Abstract
Objective.
The pathogenesis of eosinophilic granulomatosis with polyangiitis (EGPA) remains poorly understood, and may overlap with eosinophilic asthma and primary hypereosinophilic syndrome (HES). The aim of this study was to analyse a panel of serum cytokines and chemokines as markers of disease activity in patients with these conditions.
Methods.
The levels of 54 cytokines and chemokines were measured in the sera of 40 patients with active EGPA, 10 of these patients during inactive disease, 6 patients with HES, 8 with asthma, and 10 healthy controls. Serum cytokine/chemokines measured included interleukin (IL)-1α, 1β, 3, 4, 5, 6, 8, 13, 15, 17A, 17E(25), 18, 23 and 33, soluble IL-2 receptor alpha, eotaxin-1 (CCL11), -2 (CCL24) and -3 (CCL26), macrophage-derived chemokine (MDC/CCL22), macrophage inflammatory protein (MIP)-1a and -1b, and tumour necrosis factor (TNF)-α. Results were compared between disease and control groups using regression analysis, with Bonferroni correction for multiple comparisons (significant p-value ≤0.00093).
Results.
Significant differences were observed only in serum levels of MDC, IL-8, MIP-1a and -1b, TNF-α, each of which were lower in patients with active EGPA than in healthy controls (p<0.0001). Differences between patients with active disease and other disease groups did not reach significance. Paired comparisons between sera from patients with active or inactive EGPA showed no significant difference for any of the studied cytokines or chemokines.
Conclusion.
No clear difference in the serum levels of measured cytokines and chemokines helped distinguish between active EGPA or inactive EGPA, or other disease or control groups.
Keywords: eosinophilic granulomatosis with polyangiitis, cytokines, chemokines, asthma
Introduction
The main characteristics of eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss) include asthma with eosinophilia and vasculitic manifestations such as purpura. Early diagnosis of EGPA and the differentiation between EGPA and primary hypereosinophilic syndrome (HES) can be challenging but is important, as their therapeutic management and outcomes differ (1).
A few studies suggested that patients with EGPA may be distinguished from those with HES or eosinophilic asthma based on parameters other than ANCA, present in only 40% of EGPA patients, and histological evidence of vasculitis (1, 2). Immunophenotyping, T-cell clonal and cytogenetic studies, and molecular analyses to detect Fip1-like 1 (FIP1L1)–platelet-derived growth factor receptor-a (PDGFRA) gene fusion in the blood or bone marrow may help identify some patients with HES (3, 4). Studies on several serum eosinophilderived proteins and cytokines have led to variable, sometimes discordant, results (3, 5–11).
The aim of this study was to analyse a large panel of cytokines and chemokines in patients with EGPA, active or in remission, asthma or HES and controls.
Materials and methods
The levels of 54 cytokines and chemokines were measured in 40 patients with EGPA (including 10 tested twice, during active then inactive disease phases or the reverse), 6 patients with HES, 8 with asthma and 10 healthy controls.
Patients and controls
Patients with EGPA enrolled in this study have been previously entered in the Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study, and satisfied the Chapel Hill nomenclature and/or the American College of Rheumatology classification criteria for EGPA (12, 13). At the time of this study, 170 EGPA patients had been enrolled in the VCRC Study; 40 of them with serum samples collected during active disease, as assessed by the site investigators at each visit (14), were randomly selected. Serum samples collected at the time of a visit in remission were also used for 10 of these 40 patients.
Controls included subjects who had no vasculitis and followed in the Respirology and Immunology Clinic (Hospital, Hamilton) with i) asthma (n=8), previously documented with pulmonary function tests; and ii) HES (n=6). Patients with HES fulfilled the Chusid criteria for diagnosis of idiopathic HES, needed to have blood eosinophil count >1,500 per microliter for ≥6 months and eosinophilia-related organ involvement or dysfunction, with no other identifiable secondary cause of eosinophilia (15). Healthy controls (n=10) were volunteers, non-smokers, with no abnormality on metacholine test (done for another reason than the study), and not on any glucocorticoids or other immunosuppressive treatment. Local IRBs (Mount Sinai Hospital, Toronto and St. Joseph’s healthcare center, Hamilton) approved the study and informed consents were obtained from all participants in the study.
