Abstract
Inflammation plays a key role in the pathogenesis of the major depressive disorder. Namely, neuroinflammation can induce the production of neuroactive metabolites that interfere with N-methyl-D-aspartate receptors (NMDAR)-mediated glutamatergic neurotransmission and contribute to depressive-like behaviour. On the other hand, mammalian target of rapamycin (mTOR) activity with synaptogenic effects is the main mediator of antidepressant effects of several potent NMDAR antagonists. In this study, we investigated the specific role of GluN2A subunits of NMDAR on the activity of mTOR signaling and behaviour in lipopolysaccharide (LPS)-induces model of depression. The results showed that mice lacking GluN2A subunit did not display depressive-like behavior after the immune challenge, opposite to LPS-treated wild-type mice. Specifically, in GluN2A knockout mice, we estimated the activity of the mTOR pathway in the hippocampus and prefrontal cortex (PFC) by measuring synaptic levels of upstream regulators (p-Akt, p-ERK, and p-GSK3β) and downstream effectors (pmTOR, and p-p70S6K) of mTOR activity. In addition, we assessed the changes in the levels of two important synaptic markers, GluA1 and PSD-95. Contrary to downregulated mTOR signaling and decreased synaptic markers in LPS-treated wildtype animals, the resilience of GluN2A KO mice to depressive-like behaviour was paralleled with sustained mTOR signaling activity synaptic stability in hippocampus and PFC. Finally, we disclosed that resistance of GluN2A knockouts to LPS-induced depressive-like behavior was ERK-dependent.
These findings demonstrate that GluN2A-ERK-mTOR signaling is a vulnerability factor of inflammation-related depressive behaviour, making this signaling pathway the promising target for developing novel antidepressants.
Keywords: GluN2A knockout mice, synaptosomes, mTOR signaling, glutamatergic neurotransmission, LPS-induced depression
1. Introduction
Neuroinflammation is well recognized as a major contributor to several neurological and psychiatric disorders, including depression [1]. One of the best characterized animal models for investigating the relationship between depression and an immune response is the lipopolysaccharide (LPS)-induced model of depression. LPS provokes the tryptophan–kynurenine metabolic pathway dysregulation and the dominant production of quinilinic acid, an N-methyl-D-aspartate receptors (NMDAR) agonist. These neuroactive metabolites could contribute to depressive-like behaviour via affecting NMDAR-mediated glutamatergic neurotransmission [2, 3].
Currently, there is compelling evidence that a non-selective NMDAR antagonist, ketamine, has the rapid antidepressant effect, which correlates with acute dissociative side effects, limiting the widespread clinical use of this very potent antidepressant [4]. Ketamine’s antidepressant effects are mediated by mammalian target of rapamycin (mTOR) complex 1 (mTORC1) signaling that elicits translation of synaptic proteins and thereby promotes synaptogenesis [5, 6]. Furthermore, the mTOR role in the pathophysiology of depression was supported by post-mortem studies showing robust deficits in mTOR signaling in the prefrontal cortex (PFC) of subjects diagnosed with major depressive disorder (MDD) [7]. Similarly, the studies on depressed rodents showed reduced mTOR activity in the PFC and hippocampus [8, 9]. Although recent preclinical research has focused on unraveling the molecular mechanisms underlying the unique antidepressant actions of ketamine, it is still controversial whether its antidepressant effect depends on subtypes of NMDARs.
NMDARs are tetramers composed of two obligatory GluN1 subunits, associated with two GluN2 regulatory subunits (GluN2A-D). Heterogeneity in NMDA receptors subunit composition determines their biophysical properties and function. Specifically, GluN2A-containing NMDARs are highly expressed in the synapsis of the mammalian adult forebrain and mediate fast neurotransmission [10]. In addition, the expression of GluN2A-containing NMDAR increases with development and is involved in cortical network refinement during development via its actions on structural and functional synaptic plasticity [11, 12].
In our previous article [13], we briefly disclosed that GluN2A subunit plays a specific role in emotional behaviour by potentiating synaptic stabilization via sustaining levels of polysialylated form of neural cell adhesion molecule (PSA-NCAM) in the PFC and hippocampus and by increasing hippocampal brain-derived neurotrophic factor (BDNF) signaling in a neuroinflammation-induced model of depression. In this study, we further explored the contribution of GluN2A subunits of NMDAR to mTOR signaling activity and regulation of depressive behaviour. Namely, we hypothesized that GluN2A subunit affects synaptic formation in the neuroinflammation-induced model of depression through mTOR signaling and its upstream regulatory system. In order to assess our hypotheses, we used GluN2A knockout (KO) mice to estimate animal behaviour and activity of mTOR pathway in hippocampus and PFC by measuring synaptic levels of phospho-protein kinase B (p-Akt), phospho-extracellular signal-regulated kinase (p-ERK), and phospho-beta-isoform of glycogen synthase kinase 3 (p-GSK3β), as upstream mTOR regulators, phospho-m-TOR (p-mTOR) and phosphoribosomal protein S6 kinase (p-p70S6K), which are downstream indicators of mTOR activity, as well as levels of two important synaptic markers, GluA1 and postsynaptic density protein 95 (PSD-95).
2. Materials and methods
2.1. Animals and Housing
GluN2A KO mice with aC57BL/6 background were generated as previously described [14] and purchased from Jackson Laboratories. In our laboratory, heterozygous mutant mice for the GluN2A-coding gene Grin2 was bred to produce GluN2A KO and wild-type (WT) mice. Mouse genomic DNA from the tail was used for genotyping GluN2A KO, and WT animals as previously described [15].
Subjects used in experiments were adult (> 8 weeks old) male WT and GluN2A KO mice. Mice were housed individually in standard cages in a temperature and humidity-controlled vivarium under a reverse 12 h light/dark cycle with access to food and water ad libitum. All animal procedures in this study were approved by the Ethical Committee of VINCA Institute of Nuclear Sciences (Application No. 4/2015; 323–07-04657/2015–05/3) according to the guidelines of the EU Directive 2010/63/EU.
2.2. Drugs administration
In LPS-treated groups, mice were i.p injected with a single dose of LPS 0.83 mg/kg previously dissolved in sterile, pyrogen-free physiological saline (Escherichia coli 005: B5, No. L-2880 Sigma-Aldrich, St. Louis, MO, USA). The dose of LPS (0,83 mg/kg) was chosen for its ability to induce the acute sickness response and subsequent depressive-like behaviours across the time points examined here [3, 16]. The vehicle (VEH) groups were treated with the mass-adjusted volume of physiological saline. In the present study, behavioural tests were performed 24 hours after the LPS challenge.
