Abstract
Issues.
The Cannabis sativa L. plant contains hundreds of phytocannabinoids, but putatively of highest importance to public health risk is the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC), which is associated with risk for cannabis use disorder, affective disturbance, cognitive harm and psychomotor impairment. Recently, there has been an increase in the use and availability of concentrated cannabis products (or ‘concentrates’) that are made by extracting cannabinoids from the plant to form a product with THC concentrations as high as 90–95%. These products are increasingly popular nationwide. The literature on these widely available high potency concentrates is limited and there are many unknowns about their potential harms.
Approach.
This review covers the state of the research on cannabis concentrates and behavioural health-related outcomes and makes recommendations for advancing the science with studies focused on accurately testing the risks in relation to critical public and behavioural health questions.
Key Findings.
Data point to unique behavioural health implications of concentrate use. However, causal, controlled and representative research on the effects of cannabis concentrates is currently limited.
Implications.
Future research is needed to explore chronic, acute and developmental effects of concentrates, as well as effects on pulmonary function. We also highlight the need to explore these relationships in diverse populations.
Conclusion.
While the literature hints at the potential for these highly potent products to increase cannabis-related behavioural health harms, it is important to carefully design studies that more comprehensively evaluate the impact of concentrates on THC exposure and short- and long-term effects across user groups.
Keywords: cannabis concentrate, marijuana, delta-9-tetrahydrocannabinol, tetrahydrocannabinol
Introduction
The Cannabis sativa plant contains hundreds of phytocannabinoids, but delta-9-tetrahydrocannabinol (THC) is the cannabinoid most associated with public-health risks, such as cannabis use disorder (CUD), affective disturbance, cognitive harm and psychomotor impairment [1]. Over the last several decades, the potency of THC in recreational cannabis has increased substantially in the USA and worldwide [2–4]. There has also been an increase in the availability of concentrated cannabis (or ‘concentrates’), which are made by extracting cannabinoids from the plant to form a product with THC concentrations that typically range from 52 to 69% THC, but can be as high as 90–95% [5–7]. In contrast, the average potency of state marketplace flower products falls in the range of 16–21% THC [7–9]. Although we are lacking high-quality, nationally or internationally representative data on the prevalence of concentrate use, state-level data from both Colorado and Washington suggest that concentrates are increasingly popular [10,11]. For example, striking point-of-sale data provided by Colorado recreational and medical dispensaries indicate that sales of concentrates rose 444% during the first 4 years of legalisation (2014–2017) (Note: The State of Colorado Marijuana Enforcement Division changed their reporting metrics after 2017; more current data on increases in concentrates sales are not publicly available.) [12].
Concentrates are projected to be one of the fastest growing categories of cannabis products nationally and globally, with sales estimated to reach between $13 and $15 billion annually in the next 2 years [13]. It is unsurprising that the past 5 years have seen a call for increased monitoring of concentrate use worldwide, as well as research concerning methods of concentrate administration, motivations for use and short- and long-term effects and potential harms [10,14]. However, barriers to research with these cannabis products have not allowed a full assessment of risks, leaving a major gap in our ability to quantify the impact of these high potency products. The research gap is in part due to the relative novelty of wide-spread availability of these concentrated forms of cannabis and in part due to conflicts between federal and state laws in regards to the legality of these products, which prevent researchers, even in states where such products are sold and marketed, from freely conducting research studies on cannabis concentrates [15]. Below we review the state of the research on cannabis concentrates and behavioural health-related outcomes and make recommendations for advancing the science to accurately quantify risks and improve public health regulations surrounding concentrate use.
Methods
Between January and October 2020, separate literature searches were conducted utilising Google Scholar, Proquest, PsychInfo and a pre-existing cloud-based Mendeley citation repository/library with search terms separately or in combination (described in Appendix S1, Supporting Information). For example, a search may have consisted of ‘high potency’ alone or in combination with ‘cannabis’ or ‘marijuana’. All papers identified as potentially relevant to concentrates were added to the Mendeley shared library and coded with the tag ‘concentrate’. One hundred and eighty-five unique papers/resources were included as long as they presented concentrate-relevant findings that were not previously accounted for and/or were the most up-to-date review or meta-analysis in a specific topic area. Based on this process, the authors convened to categorise included studies through consensus and thus identified three overarching areas to review: production/extraction/administration; pharmacology and biomechanisms and behavioural health effects. Behavioural health effects were subdivided further into six primary topical areas: intoxication and impairment; mood and affective regulation; cannabis use disorder; adolescent concentrate use; associations with psychosis; and sex differences. Finally, based on our overall interpretation of the extent literature, the authors propose specific research areas of greatest need and promise for future directions in cannabis concentrate research.
Production, Extraction and Administration
Production and extraction
There are several approaches to producing concentrates, usually involving solvent-based or so-called solventless extraction to isolate THC from raw cannabis [16]. In solvent-based extraction, liquid butane passes through plant material and is then extracted or evaporated from the solution, resulting in a high concentration of THC [6]. In ‘solventless’ (Note: Despite the fact that water is in fact an inorganic solvent, this type of extraction method is nonetheless referred to as ‘solventless’ by industry standard.) extraction, plant matter is agitated (sometimes using ice-cold water, which causes trichomes to become brittle and break off) and sifted through various filters. This isolates the high-potency plant material, which is then pressed between heated plates in a hydraulic press [6]. There are other less-common extraction methods, most notably CO2 and dry processing methods involving dry ice that freezes trichome structures within the plant [6]. Figure 1 shows the extraction, production and administration process for cannabis concentrates.
Figure 1.
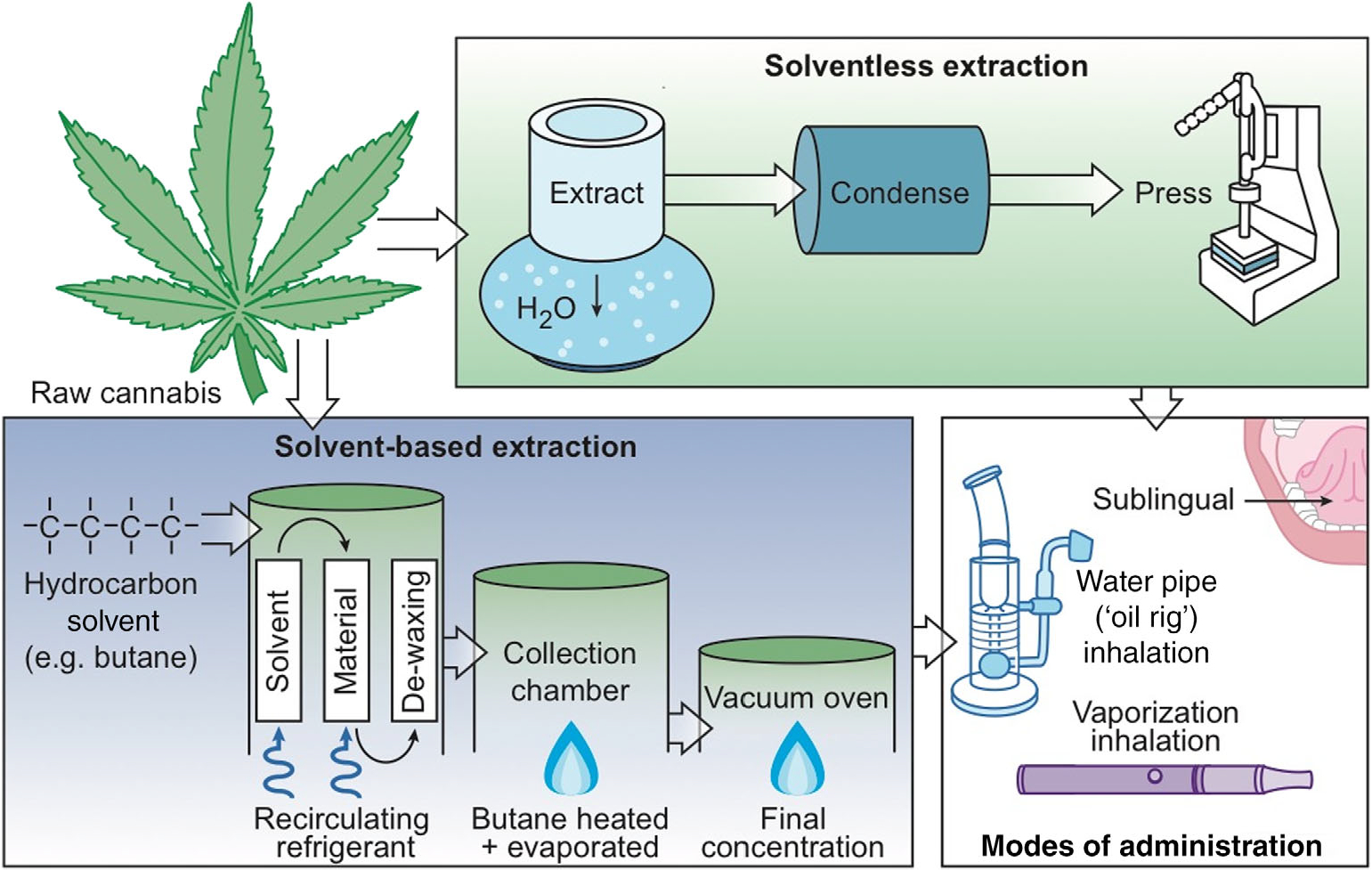
Extraction, production and administration of cannabis concentrates. In solvent-based extractiona (‘Solvent-based extraction’ box), liquid butane passes through organic plant material and is then either extracted or evaporated from the solution, resulting in a high concentration of THC. Increasingly the cannabis industry is moving towards ‘solventless’ extraction (‘Solventless extraction’ box), in which plant matter is agitated then sifted through various filters with the use of cold water, isolating high potency plant rosins that are then pressed between two heated plates. Concentrates can be administered (‘Modes of administration’ box) orally (sublingual) or via inhalation using a water pipe or vaporiser. aDespite the fact that water is in fact an inorganic solvent, extraction methods that utilise water as opposed to a hydrocarbon solvent are nonetheless referred to as ‘solventless’ by industry standard.
