly with B. henselae.
The same PCR product was inserted upstream of gfpmut3 in the promoterless vector pANT3 (19), and the new plasmids were used to transform E. coli JM109. Sequencing of selected clones was performed to confirm promoter orientations in the forward (pVBGFPF) and reverse (pVBGFPR) directions. Replication of plasmids in E. coli JM109 is believed to improve the subsequent transformation efficiency of B. henselae, perhaps by providing a restriction or modification that stabilizes plasmid DNA after entry into B. henselae (25). The plasmids were isolated from E. coli and used to transform B. henselae 882Str by electroporation. Transformants were selected on chocolate-kanamycin agar and were confirmed as B. henselae by PCR amplification of the htrA gene, and the presence of the plasmid was confirmed by PCR amplification of the kanamycin marker. Reporter constructs grown on chocolate-kanamycin agar were initially analyzed by direct fluorescence microscopy, and no production of GFP was noted (data not shown). Because the vir promoters of A. tumefaciens are induced by exposure to plant phenolic compounds (34), it is possible that a B. henselae virB promoter might require an activation signal. Since endothelial cells represent a natural target cell of B. henselae infection in humans, cocultivation of B. henselae with endothelial cells was performed to determine if these cells may serve as a suitable stimulus for virB activation.
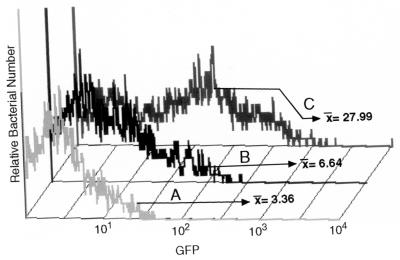
The reporter constructs were incubated with HMEC-1 cells and analyzed by flow cytometry. B. henselae-infected HMEC-1 cell cultures were treated with gentamicin, and HMEC-1 cells were lysed prior to flow cytometry to select for bacteria that have entered the endothelial cell. B. henselae pVBGFPF/882Str produced more GFP (Fig. 2C, mean fluorescence intensity of 27.99) than B. henselae pVBGFPR/882Str (Fig. 2B, geometric mean fluorescence intensity of 6.64) or B. henselae pANT/882Str (Fig. 2A, mean fluorescence intensity of 3.36). The flow-cytometric analysis indicates a fourfold increase of GFP expression from this promoter region in the forward orientation over that in the reverse orientation and eightfold more than the vector alone. This confirmed that the region is capable of initiation of transcription of gfpmut3 in B. henselae bacteria when cultured with HMEC-1. To examine and compare individual bacterial cells cultured with HMEC-1 with bacteria grown in media alone, digital analysis of fluorescence microscopy was performed.
FIG. 2.
Flow cytometry of GFP reporter constructs B. henselae pANT3/882Str (A), B. henselae pVBGFPR/882Str (B), and B. henselae pVBGFPF/882Str after coculture with HMEC-1 for 24 h (C). Samples were analyzed by flow cytometry as described in Materials and Methods. The geometric mean of the fluorescence intensity for each of the samples is designated by the x value.
Digital analysis of GFP induction by B. henselae promoter constructs.