For the i.c.v. treatment with the MAPK/ERK kinase (MEK) inhibitor 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene (U0126) (Promega, Madison, WI), drug was dissolved in 100% DMSO to concentration 10mM. Before the treatment, UO126 was diluted with artificial cerebrospinal fluid (aCSF) containing (mM): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 MgCl2, 10 glucose, and 2 CaCl to yield a final concentrations of 5mM (in 50% DMSO) [17] or vehicle (aCSF in 50% DMSO).
2.3. Experimental design
During this study, we performed two experiments.
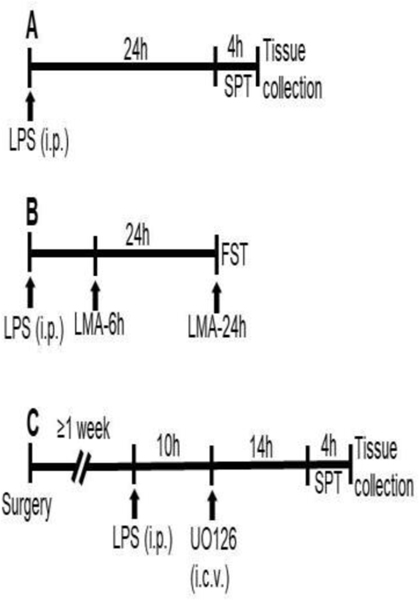
In the first experiment, we used male WT mice treated with saline (group I) or LPS (group II) and male GluN2A KO mice treated with saline (group III) or LPS (group IV). All animal groups were treated i.p. at 8:00 am. In this experiment, the first set of animals was used for a non-invasive sucrose preference test (SPT), performed 24–28 h after the LPS treatment, and immediately after testing, all animals were sacrificed by cervical dislocation. The brain structures of interest were extracted, frozen in liquid nitrogen, and stored at −80°C until sample preparation (Fig. 1A). The second set of animals was used to examine locomotor activity and the Forced Swim Test (FST). Specifically, locomotor activity was examined 6 and 24h after the LPS treatment, and FST was performed 26h after (Fig. 1B).
Fig. 1. Experimental design.
The schedule of treatment and behavioural evaluations (A, B and C).
In the second experiment, we used male GluN2A KO animals, which we subjected to stereotaxic surgery. Before the surgery, the mice were anesthetized with 1.2% Avertin anesthesia (0.4 ml per mouse) and fixed in a stereotaxic apparatus. A small incision in the scalp was made to localize the bregma, and a hole was drilled into the skull. Double cannulae were implemented into both lateral brain ventricles (−0.3 mm rostral, 1.0 mm lateral, 2.0 mm ventral to bregma; unilateral) and affixed to the skull by dental cement. After surgery, mice were housed individually and allowed to recover for seven days.
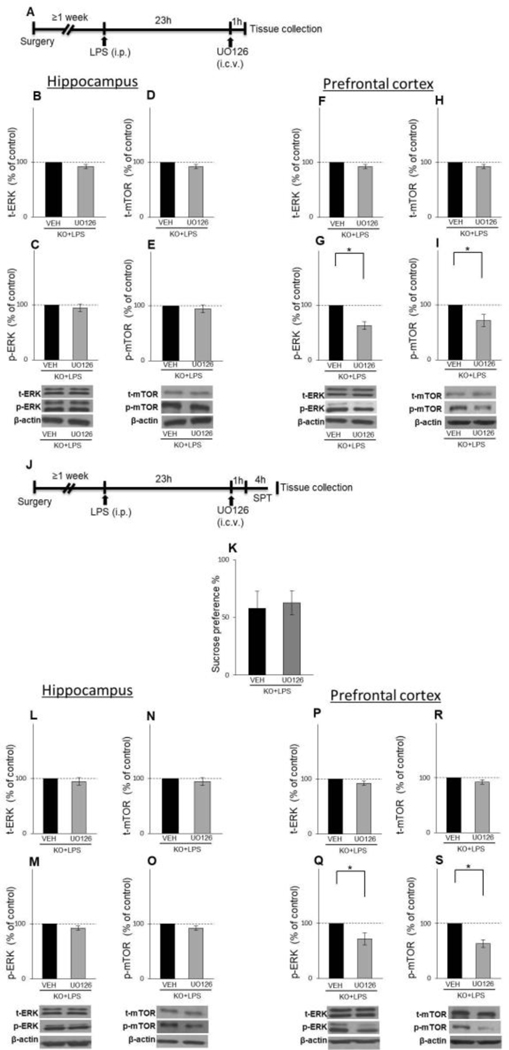
After recovery, animals were treated with LPS in the same way as in the first experiment. Before i.c.v. injection, mice were exposed to light Isoflurane anesthesia, the cap and the dummy were removed, and UO126 or vehicle was injected bilaterally, 0.5μl on each side given over 1min. After i.c.v. infusions, the injection cannula was kept in place for another 30 seconds to allow diffusion of chemicals. Animals were subjected to behavioural test (SPT), performed 24–28h after LPS treatment, after which mice were sacrificed, and brains were removed and processed, as described above (Fig. 1C, 6A, and J).
Fig. 6.
Effects of UOl26, 1 and 4 h after its administration, on behaviour and activity of ERK and mTOR in LPS-treated GluN2A KO animals in hippocampus and PFC The schedule of treatment and behavioural evaluations (A and J). The levels of total and phosphorylated ERK and mTOR in the hippocampal (B-E) and PFC (F-l) synaptosomal fraction of male LPS-treated GluN2A KO mice 1 h after i.c.v infusion with vehicle (VEH) or UOl26 (n = 4). Mean sucrose preference (%) of male LPS-treated GluN2A KO mice 4 h after Lc.v infusion with vehicle (VEH) or UOl26 (n = 8) (K). The levels of total and phosphory lated ERK and mTOR in the hippocampal (L-O) and PFC (P-S) synaptosomal fraction of male LPS-treated GluN2A KO mice 4 h after i.c.v infusion with vehicle (VEH) or UOl26 (n = 4). Values are presented as mean ± SEM % of control values. Statistically significant differences are given as p < 0.05. *VEH vs. UOl26.
2.4. Behaviour test and locomotor activity
Locomotor activity.
