Abstract
Background
Circular RNAs (circRNAs) are transcribed by RNA polymerase II and are mostly generated by the back-splicing of exons in the protein-coding gene. Massive circRNAs are reported to be differentially expressed in different species, implicating their prospects as aging biomarkers or regulators in the aging progression.
Methods
The possible role of circRNAs in aging and longevity was reviewed by the query of circRNAs from literature reports related to tissue, organ or cellular senescence, and individual longevity.
Results
A number of circRNAs have been found to positively and negatively modulate aging and longevity through canonical aging pathways in the invertebrates Caenorhabditis elegans and Drosophila. Recent studies have also shown that circRNAs regulate age-related processes and pathologies such various mammalian tissues, as the brain, serum, heart, and muscle. Besides, three identified representative circRNAs (circSfl, circGRIA1, and circNF1-419) were elucidated to correlate with aging and longevity.
Conclusion
This review outlined the current studies of circRNAs in aging and longevity, highlighting the role of circRNAs as a biomarker of aging and as a regulator of longevity.
Keywords: Aging, longevity, circular RNA, biomarkers, microRNAs, age-related diseases
1. INTRODUCTION
Circular RNAs (circRNAs) are a class of covalently closed, single-stranded non-coding RNAs transcribed by RNA polymerase II [1, 2]. CircRNAs are assumedly generated by the back-splicing of exons in protein-coding genes widespread in the eukaryotic genome [3-5]. They have been discovered in many species, including flies, worms, mouse, and human [3, 6]. Over the last few decades, a great many biological properties of circRNAs have been uncovered, such as extensive-expression, high conservation, tissue specificity, cell specificity and developmental stage-specific expression patterns [7], and they are involved in the regulation of alternative splicing, transcription, and chromatin looping in the nucleus [8]. Currently, some studies suggest that circRNAs serve as miRNA or mRNA sponges to interact with many different RBPs, enhance protein function, act as scaffolds to mediate complex formation between specific enzymes and substrates and recruit proteins to specific locations [9]. Furthermore, a few circRNAs undergo cap-independent translation and encode a protein or peptide under specific conditions [4, 5, 8, 10-12]. Hitherto, the biogenesis, biology and characterization of circRNAs have been elucidated in detail in the previous publications [3, 8, 9], which are crucially functional in such many cellular processes and physiological regulation as immune systems, tumorigenesis and neurogenesis [3, 8, 13-16]. The linear RNAs can be degraded by RNase R where the circRNA can be retained [17]. The high stability of circRNAs is one of the important causes contributing to the disease occurrence. Recently, the overall accumulation of circRNAs has been implicated in a wide variety of aging tissues [18]. More importantly, a certain correlation of circRNAs have been found with brain aging, bone aging, muscle aging, skin aging, and reproductive aging through various functions [18]. These all strengthen the hypothesis that circRNAs may be vital factors for aging and age-related diseases, of which the proposed biological functions and the potential mechanisms in aging and longevity are discussed in this review.
2. THE COMPREHENSIVE PROFILING OF CIRCRNA EXPRESSION DURING AGING
RNA-seq has the advantage of providing both expression data for coding and non-coding RNAs, which was the preferred method for discovering novel circRNAs and compressively was used for circRNAs profiling studies [9]. Moreover, microarray assay is also a viable alternative to identify circRNAs profiles in the studied tissues [9]. Currently, the aging-associated circRNAs expression profiles of multiple tissues derived from multiple species have been elucidated in the aging process, including the diverse expression abundance of circRNAs in various tissues or organs and the expression differences at different time points, namely spatio-temporal specificity. Given the importance of elaborating the role of circRNAs in aging, we summarized and analyzed circRNAs changes with age in various organisms and organs (Table 1 and Fig. 1).
Table 1.
Summary of circRNA sequencing of aging tissues from different species.
| Refs. | Species | Method | Age | Objects | Number |
|---|---|---|---|---|---|
| [18] | Drosophila flies | RNA-seq | Young (10d), middle-aged (30d), aged (50d) | Brain, gut, thorax, and fat | 1,182 |
| [19] | Caenorhabditis elegans | RNA-seq | L4-larval stage (L4), 1d, 7d, 10d | Whole worms | 1166 |
| [20] | Mouse | RNA-seq | Young (1m), aged (22m) | Cortex, hippocampus, heart | 6,791 |
| [21] | Mouse | RNA-seq | Young (3m), aged (18m) | Skeletal muscle | 4,336 |
| [22] | Rat | RNA-seq | 1d rat for senescent astrocyte preparation |
Senescent astrocyte | 7,376 |
| [23] | Rat | RNA-seq | Juvenile (2w), adolescence (6w), middle-aged (21w), aged (104w) |
Brain, muscle, heart, liver, lung, kidney, testes, thymus | 5,058 |
| [24] | Tree shrew | RNA-seq | Infant (47–52d), young (15–18m), old (78–86 m) | Hippocampus, cerebellum | 35,007 |
| [25] | Porcine | RNA-seq | Young (180 d), old (8 y) | Ovarian | 116 |
| [17] | Rhesus macaque | RNA-seq | Middle-aged (10y), aged (20y) | PFC, OC, CA1, PCC, PC, TC, DG, CB | 52,828 |
| [26] | Rhesus macaque | RNA-seq | Young (0.005-6y), middle-aged (11.3-16.93), aged (25.7-40.9) | Skeletal muscle | 12,007 |
| [27] | Human | RNA-seq | 25y,26y,28y,44y,45y,46y | Ovarian | 48,220 |
| [28] | Human | RNA-seq | Young(30–36y), aged(86–95y) | Peripheral blood | 2,207 |
| [29] | Human | RNA-seq | Young (30–32 y), old (80–85 y) | Serum | 133 |
Note: prefrontal cortex (PFC), posterior cingulate cortex (PCC), temporal cortex (TC), parietal cortex (PC), occipital cortex (OC), hippocampus (CA1), and dentate gyrus (DG), and cerebellar cortex (CB). day (d), month (m), year (y), week (w).