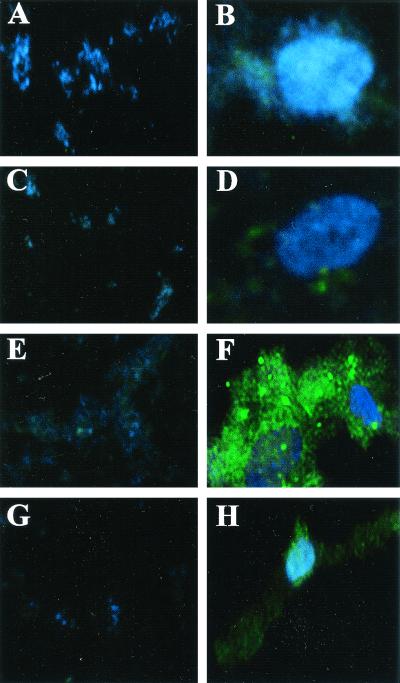
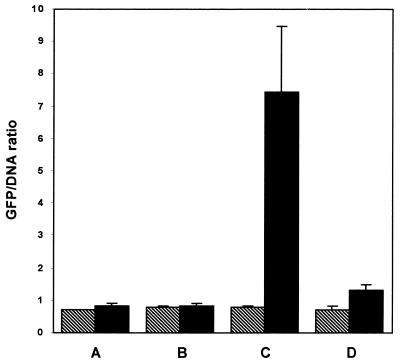
All B. henselae reporter constructs were incubated with HMEC-1 cells or in media alone on eight-well culture slides and analyzed by digital imaging as described in Materials and Methods. Coculturing of B. henselae pVBGFPF/882Str with HMEC-1 cells resulted in intracellular aggregates of bacteria producing greater amounts of GFP (Fig. 3F) than when they were incubated in media alone (Fig. 3E). B. henselae pANT3/882Str (Fig. 3A and B) and B. henselae pVBGFPR/882Str (Fig. 3C and D) produced little or no GFP in either the presence or absence of HMEC-1 cells. Intensely fluorescing pockets containing large numbers of B. henselae bacteria can be seen within HMEC-1 cells (Fig. 3F). These large aggregates of intracellular bacteria surrounded by a host cell membrane, termed invasomes, have been well documented (11, 12). To normalize reporter gene fluorescence to the amount of bacterial DNA, gfpmut3 expression is reported as a ratio of GFP (green) to DNA, visualized by DAPI staining (blue), as previously described (25). Intracellular B. henselae pVBGFPF/882Str from HMEC-1 cells produced almost ninefold more GFP than when incubated in media alone (Fig. 4C) and almost ninefold more than B. henselae pVBGFPR/882Str (Fig. 4B) and B. henselae pANT3/882Str (Fig. 4A) cultured in either media alone or with HMEC-1.
FIG. 3.
Fluorescence micrographs of B. henselae GFP reporter constructs. Panels A, C, E, and G show B. henselae GFP reporter constructs cultured in media alone. Panels B, D, F, and H show B. henselae GFP reporter constructs cocultured with HMEC-1. Reporter constructs shown are B. henselae pANT3/882Str (A and B), B. henselae pVBGFPR/882Str (C and D), B. henselae pVBGFPF/882Str (E and F), and B. henselae pVBDEL/882Str (G and H). Host cell and bacterial DNA is stained with DAPI. Overall magnification in each panel is ×400.
FIG. 4.
Digital analysis of fluorescence microscopy showing induction of GFP reported as a ratio of GFP (green fluorescence) to DNA (blue fluorescence). Promoter constructs were cultured in media alone and with HMEC-1 as indicated for 24 h before images were captured by digital fluorescence imaging. B. henselae clones were incubated in cell culture media alone (▧) or cocultured with HMEC-1 (▪). B. henselae GFP reporter constructs analyzed were B. henselae 882Str/pANT3 (A), B. henselae pVBGFPR/882Str (B), B. henselae pVBGFPF/882Str (C), and B. henselae pVBDEL/882Str (D).
An additional reporter construct was prepared that lacked 87 bp from the 5′ end of the promoter region including the sequence with homology to the A. tumefaciens vir box (Fig. 1). A PCR product was generated using the primers PVirDel and PVirR (Fig. 1) and inserted in the direction of gfpmut3 in pANT3 to generate pVBDEL. Intracellular B. henselae pVBDEL/882Str from HMEC-1 cells (Fig. 3H) appeared to produce slightly more GFP than when cultured in media alone (Fig. 3G), but the rise is not significant and is still sixfold less than B. henselae pVBGFPF/882Str cultured with HMEC-1 (Fig. 4). These data indicate that the 362-bp region upstream of the virB operon is able to function as a promoter in B. henselae and is induced inside HMEC-1. Furthermore, an 87-bp sequence located on the 5′ end of this region is required for both transcription initiation and intracellular induction.
