Abstract
Isodon suzhouensis from Suzhou, China, is a traditional Chinese herb with wide applications in medicine and food. The antioxidant activity against oxidative stress of the leaves of Isodon suzhouensis is a myth since long and is not explored earlier thoroughly. The present study is focused to explore the active components in Isodon suzhouensis leaf extracts responsible for antioxidant effects against oxidative stress and the potential mechanism of this activity. We obtained the chromatograms of Isodon suzhouensis leaf extracts by the high-performance liquid phase (HPLC) for possible detection of antioxidant constituents. Some compounds in Isodon suzhouensis leaf extracts were then further assessed through the luminol luminescence mechanism combined with HPLC analysis as well as with SwissTargetPrediction database that helped to screen out the other constituents. The targets for effects against oxidative stress were then further screened through the GeneCards database, and the PPI network was constructed. The targets were analyzed by GO and KEGG using the David database. The obtained results were then further studied by employing in vitro experimentation and protein expression analyses by Western blotting. It is found that Isodon suzhouensis leaf extracts contain rutin, isoquercetin, glaucocalyxin A, glaucocalyxin B, and other compounds with antioxidant activity. The activity map of the free radical scavenging signals from Isodon suzhouensis showed a strong ability to scavenge free radicals with the highest capacity of glaucocalyxin B followed by isoquercetin succeeding the glaucocalyxin A supervening the rutin. Further network pharmacological analyses and in vitro experimentation showed that Isodon suzhouensis leaf extracts interfere with TNF and the p38 MAPK signaling pathway for antioxidant effects against oxidative stress. Conclusively, it is found that Isodon suzhouensis leaf extracts possess strong antioxidant potential via targeting TNF and p38 MAPK signaling pathways against oxidative stress, providing scientific foundation for further studies on Isodon suzhouensis for the further therapeutic approach.
1. Introduction
Oxidative stress is a manifestation of the imbalance between oxidative and antioxidant metabolism. It is a serious metabolic disorder caused by the increase of reactive oxygen species (ROS), resulting in a certain degree of injury.1,2 In this state, a large number of free radicals attack the body cells, causing apoptosis, necrosis, and several other ailments.3 At the same time, the increase of ROS in the body can activate the signal pathway sensitive to oxidative stress and further cause cell damage.4 Recent studies have shown that the abnormal expression of free radicals often leads to diseases and cancer, aging, and many other diseases.5,6 However, antioxidants can effectively overcome the harm caused by too many free radicals. Therefore, the antioxidant properties of substances have become one of the mainstream research and development directions, and it is a need of time to find effective and safe natural antioxidants.7,8
Isodon suzhouensis is a perennial herb of the genus Camellia from the Labiatae family.9 It mainly grows in the natural ecological environment areas of the mountain forests and hillsides in Suzhou and other places of China.10 The local people in Suzhou call it “Wangzaozi” or “Wangsaozi”. Isodon suzhouensis contains a variety of effective medicinal ingredients, including flavonoids, terpenes, and other substances.11 It is popular among people because of its antibacterial, anti-inflammatory, anti-tumor, antithrombotic, body-calming, and detoxicating functions.12,13 Natural plant extracts are rich in active ingredients such as phenols and flavonoids, which are relatively safe and are regarded as an important source of exogenous antioxidant substances.14−16 Therefore, it is of great significance to study the antioxidant activity of Isodon suzhouensis and its intervention on oxidative stress.
Network pharmacology is the use of modern electronic information technology and then establishing a “drug-disease-target-pathway” network relationship. It is of great importance to predict and enrich the potential targets and pathways of traditional Chinese medicine in the treatment of diseases.17 It can reflect the complex interaction between biological macromolecules and chemical components to a great extent. The more effective components of traditional Chinese medicine can be screened out through network pharmacology, which can provide a basis for more targeted traditional Chinese medicine research.18 In addition, high-performance liquid phase (HPLC) has the characteristics of efficient separation, which, combined with the advantages of a wide linear range and high sensitivity of CL, has become effective to trace or even ultratrace analysis technology.19
In light of these facts, we designed our present study in which we first used the HPLC-CL technique to analyze the active antioxidant components in the leaves of Isodon suzhouensis and further analyzed the related effects on disease targets through network pharmacology and then used in vitro cell experiments to verify the protective effect of Isodon suzhouensis leaf extracts on cells under oxidative stress. The objective of the study was to explore the components from Isodon suzhouensis leaf extracts against oxidate stress and its potential mechanism for its potential therapeutic utility.
2. Materials and Methods
2.1. Materials and Reagents
Isodon suzhouensis leaves were supplied by Suzhou Lvyuan traditional Chinese Medicine Technology Co., Ltd. (Suzhou, China). Acetonitrile, 95% ethanol, luminol, H2O2, and methanol were purchased from Aladdin. Penicillin–streptomycin solution and fetal bovine serum were obtained from Biological Industries (Beit-Haemek, Israel). The Dulbecco’s modified Eagle medium (DMEM) medium was purchased from Gibco (Grand Island, NY, USA). The BCA protein quantification kit, SOD assay kit, MDA assay kit, and RIPI lysate were purchased from Beyotime (Shanghai, China). Antibodies against p-p38, β-actin, and HRP-labeled goat anti-rabbit were purchased from Bioworld. Antibodies for p38 and TNF-α were obtained from Proteintech (Wuhan, China). Cigarettes are purchased locally, and the oil content of each cigarette was 10 mg, the nicotine content in the smoke was 0.9 mg, and the carbon monoxide in the smoke was 12 mg. For comparison, standard compounds of more than 98% purity including glaucocalyxin A, glaucocalyxin B, isoquercetin, and rutin were obtained from Shanghai Chen Gong Biotechnology Co., Ltd.
