Abstract
Brain ischemia, also known as ischemic stroke, occurs when there is a lack of blood supply into the brain. When an ischemic insult appears, both neurons and glial cells can react in several ways that will determine the severity and prognosis. This high heterogeneity of responses has been a major obstacle in developing effective treatments or preventive methods for stroke. Although white matter pathophysiology has not been deeply assessed in stroke, its remodelling can greatly influence the clinical outcome and the disability degree. Oligodendrocytes, the unique cell type implied in CNS myelination, are sensible to ischemic damage. Loss of myelin sheaths can compromise axon survival, so new Oligodendrocyte Precursor Cells are required to restore brain function. Stroke can, therefore, enhance oligodendrogenesis to regenerate those new oligodendrocytes that will ensheath the damaged axons. Given that myelination is a highly complex process that requires coordination of multiple pathways such as Sonic Hedgehog, RTKs or Wnt/β-catenin, we will analyse new research highlighting their importance after brain ischemia. In addition, oligodendrocytes are not isolated cells inside the brain, but rather form part of a dynamic environment of interactions between neurons and glial cells. For this reason, we will put some context into how microglia and astrocytes react against stroke and influence oligodendrogenesis to highlight the relevance of remyelination in the ischemic brain. This will help to guide future studies to develop treatments focused on potentiating the ability of the brain to repair the damage.
Keywords: Cerebral ischemia, stroke, oligodendrocyte, oligodendrocyte precursor cell, remyelination, glial cells
1. INTRODUCTION
The term stroke refers to a phenomenon in which abnormal flow of blood into the brain causes cell death. According to World Health Organization, stroke is a leading cause of long-term disability and mortality after ischaemic heart disease, with more than 15 million people suffering an episode of stroke annually. Thus, 5 million people have been estimated to die and the other 5 million are left permanently disabled worldwide. The probabilities of suffering a stroke are greatly influenced (~ 90%) by risks attributed to modifiable factors such as high blood pressure, obesity or smoking-related to air pollution [1, 2]. Although the causes of brain ischemia are heterogeneous and not fully understood (30-40% are considered cryptogenic), the restriction of blood supply produces a lack of oxygen and nutrients needed for cellular metabolism, as well as accumulation of waste products [3, 4]. If this scenario is sustained over a period of time, the clinical outcome will worsen due to blood-brain barrier (BBB) dysfunction, vasogenic brain edema and neuronal death [5, 6].
Brain ischemia has attracted the attention of multiple research groups who have put efforts into elucidating what cellular and molecular processes are taking place in the ischemic brain and how to stop it or prevent it. To accomplish this challenge, scientists have developed numerous animal models, each with its own strengths and limitations, to reproduce the heterogeneous nature of the stroke. From all of them, middle cerebral artery occlusion (MCAO) in rodents has been widely studied to produce focal cerebral ischemia [7], as it mimics a localized occlusion of a cerebral artery either by an embolus or local thrombosis [8, 9]. Using both human samples and MCAO models, scientists have been able to describe two areas surrounding the vessel occlusion: the ischemic core and the penumbra. The ischemic core covers the area directly affected by the occlusion and it is where neuronal death mostly occurs. It is usually located in the caudate/putamen and the parietal cortex and its size is proportional to the occlusion time. Second, the penumbra surrounds the ischemic core, where neuronal damage of varying degrees can be found but, most importantly, where there is potential for functional recovery (Fig. 1).
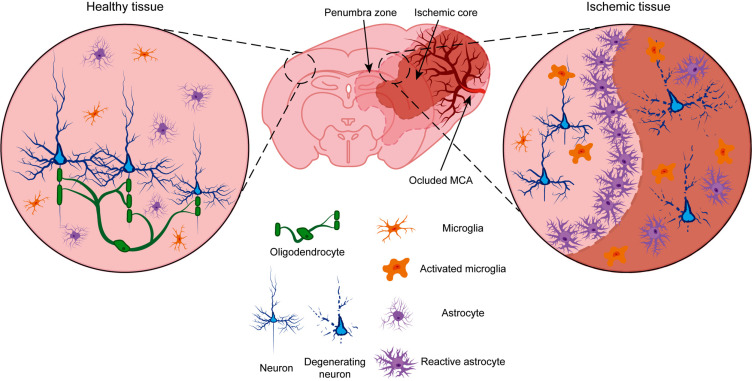
Fig. (1).
Schematic representation of MCAO brain ischemia model. In a physiological situation (left), healthy neurons (blue) are covered by oligodendrocytes (green). Astrocytes (violet) and microglia (orange) have resting morphologies. When middle cerebral artery (MCA) is occluded (right), we can distinguish two zones: the ischemic core, where we can find degenerating neurons, and the penumbra zone, with slightly damaged neurons. Oligodedrocytes are affected by the ischemic insult and myelin is lost. Activated microglia adopt ameboid morphologies around neurons and modulate the inflammatory response. Reactive astrocytes accumulate around the ischemic core to form the glial scar and phagocyte cellular debris to prevent further extension of the damage.
From a cellular point of view, neurons are energetically demanding cells that require vast amounts of oxygen and nutrients to work properly. The brain uses 20% of all oxygen consumed by the body, although it only constitutes 2% of its mass. Without a constant supply of oxygen and energy, like in stroke, neuronal function is compromised [10]. When an artery is obstructed, oxygen and nutrient supplies are largely blocked, which induces a sequence of harmful processes known as the “ischemic cascade” [11]. The cascade is triggered mainly by the lack of ATP due to the oxygen deficit. Without energy, neurons in the ischemic core are unable to maintain a proper transmembrane gradient, which in turn induces depolarization. Released glutamate cannot be taken up by either neurons or astrocytes, leading to excessive activation of glutamate receptors like N-Methyl-D-Aspartate Receptor (NMDAR) that allow the entrance of Ca2+ [12], which trigger more glutamate release, and the cycle repeats. Excess calcium enhances the generation of reactive oxygen species (ROS) and triggers apoptotic and necrotic events [13]. Neurons in the ischemic core die from swelling and disruption of their plasma membrane, which derives from neuroinflammation [14, 15]. In the penumbra, cells are still metabolically active even though they receive no electric signals from the ischemic core. Since they receive deleterious signals from the dead neurons of the ischemic core, they will die unless they get rescued [12, 16]. One essential component that contributes to neuronal survival is myelin [17, 18], which is produced by Oligodendrocytes (OLs) in the Central Nervous System (CNS) [19-22]. Myelin sheaths will speed up action potential transmission, reduce neuronal energetic requirements and provide metabolic support to neurons [23-27]. In fact, loss of myelin is considered an important factor that worsens the prognosis of ischemic stroke and other pathologies [28-30].
In stroke, both OLs and Oligodendrocyte Progenitor Cells (OPCs) are also affected [31-34]. During the ischemic cascade, glutamate receptors expressed by OLs are overactivated, leading to excitotoxicity [35, 36]. In addition, inflammatory cytokines released by activated microglia and macrophages contribute to OL death: IFN-γ leads to OL demyelination and apoptosis [37] while Tumor Necrosis Factor-α (TNF-α) also inhibits the proliferation and differentiation of OPCs [38-40]. In addition, rodent models of MCAO show lower staining against myelin basic protein (MBP) and a decrease in the number of OLs [41, 42]. After stroke, brain repair mechanisms will try to restore the lost connections. Sprouting axons must be adequately isolated by new myelin sheaths, which cannot be formed by injured OLs. In the adult brain, OPC niches can be found in the corpus callosum, the striatum and the cortex [43], all of them derived from Neural Progenitor Cells (NPCs) found in the sub-ventricular zone (SVZ) of the lateral ventricles [44-47]. Recent studies have described that OPCs can be generated and mobilized in response to ischemic insults [48]. In fact, brain ischemia induces oligodendrogenesis in order to create new mature OLs from OPCs [49]. Preclinical studies show that enhancement of endogenous oligodendrogenesis in ischemic brain reduces neurological deficits and improves general outcome [50, 51].
In this review, we will focus on the repercussions of stroke in myelin and how remyelination could be achieved by promoting different signaling pathways. In addition, we will summarize the role of each glial cell after ischemic stroke to modulate remyelination.
2. PATHWAYS INVOLVED IN MYELINATION: SONIC HEDGEHOG, RECEPTOR-TYROSINE KINASES (RTKS) AND WNT PATHWAY
2.1. Sonic Hedgehog
The Sonic Hedgehog (Shh) pathway was discovered more than 30 years ago and has been established as a regulator of OL and myelin production [52, 53]. When the pathway is off, the 12-pass transmembrane protein Patched (Ptc) acts, inhibiting Smoothened (Smo), a G-protein coupled receptor. Hedgehog proteins binding to Ptc suppress its inhibition over Smo, which transduces the signaling cascade via the transcription factors of the Glioma-associated oncogene (Gli) family [54]. Parallelly, Smo can act in a Gli-independent manner, known as the “non-canonical” route [55] (Fig. 2). Shh was discovered to be both sufficient and necessary for the expression of the Oligodendrocyte Transcription Factors 1 (Olig1) and 2 (Olig2), which are indeed markers of the OL lineage. Zebrafish lacking Smo exhibited an almost complete lack of OLs due to the absence of Shh signaling [56, 57]. Diverse demyelination models have shown Shh implication in myelin repair: Shh promoted proliferation and differentiation of OPCs [58] while Smo inhibition aggravated demyelinating phenotype [59]. In addition, it is worth noting that transient Gli1 transcription was observed in oligodendroglial cells during the step of OPC differentiation [58]. Regarding the contribution of Shh in oligodendrogenesis after brain ischemia, stroke upregulated Shh in NPCs from the SVZ [60, 61]. Administration of cerebrolysin, a mix of neurotrophic peptides that activate Shh, enhanced neurological outcomes by increasing oligodendrogenesis. Furthermore, inhibition of Shh with cyclopamine abolished the effects of cerebrolysin, proving the importance of Shh in recovery after brain ischemia [62]. Still, not only the canonical Shh pathway is important in myelin repair. Blockade of Gli1 in NPCs preferentially fated them towards oligodendroglial linage in a context of high Shh signaling [63]. In addition, cuprizone-induced demyelination, recruited Gli1+ NSCs to the corpus callosum, where they were preferentially differentiated into OLs concomitantly with Gli1 downregulation [64]. In the same model, activation of Smo by microinjection of a Smo agonist into the corpus callosum increased cell proliferation and enhanced remyelination [54, 64]. All the data above strongly suggest a role for Shh pathway in remyelination after ischemic stroke.
