Abstract
Psychiatric drugs have primacy for off-label prescribing. Among those, selective serotonin reuptake inhibitors (SSRIs) are highly versatile and, therefore, widely prescribed. Moreover, they are commonly considered as having a better safety profile compared to other antidepressants. Thus, when it comes to off-label prescribing, SSRIs rank among the top positions. In this review, we present the state of the art of off-label applications of selective serotonin reuptake inhibitors, ranging from migraine prophylaxis to SARS-CoV-2 antiviral properties. Research on SSRIs provided significant evidence in the treatment of premature ejaculation, both with the on-label dapoxetine 30 mg and the off-label paroxetine 20 mg. However, other than a serotoninergic syndrome, serious conditions like increased bleeding rates, hyponatremia, hepatoxicity, and post-SSRIs sexual dysfunctions, are consistently more prominent when using such compounds. These insidious side effects might be frequently underestimated during common clinical practice, especially by non-psychiatrists. Thus, some points must be addressed when using SSRIs. Among these, a psychiatric evaluation before every administration that falls outside the regulatory agencies-approved guidelines has to be considered mandatory. For these reasons, we aim with the present article to identify the risks of inappropriate uses and to advocate the need to actively boost research encouraging future clinical trials on this topic.
Keywords: SSRI, premature ejaculation, off-label, antidepressants, PSSD, hypersexuality, paraphilic disorders
1. INTRODUCTION
A large body of literature shows that when it comes to off-label prescribing, psychiatric drugs rank first [1-3]. It is well known that the term off-label does not necessarily mean illicit or improper use. Indeed, most of the time an off-label application implies a certain drug to be used with a different purpose, frequency, dosage, duration, or even protocol of administration [4].
The Food and Drug Administration (FDA) in the U.S. and the European Medicines Agency (EMA) in Europe approve the correct use of a drug, and pharmaceutical companies cannot sell or promote products that fall outside the FDA or EMA guidelines. Nevertheless, physicians are certainly allowed to prescribe drugs for off-label uses according to the latest evidence in the literature and to their knowledge and belief. For this reason, the absence of a specific indication has not to be considered a hurdle when prescribing a certain therapy. Deciding which medication to give is a process that has to be done considering every potential risk and benefit for the patient and has to be supported by the best available evidence.
Selective serotonin reuptake inhibitors (SSRI) act by selectively blocking the serotonin (5-hydroxytryptamine-5-HT) transporter, causing the levels of this neurotransmitter to increase both at the somatodendritic and the presynaptic end of the neuron [5]. FDA- and EMA-approved indications for SSRIs include major depression, generalized anxiety disorder, panic disorder, obsessive-compulsive disorder (OCD), bulimia nervosa, and post-traumatic stress disorder (PTSD) (all the local agencies were not cited, as importance was given only to the continental ones) [6]. Serotonin imbalances have also been hypothesized in women with premenstrual syndrome and premenstrual dysphoric disorder. Indeed, to date, SSRIs are considered first-line therapy, as FDA has approved fluoxetine, paroxetine, and sertraline for the treatment of such conditions [7]. Although SSRIs were originally designed to treat depression, later on, they became one of the most prescribed medicines, taking also into account their off-label applications [8, 9]. This is mainly because they are perceived as having a small number of side effects and an overall better safety profile than other antidepressants [10, 11].
Nevertheless, although SSRIs are commonly considered well-tolerated, they might be responsible for a plethora of adverse events, ranging from nausea or headache to sexual dysfunctions, tremors, dry mouth, anxiety, and restlessness [12].
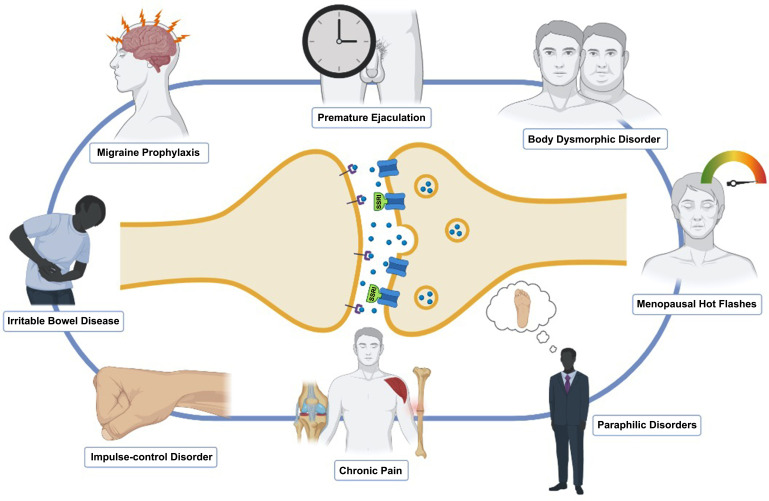
On average, a consistent number of antidepressants is prescribed by general practitioners (GPs), with most of them being administered for treating depression, and a minority for the treatment of other disorders [13, 14]. The reasons for this have to be found in few factors that improved antidepressant prescription, i.e. the increased patients’ care-seeking behavior, and most of all, the introduction of SSRIs in the 1980s [15]. A recent article pointed out how factors influencing GPs when using antidepressants may be generalized in the professional imperative of ‘doing the right thing’. Nevertheless, a wide number of primary care doctors was not aware that increased doses were not necessarily supported by higher efficacy, having low insights on drug guidelines and limitations [16]. Indeed, although to date no evidence shows that GP-prescribed drugs come with more side effects, we might infer that physicians need to pay extra caution when handling these drugs, mainly if they use them outside the guidelines of the regulatory agencies. However, even though non-psychiatrists may jump to the wrong conclusions that SSRIs are safe drugs and therefore easy to use for off-label applications, it is not hard to understand how efficacious they might be in multiple conditions (Fig. 1).
Fig. (1).
Off-label uses of SSRIs.
Although a metanalytic approach would have been desirable, the presence of a great variety of different outcome measures has made a statistical analysis impossible. Migraine, body dysmorphic disorder (BDD), impulse control disorder (ICD), irritable bowel syndrome (IBS), paraphilias, hypersexuality, and above all, premature ejaculation (PE) provided the best evidence both in literature and in clinical practice, as they are most commonly off-label prescribed. Therefore, the scope of the present article is to carefully review the literature on such off-label uses of SSRIs in order to show state of the art, the related lights, the numerous shadows, and encourage future clinical trials to further illuminate this topic.
2. MIGRAINE PROPHYLAXIS
Migraine is a medical condition that affects a huge amount of people worldwide and represents a significant burden in terms of disability and reduced quality of life [93]. Migraine is a type of headache that recurs periodically and it features a list of severe symptoms, such as photophobia, phonophobia, and nausea [94]. Dealing with the acute phase of this headache requires the use of triptans, non-steroidal anti-inflammatory drugs (NSAIDs), and antiemetic medicines.In the long run instead, preventing migraine attacks requires different approaches, like beta-blockers, antiepileptic drugs, botulinum toxin, antidepressants, monoclonal antibodies, etc. [95-98].
The theory of impaired levels of serotonin appears to be behind the pathogenesis of migraine, as these patients seem to have constant low levels of this neurotransmitter with fleeting upregulations during attacks [99, 100]. Although the serotonin theory is well established in the literature, SSRI did not show promising results compared to other antidepressants, such as TCAs [82]. Fluoxetine was the most used SSRI in randomized controlled trials (RCTs), with a dosage ranging from 20 to 40 mg/day and an average protocol duration of 1,5 months (Table 1) [17, 19, 22, 101, 102]. Only one RCT performed a comparison between amitriptyline and amitriptyline augmented with fluoxetine [20]. However, none of the present studies reported significant differences between groups.
Table 1.
List of Randomized Controlled Trials (RCT), Open-label Studies (OLS), and Retrospective Studies (RS).
| Authors | Year | Disorder | N | Drug (Posology) | Protocol | Study Type | Main Outcome Measures | Main Findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adly et al. [17] | 1992 | M | 32 | flx (20 mg/d) vs. placebo | 8 weeks | RCT | ZSDS; 1-10 headache pain scale | Significant reduction in headache pain scores compared to placebo | ||||||
| Bank [18] | 1994 | M | 32 | ami (25 mg) vs. flv (50 mg) | 12 weeks | RCT | HI | Both treatments were effective; flv reported fewer side effects | ||||||
| d’Amato et al. [19] | 1999 | M | 52 | flx (20 mg/d) vs. placebo | 24 weeks | RCT | Total pain index | Significant reduction in total pain index after the third month in the active group | ||||||
| Krymchantowski et al. [20] |
2002 | M | 39 | ami (8-40 mg/d) vs. ami+flx (8-40/mg/d) | 9 weeks | RCT | HI | Headache improvement; no difference between groups | ||||||
| Landy et al. [21] | 1999 | M | 27 | srt (50 mg/d) vs. placebo | 12 weeks | RCT | HI | Improvement was not significantly different from baseline in the active group | ||||||
| Steiner et al. [22] | 1998 | M | 65 | S-flx (40 mg) vs. placebo | 4-8 weeks | RCT | Primary: attack frequency; secondaries: migraine-days per months; PGIDS | The active group showed a reduction of 0.6 headache days per month | ||||||
| Tarlaci [23] | 2009 | M | 93 | vlx (75-150 mg/d) vs. esc (10-20 mg/d) | 12 weeks | Prospective study | HADS; BDI; headache intensity, frequency, and duration; analgesic intake; MIDAS; VAS; LWDE; | Monthly migraine frequency, duration, and intensity were decreased in the esc group, but not to the same extent as the vlx group | ||||||
| Hollander et al. [24] | 1999 | BDD | 40 | clo (25-250 mg/d) vs. des (25-250 mg/d) | 16 weeks | RCT | BDD-YBOCS; BDD-NIMH; CGI | Clo was superior to des in all outcome measures | ||||||
| Perugi et al. [25] | 1996 | BDD | 15 | flv (100-300 mg/d) | 10 weeks | OLS | HSCL-90; BDSS; CGI | Several outcome measures showed improvements | ||||||
| Phillips [26] | 2006 | BDD | 15 | esc (10-30 mg/d) | 12 weeks | OLS | BDD-YBOCS; CGI; HAMD-24; GAF; SOFAS; MOS; QLES-Q | Depressive symptoms, delusionality, functioning and quality of life significantly improved. | ||||||
| Phillips and Najjar [27] | 2003 | BDD | 15 | cit (20-60 mg/d) | 12 weeks | OLS | BDD-YBOCS; CGI; BABS | Improvements on all main outcome measures | ||||||
| Phillips et al. [28] | 2002 | BDD | 74 | flx (20-80 mg/d) vs. placebo | 12 weeks | RCT | BDD-YBOCS; CGI; BABS; BDD-NIMH; HAMD-17 | Active group showed improvements in BDD-YBOCS, both in delusional and non-delusional patients. | ||||||
| Phillips et al. [29] | 2016 | BDD | 100 | esc (10-30 mg/d) / esc (30 mg/d) vs. placebo | 14 weeks / 24 weeks | OLS / RCT | BBD-YBOCS; CGI; BABS; HAMD-17; RIFT; QLESQ-SF; BDD-PSRS | The time to relapse for patients who responded to and continued taking escitalopram was less than patients who responded to escitalopram but stopped the treatment | ||||||
| Phillips et al. [30] | 1998 | BDD | 30 | flv (50 mg/d) | 16 weeks | OLS | BDD-YBOCS; CGI; HAMD-17; BABS | Improvements on all main outcome measures | ||||||
| Stasi et al. [31] | 2019 | IBS | 50 | par (5-20 mg/d) | 16 weeks | OLS | IBS-SSS; SCID IV; CGI; HAM-A; MADRS; SCL-90; QLES-Q; ASEX | Significant improvement in both gastrointestinal and psychiatric symptoms | ||||||
| Tadyon Najafabadi et al. [32] | 2019 | IBS | 66 | flx (20 mg/d) vs. saffron (15 mg/d) | 6 weeks | RCT | IBS-QoL; HADS | Both scores improved significantly from baseline, although no statistical difference was reported between the two interventions. | ||||||
| Abu El-Hamd and Abdelhamed [33] | 2018 | PE | 150 | par (30 mg/d) vs. dapo (30 mg on-demand) vs. dapo+sild (30+50 mg/d) vs. sild (50 mg/d) vs. placebo | 6 weeks | RCT | IELT; PEDT; patient satisfaction score | IELT, satisfaction score, and PEDT improved in all active groups; dapo+sild had the best IELT improvement | ||||||
| Akgul et al. [34] | 2008 | PE | 101 | srt (50 mg/d) vs. cit (20 mg/d) | 8 weeks | RCT | IPE | Cit and srt showed both a statistical improvement in IPE, without any significant difference between groups | ||||||
| Atmaca et al. [35] | 2002 | PE | 26 | cit (20-60 mg/d) vs. placebo | 8 weeks | RCT | IELT; CGI; YSFI-II | Citalopram group reported a statistically significant increase in the IELT than that of the placebo group | ||||||
| Balci et al. [36] | 2019 | PE | 170 | par (20 mg/d) vs. flx (20 mg/d) vs. dapo (30 mg/d) | 4 weeks | RCT | ILT; PEP; CGI | Par was better than other drugs, whereas dapo had fewer side effects | ||||||
| Gameel et al. [37] | 2013 | PE | 150 | tra (50 mg on-demand) vs. sild (50 mg/d) vs. par (20 mg/d) vs. lido (2.5%) vs. placebo | 4 weeks | RCT | IELT; patient satisfaction score | Tramadol had the longer mean IELT value; sild group reported significantly better satisfaction scores | ||||||
| Gong et al. [38] | 2011 | PE | 80 | par (20 mg/d) vs. placebo | 4 weeks | RCT | IELT; patient satisfaction score | Significantly prolonged IELT and increased patient satisfaction score | ||||||
| Haensel et al. [39] | 1998 | PE | 40 | flx (5-10 mg/d) vs. placebo | 12 weeks | Randomized, double-blind, crossover study | IELT | The latency to ejaculation increased in patients with PE and erectile dysfunction | ||||||