THC is frequently the focus of extraction, but other cannabinoids, such as cannabidiol (CBD), can be extracted and concentrated via these methods. The average THC potency of cannabis concentrate has been reported at 52% [17], but can be over 70–95% [5,6,17,18]. Differences in concentrate production methods result in a variety of product consistencies, producing a range of materials and product types [19], including butane hash oil, wax, live resin, shatter (created via solvent-based extraction) and hash and bubble oil (created via water-based, ‘solventless’ extraction) [6,19,20].
Concentrate contaminants and other production risks.
While regulated legal markets have firm guidelines for testing and identification of contaminants in cannabis products, there are still risks associated with concentrate production, including high levels of contaminants and flash fires or explosions during production [16], especially outside of legal markets. Raber and colleagues compared solvent-extracted concentrates with water-extracted (‘solventless’) concentrates and found that while there was no significant difference in potency between the two concentrate types, over 80% of the solvent-extracted samples were contaminated by residual solvents (e.g. isopentane), pesticides (e.g. paclobutrazol) [6], methacrolein and benzene (formed through the degradation of terpenes) [19]. A review of potency and contamination studies revealed that naturally occurring contaminants, such as fungi, mould and bacteria, have also been found in concentrate samples at unsafe levels, as well as other contaminants, such as heavy metals leached from soils, pesticides and fertilisers [19,21]. While these problems exist across forms of cannabis, they can be exacerbated in concentrate products. Furthermore, it is challenging to compare levels of toxicity across classes of contaminants due to variations in dosing and concentration. For instance, a high level of mould intoxication may or may not be more dangerous than a low level of heavy metal contamination. Unfortunately, while the literature is growing on this important topic, most work focuses on a specific class of contaminant and more data are needed in order to make cross-class comparisons.
Administration of concentrates
These concentrate product formulations are linked with various methods of administration. Concentrates can be consumed orally (sublingual) but are most often consumed via inhalation using a water pipe or vaporiser, providing the fastest onset of effects (typically within 90 s), the shortest duration of peak effects (60–90 min) [22,23], and are typically referred to as dabbing or taking a dab. Administration via a water-pipe, often referred to as an oil rig, involves contact between the concentrate and a small heated surface, referred to as a nail [6,19]. The small surface can be electronically heated (using an e-nail) to regulate temperature, or alternatively made of titanium, ceramic or quartz and manually heated using a blowtorch [6,19]. Similar to concentrate extraction methods, these methods of administration can be dangerous, with some cases of acute lung injury [24–28], as well as injury due to explosion or fire [29–31].
Inhalation via vaporisation is a similar process converting cannabinoids to vapor through conductive (direct contact between cannabis and heating element) or convective (heated air passes over cannabis) heating. Although data are preliminary, certain forms of vaporisation are perceived to have fewer negative effects compared to smoking, such as sore throat, chest tightness, coughing and wheezing or risk for cancer [32]. Vaporiser technologies attempt to reduce the respiratory risks of inhalation through processes that heat the entire plant without igniting it, and thus release cannabinoid in a vapor containing fewer combustion by-products. A handful of studies have attempted to isolate the respiratory risk associated with vaporisers and demonstrate some reduction of harms [33–37]. However, inhalation via ‘vape pens’ may introduce additional risks as highlighted by the current rise in vaping-related adverse medical outcomes, including e-cigarette, or vaping, product use-associated lung injury (EVOLI). Although e-cigarette use and vitamin E additives are of pervasive concern in relation to EVOLI, around 85% of EVOLI patients report vaping products containing THC [38,39]. In addition, there are potential pulmonary risks of inhaling oil-based and extracted cannabis products that may contain even trace amounts of containments. For example, a recent case study documented an incidence of severe pneumonitis with acute hypoxic respiratory failure in a healthy 18-year-old female concentrate user, where regular concentrate use/butane hash oil inhalation over 3 years (reported dosage of 1–2 g per day) was reported as the cause of her lung injury [24]. In sum, the increased availability of these high potency products has produced a shift in production methods and in the available modes of cannabis administration, which may impact the risks of consumption in users of cannabis concentrate.
Pharmacology and Biomechanisms of Cannabinoids in Concentrates
Regardless of the mode of administration, THC is an exogenous cannabinoid that interacts with the body’s endocannabinoid system (eCS), an internal homeostatic system associated with immune and stress responses as well as many other physiological processes, such as appetite, mood and cognition [40–42]. The eCS includes endogenous cannabinoid neurotransmitters (anandamide and 2-Arachidonoylglycerol) and exogenous cannabinoids that come from the cannabis plant, both of which bind to cannabinoid receptors CB1 and CB2 [43].
THC is a partial agonist for CB1 and CB2 receptors, and THC has less efficacy at CB1 and CB2 receptors compared to synthetic full receptor agonists (e.g. CP55940 and WIN55212), thus its activity at these receptors depends on density of receptors, concentration of endogenous agonists and receptor-ligand coupling efficiencies (i.e. it can act as an antagonist in some tissues) [44–46]. Further, THC has a stimulating effect on dopamine release [46–50]. CB1 receptors exhibit significant density in brain regions that are known to be involved with reward and addiction as well as cognitive function (e.g. amygdala, prefrontal cortex, cingulate cortex, nucleus accumbens, ventral tegmental area and others) [44–46], highlighting THC’s role in the psychoactive and sometimes addictive effects of cannabis in humans. Research demonstrating that THC and synthetic CB1 receptor agonists are self-administered in animals [45], as well as evidence that CB1 blockade prevents intoxication [51], suggests that these processes alter brain reward function in a dose-dependent fashion. Studies also demonstrate the downregulation of cortical CB1 receptors in chronic cannabis users acutely, but that CB1 activity returned to normal levels after a 4-week cannabis abstinence period [52]. These findings suggest chronic cannabis use may cause changes in CB1 receptor density, which is potentially related to tolerance and dependence. However, the time course and severity of these underlying neural responses and their links to long-term cannabis use behaviours among concentrate users remain unstudied.