2.2. HPLC-CL Analysis
2.2.1. Sample and Solution Preparation
Preparation of sample solution: dried Isodon suzhouensis leaves were crushed into powder in a traditional Chinese medicine grinder and the powder was sifted through 80 mesh, as well as 2.0 g of Isodon suzhouensis leaf powder was precisely weighed and placed in a conical bottle with a stopper. It was then further treated ultrasonically with 95% ethanol 20 mL at 40 °C for 50 min and then filtered. After each end, the filter residue was filtered and 20 mL of 95% ethanol was added to continue to extract twice with the same method. Finally, the filtrates were combined and fixed to 100 mL using 95% ethanol. The collected samples were processed by filtration through a 0.45 μm microporous membrane; then, a part of them was injected into HPLC-CL for analysis and the other part was subjected to rotary evaporation, and the obtained concrete was weighed and stored away from light for cellular experiments.
Preparation of standard solutions of glaucocalyxin B: The standard 4.235 mg standard of glaucocalyxin B was precisely weighed, dissolved in ethanol, and then, a fixed volume was put into a 10 mL volumetric flask, and the concentration of 0.4235 mg/mL standard solution was obtained. It was labeled and stored in a refrigerator at 4 °C, avoiding light exposure.
Preparation of standard solutions of rutin: The standard solution of rutin was prepared by precisely weighing it to 3.424 mg, dissolved in ethanol, and then put into a 10 mL volumetric flask to prepare the rutin standard solution of 0.3424 mg/mL concentration. It was labeled and stored in a refrigerator at 4 °C, avoiding light exposure.
Preparation of standard solutions of glaucocalyxin A: The standard solution of glaucocalyxin A was prepared by precisely weighing 3.660 mg of glaucocalyxin A and dissolving it in ethanol, and then, a fixed volume was put into a 10 mL volumetric flask to make the standard solution of glaucocalyxin A with 0.366 mg/mL. It was labeled and stored in a refrigerator at 4 °C, avoiding light exposure.
Preparation of standard solutions of isoquercitrin: The isoquercitrin 2.176 mg standard solution was prepared by precisely weighing and dissolving in ethanol; then a fixed volume was put into the 10 mL volumetric flask, and the concentration of 0.2176 mg/mL of isoquercitrin standard solution was prepared. It was labeled and stored in a refrigerator at 4 °C, avoiding light exposure.
Preparation of mixed standard solution: The four single standard solutions prepared above were taken, 1 mL of each solution was taken and put into a 10 mL volumetric flask, and then the volume was fixed. A mixed standard solution of rutin with a concentration of 0.03424 mg/mL, glaucocalyxin B with a concentration of 0.04235 mg/mL, glaucocalyxin A with a concentration of 0.0366 mg/mL, and isoquercitrin with a concentration of 0.02176 mg/mL was prepared.
Preparation of Luminol–H2O2 solution: preparation of 0.5% hydrogen peroxide: 0.5 mL of H2O2 was diluted to 100 mL; preparation of luminol test solution: 0.2320 g of luminol was precisely weighed and dissolved in 0.1 mol/L NaOH with carbonic acid buffer solution to a fixed volume to 1 L; preparation of 0.5% hydrogen peroxide: 0.5 mL of H2O2 was diluted to 100 mL.
2.3. HPLC Conditions
The model of HPLC equipment is LC-20 AD (Shimadzu, Japan). The separation was performed on a ShimPackODSC18 (250 mm × 4.6 mm, 5 μm) column, with acetonitrile (A) and 0.5% acetic acid solution (B) as the mobile phase for binary gradient elution. The total flow rate was 0.8 mL/min, the column temperature was 30 °C, and the detection wavelength was 250 nm. For the detection solution and standard mixed solution, the binary gradient elution time procedure of WZZE is shown in Table 1.
Table 1. Gradient Elution Conditions.
| T (min) | acetonitrile (A) | 0.5% acetic acid (B) |
|---|---|---|
| 0 | 10 | 90 |
| 10 | 12 | 88 |
| 20 | 16 | 84 |
| 25 | 25 | 75 |
| 35 | 40 | 60 |
| 45 | 22 | 78 |
2.4. Establishment of the HPLC-CL System
The system is composed of an HPLC device, and a chemiluminescence detection system device connected by a tee joint and a polytetrafluoroethylene tube. It includes the composition of the relevant parts of the liquid phase device: high-pressure transfer pump, chromatographic column, automatic sampler, and chemiluminescence device (BPCL-1-ZD, Institute of Biophysics, Chinese Academy of Sciences): peristaltic pump, detection cell, photomultiplier tube, and so on; refer to Figure 1 for details. The main principle is that after the sample is separated by a chromatographic column, each active component is mixed with the working liquid of the light-emitting device behind the column, the detector can synchronously record the chromatographic signal and the luminous signal, and the active substance that can scavenge free radicals is converted into an electrical signal, which appears in the map in the form of an inverted peak.
Figure 1.
HPLC-CL system device diagram.
