Abstract
Infection of rat prostates with cytotoxic necrotizing factor type 1 (CNF1)-positive uropathogenic Escherichia coli caused more inflammation-mediated morphological and histological tissue damage than did infection with isogenic CNF1-negative mutants. These striking differences occurred despite the finding that bacterial counts for the strain pairs were indistinguishable. We conclude that CNF1 contributes to E. coli virulence in a model of acute prostatitis. To our knowledge, the results of this study provide the first demonstration of a role for any uropathogenic E. coli virulence factor in acute prostatitis.
Symptoms of prostatitis occur in up to one-half of all men at some time during their lifetimes, and these clinical manifestations are the most common source of urologic complaints in men younger than 50 years of age. Bacterial prostatitis, characterized by symptoms of urinary tract infection (UTI), accounts for 5 to 10% of all prostatitis cases. The primary etiological agent of bacterial prostatitis is Escherichia coli. Many of the disease-causing E. coli isolates express one or more of several virulence factors that include cytotoxic necrotizing factor type 1 (CNF1), hemolysin, and P fimbriae. Indeed, epidemiological studies have linked CNF1 with strains that cause prostatitis, as well as uncomplicated UTI in women. Specifically, Mitsumori et al. reported that 64% of prostatitis patient isolates were cnf1+ (18), and Andreu and colleagues found that the percentages of cnf1+ prostatitis, pyelonephritis, and cystitis patient isolates were 63, 48, and 44%, respectively (1). In accordance with the findings of Andreu et al., Terai and colleagues noted that 44% of E. coli isolates from patients with ascending urethral bacterial prostatitis were cnf1+ (27).
CNF1 is a chromosomally encoded uropathogenic E. coli (UPEC) toxin that catalyzes the deamidation of the small GTPases RhoA, Rac, and Cdc42 (8, 9, 15, 26). Deamidation of the GTPases renders these proteins constitutively active, an occurrence that in most cells leads to formation of actin stress fibers, lamellipodia, and filopodia. HEp-2 cells, which have been used as the prototypic cell line for evaluation of CNF1 toxicity, not only display actin stress fibers but also become multinucleated (3, 6, 24). Moreover, CNF1 has been reported previously to mediate a spectrum of additional phenotypic effects on cultured cells that include enhancement of phagocytosis in epithelial cells (5, 7) and reduction of CR3 receptor-dependent phagocytosis in monocytes (2, 5, 7). CNF1 also inhibits wound repair in T24 bladder cells and Hs 738 fibroblast cells (11), kills 5637 bladder cells through an apoptotic mechanism (17), effaces the brush border of T84 cells, and decreases the degree to which polymorphonuclear leukocytes migrate across a monolayer of those intestinal cells (10). Thus, CNF1 affects a variety of cellular functions in vitro, presumably through activation of the Rho GTPases.
Recently, we determined that CNF1 produced by UPEC contributes to the virulence of those organisms in a mouse model of ascending UTI (23). We found that CNF1 expression provides a selective net growth advantage to the bacterium in the urinary tracts of the mice (particularly the bladders), as suggested in single-strain challenge studies and as demonstrated in mixed-culture experiments with a CNF1-positive strain and its CNF1-negative isogenic mutant. Further, we discovered that the production of CNF1 by an infecting UPEC strain evoked an inflammatory response in the bladders of the animals that was more intense than the response in bladders from animals inoculated with the isogenic CNF1-negative mutant even when bacterial counts were similar. In that same investigation, we also showed that human polymorphonuclear leukocytes kill CNF1-negative mutants more efficiently than they do the cognate CNF1-positive UPEC strain (23). In the study described here, we compared the effects of a CNF1-producing strain with those of its CNF1-negative isogenic mutant in a rat model of acute bacterial prostatitis (4, 14, 19). We consider that this rat model provides a good representation of the analogous disease process in humans because the rat prostate has many morphological similarities with the human prostate and because the histology displayed in human prostatitis (Fig. 7.3 and 7.4 in reference 4) bears a very close resemblance to that of rat prostatitis (Fig. 11.1b and c in reference 4). Additionally, the initiation of infection in the rat is via an ascending route through the urethra, a path that is believed to be the major means by which men become infected.