To confirm that a decrease in locomotor activity, as a consequence of sickness behaviour induced by LPS, is recovered at the time of behavioural tests, we examined the motor activity of animals 6 and 24 h after treatment. Each mouse was placed in a new home cage without bedding or litter and divided into four equal areas. Mice were placed in the center of the cage, and their motor performance was recorded and analyzed during the 5 min test period using the TSE VIDEOMOT 2 software (version 5.75; TSE Systems, Bad Homburg, Germany). Locomotor activity of animals was expressed as a number of quadrant entries during the test period.
Forced Swim Test.
FST previously described by O’Connor and colleagues, 2009 was used [3]. Briefly, animals were placed into a large cylinder (diameter: 23 cm; height: 31 cm) filled with 24 ± 1°C water to a depth of 30 cm for a 6-min period. The water was changed between two testing sessions. During the test, the mice were video recorded from above, and the duration of immobility was automatically measured over the last five minutes of the test period. Immobility was defined as the lack of motions of normal escape behaviours (swimming, climbing, and diving) except those movements necessary to keep animals afloat. In order to measure immobility, we used the mobility function of the TSE VIDEOMOT 2 software (version 5.75; TSE Systems, Bad Homburg, Germany). Briefly, mice are recognized in contrast from their background and tracked in two dimensions as the surface area of the detectable object (mouse) moves within the predefined arena. Mobility is defined as the displacement of detectable surface area (mouse) over time and is averaged over 3 sample intervals to reduce error generated by sharp movements or missing frames in the digital record.
Sucrose preference test
In order to verify how LPS treatment affects depressive-like behaviour, we used SPT to estimate anhedonia in treated animals. The SPT consisted of two phases, the familiarization, and the test phase. During the familiarization phase, one week before the test phase, mice were habituated to drink 1% sucrose solution. Animals were daily exposed to a 2 ml syringe containing a freshly prepared 1% sucrose solution and an identical syringe containing tap water, placed one beside the other for 4 hours. The test phase started 24h after the LPS injection and lasted the following 4 h. Training and testing occurred between 8:00 am and 12:00 pm. Fluid consumption (grams) was measured by weighing syringes before and after the session on testing day. The sucrose preference (%) was calculated as sucrose intake (g)/total fluid consumption (g) ratio [16].
2.5. Synaptosome preparation for Western-blot detection of proteins
The crude synaptosomal fraction was prepared from dissected hippocampi and PFC as previously described [18]. Briefly, tissue was immersed in homogenization buffer (0.32 M sucrose, 20 mM HEPES (pH 7.3), 1 mM EDTA, 1 protease inhibitor cocktail, 5 mM NaF, and 1 mM NaVO3) and disrupted by 8 strokes in a Teflon-coated homogenizer and subsequently centrifuged at 948 RCF (Rotor:JA-20, Beckman J2–21 Operation) for 10 min. Supernatants were further processed by centrifugation at 17418 RCF (Rotor:JA-20, Beckman J2–21 Operation) for 10 min to obtain a pellet with crude synaptosomes. In the next step, pellets were sonicated in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF and 1 protease inhibitor cocktail.
Protein concentration was determined by the method of Markwell [19], and samples were incubated for 5 min at 100ºC in an appropriate amount of denaturing buffer according to Laemmli [20]. Forty μg of synaptic proteins were subjected to Sodium dodecyl-sulfate polyacrylamide gel electrophoresis using 7.5 %,10%, or 12% gels and subsequently transferred onto polyvinylidene fluoride membrane (Immobilon-P membrane, Millipore) using a blot system (Transblot, Bio-Rad). After protein transfer, the membrane was blocked with 5% non-fat milk in phosphate-buffered saline (PBS) for 1 hour at room temperature and then incubated with primary antibodies diluted as indicated (Table 1.) in 5% non-fat milk in PBS-Tween (PBS-T). As a loading control, we used β-actin primary antibody (Abcam, Cambridge, UK). After overnight incubation in primary antibodies, membranes were washed three times in PBS-T, and blots were developed with appropriate horseradish peroxidase (HRP)-labelled secondary antibodies (donkey anti-goat HRP (1:3000), goat anti-mouse HRP (1:5000) or mouse anti-rabbit HRP (1:3000), Santa Cruz Biotechnology). Immuno-reactive bands were visualized using enhanced chemiluminescent reagent (Pierce), SuperSignal Pico Chemiluminescent Substrate (Thermo Scientific), or SuperSignal Femto Maximum Sensitivity Substrate (Thermo Scientific) and exposed to X-ray film (Fuji Photofilm, Bedfordshire, UK or Amersham Hyperfilm ECL).
Table 1.
Western Blot primary antibodies and dilutions used in experiments.
| Name of antibody | Company | Source | Mono/Polyiclonal | Dilution for WB |
|---|---|---|---|---|
| t-ERK | Cell Signaling | Rabbit | Polyclonal | 1:1000 |
| p-ERK(Thr202/Tyr 204) | Cell Signaling | Rabbit | Polyclonal | 1:1000 |
| t-Akt | Santa Cruz | Rabbit | Polyclonal | 1:500 |
| p-AkT (Ser 473) | Santa Cruz | Mouse | Monoclonal | 1:1000 |
| t-GSK3β | Santa Cruz | Rabbit | Polyclonal | 1:500 |
| p-GSK3β (Ser 9) | Santa Cruz | Mouse | Monoclonal | 1:250 |
| t-mTOR | Santa Cruz | Mouse | Monoclonal | 1:200 |
| p-mTOR( Ser 2448) | Santa Cruz | Rabbit | Polyclonal | 1:500 |
| t-p70S6K | Santa Cruz | Mouse | Monoclonal | 1:500 |
| p-p70S6K | Santa Cruz | Goat | Polyclonal | 1:250 |
| PSD-95 | Santa Cruz | Rabbit | Polyclonal | 1:250 |
| GluA1 | Santa Cruz | Mouse | Monoclonal | 1:500 |
| β-Actin | Abcam | Rabbit | Polyclonal | 1:10000 |
The chemiluminescent signals from immunoblots were detected using Image J analysis PC software (NIH, Bethesda, MD) for quantification densitometry of protein bands on X-ray film. Amounts of all analyzed proteins were normalized to β-actin levels.
2.6. Statistical analysis
Analysis was performed using STATISTICA 7 software. According to experimental design, behavioural and biochemical data were statistically analyzed using two-way ANOVA and post hoc Tukey test or student t-test. The data are presented as a mean ±standard error of the mean (SEM), and statistical significance was accepted at p < 0.05.