2.5. Network Pharmacology Analysis
The secondary structures of rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B were predicted by Pubchem database (https://pubchem.ncbi.nlm.nih.gov/), and the targets of these compounds were predicted by SwissTargetPrediction database (http://www.swisstargetprediction.ch/). Using the Genecards database (http://genecards.org/) to search with “antioxidant” as the keyword, the relevant targets of antioxidation were obtained, and the targets corresponding to the active components of the four compounds in Isodon suzhouensis were intersected, and the potential targets of the four compounds interfering with antioxidation were obtained. The target data were imported into David database (https://david.ncifcrf.gov/list.jsp) for GO (Gene Ontology) analysis and KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis. Taking P < 0.05 as the screening condition, the top 10 items in GO analysis and the top 20 items in KEGG analysis were drawn for visual analyses.20
2.6. Cell Experiment
2.6.1. Cell Culture and Treatment
RAW264.7 was placed in DMEM medium containing 10% fetal bovine serum and 1% double antibody and was routinely cultured in a cell incubator at 37 °C and 5% CO2. The cigarette smoke was sucked by a syringe and injected into the flask of 20 mL 10% FBS DMEM. The flask was shaken to make it dissolve completely. The pH was adjusted to about 7.4 by NaOH. The obtained solution was sterilized by a 0.22 μm filter membrane and used as the raw solution of 100% CSE. In addition, the extract from the leaves of Isodon suzhouensis used in the HPLC experiment was rotated and evaporated to the shape of concrete and dried in a water bath to get the extract powder of Isodon suzhouensis and dissolve to get WZZE. When the RAW264.7 cells grew to more than 80% of the Petri dish area, the cells were digested and centrifuged, and 1 × 106 cells per dish were inserted into the cell Petri dish. The control group, model group (CSE group), and administration group (CSE + WZZE 10, 50, 100 μg/mL) were set up and cultured again at 37 °C and 5% CO2 for 24 h in the cell culture box. (The final concentration of CSE was 10%, and this concentration of CSE was verified by us to be able to produce some oxidative damage to the cells while maintaining their relative viability.)
2.7. ROS Detection
The cells were treated according to the above groups; after 24 h, the cells were washed with PBS 3 times. The DCFH-DA DMEM medium containing 10 μmol/L was added to each group. The level of ROS was observed under a fluorescence microscope after being cultured in the incubator for 30 min.
2.8. SOD and MDA Detection
The cells treated with CSE, WZZE, or rutin were collected and centrifuged at a rotational speed of 12,000 g. The supernatant was taken and the activities of SOD and content of MDA were detected strictly according to the operation steps of the kit.
2.9. Western Blot
RAW246.7 cells in the logarithmic growth phase were cultured in a 6 cm Petri dish. Twenty-four hours after administration, an appropriate amount of lytic solution was added. After 10 min, it was placed at −80 °C, and the cells were collected in a curette dish and placed in a refrigerator at 4 °C for 30 min. The supernatant was collected by centrifugation at 12,000 rpm for 10 min, and the protein was quantified by the BCA method. The protein was separated by 12% SDS-PAGE electrophoresis, transferred to a membrane, sealed with 5% BSA at room temperature for 3 h, incubated with the first antibody at 4 °C overnight, washed with TBST 3 times, (for 8 min every time), and incubated with the second antibody at room temperature for 1 h 8 min 3 times, added enhanced chemiluminescence (ECL) solution, and the target protein was detected by an e-BLOT Touch Imager (e-BLOT, Shanghai, China).
2.10. Statistical Analysis
Prism Graphpad 8 is used for statistical analysis, which is expressed by mean ± SEM. Student T test was used for comparison between the two groups, and univariate ANOVA analysis of variance was used for comparison between multiple groups. The P value of less than 0.05 was taken as a significant statistical difference.
3. Results
3.1. Method Validation
We took the stock solutions of different standards that have been configured, diluted them with gradient concentration to obtain 5 standard solutions for each component, injected the samples for detection, and performed linear regression on the content of standard (mg/mL) (X) with the chromatographic peak area (Y).
The sample solution was injected into the sample solution three times at 0, 4, 8, 12, and 24 h respectively. The contents of rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B in the samples were determined according to the chromatographic conditions described earlier. The results showed that the relative standard deviation (RSD) of the relative peak area of glaucocalyxin A, glaucocalyxin B, rutin, and isoquercitrin was 1.28, 1.72, 0.98, and 1.15%, respectively, indicating that the sample solution was stable within 24 h.
Twenty μL of rutin (0.3424 mg/mL), isoquercitrin (0.2176 mg/mL), glaucocalyxin B (0.0042 mg/mL), and glaucocalyxin A (0.0366 mg/mL) were measured. According to the previous chromatographic conditions, the total elution time was 60 min, and the peak area was determined by continuous injection 5 times. After calculation, the RSD values obtained were 0.19, 1.24, 0.56, and 1.12%, respectively, and the corresponding characteristics and number of chromatographic peaks were found with no significant change, indicating that the precision of the instrument is good.
Two grams of the same batch of samples with known content were precisely weighed, and three different doses of rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B were precisely added, and a total of 4 samples, using the chromatographic conditions mentioned above, were evaluated. The recoveries of isoquercitrin, rutin, glaucocalyxin A, and glaucocalyxin B were 101.1% (RSD 2.5%), 99.5% (RSD 2.1%), 100.8% (RSD 1.8%), and 102.2% (RSD 2.3%), respectively.