Groups of six male rats were anesthetized with 4% halothane, catheterized, and infected with 2 × 105 CFU of UPEC strain CP9 (an O4:H5:K54, hemolysin-positive, CNF1-positive isolate from the blood of a patient with pyelonephritis [25]) or its CNF1-negative isogenic mutant CP9cnf1 (23) via urethral catheter. Complementation of CP9cnf1 by transformation with a cnf1-expressing plasmid was previously demonstrated in the mouse UTI model (23). Forty-eight hours later, the rats were euthanatized by CO2 asphyxiation and their prostate glands were removed for analysis. CP9 and CP9cnf1 infected the prostate equally well. Prostates infected with CP9 contained an average of 1.3 × 106 CFU/mg of tissue, while those infected with CP9cnf1 contained 7.4 × 105 CFU/mg of tissue. This slight difference in bacterial numbers was not statistically significant. The experiment was repeated three times with different doses of CP9 or CP9cnf1 with comparable results (data not shown). Cystitis patient isolate C85 (O2:H−, hemolysin-positive, CNF1-positive cystitis isolate [28]) and the isogenic mutant, C85cnf1 (23), also reached equivalent levels of colonization in the prostate (7.1 × 104 and 4.9 × 104 CFU/mg of tissue, respectively), although total bacterial numbers were less than those achieved with CP9 and its mutant. Thus, unlike our findings in the mouse UTI model with two of three CNF1-positive and isogenic CNF1-negative strain pairs, the wild-type CNF1 isolate did not appear to possess an in vivo growth advantage in this model.
Next, we tried a mixed-infection experiment with a Lac-negative, CNF1-positive derivative of CP9 (CP9lacZ) and CP9cnf1 (Lac positive, CNF1 negative). A similar mixed-infection study was done in the mouse UTI model to conclusively demonstrate the growth advantage of the CNF1-positive strain (23). Here, five rats were inoculated with a mixture that contained 2 × 107 CFU of each strain and were euthanatized 2 days later. Prostates were removed, homogenized, and serially diluted and plated for colony counts. Unlike the mouse UTI model, no difference in colony counts between the strains 2 days after inoculation was seen (9.3 × 105 CFU/mg of tissue for CP9lacZ-infected rats versus 6.7 × 105 CFU/mg of tissue for CP9cnf1-infected rats). Thus, CNF1 does not appear to play a role in colonization of the prostate by a UPEC strain. One proviso of this conclusion is that the prostate, unlike the bladder, is composed of a number of branched acini that make removal of bacteria more difficult than in the bladder. Indeed, 50% of rats infected with E. coli in this model will go on to produce chronic infections of the prostate (4, 19). Therefore, the influence of CNF1 on colonization of the prostate may not be readily demonstrable in this model.
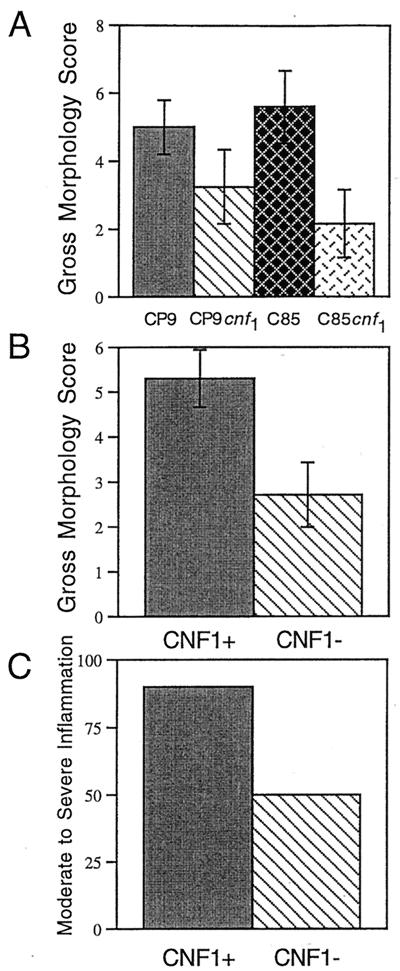
Prior to homogenization of the prostates for colony counts, the gross morphologies of all infected prostates (n = 6) were scored. Infected prostates were observed for overt signs of inflammation, including edema, congestion, and hyperemia, and given a score of 0 to 3 for each of these conditions (0 = none; 3 = the maximum score). The three scores were then totaled for a final score of 0 to 9. Examination of the gross morphologies of the infected prostates as well as the gross pathology score revealed differences between the CNF1-positive and CNF1-negative strains. CNF1 production by strains CP9 and C85 promoted an increase in the severity of both edema and hyperemia in the prostates of rats (data not shown). Prostates of animals infected with CP9 exhibited a trend toward severe tissue damage, while those infected with CP9cnf1 had more moderate damage (Fig. 1A). Similarly, those animals infected with C85 had statistically more severe damage to the prostate than did those infected with C85cnf1 (one-way analysis of variance, P = 0.015) (Fig. 1A). When the data from the two CNF1-positive strains were combined, the wild-type strains caused statistically significantly more damage than did the isogenic mutants (P < 0.05) (Fig. 1B). Thus, 90% of animals infected with CNF1-positive strains had moderate to high inflammation evident upon examination of the gross morphology of the prostate (Fig. 1C). Animals infected with CNF1-negative bacteria were less likely to exhibit moderate to high inflammation (50% of all animals [Fig. 1C]).