| Hamidi-Madani [40] | 2018 | PE | 150 | tra (50 mg on-demand) vs par (20 mg on-demand) vs. placebo | 12 weeks | RCT | IELT; PEP; | Mean IELT and PEP improved in all groups | ||||||
| Hosseini and Yarmohammadi [41] | 2007 | PE | 91 | flx (20 mg/d + 20 mg on-demand) vs. flx (20 mg/d) + sild (50 mg on-demand) | 16 weeks | RCT | IELT; mean intercourse satisfaction | Flx + sild were better in improving IELT and patient satisfaction | ||||||
| Jenkins et al. [42] | 2019 | PE | 130 | flx (20-40 mg/d) vs. | 12 months | RS | IELT; self-rated control over ejaculation; personal and partner distress due to PE | Significant improvement realized in IELT, ejaculatory control, and distress levels for both men and their partners | ||||||
| Kara et al. [43] | 1996 | PE | 17 | flx (20-40 mg/d) vs. placebo | 4 weeks | RCT | IELT | Significant improvement IELT scores in the active group | ||||||
| Kim [44] | 1998 | PE | 36 | flx (40 mg/d) vs. srt (100 mg/d) vs. clo (50 mg/d) vs. placebo | 4 weeks | RCT | IELT | Clomipramine and sertraline were better than fluoxetine or placebo in increasing IELT | ||||||
| Madeo [45] | 2008 | PE | 48 | flx (20 mg/d) cit (20-40 mg/d) vs. placebo | 4 weeks | RCT | TPE; MELT; IIEEF; GRISS | TPE was not statistically different in the active groups compared to placebo. MELT was significantly improved only in the cit group; cit and flx did not affect sexual desire | ||||||
| Manasia et al. [46] | 2003 | PE | 80 | flx (90 mg/d) vs. flx (20 mg/d) | 12 weeks | RCT | IELT | Both improved IELT without statistical differences between groups | ||||||
| Mattos et al. [47] | 2008 | PE | 60 | flx (90 mg/w) + placebo vs. tad (20mg on-demand) + flx (90mg/w) vs. tad (20 mg on-demand) + placebo vs. placebo + placebo | 12 weeks | RCT | IELT | Each active group reported a statistical improvement in IELT; flx + tad was better compared to the other groups | ||||||
| Nada et al. [48] | 2012 | PE | 60 | esc (10 mg/d) vs. cit (20 mg/d) | 6 weeks | RCT | CIPE | Both treatments were equally effective on the outcome measure, without any statistical difference | ||||||
| Nada et al. [49] | 2009 | PE | 30 | esc (10 mg/d) vs. placebo | 4 weeks | RCT | IELT; CIPE (modified) | Outcome measures were significantly improved in the active group | ||||||
| Novaretti et al. [50] | 2002 | PE | 55 | flx (20 mg/d) vs. placebo | 20 weeks | RCT | HAM-A; IELT; patient satisfaction | Both IELT and satisfaction improved in the active group | ||||||
| Rezakhaniha and Sirousbakht [51] | 2010 | PE | 77 | flx (40 mg/d) vs. cit (40 mg/d) | 4 weeks | RCT | IELT | Both drugs improved IELT, without showing any statistical difference between the two groups | ||||||
| Safarinejad [52] | 2006 | PE | 340 | dapo (60 mg on-demand) vs. par (20 mg/d) vs. placebo | 12 weeks | RCT | IIEF; IELT; mean intercourse satisfaction | All outcome measures improved in the active groups; par was significantly better than dapo in improving the intercourse satisfaction | ||||||
| Safarinejad [53] | 2007 | PE | 276 | esc (10 mg/d) vs. placebo | 12 weeks | RCT | IELT; IIEF; Meares-Stamey test | Improvements in IELT and sexual satisfaction domains of IIF in the active group | ||||||
| Safarinejad and Hosseini [54] | 2006 | PE | 58 | cit (20 mg/d) vs. placebo | 6 months | RCT | IELT; IIEF; Meares-Stamey test | Cit was statistically better at improving IEALT and intercourse satisfaction compared to placebo | ||||||
| Sunay et al. [55] | 2011 | PE | 90 | par (20 mg/d) vs. acupuncture vs sham-acupuncture (placebo) | 4 weeks | RCT | IELT; PEDT | Paroxetine was significantly better to improve PEDT and IELT scores; acupuncture was better than placebo | ||||||
| Vahid et al. [56] | 2008 | PE | 80 | cit (20 mg on-demand) vs. placebo | 4 weeks | RCT | IELT; CIPE | Patients treated with on-demand cit showed a statistically greater improvement in IELT and CIPE score compared to placebo | ||||||
| Waldinger et al. [57] | 1998 | PE | 60 | flx (20 mg/d) vs. flv (100 mg/d) vs. par (20 mg/d) vs. srt (50 mg/d) vs. placebo | 6 weeks | RCT | IELT | Compared with baseline, paroxetine had the strongest delay in ejaculation, followed by fluoxetine and sertraline | ||||||
| Waldinger et al. [58] | 2001 | PE | 30 | par (20 mg/d) vs. cit (20 mg/d) | 5 weeks | RCT | IELT | Par improved IELT more than cit, whose action was defined as mild | ||||||
| Ylmaz et al. [59] | 1999 | PE | 40 | flx (20 mg/d) vs. placebo | 4 weeks | RCT | IELT; penile sensory threshold values; sacral evoked response variables; somatosensory evoked potentials; | IELT and penile sensory threshold were significantly improved in the active group | ||||||
| Coleman et al. [60] | 1992 | PAR | 16 | flx (20 mg/d) | up to three years | RS | YBOCS; HAM-A; HAM-D | Reduction of scores in all outcome measures | ||||||
| Greenberg et al. [61] | 1996 | PAR | 58 | flv; flx; srt | 12 weeks | RS | CGI; patients reported symptoms, fantasies, and urges | The severity of fantasies decreased; no significant differences in the reported efficacy between drugs | ||||||
| Kafka [62] | 1994 | PAR | 24 | srt (100 mg/d); flx (50 mg/d) | 17-18 weeks | OLS | SOI; TSO; self-reported average time per day spent in sexual fantasies | In those who responded to srt: improvement in TSO and average time; in those who did not respond to srt and who were subsequently put on flx: 6/9 showed clinical improvement | ||||||
| Kafka [63] | 1991 | PAR | 9 | flx; imi; lithium | - | OLS | IDD; SOI | Reduction of paraphilic sexual behavior | ||||||
| Kafka and Hennen [64] | 2000 | PAR | 26 | flx (50 mg/d) vs. flv (100 mg/d) vs. par (35 mg/d) vs. srt (110 mg/d) | 8 weeks | OLS | SOI; patients reported symptoms | Significant reduction of paraphilic total sexual outlet and time spent in paraphilic behaviors with fluoxetine in 22 patients | ||||||
| Kafka and Prentky [65] | 1992 | PAR | 30 | flx (40 mg/d) | 4-6 weeks | OLS | Sexual behavior and patients reported urges and fantasies | Significant reduction of paraphilic sexual behavior, hypersexuality, and depression | ||||||
| Kafka and Prentky [66] | 1992 | PAR | 20 | flx (20-60 mg/d) | 12 weeks | OLS | Sexual behavior and patients reported urges and fantasies | Reduction of paraphilic behavior | ||||||
| Kraus et al. [67] | 2007 | PAR | 16 | par (10-20 mg/d); cit (20-40 mg/d); srt (50-100 mg/d) | 12 weeks | RS | Patients reported symptoms, fantasies, and urges | Reduction of paraphilic sexual behavior | ||||||
| Stein et al. [68] | 1992 | PAR | 3 | flx (60-80 mg/d) | 2-10 months | OLS | Sexual behavior and patients reported urges and fantasies | No improvement in sexual behavior | ||||||
| LaCroix [69] | 2012 | HF | 205 | esc (10-20 mg/d) vs. placebo | 8 weeks | RCT | Frequency and severity of vasomotor symptoms; MENQOL | Significantly greater scores in total MENQOL, Vasomotor, Psychosocial and Physical subdomains; no improvement in the Sexual Function subdomain; improvement in PEG scores as well | ||||||
| Barton et al. [70] | 2010 | HF | 254 | cit (10 mg/d) vs. cit (20 mg/d) vs. cit (30 mg/d) vs. placebo | 6 weeks | RCT | Change from baseline to 6 weeks in the hot flash score; HFRDI; | Significant improvement in hot flushes with citalopram compared to placebo, without statistical differences between active groups | ||||||
| Flevari et al. [71] | 2017 | VVS | 106 | flx (10-40 mg/d) vs. placebo | 1 year | RCT | Syncope recurrence; ASI | Flx is significantly superior in patients with VVG scoring positive for anxiety tracts compared to placebo | ||||||
| Kadri et al. [72] | 2017 | NCS | 186 | cit (5-10 mg/d) | 1 month | RS | WBs | Cit seems to have a desirable effect on syncope and patients’ well-being | ||||||
| Coccaro and Kavoussi [73] |
1997 | IED-BPD | 40 | flx (20-60 mg/d) vs. placebo | 12 weeks | RCT | MOAS; CGI | Improvement of the main outcome measures | ||||||
| Coccaro et al. [74] | 1997 | AGG | 15 | flx (20-40 mg/d) vs. placebo | 12 weeks | RCT | MOAS | The active group reported a reduction in aggression scores | ||||||
| Coccaro et al. [75] | 2009 | IED | 100 | flx (20-60 mg/d) vs. placebo | 14 weeks | RCT | MOAS; CGI | Significant improvement of main outcome measures; flx has anti-aggressive properties | ||||||
| George et al. [76] | 2009 | AGG (partner abuser) | 60 | flx (10-40 mg/d) vs. placebo | 12 weeks | RCT | MOAS | Mild significant drug effect in reducing aggressivity | ||||||
| Koran et al. [77] | 2007 | Kleptomania | 24 | esc (10-20 mg/d) | 24 weeks | OLS | Theft per week; CGI | No significant effect of the drug could be seen | ||||||
| Lee et al. [78] | 2008 | AGG (partner abuser) | 26 | flx (20-60 mg/d) vs. placebo | 12 weeks | RCT | MOAS | No drug-placebo difference | ||||||
| Reist et al. [79] | 2003 | IED | 25 | cit (20-60 mg/d) | 8 weeks | OLS | MOAS | Statistically significant improvements in the main outcome measures | ||||||
| Vartiainen et al. [80] | 1995 | AGG-S | 19 | cit (20-60 mg/d) vs. placebo | 6 months | Double‐blind cross‐over study | BPRS; CGI; SDAS; GAS | The active group reported a lower frequency of aggressive events | ||||||
Abbreviations: AGG-Aggressiveness; AGG-P-Aggressiveness-Psychopathy; AGG-S-Aggressiveness-Schizophrenia; ami-amitriptyline; ASEX-Arizona Sexual Experience Scale; ASI-Anxiety Sensitivity Index; BABS-Brown Assessment of Belief Scale; BDD-Body Dysmorphic Disorder; BDD-NIMH-National Institute of Mental Health Global Obsessive-Compulsive Scale; BDD-PSRS-Psychiatric Status Rating Scale for Body Dysmorphic Disorder; BDD-YBOCS-Yale-Brown Obsessive-Compulsive Scale Modified for Body Dysmorphic Disorder; BDDSS-Body Dysmorphic Disorder Symptom Scale; BDI-Beck Depression Inventory; BIS-11-Baratt Impulsivity Scales; BPD-Borderline Personality Disorder; BPRS-Brief Psychiatric Rating Scale; CBCL-Child Behavioral Checklist; CIPE-Chinese Index of Premature Ejaculation; cit-citalopram; clo-clomipramine; CP-Chronic Pain; CR-Case Report; dapo-dapoxetine; des-desimipramine; dul-duloxetine; esc-escitalopram; FIQ-Fibromyalgia Impact Questionnaire; flv-fluvoxamine; flx-fluoxetine; GAF-Global Assessment of Functioning; GAS-Global Aggression Scale; GHQ-General Health Questionnaire; GRISS-Golombock Rust Inventory of Sexual Satisfaction; HADS-Hospital Anxiety and Depression Scale; HAM-A-Hamilton Rating Scale for Anxiety; HAMD-17-Hamilton Depression Scale-17 items; HAMD-24-Hamilton Depression Scale-24 items; HF-Hot Flushes (in menopause); HFRDI-Hot Flushes Related Daily Interference; HI-Headache Indices; HOS-Hostility; HSCL-90-Hopkins Symptoms Check List; IBS-Irritable Bowel Syndrome; IBS-QoL-IBS Quality of Life Questionnaire; IBS-SSS-IBS Symptom Severity Score; IDD-Inventory to Diagnose Depression; IED-Intermittent Explosive Disorder; IELT-Intravaginal Ejaculation Latency Time; IIEF-International Index of Erectile Function; imi-imipramine; IPE-Index of Premature Ejaculation; lido-local lidocaine gel; M-Migraine; MADRS-Montgomery and Asberg Depression Rating Scale; MDI-Major Depression Inventory; MELT-Masturbation Ejaculation Latency Time; MENQOL-Menopause-Specific Quality of Life Questionnaire; mia-mianserine; MIDAS-Migraine Disability Assessment; MOAS-Modified Overt Aggression Scale; MOS-Medical Outcomes Study health survey short form 36; MOSPM-Medical Outcomes Study Pain Measures; mrz-mirtazapine; NCS-Neurocardiogenic Syncope; NIH-CPSI-National Institutes of Health-chronic prostatitis symptom index; OLS-Open Label Study; OSA-Obstructive Sleep Apnea; PAR-Paraphilias; par-paroxetine; PDE5i-Type 5 Phosphodiesterase Inhibitors; PE-Premature Ejaculation; PEDT-Premature Ejaculation Diagnostic Tool; PEG-Pain Intensity and Interference Scale; PEP-Premature Ejaculation Profile; PGIDS-Patient’s Global Impression of Disease Severity; PHQ-15-Patient Health Questionnaire 15 items; PMDD-Premenstrual Dysphoric Disorder; PMS-Premenstrual Syndrome; PS-Pain Score; PSAP-Point Subtraction Aggression Paradigm; QLESQ-SF-Quality of Life Enjoinment and Satisfaction Questionnaire Short Form; RCT-Randomized Clinical Trial; RIFT-Range of Impairment Functioning Tool; RS-Retrospective Study; rsp-risperidone; SAIB-Scale for the Assessment of Illness Behaviour; SBS-Single Blind Study; SCID IV-Structured Clinical Interview for DSM-IV Patient Edition; SCL-90-Symptom Check List; SD-Somatoform Disorder; SDAS-Social Dysfunction and Aggression Scale; SDS-Sheehan Disability Scale; SGA-Subjective Global Assessment; sild-sildenafil citrate; SNRI-Serotonin Norepinephrine Reuptake Inhibitor; SOFAS-Social and Occupational Functioning Assessment Scale; SOI-Sexual Outlet Inventory; SR-Stroke Recovery; SRM-Serotonin Receptor Modulators; srt-sertraline; SSRI-Selective Serotonin Reuptake Inhibitor; TA-Topical Anesthetic cream; tad-tadalafil; TCA-Tricyclic Antidepressant; TeCAs-Tetracyclic Antidepressants; TESS-Treatment Emergent Symptom Scale; TPE-Test of Penile Erection; tra-tramadol; TSO-Total Sexual Outlet; VAS-Visual Analog Scale; vlx-venlafaxine; VVS-Vasovagal Syncope; WBs-Well-being score; YSFI-II- Yonsei Sexual Function Inventory-II; zim-zimelidine; ZSDS-Zung's Self-Rating Depression Scale.