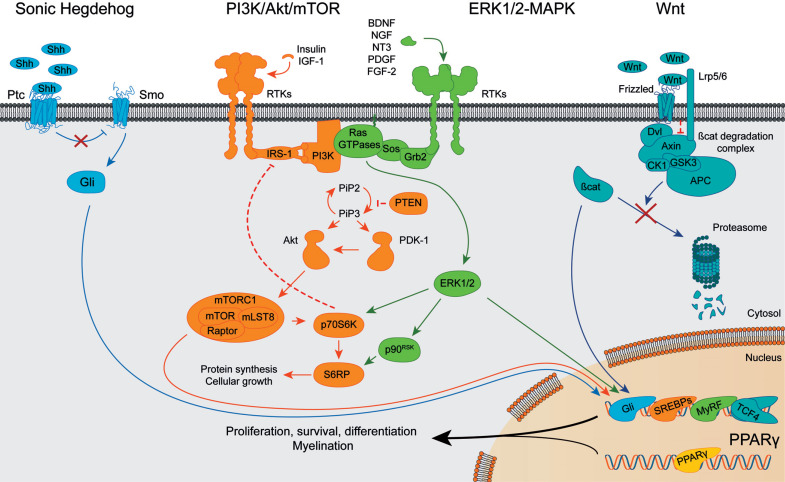
Fig. (2).
Schematic diagram of Shh, PI3K/Akt/mTOR, ERK1/2-MAPK, Wnt/β-catenin and PPARγ signaling pathways. Key elements of each pathway are shown when each route is active. Arrowheads indicate positive interactions while bars show inhibitory signals. Abbreviations: Shh: Sonic Hedgehog, Ptc: Patched, Smo: Smoothened, Gli: Glioma-associated oncogene family. BDNF: brain-derived neurotrophic factor, NGF: nerve growth factor, NT3: neurotrophin 3, RTKs: receptors tyrosine kinase, IRS-1: insulin receptor substrate 1, PI3K: phosphoinositide-3 kinase, PIP2: phosphatidylinositol (4,5)-biphosphate, PIP3: phosphatidylinositol (3,4,5)-triphosphate, PTEN: phosphatase and tensin homolog, PDK-1: 3-phosphoinositide-dependent protein kinase, Akt: protein kinase B, mTOR: mammalian target of rapamycin, mLST8: target of rapamycin complex subunit LST8, Raptor: regulatory associated protein of mTOR, p70S6K: Ribosomal protein S6 kinase beta-1, S6RP: Ribosomal protein S6, SREBPs: Sterol regulatory element-binding proteins. PDGF: platelet-derived growth factor, FGF-2: fibroblast growth factor 2, Grb2: growth factor receptor-bound protein 2, Sos: son of sevenless, ERK1/2: extracellular signal-regulated kinases 1 and 2, p90RSK: p90 ribosomal S6 kinase, MyRF: Myelin Regulatory Factor. Lrp5/6: Lipoprotein receptor-related proteins 5/6, Dvl: disheveled, GSK3: glycogen synthase kinase 3, CK1: casein kinase 1, APC: adenomatous polyposis coli, β-cat: β-catenin, TCF4: transcription factor 4. PPARγ: peroxisome proliferator-activated receptor γ.
2.2. Receptor-tyrosine Kinases (RTKs)
Many tyrosine kinase receptors (RTKs) have a role in the regulation of OPC differentiation, myelination and remyelination. They can be activated by a variety of growth factors, like Insulin/IGF-1, brain-derived neurotrophic factor (BDNF), platelet-derived growth factor (PDGF), fibroblast growth factor-2 (FGF-2) and neurotrophins. Upon stimulation, they can trigger activation of several signaling pathways, such as phosphatidyl inositol-3-phosphate kinase (PI3K) class I /Akt/ mechanistic target of rapamycin (mTOR) and Ras/Mitogen-Activated Protein Kinases (MAPK).
The PI3K/Akt/mTOR pathway is well described for its role in the regulation of OPC differentiation, myelination and remyelination [65, 66]. PI3K/Akt/mTOR pathway starts at the cell surface, where a variety of growth factors like Insulin/IGF-1, brain-derived neurotrophic factor (BDNF) or neurotrophin 3 (NT3) bind tyrosine kinase receptors that activate PI3K-Class I. This lipid kinase catalyzes the conversion of phosphatidylinositol (4,5)-biphosphate (PIP2) to generate phosphatidylinositol (3,4,5)-triphosphate (PIP3). 3-phosphoinositide-dependent kinase 1 (PDK1) and Akt are then bound to the membrane and activated, allowing further transduction of the signal by the multiple effectors of Akt. In myelination, Akt principal target is mTORC1, which in turn mediates protein and lipid biosynthesis (Fig. 2). Use of either constitutively active models of Akt or knockdowns of phosphatase and tension homolog (PTEN) lead to discover a pathological increase in myelin sheath thickness and myelin proteins levels [67, 68]. Akt downstream effector, mTOR, is key for proper myelin development as well [69, 70]. Inhibition of mTOR with rapamycin reduced the expression of myelin proteins and generated a phenotype of hypomyelination. These effects were mediated in part because mTOR is also a regulator of lipid biosynthesis via the sterol regulatory element-binding proteins (SREBPs) [71].
Another related pathway to RTKs is the Ras/MAPK pathway. As a general rule, RTK activation phosphorylate and stimulate proteins such as Grb2 and SOS 1/2. This RTK-Grb2-SOS activation stimulates GTP binding to Ras proteins, which trigger the MAPK/extracellular signal-related kinases 1 and 2 (Erk1/2) through the Raf-MEK complex [72]. It is important to remember that Ras has a direct interaction with PI3K class I, which makes both pathways directly intermixed [73, 74].
Erk1/2-MAPK signaling is crucial for oligodendroglial development, proliferation, survival, differentiation and myelination [65, 75]. Once active, Erk1/2 are translocated to the nucleus to regulate the expression of the master transcriptional regulator of critical myelin genes MyRF (Fig. 2) [76]. Lack of Erk1/2 resulted in significant hypomyelination, while overexpression of some proteins of the pathway derived in hypertrophic myelin sheaths and upregulated expression of myelin proteins [77]. Both pathways PI3K-Akt and Ras/MAPK exhibit similar phenotypes when altered because there is active crosstalk between them through proteins like insulin receptor substrate 1 (IRS-1) or ribosomal protein S6 kinase beta-1 (p70S6K). For instance, p70S6K can inhibit IRS-1 as a negative feedback loop against overactivation [78, 79]. On the other hand, relevant members of the Ras family in OLs are R-Ras1 and R-Ras2. Mice lacking R-Ras1/2 showed lower levels of Akt and ERK signaling together with an hypomyelinating phenotype and axonal degeneration [80, 81]. PI3K/Akt/mTOR and Erk1/2-MAPK cooperation in myelination has been extensively studied [82], however, few studies have shown their potential role after stroke. In a rat model of MCAO, phospho-Akt levels rise, followed by an increase in phospho-ERK, but only after reperfusion [83]. In addition, the use of Bisperoxovanadium (BpV), a PTEN inhibitor, increased phospho-Akt and phospho-ERK1/2 levels, which was associated with a neuroprotective effect [84]. Treatment with WIN55, 212-2, a cannabinoid, resulted in enhanced OPC proliferation and remyelination in ischemic lesions by activation of ERK1/2 [85]. Interestingly, an in vitro study pointed out that following ischemic insult, Thymosin beta 4 can guide NPCs from the SVZ to differentiate towards the oligodendroglia lineage [86]. In addition, enhanced proliferation of NPCs from the SVZ was observed [87] and increased OPC proliferation and OL differentiation after ischemia through MAPK dependent mechanisms [88].
2.3. Wnt Pathway
The wingless integration site (Wnt) intracellular β-catenin signaling cascade is a negative regulator of OPC differentiation, myelination and remyelination [65, 66]. Canonical Wnt pathway starts with the extracellular binding of Wnt proteins to Frizzled receptors at the cell surface. The activated receptor is then able to bind and repress the β-catenin degradation complex, formed by adenomatous polyposis coli (APC), Axin, glycogen synthase kinase 3Β (GSK-3B) and casein kinase 1 (CK1). The activation of the Wnt complex allows β-catenin to stop being degraded and enter the nucleus to activate gene expression by interacting with intranuclear T-cell factors/lymphoid enhancers factors (TCF/LEF) (Fig. 2) [89]. The first studies that connected myelination with this pathway were performed in mice using dominant-active forms of β-catenin [90, 91]. These mice showed a severe hypomyelination phenotype, displaying lower myelin protein levels, a decrease in myelin sheath thickness and lower numbers of mature OLs. Another study found increased levels of OPCs after addition of Wnt3a, which corroborated the inhibitory role of this pathway on myelination [92]. In addition to the Wnt canonical signaling, non-canonical pathways also exist: tissue inhibitor of metalloproteinase (TIMP)-1 is both a metalloproteinase inhibitor and a trophic factor that enhances myelination and remyelination through Akt activation, which directly promotes β-catenin signaling in OPCs [93]. However, the role of Wnt in ischemic OLs has not been exhaustively studied. Some studies point out that Wnt signals released from OPCs may be important to protect and restore the blood-brain barrier after ischemic insults [94] while others suggested that oligodendroglia stimulates angiogenesis by releasing vascular-endothelial growth factor (VEGF) in a Wnt-independent manner [95].