3. Results
3.1. Effects of LPS on locomotor activity and behaviour
Locomotor activity –
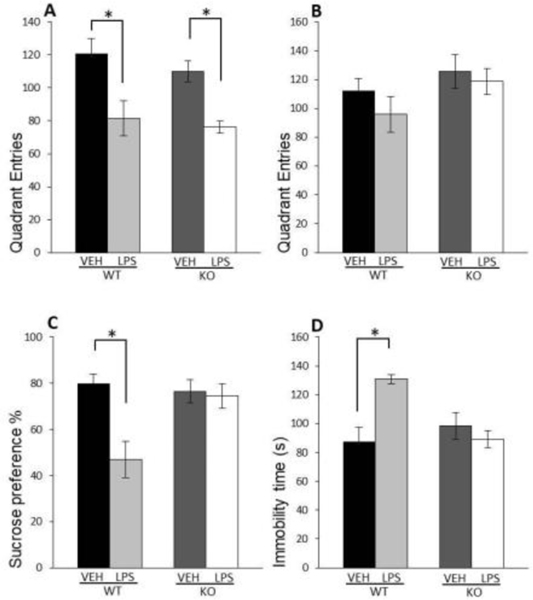
We determined alterations in the locomotor activity to better address LPS-induced sickness behaviour in mice, often associated with hypolocomotion [21]. We found that LPS decreased locomotor activity in WT as well as in GluN2A KO mice 6 h after the treatment (LPS: F(1,21)=20.51; p=0.00018) (Fig. 2A), but 24 hours after administration of LPS, there were no changes in locomotor activity between LPS-treated mice and untreated controls (Fig. 2B).
Fig. 2. Effects of LPS on locomotor activity and behaviour.
Mean number of quadrant entries in locomotor activity test of male wild-type (WT) and GluN2A knockout (KO) mice 6 h (A) and 24 h (B) upon LPS treatment compared to controls (VEH). Mean sucrose preference (%) of male WT and GluN2A KO mice upon LPS treatment compared to controls (VEH) (Q. Mean time spent immobile in the forced swim test of male WT and GluN2A KO mice upon LPS treatment compared to controls (VEH) (D). Values are presented as mean ± SEM % of control values (n = 10). Statistically significant differences are given as p < 0.05. *VEH vs. LPS, #WT vs. KO.
Sucrose preference test –
Evaluating the animals’ interest in a sweet-tasting sucrose solution, we found that LPS treatment induced a decrease in the consumption of sucrose solution only in WT mice (LPS x genotype interaction: F(1,26)=11.94; p=0.00016) (Fig. 2C) while in GluN2A KO animals, LPS treatment did not cause anhedonia.
Forced Swim test –
LPS treatment increased immobility time in WT animals relative to vehicle (LPS x genotype interaction: F(1,20)=12.98; p=0.003) (Fig. 2D) while in the GluN2A KO animals, LPS failed to affects the duration of immobility time in FST.
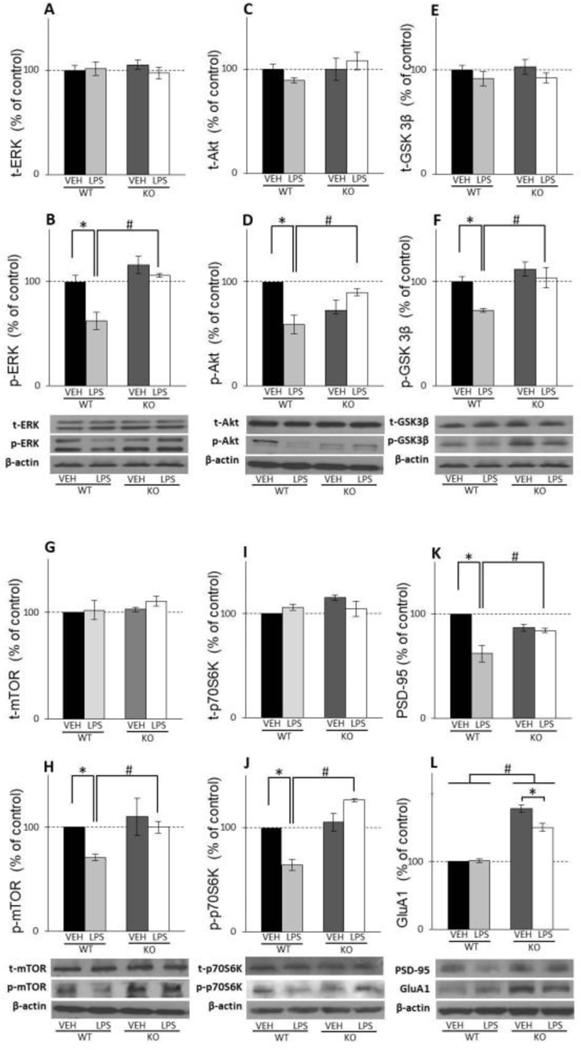
3.2. Effects of LPS on upstream regulators of mTOR activity, Akt, ERK and GSK3β
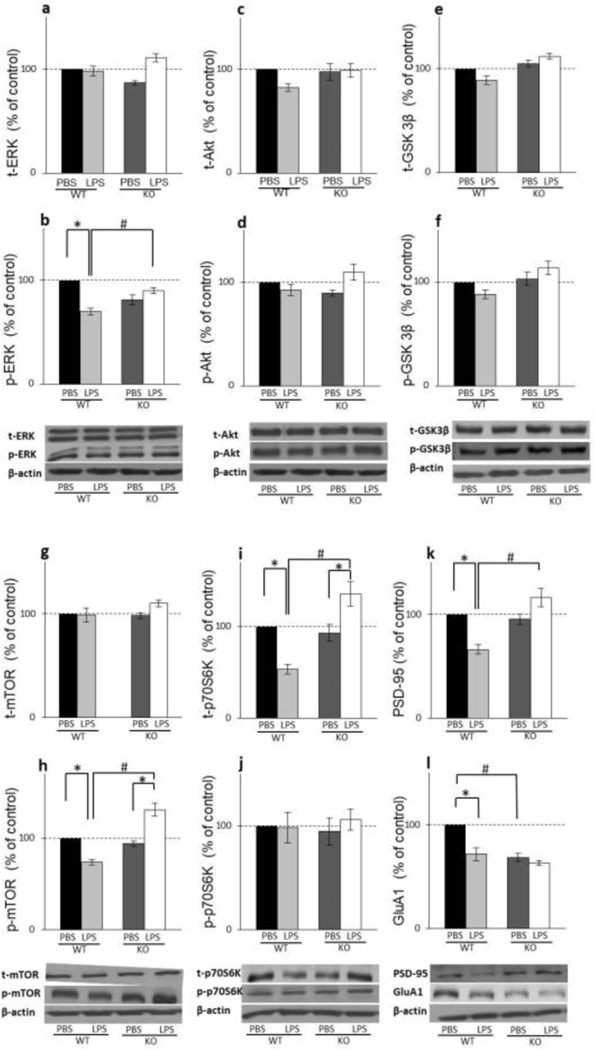
In the hippocampus of WT mice, LPS treatment significantly decreased p-ERK levels (LPS x genotype interaction: F(1,20)=76.68; p=0.00029) (Fig. 3B) while its total levels stayed unaltered upon LPS challenge (Fig. 3A). Active and total forms of other two kinases that affect mTOR activation, p-Akt and p- GSK3β, were not altered by LPS treatment in WT animals (Fig. 3C, E, D, and F). In GluN2A KO mice, LPS affected neither total nor phosphorylated form of any of these three upstream mTOR kinases (Fig. 3A–F).
