Abstract
Background
This is an update of the Cochrane Review last published in 2017. Survivors of stroke due to intracerebral haemorrhage (ICH) are at risk of major adverse cardiovascular events (MACE). Antithrombotic (antiplatelet or anticoagulant) treatments may lower the risk of ischaemic MACE after ICH, but they may increase the risk of bleeding.
Objectives
To determine the overall effectiveness and safety of antithrombotic drugs on MACE and its components for people with ICH.
Search methods
We searched the Cochrane Stroke Group Trials Register (5 October 2021). We also searched the Cochrane Central Register of Controlled Trials (CENTRAL: the Cochrane Library 2021, Issue 10), MEDLINE Ovid (from 1948 to October 2021) and Embase Ovid (from 1980 to October 2021). The online registries of clinical trials searched were the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (5 October 2021). We screened the reference lists of included randomised controlled trials (RCTs) for additional, potentially relevant RCTs.
Selection criteria
We selected RCTs in which participants with ICH of any age were allocated to a class of antithrombotic treatment as intervention or comparator.
Data collection and analysis
In accordance with standard methodological procedures recommended by Cochrane, two review authors assessed each selected RCT for its risk of bias and extracted data independently. The primary outcome was a composite of MACE, and secondary outcomes included death, individual components of the MACE composite, ICH growth, functional status and cognitive status. We estimated effects using the frequency of outcomes that occurred during the entire duration of follow‐up and calculated a risk ratio (RR) for each RCT. We grouped RCTs separately for analysis according to 1) the class(es) of antithrombotic treatment used for the intervention and comparator, and 2) the duration of antithrombotic treatment use (short term versus long term). We pooled the intention‐to‐treat populations of RCTs using a fixed‐effect model for meta‐analysis, but used a random‐effects model if RCTs differed substantially in their design or there was considerable heterogeneity (I2 ≥ 75%) in their results. We applied GRADE to assess the certainty of the evidence.
Main results
We identified seven new completed RCTs for this update, resulting in the inclusion of a total of nine RCTs based in secondary care, comprising 1491 participants (average age ranged from 61 to 79 years and the proportion of men ranged from 44% to 67%). The proportion of included RCTs at low risk of bias, by category was: random sequence generation (67%), allocation concealment (67%), performance (22%), detection (78%), attrition (89%), and reporting (78%).
For starting versus avoiding short‐term prophylactic dose anticoagulation after ICH, no RCT reported MACE. The evidence is very uncertain about the effect of starting short‐term prophylactic dose anticoagulation on death (RR 1.00, 95% CI 0.59 to 1.70, P = 1.00; 3 RCTs; very low‐certainty evidence), venous thromboembolism (RR 0.84, 95% CI 0.51 to 1.37, P = 0.49; 4 RCTs; very low‐certainty evidence), ICH (RR 0.24, 95% CI 0.04 to 1.38, P = 0.11; 2 RCTs; very low‐certainty evidence), and independent functional status (RR 2.03, 95% CI 0.78 to 5.25, P = 0.15; 1 RCT; very low‐certainty evidence) over 90 days.
For starting versus avoiding long‐term therapeutic dose oral anticoagulation for atrial fibrillation after ICH, starting long‐term therapeutic dose oral anticoagulation probably reduces MACE (RR 0.61, 95% CI 0.40 to 0.94, P = 0.02; 3 RCTs; moderate‐certainty evidence) and probably reduces all major occlusive vascular events (RR 0.27, 95% CI 0.14 to 0.53, P = 0.0002; 3 RCTs; moderate‐certainty evidence), but probably results in little to no difference in death (RR 1.05, 95% CI 0.62 to 1.78, P = 0.86; 3 RCTs; moderate‐certainty evidence), probably increases intracranial haemorrhage (RR 2.43, 95% CI 0.88 to 6.73, P = 0.09; 3 RCTs; moderate‐certainty evidence), and may result in little to no difference in independent functional status (RR 0.98, 95% CI 0.78 to 1.24, P = 0.87; 2 RCTs; low‐certainty evidence) over one to three years.
For starting versus avoiding long‐term antiplatelet therapy after ICH, the evidence is uncertain about the effects of starting long‐term antiplatelet therapy on MACE (RR 0.89, 95% CI 0.64 to 1.22, P = 0.46; 1 RCT; moderate‐certainty evidence), death (RR 1.08, 95% CI 0.76 to 1.53, P = 0.66; 1 RCT; moderate‐certainty evidence), all major occlusive vascular events (RR 1.03, 95% CI 0.68 to 1.55, P = 0.90; 1 RCT; moderate‐certainty evidence), ICH (RR 0.52, 95% CI 0.27 to 1.03, P = 0.06; 1 RCT; moderate‐certainty evidence) and independent functional status (RR 0.95, 95% CI 0.77 to 1.18, P = 0.67; 1 RCT; moderate‐certainty evidence) over a median follow‐up of two years.
For adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a clinical history of prior ICH, there was no evidence of an effect of long‐term cilostazol compared to aspirin on MACE (RR 1.33, 95% CI 0.74 to 2.40, P = 0.34; subgroup of 1 RCT; low‐certainty evidence), death (RR 1.65, 95% CI 0.55 to 4.91, P = 0.37; subgroup of 1 RCT; low‐certainty evidence), or ICH (RR 1.29, 95% CI 0.35 to 4.69, P = 0.70; subgroup of 1 RCT; low‐certainty evidence) over a median follow‐up of 1.8 years; all major occlusive vascular events and functional status were not reported.
Authors' conclusions
We did not identify beneficial or hazardous effects of short‐term prophylactic dose parenteral anticoagulation and long‐term oral antiplatelet therapy after ICH on important outcomes. Although there was a significant reduction in MACE and all major occlusive vascular events after long‐term treatment with therapeutic dose oral anticoagulation for atrial fibrillation after ICH, the pooled estimates were imprecise, the certainty of evidence was only moderate, and effects on other important outcomes were uncertain. Large RCTs with a low risk of bias are required to resolve the ongoing dilemmas about antithrombotic treatment after ICH.
Keywords: Adult, Aged, Humans, Male, Middle Aged, Anticoagulants, Anticoagulants/adverse effects, Atrial Fibrillation, Cerebral Hemorrhage, Fibrinolytic Agents, Fibrinolytic Agents/adverse effects, Platelet Aggregation Inhibitors, Platelet Aggregation Inhibitors/adverse effects, Stroke, Stroke/drug therapy
Plain language summary
Drugs to prevent clots after bleeding in the brain
Review question What are the benefits and risks of drugs used to prevent clots (known as 'antithrombotic drugs') in the short term and long term after a stroke due to bleeding in the brain (known as 'brain haemorrhage')?
Background People with stroke due to brain haemorrhage are more likely to develop clots in their blood vessels than people without brain haemorrhage. Immobility early after the stroke can cause clots in the veins of the legs and pelvis. Patients' underlying medical conditions in both the short and long term after the stroke can also cause clots in the arteries of the lungs, brain, heart, legs or other organs. These clots can cause serious illness or death. Antithrombotic drugs can prevent clots. However, these drugs can also lead to bleeding problems, which can cause serious illness or death. Whether antithrombotic drugs benefit or harm patients after brain haemorrhage is unknown. This is an update of a Cochrane Review, which was last published in 2017.
Study characteristics We updated our extensive searches for randomised controlled trials, which are the fairest tests of treatment, in October 2021. We found nine trials, which included 1491 people with a brain haemorrhage in the past. Four trials studied short‐term use of injected blood thinning drugs (known as 'anticoagulants') in immobile brain haemorrhage survivors. Three trials studied long‐term use of oral anticoagulants in brain haemorrhage survivors with an irregular heart beat (known as 'atrial fibrillation'). One trial studied long‐term use of oral blood thinning drugs (known as 'antiplatelet drugs') after brain haemorrhage, and another trial compared two different types of antiplatelet drug.
Key results We did not identify significant benefits or risks of short‐term injected anticoagulants or long‐term oral antiplatelet drugs. Although long‐term oral anticoagulants reduced the risk of any major bleeding or clotting event in brain haemorrhage survivors with atrial fibrillation, the findings were not precise, and we were only moderately certain about the evidence. We did not identify significant differences in benefits or risks when comparing two long‐term antiplatelet drugs (cilostazol versus aspirin) for people who had both a stroke or mini‐stroke due to clotting and a brain haemorrhage in the past.
Conclusion Although antithrombotic drugs appear to be promising after brain haemorrhage, we cannot be certain on the basis of the trials that have been done so far. Larger trials are needed to be sure about the effects of these drugs after brain haemorrhage. Eight ongoing trials will help resolve these uncertainties if they recruit large numbers of participants.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Starting short‐term prophylactic dose anticoagulation compared to avoiding anticoagulation for survivors of stroke due to intracerebral haemorrhage.
| Starting short‐term prophylactic dose anticoagulation compared to avoiding anticoagulation for survivors of stroke due to intracerebral haemorrhage | ||||||
| Patient or population: survivors of stroke due to intracerebral haemorrhage Setting: secondary care Intervention: starting short‐term prophylactic dose anticoagulation Comparison: avoiding anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with avoiding anticoagulation | Risk with starting short‐term prophylactic dose anticoagulation | |||||
| MACE ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Death assessed with: clinical assessment follow‐up: 90 days | 175 per 1000 | 175 per 1000 (103 to 297) | RR 1.00 (0.59 to 1.70) | 258 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Venous thromboembolism assessed with: clinical assessment follow‐up: 90 days | 142 per 1000 | 119 per 1000 (72 to 195) | RR 0.84 (0.51 to 1.37) | 333 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | |
| Intracerebral haemorrhage (ICH) assessed with: brain imaging and clinical assessment follow‐up: 90 days | 103 per 1000 | 25 per 1000 (4 to 143) | RR 0.24 (0.04 to 1.38) | 119 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,e | |
| Functional status: mRS 0‐2 assessed with: clinical assessment follow‐up: 90 days | 143 per 1000 | 290 per 1000 (111 to 750) | RR 2.03 (0.78 to 5.25) | 73 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,e | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424458755853554175. | ||||||
a The included RCTs have serious risk of bias due to lack of blinding to treatment, and random sequence generation and allocation concealment not being adequately described. b The optimal information size (OIS) criterion is not met. The sample size of this study is probably lower than the minimum number of participants required for a trial adequately powered to identify a statistically significant difference for this outcome. c Very small pooled sample size d Low pooled sample size and event rate e Extremely small pooled sample size
Summary of findings 2. Summary of findings table ‐ Starting long‐term therapeutic dose oral anticoagulation compared to avoiding anticoagulation for survivors of stroke due to intracerebral haemorrhage with atrial fibrillation.