Downstream the pathway, early studies suggested that Transcription Factor 7-like 2 (TCF7l2, also known as TCF4) expression was correlated with immature OLs [90]. However, later on, was described that a proper balance of expression between TCF7l2, β-catenin and other members of the pathway is essential to regulate OL lineage development [66, 96-98]. Indeed, TCF7l2 may have additional roles independent of β-catenin signaling: new studies have pointed out that TCF7l2 expression was upregulated at a specific point of OL maturation, just after OLs became mature [99]. Supporting these observations, two more research groups later stated that TCF7l2 binds different partners depending on the developmental stage. In an immature state, TCF7l2 can be found bound to β-catenin to repress differentiation. However, upon differentiation signals, TCF7l2 switches partners with Kaiso (also known as ZBTB33), a corepressor that facilitates repression of differentiation inhibitors. In addition, a recent study described that TCF7l2 directly binds to a regulatory region of BMP4 to inhibit its expression, as BMPs are negative regulators of OL differentiation [100, 101]. In later phases, TCF7l2 associates with Sox10 to accomplish a successful maturation and remains active in fully mature OLs, although at lower levels [98, 102].
3. ADDITIONAL KEY ELEMENTS IN OLIGODENDROGENESIS
Apart from the traditional signaling pathways involved in OL differentiation and myelination, additional elements have been found to participate in OL proliferation and maturation after brain injury. Among them, Peroxisome proliferator-activated receptor γ (PPARγ) or microRNAs (miRNAs) may be an interesting target for future therapies.
PPARγ is a transcription factor that regulates the expression of a wide variety of genes, many of them with high relevance in stroke [31]. If brain injury occurs, PPARγ is a master gatekeeper of cytoprotective responses [103]. Mice lacking PPARγ exhibited greater brain damage and oxidative stress after MCAO [104], which was recovered by administration of PPARγ agonists prior to or after stroke [31, 105]. Using these kinds of agonists less loss of white matter integrity was reported after MCAO [105, 106]. Although this effect was partly due to enhanced microglial M2 polarization [105], PPARγ activation in NSCs can guide them towards oligodendroglial differentiation [107, 108] and contribute to OPC differentiation in both developmental [109] and post-injury myelination [105] (Fig. 2). It is thought that PPARγ beneficial effects may be due to its importance in lipid metabolism, reduction of oxidative stress vulnerability and enhancement of mitochondrial function during OL process formation [109-111]. However, negative effects of PPARγ agonists in OPCs have also been described depending on the context of OPC stage [31] and need to be further addressed.
Other key elements in OL differentiation are microRNAs (miRNAs). miRNAs are small non-coding RNA molecules of approximately 20 nucleotides long. Their function is to suppress protein translation by binding to the 3´-untranslated region of mRNAs in a specific manner. Growing evidence indicates that miRNAs are important for a proper CNS development, with roles in neuronal and glial differentiation, synaptic plasticity and general homeostasis [112]. First reports linking miRNAs to OLs came from experiments where Dicer, an essential element for miRNA biogenesis, was conditionally ablated in Olig2+ or in proteolipid protein (PLP)+ lineage cells [113-115]. In both cases, Dicer suppression led to a reduction in the myelination degree. Studies on miRNA expression profiles revealed that miR-219 and miR-338 promoted OL differentiation by repressing genes such as PDGFRα, Sox6, Zfp238, FoxJ3 and Hes5. However, miRNAs may have dual roles in regulating OL differentiation. Some miRNAs, like miR-145-5p, prevent OPC differentiation by repressing the myelin regulator MyRF while at the same time enhance OPC proliferation [116]. In the context of cerebral ischemia, miRNAs such as miR17-92, miR-146a, miR-9 or miR-200b, may play a role in OLs fate. Repression of miR17-92 cluster, a group of seven miRNAs, produced a decreased proliferation of progenitor cells within the SVZ after stroke, while upregulation of this cluster had the opposite effect [61]. Indeed, it is worth noting that miR17-92 effects may be mediated by crosstalk with the Shh pathway [61]. In the same way, in situ hybridization experiments reported that miR-146a was considerably upregulated in the SVZ and corpus callosum of MCAO rat models. When miR146-a was overexpressed in primary cultured NPCs or OPCs, the result was an increased generation of O4+ OPCs or enhanced expression of myelin proteins, respectively [117]. This data strongly suggested that miR-146a plays an active role in stroke-induced oligodendrogenesis. On the other hand, miR-9 and miR-200b were considerably downregulated in white matter after ischemic injury, but their overexpression suppressed OPC differentiation [118]. Another study later reported that the mentioned effects of Thymosin beta 4 on promoting oligodendrogenesis after MCAO were partially mediated by upregulation of miR-200b levels [119]. So, further studies need to be conducted in order to fully understand the potential that miRNAs may have as a therapeutic approach after stroke.
4. ROLE OF MICROGLIA AND ASTROCYTES IN CEREBRAL ISCHEMIA
Understanding intracellular pathways inside oligodendroglial cells is important to know what mechanisms drive OL regeneration and maturation after stroke, but OLs are not isolated cells in the brain. Instead, there are a lot of extracellular interactions between healthy and damaged glial cells and neurons that modulate not only oligodendrogenesis but how the brain will recover from the ischemic insult.
When the ischemic cascade, or any brain injury, takes place, astrocytes and microglia activate and react triggering a stereotypic mechanism known as reactive gliosis [120, 121]. Activated microglia and reactive astrocytes accumulate around the ischemic core, mainly in the penumbra zone. This process is followed by the release of chemokines and cytokines to regulate the inflammatory process [122]. Depending on the extent and duration of the ischemic insult, the array of secreted molecules can exacerbate the damage or as emerging studies are showing, try to repair the neuronal function [15]. Indeed, there are situations in which, if the ischemic insult is mild enough, the brain acquires a state of tolerance against further ischemic damage, mainly mediated by astrocytic and microglial adaptations [123]. These findings bring new questions about the role of glial cells within the brain and open the window for new therapies that could be focused on potentiating this self-protection phenomenon.
4.1. Microglia
Microglia are considered the immune cells of the nervous system [124] because of their ability to react to various insults, such as ischemic stroke. After an ischemic lesion, resident microglia are the first cell type to activate, migrate and proliferate in response to the chemokines released in the injury area. Clinical studies demonstrated that microglial activation is present in the brain during the acute [125] and the recovery phase after ischemic stroke [126]. In transient MCAO models, in which the lesion only lasts for a few hours after reperfusion, the number of microglial cells gradually increases over time [122] as revealed by the increased expression of Ionized calcium-binding adapter molecule 1 (Iba-1), a specific marker of microglial population. Another way to detect microglia activation is morphological changes: resting microglia has ramified processes to be in contact with their surroundings. When they are activated, they adopt a rounded morphology with large bodies and shorter dendritic arbors [127]. Transformed microglia can release a diverse range of molecules, some of which are cytotoxic and pro-inflammatory, like cytokines or ROS, while others can be neuroprotective, such as BDNF or IGF-1 [128].
Some studies have pointed out that inhibition of microglial activation enhances recovery after ischemia, while the injury resulting from ischemic insult was augmented in the presence of microglia [129, 130]. Oxidative stress is considered a key contributor to the early phase of damage: high levels of ROS affect endothelial cell function and activate microglia, both of which contribute to BBB disruption after ischemia [131]. Microglia processes towards blood vessels could be observed by two-photon imaging 72 h after MCAO [132] (Fig. 3). In addition, Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-kB), which is a ubiquitously expressed complex that controls transcription of DNA in response to stress, was up-regulated in microglia after cerebral ischemia. Even though NF-kB activation in OLs seems to be relevant to protect them against damage [133], microglial activation of NF-kB could promote transcription of matrix metalloproteinases, which are essential in the BBB disruption [134]. Co-immunostaining revealed that Iba-1+ microglia co-localized with CD31+/Gu1+/caveolin-1+/claudin-5+/podocalyxin+ blood vessels, indicating that activated microglia phagocytosed endothelial cells contribute to BBB disruption. On the other hand, inhibition of microglia activation using CX3CR1 ko mice or minocycline greatly increased blood vessel integrity [135-137]. These studies suggested that microglia activation could induce BBB disruption after brain ischemia through the disintegration of blood vessels in the penumbra [128]. This type of harmful response is usually carried out by microglia that underwent differentiation into the M1 (classical) phenotype. M1 microglia is similar to macrophages, as they are specialized in secreting pro-inflammatory molecules such as interleukin 1β (IL-1β), interleukin 6 (IL-6), nitric oxide (NO) or TNF-α, antigen-presenting capacity and immune-potentiating abilities [124, 138] (Fig. 3). On the contrary, M2 microglia is neuroprotective, anti-inflammatory and regenerative. Studies have shown that, under ischemic conditions, M2 microglia is an early response to damage that can clean cellular debris and limit brain damage. Anti-inflammatory phenotype is achieved by expression of cytokines such as interleukin 10 (IL-10), Tissue Growth Factor β (TGF-β), interleukin 4 (IL-4), IL-13 or IGF-1 that control inflammation and promote tissue repair [128]. In addition, the expression of Arginase-1 instead of NO synthase helps to switch from production of NO to the synthesis of ornithine and polyamines that will be used for the regeneration of collagen and extracellular matrix synthesis [138]. However, we still do not know which molecules or events direct microglia towards M1 or M2 polarization. It seems like it is partially dependent on different pathophysiological conditions and can be influenced by the time and severity of injury, age or microenvironment circumstances [128, 139]. Interestingly, new studies showed that mild brain damage, like a brief period of global ischemia or hypoxic conditions, could activate microglia in a way that protects the brain from further damage if an ischemic stroke occurs [140].