Fig. (1).
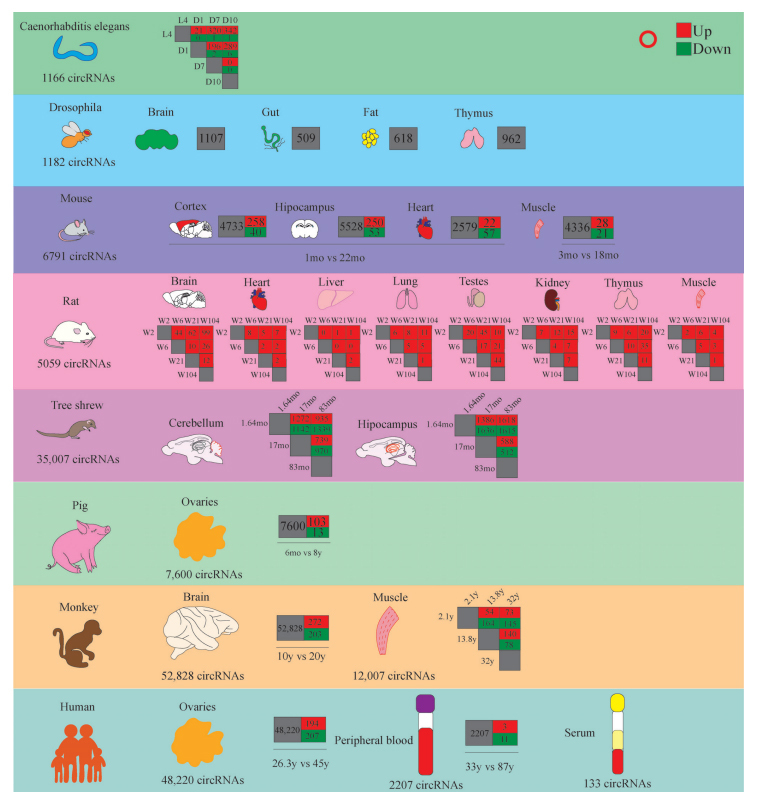
Summary of circRNA sequencing of aging tissues from different species.
2.1. Profiling of circRNA Expression During Brain Aging
Currently, the brain circRNAs expression profiles in many species, including drosophila flies, mice, rat, tree shrews and monkey were identified during aging [17-21]. In drosophila flies, the spatio-temporal specific expression manner was dissected from fly tissues grouped in the young (aged 10 days), the middle-aged (aged 30 days), and the old (aged 50 days) [18]. Of 1,182 circRNAs identified in flies, 1,159 were detected in the brain, 180 of which were brain-specific and not expressed in other tissues [18] (Table 1 and Fig. 1). The expression of global circRNAs in brain tissue increases with age as compared to other tissues [18]. Dramatically, the expression of circRNAs in dilp 2-3,5 mutant brains was also accumulated but significantly lower than that in wild-type flies, indicating that the accumulation of circRNAs was decelerated with decreasing insulin signaling.
Moreover, two studies illustrated the changes of circRNAs expression profiles during brain aging in rodents. In one study, three kinds of tissues from 1-month and 22-month-old mice, including the cerebral cortex, hippocampal formation and heart were used for transcriptome profiles [19], which uncovered 6,791 differentially expressed circRNAs across these samples. The number of circRNAs found in brain tissues is about twice more than that in heart tissue. Similar to the results obtained in drosophila flies, circRNAs tended to be significantly upregulated with age in both the cerebral cortex and hippocampal tissues, but not in the heart tissues [19]. As shown in Fig. (1), circRNAs in the cortex and hippocampal tissues showed a similar proportion of changes during aging (~5% up-regulated and ~1% down-regulated), while no bias was found in increased circRNAs abundance during cardiac aging, suggesting that the overall trend of circRNAs upregulation during aging might be brain-specific. In the cerebral cortex, altered circRNAs may be associated with synaptic structural or functional changes analyzed by functional enrichment analysis. However, the upregulated circRNAs in the aging hippocampus are associated with protein and chromosomal modifications. The above results suggested that differentially expressed circRNAs in brain tissues has regional -specificity and functional differences during aging.