| Starting long‐term therapeutic dose oral anticoagulation compared to avoiding anticoagulation for survivors of stroke due to intracerebral haemorrhage with atrial fibrillation | ||||||
| Patient or population: survivors of stroke due to intracerebral haemorrhage with atrial fibrillation Setting: Secondary care Intervention: starting long‐term therapeutic dose oral anticoagulation Comparison: avoiding anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with avoiding anticoagulation | Risk with starting long‐term therapeutic dose oral anticoagulation | |||||
| MACE (MACE) assessed with: clinical assessment follow‐up: range 1 years to 3 years | 259 per 1000 | 158 per 1000 (104 to 244) | RR 0.61 (0.40 to 0.94) | 334 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,b | |
| Death assessed with: clinical assessment follow‐up: range 1 years to 3 years | 136 per 1000 | 143 per 1000 (84 to 242) | RR 1.05 (0.62 to 1.78) | 334 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,b | |
| All major occlusive vascular events assessed with: clinical assessment follow‐up: range 1 years to 3 years | 210 per 1000 | 57 per 1000 (29 to 111) | RR 0.27 (0.14 to 0.53) | 334 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Intracranial haemorrhage assessed with: clinical assessment follow‐up: range 1 years to 3 years | 31 per 1000 | 75 per 1000 (27 to 208) | RR 2.43 (0.88 to 6.73) | 334 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,c | |
| Functional status (mRS 0‐2) assessed with: clinical assessment follow‐up: 1 years | 490 per 1000 | 480 per 1000 (382 to 607) | RR 0.98 (0.78 to 1.24) | 288 (2 RCTs) | ⊕⊕⊝⊝ Lowb,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431448658702855854. | ||||||
a All included randomised trials were open label, but these outcomes were objective. b These are the results of a small number of events being observed in three RCTs with relatively small sample sizes. c The pooled estimate was imprecise due to the small number of outcome events d All included randomised trials were open label, and this outcome was subjective
Summary of findings 3. Summary of findings table ‐ Starting long‐term oral antiplatelet therapy compared to avoiding antithrombotic therapy for survivors of stroke due to intracerebral haemorrhage.
| Starting long‐term oral antiplatelet therapy compared to avoiding antithrombotic therapy for survivors of stroke due to intracerebral haemorrhage | ||||||
| Patient or population: survivors of stroke due to intracerebral haemorrhage Setting: Secondary care Intervention: starting long‐term oral antiplatelet therapy Comparison: avoiding antithrombotic therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with avoiding antithrombotic therapy | Risk with starting long‐term oral antiplatelet therapy | |||||
| MACE assessed with: clinical assessment follow‐up: median 2 years | 228 per 1000 | 203 per 1000 (146 to 278) | RR 0.89 (0.64 to 1.22) | 536 (1 RCT) | ⊕⊕⊕⊝ Moderatea,b | |
| Death assessed with: clinical assessment follow‐up: median 2 years | 187 per 1000 | 201 per 1000 (142 to 285) | RR 1.08 (0.76 to 1.53) | 536 (1 RCT) | ⊕⊕⊕⊝ Moderatea,b | |
| All major occlusive vascular events assessed with: clinical assessment follow‐up: median 2 years | 142 per 1000 | 146 per 1000 (96 to 220) | RR 1.03 (0.68 to 1.55) | 536 (1 RCT) | ⊕⊕⊕⊝ Moderateb | |
| Intracerebral haemorrhage (ICH) assessed with: clinical assessment follow‐up: median 2 years | 86 per 1000 | 45 per 1000 (23 to 88) | RR 0.52 (0.27 to 1.03) | 536 (1 RCT) | ⊕⊕⊕⊝ Moderatea,b | |
| Fnctional status (mRS 0‐2) assessed with: clinical assessment follow‐up: 1 years | 433 per 1000 | 411 per 1000 (333 to 511) | RR 0.95 (0.77 to 1.18) | 461 (1 RCT) | ⊕⊕⊕⊝ Moderateb | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431448890153987933. | ||||||
a The included randomised trial was open label but outcomes were objective. b Only one randomised trial with 537 participants.
Summary of findings 4. Summary of findings table ‐ Cilostazol compared to aspirin for adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a clinical history of prior intracerebral haemorrhage.
| Cilostazol compared to aspirin for adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a clinical history of prior intracerebral haemorrhage | ||||||
| Patient or population: adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a clinical history of prior intracerebral haemorrhage Setting: Secondary care Intervention: cilostazol Comparison: aspirin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with aspirin | Risk with cilostazol | |||||
| MACE assessed with: clinical assessment follow‐up: median 1.8 years | 116 per 1000 | 155 per 1000 (86 to 279) | RR 1.33 (0.74 to 2.40) | 288 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |
| Death assessed with: clinical assessment follow‐up: median 1.8 years | 34 per 1000 | 57 per 1000 (19 to 168) | RR 1.65 (0.55 to 4.91) | 288 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| All major occlusive vascular events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Intracerebral haemorrhage (ICH) assessed with: clinical assessment follow‐up: median 1.8 years | 27 per 1000 | 35 per 1000 (10 to 128) | RR 1.29 (0.35 to 4.69) | 288 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| Functional status ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431449016706363235. | ||||||
a The report of the sub‐group of the PICASSO trial did not report major extracerebral haemorrhage, DVT or functional status. b Small sample size from a sub‐group of the PICASSO trial
Background
Stroke was the second‐leading cause of death worldwide in 2019, when stroke due to spontaneous (non‐traumatic) intracerebral haemorrhage (ICH) constituted 28% of all incident strokes and 48% of disability‐adjusted life years due to stroke (GBD 2019 Stroke Collaborators 2021). Half of those patients with ICH die within one year (mostly due to the ICH), leaving 20.7 million prevalent ICH survivors worldwide (GBD 2019 Stroke Collaborators 2021). ICH survivors are at high risk of major adverse cardiovascular events (MACE), such as stroke, myocardial infarction, and death due to a vascular cause. Antithrombotic drugs (Table 5) reduce the risk of MACE overall for many patients, by reducing the risk of ischaemic events despite an increase in the risk of haemorrhagic events. However, the effects of antithrombotic drugs on ICH survivors are uncertain, which makes this a common therapeutic dilemma in everyday clinical practice.
1. Non‐enzymatic antithrombotic agents with defined daily doses within group B01A of the World Health Organization Anatomical Therapeutic Chemical Classification System.
| Antithrombotic class | Antithrombotic agents |
| Vitamin K antagonists | Dicoumarol, phenindione, warfarin, phenprocoumon, acenocoumarol, ethyl biscoumacetate |
| Heparin group | Heparin, antithrombin III, dalteparin, enoxaparin, nadroparin, parnaparin, reviparin, danaparoid, tinzaparin, sulodexide, bemiparin |
| Platelet aggregation inhibitors excluding heparin | Clopidogrel, ticlopidine, acetylsalicylic acid, dipyridamole, carbasalate calcium, epoprostenol, indobufen, iloprost, abciximab, aloxiprin, eptifibatide, tirofiban, triflusal, beraprost, treprostinil, prasugrel, cilostazol, ticagrelor, cangrelor, vorapaxar, selexipag |
| Direct thrombin inhibitors | Desirudin, lepirudin, argatroban, melagatran, ximelagatran, bivalirudin, dabigatran etexilate* |
| Direct factor Xa inhibitors | Rivaroxaban*, apixaban*, edoxaban* |
| Other antithrombotic agents | Defibrotide, fondaparinux |
www.whocc.no/atc_ddd_index/?code=B01A
* Non‐vitamin K (direct) oral anticoagulants
Description of the condition
Survivors of ICH are at high risk of ischaemic and haemorrhagic MACE. Cerebral small vessel diseases, which underlie more than 85% of ICH (Samarasekera 2015), may also cause ischaemic stroke and vascular dementia. Adults with ICH are approximately 75 years old and usually have high blood pressure (BP), multiple co‐morbidities, and other risk factors for ischaemic or haemorrhagic MACE, which have already affected one third of adults before their ICH (GBD 2019 Stroke Collaborators 2021; Li 2021). Survivors of ICH are at higher future risk of ischaemic MACE than population controls (Gaist 2022; Murthy 2021). Overall, the rate of ischaemic MACE appears to exceed the rate of recurrent ICH, and these rates appear higher early after ICH (Banerjee 2020; Li 2021; Poon 2014). If ICH is in a lobar location recurrent ICH is more likely, whereas atrial fibrillation (AF) is the main risk factor for ischaemic stroke after ICH (Banerjee 2020; Casolla 2019; Li 2021; Nielsen 2022). In population‐based cohort studies of incident ICH in the UK, the annual rate of MACE ranged from 7% to 19%, dependent on survivors’ medical history (Li 2021). These rates are even higher in low‐middle income countries (Chen 2020). Most people with MACE after ICH die or are left disabled, so they cause a huge burden on health and care services worldwide (GBD 2019 Stroke Collaborators 2021; Li 2021).
Description of the intervention
The only intervention proven to reduce stroke after ICH is BP lowering (Chapman 2004), and most guidelines focus on prevention of recurrent ICH (AHA ICH guideline 2015; Canadian ICH best practice recommendation 2020; Chinese Stroke Association guidelines 2019; ESO guideline on antithrombotic treatment 2019; ESO ICH Guideline 2014; National Clinical Guideline for Stroke 2016). However, despite widespread implementation of BP lowering after ICH, which has been associated with better BP control and outcome, the annual rate of all MACE for ICH survivors has remained between 7% and 19% in the last two decades (Banerjee 2020; Casolla 2019; Chen 2020; Li 2021; Poon 2014). Therefore, better secondary prevention of all MACE after ICH is needed in standard clinical practice.
Antithrombotic drugs, which have various mechanisms of action (Table 5), are usually dichotomised into antiplatelet and anticoagulant drugs according to their use in clinical practice. These drugs reduce the risk of thrombosis and thromboembolism, but consequently increase the risk of bleeding. Anticoagulation may be prescribed in lower 'prophylactic' doses for prevention of venous thromboembolism for immobile patients, or higher 'therapeutic doses' for prevention of systemic embolism for patients in AF. The anticoagulant drugs used subcutaneously at a lower dose for prophylaxis are from the heparin group, whereas the anticoagulants used orally at a therapeutic dose for AF are the vitamin K antagonists, direct thrombin inhibitors, and direct factor Xa inhibitors (Table 5). The antiplatelet drugs used orally for prevention of occlusive vascular events are the platelet aggregation inhibitors (Table 5).
How the intervention might work
Antiplatelet drugs disrupt the formation of platelet plugs. They are used for preventing arterial thromboembolism, such as myocardial infarction or ischaemic stroke. Aspirin achieves this by inhibiting platelet activation, while clopidogrel, cilostazol and tirofiban impede aggregation.
Anticoagulant drugs disrupt the coagulation cascade to stop a fibrin mesh forming around the platelet plug and are generally used to prevent venous thromboembolism. Heparin and enoxaparin bind to a naturally occurring anticoagulant (antithrombin) to amplify its effect, while direct (non‐vitamin K) oral anticoagulants (DOACs) and warfarin directly inhibit the coagulation cascade.
Antithrombotic drugs also increase the risk of bleeding as a result of their effect on clotting and platelet aggregation.
Although antithrombotic drugs are known to be of net benefit (i.e. when considering their effects on both clotting and bleeding) in people without a history of bleeding (Antithrombotic Trialists' Collaboration 2009; Benz 2021; Hart 2007), the balance of benefit and harm is uncertain after ICH.
Why it is important to do this review
People with ICH were excluded from randomised controlled trials (RCTs) of prophylactic dose anticoagulation after acute stroke (Wang 2021), and secondary prevention of MACE with antiplatelet therapy (Antithrombotic Trialists' Collaboration 2009; Benz 2021), or therapeutic dose anticoagulation for AF (Hart 2007).
A network meta‐analysis of RCTs of pharmacological thromboprophylaxis versus intermittent pneumatic compression to prevent venous thromboembolism after ICH was unable to make meaningful comparisons between prophylactic dose anticoagulation and the current clinical standard of care with intermittent pneumatic compression (Yogendrakumar 2020).