Upregulation of the VirB protein of B. henselae by HMEC-1.
Although the promoter region of the virB operon can be activated when B. henselae is cultured with HMEC-1, the ability of endothelial cells to increase expression of VirB proteins was also investigated. The 17-kDa antigen was selected to be the marker of VirB protein expression because it is highly immunogenic in humans and the gene encoding this protein is in the center of the operon. To determine if there was any increase of expression of the 17-kDa antigen in intracellular B. henselae bacteria cultured with endothelial cells, a Western blot analysis was performed of cell lysates of B. henselae Houston-1 incubated in media alone (Fig. 5, lanes A and C) or with HMEC-1 (Fig. 5, lanes B and D) and reacted with rabbit anti-17-kDa antigen. At both 24 (Fig. 5, lanes A and B) and 48 (Fig. 5, lanes C and D) h, cocultured Houston-1 expressed two to three broad bands which reacted with the rabbit anti-17-kDa antigen serum that were absent in Houston-1 cultured in medium alone. The presence of at least two bands at this molecular mass is most likely due to the processed and unprocessed forms of the 17-kDa antigen that have been previously described (2).
FIG. 5.
Western blot of B. henselae Houston-1 cultured in media alone (lanes A and C) or with HMEC-1 (lanes B and D). Samples were cultured for 24 h (lanes A and B) or 48 h (lanes C and D). Molecular weight markers are listed at the left (given in thousands), and the various forms of the 17-kDa antigen are indicated by the arrows.
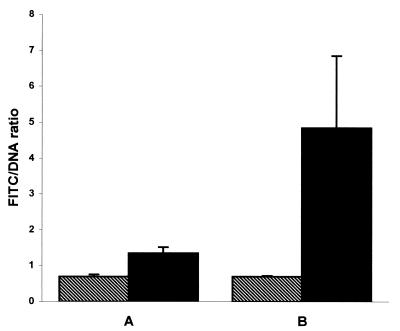
To confirm and quantify the level of expression of the 17-kDa antigen, B. henselae Houston-1 was incubated in media alone or with HMEC-1 and reacted with NRS or rabbit serum raised to the 17-kDa antigen and examined by fluorescence microscopy. Rabbit sera bound to B. henselae Houston-1 were detected by a goat anti-rabbit FITC conjugate, analyzed by digital imaging, and reported as a ratio of FITC to DNA (Fig. 6). B. henselae Houston-1 incubated in media alone reacted with anti-17-kDa-antigen serum labeled with FITC at levels similar to those seen when B. henselae Houston-1 cultured in media alone was reacted with NRS. However, FITC labeling of intracellular B. henselae Houston-1 from HMEC-1 that was reacted with anti-17-kDa-antigen serum was sevenfold greater than that of control B. henselae Houston-1 cultured in media alone and reacted with anti-17-kDa antigen serum (Fig. 6). Concentrated aggregates of intensely labeled bacteria were also observed that were similar to those seen in the GFP experiments and are again believed to influence the standard deviation. These data also agree with flow cytometry analysis of FITC-labeled B. henselae, in which the geometric mean fluorescence of intracellular B. henselae Houston-1 reacted with anti-17-kDa antigen serum was 72.03, compared to 15.54 for B. henselae Houston-1 in media alone reacted with the same serum (data not shown). All three techniques demonstrated an upregulation of the 17-kDa antigen of B. henselae Houston-1 after invasion of HMEC-1. This increase in protein expression also correlates with the induction of the virB promoter region when B. henselae GFP reporter constructs were cultured with HMEC-1.
FIG. 6.
Digital analysis of fluorescence microscopy using rabbit antiserum and FITC labeling of B. henselae Houston-1 cultured in media alone (▧) or with HMEC-1 (▪) for 5 days. Cultures were reacted with NRS (A) or with rabbit serum specific for the 17-kDa protein (B). Samples were reacted with antiserum, stained with FITC, and examined by fluorescence microscopy. Digital images were captured and reported as a ratio of FITC (green fluorescence) to DNA (blue fluorescence).