Sertraline, fluvoxamine, and escitalopram were also administered in RCTs but did not show encouraging findings [18, 21, 23]. Paroxetine seems to reduce headache days in two case reports; however, larger randomized and placebo-controlled clinical trials are needed to draw more consistent conclusions [103, 104].
The present findings do not allow to determine the efficacy of SSRIs in the prevention of migraine attacks. Indeed, the American Academy of Neurology/American Headache Society guidelines registered both fluoxetine and fluvoxamine with “level U”, meaning that there are inadequate or conflicting data to support or refute medication use [105].
In addition, extra care has to be taken into account when prescribing these drugs, as a consistent number of SSRI-induced headaches has been described in the literature [106-110].
3. BODY DYSMORPHIC DISORDER (BDD)
Among psychiatric conditions, BDD is an extremely disabling illness with a very poor quality of life. BDD is characterized by debilitating preoccupations on single or multiple parts of the body (e.g. face, nose, ears, hairs, etc.). Such obsessions are frequently associated with compulsive counter behaviors, which most often represent a significant hurdle in everyday life activities [111]. BDD may feature delusional beliefs, that usually are associated with poorer patient outcomes, especially if accompanied by the absence of insight [112].
Treatment-wise, BDD may be approached through non-pharmacological and pharmacological therapies. Among the firsts, cognitive behavioral therapy (CBT) is considered a gold standard and has shown important results either as a stand-alone treatment or together with pharmacotherapy [113, 114]. In these regards, the serotoninergic signaling seems to be relatively impaired in BDD, as few trials have shown SSRIs to significantly impact the disease course. However, proves of their efficacy mostly rely on scarce evidence, as only three RCTs have been performed so far. Phillips and colleagues conducted a randomized, placebo-controlled trial where they found that patients treated with fluoxetine 20 mg, increased up to 80 mg, had better outcome scores than the control group [28]. The same authors pointed out also that the time to relapse for patients who responded to and continued taking escitalopram was longer than patients who responded to escitalopram but stopped the treatment [29]. Although clomipramine is not an SSRI, it is a powerful serotonin reuptake inhibitor; indeed, Hollander et al. showed that BDD patients treated with clomipramine had better outcome scores than those who received desipramine, a TCA without a serotoninergic action [24].
In addition to these RCTs, four open-label studies showed that escitalopram, citalopram, and fluvoxamine improved BDD and correlated symptoms in more than half of the patients [25-27, 30].
Although very limited evidence has been provided so far, SSRIs represent the best treatment option, probably because they act on the obsessive features of BDD. However, further randomized, placebo-controlled trials with standardized conditions and larger number of patients will be required to draw more consistent conclusions.
4. IMPULSE-CONTROL DISORDER (ICD)
Impulsivity is defined as the tendency to act abruptly without any sign of reflection or forethought. ICD is listed on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), featuring kleptomania, pyromania, intermittent explosive disorder (IED), and pathological gambling [115]. Research shows that serotonin might play a key role as a modulator of impulsive behaviors on the prefrontal cortex, an important hub for cognitive behavior and therefore, for the regulation of aggressiveness [116]. However, although findings are encouraging, to date, evidence remains scarce, mostly because the dogma that the activity of the 5-HT system is inversely related to impulsive-aggressive behavior seems outdated now [117-119]. Among ICDs, prior research on IED and impulsive aggressiveness reports the highest number of studies with SSRIs showing to improve all main outcome measures. However, as of now, only three RCTs exist in the literature, with fluoxetine 20-60 mg/day for an average of 12 weeks being the most common protocol of administration [73-75], and only one open-label study with citalopram 20-60 mg/day for 8 weeks [79]. SSRIs were also used to treat impulsive aggressivity in special populations, showing positive results in patients with schizophrenia [80] and only a few to moderate improvements in partner abusers (as study populations were exiguous and only one trial reported mild drug-placebo differences in the outcome measures-for details, see Table 1) [76, 78]. Results on kleptomania, defined as the urge to pointlessly steal items, are very weak and conflicting, as only one open-label study [77] and two case reports [120, 121] on escitalopram, paroxetine, and fluvoxamine have been published to date. Similarly, a systematic review on compulsive shopping analyzed four RCTs and stated that results were of mixed quality and no consistent conclusion on the effectiveness of SSRIs could be drawn [92].
Results summarized so far preclude the possibility to establish whether SSRIs have therapeutic efficacy in ICD. More trials with larger populations are needed to shed a brighter light on this topic.
5. IRRITABLE BOWEL SYNDROME (IBS)
IBS is a functional disorder that features diarrhea, constipation, bloating, and abdominal pain. The etiology is multifactorial, as immune, genetic, and, most of all, psychological factors concur to the onset of symptoms [122]. Currently, diagnosis is made through the Rome IV diagnostic criteria [123]. Therapeutic approaches comprise rifaximin, 5-HT3 antagonists, antispasmodic agents, loperamide, and antidepressants [124]. Although TCAs proved to be the most effective antidepressants, SSRIs showed encouraging results in the treatment of IBS. Indeed, in a recent review, the authors analyzed eight RCTs on SSRIs compared with placebo and concluded that in half of the studies the active group reported significant improvements in IBS symptoms [84]. However, most of the clinical trials on TCAs summarized in this review showed a major impact on global symptom relief. Recently, two clinical trials with paroxetine 5-20 mg/daily [31] and fluoxetine 20 mg/daily [32], with 150 and 61 subjects respectively, reported significant improvements in both patient-reported gastrointestinal symptoms and clinical evaluations.
SSRIs showed promising results also on a pediatric population suffering from functional abdominal pain. A recent review article found three studies (one open-label, one RCT, and one retrospective study) where the authors reported positive findings [83]. However, the evidence is still not consistent and future trials will be needed to assess the efficacy of SSRIs in such a population more appropriately.
The evidence summarized so far strengthens the concept that SSRIs can be used as a complementary treatment option, as they showed improved gastrointestinal symptoms in patients with IBS. Indeed, a proof of concept of this was given by a recent article, where authors administered either sertraline 10 mg/kg/day, nortriptyline 10 mg/kg/day, or a phosphate buffer (placebo) to rats exposed to chronic stress. They showed that while chronic stress-induced neurons to increase in the myenteric plexus, sertraline and nortriptyline prevented cellular hyperplasia of the plexus [125]. Nevertheless, positive clinical results have to be addressed also to the impact of SSRIs on co-occurring psychiatric symptoms that frequently worsen the disease course. Moreover, a large body of literature stresses how serotonin has a major role in the pathophysiology of IBS, as disrupted 5-HT signaling may lead to either diarrhea or constipation [126]. In this respect, Lin et al. investigated the relationship between the use of SSRIs and the consequent onset of IBS in a population-based cohort study in Taiwan. The authors found that patients treated with SSRIs had a greater risk of developing IBS compared to SSRI non-users [127].
Therefore, despite the encouraging findings, data so far remain scarce. For this reason, more randomized, double-blinded, placebo-controlled clinical trials with greater populations are needed to definitively assess the effectiveness of SSRIs in IBS.
6. PARAPHILIAS AND HYPERSEXUALITY
A number of authors have shown important evidence on the use of SSRIs in the treatment of both paraphilic disorders and hypersexuality.
According to the DSM-5, paraphilias are defined as ‘recurrent, intense, sexually arousing fantasies, sexual urges, or behaviors involving sexual activity’ with non-human objects, children, nonconsenting people or consisting in suffering or humiliating oneself or one’s partner [115]. DSM-5 lists eight categories of paraphilic disorders (exhibitionistic, fetishistic, frotteuristic, paedophilic, sexual masochism, sexual sadism, voyeuristic, and transvestic disorder) with an extra one merging all the residuals (e.g., necrophilia, coprophilia, telephone scatology, etc.).
Unlike other psychotropic drugs, whose level of evidence has been classified as “E”, SSRIs proved significant therapeutic effects in patients affected by paraphilic disorders [128]. This may be explained by multiple reasons. First, evidence has shown that enhanced serotoninergic levels are able to dampen sexual drive both in human and in animal models [129]. Secondly, SSRIs are approved for OCD [130]; thus, it easy to understand how they might treat ‘compulsive’ features in paraphilias as well as in hypersexuality [131]. Lastly, SSRIs are proven both to increase the level of brain-derived neurotrophic factor and bind to its receptor, promoting neuroplasticity and therefore a possible enhanced capability of changing maladaptive behaviors, leading eventually to a better clinical outcome [132, 133]. In this regard, further studies on pharmacological treatment with SSRIs should be performed to establish the boundaries between normal and pathological sexuality in these patients.
Among SSRIs, fluoxetine showed the strongest evidence, with ten case reports, six open-label, and two retrospective studies. Protocols consisted of the administration of a mean dose of 40 mg/day, gradually increasing it until the remission of symptoms, for 4-6 weeks. Patients were treated for different forms of disorders, i.e. pedophilia [134, 135], exhibitionism [136, 137], fetishism [138], voyeurism [139], frotteurism [140, 141], and various paraphilias [60-66, 68, 142]. Fluoxetine was able to reduce paraphilic fantasies or conducts in the majority of studies; however, in one case study, the authors did not observe a reduction of paraphilic behaviors [68].
Evidence on sertraline showed positive results in three case reports, two open-label trials, and two retrospective studies, meaning that both fantasies and behaviors were reduced. The mean dose was 100 mg/daily for a mean duration of treatment of 17 weeks. Participants were treated for diverse conditions, such as pedophilia [143, 144], transvestic disorder [145, 146], frotteurism [147], voyeurism [148], and various paraphilias [61, 62, 64, 67] (Table 1).
Compared to fluoxetine and sertraline, evidence on fluvoxamine, paroxetine, and citalopram is scarce. Fluvoxamine showed positive findings in one case report [149] and two open-label trials [61, 64]. Encouraging results were reported also in one retrospective study [67], one open-label study [64], and two case reports, where the authors used paroxetine 20 mg/day to treat sexual disinhibition [150], and paroxetine 10 mg/day increasing up to 20 mg/day to treat compulsive voyeurism and exhibitionism [151].
The only controlled study was performed using citalopram, where the authors administered 20-60 mg/day vs. placebo in 28 homosexual men with compulsive sexual behavior for 12 weeks [152]. Moreover, this was the only trial, where a standardized outcome measure was used, i.e., the Yale-Brown OCD scale. In this study, the frequency of masturbation, sexual drive, and pornography use were significantly reduced in all participants.
Results summarized so far do not allow us to draw specific and solid conclusions on the effectiveness of SSRIs in paraphilic disorders. Indeed, RCTs with a standardized approach are lacking, as outcome measures like fantasies or behaviors are poorly quantifiable. Moreover, people were not treated for one condition, but many, and most of them were comorbid with other disorders, such as hypersexuality or depression. Lastly, the number of enrolled patients was exiguous. A mean of 30 participants, with 58 in only one study, was in fact reported in the open-label trials. The only double-blind clinical trial was conducted on patients whose primary feature was OCD; it is, therefore, difficult to generalize such findings to individuals with paraphilic disorders.
To date, the gold standard for paraphilic disorders remains gonadotropin-releasing hormones (GnRH)-agonists. However, the World Federation of Societies of Biological Psychiatry recommends the use of SSRIs in mild paraphilic disorders that did not respond to CBT and that have comorbidity with OCD [128].
Sexual addiction, also known as hypersexuality, is defined as compulsive sexual behavior, in which patients crave 7 or more orgasms per week, for at least 6 months, or more than one orgasm per day, for 1 year [153]. Hypersexuality might also be secondary to many neuropsychiatric disorders, such as bipolar disorder [154], frontotemporal and Alzheimer’s dementia [155] or autism [156]. The neuroscience behind it indicates a disruption in the mesolimbic dopaminergic reward pathway as a primary etiology [157]. However, as mentioned above, serotonin plays a key role in treating the compulsive features of psychiatric disorders. Besides, many patients have depressive and/or anxious comorbidities. For these reasons, SSRIs may have a place of choice when dealing with hypersexuality. On the other hand, the relationship between post-traumatic stress symptoms and hypersexual behavior was demonstrated, and the SSRIs treatment could be useful to cope with these two comorbid conditions [131].