In the other study, a deep-total RNA sequencing of 8 organs from both male and female rats at four developmental stages, juvenile (aged 2 weeks), adolescence (aged 6 weeks), adult (aged 21 weeks) and elder (aged 104 weeks) were used to provide a comprehensive circRNAs expression profile (Table 1 and Fig. 1). A total of 5,058 distinct circRNAs from 2,578 genes were detected and 640 significantly differentially expressed circRNAs were identified in 8 organs during aging. Among them, 65.8% (3,329/5,058) circRNAs were found with organ specificity. Of these 8 organs, the brain was ranked first with 1,167 (50%) specific circRNAs. Unexpectedly, the high expression abundance of circRNAs in brain tissues was consistent with findings from human, mouse and fly [18-20]. The circRNAs levels in the rat brain and kidney gradually increased during aging. It was found that the temporal expression pattern of circRNAs in the brain (253/39.5%) was most prominent. CircRNAs accumulation in brain tissue with age is largely independent of linear RNA expression of host genes, suggesting that circRNAs might play an independent biological role in the central nervous system during aging [19, 20].
The tree shrews (Tupaia belangeri) have been widely used in nervous system research, including brain development, Alzheimer's disease and aging, due to their small size, short reproductive cycle and high brain weight ratio. Using hippocampus and cerebellum samples, 35,007 circRNAs were identified in the infant (aged 47–52 days), young (aged 15–18 months), and old (aged 78–86 months) tree shrews revealed by RNA-seq [21]. A total of 983 circRNAs were expressed exclusively in the hippocampus, and 1,009 were expressed only in the cerebellum [21]. As shown in Fig. (1), from the infant group to the old group, 3.9% (youth>infant), 4.1% (old>infant), 2.9% (old>youth) circRNAs were significantly upregulated with age, respectively, and 3.5% (youth<infant), 2.9% (old<infant), 2.2% (old<youth) circRNAs were significantly downregulated in the cerebellum, respectively. In the hippocampus, 5.0% (youth>infant), 5.3% (old>infant), and 1.7% (old>youth) were significantly upregulated with age, and 4.3% (youth<infant), 5.3% (old<infant), and 1.9% (youth>old) were significantly downregulated with age, respectively [21]. Moreover, 83.1% circRNAs in the tree shrew share homology with humans, suggesting that as a reliable animal model, comprehensive expression profiles of circRNAs in the tree shrew brain during postnatal development and aging may be conducive to elucidating the functions of circRNAs in brain aging and age-related diseases.
Through deep-sequencing RNA samples from eight regions of the cerebral cortex, including prefrontal, posterior cingulate, temporal, parietal, occipital, hippocampus, dentate gyrus, and cerebellar of the monkeys (rhesus macaque) between two cohorts (the youth: 10 years old and the old: 20 years old), the profiling of circRNAs expression in the brain tissues during aging was systematically determined (Table 1 and Fig. 1), and a total of 52,828 distinct circRNAs candidates were detected [17]. Bioinformatics analysis demonstrated that the circRNA homologues between macaque and humans were much higher than that between macaque and mouse, and only 32.54% circRNAs were expressed in all three species [17]. Besides, about 40% circRNAs in macaque could not be mapped to the human circRNAs database, suggesting the inherent evolutionary feature of circRNAs. The functional enrichment analysis of circRNAs host genes showed that important neuronal-related pathways were enriched, such as Long-term potentiation (LTP), Glutamatergic synapse, Dopaminergic synapse, and Synaptic vesicle cycle. Of all the circRNAs, 475 circRNAs were age-specific and more aging-specific circRNAs were identified, with 272 ones specific for the old and 203 ones specific for the young, indicating that the abundance of circRNAs expression increased during aging.
