Abstract
Uncoupling protein 2 (UCP2) is a mitochondrial protein that acts as an anion carrier. It is involved in the regulation of several processes, including mitochondrial membrane potential, generation of reactive oxygen species within the inner mitochondrial membrane and calcium homeostasis. UCP2 expression can be regulated at different levels: genetic (gene variants), transcriptional [by peroxisome proliferator-activated receptors (PPARs) and microRNAs], and post-translational. Experimental evidence indicates that activation of UCP2 expression through the AMPK/PPAR-α axis exerts a protective effect toward renal damage and stroke occurrence in an animal model of ischemic stroke (IS) associated with hypertension. UCP2 plays a key role in heart diseases (myocardial infarction and cardiac hypertrophy) and metabolic disorders (obesity and diabetes). In humans, UCP2 genetic variants (-866G/A and Ala55Val) associate with an increased risk of type 2 diabetes mellitus and IS development. Over the last few years, many agents that modulate UCP2 expression have been identified. Some of them are natural compounds of plant origin, such as Brassica oleracea, curcumin, berberine and resveratrol. Other molecules, currently used in clinical practice, include anti-diabetic (gliptin) and chemotherapeutic (doxorubicin and taxol) drugs. This evidence highlights the relevant role of UCP2 for the treatment of a wide range of diseases, which affect the national health systems of Western countries. We will review current knowledge on the physiological and pathological implications of UCP2 with particular regard to cardiovascular and metabolic disorders and will focus on the available therapeutic approaches affecting UCP2 level for the treatment of human diseases.
Keywords: UCP2, stroke, cardiovascular disease, diabetes, obesity, therapeutics
1. INTRODUCTION
The uncoupling proteins (UCPs) family includes four components [1, 2]. UCP1, or thermogenin, was the first member of the family to be discovered in hibernators and small rodents, where UCP1 is highly expressed in the mitochondria of brown adipose tissue (BAT). Herein, UCP1 plays a physiological role in body temperature regulation by fat storage and mobilization control [3, 4]. UCP1 knock-out mice are unable to regulate the body temperature [5]. The other components of UCPs family (UCP2-UCP5) were characterized later on [6, 7]. UCP2 is ubiquitously expressed. UCP3 is more expressed in the skeletal muscle. UCP4 and UCP5 are present in the brain. The different tissue expression of UCPs reflects different functions. In fact, whereas UCP1 acts as a thermogenesis regulator in the BAT of vertebrates [8], UCP2 uncouples the proton flux and the ATP (adenosine triphosphate) synthesis and converts the energy generated in this process into heat instead of ATP. Moreover, the UCP2-UCP5 members of the family have a physiological role as reactive oxygen species (ROS) scavengers and regulators of ATP-dependent processes, including secretion [7, 9]. Interestingly, UCP2 and UCP3 knockout mice show increased ROS production [10]. This evidence has important implications for human health. In fact, it is known that high concentrations of ROS contribute to the development of several diseases [10]. As a matter of fact, evidence obtained in both animal models and humans demonstrates that UCPs are involved in the pathogenesis of metabolic, neurodegenerative and cardiovascular diseases. High levels of UCP2 exert a protective effect in atherosclerosis [11], heart diseases [myocardial ischemia/reperfusion (I/R) injury] [11] and ischemic stroke (IS) [12]. Consistently, a reduced expression of UCP2 correlates with increased renal and cerebrovascular damage in the stroke-prone spontaneously hypertensive rat (SHRSP) model [13, 14]. On the other hand, increased UCP2 expression inhibits insulin secretion from pancreatic β-cells and favors the development of type 2 diabetes mellitus (T2DM).
In this review, we describe the main physiological and pathological implications of UCP2 with a focus on its involvement in major cardiovascular and metabolic disorders, based on both experimental and human evidence. Furthermore, we discuss the therapeutic implications of UCP2 and the compounds available for the clinical use affecting UCP2 level.
2. PHYSIOLOGICAL FUNCTIONS OF UCP2
The sequence of human UCP2 is very similar to that of rat and mouse (99% and 95% of homology, respectively) [15]. The strong conservation of the gene sequence between the different species indicates that this protein exerts an important physiological role in all organisms. Transcription and translation, as well as UCP2 activity, may be controlled by many stimuli such as free fatty acids (FFA), retinoic acid, lipopolysaccharides, superoxide anion and hormones, including leptin and thyroid hormones [16, 17]. The main physiological role of UCP2 is related to the control of mitochondrial ROS production [9, 18]. Additional evidence shows that UCP2 plays an important role in the process of oxidative phosphorylation, in the regulation of ATP synthesis, in the maintenance of redox potential membrane, in the control of calcium homeostasis, cell apoptosis, and death [17, 19].
2.1. Oxidative Phosphorylation, ATP Synthesis and ROS Production
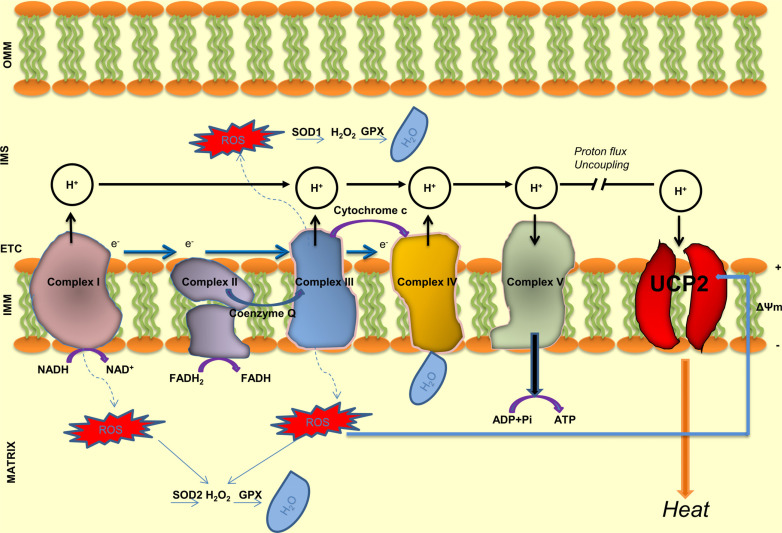
Oxidative phosphorylation is a complex process that, by a series of chemical reactions, generates highly energetic molecules of ATP. This process takes place within the inner mitochondrial membrane through the electron transport chain (ETC) made by five enzymatic complexes (labeled I to V) (Fig. 1). During this process, a flow of electrons is generated by electron transporters starting from NADH (Complex I) or FADH2 (Complex II). The Complex III (cytochrome oxidoreductase) and the Complex IV (cytochrome c oxidase) also pump protons through the membrane in the intermembranes space. The electrochemical gradient, together with the return of protons across the inner membrane, activates the last enzyme of the chain, the ATP synthase (Complex V). The latter produces ATP through the reaction ADP + Pi + 3H+ ⇌ ATP+H2O. The amount of ATP molecules generated from glucose catabolism varies across species and is related to various cellular processes. Under physiological conditions, when the energy demand of the cells is low, UCP2 dissociates the oxidation of different substrates (NADH, coenzyme Q, cytochrome c) from ADP phosphorylation. It consequently reduces the ATP synthesis and the electrochemical membrane potential (ΔΨ) [20]. UCP2, by decreasing ΔΨ, protects the cells from ROS damage and death. The energy generated in this process is dissipated in the form of heat [21]. As a consequence of the reduced ATP synthesis by UCP2, pancreatic β-cells from UCP2 knockout mice show an increased ATP, ATP/ADP ratio and insulin secretion, the latter due to the blockade of ATP-sensitive channels and the opening of the voltage-dependent Ca2+ channel involved in insulin secretion [22-24].
Fig. (1).
Schematic representation of electron transport chain (ETC), mitochondrial enzymatic complexes and UCP2 functions. During the respiratory process, the energy generated from the oxidation of various substrates including glucose, is conserved in reduced molecules such as NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide). Electron transporters and protonic pumps create a negative membrane potential and a proton-motive force across the inner mitochondrial membrane which activate the last enzyme of the chain, the ATP synthase. The latter, by the reaction between ADP (adenosine diphosphate) and inorganic phosphate (Pi) generates ATP (adenosine triphosphate). When the energy requirement of the cell is reduced, protons can return into the mitochondrial matrix across the UCP2 protein with reduction of ATP synthesis and heat release. ROS generated during the respiratory process are first reduced at H2O2 and then at H2O from several antioxidant enzymes such as SOD1 (superoxide dismutase 1), SOD2 (superoxide dismutase 2) and GPx (glutathione peroxidase).