Although THC likely exerts the majority of its psychoactive effects through its actions at CB1, THC also acts as a partial agonist at CB2 receptors. CB2 receptors have been found in low levels in reward-related brain regions, where their activation appears to reduce dopaminergic activity [53,54]. CB2 receptors are also thought to be related to immune and inflammatory functions in the brain and the body [45,55–57], and in the maintenance and proliferation of neural cells [57,58]. In contrast to CB1, which is abundantly expressed on neurons throughout the central nervous system [59], CB2 is mainly distributed in peripheral and immune cells [60].
Finally, there are a number of other minor cannabinoids, such as CBD that are found in recreational cannabis products, including concentrates. CBD has numerous purported mechanisms of action in the brain and body, and we do not yet have a complete understanding of its effects. Unlike THC, CBD appears to have low affinity for endogenous cannabinoid receptors, but is able to antagonise CB1 and CB2 receptor agonists [61], and acts as a negative allosteric modulator of CB1 [62]. In addition, some data indicate the CBD may inhibit the metabolism and effects of THC [44,63–66]. Other cannabinoids and terpenes have also been proposed to have varying synergistic relationships [66]. Overall however, research on the ‘entourage effects’ [67] of these numerous components of cannabis is still nascent, particularly for concentrates.
The existing data on THC binding to cannabinoid receptors have largely been conducted in animal models and in the context of lower potency cannabis. It is possible, but currently unknown, whether the acute or chronic administration of concentrates exacerbates these well-established CB1 or CB2 receptor-based mechanisms and further whether any meaningful pharmacological differences exist across the various concentrate formulations and modes of administration described above.
Behavioural Health Effects of Concentrate Use
Intoxication and impairment
Acute effects of THC can include intoxication, increases in subjective reward and impairment to executive function, impulse control, attention and psychomotor function [68–70]. Together these effects can increase risky behaviour during intoxication [71,72]. For example, research demonstrates that exposure to higher doses of THC is associated with ‘riskier speed-accuracy trade-offs’ [55] and premature responses [56] in laboratory-based cognitive tasks. At present, there are limited data concerning the effects of cannabis concentrate products on subjective intoxication and impairment within neurocognitive and psychomotor performance domains. The potential for increased risk-taking combined with acute impairment is concerning from a public health standpoint, particularly regarding driving under the influence of cannabis. However, concentrates may or may not intensify these acute risks, due to tolerance effects, and it is unclear whether accurate inferences about the effects of concentrates can be made from studies of the effects of lower potency cannabis products on intoxication and impairment.
Some studies testing primarily cannabis flower have demonstrated a relationship between blood THC concentration and psychomotor or driving-simulation performance, but results have been mixed [70,73,74]. It is worth noting, however, that the THC blood levels in these studies are considerably lower than the blood levels observed in concentrates users. For example, in one study exploring the correlation between THC-positive urine samples and field sobriety tests, the mean THC concentration was 9 ng/mL (range 2–60 n/mL) [74], whereas blood-THC immediately after concentrate use has been reported at an average of 321 n/mL [23]. Other data suggest that cannabis users can at least partially self-titrate their use and modulate THC exposure acutely according to the THC concentration of the particular product [23,75]. For example, in Bidwell et al. [23], users of both 70 and 90% THC concentrates showed similar THC blood levels. However, there may be a limit to the role that self-titration can play among users of very high potency products. In this study, blood levels after use of concentrates (either 70 or 90% THC) were over two-times higher than blood levels after use of flower products ranging from 16 to 24% THC (i.e. mean plasma THC levels were 321 ng/mL in concentrate users and 140 ng/mL in flower users).
Quantifying the extent of THC exposure (via well-timed cannabinoid biomarkers) that occurs during both acute and chronic concentrate use is critical to evaluating their effects. It is unclear whether, or to what extent, putatively higher THC blood levels after use of high-potency cannabis actually increases risks for acute impairment and/or promotes risk-taking behaviour compared to average potency products. Bidwell et al. took a novel approach to this question, leveraging a mobile pharmacology laboratory to collect some of the first observational data quantifying the acute effects of concentrate use [23]. In this naturalistic acute concentrate administration study, concentrate users achieved significantly higher blood-THC levels (321 ng/mL in concentrate users compared to 140 ng/mL in flower users), but demonstrated similar intoxication, cognitive and psychomotor effects compared to flower cannabis users. These data suggest several possible interpretations: (i) concentrate users may develop significant tolerance to the effects of THC; (ii) cannabinoid receptors may become saturated with THC (a CB1 partial agonist) at higher levels, beyond which there is a diminishing impact of additional THC [76]; and (iii) there may be individual differences among users in terms of metabolism or sensitivity to cannabis, which might be related to genetics or other pre-existing biological differences [77,78].
Tolerance
It is important to note that the objective quantification of THC exposure may not directly link with acute intoxication and impairment, likely due to tolerance that develops to the effects cannabis among regular and/or heavy cannabis users [52,79]. Consistent with rodent studies showing CB1 receptor downregulation following chronic cannabis exposure [80], a human positron emission tomography study indicated that human subjects who chronically smoke cannabis experience selective downregulation of CB1 receptors in cortical brain areas, and that CB1 receptor density can return to normal following 4 weeks of monitored cannabis abstinence [52]. In addition, a systemic review of the human evidence on the development of tolerance following regular cannabis use showed that regular cannabis users experience behavioural and physiological tolerance with repeated cannabis exposure (with cognitive performance showing the highest degree of tolerance), and that the acute intoxicating, psychotomimetic and cardiac effects of cannabis show partial tolerance [81]. Thus, it will be important to dissect the possible impact of tolerance among regular consumers of high potency cannabis products and accurately evaluate public health risks and time course of intoxication via concentrates in different types of users (e.g. occasional vs. regular). For example, in the Bidwell study [23], a brief psychomotor battery was administered several times after acute concentrate or cannabis flower use to explore the time course of cognitive and motor impairment. Both concentrate and flower users showed significant psychomotor impairment in several domains (e.g. balance, reaction time) immediately after consuming cannabis, but their performance returned to baseline after 1 h [23,82]. These findings suggest that even if overall tolerance develops among high-potency users, regular (weekly to daily) users still experience initial impairment in select psychomotor domains immediately after use, which warrants further investigation.
Mood and affective regulation
Whereas cannabis use is broadly associated with cognitive impairment, its relationship with mood is more complex. Cannabis has robust, acute effects on positive mood and decreased anxiety, including subjective reward, such as ‘high’, ‘liking’ and ‘mellow’ [72,83–87], and these more positive mood effects appear to be THC dose dependent and are unlikely due to expectancies [88]. A recent naturalistic study of cannabis use and effect suggested that while using acutely reduced symptoms of depression, anxiety and stress, long-term use exacerbated depression [89]. Emerging data address the acute impacts of concentrates on positive and negative mood and suggest that flower cannabis may produce more consistent positive mood, but that acute negative mood effects may be minimal and similar for both concentrates and flower [90]. However, some studies have shown that the acute negative effects of THC, such as unpleasant drug effects, confusion, sedation and dysphoria, are also dose-dependent [72,91], suggesting that individuals who use more potent products (e.g. concentrates) may be more likely to experience these negative effects. This may be a particular risk among less experienced users. Cannabis use is also associated with longer-term negative effects on emotional lability, including depressed mood and anger/hostility, regardless of whether onset is in adolescence or adulthood [92,93]. Further, cannabis use patterns may generally disturb affect with greater levels of use associated with long-term effects on psychiatric conditions [94–96]. It is possible that high potency cannabis exacerbates these short- and long-term relationships and further studies are needed to dissect any causal role of high potency products in affective disturbance.
Cannabis use disorder and related clinical phenomena
Increased THC potency cannabis has been associated with higher tolerance, physiological dependence and greater withdrawal symptoms [18,97–99]. Similarly, individuals using high THC potency cannabis appear to be at a greater risk for developing a CUD [100], highlighting concerns about increased clinical risk. Notably, one recent study found that higher cannabis potency increased the risk of CUD development and accelerated the progression of symptom onset, such that using higher potency products at the onset of cannabis use was correlated with quadruple the risk of developing CUD symptoms within the first year [100].