2.2. Profiling of circRNA Expression in Skeletal Muscle During Aging
There are many studies illustrating the changes of circRNA expression in skeletal muscle from mice, rats, and monkeys during aging [20, 22, 23], which revealed the smallest number of muscle-specific circRNAs compared to the brain, testis, thyroid, heart, and liver [20]. Guo et al. applied RNA-seq to evaluate the circRNAs profiles for quadriceps femoris muscles in three groups, including sedentary young mice, aging mice, and aging mice with aerobic exercise (Table 1 and Fig. 1), and 4,336 circRNAs in total were identified in three groups [22]. There were 49 differentially expressed circRNAs (28 up-regulated and 21 down-regulated) in the older group when compared to the youth group, however, aerobic treadmill training led to a significant change of circRNAs expression profile in aging mice, in which 10 were up-regulated and 11 were down-regulated [22]. Among these, circATP9b and circBBS9 expression significantly decreased in the aging group compared to the youth group, while aerobic treadmill training reversed their expression. In skeletal muscle of monkeys ranging from 0.003 to 40.9 years old, researchers have identified and annotated 12,007 circRNAs among the young (aged 0.003 to 6 years), the middle-aged (aged 11.3 to 16.9 years), and the old (25.7 to 40.9 years) [23], among which most muscle-related circRNAs did not change with age, while only 19 circRNAs were downregulated with age. In conclusion, despite a large amount of circRNAs existing in the muscle, the abundance of these circRNAs was generally low, and only a small number of circRNAs changed with age. Only a few studies have focused on circRNA expression changes in aging muscle, and clear mechanistic studies are still absent. As these studies implied, it will be of great interest to investigate the circRNAs-related mechanisms of muscle with age, especially in bigger animals like aged monkeys and humans. In addition, skeletal muscle is innervated by the nervous system, and it is also critical to study the link between skeletal muscle and the nervous system.
2.3. Profiling of circRNA Expression in Ovarian During Aging
Women have been delaying pregnancy unintentionally or intentionally in the past few decades and even in the future. Thus, the decrease in reproductive performance along with ovarian aging is mainly manifested by a gradual reduction in the number and quality of oocytes becomes the major challenge in women’s reproductive health [24-26]. Since the decrease in ovarian follicular reserve was nonlinear and accelerated with age [24, 25, 27]. In recent years, widespread attention has been paid to circRNAs in varieties of preclinical fields, including ovarian aging [28].
In a recent study depicting the transcriptional expression changes of ovarian during aging by using old pig and young pig samples [25], a total of 20,357 mRNAs, 4,879 lncRNAs, 1,196 miRNAs and 7,600 circRNAs were identified from four samples (Table 1 and Fig. 1), among which 116 circRNAs (103 up-regulated and 12 down-regulated) were differentially expressed during ovarian aging. Bioinformatics analysis demonstrated that transmembrane receptor protein, serine/threonine kinase activity, in utero embryonic development, reproductive process, ovarian cumulus expansion, and ovulation cycle were significantly enriched by those differentially expressed circRNAs [25]. In addition, another publication demonstrated circRNA expression profiles of ovarian from young (aged 26.3 years) and aging (aged 45 years) human patients [28]. Among 48,220 identified circRNAs, 194 circRNAs were significantly up-regulated and 207 circRNAs were down-regulated during aging [28], which were significantly enriched in the metabolic process, regulated secretory pathway, oxidation-reduction process, steroid hormone biosynthesis, and insulin secretion pathways [28]. The number of total human circRNAs and differentially expressed circRNAs was significantly larger than that of pigs, especially downregulated differentially expressed circRNAs. Furthermore, the functional enrichment outcomes of these differentially expressed circRNAs also varied significantly between the two species. Overall, these studies suggested the abundance of circRNAs and differential expression involved in the ovary aging process and might play an important role in the development and progression of ovarian senescence.
2.4. Expression Profiling of circRNAs in Peripheral Blood and Serum During Aging
A great number of studies have shown that aging remains the most crucial hazard factor for cardiovascular, cerebrovascular and neurodegenerative diseases [29, 30]. The difficulty in obtaining human aging organ samples and the significant increase in the aging population highlights the urgent need to discover disease-related circulating factors that may serve as potential biomarkers or therapeutic targets for aging and/or age-related diseases [30]. Increasing evidence shows that circulating factors play an important role in regulating organ or cell function during aging, and the discovery of circulating RNAs or proteins in human body fluids like the serum, has aroused great interest in whether these molecules can be used as disease biomarker or therapeutic targets [30].
In a study of the human serum, 133 circRNAs were identified in the serum (Table 1 and Fig. 1). There were 3 circRNAs with a higher number of reads, hsa-circ-0001305 (circ1305), hsa-circ-0000722 (circ722) and hsa-circ-0001445 (circ1445). Among these circRNAs, intriguingly, circ1305 and circ722 were significantly upregulated and circ1445 was significantly downregulated in old individuals compared with the young [30]. In another study, a total of 2,207 circRNAs were identified in human peripheral blood [31] (Table 1 and Fig. 1). Of these, only 184 circRNAs were found in young and old blood samples, 431 were expressed only in young samples and 1592 ones in old samples [31]. These results indicated the limited amount of circRNAs in serum and the limitations in uncovering age-related biomarkers in serum. Despite a higher abundance of circRNA expression in the peripheral blood, it is doubtful whether a large number of blood cells in peripheral blood as independent systems can respond to changes in other systems.