Fig. 3. Effects of LPS on upstream and downstream mTOR signalling in hippocampus.
The levels of total and phosphoiylated ERK (A.B), Akt (C,D), GSK3β (E,F), mTOR (G,H), p70S6K CLJ) and the levels of PSD-95 (K) and GluAl(L) in the hippocampal synaptosomal fraction of male wild-type (VAT) and GluN2A knockout (KO) mice upon LPS treatment compared to controls (VEH). Values are presented as mean ± SEM % of control values (n = 10). Statistically significant differences are given as p < 0.05. *VEH vs. LPS, #WT vs. KO.
In PFC, LPS treatment decreases phosphorylation levels of all three kinases, p-ERK, p- Akt and p- GSK3β but only in WT mice (LPS x genotype interaction: F(1,8)=5.35; p=0.0038 for p-ERK; LPS x genotype interaction: F(1,18)=17.83; p=0.019 for p-Akt; genotype: F(1,12)=13.19; p=0.013 for GSK3β) (Fig. 4B, D and F) with no influence on its total levels (Fig. 4A, C and E). In GluN2A KO mice, LPS treatment produced no effect at the levels of ERK, Akt, and GSK3β, as well as their active, phosphorylated forms (Fig. 4A–F).
Fig. 4. Effects of LPS on upstream and down-stream mTOR signalling in PFC.
The levels of total and phosphorylated ERK (A.B). Akt (C,D), GSK3β (E.F). mTOR (G.H). p70S6K (U) and the levels of PSD-95 (K) and GluAl(L) in PFC synaptosomal fraction of male wild-type (WT) and GluN2A knockout (KO) mice upon LPS treatment compared to controls (VEH). Values are presented as mean ± SEM % of control values (n = 10). Statistically significant differences are given as p < 0.05. *VEH vs. LPS, #WT vs. KO.
3.3. Effects of LPS on mTOR activity and downstream p70S6K
In the hippocampus, LPS treatment decreased p-mTOR levels in WT mice and increased its levels in GluN2A KO mice (LPS x genotype interaction: F(1,20)=57.20; p=0.00017) (Fig. 3H) while neither treatment nor genotype had any significant effect on total mTOR levels (Fig. 3G). Levels of the mTOR effector, phosphorylated p70S6K, remain unchanged by treatment in WT as well as in GluN2A KO animals (Fig. 3J), while LPS treatment decreased total levels of this kinase in WT animals and increased its levels in GluN2A KO mice (LPS x genotype interaction: F(1,12)=21.01; p=0.029) (Fig. 3I).
In the PFC, LPS treatment decreased p-mTOR levels in WT mice (LPS: F(1,12)=12.75; p=0.012;) while in the GluN2A KO animals treatment did not affect p-mTOR levels (genotype: F(1,12)=12.61; p=0.0013) (Fig. 4H). Levels of the total form of m-TOR kinase were altered neither by treatment nor genotype (Fig. 4G). LPS treatment decreased phosphorylated form of p70S6K kinase only in WT animals (LPS x genotype interaction: F(1,12)=30.75; p=0.0002) (Fig. 4J), while in GluN2A KO animals, its levels were unchanged by treatment. On the other hand, neither treatment nor genotype had effects at the levels of PFC total p70S6K (Fig. 4I).
3.4. Effects of LPS on synaptic PSD-95 and GluA1 levels
Our results revealed that in the hippocampus, LPS treatment decreased the levels of GluA1 and PSD-95 in WT animals (LPS x genotype interaction: F(1,12)=26.43; p=0.00025 for PSD-95; LPS: F(1,12)=25.25; p=0.0005 for GluA1) (Fig. 3K and L) but did not affect its levels in GluN2A KO mice. Furthermore, the levels of GluA1 were significantly higher in GluN2A KO mice comparing to WT animals (genotype: F(1,12)=35.03, p=0,0007) (Fig. 3L).
In PFC, LPS treatment did not affect the levels of GluA1 in WT animals but decreased its levels in GluN2A KO mice (LPS x genotype interaction: F(1,42)=12.55; p=0.032) (Fig. 4L). Interestingly, the levels of GluA1 were significantly higher in GluN2A KO mice comparing to WT animals (genotype: F(1,42)=251.37, p=0,0017) (Fig. 4L). On the other hand, the treatment decreased the levels of PSD-95 in WT animals but did not affect its levels in GluN2A KO mice (LPS x genotype interaction: F(1,8)=15.46; p=0.032) (Fig. 4K).
