Abstract
Poloxamer 188 (P188) is an FDA-approved biocompatible block copolymer composed of repeating units of Poly(Ethylene Oxide) (PEO) and poly(propylene oxide) (PPO). Due to its amphiphilic nature and high Hydrophile-Lipophile Balance (HLB) value of 29, P188 is used as a stabilizer/emulsifier in many cosmetics and pharmaceutical preparations. While the applications of P188 as an excipient are widely explored, the data on the pharmacological activity of P188 are scarce. Notably, the neuroprotective potential of P188 has gained a lot of interest. Therefore, this systematic review is aimed at summarizing evidence of neuroprotective potential of P188 in CNS disorders. The PRISMA model was used, and five databases (Google Scholar, Scopus, Wiley Online Library, ScienceDirect, and PubMed) were searched with relevant keywords. The search resulted in 11 articles, which met the inclusion criteria. These articles described the protective effects of P188 on traumatic brain injury or mechanical injury in cells, neurotoxicity, Parkinson’s disease, Amyotrophic lateral sclerosis (ALS), and ischemia/ reperfusion injury from stroke. All the articles were original research in experimental or pre-clinical stages using animal models or in vitro systems. The reported activities demonstrated the potential of P188 as a neuroprotective agent in improving CNS conditions such as neurodegeneration.
Keywords: Poloxamer 188 (P188), central nervous system (CNS), neuroprotection, neurodegenerative diseases, brain, trauma
1. INTRODUCTION
Disorders of the Central Nervous System (CNS) or neurological diseases are serious and prevalent health concerns that remain poorly treated. There is an increase in the prevalence of many neurologic conditions, especially neurodegenerative disorders, as the population age. For instance, a meta-analysis of worldwide data revealed a prevalence of 40.51 per 100,000 in individuals 40-49 years of age, 106.67 per 100,000 in the age group 50-59 years, 428.48 per 100,000 in 60-69 years of age, 1,086.54 per 100,000 in 70-79 years of age, and 1,902.98 per 100,000 in individuals over age 80 years [1]. Moreover, CNS disorders pose a global health burden. The prevalence, deaths, Years of Life Lost (YLLs), Years Lived with Disability (YLDs), and Disability-Adjusted Life-Years (DALYs) by age and sex have been estimated from 195 countries from 1990 to 2016 for 15 neurological disorder categories, including brain and spinal cord trauma. With 9.0 million (95% Uncertainty Interval [UI] 8.8-9.4) deaths or 16.5% (16.1-17.0) of total global deaths, neurological disorders are the second leading cause of death after heart disease and with 276 million (247-308) DALYs and 11.6% (10.7-12.4) of global DALYs, posing as the leading cause of disability [2]. CNS disease is an overarching term for a group of disorders ranging from ischemic, haemorrhagic, neurodegenerative, inflammatory to tumours, and neurodevelopmental disorders. To date, despite the use of various treatment strategies, the therapeutic success has been suboptimal mainly due to the failure of many therapeutic agents in crossing CNS barriers such as the blood-brain barrier and reaching the target sites. Hence, discovering novel bioactive molecules and developing them as therapeutic agents or repurposing FDA-approved drugs/materials to treat CNS disorders is of great interest.
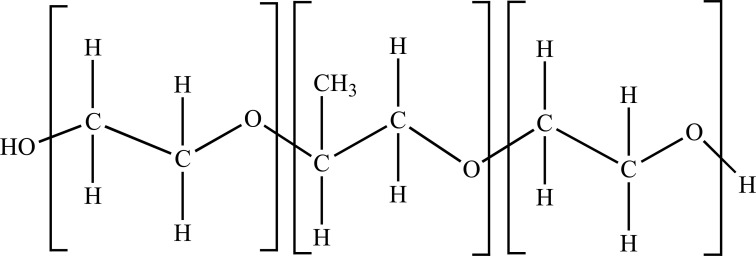
Poloxamers, commonly abbreviated with “P” followed by three digits, are synthetic tri-block copolymers composed of a central hydrophobic chain of polyoxypropylene with two hydrophilic chains of polyoxyethylene and a weight ratio of 4:2:4. The molecular structure results in an amphiphilic surface copolymer [3]. The large poloxamer family with distinct characteristics was generated in the 1950s and made commercially available via BASF Corporation, bearing the proprietary name, Pluronic® block polymers. The molecular properties of poloxamer assemblies and their adsorption properties have been utilized in many applications such as cosmetics and emulsion formulation, effective dispersants for inks/pigments, and versatile anti-biofouling coatings [4]. In the family, Poloxamer 188 (P188) is a biocompatible and non-ionic linear copolymer (Fig. 1). The P188 molecule is composed of two blocks with 38 moieties of hydrophilic polyoxyethylene, flanking one block of 29 moieties of hydrophobic polyoxypropylene. It has a molecular weight of 8400 Daltons, with an HLB value of 29, and is also referred to as PLURONIC F68, FLOCOR, RheothRx, and Kolliphor® P 188 [5]. The FDA approved the copolymer nearly 50 years ago as a therapeutic reagent to reduce viscosity in the blood before transfusions.
Fig. (1).
Structure of Poloxamer 188, with two hydrophilic ends and a hydrophobic core in the middle.
Currently, available literature depicts that P188 has a half-life of 18 hours after injection in humans, and it has been found to be safe when given for up to 72 hours [6]. Furthermore, P188 has a well-tolerated profile upon repeated exposure and is a common component used in many over-the-counter products such as laxatives, toothpaste, and mouthwash. Also, the surfactant properties of P-188 make it highly desirable in cosmetic, pharmaceutical, and industrial applications. There are well-documented in vitro and in vivo studies, which has demonstrated some of the biological effects of P188, especially on its ability to repair damaged cell membranes and sealing defects after various injuries such as skeletal muscle cell membranes rupture induced by ischemia-reperfusion injury [7] and electroporation [8]. Although the mechanism is not entirely understood, its bioactivity could be due to its ability to intercalate with the membrane bilayers and may act by increasing lipid packing density. Most of the available literature also describes the link between the development and evaluation of poloxamer-based formulations or nano-formulations on their usefulness in formulating therapeutic delivery systems and how poloxamers are made amenable to multiple processing routes, serving specific purposes. Poloxamers have been developed into various delivery systems such as hydrogels [9], core-shell vehicles [10], or poloxamer-modified hydrophobic particles [11]. Noticeably, most poloxamers-based studies used other variants of poloxamers in the family, such as Poloxamer 407.