3. THE CRUCIAL CIRCULAR RNA IN THE AGING AND LONGEVITY
3.1. The circSFL in the Aging and Longevity
Plenty of evidence indicated that the nutrient-sensing insulin/insulin-like growth factor signaling pathway is a vital regulator of metabolism and aging [18, 32]. Researches have shown that circSFL transcribed by the sulfate less (SFL) gene was highly and specifically upregulated in all tissues of several long-lived insulin mutants flies (such as long-lived dilp 2-3,5 flies), and overexpression of circSFL alone was sufficient to extend the lifespan in wild-type flies [18] (Fig. 2). Startlingly, circSFL was found to be translated in vivo, and the significantly increased levels of circSFL-derived peptide in dilp 2–3,5 mutants was sufficient to increase lifespan [18]. However, the specific mechanism underlying circSFL protein's function extending lifespan is still little known. Besides, overexpression of the SFL full-length protein also affects the lifespan. It was found that the SFL linear transcript encodes for an N-deacetylase/N-sulfotransferase (Ndst), which catalyzes the synthesis of heparan sulfate in the Golgi apparatus, and the heparan sulfate proteoglycan Dally is a key downstream target of SFL-mediated lifespan [18]. However, whether the circSFL peptide and the full-length SFL protein influence the lifespan through the same or independent mechanisms remains obscure.
Fig. (2).
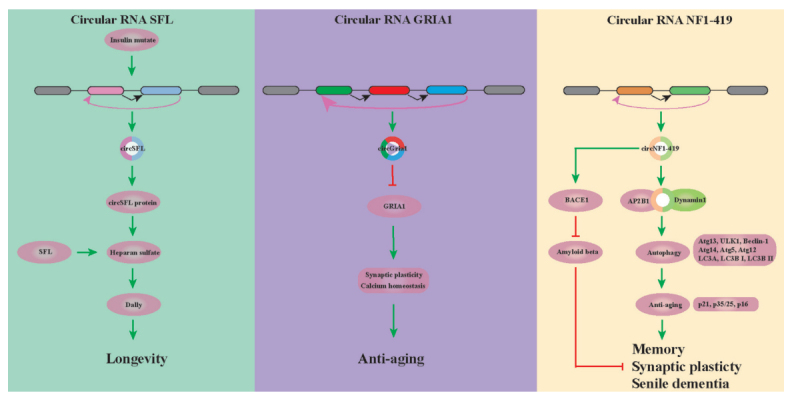
Key circRNAs that regulate senescence and longevity.
3.2. The circGRIA1 in the Aging and Longevity
CircGRIA1 was reported to be transcribed from Gria1 gene and upregulated in the brain samples of 20 year-old male rhesus macaque (macaca mulatta) [33]. It's worth noting that the expression of circGRIA1 increased in the hippocampus samples of older male macaques was negatively correlated with the expression of its host mRNA GRIA1 [33]. Nuclear circGRIA1 was able to bind the promoter region of GRIA1 and strongly downregulated GRIA1 genetic transcription in the hippocampal neurons of male instead of female macaque, and importantly, this binding capacity was significantly stronger in 20-year-old brain tissues than that in 10-year-old brain tissues [33]. A variety of synaptic related molecules showed significant decreases in the brain tissues during aging [33-35]. Knockdown of circGRIA1 significantly increased the levels of synapsin-I (presynaptic), with fewer alterations to PSD95 (postsynaptic) in the hippocampal neurons of the old male macaque [33]. Accumulating evidence suggested that the most common age-related structural changes experienced by nerve cells are represented by reduced dendrite number and length, loss of dendritic spines, decreased number of axons, the obvious loss of synapses, decreased synaptic connectivity and synaptic function, which are externalized as behavioral disorders and cognitive decline with normal aging [36-38]. Apart from circGRIA1 levels affecting the long-term potentiation (LTP) and miniature excitatory postsynaptic current (mEPSCs) in male neurons, circGRIA1 could affect the intracellular calcium concentrations in primary hippocampal neurons of male fetal macaques instead of females [33]. These strengthen that circGRIA1 significantly affects the synaptic plasticity in aging brain tissues (Fig. 2). However, sex differences revealed in the role of circGRIA1 are worth pondering whether sex hormones may play a pivotal role in the progression of aging.
3.3. The circNF1-419 in the Aging and Longevity
In a recent study unveiling the expression profiling of astrocytes from aging rats, circNF1-419, out of identified 7376 circRNAs, was demonstrated to regulate autophagy through the PI3K-I/Akt-AMPK-mTOR and PI3K-I/Akt-mTOR signaling pathways in astrocytes [39] (Fig. 2). In addition, overexpression of circNF1-419 in mouse cerebral cortex accelerated autophagy activity by binding the proteins Dynamin-1 and Adaptor protein 2 B1 (AP2B1), which further delayed senile dementia by regulating aging-related markers (p21, p35/25, and p16) and inflammatory factors (TNF-α and NF-κB), and reducing the expression of Alzheimer’s disease marker proteins (Tau, p-Tau, Aβ1-42, and APOE) [39] (Fig. 2). These findings provide important insights into the potential role of circNF1-419 in the diagnosis and treatment of dementia.