DISCUSSION
Type IV secretion systems are elaborate mechanisms that some gram-negative bacteria utilize to export products outside the bacterial cell to target host cells. The type IV system is a piliated multiprotein channel that spans both inner and outer bacterial membranes and has been identified in a number of animal, plant, and human pathogens. This secretion system is able to transport diverse types of macromolecules including both DNA and protein. The type IV system of Brucella suis is coded by the virB operon and is required for survival and replication within the host macrophage (13, 31). The ptl operon is required for the exportation of the pertussis toxin of Bordetella pertussis (41), and the virB operon of A. tumefaciens, the most characterized of the type IV systems, is responsible for the delivery of the tumor-inducing DNA (T-DNA) to plant cells (for a review, see reference 7). The virB operon of B. henselae encodes 10 genes, of which 8 have significant homology to the components of the type IV secretion system of A. tumefaciens (22, 29).
Several of the genes of the B. henselae virB operon also contain functional domains that are found in the A. tumefaciens virB operon. The Walker A NTPase binding domain of VirB4 and VirB11 and the peptidase cleavage site of VirB2 are found in both organisms (29). Although VirB2 of A. tumefaciens is posttranslationally processed at this peptidase cleavage site (16, 18), no evidence of B. henselae VirB2 processing has been reported. It is interesting that the virB5 gene has been replaced by the gene coding for the immunodominant 17-kDa antigen of B. henselae. In A. tumefaciens both VirB2 and VirB5 are subunits of the conjugative pilus (28). The presence of a signal sequence in the 17-kDa antigen suggests that this protein is localized to the membrane of the bacterium (2). Strong immunoreactivity of patient sera to the 17-kDa antigen further strengthens the possibility that this protein is exposed on the outer surface of the bacterium, possibly in conjunction with pili.
A 14-bp section of the B. henselae virB promoter region shares homology to the −35 promoter sequence (vir box) of the A. tumefaciens vir promoters (Fig. 1). The lack of GFP production by B. henselae pVBDEL/882Str (Fig. 3 and 4) demonstrates that an 87-bp region containing this sequence is required for virB promoter activity in B. henselae. The vir box is highly characterized in A. tumefaciens and is present in each of the promoters of the Ti (tumor inducing) plasmid vir operons (10). The A. tumefaciens vir box acts as a −35 promoter sequence and facilitates the binding of phosphorylated VirG, which is the activator protein of a two-component regulation system (9, 15, 20, 23, 33). The binding of VirG to the vir box is required to allow the alpha subunit of RNA polymerase to bind, form the holoenzyme, and initiate transcription (20). In addition, the vir boxes of B. henselae and A. tumefaciens share an A-T-rich region in the center of the sequence which is common to activator binding regions of other two-component regulatory systems and is believed to be necessary in DNA-protein interactions (15). Further experiments are under way to determine the role of the putative vir box in transcription and intracellular induction of the virB operon of B. henselae.
The possibility of other promoter regions initiating transcription of individual or multiple virB genes has not been eliminated. However, no additional known promoter sequences upstream of the individual B. henselae virB genes have been described (2, 22, 29). The absence of sizable intergenic regions between most B. henselae virB genes, considered together with the colinear orientation of the B. henselae virB genes compared to the A. tumefaciens virB operon, suggests that the B. henselae virB operon is polycistronic and is transcribed from a single promoter.