Mitochondria are the major source of ROS (mainly superoxide or hydrogen peroxide) in the aerobic cells [25]. They are generated at several sites of ETC, although the two main sites are Complex I (on the matrix side) and Complex III (on both the intermembrane and the matrix side) (Fig. 1) [9]. At physiological concentration, ROS act as signaling factors, whereas high levels exert detrimental effects. Superoxide anion (O2-) is a dangerous element that rapidly interacts with the lipid component of the cell membrane, causing lipid peroxidation, protein and DNA damage. ROS level is kept under the control of few antioxidant systems such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR). ROS level is also influenced by ΔΨ [17] and is modulated by UCP2 activity [18]. UCP2, by subtracting electrons from ETC, reduces superoxide formation and limits the cell damage. Animal models lacking UCP2 show higher ROS level. Conversely, overexpression of UCP2 exerts a protective function against the oxidative stress damage [18, 26].
2.2. Calcium Regulation
The calcium ion (Ca2+) is an important intracellular messenger, which acts as a regulator of cellular processes including the neurotransmitters release from neurons and the contraction of all muscle cell types. In addition, it is a fundamental cofactor of many enzymes. Ca2+ is also important for maintaining the difference in potential across membranes of excitable cells. Mitochondria and the endoplasmic reticulum sequester Ca2+ to ensure that homeostasis is maintained [27]. Cytosolic Ca2+ level is directly related to ΔΨ. Since UCP2 is involved in the regulation of ΔΨ, it can influence the calcium dynamic. In normal conditions, ΔΨ equals 30mV. However, this value can undergo small changes and it can alter important cellular functions in the presence of increased UCP2 level [28]. A drastic reduction of Ca2+ can influence cell signaling. On the other hand, a massive flow of Ca2+ in the cytosol can increase neuronal excitotoxicity, as it happens following IS or in neurodegenerative diseases [29, 30]. An excess of Ca2+ can induce apoptosis and necrosis [31].
2.3. Regulation of Cell Apoptosis and Death
The mitochondrion and some of the mitochondrial proteins play a key role in the control of apoptosis and cell death. Several studies demonstrated that UCP2 regulates mitochondrial apoptotic signaling. A recent study performed in a cerebral ischemia/reperfusion (I/R) mouse model demonstrated that UCP2 deletion exacerbated cell apoptosis [32]. Conversely, in the middle cerebral artery occlusion (MCAO) rat model, UCP2 inhibits cell death linked to mitochondrial dysfunction by inducing depolarization of the mitochondrial inner membrane, subsequent Ca2+ intake and ROS reduction [17, 33]. On the other hand, overexpression of UCP2 inhibits mitochondrial death pathway in primary cultures of neonatal rat cardiac ventricular myocytes [34].
3. IMPLICATIONS OF UCP2 IN PRE-CLINICAL MODELS AND HUMAN DISEASES
UCP2 is implicated in a wide range of physiological and pathological conditions including aging [35] obesity [36], inflammation [26], cerebral and myocardial ischemia [14, 37], hyperinsulinemia [36, 38]. With regard to aging, we reported an age-related decrease of UCP2 expression in the brain, heart and kidneys of the SHRSP model, likely involved into the higher predisposition to the hypertensive organ damage development of this strain [39]. Ren et al. also highlighted the relationship between UCP2, aging and cardiac dysfunction. UCP2 level decreased with advanced aging along with abnormal autophagy, mitophagy, and mitochondrial integrity. UCP2 level was lower in isolated cardiomyocytes from old mice compared to young ones [40, 41]. Moreover, aging was reported to suppress mitochondrial proteins, including UCP2, with a more pronounced effect in the aged mitochondrial aldehyde dehydrogenase (ALDH2) deleted mouse hearts [42]. This evidence clearly implies that increased ROS level, along with decreased UCP2 activity, contribute to the aging process.
3.1. UCP2 and IS
IS is the most common form of stroke and it results from cerebral blood vessel occlusion by an embolus or a thrombus. The initial event is followed by depolarization of neuronal membrane, ion imbalance, such as K+ and Ca2+, and a massive release of glutamate from the presynaptic vesicles into the extracellular space. Higher Ca2+ level increases both ROS production and caspase activation causing degradation of membrane phospholipids, structural proteins and nucleic acids. An increase of glutamate level, instead, contributes to excitotoxic neuronal death through overstimulation of glutamate receptors. All these events contribute to amplify cellular damage [43-45].
The upregulation of UCP2 exerted a neuroprotective role in the MCAO mouse and rat models, mainly due to its ability to keep low the depolarization level of the mitochondrial inner membrane (approximately 10 mV). As a consequence, the ROS production, the calcium uptake and the induction of cell death-inducing factors (caspases and other apoptosis mediators) decreased [30]. Conversely, low UCP2 expression induced a membrane hyperpolarization with an increase of ΔΨ and consequent ROS accumulation [26, 33].
We previously demonstrated that both cerebral and renal expression of UCP2 were downregulated in the SHRSP model when fed with a stroke permissive high-sodium/low potassium diet (Japanese-style diet, JD), as compared to the stroke-resistant strain (SHRSR). In this experimental condition, we found that UCP2 downregulation was induced by an upregulation of the microRNA-503 [37]. The treatment of JD-fed SHRSP with a Brassica oleracea (BO) sprout extract or with fenofibrate, a drug used to treat abnormal blood lipid levels, significantly decreased brain expression of mir-503, rescued UCP2 expression and consequently reduced ischemic brain damage [37, 46, 47]. Interestingly, the epigenetic control of UCP2 plays a role also in the kidney of JD-fed SHRSP, where renal damage precedes stroke events. In fact, we demonstrated that renal UCP2 expression was regulated by microRNA-34a and microRNA-24 [48] (Table 1).
Table 1.
Pathological implications of UCP2 in animal models.
| Animal Model | UCP2 Modulation | Cellular Effects | Phenotypes | Refs. |
|---|---|---|---|---|
| Stroke | ||||
| MCAO mouse | downregulation | ↑membrane hyperpolarization ↑ΔΨ ↑ROS |
Ischemic stroke | [26] |
| JD-fed SHRSP | downregulation | ↑ROS ↑inflammation |
Ischemic stroke | [38, 48] |
| Heart diseases | ||||
| I/R rat model | downregulation | ↑ROS ↑cell death ↓autophagy |
Cardiac ischemia | [34, 61] |
| Wistar rat running on a treadmill | downregulation | ↑extracellular matrix remodeling genes ↑cytoskeletal genes ↑protein synthesis genes ↑inflammatory genes |
Compensated cardiac hypertrophy | [62] |
| Wistar rat with ligation of left anterior coronary artery | upregulation | ↑cytoskeleton/extracellular matrix organization genes ↑cell death/apoptosis genes ↑cell growth/proliferation genes ↓β-oxidation of fatty acids genes |
Decompensated cardiac hypertrophy | [62, 63] |
| ET(A) receptor cardiac-specific knockout mice exposed to cold stress | upregulation | ↑oxidative stress ↑apoptosis ↑autophagy |
Cold stress-induced cardiac hypertrophy | [64, 65] |
| Obesity, diabetes, metabolic syndrome | ||||
| UCP2 knockout mice | downregulation | ↑insulin secretion | Obesity | [23] |
| UCP2 obese mice | upregulation | β-cell dysfunction ↓insulin secretion |
Diabetes | [23] |
| MS rat model treated with natural compounds | upregulation | ↑PPAR-α ↓oxidative stress |
↓dyslipidemia ↓blood pressure level ↓insulin resistance ↓obesity risk |
[89, 91] |
Abbreviations: MCAO=middle cerebral artery occlusion; SHRSP=stroke-prone spontaneously hypertensive rat; JD=Japanese diet; (ΔΨ)=membrane electrical potential; I/R=ischemia/reperfusion; PPAR-α=type α-peroxisome proliferator-activated receptor.
Several studies showed that the antioxidant activity of UCP2 was mediated by the peroxisome expression of many genes. In particular, PPAR-α activation by fenofibrate, BO juice and curcumin administration exerted a protective role toward stroke and renal damage in JD-fed SHRSP [49, 50]. Activation of PPAR-γ can also modulate UCP2 expression in different tissues [51]. At the brain level, several PPAR-γ agonists, including troglitazone, an antidiabetic drug that improves insulin resistance in peripheral tissues, led to an increase of UCP2 level, improved the in vitro motoneurons survival [52] and the outcome following cerebral ischemia [53, 54]. This evidence confirmed that PPARs play a fundamental role in modulating the beneficial effects of UCP2 [46].
The role of UCP2 has also been investigated in relation to stroke in humans. UCP2 and UCP5 expression was analyzed in brain ischemic lesions where they appeared significantly elevated as compared to the intact area. The upregulation of both UCPs was considered as an adaptive response of neuronal cells to the ischemic insult [55].