Hence, there exists a literature gap on the effectiveness of P188 in terms of neuroprotection and its association with various CNS disorders in in vitro and in vivo models. Despite the initial successes in laboratories showing the benefits of P188 on neuronal cell-based and animal models, more research is needed to relate with CNS disorders and facilitate translation into human studies as well as elucidating the mechanisms involved. In this systematic review, we aim to address this by exploring and reviewing available literature systematically regarding the utility of P188 in CNS-associated disorders and its neuroprotective potential in order to understand the potential of P188 better as a therapeutic option for severe CNS disorders.
2. MATERIALS AND METHODS
2.1. Search Technique
An extensive literature search was done to conduct a systematic review based on the PRISMA guidelines [12]. The literature search is aimed to summarize the effects of Poloxamer 188 on the central nervous system and related diseases. Five electronic databases were used, namely PubMed, Wiley Online Library, Scopus, ScienceDirect, and Google Scholar, to identify relevant articles. The results were then filtered to include studies between January 2004 and October 2020 to include more recent publications in this review while minimizing the possibility of unintentional exclusion of older studies. The following keywords were searched individually and in combination with the Poloxamer 188: brain, trauma, CNS, neurological disorder, neurodegenerative disease, neuroprotection. Search results from PubMed were exported as nbib files, SCOPUS, ScienceDirect, and Wiley Online Library were individually exported as RIS files, and Google Scholar results were exported using Harzing’s Publish or Perish 7 into RIS files. All the exported files were then imported into EndNote X9.2 to generate a library and exported as a text file in the EndNote Export style. The text file was then imported into Rayyan [13]. A list of unique entries was generated (identified duplicates by the software were automatically excluded), and the search results were screened for their relevance based on their title and abstract.
2.2. Study Selection and Exclusion/Inclusion Criteria
The search was limited to a few criteria; articles published in the English language and original research articles were only included in this review. Abstracts of symposiums and conferences, review articles, books, and patents were excluded mainly due to inadequate information for in-depth evaluation and comparison. Articles that are not relevant to CNS diseases were excluded.
2.3. Data Extraction
The data was obtained, and the titles, as well as abstracts of each article, were compared. The comparison allowed the deletion of any data duplication. PRISMA statement was used to understand and improve the reporting of systematic reviews and meta-analyses related to the use of Poloxamer 188 in CNS-related disorders. A flow diagram was prepared in accordance with the guidelines of PRISMA Transparent reporting of systematic reviews and meta-analyses [12].
3. RESULTS AND DISCUSSION
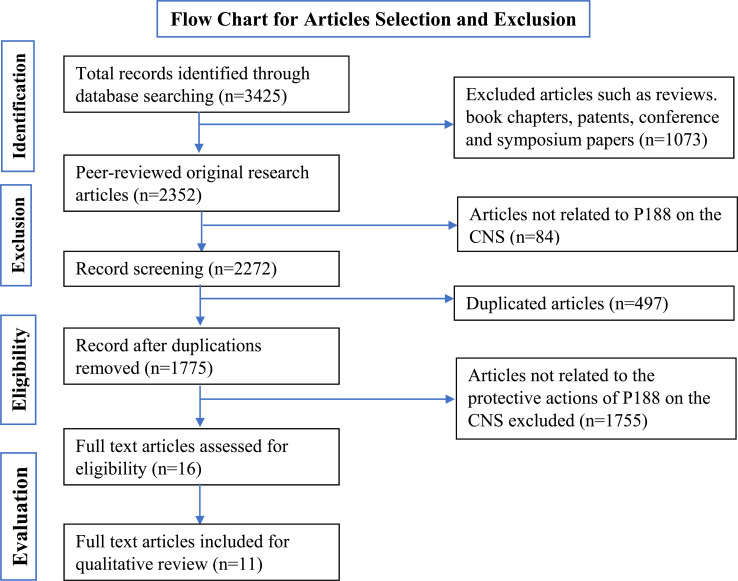
The search based on the keywords mentioned in the methodology yielded 3425 records. Of the 3425 records, 2987 were from Google Scholar, 108 from PubMed, 30 from ScienceDirect, 300 from Wiley Online Library, and zero (0) from SCOPUS. The records were then subjected to exclusion; such as reviews, book chapters, patents, conference, and symposium papers to which 1073 articles were removed. Out of the 2352 original research articles, it was found that 84 of them were not relevant to the aim of the review and did not delineate a clear relationship of the effect of P188 on CNS, and thus were excluded. Further exclusion criteria were applied, which resulted in the removal of 497 duplicates and 1755 articles, which did not have a clear investigation on the protective effects of P188 on the CNS (Fig. 2). The remaining 16 records underwent a full-text evaluation, and five records were removed, whereas 11 other records were found to be relevant.
Fig. (2).
Flow chart of the study selection criteria based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Thus, 11 articles were identified for critical appraisal. They are compiled in Table 1 and discussed in this review. Based on the inclusion criteria [14-24], the final studies acquired were all original articles and included data from mammalian cell culture models and animal models. Potential protective actions of P188 on various models of the CNS were discussed as the secondary aim of this systematic review, whereas our primary aim was focused on determining the relationship between the two. The summary of evidence is illustrated in Table 1, with key components stated for comparison between studies.
Table 1.