3.2. HPLC-CL Analysis
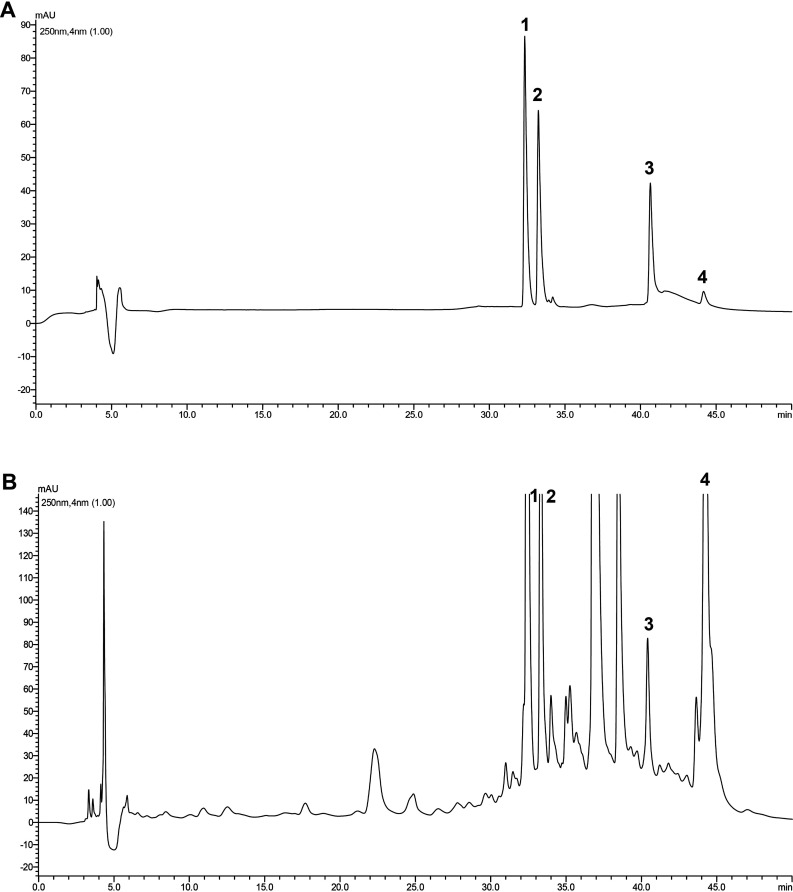
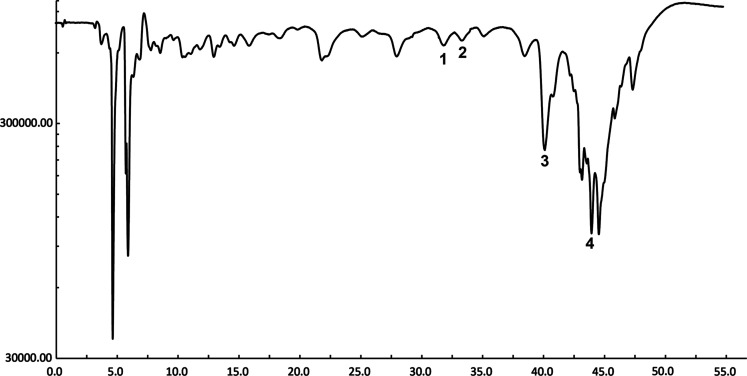
As the first step, the Suzhouensis leaf extracts were studied to obtain the peaks on graph (Figure 2B) as raw samples which were then further compared with the determined standards (rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B mixed standard HPLC graph), and they were compared and thoroughly identified, as shown in Figure 2A. It was found that signal peaks of 1, 2, and 4 were stronger and peak 3 was relatively weak in the HPLC graph plot of WZZE. The substances corresponding to peaks 1, 2, 3, and 4 were rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B. After the sample was separated by the chromatographic column, the activity spectrum 3 corresponding to the free radical scavenging signal of WZZE was synchronously recorded by HPLC-CL. It was found that the corresponding CL spectra of peaks 3 and 4 had a strong ability of scavenging free radicals, while peaks 1 and 2 were weak and concentrated within 30–45 min (Figure 3), indicating that glaucocalyxin A in WZZE has a strong ability of scavenging free radicals. At the same concentration, according to the above chromatographic conditions, the peak area of isoquercitrin was the largest, followed by those of glaucocalyxin B, rutin, and glaucocalyxin A being smaller. Combined with the free radical scavenging ability shown in Figure 3, it was deduced that the antioxidant activity pattern among these four compounds was glaucocalyxin B > isoquercitrin > glaucocalyxin A > rutin.
Figure 2.
HPLC diagram of mixed standard and leaves of Isodon suzhouensis. (A) HPLC chromatograms of standard substances, 1–4 were rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B, respectively. (B) HPLC chromatogram of Isodon suzhouensis leaves, 1–4 were rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B, respectively.
Figure 3.
Chemiluminescence map of Isodon suzhouensis leaves, 1–4 were rutin, isoquercitrin, glaucocalyxin A, and glaucocalyxin B, respectively.
3.3. Content Determination
For content determination, we obtained four linear regression equations about chromatographic peak areas and standard content curves, as shown in Table 2. It was found that there was a good linear relationship between the rutin concentration and the peak area at 35–340 μg/mL, isoquercitrin at 25–200 μg/mL, the glaucocalyxin A concentration and the peak area at 40–350 μg/mL, and glaucocalyxin B at 45–420 μg/mL. The content of each substance in the sample was obtained by calculation. According to the linear relationship equation and the peak area of the sample, the content of each substance in the standard was calculated. The results are shown in Table 3.
Table 2. Linear Relationship.
| compound | linear relationship | r2 | linear range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| rutin | Y = 12791289.523X + 198999.733 | 0.999 | 35–340 | 0.121 | 0.360 |
| isoquercitrin | Y = 46495647.521X + 176085.449 | 0.999 | 25–200 | 0.501 | 1.511 |
| glaucocalyxin A | Y = 12782983.607X + 480015.667 | 0.996 | 40–350 | 0.131 | 0.402 |
| glaucocalyxin B | Y = 16993892.388X + 11168.291 | 0.999 | 45–420 | 0.114 | 0.330 |
Table 3. Contents of Various Substances in Leaves of Isodon suzhouensis.
| batch number | rutin (mg/g) | isoquercitrin (mg/g) | glaucocalyxin A (mg/g) | glaucocalyxin B (mg/g) |
|---|---|---|---|---|
| 20210603 | 1.43909 | 0.99037 | 1.28695 | 2.04525 |
| 20210604 | 1.47087 | 0.98927 | 1.32558 | 2.06985 |
| 20210605 | 1.46943 | 0.99135 | 1.29615 | 2.07233 |
| average content | 1.459797 | 0.99033 | 1.302893 | 2.062477 |
3.4. Target Prediction of WZZE
The four identified compounds were added for prediction by the SwissTargetPrediction database, and a total of 185 targets were selected; these were then further cross-matched with 4285 targets related to antioxidation by the GeneCards database. The four compounds in the leaves of Isodon suzhouensis were intersected with the targets related to antioxidation by Venn drawing software. The 131 targets were then further shortlisted, as shown in Figure 4.