Several studies have concluded that the use of high-potency cannabis is associated with greater dependence and withdrawal in comparison to low-potency cannabis [16,18,29,97,98,100–102]. While these findings are consistent, this relationship has not been proven to be strictly causal [101] and several studies have found that cannabis concentrate users experience no more behavioural problems than do flower users after accounting for frequency of use [5,18]. However, current data in concentrate users capture these clinically relevant metrics very generally, typically through surveys, and do not shed light on fluctuations in withdrawal and other behaviours in relation to individual use patterns and abstinence behaviours. If concentrate users do experience more severe withdrawal, this may be one potential mechanism underlying an increased risk for CUD in concentrate users. As a result of more severe withdrawal, concentrate users may be more motivated to resume cannabis use following abstinence to suppress withdrawal symptoms, which could ultimately lead to more regular, heavy and persistent patterns of use. Alternatively, concentrate use may be associated with elevated affective and physiological symptoms that mirror withdrawal more chronically and fluctuate little in response to overnight abstinence and reinstatement. Given the potential clinical significance of withdrawal symptoms in the development of CUD, further characterising these symptoms under controlled conditions in concentrate users and comparing them to users of other cannabis forms is a next critical step.
Adolescent concentrate use
There are heightened concerns about use of high potency cannabis and cannabis-related consequences in adolescents, especially given that while other forms of substance use among adolescents have decreased, cannabis use rates remain high [103]. Recent research suggests that the risk for conversion from occasional to frequent cannabis use among adolescents is greatest among concentrate users [104]. This finding may be especially concerning in light of results from another study reporting 24% lifetime concentrate use among over 47 000 adolescents [105]. Novel forms of administration—particularly vaporisation—appear to be increasingly popular among adolescents and may be partially driving increases in concentrate use among this age group [103]. Current data also suggest that adolescent concentrate use also is associated with greater risk for other illicit substance use or substance use problems [103].
Among adolescents, there is the potential for THC to impact brain development [70] and concern that adolescents may be vulnerable to cognitive and behavioural health impairments from cannabis use [106,107]. The brain undergoes a number of critical developmental changes during adolescence, some of which appear to involve alterations within the eCS. In particular, the adolescent brain expresses a high density of cannabinoid receptors, resulting in greater susceptibility to the neurotoxic effects of cannabis during adolescence [108–113]. CB1 receptors are particularly abundant during adolescence, and animal models suggest chronic activation of CB1 in adolescence confers anxiety- and depression-like effects, as well as cognitive impairment [114,115]. The impact of early initiation of use and exposure to concentrates on these neurobiological and neurobehavioral pathways is unknown over the longer term.
Associations with psychosis
Some existing literature has provided evidence supporting a relationship between cannabis use and psychosis, particularly among high-potency users [116–124]. Recent high profile reviews have concluded that there is sufficient evidence for an association between dose-dependent cannabis use and psychosis, but insufficient evidence supporting causality [125,126]. The dose-dependent nature of these aggregate findings would suggest the possibility of more severe associations among psychosis and use of concentrates, as well as for more chronic users who are consuming greater and more frequent doses, but little to no research has been done specifically on a large sample of users who exclusively use concentrates in relation to psychosis. Other studies have shown CBD may mitigate some of these psychotogenic effects [64,127–129]. The causality behind this association is still unclear, but it is likely that THC potency and quantity of other cannabinoids (e.g. CBD) is a relevant factor in unpacking these associations with psychosis.
Sex differences
Males report greater prevalence of cannabis use generally and concentrate use specifically compared to females [5,130–132], although the sex gap has narrowed in recent years [133]. The limited existing studies in this area point to significant sex differences in the rate of development of cannabis dependence [134], severity of withdrawal [135] and cannabis use patterns (e.g. method of consumption, motives for use) [136]. It has been well-established that although the prevalence of cannabis use is lower among females, female users experience a faster rate of development of cannabis dependence as compared to males [134], a finding has come to be known as the ‘telescoping effect’. Female cannabis users also demonstrate a higher rate of withdrawal and tend to experience greater severity of certain withdrawal symptoms compared to men [135]. Among treatment-seeking cannabis users, women report higher overall withdrawal scores, greater negative affect symptoms (i.e. irritability, restlessness, increased anger, violent outbursts), more nausea and stomach pain, a greater number of withdrawal symptoms and greater symptom severity compared to men [137].
The existing literature hints at critical sex differences in the development and function of the eCS and in responses to cannabis [138–141], which may underlie increased withdrawal in female users [142]. In one study, male rats were shown to have higher CB1 density in the brain compared to females [143–146], and this finding has been replicated in humans [147]. However, females may have more efficient CB1 receptors [143,144]. Notably, several positron emission tomography studies in humans have demonstrated that women show greater CB1 availability compared to men [148,149]. In addition, CB1 levels in the female rat brain have been shown to fluctuate across the oestrous cycle [150], and peak CB1 levels are reached earlier in development in female rats compared to male rats [151]. Female adolescent rats also show greater CB1 receptor desensitisation in response to repeated injections of THC compared to male adolescent rats [144]. Based on preclinical findings demonstrating sexual dimorphism of the eCS, it has been suggested that the increased rate of dependence and withdrawal severity observed in female cannabis users compared to male cannabis users may be due to differential desensitisation of CB1 or alterations in endocannabinoid tone [135]. Further, one recent human study found that women (compared to men) consume less of a higher-potency cannabis strain under laboratory conditions, yet experience similar psychological and behavioural outcomes, suggesting potential sex differences in THC pharmacology and/or eCS functioning [152]. It is presently unclear whether the behavioural differences produced by sexual dimorphism within the eCS are exacerbated by high potency cannabis. It will be especially important to determine whether female concentrate users are at elevated risk.
Future Directions for Concentrate Research
Pulmonary function
As the literature suggests that cannabis use is associated with acute and chronic respiratory symptoms [24,99,102], it is important to evaluate any change in these risks in relation to concentrate use. For example, there may be long-term health risks associated with inhaling chemicals like benzene and rust from oxidised metal parts as a result of heating ‘oil rig’ devices, because these chemicals are released at higher temperatures in the process of ‘dabbing’ [10]. These concerns are highlighted by the rise in vaping-related medical issues and underscore the need for more data on the impact of inhaling concentrates and various modes of administration on pulmonary and other health functions.
Acute and short-term effects
Some evidence suggests that the greater potency of today’s cannabis, compared to earlier decades, may lead to significantly greater levels of intoxication and harm [70]. Currently, little is known about motivations that are driving increased use of concentrates and whether they are related to perceived effects of concentrates, with few studies examining motivation either directly or indirectly [132,153]. The literature is also limited in that it may either under or over-estimate the effects users experience in the real world in relation to concentrates. Only a few previous studies have examined the effect of high potency cannabis on subjective intoxication and various forms of cognitive or psychomotor impairment. Even fewer studies have directly examined the impact of high potency concentrates, which may be altered by tolerance processes, saturation of CB1 receptors and individual pre-existing differences among concentrate users. Well-controlled human trials (in both occasional and regular users) and targeted preclinical and mechanistic studies are necessary to dissect these possibilities. Future studies should explore the effects of high-potency cannabis concentrates on intoxication and psychomotor impairment in different types of users, particularly those related to driving, given that cannabis is the most frequently reported drug in connection with motor vehicle accidents [70]. Federal restrictions on cannabis research largely prevent researchers from studying legal-market cannabis products in the laboratory. As a Schedule I drug, the only federally legal source of cannabis for research is the National Institute on Drug Abuse drug supply program. There are no cannabis concentrates available through this program. Currently, the only federally compliant option for researchers is naturalistic, observational designs [23]. However, these designs are limited in that participants cannot be randomly assigned to use certain cannabis strains, and researchers have limited control over the amount of cannabis that participant choose to use during laboratory assessments. Changes in federal policies to allow researchers access to cannabis concentrates for human and animal studies are critical to advancing the science.