Summary of studies on the potential benefits of P188.
| Refs. | Study Design and Model | Testing Dose | Administration Route | Pharmacological Actions | Summary of Findings |
|---|---|---|---|---|---|
| [14] | • In vitro embryonic day 14 rat fetal ventral mesencephalic cells • In vivo 25 female Wistar rats with hemiparkinsonism |
• In vitro 200 µM • In vivo Treatment group: 400000 embryonic day 14 rat ventral mesencephalic cells (single-cell suspension in the presence of P188, 200 µM) Control group: 400 000 cells that were not exposed to P188 |
• In vitro N/A • In vivo Intrastriatal injections/ transplantation for in vivo studies |
P188 protects fetal dopaminergic cells by increasing cell survival and enhances striatal reinnervation and dopaminergic fiber outgrowth into the transplanted striatum in parkinsonian rats | P188 can be an essential adjunct to improve the clinical efficacy of neural transplantation for Parkinson’s disease |
| [15] | • In vitro Human neuroblastoma (SH-SY5Y) cells • In vivo 4 groups x 16 mice in each group, adult male C57BL/6 mice (n = 64) |
• In vitro Pre-treatment with P188 10−6-10−3M for 1 h before 1-methyl-4-phenylpyridinium (MPP+, 100 μM treatment) • In vivo P188 (0.4 g/kg or 0.8 g/kg) 30 min after MPTP administration in the first 5 days and twice a week in the next 21 days |
• In vitro N/A • In vivo Injection via the tail vein |
P188 rescued MPP+-induced lysosomal dysfunction and impaired autophagy flux in SH-SY5Y cells. Also, P188 restored lysosomal membrane integrity in sub-acute MPTP mouse model and MPP+-treated SH-SY5Y cells. P188 ameliorated α-synuclein accumulation and behavioural impairment in chronic MPTP mouse model |
P188 offers neuroprotection against DA neuron damage and decreased protein level aggregation of α-synuclein. P188-mediated lysosomal membrane integrity restoration could be a therapeutic intervention for PD and related neurodegenerative diseases |
| [16] | • In vivo Mature male CD1 mice (n = 424) |
• In vivo P188 (2, 4, 8, 16 mg/ml, dissolved in normal saline) was given 30 min before TBI. |
• In vivo Intravenous tail injection |
P188 pre-treatment attenuates TBI-induced brain edema by restoring and resealing BBB integrity, regulating AQP4 expression, and suppressing TBI-induced neural cell death/ apoptosis through the extrinsic or intrinsic pathway, and improves neurological function |
Poloxamer 188 could restore the intactness of the plasma membrane and play a cytoprotective action in plasmalemma permeability, which could be a potential target for TBI treatment |
| [17] | • In vitro PC2 cells, a subline derived from rat pheochromocytoma cell line PC12 |
• In vitro 100 μM P188 |
• In vitro N/A |
P188 demonstrated acute membrane repair and restored cell viability at 24 h post-injury. The membrane resealing property of P188 offers protection by inhibiting apoptosis and preventing necrosis | The neuroprotection offered by P188 from both necrosis and apoptosis allows it to be a potential treatment in acute membrane damage due to trauma, which leads to upstream of the many signaling cascades, ultimately contributing to subsequent pathology |
| [18] | • In vitro Mouse brain endothelial cell |
• In vitro P188, 0.5 mM |
• In vitro N/A |
P188 was demonstrated to reconstitute the BBB membrane and down-regulated the secretion of matrix metalloproteinases (MMP). P188 mitigates the BBE disruption by alleviating the loss of tight junctions |
Treatment of brain endothelial cells with P188 could serve as a potential treatment in response to traumatic brain injury by repairing the damaged brain endothelium |
| [19] | • In vivo Adult male Wistar rats (n = 23) |
• In vivo Vepoloxamer, (purified P188), 300 mg/kg, for over 60 minutes starting at 2 hours post-injury |
• In vivo Intravenous infusion into tail veins |
Initiation of Vepoloxamer treatment 2 h post-injury significantly improved functional sensorimotor recovery and spatial learning, reduced cortical lesion volume by 20%, and decreased activation of microglia/macrophages and astrogliosis in brain regions such as the injured cortex, corpus callosum, and hippocampus. Vepoloxamer treatment reduced brain and microthrombosis formation |
Vepoloxamer treatment provides neuroprotection and anti-inflammation in rats after TBI and improves functional outcomes. Further research is needed to explore the optimal dose and the mechanisms underlying the beneficial effects of vepoloxamer treatment for TBI |
| [20] | • In vitro Human neuroblastoma (SH-SY5Y) cells |
• In vitro 2 μg P188 (final concentration of 20 ng/μl) |
• In vitro N/A |
P188 promotes cell survival/ viability of oligomer-treated cells in a time-dependent manner. P188 reduces bidirectional leakage of molecules across the damaged membrane from exposure to various kinds of amyloid oligomers | P188 could potentially act as a therapeutic against neuronal membrane damage by temporarily repairing membrane defects and reinforcing the cell membrane permeabilization caused by oligomers |
| [21] | • In vivo Male B6SJL-Tg(SOD1*G93A)1Gur/J mice (n = 26) |
• In vivo Purified P188,10mM |
• In vivo Artificial cerebrospinal fluid [aCSF]) via mini-osmotic pumps. The osmotic pump (delivering 0.15 μl/h) provided 1.5 pM/h of P188 solution or aCSF for 42 days |
P188 treatment in G93ASOD1 transgenic mice ameliorates the pathology by significantly delaying the onset of symptoms, extended survival, and decreased motoneuron death | Using the P188 or a close analog, it targets the mtSOD1 misfolding-induced membrane toxicity, and this may provide a new direction for ALS treatment or may be effective in FALS patients bearing SOD1 mutations |
| [22] | • In vitro Fetal hippocampal neurons |
• In vitro 100 uM P188 |
• In vitro N/A |
P188 protects hippocampal and cerebellar neurons following severe excitotoxic and oxidative injury in vitro through membrane-targeted mechanisms, blocking lipid peroxidation and preventing the loss of intracellular contents |
P188 demonstrated direct restoration of plasma membrane integrity following a physical disruption and strong, membrane-targeted neuroprotection. P188 could be an approach to the treatment of an acute neuronal injury |
| [23] | • In vivo Female Sprague-Dawley rats (n = 30) |
• In vivo 0.2 mM P188 |
• In vivo Intravenous administration 1 hr after crush injury |
Significant improvement in axonal conduction for animals treated with P188. The segment of the axon distal to the site of injury in the P188-treated group increased in nerve fiber density. Intravenous P188 demonstrated more rapid structural and functional nerve recovery | P188 may be a therapeutic approach to treate peripheral nerve injury |