Laboratory measurements
Blood samples (one 7 ml blood vial) were collected and centrifugated on site and the serum extracted and aliquoted in labelled vials, stored at −80°C before being sent to Calgary (Multiplexing Eve technologies, Calgary, AL) for analysis. Frozen serum samples from patients with EGPA were sent from the VCRC Biospecimen Repository, and those from patients with asthma, HES and controls directly from the Hamilton clinic.
Serum cytokine/chemokine measurements were performed in duplicates using customised Human Plex Cytokine/Chemokine Panels (Multiplexing Eve technologies, Calgary, AL; discoveryassay.com) that allowed the simultaneous analysis of interleukin ((IL)-1α, 1β, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12 (p40 and p70), 13, 15, 17a, 17E(25), 18, 23 and 33, IL-1 receptor antagonist (IL-1ra), soluble CD40 ligand (sCD40L), soluble IL-2 receptor alpha (sIL-2Rα), eotaxin-1 (CCL11), -2 (CCL24) and -3 (CCL26), GRO-Pan (chemokine ligand CXCL1/2/3/GRO), macrophage-derived chemokine (MDC/CCL22), macrophage inflammatory protein (MIP)-1α and -1β, monocyte chemotactic protein-3 (MCP-3), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), interferon (IFN)-α2 and -γ, IFN-γ-inducible protein (IP)-10, FMS-like tyrosine kinase 3 ligand (Flt-3L), thymic stromal lymphopoietin (TSLP), thymus and activation-regulated chemokine (TARC), monocyte chemoattractant protein-1 (MCP-1), tumour necrosis factor (TNF)-α and -β, TNF-related apoptosis-inducing ligand (TRAIL), transforming growth factor (TGF)-α, regulated on activation, normal T cell expressed and secreted (RANTES), platelet-derived growth factor (PDGF)-AA and -BB, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) A, fibroblast growth factor (FGF)-2, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), soluble form of receptor for advanced glycation end products (sRAGE) and fractalkine (using the Human Cytokine 41-plex Discovery Assay, Custom 1-Plex Kit & Assay service; Human Cytokine Custom 7 Plex Kit & Assay Service; and the HNDGMAG-36K Human Neurodegenerative P3 Custom 2 Plex Kit & Assay Service).
Statistics
The numbers of enrolled patients and controls were based on study feasibility, with a recruitment period running from March 2012 to March 2015. Laboratory values are given as median [interquartile range (IQR)]. Cytokine/chemokine levels, according to groups (active EGPA vs. asthma, HES or controls), were compared using SAS PROC LIFEREG, which allowed for the analysis of normal and log-normal censored cytokine data. Cytokine/chemokine levels were also compared in the 10 patients with EGPA who had serum samples collected at the times of active disease and in remission, using paired Mann-Whitney tests. The Bonferroni correction was used for adjustment to p-values for multiple comparisons, with only p-values ≤0.00093 considered significant (16). Analyses were performed using SAS Software v. 9.4 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
At the time of serum sampling, for the 40 patients with EGPA with active disease (mean age 50.7±16.2 years, 17 male patients, 4 have had detectable ANCA), mean eosinophil count was 7,270±11,260/mm3; 31 were taking glucocorticoids and 22 on another immunosuppressive agent (combined with glucocorticoids in 18 of them). At the time of repeat serum sampling in remission, for 10 of these EGPA (mean age 47.6±11.4 years, 3 male patients, 1 have had detectable ANCA), 9 were on glucocorticoids, with 5 of them also on another immunosuppressive agent.