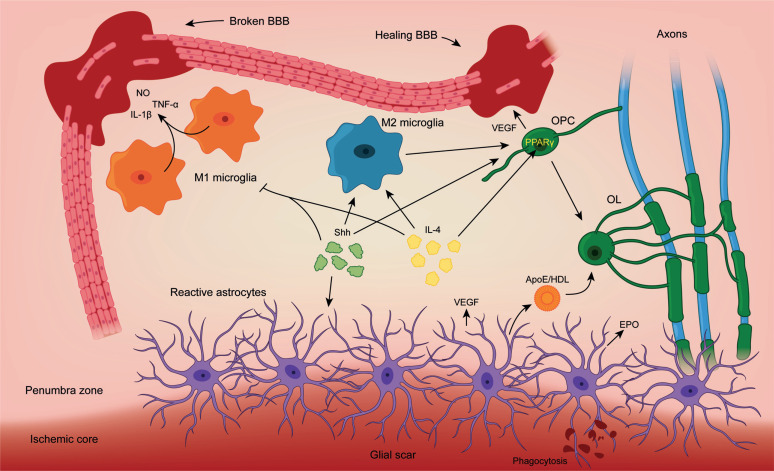
Fig. (3).
Schematic illustration of novel intercellular interactions in response to brain ischemia. Upon ischemic damage, a variety of signals is released to the medium. M1 microglia releases pro-inflammatory signals like nitric oxide (NO), tumoral necrosis factor α (TNF-α) or interleukin-1β (IL-1β) and disrupt the blood-brain barrier (BBB). These effects are mediated by NF-κΒ. M2 microglia promotes repair mechanisms and Oligodendrocyte Precursor Cell (OPC) proliferation. OPCs release vascular endothelial growth factor (VEGF) to promote angiogenesis and repair the BBB. In addition, they will differentiate into mature Oligodendrocytes (OL) to enseath damaged axons. Reactive astrocytes participate in the process by generating the glial scar to confine the ischemic core. They also provide OLs with cholesterol via Apolipoprotein E (ApoE)/ High-density lipoproteins (HDL) for myelin biogenesis and release regulatory factors like VEGF and erythropoietin (EPO). Sonic hedgehog (Shh) signals modulate glial scar formation, direct microglia towards M2 phenotype and promoting OPC proliferation. Interleukin-4 (IL-4) also guide M2 differentiation and OPC proliferation via PPARγ.
Furthermore, microglia have shown an important role in oligodendrogenesis processes after ischemia, both M1 and M2 phenotypes have demonstrated to be relevant regarding new generation of OLs [128]. Inhibition of M1 microglia activation with minocycline seems to reduce OL/OPC damage under ischemic conditions [141]. In a rat model of chronic cerebral ischemia, increased loss of OLs was coupled with activated microglia releasing TNF-α [142-144]. On the other hand, M2 microglia seems to be way more beneficial. Culture of OPCs with medium from M2 conditioned microglia enhanced OL differentiation and maturation [145]. In this same study, the use of a mouse model of multiple sclerosis showed that depletion of M2 microglia impaired OPC differentiation and blocked any possible remyelination [145]. This phenomenon could be important in physiological conditions, as activation of microglia in the SVZ of neonatal rats was found to be essential in OPCs generation [146]. A study found that IL-4 is an important mediator of these beneficial effects. It is an anti-inflammatory cytokine produced by injured neurons after stroke that polarizes microglia towards M2 phenotype [147]. IL-4 deficiency exacerbates brain damage and worsens the stroke outcome in MCAO models while intranasal delivery of IL-4 nanoparticles improved white matter integrity after stroke [148]. This same study found increased proliferation of OPCs and differentiation into mature OLs after IL-4 treatment. Interestingly, these OPCs expressed the IL-4 receptor, which tells us that this effect seems to be both dependent and independent of microglia. Oligodendroglial PPAR-γ, which we explained earlier, resulted to be the downstream effector of IL-4 signaling. This was described after seeing that knocking down OPC-PPAR-γ completely abolished IL-4 enhanced oligodendrogenesis after stroke [106] (Fig. 3).
4.2. Astrocytes
Even though microglia are the first glial cells to respond to ischemic stimulus, increased expression of glial fibrillary acidic protein (GFAP) around the ischemic lesion indicates the presence of activated astrocytes [149]. The role of astrocytes has been controversial because many studies have described detrimental roles for astrocytes in addressing brain ischemia for their ability to secrete inflammatory factors, including NO [120]. In contrast, other studies have demonstrated that reactive astrocytes are essential to minimize damage after stroke. This discrepancy is because activated astrocytes migrate and proliferate around the penumbra zone to confine the ischemic core and inhibit its expansion. This process generates the glial scar (Fig. 3), a phenomenon that protects the brain from further damage by restoring the physical and chemical integrity of the CNS at the cost of losing potential for recovery [150]. In this sense, reactive astrocytes are interconnected by molecules like connexins, channel-forming proteins that allow cell-cell communication through the cytoplasm. These connections generate a dense network that allows astrocytes to buffer and redistribute harmful molecules. However, connexins can also have detrimental roles, as diffusion of glutamate or ATP through these channels can enhance damage under ischemic, inflammatory or neurodegenerative conditions [151]. Specifically, in vivo studies have shown that specific deletion of astrocytic connexins enhances tissue recovery after damage: knocking-out connexin43 in astrocytes reduces glial activation and enhances OL maturation and remyelination after demyelination [152]. However, inhibition of connexins could also affect physiological processes, so future therapies based on pharmacological modulation of connexins require further studies.
In addition, some studies suggest new neuroprotective roles of astrocytes regarding ischemic tolerance [153]. A mild non-lethal ischemic episode can produce resistance to a subsequent more severe ischemic insult [140]. Upon this challenging situation, astrocytes start to upregulate glutamate transporters to prevent glutamate excitotoxicity from dying neurons [154, 155]. They also produce a wide variety of neuroprotective molecules such as erythropoietin (EPO), VEGF and adhesion molecules and aquaporins with the aim to preserve neurons and brain function [156-159]. On the other hand, inhibition of reactive astrocytes made ischemic tolerance impossible to achieve. In addition, rapid clearance of cellular debris after brain injuries is vital to restore brain function. It has been shown that astrocytes can participate in phagocytic processes (even under physiological conditions) [160]. This data shows that endogenous remodeling mechanisms are also important after stroke or cerebral ischemia.
Interestingly, one way by which astrocytes can modulate oligodendrogenesis is through cholesterol: apart from being a risk factor in developing stroke, cholesterol is a major source of myelin and a limiting-step in myelin membrane biogenesis [161, 162] (Fig. 3). In addition, disturbances in cholesterol metabolism have been described in patients with myelin alterations such as multiple sclerosis [163]. Apolipoprotein E (ApoE) is the most abundant apolipoprotein in the brain. It helps to solubilize phospholipids and cholesterol and stabilizes high-density lipoproteins (HDL) vesicles. ATP-binding cassette transporter A1 (ABCA1) is a reverse cholesterol transporter critical for the formation of these vesicles in the brain [164]. However, plasma cholesterol cannot be taken up by the brain [165], so it is synthesized by the astrocytes and delivered to the rest of cells via the ABCA1/ApoE/HDL signaling axis. Even though OLs can synthesize cholesterol, ABCA1-/- mice showed decreased myelin density and OL numbers during developmental myelination [166]. After ischemic stroke, ABCA1-/- mice also had impaired oligodendrogenesis and lower levels of ApoE and HDL in the remaining OPCs [164]. This phenotype was related to decreased white matter remodeling and a worse functional outcome from 3 to 21 days after stroke induction. On the other hand, supplementation with human ApoE or HDL had beneficial effects on the corpus callosum and reversed the demyelination phenotype [164, 167, 168]. In conclusion, beneficial or detrimental roles of astrocytes may depend on the severity of ischemic insults, although age and environment related processes cannot be discarded to affect astrocytic functions. Modulating reactive astrocytes responses may be crucial to control ischemic damage in the brain.
CONCLUSION
During brain ischemia, a wide array of mechanisms are triggered by the brain. Some of them are beneficial and try to restore the damage done, while others compromise the recovery from the disease. This repair system involves neurons, astrocytes, microglia and OLs. Myelin loss after ischemic stroke needs new OPCs to differentiate into myelinating OLs that will restore the myelin sheaths. In this review, we focused on oligodendrogenesis and myelin regeneration after stroke, which are two extremely complex processes that require multiple and coordinated signaling pathways. We also assessed how the environment around the ischemic zone and other glial cells participate in the modulation of the ischemic process and remyelination. Future work will help to better understand the mechanisms that orchestrate remyelination following ischemic insults to generate treatments focused on enhancing oligodendrogenesis.
ACKNOWLEDGEMENTS
We thank all members of the Lab-206, at CBMSO for their support, help, and thoughtful discussions during the preparation of this work and manuscript.