| [24] | • In vitro HT22 murine hippocampal cells • In vivo Male ICR mice |
• In vitro P188 (10-4 M in PBS) • In vivo Short-term outcome experiment P188 (small, medium and large dosages were 0.2, 0.4, 0.8 g/kg body weight, respectively) Longterm-outcome experiment P188 (0.4 g/kg) 5 min before reperfusion, and thereafter, daily administration of P188 (0.4 g/kg) for three weeks post-ischemia/reperfusion |
• In vitro N/A • In vivo Short-term outcome experiment Injection via tail vein 5 min before perfusion Long-term outcome experiment Intraperitoneal injections |
P188 treatment significantly reduced the PI-positive cells with ischemia/reperfusion injury and repaired the HT22 cell membrane rupture. It also significantly decreased infarct volume, ameliorated the brain edema and neurological symptoms 24 h after ischemia/reperfusion. In the long-term outcome study, P188 markedly alleviated brain atrophy and motor impairments and elevated survival rate in 3 weeks of the post-stroke period | P188 offers long-term protection against cerebral ischemia/reperfusion injury by preserving BBB impermeability, inhibiting MMP-9, and membrane resealing |
3.1. P188 and Parkinson’s Disease
Parkinson’s Disease (PD) is a common neurodegenerative disorder of an unknown leading cause, affecting about 1% of the population over 60 years. PD involves selective degeneration of dopaminergic neurons, and affected individuals display clinical symptoms such as rest tremors, rigidity, postural instability, and bradykinesia [25].To date, there have been drug-based interventions such as levodopa for the treatment of PD. However, long-term usage of levodopa as the gold standard of therapy for PD is associated with adverse clinical symptoms such as motor fluctuations and dyskinesis. In addition to the disease progression of PD, the pharmacokinetics of levodopa as well as its efficacy decrease over time [26]. It has been found that neural transplantation, which is suggested to repair the degenerating dopaminergic nigrostriatal pathway, could be a possible new therapeutic approach for PD [27]. Clinical trials have shown that grafted neurons can survive long-term, grow, and establish functional connections in the adult human brain [28]. However, this approach has some limitations, such as a limited supply of fetal dopaminergic neurons and a low survival rate (1- 20%), which pose some significant challenges to be offered as a routine treatment option.
One of the research articles, short-listed in this systematic review, studied ways to address these limitations, especially on the low survival rate, through the adjunctive use of, Poloxamer 188 (P188) to enhance the survival and reinnervation of the fetal dopaminergic cells [14]. The study involved fetal rat dopaminergic tissues and in-vivo female Wistar rats model, respectively, to explore the potential benefits of P188 therapy. Fetal rat dopaminergic tissue was placed in media with or without P188 and then cultured for one week, and transplanted into the striatum of rats with unilateral 6-hydroxydopamine (6-OHDA)-induced lesions of the nigrostriatal dopaminergic pathway. The cells were then examined for survival rate and reinnervation of the host brain. The results showed that fetal rat dopaminergic cells that were exposed to P188 had a significant increase of 2.2-fold in the number of surviving tyrosine hydroxylase-immunoreactive cells in vitro and in vivo. Moreover, parkinsonian rats that received intrastriatal transplants of dopaminergic cells exposed to P188 demonstrated a significant increase in striatal reinnervation of 1.8-fold increase compared to non-P188-treated cells in rats. Based on these findings, the researchers believe that adjunctive use of P188 during fetal dopaminergic cell preparation for transplantation represents an effective method to increase the survival of transplanted cells, which may have vital clinical implications in a neural transplantation strategy for PD.
There is also growing evidence for lysosomal function impairment being a contributing factor to the pathogenesis of PD. It should be noted that dopaminergic neurons are highly vulnerable to aggregate proteins such as α-synuclein, which form inclusion bodies. Lysosomes are responsible for removing aggregate-prone proteins and for the clearance of damaged organelles, such as mitochondria and lysosomes themselves [29]. A study investigated the potential bioactive compounds or drugs that offer neuroprotective properties by enhancing lysosomal function in PD. It was found that P188 (in vitro administration of P188 at 10−6-10−3M) was able to rescue dopaminergic neurons damage due to neurotoxin-induced lysosomal dysfunctions and ameliorate α-synuclein accumulation and behavioural impairment in sub-acute and chronic rodent models (in vivo administration of P188 at 0.4 g/kg or 0.8 g/kg) of PD using (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) MPTP-treated SH-SY5Y cells and MPTP mice models [15]. The researchers demonstrated the critical role played by P188 in maintaining lysosomal membrane integrity by insertion into the membrane and facilitating restoration. P188 also significantly reduced cathepsins leakage. Therefore, they propose that P188-mediated lysosomal membrane integrity restoration could be a potential therapeutic intervention for PD and related neurodegenerative diseases.
3.2. P188 on Traumatic Brain Injury (TBI) and Mechanical Trauma
Traumatic Brain Injury (TBI) is an acquired brain injury resulting from sudden, external, physical damages to the brain or when a foreign object pierces through the skull and causes mechanical trauma to the brain tissue. In 2010, the Centres for Disease Control reported that TBI caused approximately 2.5 million emergency department visits, hospitalizations, and deaths in the United States [30]. In addition, survivors of TBI often possess cognitive deficits and motor dysfunctions. This is because mechanical trauma to neuronal tissues can trigger neuroinflammation, which can induce a loss in membrane integrity and endothelial cell detachment, tight junction disruption, and altered Blood-Brain Barrier (BBB) permeability [31-33]. Although membrane damage is one of the primary pathophysiological changes observed in mechanically injured cells following traumatic injury, reparative treatment or recovery of cell membrane integrity after traumatic injury could be plausible.