As shown in Figure 1 and Table I, for the 54 cytokines and chemokines measured, the only significant differences between active EGPA and the other groups were observed in serum levels of MDC, IL-8, MIP-1a, and -1b, TNF-α (p<0.0001 for the global comparison between groups), which were especially lower than in healthy controls.
Fig. 1.
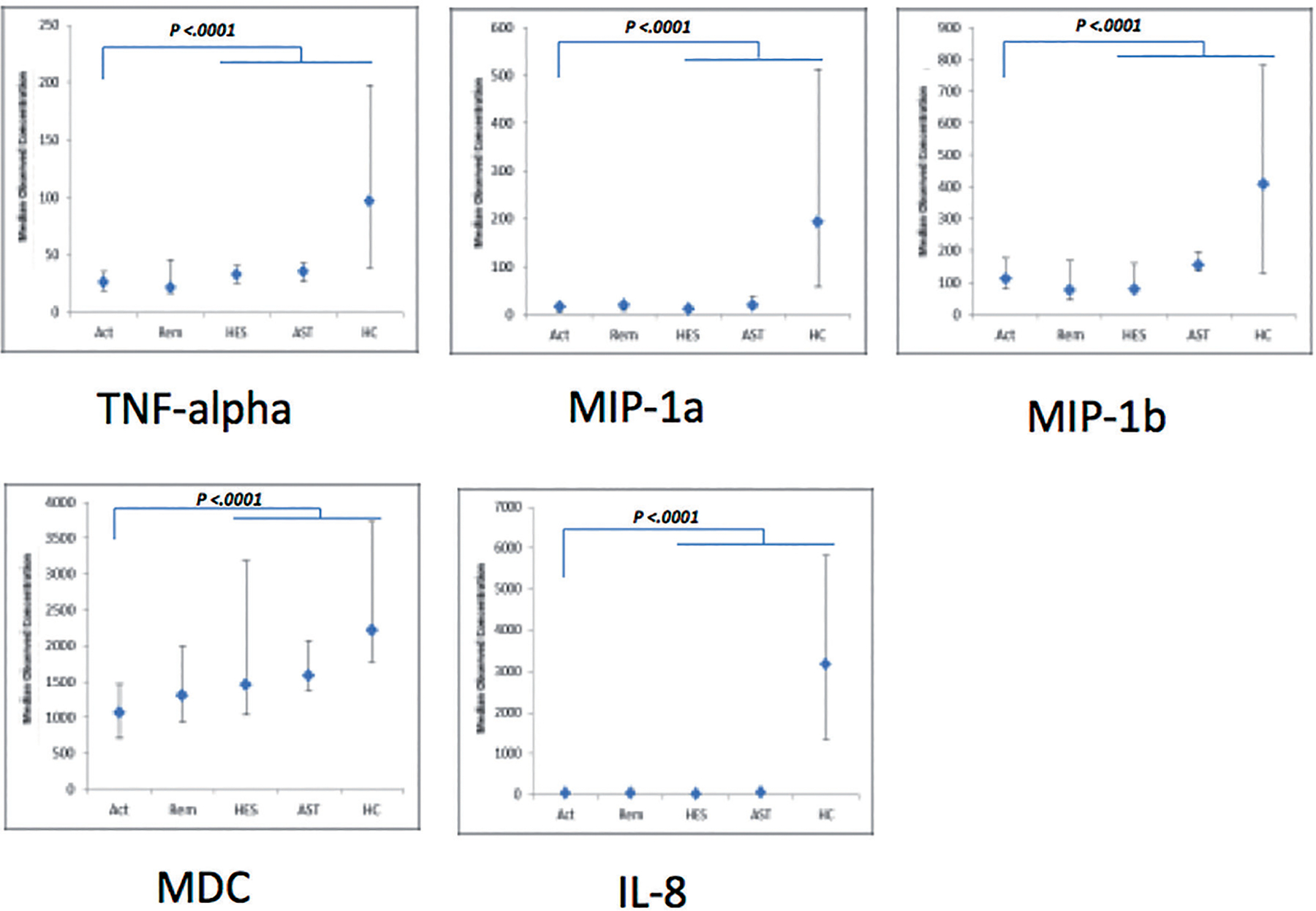
Serum levels (median [interquartile range (IQR)]; pg/ml) of the 5 cytokines and chemokines with a significant overall difference (p ≤0.00093) between patients with active EGPA [Act] and asthma [AST], hypereosinophilic syndrome [HES] or controls [HC]). Serum values for the 10 patients with EGPA also tested during remission [Rem] are indicated (but were compared only to the patients with active EGPA, using paired Mann-Whitney tests - cf. Table.