3.5. Effects of ERK inhibitor on behaviour and activity of mTOR signaling in LPS-treated GluN2A KO animals
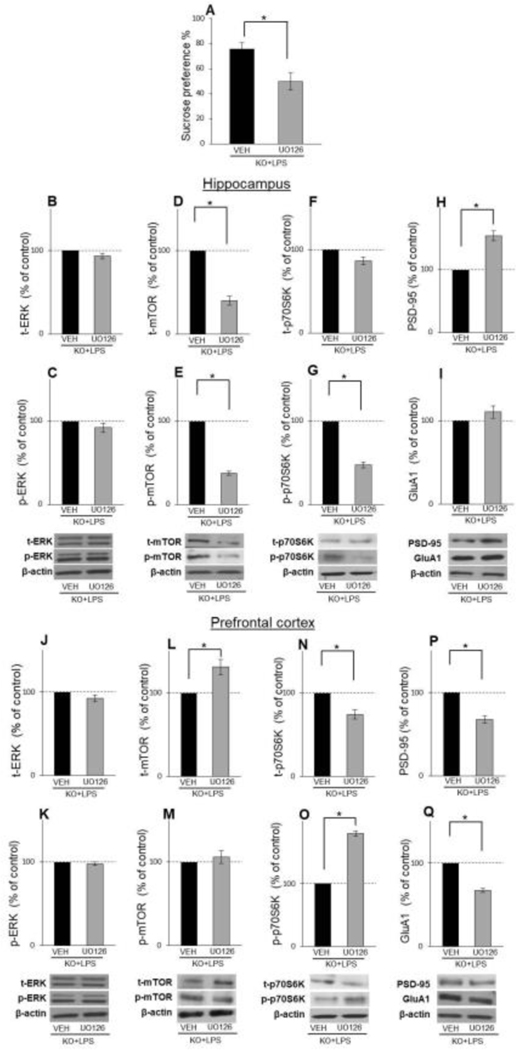
Our first experiment showed decreased p-ERK levels in both examined brain structures, in LPS-treated WT animals exhibiting depressive-like behaviour, compared to unchanged p-ERK levels in GluN2A KO animals, resilient to LPS-induced depression. These findings prompted us to hypothesize that p-ERK activation might be responsible for the downstream alteration of mTOR signaling and different behavioural response to the LPS challenge in GluN2A KO. We i.c.v infused the ERK Inhibitor (UO126) in LPS-treated GluN2A KO mice to test this hypothesis, and 18h after inhibitor administration [16], we quantified the effects of the drug on behaviour, ERK activation, and downstream mTOR signaling.
UO126 treatment induced anhedonia-like behaviour in LPS challenged GluN2A KO mice since UO126 significantly decreased the consumption of sucrose solution compared to the vehicle-infused GluN2A KO animals (t(18)=3.19; p= 0.005) (Fig. 5A).
Fig. 5.
Effects of U0126, 18 h after its administration, on behaviour and activity of mTOR signalling in LPS-treated GluN2A KO animals in hippocampus and PFC. Mean sucrose preference (%) of male LPS-treated GluN2A KO mice 18 h after i.c.v infusion with vehicle (VEH) or U0126 (n = 10) (A). The levels of total and phosphory lated ERK, mTOR, p70S6K and the levels of GluAl and PSD-95 in the hippocampal (B-l) and PFC (j-Q) synaptosomal fraction of male LPS-treated GluN2A KO mice 18 h after Lc.v infusion with vehicle (VEH) or U0126 (n = 8). Values are presented as mean ± SEM % of control values. Statistically significant differences are given as p< 0.05. *VEH vs. U0126.
In hippocampal synaptosomes, phosphorylated and total levels of ERK were unchanged, 18h after i.c.v infusion of ERK inhibitor (Fig. 5C and B), while the levels of p-mTOR, total mTOR and p-p70S6K were decreased in inhibitor-treated GluN2A KO mice (t(9)=35.62; p= 0.00012 for p-mTOR; t(8)=11.12; p= 0.0004 for t-mTOR t(6)=17.46; p= 0.00002 for p-p70S6K) (Fig. 5E, D and G), with unchanged levels of t-p70S6K (Fig. 5F). Interestingly, while the levels of GluA1 were unchanged (Fig. 5I), UO126 increased PSD-95 levels in GluN2A KO animals (t(7)=7.96; p= 0.00094 for PSD-95) (Fig. 5H).
As in hippocampus, UO126 also did not affect phosphorylated and total ERK levels in PFC (Fig. 5K and J), 18h after administration, but inhibitor did decrease levels of synaptic proteins PSD-95 and GluA1 in LPS-treated GluN2A KO animals with anhedonia-like symptoms (t(7)=9.16; p= 0.00038 for PSD-95; t(6)=16.96; p= 0.003 for GluA1) (Fig. 5P and Q). Regarding the effect of UO126 treatment on mTOR and p70S6K phosphorylation status, we detected changes only in p70S6K, which phosphorylated forms were increased in UO126-infused GluN2A KO animals (t(6)=22.53; p= 0.0001) (Fig. 5O). As for total levels of these two kinases, levels of mTOR were increased while the levels of p70S6K were decreased in ERK inhibitor-treated GluN2A KO mice (t(8)=3.43; p= 0.0089 for t-mTOR; t(6)=5.65; p= 0.00013 for t-p70S6K) (Fig. 5L and N).
In order to examine if ERK inhibition occurred in time points earlier than 18h after UO126 injection, we performed experiments in which we evaluated UO126 biochemical and behavioural effects on LPS-treated GluN2A KO animals 1 and 4 hours after inhibitor administration [22]. We detected no alterations in the total or phosphorylated forms of ERK and mTOR levels in the hippocampus in any examined time point upon UO126 injection (Fig 6B–E and 6L–O). On the other hand, in PFC, levels of total ERK and mTOR were unaltered upon UO126 treatment in both examined time points (Fig. 6F, H, P and R), but p-ERK levels, as well as levels of p-mTOR, were decreased 1h (t(8)=4.99; p= 0.0016 for p-ERK; t(6)=7.79; p= 0.002 for p-mTOR) (Fig. 6G and I) and 4h (t(8)=9.13; p= 0.0017 for p-ERK; t(6)=4.48; p= 0.0041 for p-mTOR) (Fig. 6Q and S) following UO126 administration. Interestingly, although UO126 inhibited ERK activity 4h after injection in PFC, it did not affect the behaviour of animals examined in that time point (Fig. 6K).
4. Discussion
The present study demonstrated that deletion of GluN2A subunit of NMDA receptor abrogated the development of depressive-like behaviour after LPS injection by sustaining the mTOR signaling and synaptic stability in hippocampus and PFC. We also showed that resilience of GluN2A KO to LPS-induced depressive-like behaviour was abolished by inhibition of ERK, the upstream activator of mTOR signaling, providing direct evidence of the involvement of ERK as a mediator of GluN2A effects on mTOR signaling and emergence of depressive-like behaviour after immune challenge.