Figure 4.

Venn diagram of antioxidant targets of four compounds in leaves of Isodon suzhouensis.
3.5. Go Analysis and KEGG Analysis of Antioxidation of Four Compounds in WZZE
Shortlist potential targets (131) of WZZE interfering with antioxidation were imported into David database for GO analysis and KEGG metabolic pathway enrichment analysis. The results of GO analyses (Figure 5A) showed that the biological process mainly include protein phosphorylation, response to foreign stimuli, drug response, and others. From the cellular composition, it can be inferred that these targets were mainly concentrated in the components of the plasma membrane, membrane raft, neuronal cell body, presynaptic membrane, and cell surface. Molecular functions were mainly concentrated at protein serine, threonine, tyrosine kinase activity, transmembrane, and receptor protein tyrosine kinase activity. KEGG pathway analyses showed that the main pathways focused on cancer pathways, ligand-receptor interactions that stimulate nerve tissue, calcium signaling pathways, lipid and atherosclerosis, as well as tumor necrosis factor signaling pathways (Figure 5B). These results collectively converged to appeal that WZZE may play an antioxidant role by regulating a variety of biological pathways to participate against oxidative stress pathways.
Figure 5.
GO and KEGG function enrichment analysis. (A) GO function enrichment analysis (cell component (CC), molecular function (MF), and biological process (BP)). (B) KEGG function enrichment analysis.
3.6. ROS Assay
The DCFH-DA probe method was used to detect the ROS content in cells, as shown in Figure 6. It was found that as compared to the blank group, the level of ROS in the model group was increased, and the difference was obvious. While comparing with the model group, the level of ROS in the cells treated with the WZZE medium-dose group (50 μg/mL) and high-dose group (100 μg/mL) decreased. The results showed that WZZE may effectively reduce the level of ROS in cells induced by CSE, thereby inhibiting oxidative stress and protecting cells.
Figure 6.
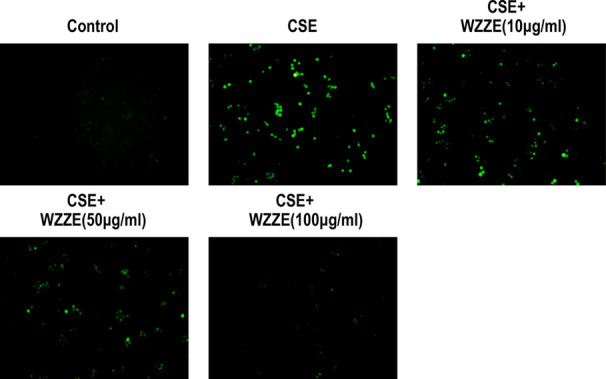
Effects of WZZE on ROS in CSE-induced RAW264.7 cells, photomicrographs were taken at 100×. Green fluorescence intensity represents the ROS content.
3.7. SOD and MDA Assay
To verify the effect of WZZE and its active ingredient rutin on the indexes related to the oxidative stress of RAW264.7 induced by CSE, we tested the SOD activity and MDA content in RAW264.7 cells exposed to CSE. As described in Figure 6A,C, the activity of SOD in RAW264.7 cells was significantly decreased under the induction of CSE; however, the expression of MDA was significantly increased. After WZZE intervention, this abnormal expression of SOD activity and MDA content were significantly reversed, and this reversal phenomenon increased with the increase of the drug concentration. In addition, rutin has the same effect as WEEZ (Figure 7B,D).
Figure 7.
Effects of WZZE and rutin on SOD and MDA in CSE-induced RAW264.7 cells. (A) Effect of WZZE on SOD activity in CSE-induced RAW264.7 cells. (B) Effect of rutin on SOD activity in CSE-induced RAW264.7 cells. (C) Effect of WZZE on MDA content in CSE-induced RAW264.7 cells. (D) Effect of rutin on the MDA content in CSE-induced RAW264.7 cells. Values are mean ± SEM of three independent experiments. ##p < 0.01, different from the values in the control group and #p < 0.05, different from the values in the control group. **p < 0.01, significantly different from the values in the CSE group and *p < 0.05, different from the values in the CSE group.
3.8. Effects of WZZE on the Expression of Signaling Pathway Proteins Related to Inflammation and Oxidative Stress
Combined with network pharmacology analyses, the effects of TNF-α, p-38, and p-p38 protein expressions in cells were detected by Western blot, and the results are shown in Figure 8. Compared with the control group, the ratio of p-p38/p38 in the model group was significantly increased while the expression level of TNF-α was significantly decreased (P < 0.01). Alternatively, upon comparison with the model group, both the middle and high doses of WZZE significantly down-regulated the ratio of p-p38/p38 and inhibition of the expression of TNF-α protein was also found (P < 0.01).
Figure 8.
Effects of WZZE on the protein expressions of TNF-α, p-38, and p-p38 in CSE-induced RAW264.7 cells. (A) Western blot analysis of TNF-α, p-p38, and p38 proteins in cells. (B) Relative protein expression of TNF-α. (C) Relative protein expression of p-p38/p38. β-actin was used as the internal reference. Values are mean ± SEM of three independent experiments. ##p < 0.01, different from the values in the control group and #p < 0.05, different from the values in the control group. **p < 0.01, significantly different from the values in the CSE group and *p < 0.05, different from the values in the CSE group.