Table I.
Main results of the logistic regression for all tested cytokines and chemokines (comparisons between values in patients with active eosinophilic granulomatosis with polyangiitis [EGPA] vs. other 3 groups – hypereosinophilic syndrome [HES], healthy controls and asthma patients).
| Group | Coefficients (intercept) | Standard error | p-value | Overall p-value | |
|---|---|---|---|---|---|
|
| |||||
| MDC* | EGPA | 6.9624 | 0.0935 | ||
| HES | 0.4851 | 0.2589 | 0.0609 | 0.0002 | |
| Controls | 0.8064 | 0.2091 | 0.0001 | ||
| Asthma | 0.5817 | 0.2290 | 0.0111 | ||
| IL-8 | EGPA | 3.5016 | 0.1596 | ||
| HES | −0.2476 | 0.4418 | 0.5752 | <0.0001 | |
| Controls | 4.1536 | 0.3568 | <0.0001 | ||
| Asthma | 0.5723 | 0.3909 | 0.1431 | ||
| MIP-1a | EGPA | 2.3123 | 0.1993 | ||
| HES | 0.2962 | 0.5496 | 0.5900 | <0.0001 | |
| Controls | 3.0718 | 0.4440 | <0.0001 | ||
| Asthma | 0.9132 | 0.4863 | 0.0604 | ||
| MIP-1b | EGPA | 4.7136 | 0.1420 | ||
| HES | 0.1706 | 0.3933 | 0.6646 | 0.0008 | |
| Controls | 1.2732 | 0.3176 | <0.0001 | ||
| Asthma | 0.5134 | 0.3479 | 0.1401 | ||
| TNF-α | EGPA | 3.2469 | 0.1197 | ||
| HES | 0.5696 | 0.3314 | 0.0857 | <0.0001 | |
| Controls | 1.2551 | 0.2676 | <0.0001 | ||
| Asthma | 0.4480 | 0.2932 | 0.1265 | ||
| Flt-3L | 0.0020 | ||||
| EGF | 0.0022 | ||||
| RANTES | 0.0022 | ||||
| IFNy | 0.0027 | ||||
| MCP 1 | 0.0028 | ||||
| IL-17A | 0.0033 | ||||
| TRAIL | 0.0033 | ||||
| TARC | 0.0045 | ||||
| sCD40L | 0.0061 | ||||
| IL-4 | 0.0143 | ||||
| IL-1a | 0.0172 | ||||
| IL-6 | 0.0382 | ||||
| IL-15 | 0.0432 | ||||
| IFNa2 | 0.0527 | ||||
| GCSF | 0.0646 | ||||
| sVCAM1 | 0.0670 | ||||
| sIL2Ra | 0.0775 | ||||
| GM-CSF | 0.0905 | ||||
| TNF-β | 0.0914 | ||||
| MCP 3 | 0.0942 | ||||
| IL-13 | 0.1142 | ||||
| IL-12P40 | 0.1262 | ||||
| IL-3 | 0.1334 | ||||
| IP10 | 0.1459 | ||||
| Eotaxin 1 | 0.1623 | ||||
| IL-5 | 0.2068 | ||||
| Fractalkine | 0.2699 | ||||
| PDGF AA | 0.3184 | ||||
| IL-12P70 | 0.3255 | ||||
| IL-1β | 0.3673 | ||||
| TGF-α | 0.3816 | ||||
| Eotaxin 3 | 0.4991 | ||||
| sICAM-1 | 0.4992 | ||||
| GRO pan | 0.5152 | ||||
| IL-23 | 0.5305 | ||||
| IL-1RA | 0.6700 | ||||
| IL-17E | 0.6758 | ||||
| TSLP | 0.7127 | ||||
| IL-33 | 0.7218 | ||||
| FGF 2 | 0.7317 | ||||
| IL-9 | 0.7586 | ||||
| IL-18 | 0.8022 | ||||
| VEGF A | 0.8363 | ||||