Nevertheless, as of now, evidence remains scarce and poorly conclusive. Apart from the double-blind clinical trial using citalopram [152], only eleven case reports are present in the literature [158-168], with citalopram and fluoxetine 20 mg/day being the most common protocols of administration. Furthermore, many patients were treated with an SSRI in augmentation with other drugs, such as naltrexone or risperidone, meaning that further analyses are needed to assess which active principle was more effective.
In conclusion, the evidence on both paraphilic disorder and hypersexuality is limited to one clinical trial and few case reports. For this reason, larger cohorts and more standardized protocols will be required to draw more specific conclusions and therefore set solid guidelines for clinical practice.
7. PREMATURE EJACULATION (PE)
Amongst sexual dysfunctions and complaints, PE is one of the most common and highly prevalent sexual problems worldwide [169]. PE is a tridimensional condition defined as i) inability to control ejaculation, ii) ejaculation that occurs less than or within one-three minutes of vaginal penetration, iii) consequent couple’s distress [170, 171]. To date, a consistent amount of approaches can be used to treat PE, with most of the guidelines recommending pharmacotherapy as the gold standard (level of evidence 1a) [171-173], possibly integrated by counseling and, in selected patients, by cognitive-behavioral therapies [174]. In this respect, multiple choices are available, ranging from topical anesthetics to SSRIs [86, 175]. Over time, a large body of literature has shown significant evidence on the use of long-acting selective serotonin reuptake inhibitors (i.e. paroxetine [33, 37, 38, 40, 52, 55, 57, 58, 88], fluoxetine [39, 41-47, 50, 57, 59, 176], sertraline [87], fluvoxamine [57], citalopram [34, 35, 45, 51, 54, 56, 177], and escitalopram [48, 49, 53]) in sexual medicine. The rationale behind this is explained by the fact that serotonin plays a key role in regulating ejaculatory reflex, as a higher tone may lead to delayed ejaculation or even anejaculation and/or anorgasmia [178]. However, although the efficacy in delaying intravaginal ejaculation latency time (IELT) was high, the number of patients enrolled in these studies is consistently low, ranging from 25 up to a maximum of 276 individuals. For these reasons, data on long-acting SSRIs, although interesting, are scarce and studies with bigger cohorts will be needed in the future. Therefore, the appearance of the first and unique approved on-demand, short-acting, sexuality-sparing SSRI, named dapoxetine has to be considered welcome.
Dapoxetine has a mean half-life of 1.3-1.5 h, which makes it highly suitable for on-demand purposes. A recent metanalysis on 11 RCTs concluded that both dapoxetine 30 and 60 mg regimens are highly effective in increasing IELT and patient-based impression scores, such as perceived control over ejaculation and patient-reported global impression of change [85]. Moreover, the authors found that this effect was dose-dependent as a higher posology was associated with better outcomes as well as more adverse effects (AEs). The incidence of AEs was reasonably low and much lower than that of SSRIs chronically used. In particular, no suicides have been recorded under dapoxetine, even at the highest dosage, and the presence of sexual side effects (e.g., hypoactive sexual desire disorder, erectile dysfunction, delayed ejaculation, anejaculation, and anorgasmia) was significantly lower in comparison with other SSRIs. For this reason, dapoxetine has been defined as the unique erection-sparing SSRI [179]. The very short half-life, the consequent inability to cross the blood-brain barrier, and the incapacity to directly modify mood are likely to be considered the reasons for having the best safety profile within the SSRIs.
In conclusion, based on the fact that RCTs on dapoxetine are the largest, with up to 6000 participants, when it comes to the clinical practice, dapoxetine 30 mg has to be considered the best choice compared to other long-acting SSRIs such as paroxetine 20 mg [86].
8. OTHER OFF-LABEL APPLICATIONS OF SSRIs
The catalog of SSRIs off-label uses is not limited to the ones mentioned above. A consistent body of literature stresses the importance of SSRIs in the treatment of hot flashes in menopause, with the best evidence coming from paroxetine [89], escitalopram [69], and citalopram [70].
On the other hand, research on the use of SSRIs in the treatment of autism spectrum disorders (ASD), in which pharmacotherapies are commonly thought of as auxiliary to behavioral interventions, has proven to be inconclusive. Although many core features of ASD, such as obsessive-compulsive behavior, impairment in social interaction, and mood, may be attributed to serotonin alterations, no evidence of effectiveness has been found in children yet, with only poor data supporting the use of SSRIs in adults [180].
The rationale behind the use of SSRIs in the treatment of chronic pain (CP) consists in the attempt to modulate the inhibitory descending fibers that originate from the brainstem and that suppress pain neurotransmission by releasing 5-HT. In fact, dysfunction of these systems is likely to induce impaired descending serotonin antinociceptive pathways that are responsible for pain chronicization [181]. A recent review article showed that 36 RCTs on SSRIs have been carried out, with escitalopram, fluoxetine, and fluvoxamine showing the most encouraging results (Table 2) [81]. These findings seem to open new promising scenarios for treating chronic pain. Fluoxetine, for example, has been inserted in the Canadian guidelines for fibromyalgia at the dosage of 20-40 mg [182]. However, a few points must be addressed. Since multiple different conditions meet CP criteria, the lack of standardized protocols will not allow more consistent conclusions to be drawn. Furthermore, larger populations and a longer duration of the treatment will be required, as chronic pain illnesses often last a consistent amount of time.
Table 2.
List of reviews and meta analysis.
| Authors | Year | No. of Reviewed Studies | Disorder | Drug(s) | Main Findings |
|---|---|---|---|---|---|
| Patetsos and Hojales-Araujo [81] |
2016 | 36 | CP | SSRI | SSRIs seem to affect most of CP conditions |
| Burch [82] | 2019 | 27 | M | SSRIs; SNRIs; TCAs; mrz | SSRI do not show evidence of efficacy compared to other antidepressants |
| Bonilla and Nurko [83] |
2018 | 4 | IBS (children) |
cit; ami; | Evidence of the efficacy of antidepressants on a pediatric population is not consistent yet |
| Kułak-Bejda et al. [84] |
2017 | 29 | IBS | TCAs; mia; SSRIs; SNRIs |
All antidepressants are effective in improving IBS symptoms compared to placebo; TCAs were more effective than SSRIs |
| Du et al. [85] | 2019 | 11 | PE | dapo | On-demand use of dapoxetine is effective in the treatment of PE, with 60 mg showing better results than 30 mg |
| Jian et al. [86] | 2018 | 40 | PE | (short- and long-acting) SSRIs; PDE5i; tra; TAs | dapo 30 mg on-demand was the best treatment option |
| Yi et al. [87] | 2019 | 12 | PE | srt | Srt significantly delayed IELT after 4, 6 and 8 weeks of treatment. In addition, patient-reported and spouse-reported sexual satisfaction rates were also improved. |
| Zhang et al. [88] | 2019 | 19 | PE | par | Par was the most effective drug to increase IELT compared to other SSRIs such as fluoxetine and escitalopram. |
| Riemma et al. [89] | 2019 | 5 | HF | par | The active groups reported a significant reduction in hot flushes compared to placebo, showing that low-dose paroxetine is an effective treatment |
| Appleton [7] | 2018 | 16 | PMS/PMDD | SSRIs | SSRIs are the first-line therapy in PMS/PMDD |
| AbdelFattah et al. [90] |
2020 | 8 | OSA | TCAs; TeCas; SSRIs; SRM | Only mirtazapine and trazodone showed a significant improvement in OSA gravity indices; |
| Legg et al. [91] | 2019 | 63 | SR | SSRIs | There is no reliable evidence that SSRIs for SR should be used in the clinical practice |
| Hague et al. [92] | 2016 | 29 | Compulsive Buying | complete pharmacotherapy |
Results on SSRIs were of mixed quality |
Abbreviations: ami-amitriptyline; cit-citalopram; CP-Chronic Pain; dapo-dapoxetine; HF-Hot Flushes (in menopause); IBS-Irritable Bowel Syndrome; M-Migraine; mia-mianserine; OSA-Obstructive Sleep Apnea; par-paroxetine; PDE5i-Type 5 Phosphodiesterase Inhibitors; PE-Premature Ejaculation; PMDD-Premenstrual Dysphoric Disorder; PMS-Premenstrual Syndrome; SNRI-Serotonin Norepinephrine Reuptake Inhibitor; SR-Stroke Recovery; SRM-Serotonin Receptor Modulators; srt-sertraline; SSRI-Selective Serotonin Reuptake Inhibitor; TCA-Tricyclic Antidepressant; TeCAs-Tetracyclic Antidepres.
SSRIs are also known to increase REM latency and suppress REM sleep; moreover, they are considered as a first-line treatment for core symptoms of post-traumatic stress disorder. For these reasons, they have been proposed to treat reoccurring nightmares in patients following a traumatic event. Research reports some improvements in sleep quality; however, the literature on PTSD-related nightmares remains inconsistent, with little evidence coming only from paroxetine and citalopram [183].
Negative conclusions were also reported for the use of paroxetine 20 mg or 40 mg in the treatment of obstructive sleep apnea, although serotonin has a pivotal role in exciting the motor innervation of the upper airway muscles [90].
Even though preliminary data on the use of SSRIs for stroke recovery were encouraging, recent findings reported no consistent evidence that they should be used in clinical practice [91].
The consensus is still lacking on the effectiveness of SSRIs on neurocardiogenic syncope. Although recent evidence pointed out positive results of fluoxetine [71] and citalopram [72], more randomized clinical trials are needed to come to more consistent conclusions.
The pharmacotherapy of borderline personality disorder oftentimes involves the administration of SSRIs to treat the core symptoms of this condition, such as anger, anxiety, depression, mood swings, etc. However, a recent review article stated that only three RCTs are present in the literature and therefore there is an urgent need for more trials [184].
The serotonin system is a treatment target of disordered eating behaviors such as food addiction, compulsive grazing, and night eating syndrome in the context of the obesity epidemic [185, 186]. These maladaptive eating patterns are characterized by loss of control on (or regarding) food consumption, impulsivity, and may be associated with body image dissatisfaction [187, 188]. Accordingly, SSRIs administration was suggested as a potential treatment strategy, particularly in combination with psychotherapies [189].
Interestingly, SSRIs might be having an important role in the management of the recently discovered SARS-CoV-2-derived disease. In particular, fluoxetine might be responsible for a plethora of antiviral mechanisms, namely controlling a neuroendocrine response, which would lead in turn to an immune modulation against the infection, or, through its interaction with ACE2 receptors, competing with the virus to enter the cell [190]. Moreover, recent evidence has pointed out how fluoxetine has lysosomotropic properties, inhibiting endosomal maturation and therefore disrupting the viral intracellular trafficking [191]. However, to date evidence comes only from in vitro or model system studies and, thus, so far no clinical data are available to support such an effect. For these reasons, these remain only but mere speculations.sants.
CONCLUSION
SSRIs are widely prescribed drugs, mostly because of their off-label applications. Nonetheless, only a little research has been carried out on off-label prescribing of SSRIs and antidepressants in general. A recent study by Wong and colleagues found that 29% of antidepressant prescriptions were off-label, of which 21.8% were SSRIs [9]. Chen et al. stated that 75% of the study population reported the administration of at least one antidepressant off-label [192]. On the other hand, three studies reported that 26% [3], 31% [1], 18% [193] of antidepressant prescriptions were off-label, however without focusing on drugs for depression in particular. To the bestour knowledge, only these works exist in the literature, showing highly heterogeneous results. In addition, reviews on SSRI off-label uses are lacking as the last one was published only in 2003 [194].
The present article shows evidence of the applications of SSRIs on a great variety of conditions. These span from migraine prophylaxis to hypersexuality, with the best evidence coming from their use in the treatment of PE, chronic pain, and paraphilias (not including FDA- and EMA-approved disorders). Such results suggest that SSRIs are extremely versatile drugs. Moreover, as opposed to many other active principles like benzodiazepines or ketamine, they are not addictive. For these reasons, SSRIs are considered as easy to handle and they are routinely prescribed by GPs, often without solid scientific evidence [9]. Indeed, when it comes to off-label prescribing, it might be problematic for GPs to keep track of recently approved indications for different disorders, especially when pharmaceutical companies encourage physicians to prescribe drugs outside the guidelines of the regulatory agencies [195, 196]. Besides, a common belief is that antidepressants, and SSRIs in particular, may be easily interchangeable within the same class [9].
However, although they were considered as having a better safety profile compared to TCAs, with less life-threatening events and overall side effects, recent evidence is pointing towards an opposite situation, with a higher hazard risk of SSRIs over their predecessors in some cases [197]. Indeed, other than a serotoninergic syndrome, serious conditions like increased bleeding rates or hyponatremia are consistently more prominent when using SSRIs. In 2004, FDA warned of a higher risk of suicidality associated with the use of SSRI, especially in children and adolescents [198]. Although nowadays this warning has been intensively discussed, physicians have to consider that a mood disorder may hide behind a given non-psychiatric condition and concur to both the onset and worsening of the condition itself. Indeed, SSRIs, causing a consistent mind activation, with symptoms of restlessness, irritability, or emotional liability may lead to significant state changes that foster suicidality, especially in people with a misdiagnosed bipolar disorder [199].
Recently, major concerns have been raised over a treacherous condition defined as post-SSRI sexual dysfunction (PSSD). PSSD is a set of sexual adverse effects featuring pleasure-loss, genital anesthesia, weak-orgasm or anorgasmia, lower sex-drive, that may arise when SSRIs are being administered and endure after their discontinuation [200]. Many etiologies have been addressed to PSSD, from serotonin neurotoxicity to epigenetic modifications [201]; however, the diagnosis is still largely challenging and requires ruling out all other possible causes of sexual dysfunctions. Moreover, no effective treatment has been proposed yet, with phototherapy and low-laser irradiation showing only but mild results [200].