A systematic review and meta‐analysis of cohort studies of patients with any type of intracranial haemorrhage (i.e. intracerebral, subarachnoid, or subdural haemorrhage) found lower risks of ischaemic MACE (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.48 to 0.79) and no evidence of a difference in haemorrhagic MACE risk (RR 0.84, 95 %CI 0.47 to 1.51) associated with resumption compared with avoidance of antiplatelet therapy (Ding 2018).
A systematic review and meta‐analysis of cohort studies of patients with spontaneous intracranial haemorrhage and AF comparing oral anticoagulation with either antiplatelet agents or no antithrombotic therapy mostly found associations between oral anticoagulation and lower risks of ischaemic MACE, but no significant change in the risk of haemorrhagic MACE, although these studies are susceptible to selection bias (Korompoki 2017).
The evidence available has left guidelines unable to recommend antithrombotic drugs after ICH (AHA ICH guideline 2015; Canadian ICH best practice recommendation 2020; Chinese Stroke Association guidelines 2019; ESO ICH Guideline 2014; ESO guideline on antithrombotic treatment 2019; National Clinical Guideline for Stroke 2016). Consequently, there has been variation in clinical practice, evident from the proportion of patients starting antithrombotic drugs after ICH varying between 11% to 45% in different countries (Pasquini 2014).
In the most recent version of this Cochrane Review, we analysed two RCTs including 121 participants, and concluded that there was insufficient evidence from RCTs to support or discourage the use of antithrombotic treatment after ICH (Perry 2017). However, several RCTs have been published since the first version of this review, therefore we performed this update.
Objectives
To determine the overall effectiveness and safety of antithrombotic drugs on major adverse cardiovascular events (MACE) and its components for people with ICH.
Methods
Criteria for considering studies for this review
Types of studies
We sought all randomised controlled trials (RCTs) that made comparisons of starting versus avoiding antithrombotic drugs, or direct comparisons of different antithrombotic classes or agents after stroke due to intracerebral haemorrhage (ICH). We included RCTs published in any language and planned to arrange translation where the language of publication was not English.
Types of participants
Eligible patients survived spontaneous ICH in the brain parenchyma diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) scan. We included patients regardless of whether they were on antithrombotic therapy at the time of ICH.
Types of interventions
We sought RCTs that compared starting any antithrombotic drug with avoiding antithrombotic treatment, as well as RCTs that compared different antithrombotic classes or agents (Table 5). We placed no constraints on dosage, route of administration, or duration of administration. We separated RCTs investigating short‐term treatment (e.g. with low‐molecular‐weight heparin (LMH) or unfractionated heparin (UFH) to prevent venous thromboembolism early after ICH onset) from those RCTs investigating long‐term secondary prevention (e.g. with oral anticoagulant or antiplatelet drugs).
If the effects of antithrombotic drugs might have been confounded by the administration of another active drug to participants (e.g. if allocation to this additional treatment was not evenly distributed between groups in an RCT, or was not randomly allocated), we planned to explore this in our risk of bias assessment and in sensitivity analyses.
Types of outcome measures
We restricted inclusion to RCTs reporting our clinical primary or secondary outcomes (after seeking information about these outcomes if they were not included in the report of the RCT).
Primary outcomes
Composite outcome of all serious vascular events, commonly known as MACE (which we defined as ischaemic stroke, myocardial infarction, other major ischaemic event, ICH, major extracerebral haemorrhage, or vascular death) during the scheduled follow‐up period.
Secondary outcomes
Death during the scheduled follow‐up period.
The individual components of the composite MACE primary outcome: ischaemic stroke, myocardial infarction, other major ischaemic event (deep vein thrombosis, pulmonary embolism or venous thromboembolism), ICH, major extracerebral haemorrhage, and vascular death.
Growth of ICH (as classified/reported by each RCT).
Functional status (where measured using validated scales, such as the modified Rankin Scale) at the end of the scheduled follow‐up period.
Cognitive status (where measured using validated scales, such as the Montreal Cognitive Assessment or the Mini‐mental State Examination) at the end of the scheduled follow‐up period.
Search methods for identification of studies
We searched for RCTs in any language and planned to arrange for the translation of relevant articles where necessary.
Electronic searches
We designed a search strategy with the help of the Cochrane Stroke Group’s Information Specialist, who ran the searches on the 5 October 2021.
We searched the following electronic databases:
Cochrane Stroke Group Trials Register;
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 10) in the Cochrane Library;
MEDLINE Ovid, 1946 to October 2021;
Embase Ovid, 1946 to October 2021.
We searched the following online registries of clinical trials:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 5 October 2021);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch; searched 5 October 2021);
In the previous version of this review, we searched the Stroke Trials Registry of the Internet Stroke Center (www.strokecenter.org/trials/) on 2 March 2017.
Searching other resources
We screened the reference lists of relevant studies to identify RCTs for potential inclusion in the review. In the event that we could not locate the full text of a RCT, or required a specific data extract, we contacted the researchers or the relevant data sharing platform.
Data collection and analysis
We imported the results of the searches into Covidence, removed duplicates, and applied the following methods to select, extract and analyse data. Review author RASS was not involved in decisions about included studies for which he was the Chief Investigator (RESTART 2019 and SoSTART 2021); decisions about these studies were made by review authors AC, CC, and co‐authors without a competing interest.
Selection of studies
Two review authors (AC and CC) independently screened titles and abstracts and full texts for eligibility, resolving conflicts by discussion with a third review author to reach consensus.
We retrieved full‐text articles for potentially eligible references, and the same three review authors independently screened these full‐text articles to identify RCTs for inclusion, and identified and recorded reasons for the exclusion of ineligible studies. We resolved any disagreements through discussion. We collated multiple reports of the same RCT, and chose the report at lowest risk of bias, so that each RCT was the unit of interest.
We recorded the cumulative results of the searches and the selection process (i.e. including the prior version of this review Perry 2017) and completed a flow diagram (Figure 1).
1.
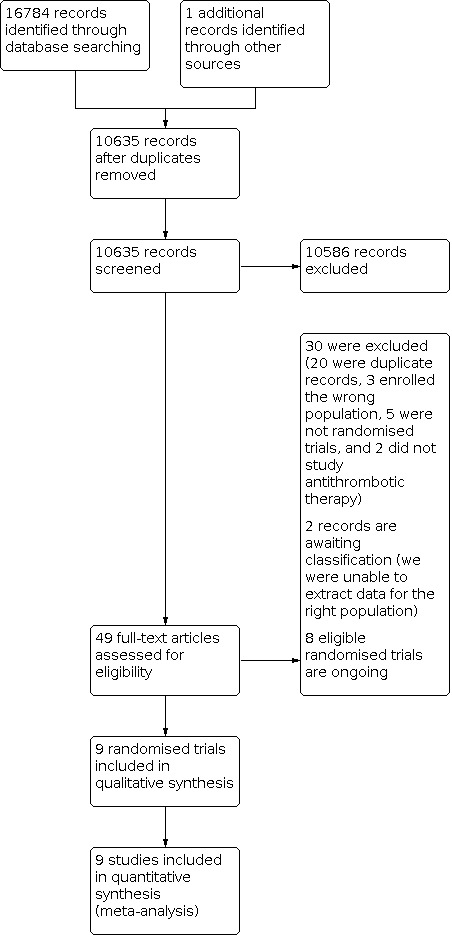
Flow diagram of study screening, eligibility assessment and inclusion
Data extraction and management
We created and independently completed an electronic data collection form in Microsoft Word (which had been piloted and used for the prior version of this review) for each included RCT. Two review authors (CC and AC) extracted data from included studies independently on methods, risk of bias, participant characteristics, intervention, comparator, and outcomes. We extracted outcome event frequencies without the need for transformation. Another review author checked all data extraction, queried errors, and agreed the final results in discussion with CC and AC.
We sought and obtained unpublished data from one RCT (NASPAF‐ICH 2020). We sought the data on a subgroup of participants with prior ICH from another RCT (ELDERCARE‐AF 2020), but data sufficient for analysis were not provided by the study Sponsor via the Vivli data sharing platform in time for inclusion in this review update.
Assessment of risk of bias in included studies
Two review authors (AC and CC) assessed risks of bias for each study independently using the RoB 1 tool and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Another review author reviewed all classifications, and achieved consensus via discussion. We assessed the risks of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other potential bias
We graded the risk of bias for each domain as high, low or unclear and provide information from the study report, together with a justification for our judgement, in the risk of bias tables.
Measures of treatment effect
We converted categorical data using numeric frequencies of outcomes and group sizes to estimates of effect using the risk ratio (RR), without adjustment.
Unit of analysis issues
Repeated observations on participants
We planned to analyse functional and cognitive status at end of follow‐up or a period of follow‐up available for all RCTs being pooled, and not to use repeat observations during follow‐up. If one RCT (or only a few RCTs) had a much longer period of follow‐up than the majority of RCTs (e.g. two years compared with six months in the majority of trials) we intended to perform a sensitivity analysis using the six‐month observations from all RCTs.
Events that may recur
We analysed the first event in all participants, and not later events.
Multiple intervention groups
Where a RCT contained multiple treatment groups that were all compared with just one control group, we intended to ensure that the control group was shared between the multiple treatment groups by dividing it into the appropriate number of subgroups and conducting separate, independent comparisons.
Dealing with missing data
We intended to contact RCT authors for unpublished data if relevant data were missing. If only a minority (less than half) of data were missing, we planned to ignore the missing data and perform a 'complete set analysis'. If more substantial amounts of data were missing, and there was a chance that data were not missing at random, we planned to perform sensitivity analyses assuming both a best‐case and a worst‐case scenario, or apply statistical imputation or models, or both, to account for the missing data. A best‐case scenario meant that we assumed that all missing data in the intervention group represent good outcomes and all missing data in the control group represent poor outcomes. A worst‐case scenario meant that we assumed that all missing data in the intervention group represent poor outcomes and all missing data in the control group represent good outcomes.
Assessment of heterogeneity
We investigated heterogeneity between included RCTs using the I2 statistic. We interpreted this value using the guide provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022):
0% to 74%: inconsiderable heterogeneity
75% to 100%: considerable heterogeneity
If we observed considerable heterogeneity in our data, we intended to use sensitivity analysis to elucidate which factors might explain the heterogeneity.
Assessment of reporting biases
We included all published and unpublished data and secondary publications from RCTs. If we had included a sufficient number of RCTs (more than 10), we planned to assess the likelihood of reporting biases through the use of a funnel plot.
Data synthesis
We included RCTs regardless of their risk of bias. If RCTs were sufficiently similar, we planned to conduct a meta‐analysis by pooling the appropriate data using RevMan Web (RevMan Web 2022). We calculated the RR for each outcome from data extracted from included RCTs using a fixed‐effect model, or a random‐effects model if there was considerable qualitative or quantitative heterogeneity (I2 ≥ 75%).
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses, which could modify the effects of antithrombotic drugs on ischaemic or haemorrhagic clinical outcome events.