Evaluating developmental effects
Data are lacking on the long-term clinical, affective and behavioural effects of concentrates in vulnerable populations. In particular, high-potency cannabis may exacerbate cannabis-related consequences in vulnerable users, such as adolescents, those exposed prenatally and individuals at risk for psychosis. Recent increases in vaping and dabbing of high potency formulations in adolescent and other vulnerable populations [154] bring greater potential for adverse effects. Data are needed to evaluate and quantify these developmental and clinical processes and assess long-term impacts of concentrate use. Genetically informed, prospective studies and biobehavioral studies that quantify short- and long-term THC exposure objectively, through well-timed assaying of cannabinoid biomarkers, are critical to making strong links among high potency products and developmental risks. For example, the ongoing Adolescent Brain and Cognitive Development study (abcdstudy.org), the largest longitudinal study of brain development and child health in the USA, is a multi-site effort funded by the National Institute on Drug Abuse designed to collect longitudinal and family-based data that will provide invaluable insight into addressing these questions.
Evaluating chronic effects on dependence and withdrawal
Greater THC exposure, tolerance and CUD have been observed in concentrate users, but few studies have evaluated these processes under controlled conditions and while carefully matching for frequency of cannabis use. It may be the case that high frequent use of cannabis, regardless of the form or potency, is the driver of these associations and this question needs to be carefully assessed. In addition, higher levels of withdrawal and affective disturbance have also been observed broadly in concentrate users, suggesting that concentrate users may experience greater difficulty in achieving cannabis abstinence and experience greater withdrawal and negative affect when reducing their use. Cannabis withdrawal and related elevated affective symptomatology are directly linked to reuptake of cannabis use during quit attempts and are a key contributor to CUD [155], and thus represent clinically relevant—but insufficiently-characterised—constructs, in need of considerable further study in relation to concentrate use.
The role of CBD in mitigating effects of high potency THC
Given that THC is known to be the putatively harmful component of cannabis and that its agonism of the CB1 receptor is thought to underlie many of the intoxicating, impairing and negative affect inducing actions of THC [48,52,71,156,157], it has been proposed that a non-intoxicating agent that weakly agonises CB1 could be useful for mitigating cannabis-related harms [158,159]. CBD is a non-psychoactive compound expressed in the Cannabis plant, which has a low affinity for CB1, and is thought to exert a number of potentially beneficial actions throughout the central nervous system and periphery [160]. Some controlled studies in humans suggest that CBD may attenuate the acute negative effects of THC on affect, intoxication [161] and cognition [162–165], and may also attenuate the subjective effects of THC [166,167]. Conversely, another study found that oral administration of CBD does not serve to reduce the subjective or reinforcing effects of THC in smoked cannabis [168]. Several other studies have found that Nabiximols, a medication that combines THC and CBD in a 1:1 ratio, was effective in reducing cannabis withdrawal, including negative affective symptoms, in a sample of individuals with cannabis dependence [169–171]. These limited and mixed findings underscore the need for further research on the effects of CBD, alone and combination with low potency THC, among concentrate users. Given that CBD products are widely available and used in medical and recreational cannabis markets throughout the USA, research into the potential for CBD to reduce the harms of high potency THC use has particular public health relevance.
Diversifying concentrate research
Across all domains of human cannabis research, the demographics are largely homogenous, favouring young white males. While this review does not make a systematic analysis of the proportion of participants by race and sex, the experimental and case study/case control studies cited here roughly average 71.8% male and 64.7% white. In a recent JAMA Open Network meta-analysis of cannabis use symptoms among 23 518 regular and dependent users, the average age of participants was 29.9 years, 69% were male and 72% were white [172], despite growing evidence that older adults are increasingly using cannabis. These estimates may also not be representative of the true diversity of the work given that 47.9% of studies did not report ethnicity. The criminalisation of cannabis use has negatively affected communities of colour disproportionately, and Black and Hispanic communities in particular, and thus may contribute to the lack of research on cannabis among these groups [173–178]. One study (89% White) found that cannabis-related stigma was negatively associated with willingness to participate in cannabis-related research [179]. While reporting results with racial and ethnic categories can be problematic and potentially reinforce racial inequity in research, the lack of reporting and representation also perpetuates an evidence base, which does not have validity across diverse groups [180,181].
Sex differences among males and females in regard to concentrate use, cannabis withdrawal and underlying affective and psychomotor processes have not yet been well-characterised [142]. Over the past decade, the prevalence of cannabis use has doubled in women [133], suggesting that women as a demographic group have evidenced important changes in cannabis use during a key period of shifting policy and legal availability of cannabis. Translational studies are needed to explore the potential for a stronger ‘telescoping effect’ to CUD and the potential for increased withdrawal severity in female concentrate users. Further, there is particular need for additional research regarding sex differences in psychiatric comorbidities. Overall, the historic criminalisation of cannabis use for Black, Indigenous and people of colour groups in combination with the tendency for biomedical research to underrepresent females and people of colour has likely contributed to a lack of diverse representation in cannabis research. These issues point to the need for cannabis research to become more inclusive across ethnicity and sex, which would greatly improve the validity and generalisability of any findings.
Conclusion
Policy makers, scientists and the public currently are in need of high quality, well-controlled scientific studies to quantify and understand the potentially harmful effects of high THC concentrate cannabis products that are growing in popularity. At present, we do not have sufficient data to make accurate conclusions about the severity of risks of these products. Gaps in the literature on their acute and long-term effects on human health need to be addressed in order to generate information about the effects of concentrates, in particular whether, and which forms, of these products are most harmful. Mechanistic and preclinical work with direct translational implications are also needed to advance the science of cannabis concentrates. An evidence base clearly describing the potential harms of specific compositions of concentrates and underlying neurobiological and behavioural mechanisms of their effects is critical to harm reduction efforts.
Supplementary Material
Acknowledgements
The authors would like to thank Carter Casad, Diantha H. T. La Vine, Mae Jones and Julia Squeri for their contributions to this manuscript. Funding for this study was provided by CDPHE 17-96947 (PI: Bidwell).
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Appendix S1. Literature search terms.
References
- [1].Volkow ND, Swanson JM, Evins AE et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a rview. JAMA Psychiat 2016;73:292–7. [DOI] [PubMed] [Google Scholar]
- [2].Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev 2012;5:32–40. [DOI] [PubMed] [Google Scholar]
- [3].ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry 2016;79:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Potter DJ, Hammond K, Tuffnell S, Walker C, Di Forti M. Potency of Δ9-tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: implications for public health and pharmacology. Drug Test Anal 2018;10:628–35. [DOI] [PubMed] [Google Scholar]
- [5].Bidwell LC, YorkWilliams SL, Mueller R, Bryan AD, Hutchison KE. Exploring cannabis concentrates on the legal market: user profiles, product strength, and health-related outcomes. Addict Behav Rep 2018;8:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raber JC, Elzinga S, Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J Toxicol Sci 2015;40:797–803. [DOI] [PubMed] [Google Scholar]
- [7].Orens A, Light M, Lewandowski B, Rowberry J, Saloga C. 2017 Market Update. Market Size and Demand for Marijuana in Colorado. Denver, Colorado; 2018. [Google Scholar]
- [8].Vergara D, Bidwell LC, Gaudino R et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep 2017;7:46528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G. Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state. Addiction 2017;122:2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stogner JM, Miller BL. The dabbing dilemma: a call for research on butane hash oil and other alternate forms of cannabis use. Subst Abus 2015;36:393–5. [DOI] [PubMed] [Google Scholar]
- [11].Committee on the Health Effects of Marijuana, National Academies of Sciences, Engineering and Medicine.The health effects of Cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: National Academies Press, 2017. (accessed 15 February 2018). [PubMed] [Google Scholar]
- [12].Marijuana Sales Reports. Department of Revenue. Available at: https://cdor.colorado.gov/data-and-reports/marijuana-data/marijuana-sales-reports (accessed 28 January 2021).