In recent years, some human studies identified different single nucleotide polymorphisms (SNPs) within UCP2 in relation to stroke occurrence. The -866G>A (rs659366) is a functional SNP located within the promoter region of UCP2, probably at the level of one or more transcriptional factors binding sites [56]. The A allele variant at -866 position is associated with increased UCP2 expression. Many studies demonstrated that the G allele is associated with lower UCP2 expression and higher ROS accumulation [57]. This gene variant was related to an increased risk of IS in Chinese women with T2DM in a 4-year prospective study [58, 59]. In a recent study, the relationship between this SNP and the functional prognosis was investigated in patients with embolic IS after early recanalization. Patients carrying the AA genotype showed a better functional outcome compared to patients harboring the AG or GG genotypes. This result suggests that the AA genotype acts as an independent marker of favorable prognosis in IS patients after recanalization of proximal MCAO [60] (Table 2).
Table 2.
Main UCP2 SNPs and their pathological implications in human diseases.
| SNP | Molecular Effects | Clinical Phenotypes | Refs. |
|---|---|---|---|
| -866G>A SNP | |||
| A allele | ↑ UCP2 ↓ROS | [80] | |
| G allele | ↓ UCP2 ⬆ROS | [57] | |
| GG genotype | ↑ plasmatic hs-CRP | ↓ T2DM in obesity | [57, 80] |
| GA genotype | ⬆ myeloperoxidase level | CAD (reduced post-MI survival in T2DM) ⬆Obesity and metabolic abnormalities in NAFLD patients |
[69, 70] [92] |
| AA genotype | ⬆ myeloperoxidase level | Associated with better functional outcome in IS patients after recanalization of proximal MCAO CAD (reduced post-MI survival in T2DM) |
[60] [69, 70] |
| 45 bp Ins/Del | |||
| I/I genotype | Heart rate variability ⬆blood pressure | [71] | |
| D/D genotype | ↑adiponectin level ↓leptin ↓TNFα ↓IL-6 levels after exercise training |
Obesity, MS | [93] |
| Ala55Val SNP C>T | |||
| C allele carriers | ↑Obesity, ↑T2DM | [83] | |
Abbreviations legend: SNP=single nucleotide polymorphism; hs-CRP= high-sensitivity-CRP, CAD=coronary artery disease; T2DM=type 2 diabetes mellitus; NAFLD= non-alcoholic fatty liver disease; MS= metabolic syndrome.
3.2. UCP2 and Heart Diseases
UCP2 is constitutively expressed in the heart of humans and rodents and its expression level depends on the plasma FFA concentration. The overexpression of UCP2 by adenoviral construct in primary cultures of neonatal rat cardiomyocytes protected them from the damage related to increased oxidative stress [34]. During myocardial infarction (MI), the same detrimental processes occurring in IS, such as increased oxidative stress and cell death, are detected. Wu et al. demonstrated that UCP2 protects the heart from myocardial I/R injury via induction of mitophagy, the selective form of autophagy for mitochondria. In fact, in preconditioning myocardial I/R performed in rats, UCP2 was upregulated in association with an increase of microtubule-associated protein-light chain 3 (LC3) and a decrease of p62, two markers of autophagy, indicating a strong activation of this process. In addition, mitochondrial dysfunction and cardiomyocytes death were reduced. By contrast, the protective effect of UCP2 in cardiomyocytes was abolished by mdivi-1, a specific inhibitor of dynamin 1-like, resulting in the inhibition of mitochondrial fission-induced autophagy [61].
UCP2 expression is differently modulated in cardiac hypertrophy. In this regard, Strøm et al. reported that Wistar rats subjected to running on a treadmill developed physiological adaptive hypertrophy with an increase of left and right ventricular mass, as compared to sedentary rats. Furthermore, gene expression profiling analysis of total RNA from hearts revealed that UCP2 was downregulated in this experimental context since ATP demand increased during exercise. In contrast, UCP2 was upregulated in the pathological or maladaptive hypertrophy resulting from MI induced by ligating the left coronary artery [62]. This evidence suggests that the downregulation of UCP2 acts as an adaptive response in compensated hypertrophy, whereas its upregulation is associated with the detrimental effects in maladaptive hypertrophy [62, 63]. Cardiac hypertrophy may also be induced in experimental animal models by exposure to low temperatures (4°C) along with increased cellular oxidative stress, apoptosis and autophagy. In this experimental condition, UCP2 is upregulated as a likely consequence of its role in thermogenesis regulation in specific conditions [64, 65] (Table 1).
UCP2 is also upregulated in animal models of cardiomyopathy induced by chronic β-adrenergic receptor stimulation. In fact, UCP2 upregulation may be partially reverted by β-adrenergic receptor blockade or by angiotensin converting enzyme inhibition [66, 67]. In addition, UCP2 deleted mice exposed to pulmonary arterial banding appeared significantly protected against pressure overload-induced right heart failure as compared to UCP2 wild type mice [68].
In humans, the -866 AA and GA genotypes are associated with reduced survival and higher levels of myeloperoxidase post-MI in diabetic patients [69]. This SNP is associated with coronary artery disease also in young South African Indians [70]. Of interest, the 45 bp insertion/deletion within exon 8 of UCP2 was related to heart rate variability and high blood pressure level in Japanese men [71] (Table 2).
3.3. UCP2 in Thermogenesis, Obesity, T2DM and Metabolic Syndrome
Soon after its discovery, UCP2 was mainly interpreted as a thermogenic protein involved in the regulation of energy expenditure and obesity [10]. In fact, FFA increases the expression of UCP2 skeletal muscle mRNA, implying that UCP2 is somehow involved in fatty acid metabolism. Later on, it was understood that, differently from UCP1, UCP2 is not normally thermogenic and can be involved in this process only under specific conditions [10, 64, 65].
Obesity and T2DM are two strictly related pathological conditions and their prevalence is increasing worldwide [72]. UCP2 is involved in the development of obesity as well as of diabetes. Zhang et al. demonstrated that UCP2 acts as a negative regulator of insulin secretion playing a key role in the determination of pancreatic β-cell dysfunction and consequently in the development of obesity and diabetes in experimental conditions [23] (Table 1). Consistently, when UCP2 expression was suppressed by microRNA-15a in mouse pancreatic β-cells, an increase in insulin secretion was observed [73]. UCP2 is downregulated in the streptozotocin (STZ)-induced diabetic mouse model. This model develops cardiomyopathy, a drastic reduction of mitochondrial ΔΨ and an increase of cell death. The overexpression of ALDH2 in STZ-induced diabetic mice exerted beneficial effects on cardiac structure and function, mitochondrial function and cell survival. UCP2 levels were preserved in STZ-ALDH2 transgenic mice as compared to STZ mice [42, 74].
The correlation between the -866G/A SNP and a greater risk of developing diabetes in the presence of obesity has been established in humans [75]. The wild-type G allele of the -866G/A SNP was associated with reduced UCP2 mRNA expression in adipose tissue, reduced transcriptional activity in vitro and increased risk of obesity in vivo. Kempler et al. showed that the pancreatic transcription factor paired box protein (PAX6) preferentially binds and activates the UCP2 promoter in the presence of the wild-type G allele in the insulinoma cells (INS1-E) derived from rat pancreatic β-cells. In the same study, the correlation between UCP2 genotypes and the pancreatic β-cells function was analyzed in 39 non-diabetic obese patients. Subjects carrying the G allele displayed a greater disposition index, the product of insulin sensitivity (SI) and acute insulin response (AIR) to glucose, as compared to subjects carrying the A allele variant. In addition, obese subjects with and without T2DM and the different UCP2 genotypes were compared. This analysis revealed that the G allele was associated with a twofold reduction of T2DM risk in obese subjects compared to carrier of the A allele variant. Additional case/control and meta-analysis studies confirmed the relationship between -866G/A, obesity and T2DM in several human populations such as Finnish [76], Iranian [77], Spanish [78] and Danish [79] populations. The wild type G allele within the UCP2 promoter, although frequently encountered in obese subjects, protected them from T2DM development [80] (Table 2). A more recent meta-analysis confirmed that -866G/A SNP is significantly associated with T2DM especially in Asian populations [81].
Yang et al. observed that -866G/A SNP, in association with the Trp64Arg mutation of the β3-adrenergic receptor gene, was related to the therapeutic efficacy of rosiglitazone, an anti-diabetic drug, in Chinese T2DM patients [82].
Finally, the Ala55Val SNP (rs660339), a missense variant located within the exon 4 of UCP2 (where there is a change of C to T in the position 164 of the transcript), is commonly associated with higher risk of obesity and higher incidence of T2DM [83] (Table 2). On the other hand, additional studies reported that this SNP was associated with an increased insulin secretion in European-American women [84] and with a reduced risk of T2DM in Asian Indians [85].