-
Participants
age
sex
stroke severity
Different classes of antithrombotic drugs (antiplatelet versus anticoagulant)
Different intensities of antithrombotic treatment (low dose versus high dose)
Different times of starting treatment (e.g. within one month of ICH versus later, or within 10 to 30 weeks or later)
Participants who were on antithrombotic treatment before ICH (re‐starters) versus participants who were not receiving these treatments before ICH (starters)
Different levels of risk for future ischaemic events (e.g. because of differences in age, sex, history of hypertension, history of AF (with further stratification by the CHA2DS2‐VASc score))
Different levels of risk for future ICH (lobar versus non‐lobar ICH location)
Biomarkers of bleeding or clotting risk on brain computed tomography (CT) or magnetic resonance imaging (MRI) (e.g. brain microbleeds on MRI)
Sensitivity analysis
If there was considerable qualitative or quantitative evidence of heterogeneity, we planned to use sensitivity analysis to investigate how the results differed when we excluded RCTs (e.g. those with a high risk of bias). We planned to perform other sensitivity analyses to explore reasons for heterogeneity, for example, where there is an active co‐intervention other than an antithrombotic drug that is not balanced by the randomisation process.
Summary of findings and assessment of the certainty of the evidence
Outcome importance: Given the number of pre‐specified outcomes and comparisons in this review, we prioritised the five following clinically important outcomes for reporting in the Summary of findings tables and abstract: (1) MACE, (2) death, (3) major occlusive vascular events, (4) ICH, and (5) functional outcome.
Summary of findings: we created summary of findings tables using the GRADEpro Guideline Development Tool (GRADEpro GDT).
Certainty of the evidence: we classified the certainty of the evidence as being 'high', 'moderate', 'low', or 'very low', based on the presence and extent of the following five criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022):
limitations in the design and implementation of the contributing trials;
indirectness of evidence;
unexplained heterogeneity of results;
imprecision of results;
high probability of publication bias.
We provided justification in the footnotes when we downgraded the certainty from 'high'.
Results
Description of studies
After screening 10,635 records (Figure 1), we excluded 10,586, assessed 49 full‐text articles (20 of which were duplicate records), excluded 12 RCTs (Excluded studies), identified eight ongoing RCTs (Ongoing studies), and included nine RCTs in qualitative and quantitative syntheses (Included studies).
Results of the search
We included nine parallel‐group RCTs in our review (APACHE‐AF 2021; Dickmann 1988; NASPAF‐ICH 2020; Orken 2009; PICASSO sub‐group 2020; PREVENTIHS 2020; Qian 2021; RESTART 2019; SoSTART 2021).
We grouped these RCTs for description and analysis into four groups:
short‐term prophylactic dose anticoagulation (start versus avoid);
long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid);
long‐term antiplatelet therapy (start versus avoid);
long‐term antiplatelet therapy (cilostazol versus aspirin).
Short‐term prophylactic dose anticoagulation (start versus avoid)
Dickmann 1988 took place in inpatient units in Germany. All 46 participants were administered 5000 units of heparin every eight hours, with the intervention group starting at day four after intracerebral haemorrhage (ICH) and the control group at day 10. Re‐bleeding, thrombosis, pulmonary embolism and death were recorded until day 10.
Orken 2009 took place in Turkey and randomised 75 participants 48 hours after admission to 48 mg enoxaparin per day or compression stockings. Outcomes were measured on days seven and 21.
PREVENTIHS 2020 was conducted in Italian hospitals. The trial was discontinued because of low recruitment rates, but randomised 73 patients to 4000 units of enoxaparin or no treatment. Outcomes were measured up to day 90.
Qian 2021 took place in Helsinki and randomised 139 patients to the intervention of 2000 units of enoxaparin 12 or 24 hours after ICH, or to the placebo group who received saline injections. After the initial 72 hours, both groups received treatment with the same does of enoxaparin. Outcomes were measured up to day 90 after ICH.
Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid)
NASPAF‐ICH 2020 took place in Canada. It compared the effectiveness of non‐vitamin K antagonist oral anticoagulants (NOACs) to aspirin in 30 participants with prior ICH and AF randomised in a 2:1 ratio. Outcomes were measured between 10.5 months and 2.8 years after ICH.
SoSTART 2021 took place in the UK, included 203 participants who had atrial fibrillation (AF) and recent ICH, randomly assigned them to either start or avoid an oral anticoagulant (34 (33%) of the participants in the 'avoid oral anticoagulation' group took antiplatelet monotherapy), and identified outcomes for up to three years after randomisation.
APACHE‐AF 2021 took place in the Netherlands, recruited 101 participants who had recent ICH and were already taking anticoagulation for AF, randomly assigned them to apixaban 5 mg twice daily or to avoid oral anticoagulation (26 (51%) of the participants in the 'avoid oral anticoagulation' group took antiplatelet therapy), and identified outcomes for up to three years after randomisation.
Long‐term antiplatelet therapy (start versus avoid)
RESTART 2019 took place in the UK and included 537 participants who had been taking an antithrombotic drug until the time of ICH, and randomised them to starting or avoiding antiplatelet therapy. Participants were followed up for a minimum of six months.
Long‐term antiplatelet therapy (cilostazol versus aspirin)
PICASSO sub‐group 2020 was a separate report of a subgroup of a factorial trial which took place in 67 centres in Asian countries. The subgroup included 268 patients with history of ICH, randomised to cilostazol or aspirin either with or without probucol.
Excluded studies
Excluded studies
We excluded 10 RCTs.
Boeer 1991 was an extension phase of Dickmann 1988, but randomisation was not described.
CAST 1997 and IST 1997 were two RCTs investigating the use of antiplatelet treatment after acute ischaemic stroke, and inadvertently included several hundred participants with ICH who were randomised before computed tomography (CT) had been performed to establish the pathological subtype of stroke. Data on the ICH subpopulation of these studies were reported in a systematic review (Keir 2002) and the individual patient data from IST 1997 (Sandercock 2011). These sources reported that this population included spontaneous ICH (52%) and haemorrhagic transformation of ischaemic stroke (48%), but unfortunately the chief investigator informed us that it would be impossible to isolate the participants with spontaneous ICH (IST 1997).
Frontera 2014 and Kuramatsu 2018 were not randomised.
Li 2013 assessed transfusion of frozen apheresis platelets (platelets collected by separation from whole blood) in patients with ICH and on aspirin.
Venturelli 2014 was a post‐hoc analysis of a RCT in which antithrombotic treatment was not randomly assigned, making this analysis observational in nature.
Yan 2014 included a population of patients with and without cerebral microbleeds after acute ischaemic stroke, but not ICH.
RESTART extended follow‐up was a two‐year extension of RESTART 2019, but participants and their primary care practitioners had been told the results of RESTART 2019, which put the extended follow‐up at higher risk of bias than the main report, so we included RESTART 2019 and excluded RESTART extended follow‐up.
ChiCTR2000040166 used two traditional Chinese medicines which are not proven to have antithrombotic effects and therefore did not meet the criteria for the review.
Studies awaiting classification
ELDERCARE‐AF 2020 and PRAGUE‐17 2020 were suitable for inclusion in our review, but ELDERCARE‐AF 2020 did not provide data that were sufficient for accurate analysis and the corresponding author of PRAGUE‐17 2020 was unable to provide the data for the subgroup of patients with prior ICH in time for inclusion in this review, so these studies are categorised as Studies awaiting classification.
Risk of bias in included studies
We assessed all included RCTs for their risk of bias (Figure 2).
2.

Allocation
Sixty‐seven per cent of included RCTs were at low risk of bias in random sequence generation.
Sixty‐seven per cent of included RCTs were at low risk of bias in allocation concealment.
Blinding
Twenty‐two per cent of included RCTs were at low risk of performance bias due to blinding of participants and personnel.
Seventy‐eight per cent of included RCTs were at low risk of detection bias in blinding of outcome assessment.
Incomplete outcome data
Eighty‐nine per cent of included RCTs were at low risk of attrition bias.
Selective reporting
Seventy‐eight per cent of included RCTs were at low risk of reporting bias.
Other potential sources of bias
We did not identify any other major sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Short‐term prophylactic dose anticoagulation (start versus avoid)
We included four RCTs, all of which compared a parenteral anticoagulant to no treatment, although there was considerable qualitative heterogeneity between the RCTs' interventions, comparators, and timing of assessment, so we used random‐effects models to pool their effect estimates (Dickmann 1988; Orken 2009; PREVENTIHS 2020; Qian 2021). We present our summary of findings in Table 1. We did not need to make post‐hoc decisions that could have led to selective outcome reporting.
Primary outcome
We were unable to extract data on our primary outcome and some secondary outcomes (ischaemic stroke, myocardial infarction, vascular death, and cognitive status) from any of the RCTs, and we did not succeed in obtaining these outcomes from the corresponding authors.
Secondary outcomes
We did not find significant differences with starting versus avoiding short‐term prophylactic dose anticoagulation in the secondary outcomes that were reported.
Death of any cause (23/132 versus 22/126; risk ratio (RR) 1.00, 95% confidence interval (CI) 0.59 to 1.70, P = 1.00; 3 published RCTs; 258 participants; very low‐certainty evidence; Analysis 1.1).
We considered the relevant clinically important composite of major occlusive events for this treatment comparison to be venous thromboembolism (21/171 versus 23/162; RR 0.84, 95% CI 0.51 to 1.37, P = 0.49; 4 published RCTs; 333 participants; very low‐certainty evidence; Analysis 1.4).
Intracerebral haemorrhage (ICH) (1/61 versus 6/58; RR 0.24, 95% CI 0.04 to 1.38, P = 0.11; 2 published RCTs; 119 participants; very low‐certainty evidence; Analysis 1.5).
Functional independence, graded 0‐2 on the modified Rankin Scale (mRS) score (11/38 versus 5/35; RR 2.03, 95% CI 0.78 to 5.25, P = 0.15; 1 published RCT; 73 participants; very low‐certainty evidence; Analysis 1.8).
ICH growth (8/110 versus 8/104; RR 0.96, 95% CI 0.38 to 2.41, P = 0.93; 2 published RCTs; 214 participants; Analysis 1.7).
Major extracerebral haemorrhage (1/148 versus 0/139; RR 2.77, 95% CI 0.12 to 65.82, P = 0.53; 3 published RCTs; 287 participants; Analysis 1.6).
Deep vein thrombosis (13/133 versus 12/127; RR 0.96, 95% CI 0.49 to 1.86, P = 0.9; 3 published RCTs; 260 participants; Analysis 1.2).
Pulmonary embolism (6/171 versus 13/162; RR 0.50, 95% CI 0.22 to 1.14, P = 0.1; 4 published RCTs; 333 participants; Analysis 1.3).
1.1. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 1: Death
1.4. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 4: Venous thromboembolism
1.5. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 5: Intracerebral haemorrhage
1.8. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 8: Functional status (modified Rankin Scale 0‐2)
1.7. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 7: Growth of qualifying intracerebral haemorrhage
1.6. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 6: Major extracerebral haemorrhage
1.2. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 2: Deep vein thrombosis
1.3. Analysis.

Comparison 1: Short‐term prophylactic dose anticoagulation (start versus avoid), Outcome 3: Pulmonary embolism
Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid)
We included three RCTs, all of which compared long‐term therapeutic dose of an oral anticoagulant (almost exclusively direct oral anticoagulants (DOACs)) to avoidance of anticoagulation (in which some patients took long‐term antiplatelet therapy and others avoided all antithrombotic drugs) (APACHE‐AF 2021; NASPAF‐ICH 2020; SoSTART 2021). We present our summary of findings in Table 2. We did not need to make post‐hoc decisions that could have led to selective outcome reporting.