These insidious side effects might be commonly underestimated during common clinical practice, especially by non-psychiatrists. For this reason, few considerations must be taken into account to avoid major issues when off-label prescribing SSRIs. GPs must bear in mind that, although sharing the same mechanism of action, i.e. the inhibition of 5-HT reuptake, each SSRI molecule may have additional and peculiar pharmacodynamic properties that in each individual patient could impact on the therapeutic effect in various conditions as well as on safety issues. Therefore, it would be wrong to consider SSRIs as highly interchangeable. Secondly, GPs oftentimes find it hard to interpret and review findings that are present in literature in a critical way [202]. For this reason, e-prescribing programs must be used extensively in order to warn physicians whether a certain off-label prescription is supported by solid scientific evidence or not. Lastly, since SSRIs are antidepressants and, therefore, psychiatric drugs, it would be of vital importance to request a specialistic examination before administering these medicines to patients. Indeed, to date, there is a unique scientific society, which is the Italian Society of Andrology and Sexual Medicine, that advocates the need for psychiatric consultation and for a psychometric diagnosis of depression in patients to be off-label treated with SSRIs [171]. In the US, the Affordable Care Act of 2010 encourages a higher collaboration among doctors providing multidisciplinary networks in order to share responsibilities and, therefore, provide complete and wider health care for patients [203].
In conclusion, trials on SSRIs show that they are extremely versatile drugs, providing encouraging evidence when used by experts to treat different conditions outside mood disorders. Nevertheless, they must not be considered as over-the-counter medicines and therefore freely prescribed regardless of possible serious adverse effects. For this reason, the take-home message of this literature review is that SSRIs can be considered for a wide range of conditions, with evidence coming from PE, chronic pain, and paraphilias. However, primary care physicians and doctors from other specialties must bear in mind that they are psychiatric drugs and therefore a thorough psychiatric evaluation and supervision will be needed before every off-label administration to reduce the risks for both the patient and the prescriber.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- AE
Adverse Effect
- ASD
Autism Spectrum Disorders
- BDD
Body Dysmorphic Disorder
- CBT
Cognitive Behavioral Therapy
- CP
Chronic Pain
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- GnRH
Gonadotropin Releasing Hormone
- GP
General Practitioners
- IBS
Irritable Bowel Syndrome
- ICD
Impulsive-control Disorder
- IED
Intermittent Explosive Disorder
- IELT
Intravaginal Ejaculation Time
- OCD
Obsessive-compulsive Disorder
- PE
Premature Ejaculation
- PSSD
Post-SSRI Sexual Dysfunction
- PTSD
Post-traumatic Stress Disorder
- RCT
Randomized Control Trial
- SSRI
Selective Serotonin Reuptake Inhibitor
- TCA
Tricyclic Antidepressant
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
EAJ is partially supported by the PRIN grant #2017S9KTNE_002.
CONFLICT OF INTEREST
EAJ is or has been paid speaker or consultant for Bayer, Ibsa, Lundbeck, Otsuka, Menarini, Pfizer, Schionogi. GDL has received personal fees from Angelini, FB Health, Lundbeck, Neuraxpharm, and Otsuka for speaking. TBJ, EB, CN, MT, GC and AS declare no conflict of interest.
REFERENCES
- 1.Radley D.C., Finkelstein S.N., Stafford R.S. Off-label prescribing among office-based physicians. Arch. Intern. Med. 2006;166(9):1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 2.Alexander G.C., Gallagher S.A., Mascola A., Moloney R.M., Stafford R.S. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol. Drug Saf. 2011;20(2):177–184. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eguale T., Buckeridge D.L., Winslade N.E., Benedetti A., Hanley J.A., Tamblyn R. Drug, patient, and physician characteristics associated with off-label prescribing in primary care. Arch. Intern. Med. 2012;172(10):781–788. doi: 10.1001/archinternmed.2012.340. [DOI] [PubMed] [Google Scholar]
- 4.Stafford R.S. Regulating off-label drug use--rethinking the role of the FDA. N. Engl. J. Med. 2008;358(14):1427–1429. doi: 10.1056/NEJMp0802107. [DOI] [PubMed] [Google Scholar]
- 5.Faquih A.E., Memon R.I., Hafeez H., Zeshan M., Naveed S. A review of novel antidepressants: a guide for clinicians. Cureus. 2019;11(3):e4185. doi: 10.7759/cureus.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lochmann D., Richardson T. Selective serotonin reuptake inhibitors. Handb. Exp. Pharmacol. 2019;250:135–144. doi: 10.1007/164_2018_172. [DOI] [PubMed] [Google Scholar]
- 7.Appleton S.M. Premenstrual Syndrome: Evidence-based evaluation and treatment. Clin. Obstet. Gynecol. 2018;61(1):52–61. doi: 10.1097/GRF.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 8.Poluzzi E., Piccinni C., Sangiorgi E., Clo M., Tarricone I., Menchetti M., De Ponti F. Trend in SSRI-SNRI antidepressants prescription over a 6-year period and predictors of poor adherence. Eur. J. Clin. Pharmacol. 2013;69(12):2095–2101. doi: 10.1007/s00228-013-1567-8. [DOI] [PubMed] [Google Scholar]
- 9.Wong J., Motulsky A., Abrahamowicz M., Eguale T., Buckeridge D.L., Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi: 10.1136/bmj.j603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin B. Selective serotonin reuptake inhibitors versus tricyclic antidepressants in young patients: A meta-analysis of efficacy and acceptability. Clin. Ther. 2014;36(7):1087–1095. doi: 10.1016/j.clinthera.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Coupland C., Hill T., Morriss R., Moore M., Arthur A., Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: cohort study using a primary care database. BMC Med. 2018;16(1):36. doi: 10.1186/s12916-018-1022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy T.K., Segarra A., Storch E.A., Goodman W.K. SSRI adverse events: how to monitor and manage. Int. Rev. Psychiatry. 2008;20(2):203–208. doi: 10.1080/09540260801889211. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C.F., Macdonald H.J., Atkinson P., Buchanan A.I., Downes N., Dougall N. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br. J. Gen. Pract. 2012;62(604):e773–e779. doi: 10.3399/bjgp12X658304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petty D.R., House A., Knapp P., Raynor T., Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing. 2006;35(5):523–526. doi: 10.1093/ageing/afl023. [DOI] [PubMed] [Google Scholar]
- 15.Moore M., Yuen H.M., Dunn N., Mullee M.A., Maskell J., Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009;339:b3999. doi: 10.1136/bmj.b3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson C.F., Williams B., MacGillivray S.A., Dougall N.J., Maxwell M. ‘Doing the right thing’: factors influencing GP prescribing of antidepressants and prescribed doses. BMC Fam. Pract. 2017;18(1):72. doi: 10.1186/s12875-017-0643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adly C., Straumanis J., Chesson A. Fluoxetine prophylaxis of migraine. Headache. 1992;32(2):101–104. doi: 10.1111/j.1526-4610.1992.hed3202101.x. [DOI] [PubMed] [Google Scholar]
- 18.Bánk J. A comparative study of amitriptyline and fluvoxamine in migraine prophylaxis. Headache. 1994;34(8):476–478. doi: 10.1111/j.1526-4610.1994.hed3408476.x. [DOI] [PubMed] [Google Scholar]
- 19.d’Amato C.C., Pizza V., Marmolo T., Giordano E., Alfano V., Nasta A. Fluoxetine for migraine prophylaxis: a double-blind trial. Headache. 1999;39(10):716–719. doi: 10.1046/j.1526-4610.1999.3910716.x. [DOI] [PubMed] [Google Scholar]
- 20.Krymchantowski A.V., Silva M.T., Barbosa J.S., Alves L.A. Amitriptyline versus amitriptyline combined with fluoxetine in the preventative treatment of transformed migraine: a double-blind study. Headache. 2002;42(6):510–514. doi: 10.1046/j.1526-4610.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- 21.Landy S., McGinnis J., Curlin D., Laizure S.C. Selective serotonin reuptake inhibitors for migraine prophylaxis. Headache. 1999;39(1):28–32. doi: 10.1046/j.1526-4610.1999.3901028.x. [DOI] [PubMed] [Google Scholar]
- 22.Steiner T.J., Ahmed F., Findley L.J., MacGregor E.A., Wilkinson M. S-fluoxetine in the prophylaxis of migraine: a phase II double-blind randomized placebo-controlled study. Cephalalgia. 1998;18(5):283–286. doi: 10.1046/j.1468-2982.1998.1805283.x. [DOI] [PubMed] [Google Scholar]
- 23.Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin. Neuropharmacol. 2009;32(5):254–258. doi: 10.1097/WNF.0b013e3181a8c84f. [DOI] [PubMed] [Google Scholar]
- 24.Hollander E., Allen A., Kwon J., Aronowitz B., Schmeidler J., Wong C., Simeon D. Clomipramine vs desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch. Gen. Psychiatry. 1999;56(11):1033–1039. doi: 10.1001/archpsyc.56.11.1033. [DOI] [PubMed] [Google Scholar]
- 25.Perugi G., Giannotti D., Di Vaio S., Frare F., Saettoni M., Cassano G.B. Fluvoxamine in the treatment of body dysmorphic disorder (dysmorphophobia). Int. Clin. Psychopharmacol. 1996;11(4):247–254. doi: 10.1097/00004850-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Phillips K.A. An open-label study of escitalopram in body dysmorphic disorder. Int. Clin. Psychopharmacol. 2006;21(3):177–179. doi: 10.1097/01.yic.0000194378.65460.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips K.A., Najjar F. An open-label study of citalopram in body dysmorphic disorder. J. Clin. Psychiatry. 2003;64(6):715–720. doi: 10.4088/JCP.v64n0615. [DOI] [PubMed] [Google Scholar]
- 28.Phillips K.A., Albertini R.S., Rasmussen S.A. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch. Gen. Psychiatry. 2002;59(4):381–388. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 29.Phillips K.A., Keshaviah A., Dougherty D.D., Stout R.L., Menard W., Wilhelm S. Pharmacotherapy relapse prevention in body dysmorphic disorder: a double-blind, placebo-controlled trial. Am. J. Psychiatry. 2016;173(9):887–895. doi: 10.1176/appi.ajp.2016.15091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips K.A., Dwight M.M., McElroy S.L. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J. Clin. Psychiatry. 1998;59(4):165–171. doi: 10.4088/JCP.v59n0404. [DOI] [PubMed] [Google Scholar]
- 31.Stasi C., Caserta A., Nisita C., Cortopassi S., Fani B., Salvadori S., Pancetti A., Bertani L., Gambaccini D., de Bortoli N., Dell’Osso L., Blandizzi C., Marchi S., Bellini M. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: A longitudinal assessment. J. Gastroenterol. Hepatol. 2019;34(4):713–719. doi: 10.1111/jgh.14375. [DOI] [PubMed] [Google Scholar]
- 32.Tadyon N.B. Therapeutic effects of saffron (Crocus sativus) versus fluoxetine on Irritable Bowel Syndrome: A double-blind randomized clinical trial. Adv. Integr. Med. 2019;6(4):167–173. doi: 10.1016/j.aimed.2019.01.001. [DOI] [Google Scholar]
- 33.Abu El-Hamd M., Abdelhamed A. Comparison of the clinical efficacy and safety of the on-demand use of paroxetine, dapoxetine, sildenafil and combined dapoxetine with sildenafil in treatment of patients with premature ejaculation: A randomised placebo-controlled clinical trial. Andrologia. 2018;50(1):12829. doi: 10.1111/and.12829. [DOI] [PubMed] [Google Scholar]
- 34.Akgül T., Karakan T., Ayyildiz A., Germiyanoğlu C. Comparison of sertraline and citalopram for treatment of premature ejaculation. Urol. J. 2008;5(1):41–45. [PubMed] [Google Scholar]
- 35.Atmaca M., Kuloglu M., Tezcan E., Semercioz A. The efficacy of citalopram in the treatment of premature ejaculation: a placebo-controlled study. Int. J. Impot. Res. 2002;14(6):502–505. doi: 10.1038/sj.ijir.3900918. [DOI] [PubMed] [Google Scholar]
- 36.Balci M., Atan A., Senel C., Guzel O., Aslan Y., Lokman U., Kayali M., Bilgin O. Comparison of the treatment efficacies of paroxetine, fluoxetine and dapoxetine in low socioeconomic status patients with lifelong premature ejaculation. Cent. Eur J. Urol. 2019;72(2):185–190. doi: 10.5173/ceju.2019.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gameel T.A., Tawfik A.M., Abou-Farha M.O., Bastawisy M.G., El-Bendary M.A. El-Gamasy, Ael-N. On-demand use of tramadol, sildenafil, paroxetine and local anaesthetics for the management of premature ejaculation: A randomised placebo-controlled clinical trial. Arab J. Urol. 2013;11(4):392–397. doi: 10.1016/j.aju.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong Z.Y., Tang T.L., Cui S., Wang J.Z., Deng X.Z. Oral paroxetine for premature ejaculation: a randomized controlled study. Zhonghua Nan Ke Xue. 2011;17(10):923–925. [PubMed] [Google Scholar]