LIST OF ABBREVIATIONS
- ABCA-1
ATP-binding cassette transporter A1
- APC
Adenomatous polyposis coli
- ApoE
Apolipoprotein E
- BBB
Blood-Brain Barrier
- BDNF
Brain-derived Neurotrophic Factor
- BMPs
Bone Morphogenetic Proteins
- BMP4
Bone Morphogenetic Protein-4
- Bpv
Bisperoxovanadium
- CK1
Casein Kinase 1
- CNS
Central Nervous System
- CX3CR1
CX3C chemokine receptor 1 / fractalkine receptor
- EPO
erythropoietin
- Erk1/2
Extracellular signal-related kinases 1/2
- FGF-2
Fibroblast Growth Factor-2
- GFAP
Glial Fibrillary Acidic Protein
- Gli
Glioma-associated oncogene
- GSK-3B
Glycogen Synthase Kinase 3B
- HDL
High-density Lipoproteins
- Iba-1
Ionized calcium-binding adapter molecule 1
- IGF-1
Insulin Growth Factor
- IL-1β
Interleukin-1β
- IL-4
Interleukin-4
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL-13
Interleukin-13
- IRS-1
Insulin Receptor Substrate-1
- LEF
Lymphoid Enhancer Factors
- MAPK
Mitogen-Activated Protein Kinases
- MBP
Myelin Basic Protein
- MCAO
Middle Cerebral Artery Occlusion
- miRNAs
microRNAs
- mTOR
mechanistic/mammalian Target of Rapamycin
- MyRF
Myelin Regulatory Factor
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- NMDAR
N-Methyl-D-Aspartate Receptor
- NO
Nitric Oxide
- NPCs
Neural Progenitor Cells
- NT3
Neurotrophin 3
- Olig1/2
Oligodendrocyte Transcription Factors 1/2
- OLs
Oligodendrocytes
- OPCs
Oligodendrocyte Progenitor Cells
- PDGF(R)
Platelet-derived Growth Factor (Receptor)
- PDK1
3-phosphoinositide-dependent Kinase
- PIP2
Phosphatidylinositol (4,5)-biphosphate
- PIP3
Phosphatidylinositol (3,4,5)-triphosphate
- PI3K
Phosphatidyl inositol-3-phosphate Kinase
- PLP
Proteolipid Protein
- PPARγ
Peroxisome Proliferator-activated Receptor γ
- P70S6K
Protein S6 Kinase beta-1
- Ptc
Patched
- PTEN
Phosphatase and Tensin homolog
- ROS
Reactive Oxygen Species
- RTKs
Receptor Tyrosine Kinases
- Shh
Sonic Hedgehog
- Smo
Smoothened
- SREBPs
Sterol Regulatory Element-binding proteins
- SVZ
Sub-Ventricular Zone
- TCF
T-cell Factors / Transcription Factors
- TCF4
Transcription Factor 4
- TCF7l2
Transcription Factor7-like 2
- TGF-β
Tissue Growth Factor β
- TIMP-1
Tissue Inhibitor of Metalloproteinase-1
- TNF-α
Tumor Necrosis Factor α
- VEGF
Vascular-endothelial Growth Factor
- Wnt
Wingless integration site
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the Spanish Ministry of Economy and Competitiveness (RTI2018-096303B-C33), to BC and (RTI2018-096303-B-C1), Comunidad de Madrid (CAM-Biomedicina, B2017/BMD-3700) and Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED) [PI2016/01] to FW.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O’Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Emergency and comprehensive care for stroke needed. Lancet. 2009;373(9674):1496. doi: 10.1016/S0140-6736(09)60833-3. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca A.C., Ferro J.M. Cryptogenic stroke. Eur. J. Neurol. 2015;22(4):618–623. doi: 10.1111/ene.12673. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 5.Yin K.J., Hamblin M., Chen Y.E. Non-coding RNAs in cerebral endothelial pathophysiology: Emerging roles in stroke. Neurochem. Int. 2014;77:9–16. doi: 10.1016/j.neuint.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krueger M., Bechmann I., Immig K., Reichenbach A., Härtig W., Michalski D. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2015;35(2):292–303. doi: 10.1038/jcbfm.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCabe C., Arroja M.M., Reid E., Macrae I.M. Animal models of ischaemic stroke and characterisation of the ischaemic penumbra. Neuropharmacology. 2018;134(Pt B):169–177. doi: 10.1016/j.neuropharm.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Durukan A., Tatlisumak T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007;87(1):179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Richard Green A., Odergren T., Ashwood T. Animal models of stroke: Do they have value for discovering neuroprotective agents? Trends Pharmacol. Sci. 2003;24(8):402–408. doi: 10.1016/S0165-6147(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 10.Rolfe D.F.S., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 11.Puig B., Brenna S., Magnus T. Molecular communication of a dying neuron in stroke. Int. J. Mol. Sci. 2018;19(9): ,E2834. doi: 10.3390/ijms19092834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fricker M., Tolkovsky A.M., Borutaite V., Coleman M., Brown G.C. Neuronal cell death. Physiol. Rev. 2018;98(2):813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoshnam S.E., Winlow W., Farzaneh M., Farbood Y., Moghaddam H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017;38(7):1167–1186. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- 14.Rock K.L., Kono H., Adriano A., Christina S. The inflammatory response to cell death. Annu. Rev. Pathol. Dis. 2008;3:67–97. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Alvarez M.J., Wandosell F. Stroke and neuroinflamation: Role of sexual hormones. Curr. Pharm. Des. 2016;22(10):1334–1349. doi: 10.2174/138161282210160304112834. [DOI] [PubMed] [Google Scholar]
- 16.Broughton B.R.S., Reutens D.C., Sobey C.G. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 17.Forbes T.A., Gallo V. All wrapped up: Environmental effects on myelination. Trends Neurosci. 2017;40(9):572–587. doi: 10.1016/j.tins.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergles D.E., Richardson W.D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 2015;8(2): ,a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simons M., Nave K.A. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb. Perspect. Biol. 2015;8(1): ,a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadelmann C., Timmler S., Barrantes-Freer A., Simons M. Myelin in the central nervous system: Structure, function, and pathology. Physiol. Rev. 2019;99(3):1381–1431. doi: 10.1152/physrev.00031.2018. [DOI] [PubMed] [Google Scholar]
- 21.Salzer J.L., Zalc B. Myelination. Curr. Biol. 2016;26(20):R971–R975. doi: 10.1016/j.cub.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 22.Goldman S.A., Kuypers N.J. How to make an oligodendrocyte. Development. 2015;142(23):3983–3995. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A., Sherman D.L., Brophy P.J. The axonal cytoskeleton and the assembly of nodes of ranvier. Neuroscientist. 2018;24(2):104–110. doi: 10.1177/1073858417710897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubetzki C., Sol-Foulon N., Desmazières A. Nodes of ranvier during development and repair in the CNS. Nat. Rev. Neurol. 2020. p. 1871. [DOI] [PubMed]
- 25.Bercury K.K., Macklin W.B. Dynamics and mechanisms of CNS myelination. Dev. Cell. 2015;32(4):447–458. doi: 10.1016/j.devcel.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philips T., Rothstein J.D., Philips T., Rothstein J.D. Oligodendroglia: Metabolic supporters of neurons. J. Clin. Invest. 2017;127(9):3271–3280. doi: 10.1172/JCI90610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain K.A., Sheng Z-H. Mechanisms for the maintenance and regulation of axonal energy supply. J. Neurosci. Res. 2019;97(8):897–913. doi: 10.1002/jnr.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson E.M., Geraghty A.C., Monje M. Bad wrap: Myelin and myelin plasticity in health and disease. Dev. Neurobiol. 2018;78(2):123–135. doi: 10.1002/dneu.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobson R., Giovannoni G. Multiple sclerosis - A review. Eur. J. Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 30.Jasiak-Zatonska M., Kalinowska-Lyszczarz A., Michalak S., Kozubski W. The immunology of neuromyelitis optica-current knowledge, clinical implications, controversies and future perspectives. Int. J. Mol. Sci. 2016;17(3):273. doi: 10.3390/ijms17030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai W., Yang T., Liu H., Han L., Zhang K., Hu X., Zhang X., Yin K.J., Gao Y., Bennett M.V.L., Leak R.K., Chen J. Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018;163-164:27–58. doi: 10.1016/j.pneurobio.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mierzwa A.J., Marion C.M., Sullivan G.M., McDaniel D.P., Armstrong R.C. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J. Neuropathol. Exp. Neurol. 2015;74(3):218–232. doi: 10.1097/NEN.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang T., Sun Y., Lu Z., Leak R.K., Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2017;34:15–29. doi: 10.1016/j.arr.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg G.A. Vascular cognitive impairment: Biomarkers in diagnosis and molecular targets in therapy. J. Cereb. Blood Flow Metab. 2016;36(1):4–5. doi: 10.1177/0271678X15609542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benarroch E.E. Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology. 2009;72(20):1779–1785. doi: 10.1212/WNL.0b013e3181a6b123. [DOI] [PubMed] [Google Scholar]
- 36.Saab A.S., Tzvetavona I.D., Trevisiol A., Baltan S., Dibaj P., Kusch K., Möbius W., Goetze B., Jahn H.M., Huang W., Steffens H., Schomburg E.D., Pérez-Samartín A., Pérez-Cerdá F., Bakhtiari D., Matute C., Löwel S., Griesinger C., Hirrlinger J., Kirchhoff F., Nave K.A. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91(1):119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W., Harding H.P., Ron D., Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-γ. J. Cell Biol. 2005;169(4):603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hövelmeyer N., Hao Z., Kranidioti K., Kassiotis G., Buch T., Frommer F., von Hoch L., Kramer D., Minichiello L., Kollias G., Lassmann H., Waisman A. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 2005;175(9):5875–5884. doi: 10.4049/jimmunol.175.9.5875. [DOI] [PubMed] [Google Scholar]