| IL-2 | 0.8882 | ||||
| IL-7 | 0.8890 | ||||
| PDGF BB | 0.9312 | ||||
| IL-10 | 0.9485 | ||||
| Eotaxin 2 | 0.9601 | ||||
| sRAGE | 0.9774 | ||||
see text (Methods) for full extended names of cytokines/chemokines.
Complete results with regression coefficients (intercept) for each group are listed only for the five cytokines and chemokines with a significant overall difference (p≤0.00093; complete results for all cytokines and chemokines are in online Suppl. Table I).
Paired comparisons between sera from patients with active or inactive EGPA showed no significant difference for any of the studied cytokines or chemokines (Table II).
Table II.
Results of the paired comparisons between levels of cytokines and chemokines in the 10 patients with eosinophilic granulomatosis with polyangiitis (EGPA) who had serum samples collected at times of active disease and in remission, using paired Mann-Whitney tests.
| Cytokine / chemokine | p-value | Cytokine / chemokine | p-value |
|---|---|---|---|
|
| |||
| PDGF AA | 0.00169 | IFNγ | 0.26454 |
| ARC | 0.01576 | TNF-α | 0.27956 |
| IL-12P40 | 0.02486 | IL-17E | 0.29593 |
| sIL2Ra | 0.04222 | IL-2 | 0.31585 |
| IL-23 | 0.04373 | Fractalkine | 0.32735 |
| IL-8 | 0.04452 | MCP 1 | 0.33087 |
| IL-15 | 0.04731 | MIP-1a | 0.37405 |
| IL-33 | 0.05093 | IL-13 | 0.39866 |
| GM-CSF | 0.08015 | GCSF | 0.42138 |
| sVCAM 1 | 0.09238 | IL-5 | 0.51945 |
| IFNa2 | 0.11867 | EGF | 0.52008 |
| TNF-β | 0.12970 | IL-1B | 0.53870 |
| IL-17A | 0.13732 | MCP 3 | 0.54557 |
| RANTES | 0.14018 | IL-18 | 0.59630 |
| TSLP | 0.15760 | FGF 2 | 0.64492 |
| IL-9 | 0.15770 | sCD40L | 0.67339 |
| IL-6 | 0.15822 | IL-10 | 0.70935 |
| IL-4 | 0.17291 | TRAIL | 0.79040 |
| IL-12P70 | 0.17938 | sICAM 1 | 0.81553 |
| IL-1a | 0.18349 | IL-1RA | 0.84591 |
| IP10 | 0.18436 | TGF-α | 0.88472 |
| Eotaxin 1 | 0.18738 | Eotaxin 2 | 0.88541 |
| GRO pan | 0.20302 | MDC | 0.88642 |
| IL-7 | 0.21781 | Eotaxin 3 | 0.89439 |
| Flt3L | 0.22748 | IL-3 | 0.92451 |
| sRAGE | 0.23766 | MIP-1B | 0.99501 |
| VEGF A | 0.25910 | PDGF BB | 0.99915 |
No difference reached the significant p-value of ≤0.00093, including for the five cytokines and chemokines (in bold) whose levels differed between patients with active EGPA vs. healthy controls, patients with asthma or hypereosinophilic syndrome.