Few studies have investigated various treatment approaches that permit the recovery of cell membrane integrity. Firstly, researchers, who believed that intractable brain edema was a leading cause of death following TBI, proposed that the permeability of the plasmalemma played a vital role in neural cell death. They hypothesized that P188, which has membrane sealing property, can reseal the plasmalemma, which could facilitate repair of the neurovascular unit structure, restore BBB integrity, and reduce cerebral edema after TBI [16]. The authors used a TBI mice model and measured cerebral water content to determine the brain edema profile. The mice also underwent other assessments such as neurological tests, BBB integrity assessment, number of TBI-induced neural cell death, and the expression of apoptotic pathway-associated proteins. The results showed that administration of P188, 30 minutes before TBI, restored BBB integrity and attenuated cerebral edema at 2, 4. and 8 mg/mL. Also, P188 reduced neural cell death and improved neurological outcomes in an in vivo model of TBI. Besides, P188 also regulates AQP4 expression and suppresses apoptosis via both extrinsic and intrinsic pathways. The authors concluded that plasmalemma integrity could be a necessary treatment for TBI, and P188 may be the potential drug in that aspect.
Further characterization of the cellular mechanism of P188 and its neuroprotective properties against apoptosis and necrosis in a mechanical injury was investigated using cell-based studies [17]. The results showed that P188 treatment inhibited p38 activation after injury, which confers some neuroprotective effects in abolishing necrotic cell death and decreasing apoptosis. Furthermore, it was reported that the membrane sealing properties of P188 did not only repair the initial membrane damage caused by trauma, which rescued the affected cells from acute death but also inhibited secondary signaling cascades that delayed cell death in affected and neighbouring cells. Therefore, the authors concluded that the application of P188 might hold a promising therapeutic strategy for neuronal recovery from acute and delayed injuries in TBI, TBI-induced diseases, and disorders.
A study aimed at investigating the structural and functional properties of P188 on modulating brain endothelium following mechanical trauma using an in vitro model showed that exposure to P188 recovered permeability and mitigated blood-brain-endothelium disruption [18]. In this study, the researchers cultured a monolayer of brain endothelial cells on a well-characterized synthetic membrane. Then, they quantitatively determined the permeability changes and disorganized tight junctions in response to simulated blast-induced microcavitation. The blast-induced microcavitation event increased the production of Reactive Oxygen Species (ROS) and upregulated Matrix Metalloproteinases (MMP)- 2 & 9, which degraded the tight junctions and increased BBB permeability. However, treatment with P188 (0.5 mM) prevented these events from taking place by suppressing the expressions of MMP- 2 & 9. The authors concluded that the multi-level beneficial effects of P188 could attenuate the extent of TBI injuries and damage to the brain endothelium; hence it is a potential treatment option in this condition.
The following study explored the neuroprotective effects through the administration of vepoloxamer (purified P188) as a form of acute treatment on motor and cognitive functional recovery in a TBI rat model [19]. This study demonstrated that administration of vepoloxamer 2-hours post-injury reduced the size of the lesion formed and decreased brain tissue loss after TBI, which induced functional recovery, including sensorimotor function and cognitive functions after TBI. The data showed that vepoloxamer administration improves sensorimotor and cognitive function by decreasing glial fibrillary acidic protein (GFAP+) astrocyte activation. The treated group demonstrated a dramatic decrease in activated astrocytes and microglia/macrophages, suggesting that the anti-inflammatory effects of vepoloxamer administration possibly contribute to functional recovery in TBI rats. Furthermore, the study demonstrated that P188 attenuates the damage of the brain endothelium, which leads to the reduction of blood-brain barrier permeability, haemorrhage, and microthrombosis in rats after TBI. In summary, intravenous administration of vepoloxamer 2-hours after TBI enhanced functional recovery and reduced neuroinflammation in rats, suggesting the potential value of P188 for TBI treatment.
Trauma-mediated peripheral nerve axon injury heavily involves structural alteration of the axonal plasmalemma cell membrane. Recovery of peripheral nerve function after an axonometric or neurotmetic injury is not always complete and results in increased axonal membrane permeability to electrolytes. Ultimately, this causes metabolic stress and axonal degeneration, if the axon is not sealed. Hence, the study's purpose was to determine whether P188 could facilitate recovery after a peripheral nerve injury using an established axonotmesis model [23]. Intravenous administration of P188 (0.2 mM) can accelerate distal axon recovery after sciatic nerve crush injury in female Sprague-Dawley rats. Their results have shown a significant improvement in axonal conduction for animals treated with P188, and the segment of axon distal to the site of injury in the P188-treated group demonstrated a significant increase in nerve fiber density. Hence, P188 can be a therapeutic agent for the rapid structural and functional recovery of peripheral nerve axons after trauma-mediated injury.
3.3. P188 Rescues Cells from Neurotoxicity Caused by Misfolded Proteins
Based on current literature, protein aggregation and misfolding have been shown to be critical features of various amyloid-related neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and prion diseases. Moreover, Amyotrophic Lateral Sclerosis (ALS) cases are caused by multiple mutations of the superoxide dismutase 1 (SOD1), leading to neurotoxicity. Hence, it is critical to focus on the misfolding of the mutant protein in these neurodegenerative diseases. Factors that contribute to protein aggregation and misfolding include gene mutations, dysfunctional chaperone machinery, and defective clearance system, which would lead to the accumulation of toxic species [20, 21, 34]. Indeed, misfolded and aggregated proteins that disrupt the membrane causing toxicity could be the primary target to stop the disease's onset and/or progression.