Primary outcome
We found a significant reduction in the composite primary outcome of major adverse cardiovascular events (MACE) with starting versus avoiding long‐term oral anticoagulation (26/172 versus 42/162; RR 0.61, 95% CI 0.40 to 0.94, P = 0.02; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 1: All major adverse cardiovascular events (MACE)
Secondary outcomes
The effects of starting versus avoiding long‐term oral anticoagulation for atrial fibrillation on secondary outcomes were as follows.
Death (24/172 versus 22/162; RR 1.05, 95% CI 0.62 to 1.78, P = 0.86; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.2).
Major occlusive vascular events (9/172 versus 34/162; RR 0.27, 95% CI 0.14 to 0.53, P = 0.0002; 3 RCTs; moderate‐certainty evidence; Analysis 2.5).
Intracranial haemorrhage (12/172 versus 5/162; RR 2.43, 95% CI 0.88 to 6.73, P = 0.09; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.6).
Functional independence, graded 0‐2 on the modified Rankin Scale (mRS) score (11/38 versus 5/35; RR 0.98, 95% CI 0.78 to 1.24, P = 0.87; 2 published RCTs; 288 participants; very low‐certainty evidence; Analysis 2.9).
Myocardial infarction (0/172 versus 4/162; RR 0.20, 95% CI 0.02 to 1.71, P =0.14; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.4).
Major extracerebral haemorrhage (2/172 versus 3/162; RR 0.58, 95 %CI 0.13 to 2.57, P = 0.47; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.7).
Ischaemic stroke (9/172 versus 26/162; RR 0.35, 95% CI 0.17 to 0.71; P = 0.004; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.3).
Vascular death (13/172 versus 9/162; RR 1.47, 95% CI 0.65 to 3.32, P = 0.36; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.8).
Modified Rankin Scale score 0 to 2 at one year (69/143 versus 71/145; RR 0.98, 95% CI 0.78 to 1.24, P = 0.87; 2 published RCTs; 288 participants; moderate‐certainty evidence; Analysis 2.9).
Any stroke (21/172 versus 30/162; RR 0.70, 95% CI 0.42 to 1.15, P = 0.16; 2 published and 1 unpublished RCTs; 334 participants; moderate‐certainty evidence; Analysis 2.10).
Any stroke or vascular death (25/151 versus 35/153; RR 0.72, 95% CI 0.46 to 1.15, P = 0.17; 2 published RCTs; 304 participants; moderate‐certainty evidence; Analysis 2.11).
2.2. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 2: Death
2.5. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 5: All major occlusive vascular events
2.6. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 6: Intracranial haemorrhage
2.9. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 9: Functional status (modified Rankin Scale score 0‐2) at 1 year
2.4. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 4: Myocardial infarction
2.7. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 7: Major extracerebral haemorrhage
2.3. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 3: Ischaemic stroke
2.8. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 8: Vascular death
2.10. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 10: Any stroke (ischaemic or haemorrhagic)
2.11. Analysis.

Comparison 2: Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid), Outcome 11: Any stroke (ischaemic or haemorrhagic) or vascular death
Long‐term antiplatelet therapy (start versus avoid)
We included one RCT which compared long‐term antiplatelet therapy (with aspirin ± dipyridamole ± clopidogrel) to avoidance of antithrombotic therapy in 537 participants, one of whom withdrew from follow‐up (RESTART 2019). We present our summary of findings in Table 3. We did not need to make post‐hoc decisions that could have led to selective outcome reporting.
Primary outcome
We did not find a significant difference in the composite primary outcome of MACE with starting versus avoiding long‐term oral antiplatelet therapy (54/268 versus 61/268; RR 0.89, 95% CI 0.64 to 1.22, P = 0.46; 1 published RCT; 536 participants; moderate‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 1: All major adverse cardiovascular events (MACE)
Secondary outcomes
The effects of starting versus avoiding long‐term oral antiplatelet therapy on secondary outcomes were as follows.
Death (54/268 versus 50/268; RR 1.08, 95% CI 0.76 to 1.53, P = 0.66; 1 published RCT; 536 participants; moderate‐certainty evidence; Analysis 3.2).
All major occlusive vascular events (39/268 versus 38/268; RR 1.03, 95% CI 0.68 to 1.55, P = 0.90; 1 published RCT; moderate‐certainty evidence; Analysis 3.5).
ICH (12/268 versus 23/268; RR 0.52, 95% CI 0.27 to 1.03, P = 0.06; 1 published RCT; 536 participants; moderate‐certainty evidence; Analysis 3.6).
Functional independence, graded 0‐2 on the modified Rankin Scale (mRS) score (95/230 versus 100/231; RR 0.95, 95% CI 0.77 to 1.18, P = 0.67; 1 RCT; moderate‐certainty evidence; Analysis 3.9).
Major vascular events as defined by the Antithrombotic Trialists’ Collaboration (45/268 versus 65/268; RR 0.69, 95% CI 0.49 to 0.97; P = 0.03; 1 published RCT; 536 participants; moderate‐certainty evidence; Analysis 3.12).
Major extracerebral haemorrhage (8/268 versus 3/268; RR 2.67, 95% CI 0.72 to 9.94, P = 0.14; 1 published RCT; moderate‐certainty evidence; Analysis 3.7).
Ischaemic stroke (19/268 versus 27/268; RR 0.70, 95% CI 0.40 to 1.23, P = 0.22; 1 published RCT; moderate‐certainty evidence; Analysis 3.3).
Myocardial infarction (5/268 versus 8/268; RR 0.63, 95% CI 0.21 to 1.89, P = 0.40; 1 published RCT; moderate‐certainty evidence; Analysis 3.4).
Deep vein thrombosis (6/268 versus 2/268; RR 3.00 (95% CI 0.61 to 14.73, P = 0.18; 1 published RCT; moderate‐certainty evidence; Analysis 3.10).
Vascular death (18/268 versus 27/268; RR 0.67 (95% CI 0.38 to 1.18, P = 0.16; 1 published RCT; moderate‐certainty evidence; Analysis 3.8).
All major haemorrhagic events (18/268 versus 25/268; RR 0.72 (95% CI 0.40 to 1.29, P = 0.27; 1 published RCT; moderate‐certainty evidence; Analysis 3.11).
3.2. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 2: Death
3.5. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 5: All major occlusive vascular events
3.6. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 6: Intracerebral haemorrhage
3.9. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 9: Functional status (modified Rankin Scale score 0‐2) at 1 year
3.12. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 12: Major vascular events as defined by the Antithrombotic Trialists’ Collaboration
3.7. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 7: Major extracerebral haemorrhage
3.3. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 3: Ischaemic stroke
3.4. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 4: Myocardial infarction
3.10. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 10: Deep vein thrombosis
3.8. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 8: Vascular death
3.11. Analysis.

Comparison 3: Long‐term antiplatelet therapy (start versus avoid), Outcome 11: All major haemorrhagic events
Long‐term antiplatelet therapy (cilostazol versus aspirin)
We included a subgroup of one RCT including adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a high risk of bleeding (PICASSO 2018), in which the reporting of outcomes for 288 participants with prior ICH was separate from the other participants (PICASSO sub‐group 2020). We present our summary of findings for this subgroup in Table 4. We did not need to make post‐hoc decisions that could have led to selective outcome reporting.
Primary outcome
We did not find a significant difference in the composite primary outcome of MACE with long‐term cilostazol versus aspirin (22/142 versus 17/146; RR 1.33, 95% CI 0.74 to 2.40), P = 0.34; 1 published RCT; 288 participants; low‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 1: All major adverse cardiovascular events (MACE)
Secondary outcomes
The effects of long‐term cilostazol versus aspirin on secondary outcomes were as follows.
Death (8/142 versus 5/146; RR 1.65, 95% CI 0.55 to 4.91, P = 0.37; 1 published RCT; 288 participants; low‐certainty evidence; Analysis 4.2).
ICH (5/142 versus 4/146; RR 1.29, 95% CI 0.35 to 4.69, P = 0.70; 1 published RCT; 288 participants; low‐certainty evidence; Analysis 4.5).
All major occlusive vascular events were not reported.
Functional status was not reported.
Ischaemic stroke (12/142 versus 13/146; RR 0.95, 95% CI 0.45 to 2.01, P = 0.89; 1 published RCT; 288 participants; moderate‐certainty evidence; Analysis 4.3).
Myocardial infarction (5/142 versus 0/146; RR 11.31, 95% CI 0.63 to 202.63, P = 0.10; 1 published RCT; 288 participants; moderate‐certainty evidence; Analysis 4.4).
Vascular death (3/142 versus 0/146; RR 7.35, 95% CI 0.38 to 143.61, P = 0.19; 1 published RCT; 288 participants; moderate‐certainty evidence; Analysis 4.6).
Any stroke (16/142 versus 17/146; RR 0.97, 95% CI 0.51 to 1.84, P = 0.92; 1 published RCT; 288 participants; moderate‐certainty evidence; Analysis 4.7).
4.2. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 2: Death
4.5. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 5: Intracerebral haemorrhage
4.3. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 3: Ischaemic stroke
4.4. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 4: Myocardial infarction
4.6. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 6: Vascular death
4.7. Analysis.

Comparison 4: Long‐term antiplatelet therapy (cilostazol versus aspirin), Outcome 7: Any stroke (ischaemic or haemorrhagic)
Subgroup analyses
We did not extract subgroup data from comparisons addressed by only one RCT, each of which was under‐powered to estimate overall effects. Subgroups of interest were not reported for RCTs of short‐term prophylactic dose anticoagulation. Two RCTs of long‐term therapeutic dose anticoagulation for AF reported data in subgroups (APACHE‐AF 2021; SoSTART 2021); however, the classifications of the subgroups and outcomes reported did not permit pooling of data for subgroup analysis.
Discussion
In this updated review, we found seven new completed randomised controlled trials (RCTs) for a total of nine completed RCTs including 1491 participants, addressing four distinct questions about antithrombotic treatment after: intracerebral haemorrhage (ICH):
short‐term prophylactic dose anticoagulation (start versus avoid);
long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid);
long‐term antiplatelet therapy (start versus avoid);
long‐term antiplatelet therapy (cilostazol versus aspirin).
Summary of main results
Short‐term prophylactic dose anticoagulation (start versus avoid)
No RCT reported this review's primary outcome of major adverse cardiovascular events (MACE). The evidence is very uncertain about the effect of starting short‐term prophylactic dose anticoagulation on death, venous thromboembolism, ICH and independent functional status over 90 days.
Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid)
Starting long‐term therapeutic dose oral anticoagulation for atrial fibrillation (AF) after ICH probably reduces MACE (RR 0.61, 95% CI 0.40 to 0.94, P = 0.02; 3 RCTs; moderate‐certainty evidence) and probably reduces all major occlusive events (RR 0.27, 95% CI 0.14 to 0.53, P = 0.0002; 3 RCTs; moderate‐certainty evidence), but probably results in little or no difference in death, probably increases intracranial haemorrhage, and may result in little to no difference in independent functional status.
Long‐term antiplatelet therapy (start versus avoid)
The evidence is uncertain about the effects of starting long‐term antiplatelet therapy after ICH on MACE, death, all major occlusive events, ICH, and independent functional status.
Long‐term antiplatelet therapy (cilostazol versus aspirin)
For adults within 180 days of non‐cardioembolic ischaemic stroke or transient ischaemic attack and a clinical history of prior ICH, there was no evidence of an effect of long‐term cilostazol compared to aspirin on MACE, death, or ICH. All major occlusive vascular events and functional status were not reported.