- 39.Haensel S.M., Klem T.M., Hop W.C., Slob A.K. Fluoxetine and premature ejaculation: a double-blind, crossover, placebo-controlled study. J. Clin. Psychopharmacol. 1998;18(1):72–77. doi: 10.1097/00004714-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Hamidi-Madani A., Motiee R., Mokhtari G., Nasseh H., Esmaeili S., Kazemnezhad E. The efficacy and safety of on-demand tramadol and paroxetine use in treatment of life long premature ejaculation: A randomized double-blind placebo-controlled clinical trial. J. Reprod. Infertil. 2018;19(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- 41.Hosseini M.M., Yarmohammadi H. Effect of fluoxetine alone and in combination with sildenafil in patients with premature ejaculation. Urol. Int. 2007;79(1):28–32. doi: 10.1159/000102909. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins L.C., Gonzalez J., Tal R., Guhring P., Parker M., Mulhall J.P. Compliance with fluoxetine use in men with primary premature ejaculation. J. Sex. Med. 2019;16(12):1895–1899. doi: 10.1016/j.jsxm.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kara H., Aydin S., Yücel M., Agargün M.Y., Odabaş O., Yilmaz Y. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo controlled study. J. Urol. 1996;156(5):1631–1632. doi: 10.1016/S0022-5347(01)65467-3. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.C., Seo K.K. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-blind, placebo controlled study. J. Urol. 1998;159(2):425–427. doi: 10.1016/S0022-5347(01)63940-5. [DOI] [PubMed] [Google Scholar]
- 45.Madeo B., Bettica P., Milleri S., Balestrieri A., Granata A.R., Carani C., Rochira V. The effects of citalopram and fluoxetine on sexual behavior in healthy men: evidence of delayed ejaculation and unaffected sexual desire. A randomized, placebo-controlled, double-blind, double-dummy, parallel group study. J. Sex. Med. 2008;5(10):2431–2441. doi: 10.1111/j.1743-6109.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 46.Manasia P., Pomerol J., Ribè N., Gutierrez del Pozo R., Alcover Garcia J. Comparison of the efficacy and safety of 90 mg versus 20 mg fluoxetine in the treatment of premature ejaculation. J. Urol. 2003;170(1):164–165. doi: 10.1097/01.ju.0000071040.12407.32. [DOI] [PubMed] [Google Scholar]
- 47.Mattos R.M., Marmo Lucon A., Srougi M. Tadalafil and fluoxetine in premature ejaculation: prospective, randomized, double-blind, placebo-controlled study. Urol. Int. 2008;80(2):162–165. doi: 10.1159/000112607. [DOI] [PubMed] [Google Scholar]
- 48.Nada E.A. A Comparison between citalopram and escitalopram in treatment of patients with premature ejaculation: a double-blind controlled clinical study. J. Sex. Med. 2012;9:261–262. [Google Scholar]
- 49.Nada E., Saleh R., Abu M. The use of Escitalopram in treatment of patients with premature ejaculation: A randomized, double-blind, placebo-controlled study. J. Sex. Med. 2009:6. [Google Scholar]
- 50.Novaretti J.P.T., Pompeo A., Arap S. Selective serotonin re-uptake inhibitor in the treatment of premature ejaculation. Br. J. Urol. 2002;28(2):116–122. [Google Scholar]
- 51.Rezakhaniha B., Sirosbakht S. Efficacy of selective serotonin reuptake inhibitor (SSRI) in patient with premature ejaculation. Iran. J. Reprod. Med. 2010;8(2):55–59. [Google Scholar]
- 52.Safarinejad M.R. Comparison of dapoxetine versus paroxetine in patients with premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. Clin. Neuropharmacol. 2006;29(5):243–252. doi: 10.1097/01.WNF.0000228210.12194.46. [DOI] [PubMed] [Google Scholar]
- 53.Safarinejad M.R. Safety and efficacy of escitalopram in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. J. Clin. Psychopharmacol. 2007;27(5):444–450. doi: 10.1097/jcp.0b013e31814b98d4. [DOI] [PubMed] [Google Scholar]
- 54.Safarinejad M.R., Hosseini S.Y. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int. J. Impot. Res. 2006;18(2):164–169. doi: 10.1038/sj.ijir.3901384. [DOI] [PubMed] [Google Scholar]
- 55.Sunay D., Sunay M., Aydoğmuş Y., Bağbancı S., Arslan H., Karabulut A., Emir L. Acupuncture versus paroxetine for the treatment of premature ejaculation: a randomized, placebo-controlled clinical trial. Eur. Urol. 2011;59(5):765–771. doi: 10.1016/j.eururo.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Vahid F. On-demand treatment of premature ejaculation with citalopram: A Randomized double-blind study. Acta Med. Iran. 2009;47(5):353–357. [Google Scholar]
- 57.Waldinger M.D., Hengeveld M.W., Zwinderman A.H., Olivier B. Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J. Clin. Psychopharmacol. 1998;18(4):274–281. doi: 10.1097/00004714-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Waldinger M.D., Zwinderman A.H., Olivier B. Antidepressants and ejaculation: a double-blind, randomized, placebo-controlled, fixed-dose study with paroxetine, sertraline, and nefazodone. J. Clin. Psychopharmacol. 2001;21(3):293–297. doi: 10.1097/00004714-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Yilmaz U., Tatlişen A., Turan H., Arman F., Ekmekçioğlu O. The effects of fluoxetine on several neurophysiological variables in patients with premature ejaculation. J. Urol. 1999;161(1):107–111. doi: 10.1016/S0022-5347(01)62078-0. [DOI] [PubMed] [Google Scholar]
- 60.Coleman E. An exploratory study of the role of psychotropic medications in the treatment of sex offenders. J. Offender Rehabil. 1992;18(3-4):75–88. doi: 10.1300/J076v18n03_08. [DOI] [Google Scholar]
- 61.Greenberg D.M., Bradford J.M., Curry S., O’Rourke A. A comparison of treatment of paraphilias with three serotonin reuptake inhibitors: a retrospective study. Bull. Am. Acad. Psychiatry Law. 1996;24(4):525–532. [PubMed] [Google Scholar]
- 62.Kafka M.P. Sertraline pharmacotherapy for paraphilias and paraphilia-related disorders: an open trial. Ann. Clin. Psychiatry. 1994;6(3):189–195. doi: 10.3109/10401239409149003. [DOI] [PubMed] [Google Scholar]
- 63.Kafka M.P. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J. Clin. Psychiatry. 1991;52(2):60–65. [PubMed] [Google Scholar]
- 64.Kafka M.P., Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J. Clin. Psychiatry. 2000;61(9):664–670. doi: 10.4088/JCP.v61n0912. [DOI] [PubMed] [Google Scholar]
- 65.Kafka M.P., Prentky R. A comparative study of nonparaphilic sexual addictions and paraphilias in men. J. Clin. Psychiatry. 1992;53(10):345–350. [PubMed] [Google Scholar]
- 66.Kafka M.P., Prentky R. Fluoxetine treatment of nonparaphilic sexual addictions and paraphilias in men. J. Clin. Psychiatry. 1992;53(10):351–358. [PubMed] [Google Scholar]
- 67.Kraus C., Strohm K., Hill A., Habermann N., Berner W., Briken P. Selective serotonine reuptake inhibitors (SSRI) in the treatment of paraphilia. Fortschr. Neurol. Psychiatr. 2007;75(6):351–356. doi: 10.1055/s-2006-944261. [DOI] [PubMed] [Google Scholar]
- 68.Stein D.J., Hollander E., Anthony D.T., Schneier F.R., Fallon B.A., Liebowitz M.R., Klein D.F. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J. Clin. Psychiatry. 1992;53(8):267–271. [PubMed] [Google Scholar]
- 69.LaCroix A.Z., Freeman E.W., Larson J., Carpenter J.S., Joffe H., Reed S.D., Newton K.M., Seguin R.A., Sternfeld B., Cohen L., Ensrud K.E. Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial. Maturitas. 2012;73(4):361–368. doi: 10.1016/j.maturitas.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barton D.L., LaVasseur B.I., Sloan J.A., Stawis A.N., Flynn K.A., Dyar M., Johnson D.B., Atherton P.J., Diekmann B., Loprinzi C.L. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. J. Clin. Oncol. 2010;28(20):3278–3283. doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flevari P., Leftheriotis D., Repasos E., Katsaras D., Katsimardos A., Lekakis J. Fluoxetine vs. placebo for the treatment of recurrent vasovagal syncope with anxiety sensitivity. Europace. 2017;19(1):127–131. doi: 10.1093/europace/euw153. [DOI] [PubMed] [Google Scholar]
- 72.Kadri A.N., Nusairat L., Kadri S., Alqaid A., Hernandez A.V., Kadri N.N. Effect of citalopram for the treatment of neurocardiogenic syncope. Am. J. Ther. 2019;26(3):e339–e343. doi: 10.1097/MJT.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 73.Coccaro E.F., Kavoussi R.J. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch. Gen. Psychiatry. 1997;54(12):1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 74.Coccaro E.F., Kavoussi R.J., Hauger R.L. Serotonin function and antiaggressive response to fluoxetine: a pilot study. Biol. Psychiatry. 1997;42(7):546–552. doi: 10.1016/S0006-3223(97)00309-0. [DOI] [PubMed] [Google Scholar]
- 75.Coccaro E.F., Lee R.J., Kavoussi R.J. A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J. Clin. Psychiatry. 2009;70(5):653–662. doi: 10.4088/JCP.08m04150. [DOI] [PubMed] [Google Scholar]
- 76.George D.T., Phillips M.J., Lifshitz M., Lionetti T.A., Spero D.E., Ghassemzedeh N., Doty L., Umhau J.C., Rawlings R.R. Fluoxetine treatment of alcoholic perpetrators of domestic violence: a 12-week, double-blind, randomized, placebo-controlled intervention study. J. Clin. Psychiatry. 2011;72(1):60–65. doi: 10.4088/JCP.09m05256gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koran L.M., Aboujaoude E.N., Gamel N.N. Escitalopram treatment of kleptomania: an open-label trial followed by double-blind discontinuation. J. Clin. Psychiatry. 2007;68(3):422–427. doi: 10.4088/JCP.v68n0311. [DOI] [PubMed] [Google Scholar]
- 78.Lee R., Kavoussi R.J., Coccaro E.F. Placebo-controlled, randomized trial of fluoxetine in the treatment of aggression in male intimate partner abusers. Int. Clin. Psychopharmacol. 2008;23(6):337–341. doi: 10.1097/YIC.0b013e32830fbdd2. [DOI] [PubMed] [Google Scholar]
- 79.Reist C., Nakamura K., Sagart E., Sokolski K.N., Fujimoto K.A. Impulsive aggressive behavior: open-label treatment with citalopram. J. Clin. Psychiatry. 2003;64(1):81–85. doi: 10.4088/JCP.v64n0115. [DOI] [PubMed] [Google Scholar]
- 80.Vartiainen H., Tiihonen J., Putkonen A., Koponen H., Virkkunen M., Hakola P., Lehto H. Citalopram, a selective serotonin reuptake inhibitor, in the treatment of aggression in schizophrenia. Acta Psychiatr. Scand. 1995;91(5):348–351. doi: 10.1111/j.1600-0447.1995.tb09793.x. [DOI] [PubMed] [Google Scholar]
- 81.Patetsos E., Horjales-Araujo E. Treating Chronic Pain with SSRIs: What Do We Know? Pain Res. Manag. 2016;2016:2020915. doi: 10.1155/2016/2020915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burch R. Antidepressants for preventive treatment of migraine. Curr. Treat. Options Neurol. 2019;21(4):18. doi: 10.1007/s11940-019-0557-2. [DOI] [PubMed] [Google Scholar]
- 83.Bonilla S., Nurko S. Focus on the use of antidepressants to treat pediatric functional abdominal pain: current perspectives. Clin. Exp. Gastroenterol. 2018;11:365–372. doi: 10.2147/CEG.S146646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kułak-Bejda A., Bejda G., Waszkiewicz N. Antidepressants for irritable bowel syndrome-A systematic review. Pharmacol. Rep. 2017;69(6):1366–1379. doi: 10.1016/j.pharep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Du Y., Jiang Y., Zhang J., Tian G., Zhang N., Wu D., Bai X. Efficacy and Safety of “On-Demand” dapoxetine in treatment of patients with premature ejaculation: A Meta-Analysis. Med. Sci. Monit. 2019;25:4225–4232. doi: 10.12659/MSM.913606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jian Z., Wei X., Ye D., Li H., Wang K. Pharmacotherapy of premature ejaculation: a systematic review and network meta-analysis. Int. Urol. Nephrol. 2018;50(11):1939–1948. doi: 10.1007/s11255-018-1984-9. [DOI] [PubMed] [Google Scholar]
- 87.Yi Z.M., Chen S.D., Tang Q.Y., Tang H.L., Zhai S.D. Efficacy and safety of sertraline for the treatment of premature ejaculation: Systematic review and meta-analysis. Medicine (Baltimore) 2019;98(23):e15989. doi: 10.1097/MD.0000000000015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang D., Cheng Y., Wu K., Ma Q., Jiang J., Yan Z. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2019;19(1):2. doi: 10.1186/s12894-018-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riemma G., Schiattarella A., La Verde M., Zarobbi G., Garzon S., Cucinella G., Calagna G., Labriola D., De Franciscis P. Efficacy of low-dose paroxetine for the treatment of hot flushes in surgical and physiological postmenopausal women: systematic review and meta-analysis of randomized trials. Medicina (Kaunas) 2019;55(9):E554. doi: 10.3390/medicina55090554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdel F.M.R., Jung S.W., Greenspan M.A., Padilla M., Enciso R. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to placebo. a systematic review with meta-analysis. Sleep Breath. 2020;24(2):443–453. doi: 10.1007/s11325-019-01954-9. [DOI] [PubMed] [Google Scholar]