In addition to the virB operon of A. tumefaciens, the regulation of other type IV secretion system operons has been well studied. Transcription of the ptl operon of Bordetella pertussis is under control of the ptx promoter, which is activated by a two-component regulatory system (27, 35). The ptl genes of Bordetella pertussis are immediately downstream of the ptx operon, which codes for the production of the pertussis toxin proteins (41). The promoter upstream of the ptx operon is regulated by the BvgA and BvgS two-component regulatory system (27). This same promoter and regulation system is also responsible for the transcription of the Bordetella pertussis ptl operon that codes for the type IV secretion system and is necessary to export the pertussis toxin from the cell (4). Both the ptx and ptl genes are cotranscribed as a single polycistronic mRNA from this promoter. The induction of GFP by the virB promoter region of intracellular B. henselae but not in media alone suggests that a similar activator system may also be at work in this organism. The inability of the B. henselae virB promoter sequence to induce a reporter gene in E. coli further strengthens the theory that a Bartonella-specific protein is needed for initiation of transcription. However, we have observed that the virB promoter is activated by other cell types including HeLa (epithelial origin), HGF-1 (fibroblast origin), and differentiated THP-1 (human macrophage), suggesting that induction is not endothelial cell specific (data not shown).
Coupled with the initiation of transcription by the virB promoter region, the expression of genes encoding potential surface proteins is also of interest. The 17-kDa antigen, encoded by a gene found in the middle of the virB operon of B. henselae, is expressed at low levels when grown on laboratory media. A faint immunoreactive protein at 17 kDa can be noted on Western blots of whole-cell lysates of B. henselae grown on laboratory media when they are reacted with sera from patients diagnosed with BAD. However, the 17-kDa band was considerably stronger in intracellular B. henselae Houston-1 from HMEC-1 (Fig. 5). The strongest evidence for the upregulation of the 17-kDa antigen gene by an external stimulus is the increased FITC labeling of intracellular B. henselae Houston-1 reacted with the 17-kDa-antigen-specific rabbit sera (Fig. 6). This increase of protein expression induced by human endothelial cells helps explain why the 17-kDa antigen is not expressed at high levels in bacteria grown on laboratory media yet antibodies to this protein appear at high titers in humans who have been diagnosed with BAD. This would suggest that the 17-kDa antigen is expressed by the bacterium at high levels during infection, and due to its probable presence on the cell surface it represents a good target for the humoral immune response.
It is not currently known what specific environmental stimulus is required to activate the virB operon. The results of each of the experiments described in this study show that the virB operon of B. henselae is activated in intracellular bacteria. The requirement for host cell invasion for virB activation has not yet been defined. However, experiments conducted using GFP constructs to infect HMEC-1 in the absence of gentamicin treatment resulted in the presence of multiple-cell-associated bacteria that did not exhibit fluorescence (unpublished data). Thus, invasion and not merely attachment appears to be required for induction of the virB operon of B. henselae, and it is likely that the intracellular environment of the host cell contains the specific stimulus which is necessary for induction.
B. henselae is able to adhere to and invade a variety of different cell types including epithelial and endothelial cells (5, 6, 12). However, the interaction of B. henselae with endothelial cells is most interesting and results in proliferation and ultimately angiogenesis. The proliferative activity of B. henselae has been reported as both a membrane-associated (8) and a secreted diffusible (21) factor. The specific B. henselae protein responsible for this proliferation has not yet been identified. Additionally, it is not known how the effector molecule is delivered to the host endothelial cell. If the proliferative factor is exported through a type IV secretion system, this factor may be present in membrane preparations at high enough concentrations to induce cell proliferation, as reported by Conley et al. (8), and could also be secreted from the bacterium, as reported by Maeno et al. (21). The delivery of the pertussis toxin and T-DNA by type IV secretion systems indicates that this system plays an important role in delivery of biologically significant effector molecules in other bacteria. Similarity of gene structure and regulation of the virB operon of B. henselae to those of other bacteria suggests that this operon may play a similar transport role in delivery of biologically important effector molecules to endothelial cells.