Recent scientific evidence has pointed out the essential role of the glutamatergic neurotransmission system in depression pathophysiology [23]. Indeed, in a neuroinflammation-induced model of depression, the overactivation of glutamate receptor, NMDAR, is involved in the appearance of depressive-like behaviour in animals [16, 24]. On the other hand, one of the most potent antidepressants with synaptogenic effects accomplished through activating mTOR signaling is glutamatergic non-selective NMDA receptor antagonist, ketamine [18]. Overall, there is strong support for therapeutic advances in understanding glutamatergic neurotransmitter systems and the role of the mTOR signaling pathway in depression. Thus, our study aimed to understand better the molecular mechanisms responsible for the altered behaviour of mice lacking GluN2A subunits of NMDAR in the LPS-induced model of depression.
The results of behavioural analyses performed in this study confirmed that LPS treatment induced depressive-like behaviour in WT rodents 24 h after the injection, and these data are in accordance with previous studies [25, 26]. In contrast, in GluN2A KO mice LPS did not cause depressive-like behaviour, which was clearly shown by higher consumption of sucrose and decreased immobility time in FST compared to WT counterparts. Additionally, our results demonstrated that impairment in locomotor activity of animals, 6 h post-LPS administration, subsided 24h after the treatment, which suggests that all behavioural changes can be attributed solely to depressive-like behaviour.
The molecular analyses showed that depressive-like behaviour in WT animals after the LPS challenge was linked to the downregulation of mTOR signaling. The decreased phosphorylation of mTOR in the hippocampus and PFC correlated with the decreased activity of its upstream kinases, specifically, the decreased levels of p-ERK in the hippocampus and p-ERK, p-Akt, and p-GSK3β in PFC. Further, We were able to link the diminished mTOR activity with the reduced activity of its downstream effectors, p70S6K, and decreased levels of synaptic markers, GluA1 and PSD-95, in PFC and hippocampus. These results are in accordance with previous studies showing that sustained activation of the NMDAR is associated with dephosphorylation of ERK and Akt kinase [27] and activation of GSK-3β [28]. Inhibitory action on the mTOR activity via its upstream regulators resulted in reduced synaptic protein synthesis and the development of the depressive-like phenotype [18, 29]. Our results support the role of m-TOR pathway in depression, being that WT animals with LPS-induced depressive-like behaviour exhibit impaired synaptogenesis as a consequence of mTOR pathway downregulation.
Contrary to WT mice, animals lacking GluN2A subunit of NMDA receptor, when treated with LPS, exhibited resilience to depressive-like behaviour along with unaltered or even increased mTOR activity in analyzed brain structures. Specifically, while PFC mTOR signaling stayed unchanged, p-mTOR and t-p70S6K levels were increased in the hippocampus of LPS-treated GluN2A KO animals, leading to sustained expression synaptic markers. Therefore, for the first time, our results showed that persistent mTOR activity and preserved synaptic stability upon neuroinflammation were GluN2A-dependent, which prevented the onset of depression. Also, these results suggest that pharmacological inhibition of GluN2A subunits of NMDAR could have beneficial effects in treating neuroinflammation-related depression as an alternative to ketamine with serious side effects.
To further analyze the role of mTOR signaling and its interplay with GluN2A subunit in depressive phenotype, we targeted ERK as its upstream regulator with the most prominent changes in PFC and hippocampus upon LPS in WT animals. Indeed, treatment with ERK inhibitor UO126 abrogated resilience to the depressive phenotype of GluN2A KO mice upon LPS treatment, 18h after inhibitor administration. This is in accordance with already published data indicating that ERK signaling was significantly downregulated in the PFC and hippocampus of depressed humans and animals [30, 31]. Likewise, a study reported that besides ERK inhibitors’ ability to induce depression-like behaviour, they could also block the effects of several antidepressants [32, 33].
Interestingly, we did not find that ERK activity was diminished at the 18th hour after the UO126 administration. However, we showed that in LPS-treated GluN2A KO animals, p-ERK decrease could be detected 1 and 4h after the inhibitor application in PFC. However, it did not provoke an anhedonia-like response in a sucrose preference test, as a key component of depressive-like phenotype, measured 4h after inhibitor administration. Therefore, ERK inhibition had a delayed influence on animal behaviour, since they started to display anhedonia-like response 18h after UO126 administration when ERK activity was returned to control levels. Other authors also reported the delayed effect of decreased ERK activity on behaviour since depressive-like behaviour developed 7 days following an initial decrease of the ERK activity [31, 34]. In our study, 18h after UO126 administration, we detected decreased synaptic protein levels in PFC, which could be responsible for anhedonia-like behavior in the GluN2A KO animals. Namely, decreased activation of ERK and downstream mTOR in the first four hours after UO126 administration in PFC could decrease synaptic markers evidenced after 18h, as a certain time is needed for their effects on translation to be detected.
In the hippocampus, we did not observe a decrease of ERK activity, although the downregulation of mTOR-p70S6K cascade was apparent 18h upon UO126 administration in LPS challenged GluN2A KO animals. However, the levels of synaptic marker PSD-95 were increased with sustained GluA1 levels at that time point. Elevated levels of PSD-95 could be a consequence of increased BDNF in the hippocampus of LPS-treated GluN2A KO animals that we observed in our previous study [13]. Namely, PSD-95 expression seems to be also linked to BDNF signaling since BDNF application induced a robust increase in PSD-95 [35].
Taken together, the presented results demonstrated that ERK activity is implicated in the resilience of GluN2A KO animals to depression, and GluN2A-ERK-mTOR signaling, in PFC in particular, is a vulnerability factor of inflammation-related depressive behaviour.
Our study should be interpreted considering several limitations. Here, we studied the effects of the GluN2A NMDAR subunit by its genetic ablation, which could affect the development of the glutamatergic system in these mice, possibly affecting the expression of other GluN2 subunits and thus indirectly contributing to the resilience to LPS-induced depression. Further experiments using specific pharmacological NMDAR subunit antagonists or KO mice for different NMDAR subunits could be used to define the role of each NMDAR subunit in LPS-induced depressive behaviour. Also, in this study, we characterized the role of ERK in the mTOR-dependent resilience of GluN2A KO to depression. However, it would be helpful to perform similar experiments with inhibitors of other two mTOR upstream regulatory kinases, Akt and GSK3β, and defined their influence on mTOR signaling activity and resilience of GluN2A KO to depression.
In summary, this study demonstrated that the absence of GluN2A subunit abolished depressive outcomes when challenged with LPS by sustaining mTOR pathway activity and preserving synaptic stability and that this mechanism is ERK-dependent. Therefore, our results suggest that side effects of ketamine, whose antidepressant effects are mediated through mTOR-dependent synaptic protein synthesis, could be avoided by agents that interfere more directly with the mTOR pathway or target specific NMDA receptor subunits. Thus, our findings can contribute to a better understanding of neurobiological mechanisms of depression and facilitate the discovery of more effective pharmacological treatments targeting the growth and stabilization of synaptic connections.