Discussion
This study failed to identify specific and distinct cytokine and chemokine differences between patients with EGPA or control groups, which may have provided new insights into disease pathophysiology or been of interest for diagnostic purpose. Only the levels of MDC, IL-8, MIP-1a and -1b, and TNF-α, which are mainly produced by macrophages and are pro-inflammatory (17–19), were found to differ between patients with active EGPA and controls, but no clear difference was observed between active or inactive EGPA, or versus patients with HES or asthma.
In a few previous studies with a more limited panel of cytokines or chemokines, eotaxin-1 levels were found to be increased in subjects with asthma and/or sinusitis compared with HES and normal controls, as well as serum IL-10 levels in patients with EGPA, either active or in remission compared to normal controls. Serum eosinophil cationic protein, IL-5, IL-25, RANTES, TRAIL, VEGF or eotaxin-3 (CCL26) were increased in a few studies in patients in active EGPA, but some of these were also increased in patients with asthma or other eosinophilic conditions (6–10). However, and similarly to our findings, none of the several serum biomarkers examined in Khoury et al. or Dejacco et al., including IL-10 or eotaxin-3, were found useful in differentiating between subjects with EGPA (definite or probable) and HES with asthma (3, 5). The reasons for these variable findings between studies remain unclear. Our findings could also suggest that, whereas most emphasis in the pathophysiology of EGPA has been given to eosinophils and some cytokines, more should possibly be given to macrophages and neutrophils, and treatments should not be determined based only on cytokine levels.
Caution must be exercised in interpreting the results of studies on serum levels of cytokines and chemokines in EGPA. Treatment may affect levels of several cytokines. The use of glucocorticoids was associated with a lower level of eotaxin-3 in the sera of patients with EGPA in the study by Dejacco et al., but levels of TARC were not affected in the study by Khoury et al. (3, 5). The measurements of serum cytokines and chemokines may also be technically challenging, due to conservation methods and glycoprotein degradation ex vivo. There are currently no FDA-approved cytokine measurement kit, and no international units to which existing kits could be calibrated towards (20). No reference ranges were available for the cytokines and chemokines tested with the kits used in this study. Other techniques, such as the measurement of gene expression in peripheral blood cells or single nucleotide polymorphisms, may be more reliable and suitable to analyse cytokines and chemokines in these conditions. Urine levels, rather than serum levels, of cytokines or chemokines may also be more informative, at least in patients with renal involvement. Finally, sample sizes are small, due to the rarity of EGPA, and only 10% of our EGPA patients have had detectable ANCA, which limited the power of this study and prevented us from conducting any meaningful subgroup analysis (e.g. to compare patients with EGPA on glucocorticoids or not), and some clinical information were lacking for control groups, including on treatments.
In summary, this study found no major differences in the serum levels of a large panel of cytokines and chemokines that could distinguish between patients with active or inactive EGPA, HES or asthma. Conversely, these data may suggest that clinical and biological overlaps exist between these conditions.
Supplementary Material
Acknowledgements
Dr Michael Trus, Katherine Radford and Svetlana Davydchenko (all from the Division of Respirology, St. Joseph’s Healthcare, McMaster University, Hamilton, ON, Canada) helped recruit the HES, asthma and control subjects.
Funding
This work was supported by the Vasculitis Clinical Research Consortium (VCRC), a research grant from the Arthritis & Autoimmunity Research Centre Foundation (Toronto, ON, Canada), and the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The Vasculitis Clinical Research Consortium (VCRC) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS). The VCRC is funded through collaboration between NCATS (U54 RR019497), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR057319). P. Nair was supported by the Frederick E. Hargreave Teva Innovation Chair in Airway Diseases.