FIG. 1.
CNF1-positive strains (CP9 and C85) cause more morphological damage to the prostate than do CNF1-negative strains (CP9cnf1 and C85cnf1). Male rats were inoculated with 2 × 105 CFU of either strain and euthanatized 48 h later. Prostates were removed and examined for gross morphological changes. Each prostate was given a score of 0 to 3 for edema, congestion, and hyperemia (0 was none, and 3 was the maximum score). Data are from six rats for each strain. Scores were combined to give the total score that is depicted in the graph. (A) Average gross morphology scores for each individual strain. (B) Average gross morphology scores for combined CNF1-positive and combined CNF1-negative strains. (C) Percentages of animals that displayed moderate (score of 2 to 4) to severe (score of 5 to 9) inflammation.
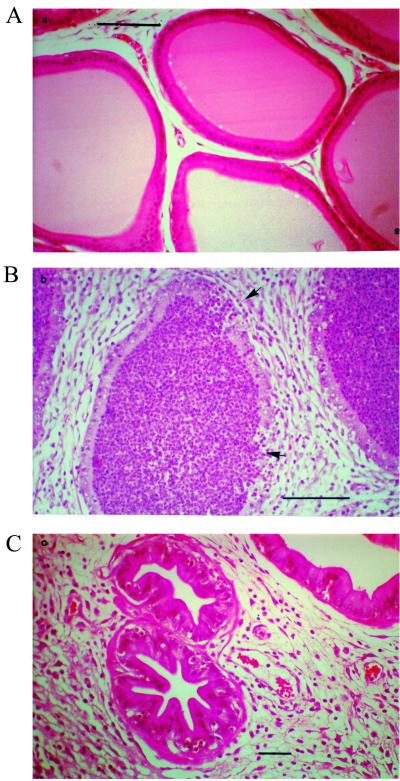
We then examined the influence of CNF1 production by UPEC on the histology of prostate tissues. One half of each prostate was fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Prostates infected with wild-type bacteria consistently showed histological evidence of more extensive and severe inflammation than did those infected with the CNF1-negative mutants (Fig. 2). The prostate is a compound gland composed of numerous acini (sac-like ducts) within stromal tissue. In normal prostates, the acini are clear of neutrophils and there is little stromal tissue surrounding the acini. Acini in prostates infected with CP9 were filled with neutrophils, and the stromal tissue was edematous (Fig. 2B). Conversely, the acini of prostates from CP9cnf1-infected animals contained many fewer neutrophils and the stromal tissue was markedly less edematous (Fig. 2C).
FIG. 2.