4. Discussion
With the advancement in the technology and analytical tools as well as the progressive detection expertise, the utilization of HPLC-CL is on rise. HPLC-CL has the characteristics advantage of good separation effect, strong selectivity, and swift analysis speed. It can quickly analyze the antioxidant activity of the substance, which not only improves the problem of poor selectivity of chemiluminescence detection but also expands its application range.21 There are several related literature reports that have employed HPLC-CL analysis technology for the detection and identification of chemical fingerprint peaks and antioxidant activity fingerprint peaks corresponding to each component in the mixed sample.22 In addition, the luminol–H2O2 system is the most widely used reaction system at present that not only generates hydrogen peroxide (H2O2) but also generate superoxide anion radicals (O2–•) and hydroxyl radicals (•OH). These radicals may scavenge biological excessive O2– in the body, and scavenging free radicals is an important manifestation of the ability to resist oxidative stress.23,24 Based on these facts, our present study was established employing the HPLC-CL method for WZZE. Our findings showed that terpenes and flavonoids had antioxidant properties by HPLC-CL combined technology, and the ability of scavenging free radicals of rutin, isoquercetin, glaucocalyxin A, and glaucocalyxin B was determined by CL spectra. At the same time, under this condition, the active substances contained in WZZE were well separated, and the content of known compounds was accurately figured out. The spectrum obtained by the combination of HPLC-CL clearly showed the anti-oxidative stress ability of active components in WZZE, which was helpful for the rapid identification of active components in WZZE.
Network pharmacology can reflect the complex interaction between biological macromolecules and chemical components to a large extent. Screening out the effective components from traditional Chinese medicine through network pharmacology can provide a basis for more targeted research on active components from traditional Chinese medicine.25 Our pathway enrichment studies showed that the main targets of the four compounds in the leaves of Isodon suzhouensis were concentrated in the TNF signaling pathway and the cancer signaling pathways. Therefore, when the body is under oxidative stress, Isodon suzhouensis may relieve symptoms through regulating these pathways.
CSE contains a variety of oxide components. When macrophages are stimulated by CSE, cell oxidation and antioxidation are out of balance and ROS expression increases, resulting in neutrophil inflammatory infiltration, leading to severe oxidative stress and inflammatory damage.26 It was found that the addition of ROS inhibitor N-acetylcysteine may significantly inhibit ROS and scavenge free radicals, thus inhibiting oxidative stress damage.27 Our results showed that CSE significantly promoted the expression of ROS, which was consistent with previous studies. However, the content of ROS was significantly inhibited by the addition of WZZE, suggesting that WZZE was quite effective against ROS scavengers to inhibit oxidative stress. SOD is the main antioxidant enzyme in cells, and its activity is directly related to the level of oxygen free radicals, and the abnormal increase of oxygen free radicals in cells will lead to the oxidation of lipids in cells, resulting in oxidative stress damage, so SOD and MDA are also used as classical markers for the detection of abnormal oxidative stress.28−31 In our study, we also detected the activity of SOD and the content of MDA in RAW264.7 cells exposed to CSE. As previously reported, CSE significantly inhibited the activity of SOD in cells and increased the content of MDA in cells. However, when WZZE was added to cells, the activity of SOD and the content of MDA were significantly reversed. Furthermore, in addition, we examined the effects of rutin (one of the four compounds we studied) on oxidative stress-related indicators in cells under oxidative stress. Interestingly, similar to WZZE, rutin also improved the activity of SOD and the content of MDA in CSE-induced RAW264.7 cells. In addition, some studies have shown that TNF-α, a classical inflammatory signal factor, not only participates in the inflammatory response but also involves in the process of oxidative stress, destroying the balance of the oxidation-antioxidant system in the body and then causing oxidative damage.32 It had been reported that oxidative stress can trigger inflammatory response and this inflammatory response may further enhance oxidative stress.33 In most diseases, oxidative stress and inflammatory response promote each other, thus promoting the development of the disease.34 Therefore, the inhibition of the inflammatory reaction may inhibit the oxidative stress reaction. Similarly, as an important process pathway of cell growth, development, proliferation, differentiation, and apoptosis, the p38 MAPK pathway was not only a regulatory inflammatory response mediator35 but also interferes with oxidative stress36 and plays an important role in the occurrence and development of disease. In view of this, we investigated the effect of WZZE on the pathway of TNF-α and p38 MAPK. Our western blot results showed that CSE significantly upregulated the expression of TNF-α and increased the ratio of p-p38/p38. After the intervention of WZZE, the expression of TNF-α decreased and the ratio of p-p38/p38 decreased significantly. It was suggested that WZZE can exert the effects of anti-inflammatory and antioxidant stress by inhibiting TNF-α and p38 MAPK signaling pathways, which was even consistent with the analysis of network pharmacology. In this study, although we evaluated the antioxidant activity of the extract and the main active components of Isodon suzhouensis, our study was limited. We only matched the strong antioxidant components of the above four. However, some compounds that still have strong antioxidation have not been explored by us, which need us to continue to study. The components of the extract of Isodon suzhouensis are complex. Obtaining more complete ingredients and antioxidant systems of Isodon suzhouensis is the top priority of our exploration.
To sum up, this study explored the antioxidant stress components and mechanism of WZZE by HPLC-CL technology, network pharmacology, and in vitro cellular studies, which provided a strong material basis and foundation for the further development of the valuable therapeutic product from Isodon suzhouensis leaves and may also provide the scientific basis for the study of the antioxidant activity of other parts of Isodon suzhouensis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82170481), Anhui Natural Science Foundation (2008085J39 and 2108085MH314), the Excellent top-notch Talents Training program of Anhui Universities (gxbjZD2022073), the Natural Science Foundation of Anhui Educational Committee (KJ2021A1108), Anhui Province Innovation Team of Authentic medicinal materials Development and High Value Utilization (2022AH010080), and Suzhou University Joint Cultivation Postgraduate Research Innovation Fund Project (2021KYCX04).