- [13].BDS Analytics. Concentrates: the hottest product category in Cannabis. Boulder, CO; 2019. Available at: https://arcviewgroup.com/research/portal/. (accessed 21 August, 2020). [Google Scholar]
- [14].Freeman TP, Swift W. Cannabis potency: the need for global monitoring. Addiction 2016;111:376–7. [DOI] [PubMed] [Google Scholar]
- [15].Hutchison KE, Bidwell LC, Ellingson JM, Bryan AD. Cannabis and Health Research: rapid Progress requires innovative research designs. Value Heal 2019;22:1289–94. [DOI] [PubMed] [Google Scholar]
- [16].Al-Zouabi I, Stogner JM, Miller BL, Lane ES. Butane hash oil and dabbing: insights into use, amateur production techniques, and potential harm mitigation. Subst Abuse Rehabil 2018;9:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lamy FR, Daniulaityte R, Zatreh M et al. “You got to love rosin: Solventless dabs, pure, clean, natural medicine.” Exploring Twitter data on emerging trends in Rosin Tech marijuana concentrates. Drug Alcohol Depend 2018;183:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loflin M, Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict Behav 2014;39:1430–3. [DOI] [PubMed] [Google Scholar]
- [19].Meehan-Atrash J, Luo W, Strongin RM. Toxicant formation in dabbing: the terpene story. ACS Omega 2017;2:6112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Daniulaityte R, Nahhas RW, Wijeratne S et al. “Time for dabs”: Analyzing Twitter data on marijuana concentrates across the US. Drug Alcohol Depend 2015;155:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of the literature. Addiction 2008;103:1100–9. [DOI] [PubMed] [Google Scholar]
- [22].Hollister LE, Gillespie HK, Ohlsson A, Lindgren J-E, Wahlen A, Agurell S. Do plasma concentrations of Δ9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol 1981;21:171S–7S. [DOI] [PubMed] [Google Scholar]
- [23].Bidwell LC, Ellingson JM, Karoly HC et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiat 2020;77:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anderson RP, Zechar K. Lung injury from inhaling butane hash oil mimics pneumonia. Respir Med Case Rep 2019;26:171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stephens D, Patel JK, Angelo D, Frunzi J. Cannabis butane hash oil dabbing induced lung injury mimicking atypical pneumonia. Cureus 2020;12:e7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kelley BP, Prakash PB. Vaping-associated lung injury: should we consider screening adolescents who vape? Clin Pediatr (Phila) 2020;59:1033–5. [DOI] [PubMed] [Google Scholar]
- [27].Tanne JH. Don’t vape, CDC says, as US lung disease epidemic grows. BMJ 2019;366:l5479. [DOI] [PubMed] [Google Scholar]
- [28].Abeles M, Popofsky S, Wen A et al. Vaping-associated lung injury caused by inhalation of cannabis oil. Pediatr Pulmonol 2020;55:226–8. [DOI] [PubMed] [Google Scholar]
- [29].Stogner JM, Miller BL. Assessing the dangers of “dabbing”: mere marijuana or harmful new trend? Pediatrics 2015;136:1–3. [DOI] [PubMed] [Google Scholar]
- [30].Alzghari SK, Fung V, Rickner SS, Chacko L, Fleming SW. To dab or not to dab: rising concerns regarding the toxicity of cannabis concentrates. Cureus 2017;9:e1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schneberk T, Sterling GP, Valenzuela R, Mallon WK. “A Little Dab Will Do Ya”: an emergency department case series related to a new form of “high-potency” marijuana known as “wax”. Ann Emerg Med 2014;64:S139. [Google Scholar]
- [32].Loflin M, Earleywine M. No smoke, no fire: what the initial literature suggests regarding vapourized cannabis and respiratory risk. Can J Respir Ther 2015;51:7–9. [PMC free article] [PubMed] [Google Scholar]
- [33].Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther 2007;82:572–8. [DOI] [PubMed] [Google Scholar]
- [34].Doblin R The MAPS/California NORML marijuana water-pipe/vaporizer study. Newsl Multidiscip Assoc Psychedelic Stud 1994;5:19–22. [Google Scholar]
- [35].Van Dam NT, Earleywine M. Pulmonary function in cannabis users: support for a clinical trial of the vaporizer. Int J Drug Policy 2010;21:511–3. [DOI] [PubMed] [Google Scholar]
- [36].Bloor RN, Wang TS, Spanel P, Smith D. Ammonia release from heated ‘street’ cannabis leaf and its potential toxic effects on cannabis users. Addiction 2008;103:1671–7. [DOI] [PubMed] [Google Scholar]
- [37].Earleywine M, Barnwell SS. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J 2007;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moritz ED, Zapata LB, Lekiachvili A et al. Update: characteristics of patients in a national outbreak of e-cigarette, or vaping, product use–associated lung injuries — United States, October 2019. MMWR Morb Mortal Wkly Rep 2019;68:985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ghinai I, Pray IW, Navon L et al. E-cigarette product use, or vaping, among persons with associated lung injury — Illinois and Wisconsin, April–September 2019. MMWR Morb Mortal Wkly Rep 2019;68:865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].García-Bueno B, Caso JR. Cannabis, cannabinoid receptors, and stress-induced excitotoxicity. In: Neuropathology of drug addictions and substance misuse, Vol. 1. Cambridge, MA: Elsevier Inc., 2016:731–7 p. Available at: 10.1016/B978-0-12-800213-1.00068-7. [DOI] [Google Scholar]
- [41].Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res 2009;60:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jager G, Witkamp RF. The endocannabinoid system and appetite: relevance for food reward. Nutr Res Rev 2014;27:172–85. [DOI] [PubMed] [Google Scholar]
- [43].Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci 2012;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015;172:737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 2015;16:579–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pertwee RG. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br J Pharmacol 2008;153:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Laviolette SR. Cannabinoid regulation of opiate motivational processing in the mesolimbic system: the integrative roles of amygdala, prefrontal cortical and ventral hippocampal input pathways. Curr Opin Behav Sci 2017;13:46–54. [Google Scholar]
- [48].D’Souza DC, Cortes-Briones JA, Ranganathan M et al. Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 2016;1:60–7. [DOI] [PubMed] [Google Scholar]
- [49].Onaivi ES, Chakrabarti A, Gwebu ET, Chaudhuri G. Neurobehavioral effects of Δ9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav Brain Res 1995;72:115–25. [DOI] [PubMed] [Google Scholar]
- [50].McAllister SD, Glass M. CB1 and CB2 receptor-mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot Essent Fat Acids 2002;66:161–71. [DOI] [PubMed] [Google Scholar]
- [51].Huestis MA, Gorelick DA, Heishman SJ et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 2001;58:322–8. [DOI] [PubMed] [Google Scholar]
- [52].Hirvonen J, Goodwin RS, Li C-T et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012;17:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang H-Y, Gao M, Shen H et al. Expression of functional cannabinoid CB 2 receptor in VTA dopamine neurons in rats. Addict Biol 2017;22:752–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang HY, Gao M, Liu QR et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A 2014;111:E5007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Aso E, Andrés-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid receptor 2 participates in amyloid-β processing in a mouse model of Alzheimer’s disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J Alzheimers Dis 2016;51:489–500. [DOI] [PubMed] [Google Scholar]
- [56].Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 2006;58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fernández-Ruiz J, Pazos MR, García-Arencibia M, Sagredo O, Ramos JA. Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol 2008;286(1–2 Suppl):S91–6. [DOI] [PubMed] [Google Scholar]
- [58].Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 2007;28:39–45. [DOI] [PubMed] [Google Scholar]
- [59].Mackie K Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 2005;168:299–325. [DOI] [PubMed] [Google Scholar]
- [60].Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–5. [DOI] [PubMed] [Google Scholar]
- [61].Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br J Pharmacol 2007;150:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br J Pharmacol 2015;172:4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Klein C, Karanges E, Spiro A et al. Cannabidiol potentiates Δ 9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218:443–57. [DOI] [PubMed] [Google Scholar]
- [64].Englund A, Morrison PD, Nottage J et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013;27:19–27. [DOI] [PubMed] [Google Scholar]
- [65].Rømer Thomsen K, Callesen MB, Feldstein Ewing SW. Recommendation to reconsider examining cannabis subtypes together due to opposing effects on brain, cognition and behavior. Neurosci Biobehav Rev 2017;80:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011;163:1344–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ben-Shabat S, Fride E, Sheskin T et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 1998;353:23–31. [DOI] [PubMed] [Google Scholar]