The specific function of each of the B. henselae virB genes is still unknown; however, the presence of an operon with significant homology to other type IV secretion systems suggests that some effector molecule is secreted from the bacteria into the environment or host cell. The expression of the virB operon is upregulated in intracellular bacteria and appears to require a Bartonella-specific component. Although the specific activator molecule is not known, the upregulation of the 17-kDa antigen demonstrates that this operon is expressed after stimulation by endothelial cells. It is tempting to couple this secretion system with the delivery of the angiogenic factor, because of the ability of endothelial cells to induce VirB expression and the ability of B. henselae to proliferate endothelial cells. Currently B. henselae virB mutants are being constructed to determine the role this operon plays in attachment, invasion, or proliferation of endothelial cells. Although the exact mechanism of angiogenesis is probably complex and requires the expression of a variety of proteins, the role of virB operon-encoded proteins in this process should be considered.
ACKNOWLEDGMENTS
This work was supported by NIH grants DA05866-03 and R29-AI38178.
We thank Anthea Lee and the laboratory of Stanley Falkow for their generous gift of pANT3. We thank Thomas Lawley of Emory University and The Biological Products Branch, Centers for Disease Control and Prevention, for providing the HMEC-1 cell line used in these studies. We also thank Sandra Resto-Ruiz for providing the E. coli pCBlacZ/CB454 and pSIR4/CB454 β-galactosidase reporter constructs.
REFERENCES
- 1.Ades E W, Candal F J, Swerlick R A, George V G, Summers S, Bosse D C, Lawley T J. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B, Lu E, Jones D, Regnery R. Characterization of a 17-kilodalton antigen of Bartonella henselae reactive with sera from patients with cat scratch disease. J Clin Microbiol. 1995;33:2358–2365. doi: 10.1128/jcm.33.9.2358-2365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson B, Sims K, Regnery R, Robinson L, Schmidt M J, Goral S, Hager C, Edwards K. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol. 1994;32:942–948. doi: 10.1128/jcm.32.4.942-948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker S M, Masi A, Liu D F, Novitsky B K, Deich R A. Pertussis toxin export genes are regulated by the ptx promoter and may be required for efficient translation of ptx mRNA in Bordetella pertussis. Infect Immun. 1995;63:3920–3926. doi: 10.1128/iai.63.10.3920-3926.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batterman H J, Peek J A, Loutit J S, Falkow S, Tompkins L S. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun. 1995;63:4553–4556. doi: 10.1128/iai.63.11.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess A W, Anderson B E. Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb Pathog. 1998;25:157–164. doi: 10.1006/mpat.1998.0223. [DOI] [PubMed] [Google Scholar]
- 7.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conley T, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 9.Das A, Pazour G J. Delineation of the regulatory region sequences of Agrobacterium tumefaciens virB operon. Nucleic Acids Res. 1989;17:4541–4550. doi: 10.1093/nar/17.12.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Stachel S, Ebert P, Allenza P, Montoya A, Nester E. Promoters of Agrobacterium tumefaciens Ti-plasmid virulence genes. Nucleic Acids Res. 1986;14:1355–1364. doi: 10.1093/nar/14.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehio C. Interactions of Bartonella henselae with vascular endothelial cells. Curr Opin Microbiol. 1999;2:78–82. doi: 10.1016/s1369-5274(99)80013-7. [DOI] [PubMed] [Google Scholar]
- 12.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 13.Foulongne V, Bourg G, Cazevieille C, Michaux-Charachon S, O'Callaghan D. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect Immun. 2000;68:1297–1303. doi: 10.1128/iai.68.3.1297-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia F U, Wojta J, Broadley K N, Davidson J M, Hoover R L. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol. 1990;136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 15.Jin S G, Roitsch T, Christie P J, Nester E W. The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990;172:531–537. doi: 10.1128/jb.172.2.531-537.