- 91.Legg L.A., Tilney R., Hsieh C.F., Wu S., Lundström E., Rudberg A.S., Kutlubaev M.A., Dennis M., Soleimani B., Barugh A., Hackett M.L., Hankey G.J., Mead G.E. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst. Rev. 2019;2019(11):CD009286. doi: 10.1002/14651858.CD009286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hague B., Hall J., Kellett S. Treatments for compulsive buying: A systematic review of the quality, effectiveness and progression of the outcome evidence. J. Behav. Addict. 2016;5(3):379–394. doi: 10.1556/2006.5.2016.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vos T. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burch R.C., Buse D.C., Lipton R.B. Migraine: epidemiology, burden, and comorbidity. Neurol. Clin. 2019;37(4):631–649. doi: 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Charles A. Migraine. N. Engl. J. Med. 2017;377(17):1698–1699. doi: 10.1056/NEJMc1711803. [DOI] [PubMed] [Google Scholar]
- 96.Huang I.H., Wu P.C., Lin E.Y., Chen C.Y., Kang Y.N. Effects of anti-calcitonin gene-related peptide for migraines: a systematic review with meta-analysis of randomized clinical trials. Int. J. Mol. Sci. 2019;20(14):E3527. doi: 10.3390/ijms20143527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Viganò A., Torrieri M.C., Toscano M., Puledda F., Petolicchio B., Sasso D’Elia T., Verzina A., Ruggiero S., Altieri M., Vicenzini E., Schoenen J., Di Piero V. Neurophysiological correlates of clinical improvement after greater occipital nerve (GON) block in chronic migraine: relevance for chronic migraine pathophysiology. J. Headache Pain. 2018;19(1):73. doi: 10.1186/s10194-018-0901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Viganò A., Toscano M., Puledda F., Di Piero V. Treating chronic migraine with neuromodulation: the role of neurophysiological abnormalities and maladaptive plasticity. Front. Pharmacol. 2019;10:32. doi: 10.3389/fphar.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deen M., Christensen C.E., Hougaard A., Hansen H.D., Knudsen G.M., Ashina M. Serotonergic mechanisms in the migraine brain - a systematic review. Cephalalgia. 2017;37(3):251–264. doi: 10.1177/0333102416640501. [DOI] [PubMed] [Google Scholar]
- 100.Altamura C., Corbelli I., de Tommaso M., Di Lorenzo C., Di Lorenzo G., Di Renzo A., Filippi M., Jannini T.B., Messina R., Parisi P., Parisi V., Pierelli F., Rainero I., Raucci U., Rubino E., Sarchielli P., Li L., Vernieri F., Vollono C., Coppola G. Pathophysiological bases of comorbidity in migraine. Front. Hum. Neurosci. 2021;15(131):640574. doi: 10.3389/fnhum.2021.640574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saper J.R., Silberstein S.D., Lake A.E., III, Winters M.E. Double-blind trial of fluoxetine: chronic daily headache and migraine. Headache. 1994;34(9):497–502. doi: 10.1111/j.1526-4610.1994.hed3409497.x. [DOI] [PubMed] [Google Scholar]
- 102.Saper J.R., Silberstein S.D., Lake A.E., III, Winters M.E. Fluoxetine and migraine: comparison of double-blind trials. Headache. 1995;35(4):233. doi: 10.1111/j.1526-4610.1995.hed3504233_1.x. [DOI] [PubMed] [Google Scholar]
- 103.Black K.J., Sheline Y.I. Paroxetine as migraine prophylaxis. J. Clin. Psychiatry. 1995;56(7):330–331. [PubMed] [Google Scholar]
- 104.Hays P. Paroxetine prevents migraines. J. Clin. Psychiatry. 1997;58(1):30–31. doi: 10.4088/JCP.v58n0106b. [DOI] [PubMed] [Google Scholar]
- 105.Silberstein S.D., Holland S., Freitag F., Dodick D.W., Argoff C., Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alemdar M., Selekler H.M. Migraine with aura triggered by citalopram. Neuropsychobiology. 2007;55(2):121–122. doi: 10.1159/000104469. [DOI] [PubMed] [Google Scholar]
- 107.Bickel A., Kornhuber J., Maihöfner C., Ropohl A. Exacerbation of migraine attacks during treatment with the selective serotonin reuptake inhibitor sertraline. A case report. Pharmacopsychiatry. 2005;38(6):327–328. doi: 10.1055/s-2005-916190. [DOI] [PubMed] [Google Scholar]
- 108.Delva N.J., Horgan S.A., Hawken E.R. Valproate prophylaxis for migraine induced by selective serotonin reuptake inhibitors. Headache. 2000;40(3):248–251. doi: 10.1046/j.1526-4610.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 109.Larson E.W. Migraine with typical aura associated with fluoxetine therapy: case report. J. Clin. Psychiatry. 1993;54(6):235–236. [PubMed] [Google Scholar]
- 110.Munera P.A., Goldstein A. Migraine and sertraline. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(10):1125–1126. doi: 10.1097/00004583-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 111.Mufaddel A., Osman O.T., Almugaddam F., Jafferany M. A review of body dysmorphic disorder and its presentation in different clinical settings. PCC.12r01464. . Prim. Care Companion CNS Disord. 2013;15(4) doi: 10.4088/PCC.12r01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mancuso S.G., Knoesen N.P., Castle D.J. Delusional versus nondelusional body dysmorphic disorder. Compr. Psychiatry. 2010;51(2):177–182. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Krebs G., Fernández de la Cruz L., Monzani B., Bowyer L., Anson M., Cadman J., Heyman I., Turner C., Veale D., Mataix-Cols D. Long-term outcomes of cognitive-behavioral therapy for adolescent body dysmorphic disorder. Behav. Ther. 2017;48(4):462–473. doi: 10.1016/j.beth.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Veale D., Anson M., Miles S., Pieta M., Costa A., Ellison N. Efficacy of cognitive behaviour therapy versus anxiety management for body dysmorphic disorder: a randomised controlled trial. Psychother. Psychosom. 2014;83(6):341–353. doi: 10.1159/000360740. [DOI] [PubMed] [Google Scholar]
- 115. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 116.New A.S., Buchsbaum M.S., Hazlett E.A., Goodman M., Koenigsberg H.W., Lo J., Iskander L., Newmark R., Brand J., O’Flynn K., Siever L.J. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology (Berl.) 2004;176(3-4):451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- 117.Coccaro E.F. Central serotonin and impulsive aggression. Br. J. Psychiatry Suppl. 1989;(8):52–62. doi: 10.1192/S0007125000291769. [DOI] [PubMed] [Google Scholar]
- 118.Toscano M., Viganò A., Puledda F., Verzina A., Rocco A., Lenzi G.L., Di Piero V. Serotonergic correlation with anger and aggressive behavior in acute stroke patients: an intensity dependence of auditory evoked potentials (IDAP) study. Eur. Neurol. 2014;72(3-4):186–192. doi: 10.1159/000362268. [DOI] [PubMed] [Google Scholar]
- 119.van der Vegt B.J., Lieuwes N., Cremers T.I., de Boer S.F., Koolhaas J.M. Cerebrospinal fluid monoamine and metabolite concentrations and aggression in rats. Horm. Behav. 2003;44(3):199–208. doi: 10.1016/S0018-506X(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 120.Chong S.A., Low B.L. Treatment of kleptomania with fluvoxamine. Acta Psychiatr. Scand. 1996;93(4):314–315. doi: 10.1111/j.1600-0447.1996.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 121.Kraus J.E. Treatment of kleptomania with paroxetine. J. Clin. Psychiatry. 1999;60(11):793. doi: 10.4088/JCP.v60n1116. [DOI] [PubMed] [Google Scholar]
- 122.Mayer E.A. Clinical practice. Irritable bowel syndrome. N. Engl. J. Med. 2008;358(16):1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and rome IV. Gastroenterology, 2016;S0016-5085(16):00223–7. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 124.Mozaffari S., Nikfar S., Abdollahi M. The safety of novel drugs used to treat irritable bowel syndrome. Expert Opin. Drug Saf. 2014;13(5):625–638. doi: 10.1517/14740338.2014.902932. [DOI] [PubMed] [Google Scholar]
- 125.Noorafshan A., Yousefi M., Hosseini L., Karbalay-Doust S. Can sertraline and nortriptyline protect the neurons in submucosal and myenteric plexuses of Rat’s Colon Against Stress? Dig. Dis. Sci. 2019;64(9):2548–2554. doi: 10.1007/s10620-019-05600-y. [DOI] [PubMed] [Google Scholar]
- 126.Stasi C., Bellini M., Bassotti G., Blandizzi C., Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech. Coloproctol. 2014;18(7):613–621. doi: 10.1007/s10151-013-1106-8. [DOI] [PubMed] [Google Scholar]
- 127.Lin W-T., Liao Y.J., Peng Y.C., Chang C.H., Lin C.H., Yeh H.Z., Chang C.S. Relationship between use of selective serotonin reuptake inhibitors and irritable bowel syndrome: A population-based cohort study. World J. Gastroenterol. 2017;23(19):3513–3521. doi: 10.3748/wjg.v23.i19.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thibaut F., Cosyns P., Fedoroff J.P., Briken P., Goethals K., Bradford J.M.W. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J. Biol. Psychiatry. 2020;21(6):412–490. doi: 10.1080/15622975.2020.1744723. [DOI] [PubMed] [Google Scholar]
- 129.Snoeren E.M.S., Veening J.G., Olivier B., Oosting R.S. Serotonin 1A receptors and sexual behavior in male rats: a review. Pharmacol. Biochem. Behav. 2014;121:102–114. doi: 10.1016/j.pbb.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 130.Turner D., Schöttle D., Bradford J., Briken P. Assessment methods and management of hypersexuality and paraphilic disorders. Curr. Opin. Psychiatry. 2014;27(6):413–422. doi: 10.1097/YCO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 131.Fontanesi L. Hypersexuality and Trauma: a mediation and moderation model from psychopathology to problematic sexual behavior. J. Affect. Disord. 2020;281:631–637. doi: 10.1016/j.jad.2020.11.100. [DOI] [PubMed] [Google Scholar]
- 132.Björkholm C., Monteggia L.M. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Casarotto P.C. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184(5):1299–1313. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bourgeois J.A., Klein M. Risperidone and fluoxetine in the treatment of pedophilia with comorbid dysthymia. J. Clin. Psychopharmacol. 1996;16(3):257–258. doi: 10.1097/00004714-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 135.Perilstein R.D., Lipper S., Friedman L.J. Three cases of paraphilias responsive to fluoxetine treatment. J. Clin. Psychiatry. 1991;52(4):169–170. [PubMed] [Google Scholar]
- 136.Bianchi M.D. Fluoxetine treatment of exhibitionism. Am. J. Psychiatry. 1990;147(8):1089–1090. doi: 10.1176/ajp.147.8.1089c. [DOI] [PubMed] [Google Scholar]
- 137.Yang F.W., Liang C.S. Paraphilias in schizophrenia: differential diagnosis and treatment with selective serotonin reuptake inhibitors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(6):1126–1127. doi: 10.1016/j.pnpbp.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 138.Lorefice L.S. Fluoxetine treatment of a fetish. J. Clin. Psychiatry. 1991;52(1):41. [PubMed] [Google Scholar]
- 139.Emmanuel N.P., Lydiard R.B., Ballenger J.C. Fluoxetine treatment of voyeurism. Am. J. Psychiatry. 1991;148(7):950. doi: 10.1176/ajp.148.7.950a. [DOI] [PubMed] [Google Scholar]
- 140.Bhatia M.S., Jhanjee A., Srivastava S., Kumar P. An uncommon case of hypersexual behaviour with frotteurism. Med. Sci. Law. 2010;50(4):228–229. doi: 10.1258/msl.2010.010103. [DOI] [PubMed] [Google Scholar]
- 141.Aguirre B. Fluoxetine and compulsive sexual behavior. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(8):943–943. doi: 10.1097/00004583-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 142.Galli V.B., Raute N.J., McConville B.J., McElroy S.L. An adolescent male with multiple paraphilias successfully treated with fluoxetine. J. Child Adolesc. Psychopharmacol. 1998;8(3):195–197. doi: 10.1089/cap.1998.8.195. [DOI] [PubMed] [Google Scholar]
- 143.Bradford J.M., Gratzer T.G. A treatment for impulse control disorders and paraphilia: a case report. Can. J. Psychiatry. 1995;40(1):4–5. doi: 10.1177/070674379504000103. [DOI] [PubMed] [Google Scholar]
- 144.Chow E.W.C., Choy A.L. Clinical characteristics and treatment response to SSRI in a female pedophile. Arch. Sex. Behav. 2002;31(2):211–215. doi: 10.1023/A:1014795321404. [DOI] [PubMed] [Google Scholar]
- 145.Rubenstein E.B., Engel N.L. Successful treatment of transvestic fetishism with sertraline and lithium. J. Clin. Psychiatry. 1996;57(2):92. [PubMed] [Google Scholar]
- 146.Cetin F.H. Inappropriate sexual behaviors associated with mental retardation or transvestic fetishism: a case study. Anadolu Psikiyatri Dergisi-Anatolian J. Psychiat. 2015;16(6):454–457. doi: 10.5455/apd.1414665797. [DOI] [Google Scholar]
- 147.Patra A.P., Bharadwaj B., Shaha K.K., Das S., Rayamane A.P., Tripathi C.S. Impulsive frotteurism: a case report. Med. Sci. Law. 2013;53(4):235–238. doi: 10.1177/0025802412474813. [DOI] [PubMed] [Google Scholar]
- 148.Ismail N., Husain R., Sidi H. Anxiety-depression psychopathology of a patient with voyeurism, major depression and premature ejaculation. Asian J. Psychiatr. 2017;18(2):278–280. [Google Scholar]
- 149.Zohar J., Kaplan Z., Benjamin J. Compulsive exhibitionism successfully treated with fluvoxamine: a controlled case study. J. Clin. Psychiatry. 1994;55(3):86–88. [PubMed] [Google Scholar]
- 150.Stewart J.T., Shin K.J. Paroxetine treatment of sexual disinhibition in dementia. Am. J. Psychiatry. 1997;154(10):1474. doi: 10.1176/ajp.154.10.1474a. [DOI] [PubMed] [Google Scholar]