The ability of P188 to repair cell membranes and rescue cell viability following permeabilization by a variety of amyloid oligomers was assessed in a cell-based study. In this study, there was a 16% increase in cell survival of SH-SY5Y cells treated with 2 µg P188 following 15 minutes incubation with Aβ42 compared to cells that were not rescued with P188 [20]. The researchers reported that exposure to amyloid oligomer caused defects in the lipid bilayer, which affected the membrane permeability, and early administration of P188 reduced the bidirectional leakage of molecules across the damaged membrane, thereby reducing membrane permeability. The researchers propose that P188 temporarily repairs these membrane defects induced by exposure to amyloid oligomers and reinforces the cell membrane by inserting itself into the damaged membrane and increasing local lipid packing density. The action temporarily plugs the defect, thereby preventing uncontrolled ionic flux and cellular contents. Subsequently, the cell's innate repair mechanisms can be triggered to patch the disrupted membrane via exocytic vesicle fusion to reseal the plasma membrane and restore surface pressure and lipid density. It was suggested that P188 could be a potential therapeutic agent for neuronal membrane damage and neurotoxicity caused by oligomer interaction with cell membranes.
Another study looked into familial ALS. Based on previous literature, mtSOD1 misfolding enables the protein to form a tetrameric channel-like structure in lipid bilayers, which can exhibit toxic channel activity, leading to motor neuronal death. The researchers have investigated the potential effects of P188 in targeting membrane toxicity caused by misfolded SOD1 [21]. Their results showed that P188 could reverse membrane toxicity and ameliorate disease in G93ASOD1 transgenic mice, an ALS mouse model. The author suggested that P188 may loosely interact with certain membrane surfaces while forming a molecular adlayer to protect the membranes from physicochemical damage. Their findings indicated the possibility of a new therapeutic approach for rescuing and protecting motor neurons in ALS from neurotoxicity induced by SOD1 oligomers through cellular membrane stabilization.
3.4. P188 Provides Neuroprotection
Excitatory amino acid receptor activation and reactive oxygen species production are essential indicators of neuronal death and may lead to loss of membrane integrity. In this study, researchers investigated the potential of P188 to offer membrane-targeted neuroprotection following different injuries or assaults [22]. Their results demonstrated that administration of P188 at 100 µM provides significant neuroprotection to the hippocampal cerebellar neurons, which have been exposed to intense excitotoxic and oxidative injury in the in vitro model. Rescued neurons showed the regular function of intracellular enzymes, cell surface receptors, voltage-gated channels, and calcium homeostasis. Apart from its insertion mechanism into the lipid bilayer, P188 also increased the surface area of the cell without receptor blockade. P188 also prevented the loss of intracellular contents by the restoration of plasma membrane integrity following plasma membrane electroporation. Furthermore, P188 demonstrated its neuroprotection by blocking lipid peroxidation. These observations enabled the researchers to believe that amphiphilic tri-block copolymers such as P188 offer potent and membrane-oriented neuroprotection, representing a new therapeutic approach.
Stroke is the abrupt blockage of constant blood flow to the brain, leading to loss of neurological functions. Out of the many forms of stroke, ischemic stroke is the most common one, which involves an acute interruption of arterial blood flow to the brain. Cell membranes will experience loss of structural integrity and then experience necrotic cell death. A study was conducted to determine if P188 offered neuroprotection on cerebral ischemia-reperfusion injury and aimed to elucidate its neuroprotective effects on maintaining the cell membrane integrity and the BBB [24]. The results demonstrated that daily intraperitoneal administration of P188 improves long-term neurological outcomes through the motor behavioural tasks assessment. The insertion property of P188 enabled it to preserve the HT22 plasma membrane and BBB integrity and demonstrate neuroprotection through reduced cerebral ischemic or oxygen-glucose deprivation/reoxygenation-induced damage by preventing activation of MMP-9 and leakage. They concluded that the neuroprotection offered by P188 involves a multi-level mechanism against ischemic tissue injury of cerebral neurons; thus, it could be an excellent therapeutic agent to explore.
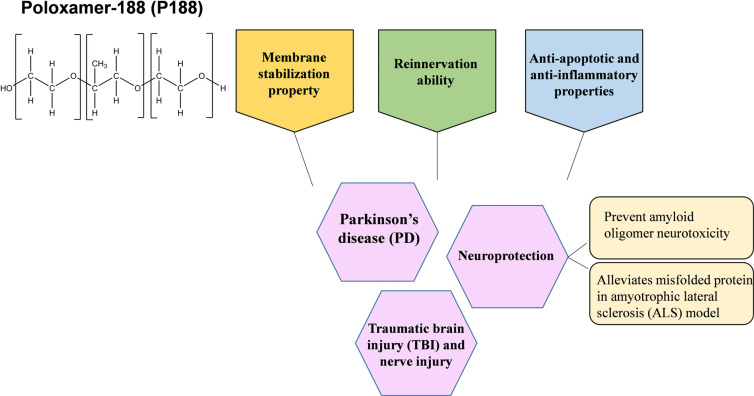
In a nutshell, the main finding of this systematic review supports a promising relationship between P188 and CNS disorders. Based on the 11 studies selected for this systematic review, a few deductions can be made. Firstly, the effects of P188 could possibly benefit patients who experience a form of CNS disorder. The compiled articles revealed improvements in their respective experimental models in different aspects such as neuroprotection and cell viability, preventing neurotoxicity induced by protein aggregation, and restoration of the synaptic network or integrity of the BBB. Secondly, the benefits of P188 seen in their studies could lie in a proposed primary mode of mechanism. The proposed mode of mechanism is that P188 has the ability to intercalate with the membrane bilayers and may act by increasing lipid packing density. Through this, its beneficial activities can be seen in terms of maintaining plasma membrane integrity and facilitating restoration in the synaptic network. Apart from its membrane stabilization property, P188 has also been said to affect specific regulatory pathways, have anti-inflammatory and anti-apoptotic characteristics, and reinnervation ability. However, more research is definitely needed to explore its list of potential bioactivities. The deductions together support the aim of this review on the relationship between P188 and CNS disorders. Overall, the potential benefits of P188 administration are summarized (Fig. 3).