Histological sections from the prostates of a control rat and rats infected with CP9 or CP9cnf1. For infected rats, animals were inoculated intraurethrally with 2 × 105 CFU of either strain and euthanatized 48 h later. Prostates were removed, fixed in formalin, and processed for histology. (A) Control prostate from uninfected rat. Acini (sacs) are open, with well-defined borders, and no neutrophils are evident within the lumens of the acini. Minimal stromal tissue surrounds the acini. (B) Prostate from rat infected with CP9. Acini are filled with neutrophils (typical of most acini observed across fields). The borders of some acini are ill defined in certain areas, a finding which supports a loss of tissue integrity (see arrows). Note the higher magnification in this panel to facilitate resolution of the borders of the acini. Stromal tissue is edematous and contains neutrophils. (C) Prostate from a rat infected with CP9cnf1. Acini are thickened and contain basal neutrophils, but no neutrophils are present within the sacs. Only a minority of acini across many fields are filled with neutrophils (not shown). Again, stromal tissue is edematous and contains neutrophils.
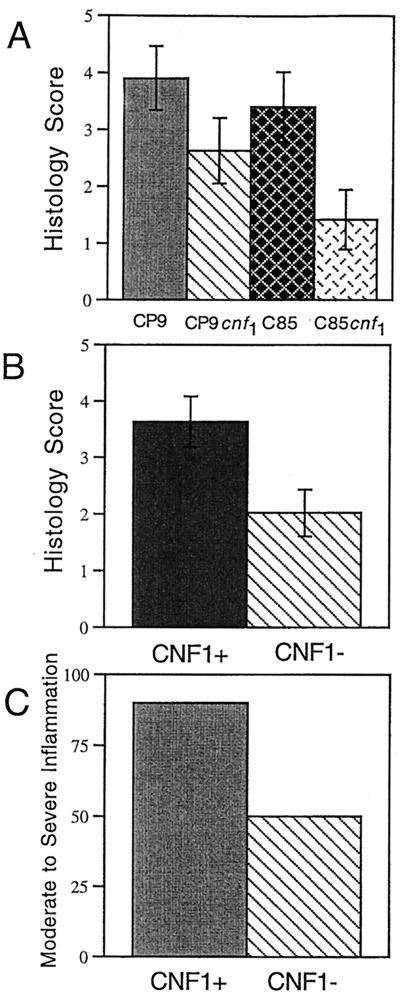
To more quantitatively measure the readily visible histological differences between prostates infected with CP9 and those infected with CP9cnf1, stained sections were scored for histological signs of inflammation: edema, hemorrhage, and leukocyte infiltration. Histological changes were scored in the same manner as for the gross morphological changes, i.e., each of the three conditions was given a score of 0 to 3. Total scores of less than 2 were classified as mild inflammation, scores of 2 to 4 were considered moderate inflammation, and scores of 5 or greater were categorized as severe inflammation. Tissue sections from prostates of animals infected with CP9 exhibited a trend toward more extensive damage, as measured by examination of the levels of edema, hemorrhage, and leukocyte infiltration, than did prostates infected with the isogenic mutant (Fig. 3A). While the histological damage caused by strain C85 was not as extensive as that caused by CP9, damage caused by strain C85 and that caused by strain C85cnf1 were statistically different (P < 0.05) (Fig. 3A). When histology data for all prostates infected with CNF1-positive bacteria were combined, these prostates were more damaged than were prostates infected with the isogenic mutants (P < 0.05) (Fig. 3B). All animals infected with strain CP9 had moderate to high inflammation in comparison to 66% of those infected with CP9cnf1 (Fig. 3C). Strain C85 caused severe damage slightly less often than did strain CP9, with 80% of wild-type- and only 33% of mutant-infected prostates manifesting moderate to high inflammation (Fig. 3C). In sum, prostates infected with either wild-type strain, CP9 or C85, had more extensive gross tissue damage, as well as more severe histological damage, than did prostates infected with either mutant strain. These results are consistent with observations made on the role of CNF1 in induction of extensive inflammation in the mouse UTI model (23). In that system, we also noted greater edema and neutrophil accumulation within bladder tissue infected with either CP9 or C85. From the previous studies with the mouse UTI model and the present data for the rat prostatitis model, we conclude that CNF1 appears to intensify the host inflammatory response while simultaneously promoting protection of the bacterium from the antibacterial effects of polymorphonuclear leukocytes.
FIG. 3.