Highlights.
LPS induced depressive-like behavior in wild type mice, but not in GluN2A −/− mice.
Resilience of GluN2A−/− mice to depression is linked to sustained mTOR activity.
mTOR-mediated synaptic stability protects GluN2A −/− mice from depression.
GluN2A-ERK-mTOR pathway is vulnerability factor of inflammation related depression.
Acknowledgements
This work was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia 451-03-9/2021-14/ 200017 and grant R01MH078064
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Miller AH, Raison CL, The role of inflammation in depression: from evolutionary imperative to modern treatment target, Nature reviews. Immunology 16(1) (2016) 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dantzer R, Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches, Current topics in behavioral neurosciences 31 (2017) 117–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R, Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice, Molecular psychiatry 14(5) (2009) 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zanos P, Gould TD, Mechanisms of ketamine action as an antidepressant, Molecular psychiatry 23(4) (2018) 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK, GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38(11) (2013) 2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sarkar A, Kabbaj M, Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats, Biological psychiatry 80(6) (2016) 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B, The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder, Progress in neuro-psychopharmacology & biological psychiatry 35(7) (2011) 1774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhu WL, Wang SJ, Liu MM, Shi HS, Zhang RX, Liu JF, Ding ZB, Lu L, Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats, Journal of psychiatry & neuroscience : JPN 38(5) (2013) 306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, Zhang HT, Cravatt BF, Liu QS, Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39(7) (2014) 1763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paoletti P, Bellone C, Zhou Q, NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease, Nature reviews. Neuroscience 14(6) (2013) 383–400. [DOI] [PubMed] [Google Scholar]
- [11].Cho KK, Khibnik L, Philpot BD, Bear MF, The ratio of NR2A/B NMDA receptor subunits determines the qualities of ocular dominance plasticity in visual cortex, Proceedings of the National Academy of Sciences of the United States of America 106(13) (2009) 5377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK, Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling, Proceedings of the National Academy of Sciences of the United States of America 100(5) (2003) 2854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Francija E, Petrovic Z, Brkic Z, Mitic M, Radulovic J, Adzic M, Disruption of the NMDA receptor GluN2A subunit abolishes inflammation-induced depression, Behavioural brain research 359 (2019) 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. , Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit, Nature 373(6510) (1995) 151–5. [DOI] [PubMed] [Google Scholar]
- [15].Gao C, Frausto SF, Guedea AL, Tronson NC, Jovasevic V, Leaderbrand K, Corcoran KA, Guzman YF, Swanson GT, Radulovic J, IQGAP1 regulates NR2A signaling, spine density, and cognitive processes, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(23) (2011) 8533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38(9) (2013) 1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Silingardi D, Angelucci A, De Pasquale R, Borsotti M, Squitieri G, Brambilla R, Putignano E, Pizzorusso T, Berardi N, ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex, Frontiers in behavioral neuroscience 5 (2011) 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists, Science 329(5994) (2010) 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Markwell MA, Haas SM, Bieber LL, Tolbert NE, A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples, Analytical biochemistry 87(1) (1978) 206–10. [DOI] [PubMed] [Google Scholar]
- [20].Laemmli UK, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227(5259) (1970) 680–5. [DOI] [PubMed] [Google Scholar]
- [21].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, From inflammation to sickness and depression: when the immune system subjugates the brain, Nature reviews. Neuroscience 9(1) (2008) 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deyama S, Ishikawa Y, Yoshikawa K, Shimoda K, Ide S, Satoh M, Minami M, Resolvin D1 and D2 Reverse Lipopolysaccharide-Induced Depression-Like Behaviors Through the mTORC1 Signaling Pathway, The international journal of neuropsychopharmacology 20(7) (2017) 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Niciu MJ, Ionescu DF, Richards EM, Zarate CA Jr., Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder, Journal of neural transmission 121(8) (2014) 907–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V, Neuroinflammation and depression: A review, The European journal of neuroscience 53(1) (2021) 151–171. [DOI] [PubMed] [Google Scholar]
- [25].Walker AK, Wing EE, Banks WA, Dantzer R, Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice, Molecular psychiatry 24(10) (2019) 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qiu J, Liu R, Ma Y, Li Y, Chen Z, He H, Chen J, Tong L, Huang C, You Q, Lipopolysaccharide-Induced Depression-Like Behaviors Is Ameliorated by Sodium Butyrate via Inhibiting Neuroinflammation and Oxido-Nitrosative Stress, Pharmacology 105(9–10) (2020) 550–560. [DOI] [PubMed] [Google Scholar]
- [27].Burket JA, Benson AD, Tang AH, Deutsch SI, NMDA receptor activation regulates sociability by its effect on mTOR signaling activity, Progress in neuropsychopharmacology & biological psychiatry 60 (2015) 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL, LTP inhibits LTD in the hippocampus via regulation of GSK3beta, Neuron 53(5) (2007) 703–17. [DOI] [PubMed] [Google Scholar]
- [29].Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B, Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress, Progress in neuro-psychopharmacology & biological psychiatry 40 (2013) 240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R, The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex, Neural plasticity 2017 (2017) 6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qi X, Lin W, Li J, Pan Y, Wang W, The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress, Behavioural brain research 175(2) (2006) 233–40. [DOI] [PubMed] [Google Scholar]
- [32].Domin H, Szewczyk B, Pochwat B, Wozniak M, Smialowska M, Antidepressant-like activity of the neuropeptide Y Y5 receptor antagonist Lu AA33810: behavioral, molecular, and immunohistochemical evidence, Psychopharmacology 234(4) (2017) 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pochwat B, Rafalo-Ulinska A, Domin H, Misztak P, Nowak G, Szewczyk B, Involvement of extracellular signal-regulated kinase (ERK) in the short and long-lasting antidepressant-like activity of NMDA receptor antagonists (zinc and Ro 25–6981) in the forced swim test in rats, Neuropharmacology 125 (2017) 333–342. [DOI] [PubMed] [Google Scholar]
- [34].Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M, Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress, Neurobiology of disease 31(2) (2008) 278–85. [DOI] [PubMed] [Google Scholar]
- [35].Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, Dent EW, BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(43) (2011) 15597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]