The presence of obesity at the abdominal level is one of the four conditions needed to diagnose metabolic syndrome (MS), along with high blood glucose, high serum triglycerides and low serum high-density lipoprotein (HDL) levels [86]. Patients with MS show a 2-fold higher risk of developing cardiovascular events, such as MI and IS, and of developing T2DM compared to subjects without MS [87]. In the MS rat model (Mets), UCP2 expression is increased in the liver, where it acts as an adaptive mechanism of protection toward obesity-related oxidative stress [88]. In this model, the administration of both resveratrol and quercetin leads to increased UCP2 and PPAR-α expression in the abdominal white adipose tissue, and it improves metabolic parameters such as dyslipidemia, central adiposity and insulin resistance [89]. Recently, Wei D et al. demonstrated that a treatment with Shexiang Baoxin Pill (SBP) (containing a mixture composed of seven raw medicinal materials including Radix ginseng and Venenum bufonis) in the MS rat model exerted a protective therapeutic effect against this disorder, probably due to the interaction of anti-inflammatory and antioxidant mechanisms which are able to improve lipid metabolism and protect mitochondrial function. In particular, the authors demonstrated that the expression level of UCP2 increased in several tissues of MS rats, especially in the heart, after SBP treatment. Conversely, the expression level of cytochrome b, the main subunit of mitochondrial complex III, was downregulated in the liver, heart, skeletal muscle and adipose tissue of MS rats. The expression of ATPase (mitochondrial Complex V) appeared to be upregulated after treatment with SBP for 12 weeks [90, 91] (Table 1).
Recently, the association of some components of MS with the -866G/A SNP has been observed in patients with non-alcoholic fatty liver disease (NAFLD). In a case/control study involving 75 patients with NAFLD and 76 healthy individuals, subjects carrying the GA genotype had higher values of central obesity indices and metabolic abnormalities compared with healthy individuals [92].
The 45 bp insertion/deletion (I/D) within the 3'-untranslated region (UTR) of UCP2 was studied in relation to some MS markers such as adiponectin, leptin, tumor necrosis factor-α (TNFα), and interleukin-6 (IL-6) levels. In a study performed in post-menopausal obese women, subjects carrying the DD genotype, but not those carrying the ID genotype, showed an increased level of adiponectin whereas leptin, TNFα, and IL-6 levels were significantly decreased after exercise training [93] (Table 2). The mechanism underlying this phenomenon remains obscure.
4. PHARMACOLOGICAL MODULATION OF UCP2
As discussed above, UCP2 expression can be regulated at multiple levels [94]. In addition, the UCP2 biological effects are tissue specific (Fig. 1). Any therapeutic approach targeting UCP2 should take into account the type of target tissue [17]. Much of the therapeutic potential of UCP2 depends on its antioxidant and anti-inflammatory properties [53, 54], which make UCP2 an attractive therapeutic tool in many diseases, including heart and brain diseases, and inflammatory disorders [17].
Several available drugs affect UCP2 expression levels, although we cannot precisely state if they directly target UCP2. Among them, the dipeptidyl peptidase-4 (DPP-4) inhibitors or gliptins, a class of oral anti-diabetic drugs, are able to restore endothelial function in hypertension by reducing oxidative stress [95]. In particular, chronic sitagliptin administration attenuated endothelium-dependent contraction (EDC) and reduced ROS level by the upregulation of Glucagon-like Peptide-1 (GLP-1)/5-adenosine monophosphate-activated protein kinase (AMPK)/UCP2 pathway and the downregulation of cyclooxygenase-2 (COX-2) expression in renal arteries from spontaneously hypertensive rats (SHR) and angiotensin II (Ang II)-infused mouse aorta. Conversely, sitagliptin did not affect EDC in Ang II-infused UCP2-KO mice. In addition, the beneficial effects of sitagliptin on endothelial function were inhibited by genipin, a UCP2 inhibitor, in renal arteries of SHR, confirming the relevance of UCP2 in this context.
Consistently, UCP2 overexpression, obtained by an adenoviral construct, improved the vascular function in the same experimental rat model. The administration of exendin-4 (Ex-4), a GPL-1 agonist, also improved renal vascular function in renal arteries of both SHR and hypertensive patients through UCP2 level increase with a parallel decrease of COX2 and ROS levels [96, 97].
Other anti-diabetic molecules, such as metformin and berberine, modulate UCP2 level. Metformin, the most common anti-diabetic drug, acts by AMPK upregulation. In fact, AMPK inhibition by compound C prevented the UCP2 increase [98]. Berberine, an isoquinolone alkaloid extracted from plants and used in traditional Chinese medicine, shows anti-diabetic and anti-atherogenic properties, improves dyslipidemia and endothelial function, and reduces the incidence of cardiovascular diseases. Berberine significantly increases UCP2 mRNA and protein level in an AMPK-dependent manner both in vitro and in a mouse model of atherosclerosis [99].
Previous studies have shown that PPAR agonists are able to modulate the UCP2 expression level. These drugs are a group of structurally diverse compounds that bind and activate PPAR receptors. Some of them, the thiazolidinediones (TZD), also known as glitazones, are potent PPAR-γ agonists [100], commonly used in the clinical practice for the treatment of diabetes and MS with beneficial effects. These drugs cause a marked reduction of FFA plasmatic level and inhibit lipolysis in T2DM patients. Plasmatic FFA reduction, in turn, leads to fat mobilization from the muscle and the liver and improves insulin sensitivity in these organs [101]. Glitazones also contribute to lower blood pressure level and improve lipid metabolism by increasing HDL cholesterol level and by reducing triglycerides level [102, 103]. Several studies revealed that the TZD administration, mainly rosiglitazone, induced a rapid increase of UCP2 both in mouse and human adipocyte cell lines [104, 105] as compared to control cells. Its effect appeared to be mediated by PPAR-γ [104, 106]. In addition, administration of rosiglitazone in Sprague-Dawley rats reduced mitochondrial hydrogen peroxide level in rostral ventrolateral medulla, a region that contains the sympathetic premotor neurons for the maintenance of vasomotor tone and of systemic arterial pressure. Rosiglitazone can exert a protective effect against oxidative stress-associated neurogenic hypertension through UCP2 upregulation and ROS reduction [107].
Additional pre-clinical and in vitro studies analyzed the effect of pioglitazone, a well-known PPAR-γ agonist, toward oxidative stress in the rat brain [97]. It was demonstrated that this drug, through PPAR-γ activation and UCP2 upregulation, attenuated oxidative stress in cerebral arteries of aging rats. They also showed that one-month oral administration of pioglitazone improved endothelium-dependent relaxation by increasing both eNOS phosphorylation and nitric oxide (NO) availability. PPAR-γ appears as a promising target for the treatment of diabetes and age-related cerebrovascular dysfunction.
No side effects dependent on UCP2 upregulation have been so far reported with the available compounds, although it is known that NO, a mediator of UCP2, may contribute to exacerbate neuronal cell dysfunction and diminish cell survival after brain injury [108]. Exaggerate NO production is also associated with acute pancreatitis in rats [109] and liver damage in mice [110].
Interestingly, some chemotherapeutic drugs, such as doxorubicin (DOX) and taxol, used for the treatment of different types of cancers, downregulate UCP2 expression by activation of c-Jun N-terminal kinase (JNK)/mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) signaling, with consequent ROS accumulation and increase of apoptosis in melanoma cells [111]. UCP2 downregulation and consequent ROS accumulation have also been associated with severe cardiotoxicity in both animal models and cardiomyocytes treated with DOX. Interestingly, administration of melatonin counteracted the cardiac toxic effects of DOX by improvement of mitochondrial damage and upregulation of UCP2 [112].
Apart from the above mentioned drugs, UCP2 expression can be modulated by nutraceuticals as documented in the SHRSP model [13, 37] and in humans [13]. Pagliaro et al. described the beneficial effects exerted in the cardiovascular system by different natural compounds such as BO, curcumin, resveratrol and berberine. These natural compounds of plant origin, through the activation of both AMPK and silent mating type information regulation-1 (SIRT-1), upregulate PPARs (mainly PPAR-𝛼 and PPAR-𝛾) leading to UCP2 increase, reduction of ROS level and of inflammation, improvement of brain ischemic injury and decrease of stroke occurrence [113]. The beneficial effects of BO in humans were tested in a small clinical trial in which broccoli extract pills and fresh broccoli were administered simultaneously for 7 days to healthy subjects. At the end of the study, a significant reduction of both total and LDL cholesterol, along with a decrease of urinary 8-isoprostanes and other markers of oxidative stress, was observed [114]. The administration of broccoli juice for 12 weeks in 32 men with hypercholesterolemia significantly reduced plasma LDL cholesterol and increased both HDL cholesterol and GPx activity, thus lowering cardiovascular disease risk [115]. Interestingly, treatment with resveratrol, known for its beneficial effects on diabetes progression, oxidative stress and lipid metabolism, exerts a protective effect towards diabetic cardiomyopathy induced by STZ in a UCP2 dependent manner [116]. In fact, resveratrol administration significantly increased UCP2 expression, reduced ROS level and increased the manganese superoxide dismutase level. Conversely, UCP2 inhibition by siRNA hinders the cardioprotective effects of resveratrol on ROS production and apoptosis level in high glucose-treated cardiomyocytes [117].