- [68].Desrosiers NA, Ramaekers JG, Chauchard E, Gorelick DA, Huestis MA. Smoked cannabis’ psychomotor and neurocognitive effects in occasional and frequent smokers. J Anal Toxicol 2015;39:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ramaekers JG, van Wel JH, Spronk DB et al. Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep 2016;6:26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med 2014;370:2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Curran V, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ 9 -tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. [DOI] [PubMed] [Google Scholar]
- [72].Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 2001;25:757–65. [DOI] [PubMed] [Google Scholar]
- [73].Busardò FP, Pellegrini M, Klein J, di Luca NM. Neurocognitive correlates in driving under the influence of cannabis. CNS Neurol Disord Drug Targets 2017;16:534–40. [DOI] [PubMed] [Google Scholar]
- [74].Declues K, Perez S, Figueroa A. A 2-year study of δ 9-tetrahydrocannabinol concentrations in drivers: examining driving and field sobriety test performance. J Forensic Sci 2016;61:1664–70. [DOI] [PubMed] [Google Scholar]
- [75].Freeman TP, Morgan CJA, Hindocha C, Schafer G, Das RK, Curran HV. Just say ‘know’: how do cannabinoid concentrations influence users’ estimates of cannabis potency and the amount they roll in joints? Addiction 2014;109:1686–94. [DOI] [PubMed] [Google Scholar]
- [76].Yao B, Mackie K. Endocannabinoid receptor pharmacology. Curr Top Behav Neurosci 2009;1:37–63. [DOI] [PubMed] [Google Scholar]
- [77].Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 a/a (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum Genet 2006;120:581–8. [DOI] [PubMed] [Google Scholar]
- [78].Cravatt BF, Giangi DK, Mayfieldt S, Boger DL, Lerner RA, Gilulat NB. Fatty-acid amides. Nature 1996;384:356–8. [DOI] [PubMed] [Google Scholar]
- [79].Bosker WM, Kuypers KPC, Theunissen EL et al. Medicinal Δ9-tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in Standard Field Sobriety Tests. Addiction 2012;107:1837–44. [DOI] [PubMed] [Google Scholar]
- [80].González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 2005;81:300–18. [DOI] [PubMed] [Google Scholar]
- [81].Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev 2018;93:1–25. [DOI] [PubMed] [Google Scholar]
- [82].Karoly HC, Milburn MA, Brooks-Russell A et al. Effects of high-potency cannabis on psychomotor performance in frequent cannabis users. Cannabis Cannabinoid Res 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology (Berl) 1988;94:206–12. [DOI] [PubMed] [Google Scholar]
- [84].Chait LD, Zacny JP. Reinforcing and subjective effects of oral Δ9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–62. [DOI] [PubMed] [Google Scholar]
- [85].Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol 2008;13:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav 1990;37:561–5. [DOI] [PubMed] [Google Scholar]
- [87].Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: an exploratory analysis. Psychopharmacology (Berl) 2009;203:511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–9. [DOI] [PubMed] [Google Scholar]
- [89].Cuttler C, Spradlin A, McLaughlin RJ. A naturalistic examination of the perceived effects of cannabis on negative affect. J Affect Disord 2018;235:198–205. [DOI] [PubMed] [Google Scholar]
- [90].Okey SA, Meier MH. A within-person comparison of the subjective effects of higher vs. lower-potency cannabis. Drug Alcohol Depend 2020;2016:108225. [DOI] [PubMed] [Google Scholar]
- [91].Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H et al. Comparison of the subjective effects of Δ9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–9. [DOI] [PubMed] [Google Scholar]
- [92].Fairman BJ, Anthony JC. Are early-onset cannabis smokers at an increased risk of depression spells? J Affect Disord 2012;138:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pahl K, Brook JS, Koppel J. Trajectories of marijuana use and psychological adjustment among urban African American and Puerto Rican women. Psychol Med 2011;41:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cornelius JR, Kirisci L, Reynolds M, Clark DB, Hayes J, Tarter R. PTSD contributes to teen and young adult cannabis use disorders. Addict Behav 2010;35:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Crippa JA, Zuardi AW, Martín-Santos R et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol Clin Exp 2009;24:515–23. [DOI] [PubMed] [Google Scholar]
- [96].Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: testing for causal associations. Addiction 2006;101:1463–72. [DOI] [PubMed] [Google Scholar]
- [97].Hall W, Degenhardt L. High potency cannabis: a risk factor for dependence, poor psychosocial outcomes, and psychosis. BMJ 2015;350:h1205. [DOI] [PubMed] [Google Scholar]
- [98].Meier MH. Associations between butane hash oil use and cannabis-related problems. Drug Alcohol Depend 2017;179:25–31. [DOI] [PubMed] [Google Scholar]
- [99].Prince MA, Conner BT. Examining links between cannabis potency and mental and physical health outcomes. Behav Res Ther 2019;115:111–20. [DOI] [PubMed] [Google Scholar]
- [100].Arterberry BJ, Treloar Padovano H, Foster KT, Zucker RA, Hicks BM. Higher average potency across the United States is associated with progression to first cannabis use disorder symptom. Drug Alcohol Depend 2019;195:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med 2015;45:3181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use – basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy 2018;52:87–96. [DOI] [PubMed] [Google Scholar]
- [103].Ryan S Use of cannabis concentrates by adolescents. In: Pediatrics, Vol. 144. Itasca, IL: American Academy of Pediatrics, 2019. [DOI] [PubMed] [Google Scholar]
- [104].Barrington-Trimis JL, Cho J, Ewusi-Boisvert E et al. Risk of persistence and progression of use of 5 cannabis products after experimentation among adolescents. JAMA Netw Open 2020;3:e1919792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Meier MH, Docherty M, Leischow SJ, Grimm KJ, Pardini D. Cannabis concentrate use in adolescents. Pediatrics 2019;144:e20190338. [DOI] [PubMed] [Google Scholar]
- [106].Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc 2007;13:807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tapert SF, Schweinsburg AD, Drummond SPA et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bossong MG, Niesink RJM. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 2010;92:370–85. [DOI] [PubMed] [Google Scholar]
- [109].Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psych 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol 2010;160:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Quinn HR, Matsumoto I, Callaghan PD et al. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 2008;33:1113–26. [DOI] [PubMed] [Google Scholar]
- [112].Rubino T, Realini N, Braida D et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 2009;19:763–72. [DOI] [PubMed] [Google Scholar]
- [113].Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev 2008;1:81–98. [DOI] [PubMed] [Google Scholar]
- [114].Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis 2010;37:641–55. [DOI] [PubMed] [Google Scholar]
- [115].Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 2016;79:578–85. [DOI] [PubMed] [Google Scholar]
- [116].Di Forti M, Morgan C, Dazzan P et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry 2009;195:488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Di Forti M, Marconi A, Carra E et al. Proportion of patients in South London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry 2015;2:233–8. [DOI] [PubMed] [Google Scholar]
- [118].Murray RM, Quigley H, Quattrone D, Englund A, Di Forti M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry 2016;15:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Di Forti M, Quattrone D, Freeman TP et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 2019;6:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Radhakrishnan R, Addy PH, Sewell RA, Skosnik PD, Ranganathan M, D’Souza DC. Cannabis, cannabinoids, and the association with psychosis. In: The effects of drug abuse on the human nervous system. Cambridge, MA: Elsevier Inc., 2013:423–74. [Google Scholar]
- [121].Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psych 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry 2011;68:555–61. [DOI] [PubMed] [Google Scholar]
- [123].Hines LA, Freeman TP, Gage SH et al. Association of high-potency cannabis use with mental health and substance use in adolescence. JAMA Psychiat 2020;77:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Murray RM, Englund A, Abi-Dargham A et al. Cannabis-associated psychosis: neural substrate and clinical impact. Neuropharmacology 2017;124:89–104. [DOI] [PubMed] [Google Scholar]
- [125].Hasan A, von Keller R, Friemel CM et al. Cannabis use and psychosis: a review of reviews. Eur Arch Psychiatry Clin Neurosci 2020;270:403–12. [DOI] [PubMed] [Google Scholar]
- [126].National Academies of Sciences. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 2017. [PubMed] [Google Scholar]