CONCLUSION AND FUTURE PERSPECTIVE
The circRNA transcriptional profiles in different species unveiled the number of circRNAs gradually increases with age. In the same species, the largest number of circRNAs and their significant differential expression were found in the brain tissues during aging, strengthening the important position and significance of the nervous system in normal physiological functions and aging processes. In addition, reproductive organs like ovaries and testis also contain large amounts of circRNAs along with significantly differential expression during aging. This suggests that the reproductive organs are also sensitive tissues in the progression of individual aging. Moreover, there is also a considerable amount of circRNAs in peripheral blood and serum and these circRNAs may be circulating markers of senescence in some tissues. However, the relatively small number of circRNAs in peripheral blood and serum enables more experimental studies to investigate whether circRNAs in peripheral blood and serum can be potentiated as an indicator of an organ or individual aging.
CircRNAs may affect the individual aging process and corresponding functional performance (e.g., cognition) through multiple functions, which include: 1) involvement of circRNAs expressed peptides in signaling transduction; 2) promoter binding of circRNAs to its transcription genes affects the production of linear transcripts; 3) binding of circRNAs to other proteins is involved in signaling transduction. The same circRNA may involve in tissue senescence and individual lifespan through different functions, and different circRNAs may also affect tissue senescence and individual lifespan through the same function. Despite much progress made, the role of circRNAs and their specific functions in aging remain largely uncovered and mysterious. Moreover, preclinical outcomes of circRNAs are difficult to be translated into clinical applications in diagnosis and treatment due to the functional complexity and operability, cost consumption, etc. Fortunately, multiple system-related organoids have been developed to capture the phenotypic and molecular heterogeneity of different organs for developmental, aging and drug studies, including skin, hepatocytes, mini-intestines, retina, brain, heart and multiple cancers [40-49]. Furthermore, studies concerning circRNA regulatory mechanism based on the human-derived organoid are warranted to update and broaden our knowledge on aging to provide an effective anti-aging strategy to individuals suffering from aging-related diseases. The rapid development of sequencing technologies and the increasing number of constructed related databases make it easier to study the role of these circRNAs in the aging process. Moreover, the emerging new models and RNA transmission systems like selective endogenous encapsidation for cellular delivery (SEND) [50] promote the clinical translation of circRNA-related preclinical research findings.
ACKNOWLEDGEMENTS
We are grateful to the members of our departments for their support and critical revision of this review.
LIST OF ABBREVIATIONS
- CircRNAs
circular RNAs
- lncRNAs
Long non-coding RNAs
- LTP
Long-Term Potentiation
- mEPSCs
Miniature Excitatory Postsynaptic Current
- miRNAs
microRNAs
- PCR
Polymerase Chain Reaction
- SEND
Selective Endogenous Encapsidation for Cellular Delivery
AUTHORS’ CONTRIBUTIONS
Rui-ze Niu drafted the manuscript. Jia Lia discussed and revised the manuscript. Both authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The consumption of experiment materials was supported by PhD Student Innovation Fund Program of Kunming Medical University, China (Grant number 2022B07).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Liang D., Tatomer D.C., Luo Z., Wu H., Yang L., Chen L.L., Cherry S., Wilusz J.E. The output of protein-coding genes shifts to circular RNAs when the pre-mRNA processing machinery is limiting. Mol. Cell. 2017;68(5):940–954.e3. doi: 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L.L., Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann K. CircRNAs in lifespan. Nat. Rev. Mol. Cell Biol. 2020;21(8):420. doi: 10.1038/s41580-020-0269-1. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y., Jin Y., Yang Y., Chen L.L., Wang Y., Wong C.C.L., Xiao X., Wang Z. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., Huang N., Yang X., Zhao K., Zhou H., Huang S., Xie B., Zhang N. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J. Natl. Cancer Inst. 2018;110(3):304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D., Yang K., Yang M. Circular RNA in aging and age-related diseases. Adv. Exp. Med. Biol. 2018;1086:17–35. doi: 10.1007/978-981-13-1117-8_2. [DOI] [PubMed] [Google Scholar]