CONCLUSION
The physiological role of UCP2 appears complex and it still needs to be fully dissected out. UCP2 expression and functions are tissue specific. Most of the beneficial properties of UCP2 depend on its role as ROS scavenger. UCP2 is also involved in the regulation of ΔΨ, ATP synthesis, calcium level and cell death.
UCP2 upregulation attenuates mitochondrial ROS production, consequently the cellular oxidative damage, and exerts protective effects in several organs. On the other hand, UCP2 inhibition protects from hyperglycemia and diabetes through the control of insulin secretion in the pancreatic β-cells.
Therefore, UCP2 behaves as a critical determinant of many human disorders such as atherosclerosis, ischemic heart disease, neurodegenerative diseases, including IS, and diabetes. Current evidence suggests that UCP2 modulation may represent a suitable tool for the treatment of several human diseases (Fig. 2).
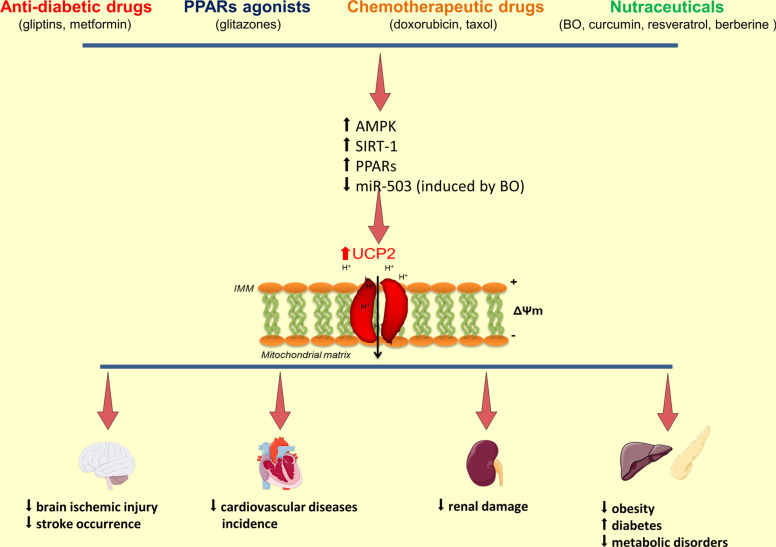
Fig. (2).
UCP2 gene/protein expression modulation in relation to cardiovascular and metabolic diseases. Schematic representation of the modulation of UCP2 gene and protein expression by known agents. Both nutraceuticals and drugs can upregulate UCP2 level mainly through the AMPK/SIRT-1/PPARs pathway. Of interest, the epigenetic regulation can also be involved, as shown upon BO extract administration (see text). Increased UCP2 levels induce membrane mitochondrial depolarization, decreased ROS production, reduced cell death and inflammatory responses. Consequently, the occurrence of cardiovascular and metabolic diseases is significantly affected.
Further experimental studies will allow improving our understanding of the molecular pathways involved in UCP2 activation. Human studies may reveal important new insights on the pathogenic role of UCP2 gene variants. The new knowledge could facilitate the identification of novel pharmacological strategies that, by controlling the expression of UCP2, can reduce the occurrence and progression of diseases in which UCP2 plays a fundamental role. In this latter regard, additional investigations are necessary to identify the exact relationship between molecular mechanisms, cellular effects and clinical consequences, along with potential caveats, upon pharmacological stimulation of UCP2.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS' CONTRIBUTIONS
Conceptualization: [RS, SR]; Writing- original draft preparation: [RS]; Writing - review and editing: [RS, MF, SR]; Literature search: [RS, MC, FB, SM]; Critical revision: [CB, FF, SR]. All authors read and approved the final version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by a grant from the Italian Ministry of Health.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Nedergaard J., Ricquier D., Kozak L.P. Uncoupling proteins: current status and therapeutic prospects. EMBO Rep. 2005;6(10):917–921. doi: 10.1038/sj.embor.7400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousset S., Alves-Guerra M.C., Mozo J., Miroux B., Cassard-Doulcier A.M., Bouillaud F., Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl. 1):S130–S135. doi: 10.2337/diabetes.53.2007.S130. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls D.G. A history of UCP1. Biochem. Soc. Trans. 2001;29(Pt 6):751–755. doi: 10.1042/bst0290751. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Bouillaud F., Couplan E., Pecqueur C., Ricquier D. Homologues of the uncoupling protein from brown adipose tissue (UCP1): UCP2, UCP3, BMCP1 and UCP4. Biochim. Biophys. Acta. 2001;1504(1):107–119. doi: 10.1016/S0005-2728(00)00241-3. [DOI] [PubMed] [Google Scholar]
- 7.Erlanson-Albertsson C. Uncoupling proteins--a new family of proteins with unknown function. Nutr. Neurosci. 2002;5(1):1–11. doi: 10.1080/10284150290007038. [DOI] [PubMed] [Google Scholar]
- 8.Gaudry M.J., Jastroch M. Molecular evolution of uncoupling proteins and implications for brain function. Neurosci. Lett. 2019;696:140–145. doi: 10.1016/j.neulet.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Mailloux R.J., Harper M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011;51(6):1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Tian X.Y., Ma S., Tse G., Wong W.T., Huang Y. Uncoupling protein 2 in cardiovascular health and disease. Front. Physiol. 2018;9:1060. doi: 10.3389/fphys.2018.01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan C., Chen X., Zhang Y., Wang W., Wang W.E., Liu Y., Cai Y., Ren H., Zheng S., Zhou L., Zeng C. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc. Disord. 2018;18(1):43. doi: 10.1186/s12872-018-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierelli G., Stanzione R., Forte M., Migliarino S., Perelli M., Volpe M., Rubattu S. Uncoupling Protein 2: a key player and a potential therapeutic target in vascular diseases. Oxid. Med. Cell. Longev. 2017;2017: ,7348372. doi: 10.1155/2017/7348372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busceti C.L., Cotugno M., Bianchi F., Forte M., Stanzione R., Marchitti S., Battaglia G., Nicoletti F., Fornai F., Rubattu S. Brain overexpression of uncoupling protein-2 (UCP2) delays renal damage and stroke occurrence in stroke-prone spontaneously hypertensive rats. Int. J. Mol. Sci. 2020;21(12): ,E4289. doi: 10.3390/ijms21124289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidaka S., Kakuma T., Yoshimatsu H., Yasunaga S., Kurokawa M., Sakata T. Molecular cloning of rat uncoupling protein 2 cDNA and its expression in genetically obese Zucker fatty (fa/fa) rats. Biochim. Biophys. Acta. 1998;1389(3):178–186. doi: 10.1016/S0005-2760(97)00188-4. [DOI] [PubMed] [Google Scholar]
- 16.Thompson M.P., Kim D. Links between fatty acids and expression of UCP2 and UCP3 mRNAs. FEBS Lett. 2004;568(1-3):4–9. doi: 10.1016/j.febslet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Mattiasson G., Sullivan P.G. The emerging functions of UCP2 in health, disease, and therapeutics. Antioxid. Redox Signal. 2006;8(1-2):1–38. doi: 10.1089/ars.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 18.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018;1859(9):940–950. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Brand M.D., Chien L.F., Ainscow E.K., Rolfe D.F., Porter R.K. The causes and functions of mitochondrial proton leak. Biochim. Biophys. Acta. 1994;1187(2):132–139. doi: 10.1016/0005-2728(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 20.Suski J., Lebiedzinska M., Bonora M., Pinton P., Duszynski J., Wieckowski M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2018;1782:357–381. doi: 10.1007/978-1-4939-7831-1_22. [DOI] [PubMed] [Google Scholar]
- 21.Zhao R.Z., Jiang S., Zhang L., Yu Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling.(Review). Int. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph J.W., Koshkin V., Saleh M.C., Sivitz W.I., Zhang C.Y., Lowell B.B., Chan C.B., Wheeler M.B. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J. Biol. Chem. 2004;279(49):51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C.Y., Baffy G., Perret P., Krauss S., Peroni O., Grujic D., Hagen T., Vidal-Puig A.J., Boss O., Kim Y.B., Zheng X.X., Wheeler M.B., Shulman G.I., Chan C.B., Lowell B.B. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. doi: 10.1016/S0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 24.Pecqueur C., Alves-Guerra C., Ricquier D., Bouillaud F. UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life. 2009;61(7):762–767. doi: 10.1002/iub.188. [DOI] [PubMed] [Google Scholar]