Overall completeness and applicability of evidence
All data were available for RCTs of short‐term prophylactic dose anticoagulation. The included RCTs were representative of clinical practice in Italy, Germany, Turkey and Finland.
Data sufficient for analysis were not provided by the authors of ELDERCARE‐AF 2020 and PRAGUE‐17 2020. ELDERCARE‐AF 2020 shared the entire trial dataset, but we were unable to extract data on the subgroup of 80 participants with ICH, which would have increased the data in our pooled analyses of the effects of long‐term therapeutic dose oral anticoagulation for AF by 80/334 (24%). The RCTs that were included reflected standard clinical practice in the UK, the Netherlands and Canada.
The number of patients with prior ICH in PRAGUE‐17 2020 was not reported and was not quantified by the corresponding author in communication with us; this RCT would have been the only RCT contributing data to a new comparison of direct oral anticoagulants (DOACs) versus left atrial appendage closure for AF after ICH.
Only one RCT addressed long‐term antiplatelet therapy after ICH, and this was based in the UK (RESTART 2019).
Only one RCT compared different types of antiplatelet therapy after ICH, and this was based in South Korea, Hong Kong, and the Philippines (PICASSO sub‐group 2020).
Quality of the evidence
The methodological quality of the included RCTs varied, and tended to be best for the RCTs comparing starting versus avoiding long‐term therapeutic dose oral anticoagulation for AF after ICH and the RCT comparing starting versus avoiding long‐term antiplatelet therapy after ICH.
Most RCTs did not blind patients and professionals to treatment allocation, so the body of evidence was most vulnerable to performance bias, although all of our outcomes were objective with the exception of functional status. The proportion of included RCTs at low risk of bias, by category was: random sequence generation (67%), allocation concealment (67%), performance (22%), detection (78%), attrition (89%), and reporting (78%).
Heterogeneity between the RCTs' results varied according to the comparisons and outcomes being analysed, but the I2 was less than 75% for all 15 analyses that pooled at least two RCTs.
The risk of bias of the included RCTs and the imprecision of individual or pooled RCTs meant that the GRADE certainty of evidence was very low for RCTs of short‐term prophylactic dose anticoagulation, low to moderate for RCTs of long‐term therapeutic dose oral anticoagulation for AF, moderate for long‐term antiplatelet therapy and low for long‐term cilostazol versus aspirin.
Potential biases in the review process
We minimised the effects of publication bias by conducting extensive searches for published and unpublished RCTs, reviewing bibliographies for additional RCTs, and by seeking missing data directly from authors.
We minimised selection bias by involving two review authors in study selection and data extraction, with arbitration and independent checking by a third review author.
We collected data for all outcomes that were reported (and added pulmonary embolism, venous thromboembolism, and various variants of the MACE composite outcome relevant to the assessment of efficacy and net benefit).
Agreements and disagreements with other studies or reviews
We identified two systematic reviews of short‐term antithrombotic treatment after ICH in RCTs (Keir 2002; Paciaroni 2011), and a third systematic review of long‐term antiplatelet therapy after ICH (Cheng 2021).
Keir 2002 included individual participant data from IST 1997 and CAST 1997 on participants with not only ICH (52%), but also haemorrhagic transformation of cerebral infarction (48%), although it did not describe results for patients with ICH alone, who are the focus of this systematic review. This review's conclusions did not differ from ours.
Paciaroni 2011 and Cheng 2021 pooled non‐randomised studies as well as RCTs, which is a less methodologically rigorous approach than pooling RCTs alone. These reviews' conclusions did not differ from ours.
Authors' conclusions
Implications for practice.
On the basis of the available evidence, we could not be certain about benefit or harm from antithrombotic treatment after intracerebral haemorrhage (ICH).
We did not identify beneficial or hazardous effects of short‐term prophylactic dose parenteral anticoagulation on our primary or secondary outcomes, so there are no implications for clinical practice.
The reduction of major adverse cardiovascular events (MACE) overall and ischaemic stroke by long‐term treatment with therapeutic dose oral anticoagulation for atrial fibrillation (AF) after intracerebral haemorrhage (ICH) provides some reassurance for the use of this treatment (Analysis 2.1). However, the pooled estimate was imprecise, none of the individual randomised controlled trials (RCTs) were conclusive, there was some heterogeneity between the RCTs, and we were only moderately certain about the evidence, and the possibility of an increase in recurrent ICH remains, so we cannot identify specific implications for clinical practice. Clinical guidelines that are being updated following the publication of these RCTs are yet to publish their recommendations for clinical practice (AHA ICH guideline 2015; ESO ICH Guideline 2014; National Clinical Guideline for Stroke 2016).
We did not identify beneficial or hazardous effects of starting versus avoiding long‐term oral antiplatelet therapy, or particular antiplatelet agents, after ICH on the primary outcome of MACE. However, the results of RESTART 2019 provide some reassurance about the hazards of antiplatelet therapy after ICH (Analysis 3.6) as well as the potential for a reduction in stroke, myocardial infarction or vascular death (Analysis 3.12; Antithrombotic Trialists' Collaboration 2009). Consequently, guidelines that followed the publication of RESTART 2019 have concluded that antiplatelet therapy may be considered for survivors of antithrombotic drug‐associated ICH (Canadian ICH best practice recommendation 2020; Chinese Stroke Association guidelines 2019).
Implications for research.
More RCTs are required to resolve the ongoing uncertainties about antithrombotic treatment after ICH. These RCTs should be at low risk of bias and sufficiently large to reliably exclude or confirm the direction and magnitude of the effects suggested by our pooled analyses of pilot phase RCTs. Even if some of these RCTs recruit an insufficient sample size to be conclusive in their own right, their data will contribute substantially to a further update of this Cochrane Review, which will increase the precision and certainty of the pooled estimates of effects.
We are not aware of any ongoing RCTs investigating the effects of starting versus avoiding short‐term prophylactic dose anticoagulation after ICH. Further RCTs seem justified if there is uncertainty in clinical practice about using prophylactic dose anticoagulation in addition to intermittent pneumatic compression in clinical practice.
Several ongoing RCTs are investigating the effects of starting versus avoiding long‐term therapeutic dose oral anticoagulation for AF after ICH (NCT03186729; NCT03243175; NCT03907046; NCT03950076; PRESTIGE‐AF). Some of these RCTs may be conclusive in their own right when completed, but together they should certainly provide sufficient data for an update of this Cochrane Review to be conclusive about our primary and secondary outcomes. Further RCTs do not appear to be required to investigate effects overall, but large numbers of participants will be required to investigate heterogeneity of effects in subgroups. The emphasis should be on completing recruitment to the ongoing RCTs as soon as possible.
Some ongoing RCTs are investigating the effects of starting versus avoiding long‐term antiplatelet therapy after ICH (NCT02966119; NCT03186729; NCT04522102; NCT04820972). However, the sample sizes of these RCTs make each of them very unlikely to be definitive about effects on our primary and secondary outcomes, and they are unlikely to be definitive when pooled, since NCT04522102 estimates that more than 4000 participants are required to generate definitive evidence about starting versus avoiding long‐term antiplatelet therapy after ICH. Therefore, a further, large, definitive RCT seems justified to investigate effects both overall and in subgroups. If starting antiplatelet therapy proves to be beneficial, then further RCTs comparing different antiplatelet agents would be justified.
Only one ongoing RCT is comparing the effects of long‐term therapeutic dose oral anticoagulation for AF after ICH to the left atrial appendage closure device (NCT03243175), although another RCT is comparing the left atrial appendage closure device to any antithrombotic prescribing strategy for the same indication (STROKECLOSE, NCT02830152). If long‐term therapeutic dose oral anticoagulation for AF after ICH is beneficial as suggested by this review (Analysis 2.1), the non‐inferiority or superiority of this device to long‐term therapeutic dose oral anticoagulation will need to be investigated.
Further updates of this Cochrane Review and the individual participant data meta‐analysis planned by the Collaboration Of Controlled Randomised trials of Oral Antithrombotic agents after intraCranial Haemorrhage (COCROACH, CRD42021246133) will be required to provide the most precise estimates of the effects of antithrombotic treatments after stroke due to ICH.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2023 | Amended | Change to open access agreement |
History
Protocol first published: Issue 4, 2016 Review first published: Issue 5, 2017
| Date | Event | Description |
|---|---|---|
| 5 October 2021 | New citation required but conclusions have not changed | Conclusions not changed |
| 5 October 2021 | New search has been performed | Literature search updated. We identified seven new trials for inclusion, bringing the total number of included trials to nine, involving 1491 participants. |
| 5 January 2021 | Amended | Date of search |
Acknowledgements
Luke A Perry, Eivind Berge (now deceased), Joshua Bowditch, Elisabeth Forfang, Ole Morten Rønning, Graeme J Hankey, Elmer Villanueva, and Rustam Al‐Shahi Salman developed, designed, and delivered the protocol for this review on 8 April 2016, and published the first version of this review on 25 May 2017.
We acknowledge and thank the Cochrane Stroke Group's Information Specialists Joshua Cheyne and his predecessor Brenda Thomas for their assistance in developing the search strategies for this review.
Special thanks to our two consumer reviewers, Odie Geiger and U Hla Htay, for generously providing their feedback on the first version of this review.
The following people conducted the editorial process for this review update
Sign‐off Editor (final editorial decision): Peter Langhorne, University of Glasgow
Managing Editor (provided editorial guidance to authors, edited the review, selected peer reviewers, and collated peer‐reviewer comments): Hazel Fraser, Cochrane Stroke
Statistical Editor (provided comments): Aryelly Rodriguez, Edinburgh Clinical trials unit (ECTU) at the University of Edinburgh
Copy Editor (copy‐editing and production): [TO BE ADDED], Cochrane Copy Edit Support.