Footnotes
Competing interests: C.A. Langford has received research grant from Glaxo Smith Kline; the other co-authors have declared no competing interests.
References
- 1.GROH M, PAGNOUX C, BALDINI C et al. : Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med 2015; 26: 545–53. [DOI] [PubMed] [Google Scholar]
- 2.COMARMOND C, PAGNOUX C, KHELLAF M et al. : Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013; 65: 270–81. [DOI] [PubMed] [Google Scholar]
- 3.KHOURY P, ZAGALLO P, TALAR-WILLIAMS C et al. : Serum biomarkers are similar in Churg-Strauss syndrome and hypereosinophilic syndrome. Allergy 2012; 67: 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VALENT P, KLION AD, HORNY HP et al. : Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130: 607–12 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DEJACO C, OPPL B, MONACH P et al. : Serum biomarkers in patients with relapsing eosinophilic granulomatosis with polyangiitis (Churg-Strauss). PLoS One 2015; 10: e0121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ZWERINA J, BACH C, MARTORANA D et al. : Eotaxin-3 in Churg-Strauss syndrome: a clinical and immunogenetic study. Rheumatology 2011; 50: 1823–7. [DOI] [PubMed] [Google Scholar]
- 7.DALLOS T, HEILAND GR, STREHL J et al. : CCL17/thymus and activation-related chemokine in Churg-Strauss syndrome. Arthritis Rheum 2010; 62: 3496–503. [DOI] [PubMed] [Google Scholar]
- 8.HELLMICH B,CSERNOK E, GROSS WL: Proinflammatory cytokines and autoimmunity in Churg-Strauss syndrome. Ann NY Acad Sci 2005; 1051: 121–31. [DOI] [PubMed] [Google Scholar]
- 9.POLZER K, KARONITSCH T, NEUMANN T et al. : Eotaxin-3 is involved in Churg-Strauss syndrome--a serum marker closely correlating with disease activity. Rheumatology 2008; 47: 804–8. [DOI] [PubMed] [Google Scholar]
- 10.CAPECCHI R, MANGANELLI S, PUXEDDU I et al. : CCL5/RANTES in ANCA-associated small vessel vasculitis. Scand J Rheumatol 2012; 41: 403–5. [DOI] [PubMed] [Google Scholar]
- 11.MONACH PA, WARNER RL, TOMASSON G et al. : Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Ann Rheum Dis 2013; 72: 1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.JENNETTE JC, FALK RJ, BACON PA et al. : 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65: 1–11. [DOI] [PubMed] [Google Scholar]
- 13.MASI AT, HUNDER GG, LIE JT et al. : The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33: 1094–100. [DOI] [PubMed] [Google Scholar]
- 14.GRAYSON PC, MONACH PA, PAGNOUX C et al. : Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford) 2015; 54: 1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CHUSID MJ, DALE DC, WEST BC, WOLFF SM: The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975; 54: 1–27. [PubMed] [Google Scholar]
- 16.HOCHBERG Y, BENJAMINI Y: More powerful procedures for multiple significance testing. Stat Med 1990;9:811–8. [DOI] [PubMed] [Google Scholar]
- 17.MANTOVANI A, GRAY PA, van DAMME J, SOZZANI S : Macrophage-derived chemokine (MDC). J Leukoc Biol 2000; 68: 400–4. [PubMed] [Google Scholar]
- 18.EMAURER M, von STEBUT E: Macrophage inflammatory protein-1. Int J Biochem Cell Biol 2004; 36: 1882–6. [DOI] [PubMed] [Google Scholar]
- 19.HERNANDEZ-PANDO R, ROOK GA: The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology 1994; 82: 591–5. [PMC free article] [PubMed] [Google Scholar]
- 20.DUPUY AM, KUSTER N, LIZARD G et al. : Performance evaluation of human cytokines profiles obtained by various multiplexed-based technologies underlines a need for standardization. Clin Chem Lab Med 2013; 51: 1385–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