- [127].Schubart CD, Sommer IEC, van Gastel WA, Goetgebuer RL, Kahn RS, Boks MPM. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res 2011;130:216–21. [DOI] [PubMed] [Google Scholar]
- [128].Morgan CJA, Gardener C, Schafer G et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med 2012;42:391–400. [DOI] [PubMed] [Google Scholar]
- [129].Leweke FM, Mueller JK, Lange B, Rohleder C. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry 2016;79:604–12. [DOI] [PubMed] [Google Scholar]
- [130].Cuttler C, Mischley LK, Sexton M. Sex differences in Cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res 2016;1:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Daniulaityte R, Lamy FR, Barratt M et al. Characterizing marijuana concentrate users: a web-based survey. Drug Alcohol Depend 2017;178:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chan GCK, Hall W, Freeman TP, Ferris J, Kelly AB, Winstock A. User characteristics and effect profile of butane hash oil: an extremely high-potency cannabis concentrate. Drug Alcohol Depend 2017;178:32–8. [DOI] [PubMed] [Google Scholar]
- [133].Hasin DS, Kerridge BT, Saha TD et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry 2016;173:588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ehlers CL, Gizer IR, Vieten C et al. Cannabis dependence in the San Francisco family study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav 2010;35:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Schlienz NJ, Budney AJ, Lee DC, Vandrey R. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep 2017;4:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Noack R, Höfler M, Lueken U. Cannabis use patterns and their association with DSM-IV cannabis dependence and gender. Eur Addict Res 2011;17:321–8. [DOI] [PubMed] [Google Scholar]
- [137].Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol 2015;23:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci 2013;92:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 2014;143:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wiley JL. Sex-dependent effects of Δ9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett 2003;352:77–80. [DOI] [PubMed] [Google Scholar]
- [141].Brents LK. Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med 2016;89:175–91. [PMC free article] [PubMed] [Google Scholar]
- [142].Khan SS, Secades-Villa R, Okuda M et al. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of alcohol and related conditions. Drug Alcohol Depend 2013;130:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rubino T, Vigano’ D, Realini N et al. Chronic Δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 2008;33:2760–71. [DOI] [PubMed] [Google Scholar]
- [144].Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol 2010;161:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mateos B, Borcel E, Loriga R et al. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. J Psychopharmacol 2011;25:1676–90. [DOI] [PubMed] [Google Scholar]
- [146].Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 2010;35:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Van Laere K, Goffin K, Casteels C et al. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. Neuroimage 2008;39:1533–41. [DOI] [PubMed] [Google Scholar]
- [148].Neumeister A, Normandin MD, Pietrzak RH et al. Elevated brain cannabinoid CB 1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry 2013;18:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Normandin MD, Zheng M-Q, Lin K-S et al. Imaging the cannabinoid CB1 receptor in humans with [11C] OMAR: assessment of kinetic analysis methods, test–retest reproducibility, and gender differences. J Cereb Blood Flow Metab 2015;35:1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].de Fonseca FR, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 1994;54:159–70. [DOI] [PubMed] [Google Scholar]
- [151].de Fonseca FR, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 1993;4:135–8. [DOI] [PubMed] [Google Scholar]
- [152].Matheson J, Sproule B, Di Ciano P et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl) 2020;237:305–16. [DOI] [PubMed] [Google Scholar]
- [153].Sagar KA, Lambros AM, Dahlgren MK, Smith RT, Gruber SA. Made from concentrate? A national web survey assessing dab use in the United States. Drug Alcohol Depend 2018;190:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Healthy Kids Colorado Survey and Smart Source Information. Department of Public Health and Environment; 2019. Available at: https://www.colorado.gov/pacific/cdphe/hkcs (accessed 1 October 2020).
- [155].Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol 2003;112:393–402. [DOI] [PubMed] [Google Scholar]
- [156].McDonald J, Schleifer L, Richards JB, De Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003;28:1356–65. [DOI] [PubMed] [Google Scholar]
- [157].Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 2009;23:266–77. [DOI] [PubMed] [Google Scholar]
- [158].Batalla A, Janssen H, Gangadin SS, Bossong MG. The potential of cannabidiol as a treatment for psychosis and addiction: who benefits most? A systematic review. J Clin Med 2019;8:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 2018;43:173–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. Dordrecht, Netherlands: Kluwer Academic/Plenum Publishers, 2004:32–83. [Google Scholar]
- [161].Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by δ9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–50. [DOI] [PubMed] [Google Scholar]
- [162].Bhattacharyya S, Morrison PD, Fusar-Poli P et al. Opposite effects of Δ−9-tetrahydrocannabinol and Cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010;35:764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Morgan CJA, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Δ9- tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology 2010;35:1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Demirakca T, Sartorius A, Ende G et al. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend 2011;114:242–5. [DOI] [PubMed] [Google Scholar]
- [165].Vann RE, Gamage TF, Warner JA et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug Alcohol Depend 2008;94:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther 1976;19:300–9. [DOI] [PubMed] [Google Scholar]
- [167].Bidwell LC, Mueller R, YorkWilliams SL, Hagerty S, Bryan AD, Hutchison KE. A novel observational method for assessing acute responses to cannabis: preliminary validation using legal market strains. Cannabis Cannabinoid Res 2018;3:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Haney M, Malcolm RJ, Babalonis S et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 2016;41:1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Allsop DJ, Copeland J, Lintzeris N et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal. JAMA Psychiat 2014;71:281–91. [DOI] [PubMed] [Google Scholar]
- [170].Trigo JM, Soliman A, Staios G et al. Sativex associated with behavioral-relapse prevention strategy as treatment for cannabis dependence: a case series. J Addict Med 2016;10:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Trigo JM, Soliman A, Quilty LC et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. Cooper ZD, editor. PLoS ONE 2018;13:e0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bahji A, Stephenson C, Tyo R, Hawken ER, Seitz DP. Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids. JAMA Netw Open 2020;3:e202370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Bender SW. The colors of Cannabis: race and marijuana. Univ Calif - Davis Law Reivew; 2016;50:689–706. [Google Scholar]
- [174].Ramchand R, Pacula RL, Iguchi MY. Racial differences in marijuana-users’ risk of arrest in the United States. Drug Alcohol Depend 2006;84:264–72. [DOI] [PubMed] [Google Scholar]
- [175].Mitchell O, Caudy MS. Examining racial disparities in drug arrests. Justice Q 2015;32:288–313. [Google Scholar]
- [176].Moran TJ. Just a little bit of history repeating: the California model of marijuana legalization and how it might affect racial and ethnic minorities. Wash Lee J Civ Rts Soc Just 2010;17:557. [Google Scholar]
- [177].Firth CL, Maher JE, Dilley JA, Darnell A, Lovrich NP. Did marijuana legalization in Washington state reduce racial disparities in adult marijuana arrests? Subst Use Misuse 2019;54:1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Tran NK, Goldstein ND, Purtle J et al. The heterogeneous effect of marijuana decriminalization policy on arrest rates in Philadelphia, Pennsylvania, 2009–2018. Drug Alcohol Depend 2020;212:108058. [DOI] [PubMed] [Google Scholar]
- [179].Hohl NM. Medical Marijuana and Stigma: A Causal Comparative Quantitative Study. Doctoral dissertation, Denver, USA: The University of the Rockies, 2018. [Google Scholar]
- [180].Ross PT, Hart-Johnson T, Santen SA, Zaidi NLB. Considerations for using race and ethnicity as quantitative variables in medical education research. Perspect Med Educ 2020;9:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Shanawani H, Dame L, Schwartz DA, Cook-Deegan R. Non-reporting and inconsistent reporting of race and ethnicity in articles that claim associations among genotype, outcome, and race or ethnicity. J Med Ethics 2006;32:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