- 39.Pang Y., Cai Z., Rhodes P.G. Effect of tumor necrosis factor-α on developing optic nerve oligodendrocytes in culture. J. Neurosci. Res. 2005;80(2):226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- 40.Shi H., Hu X., Leak R.K., Shi Y., An C., Suenaga J., Chen J., Gao Y. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp. Neurol. 2015;272:17–25. doi: 10.1016/j.expneurol.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantoni L., Garcia J.H., Gutierrez J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1646. doi: 10.1161/01.STR.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X., Pu H., Hu X., Wei Z., Hong D., Zhang W., Gao Y., Chen J., Shi Y. A post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl. Stroke Res. 2016;7(6):548–561. doi: 10.1007/s12975-016-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson M.R.L., Polito A., Levine J.M., Reynolds R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003;24(2):476–488. doi: 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 44.Nait-Oumesmar B., Picard-Riera N., Kerninon C., Decker L., Seilhean D., Höglinger G.U., Hirsch E.C., Reynolds R., Baron-Van E.A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA. 2007;104(11):4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menn B., Garcia-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fancy S.P.J., Zhao C., Franklin R.J.M. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell. Neurosci. 2004;27(3):247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Tong C.K., Fuentealba L.C., Shah J.K., Lindquist R.A., Ihrie R.A., Guinto C.D., Rodas-Rodriguez J.L., Alvarez-Buylla A. A dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Reports. 2015;5(4):461–470. doi: 10.1016/j.stemcr.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flygt J., Clausen F., Marklund N. Diffuse traumatic brain injury in the mouse induces a transient proliferation of oligodendrocyte progenitor cells in injured white matter tracts. Restor. Neurol. Neurosci. 2017;35(2):251–263. doi: 10.3233/RNN-160675. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R., Chopp M., Zhang Z.G. Oligodendrogenesis after cerebral ischemia. Front. Cell. Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L.X., Ma S.M., Zhang P., Fan Z.C., Xiong M., Cheng G.Q., Yang Y., Qiu Z.L., Zhou W.H., Li J. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PLoS One. 2015;10(3): ,e0115997. doi: 10.1371/journal.pone.0115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orthmann-Murphy J., Call C.L., Molina-Castro G.C., Hsieh Y.C., Rasband M.N., Calabresi P.A., Bergles D.E. Remyelination alters the pattern of myelin in the cerebral cortex. eLife. 2020;9:1–61. doi: 10.7554/eLife.56621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poncet C., Soula C., Trousse F., Kan P., Hirsinger E., Pourquié O., Duprat A.M., Cochard P. Induction of oligodendrocyte progenitors in the trunk neural tube by ventralizing signals: Effects of notochord and floor plate grafts, and of sonic hedgehog. Mech. Dev. 1996;60(1):13–32. doi: 10.1016/S0925-4773(96)00595-3. [DOI] [PubMed] [Google Scholar]
- 53.Pringle N.P., Yu W.P., Guthrie S., Roelink H., Lumsden A., Peterson A.C., Richardson W.D. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev. Biol. 1996;177(1):30–42. doi: 10.1006/dbio.1996.0142. [DOI] [PubMed] [Google Scholar]
- 54.Laouarem Y., Traiffort E. Developmental and repairing production of myelin: The role of hedgehog signaling. Front. Cell. Neurosci. 2018;12:305. doi: 10.3389/fncel.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferent J., Traiffort E. Hedgehog: Multiple paths for multiple roles in shaping the brain and spinal cord. Neuroscientist. 2015;21(4):356–371. doi: 10.1177/1073858414531457. [DOI] [PubMed] [Google Scholar]
- 56.Park H.C., Mehta A., Richardson J.S., Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 2002;248(2):356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- 57.Schebesta M., Serluca F.C. Olig 1 Expression identifies developing oligodendrocytes in zebrafish and requires hedgehog and notch signaling. Dev. Dyn. 2009;238(4):887–898. doi: 10.1002/dvdy.21909. [DOI] [PubMed] [Google Scholar]
- 58.Ferent J., Zimmer C., Durbec P., Ruat M., Traiffort E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J. Neurosci. 2013;33(5):1759–1772. doi: 10.1523/JNEUROSCI.3334-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonnière L., Bernard M., Van Horssen J., De Vries H.E., Charron F., Prat A. The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 60.Wang L., Zhang Z.G., Gregg S.R., Zhang R.L., Jiao Z., LeTourneau Y., Liu X., Feng Y., Gerwien J., Torup L., Leist M., Noguchi C.T., Chen Z.Y., Chopp M. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J. Biol. Chem. 2007;282(44):32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- 61.Liu X.S., Chopp M., Wang X.L., Zhang L., Hozeska-Solgot A., Tang T., Kassis H., Zhang R.L., Chen C., Xu J., Zhang Z.G. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J. Biol. Chem. 2013;288(18):12478–12488. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L., Chopp M., Meier D.H., Winter S., Wang L., Szalad A., Lu M., Wei M., Cui Y., Zhang Z.G. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke. 2013;44(7):1965–1972. doi: 10.1161/STROKEAHA.111.000831. [DOI] [PubMed] [Google Scholar]
- 63.Samanta J., Grund E.M., Silva H.M., Lafaille J.J., Fishell G., Salzer J.L. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526(7573):448–452. doi: 10.1038/nature14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez M.A., Sullivan G.M., Armstrong R.C. Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination. Neurobiol. Dis. 2018;115(115):145–156. doi: 10.1016/j.nbd.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Alcover-Sanchez B., Garcia-Martin G., Wandosell F., Cubelos B. R-Ras GTPases signaling role in myelin neurodegenerative diseases. Int. J. Mol. Sci. 2020;21(16):1–18. doi: 10.3390/ijms21165911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaesser J.M., Fyffe-Maricich S.L. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol. 2016;283(Pt B):501–511. doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flores A.I., Narayanan S.P., Morse E.N., Shick H.E., Yin X., Kidd G., Avila R.L., Kirschner D.A., Macklin W.B. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28(28):7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goebbels S., Oltrogge J.H., Kemper R., Heilmann I., Bormuth I., Wolfer S., Wichert S.P., Möbius W., Liu X., Lappe-Siefke C., Rossner M.J., Groszer M., Suter U., Frahm J., Boretius S., Nave K.A. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 2010;30(26):8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLane L.E., Bourne J.N., Evangelou A.V., Khandker L., Macklin W.B., Wood T.L. Loss of tuberous sclerosis complex1 in adult oligodendrocyte progenitor cells enhances axon remyelination and increases myelin thickness after a focal demyelination. J. Neurosci. 2017;37(31):7534–7546. doi: 10.1523/JNEUROSCI.3454-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi Q., Saifetiarova J., Taylor A.M., Bhat M.A. mTORC1 Activation by loss of tsc1 in myelinating glia causes downregulation of quaking and neurofascin 155 leading to paranodal domain disorganization. Front. Cell. Neurosci. 2018;12:201. doi: 10.3389/fncel.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figlia G., Gerber D., Suter U. Myelination and mTOR. Glia. 2018;66(4):693–707. doi: 10.1002/glia.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malumbres M., Barbacid M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 73.Sjölander A., Yamamoto K., Huber B.E., Lapetina E.G. Association of p21ras with phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 1991;88(18):7908–7912. doi: 10.1073/pnas.88.18.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Viciana P., Warne P.H., Dhand R., Vanhaesebroeck B., Gout I., Fry M.J., Waterfield M.D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370(6490):527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 75.Gonsalvez D., Ferner A.H., Peckham H., Murray S.S., Xiao J. The roles of extracellular related-kinases 1 and 2 signaling in CNS myelination. Neuropharmacology, 2016;110(Pt B):586–593. doi: 10.1016/j.neuropharm.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Ishii A., Furusho M., Dupree J.L., Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J. Neurosci. 2014;34(48):16031–16045. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii A., Fyffe-Maricich S.L., Furusho M., Miller R.H., Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 2012;32(26):8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai J., Bercury K.K., Macklin W.B. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia. 2014;62(12):2096–2109. doi: 10.1002/glia.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michel K., Zhao T., Karl M., Lewis K., Fyffe-Maricich S.L. Translational control of myelin basic protein expression by ERK2 MAP kinase regulates timely remyelination in the adult brain. J. Neurosci. 2015;35(20):7850–7865. doi: 10.1523/JNEUROSCI.4380-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanz-Rodriguez M., Gruart A., Escudero-Ramirez J., de Castro F., Delgado-García J.M., Wandosell F., Cubelos B. R-Ras1 and R-Ras2 Are essential for oligodendrocyte differentiation and survival for correct myelination in the central nervous system. J. Neurosci. 2018;38(22):5096–5110. doi: 10.1523/JNEUROSCI.3364-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alcover-Sanchez B., Garcia-Martin G., Escudero-Ramirez J., Gonzalez-Riano C., Lorenzo P., Gimenez-Cassina A., Formentini L., de la Villa-Polo P., Pereira M.P., Wandosell F., Cubelos B. Absence of R-Ras1 and R-Ras2 causes mitochondrial alterations that trigger axonal degeneration in a hypomyelinating disease model. Glia. 2021;69(3):619–637. doi: 10.1002/glia.23917. [DOI] [PubMed] [Google Scholar]