Peer reviewers (provided comments and recommended an editorial decision):
M. Irem Baharoglu MD PhD, Amsterdam UMC location University of Amsterdam, Department of Neurology, Meibergdreef 9, Amsterdam, the Netherlands
Dr Amanda Barugh, Associate Editor, Cochrane Stroke
Peter Langhorne, University of Glasgow
One reviewer provided comments but requested not be acknowledged.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Basal Ganglia Hemorrhage] explode all trees
#2 MeSH descriptor: [Intracranial Hemorrhages] this term only
#3 MeSH descriptor: [Intracranial Hemorrhage, Hypertensive] this term only
#4 MeSH descriptor: [Cerebral Hemorrhage] this term only
#5 MeSH descriptor: [Hemorrhagic Stroke] this term only
#6 (((brain* or cerebr* or cerebell* or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher*) NEAR/5 (h?emorrhag* or h?ematoma* or bleed*))):ti,ab,kw
#7 (ICH or ICHs):ti,ab,kw
#8 {or #1‐#7}
#9 MeSH descriptor: [Anticoagulants] explode all trees
#10 MeSH descriptor: [Vitamin K] explode all trees and with qualifier(s): [antagonists & inhibitors ‐ AI]
#11 MeSH descriptor: [Thrombin] explode all trees and with qualifier(s): [antagonists & inhibitors ‐ AI]
#12 MeSH descriptor: [Factor Xa] this term only
#13 MeSH descriptor: [Blood Coagulation Factors] explode all trees and with qualifier(s): [antagonists & inhibitors ‐ AI]
#14 MeSH descriptor: [Antithrombins] explode all trees
#15 MeSH descriptor: [Hirudin Therapy] this term only
#16 (anticoagul* or antithromb*):ti,ab,kw
#17 (Vitamin K antagonist* or VKA or VKAs):ti,ab,kw
#18 (direct* NEAR/3 thrombin NEAR/3 inhib*):ti,ab,kw
#19 (DTI*):ti,ab,kw
#20 ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) NEAR/3 inhib*):ti,ab,kw
#21 (activated NEAR/3 (factor X or factor 10) NEAR/3 inhib*):ti,ab,kw
#22 (acenocoumarol* or dicoumarol* or ethyl biscoumacetate* or phenprocoumon* or warfarin* or ancrod* or citric acid* or coumarin* or chromonar* or coumestro* or esculi* or ochratoxin* or umbelliferone* or dermatan sulfate* or dextran* or edetic acid* or enoxaparin* or gabexate* or heparin* or lmwh* or nadroparin* or pentosan sulfuric polyester* or phenindione* or protein c or protein s or tedelparin*):ti,ab,kw
#23 (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org 10172 or mesoglycan or polysaccharide sulphate* or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216):ti,ab,kw
#24 (Marevan or Fragmin* or Fraxiparin* or Klexane):ti,ab,kw
#25 (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin* or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil):ti,ab,kw
#26 (xabans or antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717):ti,ab,kw
#27 MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees
#28 MeSH descriptor: [Platelet Glycoprotein GPIIb‐IIIa Complex] explode all trees and with qualifier(s): [antagonists & inhibitors ‐ AI]
#29 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* or (platelet* NEAR3 inhibit*) or (thrombocyt* NEAR3 inhibit*)):ti,ab,kw
#30 (alprostadil* or aspirin* or acetylsalicylic acid or acetyl salicylic acid* or acetyl?salicylic acid or epoprostenol* or ketanserin* or ketorolac tromethamine* or milrinone* or mopidamol* or procainamide* or thiophen* or trapidil* or picotamide* or ligustrazine* or levamisol* or suloctidil* or ozagrel* or oky046 or oky‐046 or defibrotide* or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or ((glycoprotein iib* or gp iib*) NEAR/5 (antagonist* or inhibitor*)) or GR144053 or GR‐144053 or triflusal):ti,ab,kw
#31 (Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel):ti,ab,kw
#32 (Dispril or Albyl* or Ticlid* or Persantin* or Plavix or ReoPro or Integrilin* or Aggrastat):ti,ab,kw
#33 {or #8‐#32}
#34 #8 AND #33
Appendix 2. MEDLINE (Ovid) search strategy
The search consists of antithrombotics and stroke subject searches (lines 1‐5 and 6‐25) which have been linked to the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format (lines 15‐25), as referenced in the Box 3.c in the Technical Supplement to Chapter 4: Searching for and selecting studies in the Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022) (Lefebvre 2022). Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Paynter R, Rader T, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook.
1. exp basal ganglia hemorrhage/ or intracranial hemorrhages/ or cerebral hemorrhage/ or intracranial hemorrhage, hypertensive/ or hemorrhagic stroke/
2. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
3. ((h?emorrhag$ or bleed$) adj5 (stroke or apoplex$)).tw.
4. (ICH or ICHs).tw.
5. or/1‐4
6. exp anticoagulants/
7. exp Vitamin K/ai or thrombin/ai or factor Xa/ai or exp Blood coagulation factors/ai
8. exp antithrombins/ or hirudin therapy/
9. (anticoagul$ or antithromb$).tw.
10. (Vitamin K antagonist$ or VKA or VKAs).tw.
11. (direct$ adj3 thrombin adj3 inhib$).tw.
12. DTI$1.tw.
13. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj3 inhib$).tw.
14. (activated adj3 (factor X or factor 10) adj3 inhib$).tw.
15. (acenocoumarol$ or dicoumarol$ or ethyl biscoumacetate$ or phenprocoumon$ or warfarin$ or ancrod$ or citric acid$ or coumarin$ or chromonar$ or coumestro$ or esculi$ or ochratoxin$ or umbelliferone$ or dermatan sulfate$ or dextran$ or edetic acid$ or enoxaparin$ or gabexate$ or heparin$ or lmwh$ or nadroparin$ or pentosan sulfuric polyester$ or phenindione$ or protein c or protein s or tedelparin$).tw,nm.
16. (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org 10172 or mesoglycan or polysaccharide sulphate$ or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216).tw,nm.
17. (Marevan or Fragmin$ or Fraxiparin$ or Klexane).tw,nm.
18. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw,nm.
19. (xabans or antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717).tw,nm. 20. exp platelet aggregation inhibitors/ or exp platelet glycoprotein gpiib‐iiia complex/ai
21. (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj3 inhibit$) or (thrombocyt$ adj3 inhibit$)).tw.
22. (alprostadil$ or aspirin$ or acetylsalicylic acid or acetyl salicylic acid$ or acetyl?salicylic acid or epoprostenol$ or ketanserin$ or ketorolac tromethamine$ or milrinone$ or mopidamol$ or procainamide$ or thiophen$ or trapidil$ or picotamide$ or ligustrazine$ or levamisol$ or suloctidil$ or ozagrel$ or oky046 or oky‐046 or defibrotide$ or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or ((glycoprotein iib$ or gp iib$) adj5 (antagonist$ or inhibitor$)) or GR144053 or GR‐144053 or triflusal).tw,nm.
23. (Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel).tw,nm.
24. (Dispril or Albyl$ or Ticlid$ or Persantin$ or Plavix or ReoPro or Integrilin$ or Aggrastat).tw,nm.
25. or/6‐24
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. randomized.ab.
29. placebo.ab.
30. drug therapy.fs.
31. randomly.ab.
32. trial.ti.
33. groups.ab.
34. or/26‐33
35. exp animals/ not humans.sh.
36. 34 not 35
37. 5 and 25 and 36
Appendix 3. Embase (Ovid) search strategy
1. basal ganglion hemorrhage/ or brain hemorrhage/ or brain ventricle hemorrhage/ or cerebellum hemorrhage/
2. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
3. ((h?emorrhag$ or bleed$) adj5 (stroke or apoplex$)).tw.
4. (ICH or ICHs).tw.
5. 1 or 2 or 3 or 4
6. anticoagulant agent/ or antivitamin k/ or exp blood clotting inhibitor/ or exp coumarin anticoagulant/ or defibrotide/ or dextran sulfate/ or fluindione/ or glycosaminoglycan polysulfate/ or exp heparin derivative/ or lupus anticoagulant/ or phenindione/
7. (anticoagul$ or antithromb$).tw.
8. (Vitamin K antagonist$ or VKA or VKAs).tw.
9. (direct$ adj3 thrombin adj3 inhib$).tw.
10. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj3 inhib$).tw.
11. (activated adj3 (factor X or factor 10) adj3 inhib$).tw.
12. (acenocoumarol$ or dicoumarol$ or ethyl biscoumacetate$ or phenprocoumon$ or warfarin$ or ancrod$ or citric acid$ or coumarin$ or chromonar$ or coumestro$ or esculi$ or ochratoxin$ or umbelliferone$ or dermatan sulfate$ or dextran$ or edetic acid$ or enoxaparin$ or gabexate$ or heparin$ or lmwh$ or nadroparin$ or pentosan sulfuric polyester$ or phenindione$ or protein c or protein s or tedelparin$).tw.
13. (tinzaparin or parnaparin or dalteparin or reviparin or danaparoid or lomoparan or org 10172 or mesoglycan or polysaccharide sulphate$ or sp54 or sp‐54 or md805 or md‐805 or cy222 or cy‐222 or cy216 or cy‐216).tw.
14. (Marevan or Fragmin$ or Fraxiparin$ or Klexane).tw.
15. (argatroban or MD805 or MD‐805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw.
16. (xabans or antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717).tw.
17. or/6‐16
18. exp antithrombocytic agent/
19. fibrinogen receptor/dt [Drug Therapy]
20. (antiplatelet$ or anti‐platelet$ or antiaggreg$ or anti‐aggreg$ or (platelet$ adj5 inhibit$) or (thrombocyt$ adj5 inhibit$)).tw.
21. (alprostadil$ or aspirin$ or acetylsalicylic acid or acetyl salicylic acid$ or acetyl?salicylic acid or epoprostenol$ or ketanserin$ or ketorolac tromethamine$ or milrinone$ or mopidamol$ or procainamide$ or thiophen$ or trapidil$ or picotamide$ or ligustrazine$ or levamisol$ or suloctidil$ or ozagrel$ or oky046 or oky‐046 or defibrotide$ or cilostazol or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or ((glycoprotein iib$ or gp iib$) adj5 (antagonist$ or inhibitor$)) or GR144053 or GR‐144053 or triflusal).tw.
22. (Argatroban or Beraprost or Cicaprost or Cilostazol or Clopidogrel or Dipyridamole or Iloprost or Indobufen or Lepirudin or Pentosan Polysulfate or Pentoxifylline or Piracetam or Prostacyclin or Sulfinpyrazone or Sulphinpyrazone or Ticlopidine or Triflusal or Abciximab or Disintegrin or Echistatin or Eptifibatide or Lamifiban or Orbofiban or Roxifiban or Sibrafiban or Tirofiban or Xemilofiban or terutroban or picotamide or prasugrel).tw.
23. (Dispril or Albyl$ or Ticlid$ or Persantin$ or Plavix or ReoPro or Integrilin$ or Aggrastat).tw.
24. or/18‐23
25. 17 or 24
26. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 27. Randomization/ 28. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 29. control group/ or controlled study/ 30. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
31. Crossover Procedure/
32. Double Blind Procedure/
33. Single Blind Procedure/ or triple blind procedure/
34. placebo/ or placebo effect/
35. (random$ or RCT or RCTs).tw.
36. (controlled adj5 (trial$ or stud$)).tw.
37. (clinical$ adj5 trial$).tw.
38. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
39. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
40. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
41. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
42. (cross‐over or cross over or crossover).tw.
43. (placebo$ or sham).tw.
44. trial.ti.
45. (assign$ or allocat$).tw.
46. controls.tw.
47. or/26‐46
48. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
49. 5 and 25 and 47
50. 49 not 48
Appendix 4. Trials register search strategies
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) AREA[StudyType] EXPAND[Term] COVER[FullMatch] "Interventional" AND AREA[ConditionSearch] Intracerebral Haemorrhage AND AREA[StudyFirstPostDate] EXPAND[Term] RANGE[01/14/2020, 01/05/2021]
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/) Basic search: intracerebral haemorrhage OR intracerebral hemorrhage OR ICH Phases are: ALL
In the previous version of this review, we searched the Stroke Trials Registry of the Internet Stroke Center (www.strokecenter.org/trials/) on 2 March 2017.