- 7.Cai H., Li Y., Niringiyumukiza J.D., Su P., Xiang W. Circular RNA involvement in aging: An emerging player with great potential. Mech. Ageing Dev. 2019;178:16–24. doi: 10.1016/j.mad.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21(8):475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 10.Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., Fatica A., Santini T., Andronache A., Wade M., Laneve P., Rajewsky N., Bozzoni I. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell. 2017;66(1):22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., Hanan M., Wyler E., Perez-Hernandez D., Ramberger E., Shenzis S., Samson M., Dittmar G., Landthaler M., Chekulaeva M., Rajewsky N., Kadener S. Translation of CircRNAs. Mol. Cell. 2017;66(1):9–21.e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei M., Zheng G., Ning Q., Zheng J., Dong D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer. 2020;19(1):30. doi: 10.1186/s12943-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L.Y., Zhai M., Huang Y., Xu S., An T., Wang Y.H., Zhang R.C., Liu C.Y., Dong Y.H., Wang M., Qian L.L., Ponnusamy M., Zhang Y.H., Zhang J., Wang K. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019;26(7):1299–1315. doi: 10.1038/s41418-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaichian S., Shafabakhsh R., Mirhashemi S.M., Moazzami B., Asemi Z. Circular RNAs: A novel biomarker for cervical cancer. J. Cell. Physiol. 2020;235(2):718–724. doi: 10.1002/jcp.29009. [DOI] [PubMed] [Google Scholar]
- 15.Altesha M.A., Ni T., Khan A., Liu K., Zheng X. Circular RNA in cardiovascular disease. J. Cell. Physiol. 2019;234(5):5588–5600. doi: 10.1002/jcp.27384. [DOI] [PubMed] [Google Scholar]
- 16.Chen X., Yang T., Wang W., Xi W., Zhang T., Li Q., Yang A., Wang T. Circular RNAs in immune responses and immune diseases. Theranostics. 2019;9(2):588–607. doi: 10.7150/thno.29678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K., Chen D., Wang Z., Ma J., Zhou J., Chen N., Lv L., Zheng Y., Hu X., Zhang Y., Li J. Annotation and functional clustering of circRNA expression in Rhesus macaque brain during aging. Cell Discov. 2018;4(1):48. doi: 10.1038/s41421-018-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigelt C.M., Sehgal R., Tain L.S., Cheng J., Eßer J., Pahl A., Dieterich C., Grönke S., Partridge L. An insulin-sensitive circular RNA that regulates lifespan in Drosophila. Mol. Cell. 2020;79(2):268–279.e5. doi: 10.1016/j.molcel.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruner H., Cortés-López M., Cooper D.A., Bauer M., Miura P. CircRNA accumulation in the aging mouse brain. Sci. Rep. 2016;6(1):38907. doi: 10.1038/srep38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudi E., Cairns M.J. Circular RNAs are temporospatially regulated throughout development and ageing in the rat. Sci. Rep. 2019;9(1):2564. doi: 10.1038/s41598-019-38860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C., Sun X., Li N., Wang W., Kuang D., Tong P., Han Y., Dai J. CircRNAs in the tree shrew (Tupaia belangeri) brain during postnatal development and aging. Aging. 2018;10(4):833–852. doi: 10.18632/aging.101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo M., Qiu J., Shen F., Wang S., Yu J., Zuo H., Yao J., Xu S., Hu T., Wang D., Zhao Y., Hu Y., Shen F., Ma X., Lu J., Gu X., Xu L. Comprehensive analysis of circular RNA profiles in skeletal muscles of aging mice and after aerobic exercise intervention. Aging. 2020;12(6):5071–5090. doi: 10.18632/aging.102932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelmohsen K., Panda A.C., De S., Grammatikakis I., Kim J., Ding J., Noh J.H., Kim K.M., Mattison J.A., de Cabo R., Gorospe M. Circular RNAs in monkey muscle: Age-dependent changes. Aging. 2015;7(11):903–910. doi: 10.18632/aging.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younis J.S. Ovarian aging. Curr. Opin. Obstet. Gynecol. 2011;23(6):427–434. doi: 10.1097/GCO.0b013e32834b92b0. [DOI] [PubMed] [Google Scholar]
- 25.Xi X., Zou Q., Wei Y., Chen Y., Wang X., Lv D., Li P., Wen A., Zhu L., Tang G., Ma J., Li M., Li X., Jiang Y. Dynamic changes of DNA methylation and transcriptome expression in porcine ovaries during aging. BioMed Res. Int. 2019;2019:1–15. doi: 10.1155/2019/8732023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titus S., Li F., Stobezki R., Akula K., Unsal E., Jeong K., Dickler M., Robson M., Moy F., Goswami S., Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992;7(10):1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 28.Cai H., Li Y., Li H., Niringiyumukiza J.D., Zhang M., Chen L., Chen G., Xiang W. Identification and characterization of human ovary-derived circular RNAs and their potential roles in ovarian aging. Aging. 2018;10(9):2511–2534. doi: 10.18632/aging.101565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dluzen D.F., Noren Hooten N., De S., Wood W.H., III, Zhang Y., Becker K.G., Zonderman A.B., Tanaka T., Ferrucci L., Evans M.K. Extracellular RNA profiles with human age. Aging Cell. 2018;17(4):e12785. doi: 10.1111/acel.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith L.K. He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; Wheatley, E.G.; Bouchard, J.; Eggel, A.; Narasimha, R.; Grant, J.L.; Luo, J.; Wyss-Coray, T.; Villeda, S.A. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015;21(8):932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque S., Ames R.M., Moore K., Pilling L.C., Peters L.L., Bandinelli S., Ferrucci L., Harries L.W. circRNAs expressed in human peripheral blood are associated with human aging phenotypes, cellular senescence and mouse lifespan. Geroscience. 2020;42(1):183–199. doi: 10.1007/s11357-019-00120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy D.J., Gems D., Harshman L.G., Oldham S., Stocker H., Hafen E., Leevers S.J., Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 33.Xu K., Zhang Y., Xiong W., Zhang Z., Wang Z., Lv L., Liu C., Hu Z., Zheng Y.T., Lu L., Hu X.T., Li J. CircGRIA1 shows an age-related increase in male macaque brain and regulates synaptic plasticity and synaptogenesis. Nat. Commun. 2020;11(1):3594. doi: 10.1038/s41467-020-17435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu F., Cizeron M., Qiu Z., Benavides-Piccione R., Kopanitsa M.V., Skene N.G., Koniaris B., DeFelipe J., Fransén E., Komiyama N.H., Grant S.G.N. Architecture of the mouse brain synaptome. Neuron. 2018;99(4):781–799.e10. doi: 10.1016/j.neuron.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haley G.E., Kohama S.G., Urbanski H.F., Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the Rhesus macaque prefrontal cortex and hippocampus. Age. 2010;32(3):283–296. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., Gamo N.J., Yang Y., Jin L.E., Wang X.J., Laubach M., Mazer J.A., Lee D., Arnsten A.F.T. Neuronal basis of age-related working memory decline. Nature. 2011;476(7359):210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct. Funct. 2011;216(2):85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- 38.von Bohlen und Halbach. O.; Zacher, C.; Gass, P.; Unsicker, K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J. Neurosci. Res. 2006;83(4):525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- 39.Diling C., Yinrui G., Longkai Q., Xiaocui T., Yadi L., Xin Y., Guoyan H., Ou S., Tianqiao Y., Dongdong W., Yizhen X., Yang B.B., Qingping W. Circular RNA NF1-419 enhances autophagy to ameliorate senile dementia by binding Dynamin-1 and Adaptor protein 2 B1 in AD-like mice. Aging. 2019;11(24):12002–12031. doi: 10.18632/aging.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugraha B., Buono M.F., Emmert M.Y. Modelling human cardiac diseases with 3D organoid. Eur. Heart J. 2018;39(48):4234–4237. doi: 10.1093/eurheartj/ehy765. [DOI] [PubMed] [Google Scholar]
- 41.Nugraha B., Buono M.F., Boehmer L., Hoerstrup S.P., Emmert M.Y. Human cardiac organoids for disease modeling. Clin. Pharmacol. Ther. 2019;105(1):79–85. doi: 10.1002/cpt.1286. [DOI] [PubMed] [Google Scholar]
- 42.Drost J., Clevers H. Organoids in cancer research. Nat. Rev. Cancer. 2018;18(7):407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 43.Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018;19(11):671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 44.Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F., Balgobind A.V., Wind K., Gracanin A., Begthel H., Korving J., van Boxtel R., Duarte A.A., Lelieveld D., van Hoeck A., Ernst R.F., Blokzijl F., Nijman I.J., Hoogstraat M., van de Ven M., Egan D.A., Zinzalla V., Moll J., Boj S.F., Voest E.E., Wessels L., van Diest P.J., Rottenberg S., Vries R.G.J., Cuppen E., Clevers H. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1-2):373–386.e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowan C.S., Renner M., De Gennaro M., Gross-Scherf B., Goldblum D., Hou Y., Munz M., Rodrigues T.M., Krol J., Szikra T., Cuttat R., Waldt A., Papasaikas P., Diggelmann R., Patino-Alvarez C.P., Galliker P., Spirig S.E., Pavlinic D., Gerber-Hollbach N., Schuierer S., Srdanovic A., Balogh M., Panero R., Kusnyerik A., Szabo A., Stadler M.B., Orgül S., Picelli S., Hasler P.W., Hierlemann A., Scholl H.P.N., Roma G., Nigsch F., Roska B. Cell types of the human retina and its organoids at single-cell resolution. Cell. 2020;182(6):1623–1640.e34. doi: 10.1016/j.cell.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu H., Gehart H., Artegiani B. LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; van den Born, M.; Zou, C.; Quirk, C.; Chiriboga, L.; Rice, C.M.; Ma, S.; Rios, A.; Peters, P.J.; de Jong, Y.P.; Clevers, H. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175(6):1591–1606.e19. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Nikolaev M., Mitrofanova O., Broguiere N., Geraldo S., Dutta D., Tabata Y., Elci B., Brandenberg N., Kolotuev I., Gjorevski N., Clevers H., Lutolf M.P. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 2020;585(7826):574–578. doi: 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- 49.Lee J., Rabbani C.C., Gao H., Steinhart M.R., Woodruff B.M., Pflum Z.E., Kim A., Heller S., Liu Y., Shipchandler T.Z., Koehler K.R. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582(7812):399–404. doi: 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segel M., Lash B., Song J., Ladha A., Liu C.C., Jin X., Mekhedov S.L., Macrae R.K., Koonin E.V., Zhang F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373(6557):882–889. doi: 10.1126/science.abg6155. [DOI] [PMC free article] [PubMed] [Google Scholar]