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones A L, Lai E M, Shirasu K, Kado C I. VirB2 is a processed pilin-like protein encoded by the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1996;178:5706–5711. doi: 10.1128/jb.178.19.5706-5711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler J E, Quinn F D, Berger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 18.Lai E M, Kado C I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A K, Falkow S. Constitutive and inducible green fluorescent protein expression in Bartonella henselae. Infect Immun. 1998;66:3964–3967. doi: 10.1128/iai.66.8.3964-3967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohrke S M, Nechaev S, Yang H, Severinov K, Jin S J. Transcriptional activation of Agrobacterium tumefaciens virulence gene promoters in Escherichia coli requires the A. tumefaciens rpoA gene, encoding the alpha subunit of RNA polymerase. J Bacteriol. 1999;181:4533–4539. doi: 10.1128/jb.181.15.4533-4539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeno N, Oda H, Yoshiie K, Wahid M R, Fujimura T, Matayoshi S. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 22.Padmalayam I, Karem K, Baumstark B, Massung R. The gene encoding the 17-kDa antigen of Bartonella henselae is located within a cluster of genes homologous to the virB virulence operon. DNA Cell Biol. 2000;19:377–382. doi: 10.1089/10445490050043344. [DOI] [PubMed] [Google Scholar]
- 23.Pazour G J, Das A. virG, an Agrobacterium tumefaciens transcriptional activator, initiates translation at a UUG codon and is a sequence-specific DNA-binding protein. J Bacteriol. 1990;172:1241–1249. doi: 10.1128/jb.172.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resto-Ruiz S I, Sweger D, Widen R H, Valkov N, Anderson B E. Transcriptional activation of the htrA (high-temperature requirement A) gene from Bartonella henselae. Infect Immun. 2000;68:5970–5978. doi: 10.1128/iai.68.10.5970-5978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricketts W. Clinical manifestations of Carrion's disease. Arch Intern Med. 1949;84:751–781. doi: 10.1001/archinte.1949.00230050087005. [DOI] [PubMed] [Google Scholar]
- 27.Roy C R, Miller J F, Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989;171:6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Eisenlohr H, Domke N, Angerer C, Wanner G, Zambryski P C, Baron C. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J Bacteriol. 1999;181:7485–7492. doi: 10.1128/jb.181.24.7485-7492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmiederer M, Anderson B. Cloning, sequencing, and expression of three Bartonella henselae genes homologous to the Agrobacterium tumefaciens VirB region. DNA Cell Biol. 2000;19:141–147. doi: 10.1089/104454900314528. [DOI] [PubMed] [Google Scholar]
- 30.Schneider K, Beck C F. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene. 1986;42:37–48. doi: 10.1016/0378-1119(86)90148-4. [DOI] [PubMed] [Google Scholar]
- 31.Sieira R, Comerci D J, Sanchez D O, Ugalde R A. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J Bacteriol. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith K J, Skelton H G, Tuur S, Larson P L, Angritt P. Bacillary angiomatosis in an immunocompetent child. Am J Dermatopathol. 1996;18:597–600. doi: 10.1097/00000372-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Stachel S E, Nester E W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986;5:1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stachel S E, Zambryski P C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986;46:325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- 35.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 36.Stoler M H, Bonfiglio T A, Steigbigel R T, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983;80:714–718. doi: 10.1093/ajcp/80.5.714. [DOI] [PubMed] [Google Scholar]
- 37.Sweger D, Resto-Ruiz S, Johnson D P, Schmiederer M, Hawke N, Anderson B. Conservation of the 17-kilodalton antigen gene within the genus Bartonella. Clin Diagn Lab Immunol. 2000;7:251–257. doi: 10.1128/cdli.7.2.251-257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tappero J W, Mohle-Boetani J, Koehler J E, Swaminathan B, Berger T G, LeBoit P E, Smith L L, Wenger J D, Pinner R W, Kemper C A, et al. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA. 1993;269:770–775. [PubMed] [Google Scholar]
- 39.Tappero J W, Perkins B A, Wenger J D, Berger T G. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev. 1995;8:440–450. doi: 10.1128/cmr.8.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 43.Youngman P. Use of transposon and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. New York, N.Y: John Wiley & Sons; 1990. pp. 221–259. [Google Scholar]