Fig. (3).
Potential mechanisms of Poloxamer 188 in CNS disorders.
4. FUTURE DIRECTIONS
All 11 articles discussed in this systematic review presented very encouraging results regarding the potential beneficial effects of P188 on associated CNS disorders. The promising results can then prompt human studies to be conducted to learn more about P188, but this is a major limitation across all 11 articles in the pre-clinical stages of using cells and animal models. Translation into human studies would mean it has to cross the species barrier and the in vitro stages, requiring a lot of time. Nonetheless, human studies are only of significance when mechanistic aspects of P188 are fully understood in the pre-clinical stages. Studies that are utilizing animal models could also address limitations by including long-term studies to compare and uncover the long-term effects of P188 intervention and the discontinuation of the intervention. This investigation could provide crucial information for the future treatment of CNS disorders.
Another discrepancy across the articles is the usage of commercial-grade P188 and the purified P188, vepoloxamer. A study demonstrated the differential effects between commercial-grade P188 and purified P188 on renal function, whereby it was revealed that removal of low molecular weight substances is associated with substantially less renal dysfunction, and purified P188 improves renal safety profile [35]. However, there is still a scarcity of data on the differences between P188 and vepoloxamer in other diseases and CNS disorders. Hence, this warrants further investigations on the potentially different effects, potency level, and other functional characteristics between the two, in vitro and in vivo, as various manufacturing processes may lead to loss or gain in the bioactivity of P188. It would also be a fair and easy comparison when the study includes both types of P188.
Studies on the potential effects of P188 can be further improved by incorporating a more detailed time-dependent and concentration-dependent analysis. Although higher concentration may risk cellular survival, it may also exhibit unknown effects that could be note-worthy to increase the library of research on P188. If P188 is to be made into a drug that penetrates the BBB, it would be exciting and important to note any extreme effects from administration of the lowest concentration to highest concentration and the earlier point of administration to the latest from time of injury or assault. This provides more information on the extent of effectiveness before, during, and after the P188 administration. Also, regulatory and expression studies are vital. More research should explore the regulatory pathways and protein expression after P188 administration, which can further elucidate the mechanisms affected by P188 administration.
A study concluded that RheothRx (Glaxo Wellcome Inc, Research Triangle Park, NC; poloxamer 188) injection into 50 patients with sickle cell disease was well tolerated and had no clinically significant differences in adverse experiences [6]. However, there is no information on reproductive and developmental toxicity, so its use during pregnancy and lactation cannot be recommended, as well as potential drug-drug interaction has not been well documented. An updated safety and toxicity profile of P188 and its variants is also required along with its pharmacokinetics and pharmacodynamic profiles to improvise the information pool about P188. The findings provided by this systematic review establish a promising association between P188 and CNS disorders, but further studies are definitely needed to corroborate and deliver more insight into the underlying mechanism and pathophysiology of this relationship in order to advance the current approach in treating different CNS disorders.
CONCLUSION
In conclusion, the literature selected in this systematic review supports the notion of the potential benefits of P188 against CNS disorders. P188 exogenous intervention may play a major role in the alteration or regulation of CNS disorders. This evidence-based systematic review proposes that P188 could exhibit a protective role by modulating membrane permeability and integrity, alleviating downstream effects that contribute to the disease's pathology, promoting reinnervation in damaged nerves, and improving conditions like cerebral ischemia, traumatic brain injury, and Parkinson’s disease. However, this review also identifies that there is still much to discover about the mechanisms of P188 and its role in CNS disorders. Once the current limitations, seen within these studies, such as methodological insufficiencies, duration of studies, and lack of relevant scientific information on the P188 profile, are addressed overcome, a possible effective and safe treatment could potentially be developed in future studies. Hence, enhancing our present understanding of the effects of P188 in CNS disorders will greatly improve this field of research.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
PRISMA guideline were followed fot the study.
FUNDING
The project is funded by the Ministry of Higher Education, Fundamental Research Grant Scheme (FRGS/1/2020/ SKK0/MUSM/02/6).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Pringsheim T., Fiest K., Jette N. The international incidence and prevalence of neurologic conditions: how common are they? Neurology. 2014;83(18):1661–1664. doi: 10.1212/WNL.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll W.M. The global burden of neurological disorders. Lancet Neurol. 2019;18(5):418–419. doi: 10.1016/S1474-4422(19)30029-8. [DOI] [PubMed] [Google Scholar]
- 3.Moloughney J.G., Weisleder N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent Pat. Biotechnol. 2012;6(3):200–211. doi: 10.2174/1872208311206030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodratti A.M., Alexandridis P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018;9(1): ,E11. doi: 10.3390/jfb9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry D.J., Wright D.A., Lee R.C., Kang U.J., Frim D.M. Poloxamer 188 volumetrically decreases neuronal loss in the rat in a time-dependent manner. Neurosurgery. 2004;55(4):943–948. doi: 10.1227/01.NEU.0000137890.29862.2C. [DOI] [PubMed] [Google Scholar]