- 82.Ishii A., Furusho M., Macklin W., Bansal R. Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood. Glia. 2019;67(7):1277–1295. doi: 10.1002/glia.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J., Du T., Li B., Rong Y., Verkhratsky A., Peng L. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro. 2015;7(5): ,1759091415602463. doi: 10.1177/1759091415602463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu R., Tang J.C., Pan M.X., Zhuang Y., Zhang Y., Liao H.B., Zhao D., Lei Y., Lei R.X., Wang S., Liu A.C., Qin X.P., Chen J., Zhang Z.F., Wan Q. ERK 1/2 activation mediates the neuroprotective effect of BpV(pic) in focal cerebral ischemia-reperfusion injury. Neurochem. Res. 2018;43(7):1424–1438. doi: 10.1007/s11064-018-2558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun J., Fang Y., Chen T., Guo J., Yan J., Song S., Zhang L., Liao H. WIN55, 212-2 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res. 2013;1491:225–235. doi: 10.1016/j.brainres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Santra M., Chopp M., Zhang Z.G., Lu M., Santra S., Nalani A., Santra S., Morris D.C. Thymosin β 4 mediates oligodendrocyte differentiation by upregulating p38 MAPK. Glia. 2012;60(12):1826–1838. doi: 10.1002/glia.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shioda N., Han F., Fukunaga K. Role of akt and erk signaling in the neurogenesis following brain Ischemia. 1st ed. Elsevier Inc.; 2009. p. 85. [DOI] [PubMed] [Google Scholar]
- 88.Yu Y., Fu P., Yu Z., Xie M., Wang W., Luo X. NKCC1 inhibition attenuates chronic cerebral hypoperfusion-induced white matter lesions by enhancing progenitor cells of oligodendrocyte proliferation. J. Mol. Neurosci. 2018;64(3):449–458. doi: 10.1007/s12031-018-1043-0. [DOI] [PubMed] [Google Scholar]
- 89.Guo F., Lang J., Sohn J., Hammond E., Chang M., Pleasure D. Canonical Wnt signaling in the oligodendroglial lineage--puzzles remain. Glia. 2015;63(10):1671–1693. doi: 10.1002/glia.22813. [DOI] [PubMed] [Google Scholar]
- 90.Fancy S.P.J., Baranzini S.E., Zhao C., Yuk D.I., Irvine K.A., Kaing S., Sanai N., Franklin R.J.M., Rowitch D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23(13):1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feigenson K., Reid M., See J., Crenshaw E.B. III.; Grinspan, J.B. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol. Cell. Neurosci. 2009;42(3):255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 92.Azim K., Butt A.M. GSK3β negatively regulates oligodendrocyte differentiation and myelination In vivo. Glia. 2011;59(4):540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- 93.Nicaise A.M., Johnson K.M., Willis C.M., Guzzo R.M., Crocker S.J. TIMP-1 promotes oligodendrocyte differentiation through receptor-mediated signaling. Mol. Neurobiol. 2019;56(5):3380–3392. doi: 10.1007/s12035-018-1310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L., Geng J., Qu M., Yuan F., Wang Y., Pan J., Li Y., Ma Y., Zhou P., Zhang Z., Yang G-Y. Oligodendrocyte precursor cells transplantation protects blood-brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020;11(1):9. doi: 10.1038/s41419-019-2206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S., Kim B., Zhu X., Gui X., Wang Y., Lan Z., Prabhu P., Fond K., Wang A., Guo F. Glial type specific regulation of CNS angiogenesis by hifα-activated different signaling pathways. Nat. Commun. 2020;11(1):1–17. doi: 10.1038/s41467-020-15656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu H., Kesari S., Cai J. Tcf7l2 is tightly controlled during myelin formation. Cell. Mol. Neurobiol. 2012;32(3):345–352. doi: 10.1007/s10571-011-9778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weng C., Ding M., Fan S., Cao Q., Lu Z. Transcription factor 7 like 2 promotes oligodendrocyte differentiation and remyelination. Mol. Med. Rep. 2017;16(2):1864–1870. doi: 10.3892/mmr.2017.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao C., Deng Y., Liu L., Yu K., Zhang L., Wang H., He X., Wang J., Lu C., Wu L.N., Weng Q., Mao M., Li J., van Es J.H., Xin M., Parry L., Goldman S.A., Clevers H., Lu Q.R. Dual regulatory switch through interactions of Tcf7l2/Tcf4 with stage-specific partners propels oligodendroglial maturation. Nat. Commun. 2016;7:10883. doi: 10.1038/ncomms10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hammond E., Lang J., Maeda Y., Pleasure D., Angus-Hill M., Xu J., Horiuchi M., Deng W., Guo F. The Wnt effector transcription factor 7-like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/β-catenin signaling. J. Neurosci. 2015;35(12):5007–5022. doi: 10.1523/JNEUROSCI.4787-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang S., Wang Y., Zhu X., Song L., Zhan X., Ma E., McDonough J., Fu H., Cambi F., Grinspan J., Guo F. The wnt effector tcf7l2 promotes oligodendroglial differentiation by repressing autocrine bmp4-mediated signaling. J. Neurosci. 2021;41(8):1650–1664. doi: 10.1523/JNEUROSCI.2386-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feigenson K., Reid M., See J., Crenshaw E.B., III, Grinspan J.B. Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro. 2011;3(3): ,e00061. doi: 10.1042/AN20110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cantone M., Küspert M., Reiprich S., Lai X., Eberhardt M., Göttle P., Beyer F., Azim K., Küry P., Wegner M., Vera J. A gene regulatory architecture that controls region-independent dynamics of oligodendrocyte differentiation. Glia. 2019;67(5):825–843. doi: 10.1002/glia.23569. [DOI] [PubMed] [Google Scholar]
- 103.Sauer S. Ligands for the nuclear peroxisome proliferator-activated receptor gamma. Trends Pharmacol. Sci. 2015;36(10):688–704. doi: 10.1016/j.tips.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Zhao X., Strong R., Zhang J., Sun G., Tsien J.Z., Cui Z., Grotta J.C., Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J. Neurosci. 2009;29(19):6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han L., Cai W., Mao L., Liu J., Li P., Leak R.K., Xu Y., Hu X., Chen J. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–2636. doi: 10.1161/STROKEAHA.115.010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Q., Zhu W., Xu F., Dai X., Shi L., Cai W., Mu H., Hitchens T.K., Foley L.M., Liu X., Yu F., Chen J., Shi Y., Leak R.K., Gao Y., Chen J., Hu X. The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019;17(6): ,e3000330. doi: 10.1371/journal.pbio.3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kanakasabai S., Pestereva E., Chearwae W., Gupta S.K., Ansari S., Bright J.J. PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS One. 2012;7(11): ,e50500. doi: 10.1371/journal.pone.0050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan Ibrahim W.N., Tofighi R., Onishchenko N., Rebellato P., Bose R., Uhlén P., Ceccatelli S. Perfluorooctane sulfonate induces neuronal and oligodendrocytic differentiation in neural stem cells and alters the expression of PPARγ in vitro and In vivo. Toxicol. Appl. Pharmacol. 2013;269(1):51–60. doi: 10.1016/j.taap.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 109.De Nuccio C., Bernardo A., De Simone R., Mancuso E., Magnaghi V., Visentin S., Minghetti L. Peroxisome proliferator-activated receptor γ agonists accelerate oligodendrocyte maturation and influence mitochondrial functions and oscillatory Ca(2+) waves. J. Neuropathol. Exp. Neurol. 2011;70(10):900–912. doi: 10.1097/NEN.0b013e3182309ab1. [DOI] [PubMed] [Google Scholar]
- 110.Roth A.D., Leisewitz A.V., Jung J.E., Cassina P., Barbeito L., Inestrosa N.C., Bronfman M. PPAR γ activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J. Neurosci. Res. 2003;72(4):425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- 111.Bernardo A., Bianchi D., Magnaghi V., Minghetti L. Peroxisome proliferator-activated receptor-γ agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J. Neuropathol. Exp. Neurol. 2009;68(7):797–808. doi: 10.1097/NEN.0b013e3181aba2c1. [DOI] [PubMed] [Google Scholar]
- 112.Kawase-Koga Y., Otaegi G., Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009;238(11):2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nave K.A. Oligodendrocytes and the “micro brake” of progenitor cell proliferation. Neuron. 2010;65(5):577–579. doi: 10.1016/j.neuron.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 114.Dugas J.C., Cuellar T.L., Scholze A., Ason B., Ibrahim A., Emery B., Zamanian J.L., Foo L.C., McManus M.T., Barres B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65(5):597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao X., He X., Han X., Yu Y., Ye F., Chen Y., Hoang T., Xu X., Mi Q.S., Xin M., Wang F., Appel B., Lu Q.R. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65(5):612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kornfeld S.F., Cummings S.E., Fathi S., Bonin S.R., Kothary R. MiRNA-145-5p prevents differentiation of oligodendrocyte progenitor cells by regulating expression of myelin gene regulatory factor. J. Cell. Physiol. 2021;236(2):997–1012. doi: 10.1002/jcp.29910. [DOI] [PubMed] [Google Scholar]
- 117.Liu X.S., Chopp M., Pan W.L., Wang X.L., Fan B.Y., Zhang Y., Kassis H., Zhang R.L., Zhang X.M., Zhang Z.G. MicroRNA-146a Promotes Oligodendrogenesis in Stroke. Mol. Neurobiol. 2017;54(1):227–237. doi: 10.1007/s12035-015-9655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buller B., Chopp M., Ueno Y., Zhang L., Zhang R.L., Morris D., Zhang Y., Zhang Z.G. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia. 2012;60(12):1906–1914. doi: 10.1002/glia.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santra M., Chopp M., Santra S., Nallani A., Vyas S., Zhang Z.G., Morris D.C. Thymosin beta 4 up-regulates miR-200a expression and induces differentiation and survival of rat brain progenitor cells. J. Neurochem. 2016;136(1):118–132. doi: 10.1111/jnc.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burda J.E., Bernstein A.M., Sofroniew M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gomez-Nicola D., Perry V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neuroscientist. 2015;21(2):169–184. doi: 10.1177/1073858414530512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu L., Zhang K., Hu G., Yan H., Xie C., Wu X. Inflammatory response and neuronal necrosis in rats with cerebral ischemia. Neural Regen. Res. 2014;9(19):1753–1762. doi: 10.4103/1673-5374.143419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDonough A., Weinstein J.R. Neuroimmune Response in Ischemic Preconditioning. Neurotherapeutics. 2016;13(4):748–761. doi: 10.1007/s13311-016-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fumagalli M., Lombardi M., Gressens P., Verderio C. How to reprogram microglia toward beneficial functions. Glia. 2018;66(12):2531–2549. doi: 10.1002/glia.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krupiński J., Kałuza J., Kumar P., Kumar S. Immunocytochemical studies of cellular reaction in human ischemic brain stroke. MAB anti-CD68 stains macrophages, astrocytes and microglial cells in infarcted area. Folia Neuropathol. 1996;34(1):17–24. [PubMed] [Google Scholar]