CNF1-positive strains (CP9 and C85) cause more histological damage to the prostate than do CNF1-negative strains (CP9cnf1 and C85cnf1). Male rats were inoculated intraurethrally with 2 × 105 CFU of either strain and euthanatized 48 h later. Prostates were removed, fixed in formalin, and processed for histological examination. Slides were examined under light microscopy, and each prostate was given a score of 0 to 3 for edema, hemorrhage, and leukocyte infiltration (0 was none, and 3 was the maximum score). Data are from six rats for each strain. Scores were combined to give the total score that is depicted in the graph. (A) Average histology scores for each individual strain. (B) Average histology scores for combined CNF1-positive and combined CNF1-negative strains. (C) Percentages of animals that displayed moderate (score of 2 to 4) to severe (score of 5 to 9) inflammation.
Even though the presence of CNF1 appeared to augment inflammation in the bladders and prostates of the mice and rats, respectively, the impact of CP9 and C85 in the two model systems differed. C85 caused significantly more damage to the prostate than did its mutant, while the differences between CP9 and its mutant were not significant. This is in contrast with our cystitis findings, where the differences between CP9 and its mutant were greater than those between the C85 strain pair. CP9 and C85 were isolated from different sources within the body and have different repertoires of virulence factors. These variations in sources of strain isolation and virulence factor constellations may account for the observed differences between damage mediated by CP9 and that mediated by C85 in the bladder or prostate.
In these experiments, we tested an acute model of bacterial prostatitis because we had observed that CNF1 plays an early role in establishing infection and promoting inflammation in the mouse bladder (23). We did not evaluate the influence of CNF1 in chronic prostatitis. Because bacterial 16S ribosomal DNA sequences similar to those of E. coli, but not viable bacteria, have been found in the prostates of men with chronic prostatitis as well as in men with prostate cancer (13), we intend to extend our investigation on the role of CNF1 in chronic prostatitis. Furthermore, it is conceivable that CNF1, through deamidation and activation of the Rho family of small GTPases (8, 16, 26), is involved in development of prostate cancer. Constitutive activation of the Rho GTPases by CNF1 in the prostate would result in aberrant cell signaling which could, in turn, lead to transformation of the cell. Indeed, activation of the Rho GTPases has been shown to weakly transform fibroblast cell lines, and a role for RhoA and Rac1 in Ras transformation has been established elsewhere (12, 20–22). Rho GTPase signaling in prostate cell lines has not been examined, and so it is possible that CNF1-positive UPEC could contribute to the development of prostate cancer. Whether further studies support such a speculative hypothesis, the findings in this report are the first to our knowledge that show a definitive role for any UPEC virulence factor in acute prostatitis in an animal model.
Acknowledgments
This research was supported by operating grants from the National Institutes of Health (grant AI38281-05) to A. O'Brien and by the Natural Science and Engineering Research Council of Canada (NSERC) to H. Ceri. Michael Lang was supported by studentships from NSERC and the Alberta Heritage Foundation for Medical Research.
K.E.R.-L. and M.L. contributed equally to the work.
REFERENCES
- 1.Andreu A, Stapleton A E, Fennell C, Lockman H A, Xercavins M, Fernandez F, Stamm W E. Urovirulence determinants in Escherichia coli strains causing prostatitis. J Infect Dis. 1997;176:464–469. doi: 10.1086/514065. [DOI] [PubMed] [Google Scholar]
- 2.Capo C, Meconi S, Sanguedolce M-V, Bardin N, Flatau G, Boquet P, Mege J-L. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton in human monocytes: role in the regulation of integrin-dependent phagocytosis. J Immunol. 1998;161:4301–4308. [PubMed] [Google Scholar]
- 3.Caprioli A, Falbo V, Ruggeri F M, Baldassarri L, Bisicchia R, Ippolito G, Romoli E, Donelli G. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J Clin Microbiol. 1987;25:146–149. doi: 10.1128/jcm.25.1.146-149.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceri H, Olson M E, Nickel J C. Prostatitis: role of the animal model. In: Nickel J C, editor. Textbook of prostatitis. Oxford, United Kingdom: Isis Medical Media; 1999. pp. 109–114. [Google Scholar]