- 6.Adams-Graves P., Kedar A., Koshy M., Steinberg M., Veith R., Ward D., Crawford R., Edwards S., Bustrack J., Emanuele M. RheothRx (poloxamer 188) injection for the acute painful episode of sickle cell disease: A pilot study. Blood. 1997;90(5):2041–2046. doi: 10.1182/blood.V90.5.2041. [DOI] [PubMed] [Google Scholar]
- 7.Murphy A.D., McCormack M.C., Bichara D.A., Nguyen J.T., Randolph M.A., Watkins M.T., Lee R.C., Austen W.G. Jr. Poloxamer 188 protects against ischemia-reperfusion injury in a murine hind-limb model. Plast. Reconstr. Surg. 2010;125(6):1651–1660. doi: 10.1097/PRS.0b013e3181ccdbef. [DOI] [PubMed] [Google Scholar]
- 8.Lee R.C., River L.P., Pan F.S., Ji L., Wollmann R.L. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc. Natl. Acad. Sci. USA. 1992;89(10):4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie M., Betts D., Suh A., Bui K., Kim L.D., Cho H. Hydrogel-based drug delivery systems for poorly water-soluble drugs. Molecules. 2015;20(11):20397–20408. doi: 10.3390/molecules201119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung Y.W., Lee H., Kim J.Y., Koo E.J., Oh K.S., Yuk S.H. Pluronic-based core/shell nanoparticles for drug delivery and diagnosis. Curr. Med. Chem. 2013;20(28):3488–3499. doi: 10.2174/09298673113209990036. [DOI] [PubMed] [Google Scholar]
- 11.Shubhra Q.T.H., Tóth J., Gyenis J., Feczkó T. Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption. Colloids Surf. B Biointerfaces. 2014;122:529–536. doi: 10.1016/j.colsurfb.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn M., Mukhida K., Sadi D., Hong M., Mendez I. Adjunctive use of the non-ionic surfactant Poloxamer 188 improves fetal dopaminergic cell survival and reinnervation in a neural transplantation strategy for Parkinson’s disease. Eur. J. Neurosci. 2008;27(1):43–52. doi: 10.1111/j.1460-9568.2007.05991.x. [DOI] [PubMed] [Google Scholar]
- 15.Dong H., Qin Y., Huang Y., Ji D., Wu F. Poloxamer 188 rescues MPTP-induced lysosomal membrane integrity impairment in cellular and mouse models of Parkinson’s disease. Neurochem. Int. 2019;126:178–186. doi: 10.1016/j.neuint.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Bao H.J., Wang T., Zhang M.Y., Liu R., Dai D.K., Wang Y.Q., Wang L., Zhang L., Gao Y.Z., Qin Z.H., Chen X.P., Tao L.Y. Poloxamer-188 attenuates TBI-induced blood-brain barrier damage leading to decreased brain edema and reduced cellular death. Neurochem. Res. 2012;37(12):2856–2867. doi: 10.1007/s11064-012-0880-4. [DOI] [PubMed] [Google Scholar]
- 17.Serbest G., Horwitz J., Jost M., Barbee K. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. FASEB J. 2006;20(2):308–310. doi: 10.1096/fj.05-4024fje. [DOI] [PubMed] [Google Scholar]
- 18.Inyang E., Abhyankar V., Chen B., Cho M. Modulation of in vitro brain endothelium by mechanical trauma: Structural and functional restoration by poloxamer 188. Sci. Rep. 2020;10(1):3054. doi: 10.1038/s41598-020-59888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Chopp M., Emanuele M., Zhang L., Zhang Z.G., Lu M., Zhang T., Mahmood A., Xiong Y. Purified poloxamer 188. J. Neurotrauma. 2018;35(4):661–670. doi: 10.1089/neu.2017.5284. [DOI] [PubMed] [Google Scholar]
- 20.Mina E.W., Lasagna-Reeves C., Glabe C.G., Kayed R. Poloxamer 188 copolymer membrane sealant rescues toxicity of amyloid oligomers in vitro. J. Mol. Biol. 2009;391(3):577–585. doi: 10.1016/j.jmb.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Riehm J.J., Wang L., Ghadge G., Teng M., Correa A.M., Marks J.D., Roos R.P., Allen M.J. Poloxamer 188 decreases membrane toxicity of mutant SOD1 and ameliorates pathology observed in SOD1 mouse model for ALS. Neurobiol. Dis. 2018;115:115–126. doi: 10.1016/j.nbd.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks J.D., Pan C.Y., Bushell T., Cromie W., Lee R.C. Amphiphilic, tri-block copolymers provide potent membrane-targeted neuroprotection. FASEB J. 2001;15(6):1107–1109. doi: 10.1096/fj.00-0547fje. [DOI] [PubMed] [Google Scholar]
- 23.Prescher H., Ling M., Lee R. Copolymer surfactant poloxamer 188 accelerates post-axonotemetic sciatic nerve regeneration. Regen. Eng. Transl. Med. 2020 doi: 10.1007/s40883-020-00174-y. [DOI] [Google Scholar]
- 24.Gu J.H., Ge J.B., Li M., Xu H.D., Wu F., Qin Z.H. Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PLoS One. 2013;8(4): ,e61641. doi: 10.1371/journal.pone.0061641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess C.W., Hallett M. The phenomenology of Parkinson’s disease. Semin. Neurol. 2017;37(2):109–117. doi: 10.1055/s-0037-1601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewitt P.A. Levodopa for the treatment of Parkinson’s disease. N. Engl. J. Med. 2008;359(23):2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 27.Mendez I., Sanchez-Pernaute R., Cooper O., Viñuela A., Ferrari D., Björklund L., Dagher A., Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(Pt 7):1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J. Intern. Med. 2016;279(1):30–40. doi: 10.1111/joim.12415. [DOI] [PubMed] [Google Scholar]
- 29.Radulovic M., Schink K.O., Wenzel E.M., Nähse V., Bongiovanni A., Lafont F., Stenmark H. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018;37(21): ,e99753. doi: 10.15252/embj.201899753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor C.A., Bell J.M., Breiding M.J., Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill. Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettus E.H., Christman C.W., Giebel M.L., Povlishock J.T. Traumatically induced altered membrane permeability: Its relationship to traumatically induced reactive axonal change. J. Neurotrauma. 1994;11(5):507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- 33.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14(2):215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Gonzalez I., Soto C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011;22(5):482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emanuele M., Balasubramaniam B. Differential effects of commercial-grade and purified poloxamer 188 on renal function. Drugs R D. 2014;14(2):73–83. doi: 10.1007/s40268-014-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]