- 126.Gulyás B., Tóth M., Schain M., Airaksinen A., Vas A., Kostulas K., Lindström P., Hillert J., Halldin C. Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: A PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J. Neurol. Sci. 2012;320(1-2):110–117. doi: 10.1016/j.jns.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 127.Wolf S.A., Boddeke H.W.G.M., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79(79):619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 128.Ma Y., Wang J., Wang Y., Yang G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 129.Yrjänheikki J., Keinänen R., Pellikka M., Hökfelt T., Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehnardt S., Massillon L., Follett P., Jensen F.E., Ratan R., Rosenberg P.A., Volpe J.J., Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kacimi R., Giffard R.G., Yenari M.A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J. Inflamm. (Lond.) 2011;8:7. doi: 10.1186/1476-9255-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jolivel V., Bicker F., Binamé F., Ploen R., Keller S., Gollan R., Jurek B., Birkenstock J., Poisa-Beiro L., Bruttger J., Opitz V., Thal S.C., Waisman A., Bäuerle T., Schäfer M.K., Zipp F., Schmidt M.H.H. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279–295. doi: 10.1007/s00401-014-1372-1. [DOI] [PubMed] [Google Scholar]
- 133.Stone S., Jamison S., Yue Y., Durose W., Schmidt-Ullrich R., Lin W. NF-κB Activation protects oligodendrocytes against inflammation. J. Neurosci. 2017;37(38):9332–9344. doi: 10.1523/JNEUROSCI.1608-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.da Fonseca A.C.C., Matias D., Garcia C., Amaral R., Geraldo L.H., Freitas C., Lima F.R.S. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014;8(November):362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Machado L.S., Sazonova I.Y., Kozak A., Wiley D.C., El-Remessy A.B., Ergul A., Hess D.C., Waller J.L., Fagan S.C. Minocycline and tissue-type plasminogen activator for stroke: Assessment of interaction potential. Stroke. 2009;40(9):3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tikka T., Fiebich B.L., Goldsteins G., Keinänen R., Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001;21(8):2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yenari M.A., Xu L., Tang X.N., Qiao Y., Giffard R.G. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline In vivo and in vitro. Stroke. 2006;37(4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 138.Amici S.A., Dong J., Guerau-de-Arellano M. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 2017;8:1520. doi: 10.3389/fimmu.2017.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 2015;11(1):56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koizumi S., Hirayama Y., Morizawa Y.M. New roles of reactive astrocytes in the brain; an organizer of cerebral ischemia. Neurochem. Int. 2018;119:107–114. doi: 10.1016/j.neuint.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Schmitz T., Krabbe G., Weikert G., Scheuer T., Matheus F., Wang Y., Mueller S., Kettenmann H., Matyash V., Bührer C., Endesfelder S. Minocycline protects the immature white matter against hyperoxia. Exp. Neurol. 2014;254:153–165. doi: 10.1016/j.expneurol.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 142.Masumura M., Hata R., Nagai Y., Sawada T. Oligodendroglial cell death with DNA fragmentation in the white matter under chronic cerebral hypoperfusion: Comparison between normotensive and spontaneously hypertensive rats. Neurosci. Res. 2001;39(4):401–412. doi: 10.1016/S0168-0102(01)00195-X. [DOI] [PubMed] [Google Scholar]
- 143.Jalal F.Y., Yang Y., Thompson J., Lopez A.C., Rosenberg G.A. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43(4):1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K.F., Leak R.K., Gao Y., Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 2013;33(12):1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miron V.E., Boyd A., Zhao J.W., Yuen T.J., Ruckh J.M., Shadrach J.L., van Wijngaarden P., Wagers A.J., Williams A., Franklin R.J.M., Ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shigemoto-Mogami Y., Hoshikawa K., Goldman J.E., Sekino Y., Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014;34(6):2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao X., Wang H., Sun G., Zhang J., Edwards N.J., Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci. 2015;35(32):11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiong X., Barreto G.E., Xu L., Ouyang Y.B., Xie X., Giffard R.G. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42(7):2026–2032. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Preston A.N., Cervasio D.A., Laughlin S.T. Visualizing the brain’s astrocytes, 1st. Elsevier Inc.; 2019. p. 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adams K.L., Gallo V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018;21(1):9–15. doi: 10.1038/s41593-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Giaume C., Naus C.C., Sáez J.C., Leybaert L. Glial connexins and pannexins in the healthy and diseased brain. Physiol. Rev. 2021;101(1):93–145. doi: 10.1152/physrev.00043.2018. [DOI] [PubMed] [Google Scholar]
- 152.Li T., Niu J., Yu G., Ezan P., Yi C., Wang X., Koulakoff A., Gao X., Chen X., Sáez J.C., Giaume C., Xiao L. Connexin 43 deletion in astrocytes promotes CNS remyelination by modulating local inflammation. Glia. 2020;68(6):1201–1212. doi: 10.1002/glia.23770. [DOI] [PubMed] [Google Scholar]
- 153.Hirayama Y., Ikeda-Matsuo Y., Notomi S., Enaida H., Kinouchi H., Koizumi S. Astrocyte-mediated ischemic tolerance. J. Neurosci. 2015;35(9):3794–3805. doi: 10.1523/JNEUROSCI.4218-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shinozaki Y., Shibata K., Yoshida K., Shigetomi E., Gachet C., Ikenaka K., Tanaka K.F., Koizumi S. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017;19(6):1151–1164. doi: 10.1016/j.celrep.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 155.Krzyżanowska W., Pomierny B., Bystrowska B., Pomierny-Chamioło L., Filip M., Budziszewska B., Pera J. Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS One. 2017;12(10): ,e0186243. doi: 10.1371/journal.pone.0186243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ruscher K., Freyer D., Karsch M., Isaev N., Megow D., Sawitzki B., Priller J., Dirnagl U., Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: Evidence from an in vitro model. J. Neurosci. 2002;22(23):10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fang B., Li X.M., Sun X.J., Bao N.R., Ren X.Y., Lv H.W., Ma H. Ischemic preconditioning protects against spinal cord ischemia-reperfusion injury in rabbits by attenuating blood spinal cord barrier disruption. Int. J. Mol. Sci. 2013;14(5):10343–10354. doi: 10.3390/ijms140510343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hirt L., Ternon B., Price M., Mastour N., Brunet J.F., Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J. Cereb. Blood Flow Metab. 2009;29(2):423–433. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- 159.Neumann J.T., Thompson J.W., Raval A.P., Cohan C.H., Koronowski K.B., Perez-Pinzon M.A. Increased BDNF protein expression after ischemic or PKC epsilon preconditioning promotes electrophysiologic changes that lead to neuroprotection. J. Cereb. Blood Flow Metab. 2015;35(1):121–130. doi: 10.1038/jcbfm.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morizawa Y.M., Hirayama Y., Ohno N., Shibata S., Shigetomi E., Sui Y., Nabekura J., Sato K., Okajima F., Takebayashi H., Okano H., Koizumi S. Reactive astrocytes function as phagocytes after brain ischemia via abca1-mediated pathway. Nat. Commun. 2017;8(1):1–14. doi: 10.1038/s41467-017-00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schmitt S., Castelvetri L.C., Simons M. Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta. 2015;1851(8):999–1005. doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 162.Gu X., Li Y., Chen S., Yang X., Liu F., Li Y., Li J., Cao J., Liu X., Chen J., Shen C., Yu L., Huang J., Lam T.H., Fang X., He Y., Zhang X., Lu X., Wu S., Gu D. Association of lipids with ischemic and hemorrhagic stroke: A prospective cohort study among 267 500 chinese. Stroke. 2019;50(12):3376–3384. doi: 10.1161/STROKEAHA.119.026402. [DOI] [PubMed] [Google Scholar]
- 163.Berghoff S.A., Gerndt N., Winchenbach J., Stumpf S.K., Hosang L., Odoardi F., Ruhwedel T., Böhler C., Barrette B., Stassart R., Liebetanz D., Dibaj P., Möbius W., Edgar J.M., Saher G. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017;8:14241. doi: 10.1038/ncomms14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li L., Li R., Zacharek A., Wang F., Landschoot-Ward J., Chopp M., Chen J., Cui X. ABCA1/ApoE/HDL signaling pathway facilitates myelination and oligodendrogenesis after stroke. Int. J. Mol. Sci. 2020;21(12):1–18. doi: 10.3390/ijms21124369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dietschy J.M., Turley S.D. Thematic review series: Brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 166.Cui X., Chopp M., Zacharek A., Karasinska J.M., Cui Y., Ning R., Zhang Y., Wang Y., Chen J. Deficiency of brain ATP-binding cassette transporter A-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke. 2015;46(3):827–834. doi: 10.1161/STROKEAHA.114.007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cui X., Chopp M., Zhang Z., Li R., Zacharek A., Landschoot-Ward J., Venkat P., Chen J. ABCA1/ApoE/HDL pathway mediates GW3965-induced neurorestoration after stroke. Stroke. 2017;48(2):459–467. doi: 10.1161/STROKEAHA.116.015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X., Li R., Zacharek A., Landschoot-Ward J., Wang F., Wu K.H.H., Chopp M., Chen J., Cui X. Administration of downstream apoe attenuates the adverse effect of brain ABCA1 deficiency on stroke. Int. J. Mol. Sci. 2018;19(11): ,E3368. doi: 10.3390/ijms19113368. [DOI] [PMC free article] [PubMed] [Google Scholar]