- 25.Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29(3-4):222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 26.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B.S., Miroux B., Couplan E., Alves-Guerra M.C., Goubern M., Surwit R., Bouillaud F., Richard D., Collins S., Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26(4):435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls D.G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim. Biophys. Acta. 2009;1787(11):1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldeck-Weiermair M., Malli R., Naghdi S., Trenker M., Kahn M.J., Graier W.F. The contribution of UCP2 and UCP3 to mitochondrial Ca(2+) uptake is differentially determined by the source of supplied Ca(2+). Cell Calcium. 2010;47(5):433–440. doi: 10.1016/j.ceca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Takarada T., Fukumori R., Yoneda Y. Mitochondrial uncoupling protein-2 in glutamate neurotoxicity. Nippon Yakurigaku Zasshi. 2013;142(1):13–16. doi: 10.1254/fpj.142.13. [DOI] [PubMed] [Google Scholar]
- 30.Mehta S.L., Li P.A. Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J. Cereb. Blood Flow Metab. 2009;29(6):1069–1078. doi: 10.1038/jcbfm.2009.4. [DOI] [PubMed] [Google Scholar]
- 31.Orrenius S., Gogvadze V., Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 32.He M., Zhang T., Fan Y., Ma Y., Zhang J., Jing L., Li P.A. Deletion of mitochondrial uncoupling protein 2 exacerbates mitophagy and cell apoptosis after cerebral ischemia and reperfusion injury in mice. Int. J. Med. Sci. 2020;17(17):2869–2878. doi: 10.7150/ijms.49849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattiasson G., Shamloo M., Gido G., Mathi K., Tomasevic G., Yi S., Warden C.H., Castilho R.F., Melcher T., Gonzalez-Zulueta M., Nikolich K., Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003;9(8):1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 34.Teshima Y., Akao M., Jones S.P., Marbán E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003;93(3):192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno T., Miura-Suzuki T., Yamashita H., Mori N. Distinct regulation of brain mitochondrial carrier protein-1 and uncoupling protein-2 genes in the rat brain during cold exposure and aging. Biochem. Biophys. Res. Commun. 2000;278(3):691–697. doi: 10.1006/bbrc.2000.3859. [DOI] [PubMed] [Google Scholar]
- 36.Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., Bouillaud F., Seldin M.F., Surwit R.S., Ricquier D., Warden C.H. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997;15(3):269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 37.Rubattu S., Stanzione R., Bianchi F., Cotugno M., Forte M., Della Ragione F., Fioriniello S., D’Esposito M., Marchitti S., Madonna M., Baima S., Morelli G., Sciarretta S., Sironi L., Gelosa P., Volpe M. Reduced brain UCP2 expression mediated by microRNA-503 contributes to increased stroke susceptibility in the high-salt fed stroke-prone spontaneously hypertensive rat. Cell Death Dis. 2017;8(6): ,e2891. doi: 10.1038/cddis.2017.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph J.W., Koshkin V., Zhang C.Y., Wang J., Lowell B.B., Chan C.B., Wheeler M.B. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51(11):3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- 39.Rubattu S., Bianchi F., Busceti C.L., Cotugno M., Stanzione R., Marchitti S., Di Castro S., Madonna M., Nicoletti F., Volpe M. Differential modulation of AMPK/PPARα/UCP2 axis in relation to hypertension and aging in the brain, kidneys and heart of two closely related spontaneously hypertensive rat strains. Oncotarget. 2015;6(22):18800–18818. doi: 10.18632/oncotarget.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Mi S.L., Hu N., Doser T.A., Sun A., Ge J., Ren J. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 2014;71:208–220. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren J., Yang L., Zhu L., Xu X., Ceylan A.F., Guo W., Yang J., Zhang Y. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell. 2017;16(5):976–987. doi: 10.1111/acel.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Babcock S.A., Hu N., Maris J.R., Wang H., Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function. BMC Med. 2012;10:40. doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Bano D., Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38(2) Suppl.:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- 44.Sanganalmath S.K., Gopal P., Parker J.R., Downs R.K., Parker J.C., Jr, Dawn B. Global cerebral ischemia due to circulatory arrest: insights into cellular pathophysiology and diagnostic modalities. Mol. Cell. Biochem. 2017;426(1-2):111–127. doi: 10.1007/s11010-016-2885-9. [DOI] [PubMed] [Google Scholar]
- 45.Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Rubattu S., Di Castro S., Cotugno M., Bianchi F., Mattioli R., Baima S., Stanzione R., Madonna M., Bozzao C., Marchitti S., Gelosa P., Sironi L., Pignieri A., Maldini M., Giusti A.M., Nardini M., Morelli G., Costantino P., Volpe M. Protective effects of Brassica oleracea sprouts extract toward renal damage in high-salt-fed SHRSP: role of AMPK/PPARα/UCP2 axis. J. Hypertens. 2015;33(7):1465–1479. doi: 10.1097/HJH.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 47.Gallo G., Forte M., Stanzione R., Cotugno M., Bianchi F., Marchitti S., Berni A., Volpe M., Rubattu S. Functional role of natriuretic peptides in risk assessment and prognosis of patients with mitral regurgitation. J. Clin. Med. 2020;9(5): ,E1348. doi: 10.3390/jcm9051348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Castro S., Scarpino S., Marchitti S., Bianchi F., Stanzione R., Cotugno M., Sironi L., Gelosa P., Duranti E., Ruco L., Volpe M., Rubattu S. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension. 2013;61(2):534–541. doi: 10.1161/HYPERTENSIONAHA.111.00101. [DOI] [PubMed] [Google Scholar]
- 49.Hou X., Shen Y.H., Li C., Wang F., Zhang C., Bu P., Zhang Y. PPARalpha agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochem. Biophys. Res. Commun. 2010;394(3):653–659. doi: 10.1016/j.bbrc.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Gelosa P., Banfi C., Gianella A., Brioschi M., Pignieri A., Nobili E., Castiglioni L., Cimino M., Tremoli E., Sironi L. Peroxisome proliferator-activated receptor alpha agonism prevents renal damage and the oxidative stress and inflammatory processes affecting the brains of stroke-prone rats. J. Pharmacol. Exp. Ther. 2010;335(2):324–331. doi: 10.1124/jpet.110.171090. [DOI] [PubMed] [Google Scholar]
- 51.Medvedev A.V., Snedden S.K., Raimbault S., Ricquier D., Collins S. Transcriptional regulation of the mouse uncoupling protein-2 gene. Double E-box motif is required for peroxisome proliferator-activated receptor-gamma-dependent activation. J. Biol. Chem. 2001;276(14):10817–10823. doi: 10.1074/jbc.M010587200. [DOI] [PubMed] [Google Scholar]
- 52.Nishijima C., Kimoto K., Arakawa Y. Survival activity of troglitazone in rat motoneurones. J. Neurochem. 2001;76(2):383–390. doi: 10.1046/j.1471-4159.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- 53.Shimazu T., Inoue I., Araki N., Asano Y., Sawada M., Furuya D., Nagoya H., Greenberg J.H. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36(2):353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- 54.Sundararajan S., Gamboa J.L., Victor N.A., Wanderi E.W., Lust W.D., Landreth G.E. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130(3):685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Nakase T., Yoshida Y., Nagata K. Amplified expression of uncoupling proteins in human brain ischemic lesions. Neuropathology. 2007;27(5):442–447. doi: 10.1111/j.1440-1789.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 56.Dalgaard L.T., Andersen G., Larsen L.H., Sørensen T.I., Andersen T., Drivsholm T., Borch-Johnsen K., Fleckner J., Hansen T., Din N., Pedersen O. Mutational analysis of the UCP2 core promoter and relationships of variants with obesity. Obes. Res. 2003;11(11):1420–1427. doi: 10.1038/oby.2003.191. [DOI] [PubMed] [Google Scholar]