- 151.Abouesh A., Clayton A. Compulsive voyeurism and exhibitionism: a clinical response to paroxetine. Arch. Sex. Behav. 1999;28(1):23–30. doi: 10.1023/A:1018737504537. [DOI] [PubMed] [Google Scholar]
- 152.Wainberg M.L., Muench F., Morgenstern J., Hollander E., Irwin T.W., Parsons J.T., Allen A., O’Leary A. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J. Clin. Psychiatry. 2006;67(12):1968–1973. doi: 10.4088/JCP.v67n1218. [DOI] [PubMed] [Google Scholar]
- 153.Haverkos H.W., Drotman D.P. The social organization of sexuality. JAMA. 1995;274(7):535–537. doi: 10.1001/jama.274.7.535. [DOI] [PubMed] [Google Scholar]
- 154.Mazza M., Harnic D., Catalano V., Di Nicola M., Bruschi A., Bria P., Daniele A., Mazza S. Sexual behavior in women with bipolar disorder. J. Affect. Disord. 2011;131(1-3):364–367. doi: 10.1016/j.jad.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 155.Nordvig A.S., Goldberg D.J., Huey E.D., Miller B.L. The cognitive aspects of sexual intimacy in dementia patients: a neurophysiological review. Neurocase. 2019;25(1-2):66–74. doi: 10.1080/13554794.2019.1603311. [DOI] [PubMed] [Google Scholar]
- 156.Schöttle D., Briken P., Tüscher O., Turner D. Sexuality in autism: hypersexual and paraphilic behavior in women and men with high-functioning autism spectrum disorder. Dialogues Clin. Neurosci. 2017;19(4):381–393. doi: 10.31887/DCNS.2017.19.4/dschoettle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Estellon V., Mouras H. Sexual addiction: insights from psychoanalysis and functional neuroimaging. Socioaffect. Neurosci. Psychol. 2012;2:11814. doi: 10.3402/snp.v2i0.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anneser J.M., Jox R.J., Borasio G.D. Inappropriate sexual behaviour in a case of ALS and FTD: successful treatment with sertraline. Amyotroph. Lateral Scler. 2007;8(3):189–190. doi: 10.1080/17482960601073543. [DOI] [PubMed] [Google Scholar]
- 159.Herguner S., Herguner A., Cicek E. Combination of risperidone and paroxetine for inappropriate sexual behaviors in an adolescent with autism and mental retardation. Noropsikiyatri Arsivi-Arch. Neuropsychiatry. 2012;49(4):311–313. [Google Scholar]
- 160.Malladi S.S., Singh A.N. Hypersexuality and its response to citalopram in a patient with hypothalamic hamartoma and precocious puberty. Int. J. Neuropsychopharmacol. 2005;8(4):635–636. doi: 10.1017/S1461145705005493. [DOI] [PubMed] [Google Scholar]
- 161.Ozmen M., Erdogan A., Duvenci S., Ozyurt E., Ozkara C. Excessive masturbation after epilepsy surgery. Epilepsy Behav. 2004;5(1):133–136. doi: 10.1016/j.yebeh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 162.Raymond N.C., Grant J.E., Kim S.W., Coleman E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int. Clin. Psychopharmacol. 2002;17(4):201–205. doi: 10.1097/00004850-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 163.Schupak C. Case report: lamotrigine/fluoxetine combination in the treatment of compulsive sexual behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(6):1337–1338. doi: 10.1016/j.pnpbp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 164.Solanki M.S., Singh P. Right thalamic bleed resulting in hypersexuality successfully treated with Selective Serotonin reuptake inhibitor Sertraline - A case report. Asian J. Psychiatr. 2017;30:210–211. doi: 10.1016/j.ajp.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 165.Tosto G., Talarico G., Lenzi G.L., Bruno G. Effect of citalopram in treating hypersexuality in an Alzheimer’s disease case. Neurol. Sci. 2008;29(4):269–270. doi: 10.1007/s10072-008-0979-1. [DOI] [PubMed] [Google Scholar]
- 166.Kornreich C., Den Dulk A., Verbanck P., Pelc I. Fluoxetine treatment of compulsive masturbation in a schizophrenic patient. J. Clin. Psychiatry. 1995;56(7):334–334. [PubMed] [Google Scholar]
- 167.Rao V., Handel S., Vaishnavi S., Keach S., Robbins B., Spiro J., Ward J., Berlin F. Psychiatric sequelae of traumatic brain injury: a case report. Am. J. Psychiatry. 2007;164(5):728–735. doi: 10.1176/ajp.2007.164.5.728. [DOI] [PubMed] [Google Scholar]
- 168.Stein D.J., Black D.W., Shapira N.A., Spitzer R.L. Hypersexual disorder and preoccupation with internet pornography. Am. J. Psychiatry. 2001;158(10):1590–1594. doi: 10.1176/appi.ajp.158.10.1590. [DOI] [PubMed] [Google Scholar]
- 169.Jannini E.A., Lenzi A. Epidemiology of premature ejaculation. Curr. Opin. Urol. 2005;15(6):399–403. doi: 10.1097/01.mou.0000182327.79572.fd. [DOI] [PubMed] [Google Scholar]
- 170.Jannini E.A., Ciocca G., Limoncin E., Mollaioli D., Di Sante S., Gianfrilli D., Lombardo F., Lenzi A. Premature ejaculation: old story, new insights. Fertil. Steril. 2015;104(5):1061–1073. doi: 10.1016/j.fertnstert.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 171.Sansone A. Management of premature ejaculation: a clinical guideline from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Invest. 2020 doi: 10.1007/s40618-020-01458-4. [DOI] [PubMed] [Google Scholar]
- 172.Althof S.E., Abdo C.H., Dean J., Hackett G., McCabe M., McMahon C.G., Rosen R.C., Sadovsky R., Waldinger M., Becher E., Broderick G.A., Buvat J., Goldstein I., El-Meliegy A.I., Giuliano F., Hellstrom W.J., Incrocci L., Jannini E.A., Park K., Parish S., Porst H., Rowland D., Segraves R., Sharlip I., Simonelli C., Tan H.M. International Society for Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation. J. Sex. Med. 2010;7(9):2947–2969. doi: 10.1111/j.1743-6109.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 173.McMahon C.G., Jannini E., Waldinger M., Rowland D. Standard operating procedures in the disorders of orgasm and ejaculation. J. Sex. Med. 2013;10(1):204–229. doi: 10.1111/j.1743-6109.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 174.Ciocca G., Limoncin E., Mollaioli D., Gravina G.L., Di Sante S., Carosa E., Lenzi A., Jannini E.A. Integrating psychotherapy and pharmacotherapy in the treatment of premature ejaculation. Arab J. Urol. 2013;11(3):305–312. doi: 10.1016/j.aju.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abdel-Hamid I.A., Jannini E.A., Andersson K.E. Premature ejaculation: focus on therapeutic targets. Expert Opin. Ther. Targets. 2009;13(2):175–193. doi: 10.1517/14728220802663549. [DOI] [PubMed] [Google Scholar]
- 176.Forster P., King J. Fluoxetine for premature ejaculation. Am. J. Psychiatry. 1994;151(10):1523. doi: 10.1176/ajp.151.10.1523a. [DOI] [PubMed] [Google Scholar]
- 177.Waldinger M.D., Zwinderman A.H., Olivier B. SSRIs and ejaculation: a double-blind, randomized, fixed-dose study with paroxetine and citalopram. J. Clin. Psychopharmacol. 2001;21(6):556–560. doi: 10.1097/00004714-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 178.Kalejaiye O., Almekaty K., Blecher G., Minhas S. Premature ejaculation: challenging new and the old concepts. F1000 Res. 2017;6:2084. doi: 10.12688/f1000research.12150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Colonnello E. Redefining a sexual medicine paradigm: subclinical premature ejaculation as a new taxonomic entity. Nat. Rev. Urol. 2021;18(2):115–127. doi: 10.1038/s41585-020-00417-1. [DOI] [PubMed] [Google Scholar]
- 180.Howes O.D., Rogdaki M., Findon J.L., Wichers R.H., Charman T., King B.H., Loth E., McAlonan G.M., McCracken J.T., Parr J.R., Povey C., Santosh P., Wallace S., Simonoff E., Murphy D.G. Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 2018;32(1):3–29. doi: 10.1177/0269881117741766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mika J., Zychowska M., Makuch W., Rojewska E., Przewlocka B. Neuronal and immunological basis of action of antidepressants in chronic pain - clinical and experimental studies. Pharmacol. Rep. 2013;65(6):1611–1621. doi: 10.1016/S1734-1140(13)71522-6. [DOI] [PubMed] [Google Scholar]
- 182.Fitzcharles M.A., Ste-Marie P.A., Goldenberg D.L., Pereira J.X., Abbey S., Choinière M., Ko G., Moulin D.E., Panopalis P., Proulx J., Shir Y. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res. Manag. 2013;18(3):119–126. doi: 10.1155/2013/918216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.El-Solh A.A. Management of nightmares in patients with posttraumatic stress disorder: current perspectives. Nat. Sci. Sleep. 2018;10:409–420. doi: 10.2147/NSS.S166089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stoffers-Winterling J., Storebø O.J., Lieb K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr. Psychiatry Rep. 2020;22(8):37. doi: 10.1007/s11920-020-01164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mayer L.E., Walsh B.T. The use of selective serotonin reuptake inhibitors in eating disorders. J. Clin. Psychiatry. 1998;59(Suppl. 15):28–34. [PubMed] [Google Scholar]
- 186.Milano W., De Rosa M., Milano L., Capasso A. Night eating syndrome: an overview. J. Pharm. Pharmacol. 2012;64(1):2–10. doi: 10.1111/j.2042-7158.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 187.Bianciardi E., Di Lorenzo G., Niolu C., Betrò S., Zerbin F., Gentileschi P., Siracusano A. Body image dissatisfaction in individuals with obesity seeking bariatric surgery: exploring the burden of new mediating factors. Riv. Psichiatr. 2019;54(1):8–17. doi: 10.1708/3104.30935. [DOI] [PubMed] [Google Scholar]
- 188.Bianciardi E., Fabbricatore M., Di Lorenzo G., Innamorati M., Tomassini L., Gentileschi P., Niolu C., Siracusano A., Imperatori C. Prevalence of Food Addiction and Binge Eating in an Italian sample of bariatric surgery candidates and overweight/obese patients seeking low-energy-diet therapy. Riv. Psichiatr. 2019;54(3):127–130. doi: 10.1708/3181.31602. [DOI] [PubMed] [Google Scholar]
- 189.Vella S.C., Pai N.B. A narrative review of potential treatment strategies for food addiction. Eat. Weight Disord. 2017;22(3):387–393. doi: 10.1007/s40519-017-0400-2. [DOI] [PubMed] [Google Scholar]
- 190.Sheikhpour M. The current recommended drugs and strategies for the treatment of Coronavirus Disease (COVID-19). Ther. Clin. Risk Manag. 2020;16:933–946. doi: 10.2147/TCRM.S262936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Homolak J., Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents. 2020;56(2):106044. doi: 10.1016/j.ijantimicag.2020.106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen H., Reeves J.H., Fincham J.E., Kennedy W.K., Dorfman J.H., Martin B.C. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia medicaid enrollees in 2001. J. Clin. Psychiatry. 2006;67(6):972–982. doi: 10.4088/JCP.v67n0615. [DOI] [PubMed] [Google Scholar]
- 193.Walton S.M., Schumock G.T., Lee K.V., Alexander G.C., Meltzer D., Stafford R.S. Prioritizing future research on off-label prescribing: results of a quantitative evaluation. Pharmacotherapy. 2008;28(12):1443–1452. doi: 10.1592/phco.28.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stone K.J., Viera A.J., Parman C.L. Off-label applications for SSRIs. Am. Fam. Physician. 2003;68(3):498–504. [PubMed] [Google Scholar]
- 195.Chen D.T., Wynia M.K., Moloney R.M., Alexander G.C.U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidemiol. Drug Saf. 2009;18(11):1094–1100. doi: 10.1002/pds.1825. [DOI] [PubMed] [Google Scholar]
- 196.Ghinea N., Lipworth W., Kerridge I. Off-Label promotion of prescription medicine: is it ever justifiable? Ther. Innov. Regul. Sci. 2015;49(3):359–363. doi: 10.1177/2168479015570337. [DOI] [PubMed] [Google Scholar]
- 197.Wang S.M., Han C., Bahk W.M., Lee S.J., Patkar A.A., Masand P.S., Pae C.U. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med. J. 2018;54(2):101–112. doi: 10.4068/cmj.2018.54.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goodman W.K., Murphy T.K., Storch E.A. Risk of adverse behavioral effects with pediatric use of antidepressants. Psychopharmacology (Berl.) 2007;191(1):87–96. doi: 10.1007/s00213-006-0642-6. [DOI] [PubMed] [Google Scholar]
- 199.Hogg S., Ansari S., Masood Q., Agius M., Rihmer Z. Are SSRIs responsible for precipitating suicidal ideation in teenagers with ‘subsyndromal’ bipolar affective disorder who have been misdiagnosed with unipolar depression? Psychiatr. Danub. 2016;28(Suppl. 1):79–82. [PubMed] [Google Scholar]
- 200.Bala A., Nguyen H.M.T., Hellstrom W.J.G. Post-SSRI sexual dysfunction: a literature review. Sex. Med. Rev. 2018;6(1):29–34. doi: 10.1016/j.sxmr.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 201.Popova N.K., Amstislavskaya T.G. Involvement of the 5-HT(1A) and 5-HT(1B) serotonergic receptor subtypes in sexual arousal in male mice. Psychoneuroendocrinology. 2002;27(5):609–618. doi: 10.1016/S0306-4530(01)00097-X. [DOI] [PubMed] [Google Scholar]
- 202.Godwin M., Seguin R. Critical appraisal skills of family physicians in Ontario, Canada. BMC Med. Educ. 2003;3:10. doi: 10.1186/1472-6920-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mojtabai R., Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff. (Millwood) 2011;30(8):1434–1442. doi: 10.1377/hlthaff.2010.1024. [DOI] [PubMed] [Google Scholar]