Glossary
Abbreviations
- CL
Chemiluminescence
- CSE
Cigarette smoke extract
- ECL
Enhanced chemiluminescence
- GO
Gene ontology
- HPLC
High-performance liquid phase
- KEGG
Kyoto encyclopedia of genes and genomes
- MAPK
Mitogen-activated protein kinase
- RIPA
RIPA lysis buffer
- ROS
Reactive oxygen species
- RSD
Relative standard deviation
- TNF
Tumor necrosis factor
- WZZE
Isodon suzhouensis leaf extract
Author Contributions
W.W., H.L., and J.L. wrote the paper draft, G.J.K. and H.D. corrected the draft, K.Z. and Z.X. supervised the experimentators, and J.Z. and N.B. performed the experiments. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
The authors declare no competing financial interest.
References
- Homma T.; Fujii J. Emerging connections between oxidative stress; defective proteolysis; and metabolic diseases. Free Radical Res. 2020, 54, 931–946. 10.1080/10715762.2020.1734588. [DOI] [PubMed] [Google Scholar]
- Rani V.; Deep G.; Singh R. K.; Palle K.; Yadav U. C. S. Oxidative stress and metabolic disorders, Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Ma B. C.; Guan G. P.; Lv Q. Z.; Yang L. Curcumin Ameliorates Palmitic Acid-Induced Saos-2 Cell Apoptosis Via Inhibiting Oxidative Stress and Autophagy. J. Evidence-Based Complementary Altern. Med. 2021, 2021, 5563660 10.1155/2021/5563660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Lam G. Y.; Brumell J. H. Autophagy Signaling Through Reactive Oxygen Species. Antioxid. Redox Signaling 2011, 14, 2215–2231. 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- Sharma G. N.; Gupta G.; Sharma P. A Comprehensive Review of Free Radicals; Antioxidants; and Their Relationship with Human Ailments. Crit. Rev. Eukaryotic Gene Expression 2018, 28, 139–154. 10.1615/CritRevEukaryotGeneExpr.2018022258. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Z.; Hou Y. X.; Zhou W.; Keerthiga R.; Fu A. L. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng. Transl. Med. 2020, 6, e10209 10.1002/btm2.10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W.; He C. N.; Ma Y. Y.; Shen J.; Zhang L. H.; Peng Y.; Xiao P. G. Investigation of free amino acid; total phenolics; antioxidant activity and purine alkaloids to assess the health properties of non-Camellia tea. Acta Pharm. Sin. B 2016, 6, 170–181. 10.1016/j.apsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neha K.; Haider M. R.; Pathak A.; Yar M. S. Medicinal prospects of antioxidants, A review. Eur. J. Med. Chem. 2019, 178, 687–704. 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Zhai K. F.; Wang W.; Zheng M. Q.; Khan G. J.; Wang Q. B.; Chang J. W.; Dong Z.; Zhang X. T.; Duan H.; Gong Z. P.; Cao H. Protective effects of Isodon Suzhouensis extract and glaucocalyxin A on chronic obstructive pulmonary disease through SOCS3–JAKs/STATs pathway. Food Front. 2022, 10.1002/fft2.177. [DOI] [Google Scholar]
- Ma H. J.; Zhai K. F.; Han Z. B.; Zhou S. B. lsodon Suzhouensis (Lamiaceae), A New Species from Anhui. J. Suzhou Univ. 2022, 37, 41–45. [Google Scholar]
- Duan H.; Wang G. C.; Khan G. J.; Su X. H.; Guo S. L.; Niu Y. M.; Cao W. G.; Wang W. T.; Zhai K. F. Identification and characterization of potential antioxidant components in Isodon amethystoides (Benth.) Hara tea leaves by UPLC-LTQ-Orbitrap-MS. Food Chem. Toxicol. 2021, 148, 111961 10.1016/j.fct.2020.111961. [DOI] [PubMed] [Google Scholar]
- Zhai K. F.; Duan H.; Cao W. G.; Gao G. Z.; Shan L. L.; Fang X. M.; Zhao L. Protective effect of Rabdosia amethystoides (Benth) Hara extract on acute liver injury induced by Concanavalin A in mice through inhibition of TLR4-NF-kappa B signaling pathway. J. Pharmacol. Sci. 2016, 130, 94–100. 10.1016/j.jphs.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Zhao F. L.; Zhang W. J.; Meng X.; Yang X.; Yang L. Z.; Teng J. T.; Xue J. P.; Duan Y. B.; Sheng W. Antioxidant and antimicrobial properties of Isodon amethystoides (Benth.) CY Wu et Hsuan leaf extracts against agriculturally important pathogenic fungi. Biotechnol. Biotechnol. Equip. 2019, 33, 1690–1697. 10.1080/13102818.2019.1695542. [DOI] [Google Scholar]
- Akbari B.; Baghaei-Yazdi N.; Bahmaie M.; Abhari F. M. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. 10.1002/biof.1831. [DOI] [PubMed] [Google Scholar]
- Nam T. G.; Kim D. O.; Eom S. H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. 10.1007/s10068-017-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P. Q.; Du W. J.; Wang Y. Y.; Teng X. X.; Chen X. D.; Ye L. B. Total phenolic; flavonoid content; and antioxidant activity of bulbs; leaves; and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr. 2018, 7, 148–154. 10.1002/fsn3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H.; Khan G. J.; Shang L. J.; Peng H.; Hu W. C.; Zhang J. Y.; Hua J.; Cassandra A.; Rashed M. M. A.; Zhai K. F. Computational pharmacology and bioinformatics to explore the potential mechanism of Schisandra against atherosclerosis. Food Chem. Toxicol. 2021, 150, 112058 10.1016/j.fct.2021.112058. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wang Z. Y.; Zheng J. H.; Li S. TCM network pharmacology, A new trend towards combining computational; experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. 10.1016/S1875-5364(21)60001-8. [DOI] [PubMed] [Google Scholar]