- 57.Lapice E., Pinelli M., Pisu E., Monticelli A., Gambino R., Pagano G., Valsecchi S., Cocozza S., Riccardi G., Vaccaro O. Uncoupling protein 2 G(-866)A polymorphism: a new gene polymorphism associated with C-reactive protein in type 2 diabetic patients. Cardiovasc. Diabetol. 2010;9:68. doi: 10.1186/1475-2840-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chai Y., Gu B., Qiu J.R., Yi H.G., Zhu Q., Zhang L., Hu G. The uncoupling protein 2 -866G > a polymorphism is associated with the risk of ischemic stroke in Chinese type 2 diabetic patients. CNS Neurosci. Ther. 2012;18(8):636–640. doi: 10.1111/j.1755-5949.2012.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai Y., Gu B., Qiu J.R., Yi H.G., Zhu Q., Zhang L., Hu G. Effects of uncoupling protein 2 -866G/A polymorphism on platelet reactivity and prognosis in Chinese patients with type 2 diabetes and ischemic stroke. Int. J. Neurosci. 2013;123(11):752–758. doi: 10.3109/00207454.2013.798733. [DOI] [PubMed] [Google Scholar]
- 60.Díaz-Maroto Cicuéndez I., Fernández-Díaz E., García-García J., Jordán J., Fernández-Cadenas I., Montaner J., Serrano-Heras G., Segura T. The UCP2-866G/A polymorphism could be considered as a genetic marker of different functional prognosis in ischemic stroke after recanalization. Neuromolecular Med. 2017;19(4):571–578. doi: 10.1007/s12017-017-8470-x. [DOI] [PubMed] [Google Scholar]
- 61.Wu H., Ye M., Liu D., Yang J., Ding J.W., Zhang J., Wang X.A., Dong W.S., Fan Z.X., Yang J. UCP2 protect the heart from myocardial ischemia/reperfusion injury via induction of mitochondrial autophagy. J. Cell. Biochem. 2019;120(9):15455–15466. doi: 10.1002/jcb.28812. [DOI] [PubMed] [Google Scholar]
- 62.Strøm C.C., Aplin M., Ploug T., Christoffersen T.E., Langfort J., Viese M., Galbo H., Haunsø S., Sheikh S.P. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005;272(11):2684–2695. doi: 10.1111/j.1742-4658.2005.04684.x. [DOI] [PubMed] [Google Scholar]
- 63.Strøm C.C., Kruhøffer M., Knudsen S., Stensgaard-Hansen F., Jonassen T.E., Orntoft T.F., Haunsø S., Sheikh S.P. Identification of a core set of genes that signifies pathways underlying cardiac hypertrophy. Comp. Funct. Genomics. 2004;5(6-7):459–470. doi: 10.1002/cfg.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y., Li L., Hua Y., Nunn J.M., Dong F., Yanagisawa M., Ren J. Cardiac-specific knockout of ET(A) receptor mitigates low ambient temperature-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell Biol. 2012;4(2):97–107. doi: 10.1093/jmcb/mjs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong X., Liu H., He X., Sun Y., Ge W. Unraveling the mystery of cold stress-induced myocardial injury. Front. Physiol. 2020;11: ,580811. doi: 10.3389/fphys.2020.580811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaussin V., Tomlinson J.E., Depre C., Engelhardt S., Antos C.L., Takagi G., Hein L., Topper J.N., Liggett S.B., Olson E.N., Lohse M.J., Vatner S.F., Vatner D.E. Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation. 2003;108(23):2926–2933. doi: 10.1161/01.CIR.0000101922.18151.7B. [DOI] [PubMed] [Google Scholar]
- 67.Murakami K., Mizushige K., Noma T., Tsuji T., Kimura S., Kohno M. Perindopril effect on uncoupling protein and energy metabolism in failing rat hearts. Hypertension. 2002;40(3):251–255. doi: 10.1161/01.HYP.0000029094.85023.01. [DOI] [PubMed] [Google Scholar]
- 68.Esfandiary A., Kutsche H.S., Schreckenberg R., Weber M., Pak O., Kojonazarov B., Sydykov A., Hirschhäuser C., Wolf A., Haag D., Hecker M., Fink L., Seeger W., Ghofrani H.A., Schermuly R.T., Weißmann N., Schulz R., Rohrbach S., Li L., Sommer N., Schlüter K.D. Protection against pressure overload-induced right heart failure by uncoupling protein 2 silencing. Cardiovasc. Res. 2019;115(7):1217–1227. doi: 10.1093/cvr/cvz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmer B.R., Devereaux C.L., Dhamrait S.S., Mocatta T.J., Pilbrow A.P., Frampton C.M., Skelton L., Yandle T.G., Winterbourn C.C., Richards A.M., Montgomery H.E., Cameron V.A. The common G-866A polymorphism of the UCP2 gene and survival in diabetic patients following myocardial infarction. Cardiovasc. Diabetol. 2009;8:31. doi: 10.1186/1475-2840-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phulukdaree A., Moodley D., Khan S., Chuturgoon A.A. Uncoupling protein 2 -866G/A and uncoupling protein 3 -55C/T polymorphisms in young South African Indian coronary artery disease patients. Gene. 2013;524(2):79–83. doi: 10.1016/j.gene.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 71.Matsunaga T., Gu N., Yamazaki H., Tsuda M., Adachi T., Yasuda K., Moritani T., Tsuda K., Nonaka M., Nishiyama T. Association of UCP2 and UCP3 polymorphisms with heart rate variability in Japanese men. J. Hypertens. 2009;27(2):305–313. doi: 10.1097/HJH.0b013e32831ac967. [DOI] [PubMed] [Google Scholar]
- 72.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 73.Sun L.L., Jiang B.G., Li W.T., Zou J.J., Shi Y.Q., Liu Z.M. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res. Clin. Pract. 2011;91(1):94–100. doi: 10.1016/j.diabres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Ren J., Pulakat L., Whaley-Connell A., Sowers J.R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. (Berl.) 2010;88(10):993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalgaard L.T. Genetic variance in uncoupling protein 2 in relation to obesity, type 2 diabetes, and related metabolic traits: Focus on the functional -866G>A promoter variant (rs659366). J. Obes. 2011;2011: ,340241. doi: 10.1155/2011/340241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salopuro T., Pulkkinen L., Lindström J., Kolehmainen M., Tolppanen A.M., Eriksson J.G., Valle T.T., Aunola S., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Tuomilehto J., Laakso M., Uusitupa M. Variation in the UCP2 and UCP3 genes associates with abdominal obesity and serum lipids: the Finnish Diabetes Prevention Study. BMC Med. Genet. 2009;10:94. doi: 10.1186/1471-2350-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heidari J., Akrami S.M., Heshmat R., Amiri P., Fakhrzadeh H., Pajouhi M. Association study of the -866G/A UCP2 gene promoter polymorphism with type 2 diabetes and obesity in a Tehran population: a case control study. Arch. Iran Med. 2010;13(5):384–390. [PubMed] [Google Scholar]
- 78.Ochoa M.C., Santos J.L., Azcona C., Moreno-Aliaga M.J., Martínez-González M.A., Martínez J.A., Marti A. Association between obesity and insulin resistance with UCP2-UCP3 gene variants in Spanish children and adolescents. Mol. Genet. Metab. 2007;92(4):351–358. doi: 10.1016/j.ymgme.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 79.Andersen G., Dalgaard L.T., Justesen J.M., Anthonsen S., Nielsen T., Thørner L.W., Witte D., Jørgensen T., Clausen J.O., Lauritzen T., Holmkvist J., Hansen T., Pedersen O. The frequent UCP2 -866G>A polymorphism protects against insulin resistance and is associated with obesity: a study of obesity and related metabolic traits among 17 636 Danes. Int. J. Obes. 2013;37(2):175–181. doi: 10.1038/ijo.2012.22. [DOI] [PubMed] [Google Scholar]
- 80.Krempler F., Esterbauer H., Weitgasser R., Ebenbichler C., Patsch J.R., Miller K., Xie M., Linnemayr V., Oberkofler H., Patsch W. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51(11):3331–3335. doi: 10.2337/diabetes.51.11.3331. [DOI] [PubMed] [Google Scholar]
- 81.Xu L., Chen S., Zhan L. Association of uncoupling protein-2 -866G/A and Ala55Val polymorphisms with susceptibility to type 2 diabetes mellitus: A meta-analysis of case-control studies. Medicine (Baltimore) 2021;100(6): ,e24464. doi: 10.1097/MD.0000000000024464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang M., Huang Q., Wu J., Yin J.Y., Sun H., Liu H.L., Zhou H.H., Liu Z.Q. Effects of UCP2 -866 G/A and ADRB3 Trp64Arg on rosiglitazone response in Chinese patients with Type 2 diabetes. Br. J. Clin. Pharmacol. 2009;68(1):14–22. doi: 10.1111/j.1365-2125.2009.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu K., Zhang M., Cui D., Fu Y., Qian L., Gu R., Wang M., Shen C., Yu R., Yang T. UCP2 -866G/A and Ala55Val, and UCP3 -55C/T polymorphisms in association with type 2 diabetes susceptibility: a meta-analysis study. Diabetologia. 2011;54(9):2315–2324. doi: 10.1007/s00125-011-2245-y. [DOI] [PubMed] [Google Scholar]