- 5.Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E, Boquet P. Induction of phagocytic behavior in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol. 1993;9:1247–1254. doi: 10.1111/j.1365-2958.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentini C, Arancia G, Caprioli A, Falbo V, Ruggeri F M, Donelli G. Cytoskeletal changes induced in HEp-2 cells by the cytotoxic necrotizing factor of Escherichia coli. Toxicon. 1988;26:1047–1056. doi: 10.1016/0041-0101(88)90203-6. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentini C, Fabbri A, Matarrese P, Falzano L, Boquet P, Malorni W. Hinderance of apoptosis and phagocytic behaviour induced by Escherichia coli cytotoxic necrotizing factor 1: two related activities in epithelial cells. Biochem Biophys Res Commun. 1997;241:341–346. doi: 10.1006/bbrc.1997.7723. [DOI] [PubMed] [Google Scholar]
- 8.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard R, Schmidt G, Hofmann F, Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect Immun. 1998;66:5125–5131. doi: 10.1128/iai.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofman P, Flatau G, Selva E, Gauthier M, Le Negrate G, Fiorentini C, Rossi B, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66:2494–2500. doi: 10.1128/iai.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Island M D, Cui X, Warren J W. Effect of Escherichia coli cytotoxic necrotizing factor 1 on repair of human bladder cell monolayers in vitro. Infect Immun. 1999;67:3657–3661. doi: 10.1128/iai.67.7.3657-3661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger J N, Riley D E, Vesella R L, Miner D C, Ross S O, Lange P H. Bacterial DNA sequences in prostate tissue from patients with prostate cancer and chronic prostatitis. J Urol. 2000;164:1221–1228. [PubMed] [Google Scholar]
- 14.Lang M D, Nickel J C, Olson M E, Howard S, Ceri H. A rat model of experimentally induced abacterial prostatitis. Prostate. 2000;45:201–206. doi: 10.1002/1097-0045(20001101)45:3<201::aid-pros1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Lerm M, Schmidt G, Goehring U-M, Schirmer J, Aktories K. Identification of the region of Rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor 1 (CNF1) J Biol Chem. 1999;274:28999–29004. doi: 10.1074/jbc.274.41.28999. [DOI] [PubMed] [Google Scholar]
- 16.Lerm M, Selzer J, Hoffmeyer A, Rapp U R, Aktories K, Schmidt G. Deamidation of CDC42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect Immun. 1999;67:496–503. doi: 10.1128/iai.67.2.496-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills M, Meysick K C, O'Brien A D. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect Immun. 2000;68:5869–5880. doi: 10.1128/iai.68.10.5869-5880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsumori K, Terai A, Yamamoto S, Ishitoya S, Yoshida O. Virulence characteristics of Escherichia coli in acute bacterial prostatitis. J Infect Dis. 1999;180:1378–1381. doi: 10.1086/314976. [DOI] [PubMed] [Google Scholar]
- 19.Nickel J C, Olson M E, Barabas A, Benediktsson H, Dasgupta M K, Costerton J W. Pathogenesis of chronic bacterial prostatitis in an animal model. Br J Urol. 1990;66:47–54. doi: 10.1111/j.1464-410x.1990.tb14864.x. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast G C, Khosravi-Far R, Solski P A, Kurzawa H, Lebowitz P F, Der C J. Critical role for Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- 21.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 22.Qiu R-G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rippere-Lampe K E, O'Brien A D, Conran R, Lockman H A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect Immun. 2001;69:3954–3964. doi: 10.1128/IAI.69.6.3954-3964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri F M, Fiorentini C, Caprioli A, Arancia G, Falbo V, Donelli G. HEp-2 cells multinucleation induced by an E. coli cytotoxic factor. IRCS Med Sci. 1986;14:833–834. [Google Scholar]
- 25.Russo T, Guenther J E, Wenderoth S, Frank M M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol Microbiol. 1993;9:357–364. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 27.Terai A, Ishitoya S, Mitsumori K, Ogawa O. Molecular epidemiological evidence for ascending urethral infection in acute bacterial prostatitis. J Urol. 2000;164:1945–1947. [PubMed] [Google Scholar]
- 28.Yamamoto S, Tsukamoto T, Terai A, Korazono H, Takeda Y, Yoshida O. Distribution of virulence factors in Escherichia coli isolated from urine of cystitis patients. Microbiol Immunol. 1995;39:401–404. doi: 10.1111/j.1348-0421.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]