- Li Q. Q.; Shang F.; Lu C.; Zheng Z. X.; Lin J. M. Fluorosurfactant-prepared triangular gold nanoparticles as postcolumn chemiluminescence reagents for high-performance liquid chromatography assay of low molecular weight aminothiols in biological fluids. J. Chromatogr. A 2011, 1218, 9064–9070. 10.1016/j.chroma.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Wang S. J.; Liu Q. Q.; Jiang H. J.; Cheng Y. F.; Yan Y. H.; Yao H. E.; Zhang J. M.; Pei J. Active components and mechanism of Taohong Siwu Decoction in treatment of primary dysmenorrhea based on network pharmacology and molecular docking technology. Zhongguo Zhongyao Zazhi 2020, 45, 5373–5382. 10.19540/j.cnki.cjcmm.20200723.401. [DOI] [PubMed] [Google Scholar]
- Wei L. L.; Lu X. T.; Kang X. J.; Song Y. Determination of Glutathione and Cysteine in Human Breast Milk by High-Performance Liquid Chromatography with Chemiluminescence Detection for Evaluating the Oxidative Stress and Exposure to Heavy Metals of Lactating Women. Anal. Lett. 2020, 53, 2607–2618. 10.1080/00032719.2020.1750024. [DOI] [Google Scholar]
- Wang Y.; Calandra M. J.; Veazey R. Identification of four novel compounds in citrus oils via high-performance liquid chromatography; using post-column luminol-mediated chemiluminescence detection and NMR analysis. Flavour Fragrance J. 2020, 35, 616–622. 10.1002/ffj.3600. [DOI] [Google Scholar]
- Chen X. Y.; Sun-Waterhouse D.; Yao W. Z.; Li X.; Zhao M. M.; You L. J. Free radical-mediated degradation of polysaccharides, Mechanism of free radical formation and degradation; influence factors and product properties. Food Chem. 2021, 365, 130524 10.1016/j.foodchem.2021.130524. [DOI] [PubMed] [Google Scholar]
- Liu Z. Q. Bridging free radical chemistry with drug discovery, A promising way for finding novel drugs efficiently. Eur. J. Med. Chem. 2020, 189, 112020 10.1016/j.ejmech.2019.112020. [DOI] [PubMed] [Google Scholar]
- Jiao W. Y.; Mi S.; Sang Y. X.; Jin Q. X.; Chitrakar B.; Wang X. H.; Wang S. Integrated network pharmacology and cellular assay for the investigation of an anti-obesity effect of 6-shogaol. Food Chem. 2022, 374, 131755 10.1016/j.foodchem.2021.131755. [DOI] [PubMed] [Google Scholar]
- Zhang X. F.; Ding M. J.; Cheng C.; Zhang Y.; Xiang S. Y.; Lu J.; Liu Z. B. Andrographolide attenuates oxidative stress injury in cigarette smoke extract exposed macrophages through inhibiting SIRT1/ERK signaling. Int. Immunopharmacol. 2020, 81, 106230 10.1016/j.intimp.2020.106230. [DOI] [PubMed] [Google Scholar]
- Kularatne R. N.; Bulumulla C.; Catchpole T.; Takacs A.; Christie A.; Stefan M. C.; Csaky K. G. Protection of human retinal pigment epithelial cells from oxidative damage using cysteine prodrugs. Free Radical Biol. Med. 2020, 152, 386–394. 10.1016/j.freeradbiomed.2020.03.024. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y.; Yi M. H.; Huang Y. P. Oxymatrine Ameliorates Doxorubicin-Induced Cardiotoxicity in Rats. Cell. Physiol. Biochem. 2017, 43, 626–635. 10.1159/000480471. [DOI] [PubMed] [Google Scholar]
- Naresh C. K.; Rao S. M.; Shetty P. R.; Ranganath V.; Patil A. S.; Anu A. J. Salivary antioxidant enzymes and lipid peroxidation product malondialdehyde and sialic acid levels among smokers and non-smokers with chronic periodontitis-A clinico-biochemical study. J. Fam. Med. Prim. Care. 2019, 8, 2960–2964. 10.4103/jfmpc.jfmpc_438_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilona K. S.; Joanna F.; Lech S.; Maria Z.; Weronika W.; Iwona S.; Dorota W. P.; Ewa M. K. Effect of caffeine on biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. 2019, 15, 1073–1080. 10.5114/aoms.2019.85461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu-Ingok A.; Catalkaya G.; Capanoglu E.; Karbancioglu-Guler F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. 10.1002/fft2.77. [DOI] [Google Scholar]
- Zhao X. Y.; Zhang G. R.; Wu L. Z.; Tang Y. L.; Guo C. B. Inhibition of ER stress-activated JNK pathway attenuates TNF-alpha-induced inflammatory response in bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2021, 541, 8–14. 10.1016/j.bbrc.2020.12.101. [DOI] [PubMed] [Google Scholar]
- Dandekar A.; Mendez R.; Zhang K. Cross talk between ER stress; oxidative stress; and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- Jha J. C.; Ho F.; Dan C.; Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. 10.1042/CS20171459. [DOI] [PubMed] [Google Scholar]
- Xing J.; Yu Z. L.; Zhang X. Y.; Li W. Y.; Gao D. N.; Wang J.; Ma X. C.; Nie X. S.; Wang W. Epicatechin alleviates inflammation in lipopolysaccharide-induced acute lung injury in mice by inhibiting the p38 MAPK signaling pathway. Int. Immunopharmacol. 2019, 66, 146–153. 10.1016/j.intimp.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Lv W. P.; Li M. X.; Wang L. Peroxiredoxin 1 inhibits lipopolysaccharide-induced oxidative stress in lung tissue by regulating P38/JNK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1876–1883. [PubMed] [Google Scholar]