- 84.Willig A.L., Casazza K.R., Divers J., Bigham A.W., Gower B.A., Hunter G.R., Fernandez J.R. Uncoupling protein 2 Ala55Val polymorphism is associated with a higher acute insulin response to glucose. Metabolism. 2009;58(6):877–881. doi: 10.1016/j.metabol.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vimaleswaran K.S., Radha V., Ghosh S., Majumder P.P., Sathyanarayana Rao M.R., Mohan V. Uncoupling protein 2 and 3 gene polymorphisms and their association with type 2 diabetes in asian indians. Diabetes Technol. Ther. 2011;13(1):19–25. doi: 10.1089/dia.2010.0091. [DOI] [PubMed] [Google Scholar]
- 86.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014: ,943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Ford E.S., Li C., Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31(9):1898–1904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruiz-Ramírez A., Chávez-Salgado M., Peñeda-Flores J.A., Zapata E., Masso F., El-Hafidi M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011;301(6):E1198–E1207. doi: 10.1152/ajpendo.00631.2010. [DOI] [PubMed] [Google Scholar]
- 89.Castrejón-Tellez V., Rodríguez-Pérez J.M., Pérez-Torres I., Pérez-Hernández N., Cruz-Lagunas A., Guarner-Lans V., Vargas-Alarcón G., Rubio-Ruiz M.E. The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016;17(7): ,E1069. doi: 10.3390/ijms17071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu L., Sun X., Chen C., Qin Y., Guo X. Shexiang baoxin pill, derived from the traditional chinese medicine, provides protective roles against cardiovascular diseases. Front. Pharmacol. 2018;9:1161. doi: 10.3389/fphar.2018.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei D., Zheng N., Zheng L., Wang L., Song L., Sun L. Shexiang baoxin pill corrects metabolic disorders in a rat model of metabolic syndrome by targeting mitochondria. Front. Pharmacol. 2018;9:137. doi: 10.3389/fphar.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abbasalizad Farhangi M., Mohseni F., Farajnia S., Jafarabadi M.A. Major components of metabolic syndrome and nutritional intakes in different genotype of UCP2 -866G/A gene polymorphisms in patients with NAFLD. J. Transl. Med. 2016;14(1):177. doi: 10.1186/s12967-016-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lim K.I., Shin Y.A. Impact of UCP2 polymorphism on long-term exercise-mediated changes in adipocytokines and markers of metabolic syndrome. Aging Clin. Exp. Res. 2014;26(5):491–496. doi: 10.1007/s40520-014-0213-3. [DOI] [PubMed] [Google Scholar]
- 94.Donadelli M., Dando I., Fiorini C., Palmieri M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014;71(7):1171–1190. doi: 10.1007/s00018-013-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crepaldi G., Carruba M., Comaschi M., Del Prato S., Frajese G., Paolisso G. Dipeptidyl peptidase 4 (DPP-4) inhibitors and their role in Type 2 diabetes management. J. Endocrinol. Invest. 2007;30(7):610–614. doi: 10.1007/BF03346357. [DOI] [PubMed] [Google Scholar]
- 96.Lemos N.E., Dieter C., Carlessi R., Rheinheimer J., Brondani L.A., Leitão C.B., Bauer A.C., Crispim D. Renal effects of exendin-4 in an animal model of brain death. Mol. Biol. Rep. 2019;46(2):2197–2207. doi: 10.1007/s11033-019-04674-1. [DOI] [PubMed] [Google Scholar]
- 97.Liu L., Liu J., Tian X.Y., Wong W.T., Lau C.W., Xu A., Xu G., Ng C.F., Yao X., Gao Y., Huang Y. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid. Redox Signal. 2014;21(11):1571–1581. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anedda A., Rial E., González-Barroso M.M. Metformin induces oxidative stress in white adipocytes and raises uncoupling protein 2 levels. J. Endocrinol. 2008;199(1):33–40. doi: 10.1677/JOE-08-0278. [DOI] [PubMed] [Google Scholar]
- 99.Wang Q., Zhang M., Liang B., Shirwany N., Zhu Y., Zou M.H. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6(9): ,e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berger J., Moller D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 101.Olefsky J.M., Saltiel A.R. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol. Metab. 2000;11(9):362–368. doi: 10.1016/S1043-2760(00)00306-4. [DOI] [PubMed] [Google Scholar]
- 102.Gurnell M. Peroxisome proliferator-activated receptor gamma and the regulation of adipocyte function: lessons from human genetic studies. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19(4):501–523. doi: 10.1016/j.beem.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Staels B., Fruchart J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54(8):2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 104.Camirand A., Marie V., Rabelo R., Silva J.E. Thiazolidinediones stimulate uncoupling protein-2 expression in cell lines representing white and brown adipose tissues and skeletal muscle. Endocrinology. 1998;139(1):428–431. doi: 10.1210/endo.139.1.5808. [DOI] [PubMed] [Google Scholar]
- 105.Strobel A., Siquier K., Zilberfarb V., Strosberg A.D., Issad T. Effect of thiazolidinediones on expression of UCP2 and adipocyte markers in human PAZ6 adipocytes. Diabetologia. 1999;42(5):527–533. doi: 10.1007/s001250051190. [DOI] [PubMed] [Google Scholar]
- 106.Viguerie-Bascands N., Saulnier-Blache J.S., Dandine M., Dauzats M., Daviaud D., Langin D. Increase in uncoupling protein-2 mRNA expression by BRL49653 and bromopalmitate in human adipocytes. Biochem. Biophys. Res. Commun. 1999;256(1):138–141. doi: 10.1006/bbrc.1999.0303. [DOI] [PubMed] [Google Scholar]
- 107.Chan S.H., Wu C.A., Wu K.L., Ho Y.H., Chang A.Y., Chan J.Y. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ. Res. 2009;105(9):886–896. doi: 10.1161/CIRCRESAHA.109.199018. [DOI] [PubMed] [Google Scholar]
- 108.Brabazon F., Bermudez S., Shaughness M., Khayrullina G., Byrnes K.R. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS One. 2018;13(8): ,e0201878. doi: 10.1371/journal.pone.0201878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozturk F., Gul M., Esrefoglu M., Ates B. The contradictory effects of nitric oxide in caerulein-induced acute pancreatitis in rats. Free Radic. Res. 2008;42(4):289–296. doi: 10.1080/10715760801930730. [DOI] [PubMed] [Google Scholar]
- 110.Walthers E.A., Bradford C.S., Moore F.L. Cloning, pharmacological characterization and tissue distribution of an ORL1 opioid receptor from an amphibian, the rough-skinned newt Taricha granulosa. J. Mol. Endocrinol. 2005;34(1):247–256. doi: 10.1677/jme.1.01687. [DOI] [PubMed] [Google Scholar]
- 111.Selimovic D., Hassan M., Haikel Y., Hengge U.R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell. Signal. 2008;20(2):311–322. doi: 10.1016/j.cellsig.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 112.Liu D., Ma Z., Di S., Yang Y., Yang J., Xu L., Reiter R.J., Qiao S., Yuan J. AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic. Biol. Med. 2018;129:59–72. doi: 10.1016/j.freeradbiomed.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 113.Pagliaro B., Santolamazza C., Simonelli F., Rubattu S. Phytochemical compounds and protection from cardiovascular diseases: A state of the art. BioMed Res. Int. 2015;2015: ,918069. doi: 10.1155/2015/918069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clarke J.D., Riedl K., Bella D., Schwartz S.J., Stevens J.F., Ho E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J. Agric. Food Chem. 2011;59(20):10955–10963. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim S.Y., Yoon S., Kwon S.M., Park K.S., Lee-Kim Y.C. Kale juice improves coronary artery disease risk factors in hypercholesterolemic men. Biomed. Environ. Sci. 2008;21(2):91–97. doi: 10.1016/S0895-3988(08)60012-4. [DOI] [PubMed] [Google Scholar]
- 116.Bhagani H., Nasser S.A., Dakroub A., El-Yazbi A.F., Eid A.A., Kobeissy F., Pintus G., Eid A.H. The mitochondria: A target of polyphenols in the treatment of diabetic cardiomyopathy. Int. J. Mol. Sci. 2020;21(14): ,E4962. doi: 10.3390/ijms21144962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diao J., Wei J., Yan R., Fan G., Lin L., Chen M. Effects of resveratrol on regulation on UCP2 and cardiac function in diabetic rats. J. Physiol. Biochem. 2019;75(1):39–51. doi: 10.1007/s13105-018-0648-7. [DOI] [PubMed] [Google Scholar]