Data and analyses
Comparison 1. Short‐term prophylactic dose anticoagulation (start versus avoid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.59, 1.70] |
| 1.2 Deep vein thrombosis | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.49, 1.86] |
| 1.3 Pulmonary embolism | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.14] |
| 1.4 Venous thromboembolism | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.37] |
| 1.5 Intracerebral haemorrhage | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.04, 1.38] |
| 1.6 Major extracerebral haemorrhage | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [0.12, 65.82] |
| 1.7 Growth of qualifying intracerebral haemorrhage | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.38, 2.41] |
| 1.8 Functional status (modified Rankin Scale 0‐2) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.78, 5.25] |
Comparison 2. Long‐term therapeutic dose oral anticoagulation for atrial fibrillation (start versus avoid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All major adverse cardiovascular events (MACE) | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.94] |
| 2.2 Death | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.78] |
| 2.3 Ischaemic stroke | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.71] |
| 2.4 Myocardial infarction | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.71] |
| 2.5 All major occlusive vascular events | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.14, 0.53] |
| 2.6 Intracranial haemorrhage | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.88, 6.73] |
| 2.7 Major extracerebral haemorrhage | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.57] |
| 2.8 Vascular death | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.32] |
| 2.9 Functional status (modified Rankin Scale score 0‐2) at 1 year | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.24] |
| 2.10 Any stroke (ischaemic or haemorrhagic) | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.15] |
| 2.11 Any stroke (ischaemic or haemorrhagic) or vascular death | 2 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.46, 1.15] |
Comparison 3. Long‐term antiplatelet therapy (start versus avoid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All major adverse cardiovascular events (MACE) | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.22] |
| 3.2 Death | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.53] |
| 3.3 Ischaemic stroke | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.40, 1.23] |
| 3.4 Myocardial infarction | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.21, 1.89] |
| 3.5 All major occlusive vascular events | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.55] |
| 3.6 Intracerebral haemorrhage | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 1.03] |
| 3.7 Major extracerebral haemorrhage | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.72, 9.94] |
| 3.8 Vascular death | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.18] |
| 3.9 Functional status (modified Rankin Scale score 0‐2) at 1 year | 1 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 3.10 Deep vein thrombosis | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.61, 14.73] |
| 3.11 All major haemorrhagic events | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.40, 1.29] |
| 3.12 Major vascular events as defined by the Antithrombotic Trialists’ Collaboration | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.97] |
Comparison 4. Long‐term antiplatelet therapy (cilostazol versus aspirin).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All major adverse cardiovascular events (MACE) | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.74, 2.40] |
| 4.2 Death | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.55, 4.91] |
| 4.3 Ischaemic stroke | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.01] |
| 4.4 Myocardial infarction | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.31 [0.63, 202.63] |
| 4.5 Intracerebral haemorrhage | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.35, 4.69] |
| 4.6 Vascular death | 1 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.35 [0.38, 143.61] |
| 4.7 Any stroke (ischaemic or haemorrhagic) | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APACHE‐AF 2021.
| Study characteristics | ||
| Methods |
Design: randomised controlled PROBE parallel group phase 2 trial Setting: multicentre (16 hospitals) in the Netherlands Dates: 15 January 2015 to 6 July 2020 |
|
| Participants |
Sample size: 101 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age: median age was 78 years (IQR 73–83) overall; 77 years (74–83) in the intervention group and 79 years (72–83) in the comparator group Sex: 55 (54%) were men overall; 27 (54%) in the intervention group and 28 (55%) in the comparator group |
|
| Interventions |
Intervention: apixaban oral dose of 5 mg twice daily, or a reduced dose of 2.5 mg twice daily if their creatine clearance was 30 mL/min or less, or if two of three of the following criteria were present: age 80 years or older, bodyweight 60 kg or lower, or serum creatinine 133 μmol/L or greater (50 participants) Comparator: no antithrombotic treatment or oral antiplatelet treatment (acetylsalicylic acid 80 mg once daily; carbasalate calcium 100 mg once daily; clopidogrel 75 mg once daily; or a combination of dipyridamole 200 mg twice daily with either acetylsalicylic acid 80 mg once daily or carbasalate calcium 100 mg once daily) at the discretion of the treating physician. (51 participants; 26 of these received antiplatelet therapy) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Duration of follow‐up: median 1·9 years (IQR 1·0–3·1), with a total of 222 person‐years |
|
| Notes |
Declarations of interest: FHBMS reports two grants from the Dutch Heart Foundation (grant 2012T077 for this study; and grant 2019T060 outside the submitted work). DWD reports funding from the Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Sciences & Health, and unrestricted grants from Penumbra, Stryker, Medtronic, Thrombolytic Science, and Cerenovus for research outside the current work, all paid to their institution. JS reports grants to their institution outside the submitted work (H2020 programme). JMC reports research funding from Portola, Boehringer, and Bayer, outside the submitted work. HBvdW reports fees for consultancy from Bayer and LivaNova, all paid to their institution; and grants outside the submitted work (EU Horizon 2020 programme; Dutch Heart foundation; and Stryker, of which the last two are through the CONTRAST consortium). CJMK reports grants from the Dutch Heart Foundation (grant 2012T077; this study), and grants outside the submitted work: The Netherlands Organization for Health Research and Development, ZonMw (grant 015008048); support of the Netherlands Cardiovascular Research Initiative, which is supported by the Dutch Heart Foundation, CVON2015‐01: CONTRAST; and the support of the Brain Foundation Netherlands (HA2015.01.06). All other authors declare no competing interests. Sources of funding: Dutch Heart Foundation (grant 2012T077) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisat ion system. Treatment allocation was stratified by intention to start an antiplatelet agent or not in the avoid group, and subsequently based on proportional minimisation, according to age (≤75 years vs > 75 years) and location of the ICH (lobar vs non‐lobar) |
| Allocation concealment (selection bias) | Low risk | Prof Bart van der Worp confirmed that allocation was concealed by the randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, their treating physicians, and local investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome event adjudication was done by LJK and GJER masked to the patient’s identity, treatment allocation, and antithrombotic drug use |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Dickmann 1988.
| Study characteristics | ||
| Methods |
Design: parallel group RCT Setting: inpatient admissions to the neurology unit of one hospital in Göttingen, Germany Dates: not described |
|
| Participants |
Sample size: 46 participants Diagnosis: ICH Inclusion criteria: diagnosis of ICH by CT with onset 24 hours before admission Exclusion criteria: bleeding diathesis, diastolic blood pressure higher than 120 mmHg, and deep coma with clinical signs of brain herniation Age (years, mean (SD)): 62 (SD not described) (intervention group), 60 (SD not described) (comparator group) Sex: 52% female (intervention group), 48% female (comparator group) |
|
| Interventions |
Intervention: 5000 units of heparin administered subcutaneously 8‐hourly starting at day 4 (23 participants) Comparator: 5000 units of heparin subcutaneously 8‐hourly starting at day 10 (23 participants) Both groups had the same treatment otherwise, with compression stockings and physical treatment |
|
| Outcomes |
Primary outcome: not specified Secondary outcomes: not specified Outcomes: rebleeding occurring during the trial period; thrombosis of abdomen or legs at day 2 and day 10; pulmonary embolism at day 10; death Duration: the duration of follow‐up is not specified, but is assumed to be 10 days |
|
| Notes |
Declations of interest: not described Sources of funding: not described Rebleeding was not defined by the authors of this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open trial, comparing two timings of heparin |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All scintigrams were read by the same blinded investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the Methods section were reported in full in the Results section. Additionally, most expected outcomes of interest were reported in this study |
NASPAF‐ICH 2020.
| Study characteristics | ||
| Methods |
Design: randomised controlled PROBE parallel group (2:1) phase 2 trial Setting: multicentre at 8 hospitals in Canada Dates: April 2017 to May 2019 |
|
| Participants |
Sample size: 30 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age(years, mean (SD)): 77.7 (SD 9.2) intervention versus 75.8 (SD 5.5) comparator Sex (male): 12/21 (57%) intervention versus 5/9 (56%) comparator |
|
| Interventions |
Intervention: NOAC (apixaban or dabigatran or edoxaban or rivaroxaban) and BP control to target < 130/80mmHg (21 participants) Comparator: acetylsalicylic acid 81 mg/day and BP control to target < 130/80mmHg (9 participants) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Duration of follow‐up: mean 1.53 years (SD 0.54) |
|
| Notes |
Declarations of interest: Daiichi Sankyo Ltd, Bayer AG, Octapharma Canada, Portola pharmaceuticals, BMS/Pfizer, Servier Canada Inc Sources of funding: not specified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with the chief investigator confirmed that central, web‐based randomisation was used, with allocation concealment |
| Allocation concealment (selection bias) | Low risk | Personal correspondence with the chief investigator confirmed that central, web‐based randomisation was used, with allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% complete follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
Orken 2009.
| Study characteristics | ||
| Methods |
Design: parallel group RCT Setting: inpatient admissions to Sisli Etfal Education and Research Hospital, Department of Neurology in Turkey Dates: January 2006 ‐ March 2008 |
|
| Participants |
Sample size: 75 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age (years, mean (SD)): 68 ± 11 (intervention) 66 ± 10 (comparator) Sex: 56% female (intervention), 22% female (comparator) |
|
| Interventions |
Intervention: subcutaneous LMWH (enoxaparin sodium 40mg/d) (39 participants) Comparator: long compression stockings (36 participants) |
|
| Outcomes |
Primary outcome: not described Secondary outcomes: not described Outcomes
Duration of follow‐up: 21 days |
|
| Notes |
Declarations of interest: not described Sources of funding: not described Other: it is not clear whether 4 patients who were excluded because of "death before 7th day investigations" were excluded before or after randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was said to be done, "after the first 48 hours according to the order of hospital admission dates" which seems to be predictable and at high risk of bias |
| Allocation concealment (selection bias) | High risk | Randomisation was said to be done, "after the first 48 hours according to the order of hospital admission dates" which seems to be predictable and at high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intervention and comparator could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All radiologic material was prospectively evaluated by 2 radiologists ... who were blinded to the clinical findings and cranial CTs of the patients" The methods did not explicitly state that the radiologists were also blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the Methods section were reported in full in the Results section. Additionally, most expected outcomes of interest were reported in this study |
PICASSO sub‐group 2020.
| Study characteristics | ||
| Methods |
Design: randomised controlled, 2 × 2 factorial parallel group trial Setting: multicentre, 67 hospitals in three Asian countries Dates: 1 August 2009 to 31 August 2015 |
|
| Participants |
Sample size: 1534 participants overall (288 with prior ICH in the subgroup analysis included in this meta‐analysis) Diagnosis: non‐cardioembolic ischaemic stroke or transient ischaemic attack within 180 days and clinical history of prior ICH Inclusion criteria
Exclusion criteria
Age (years, mean (SD)): unknown for this subgroup with prior ICH Sex: unknown for this subgroup with prior ICH |
|
| Interventions |
Intervention: cilostazol 100 mg orally twice daily (matching the comparator) Comparator: aspirin 100 mg orally once daily (matching the intervention) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Duration of follow‐up: at least 12 months. |
|
| Notes |
Declarations of interest: SUK declares grants from Korea Otsuka Pharmaceutical Company. All other authors declare no competing interests Sources of funding: Korea Otsuka Pharmaceutical Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive web‐based system. each participant received a subject number generated by the computer of the central randomisation service and was randomly assigned (1:1:1:1) to receive cilostazol (with aspirin placebo), aspirin (with cilostazol placebo), cilostazol plus probucol (with aspirin placebo), or aspirin plus probucol (with cilostazol placebo) using centralised blocks (block size 4) stratified by centre |
| Allocation concealment (selection bias) | Low risk | The analysis of cilostazol versus aspirin (antiplatelet arm) was double‐blinded, and the interactive web response system was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Cilostazol, aspirin, and placebos—used as a double dummy—were provided every 3 months in boxes within sealed opaque envelopes, with instructions to be taken twice a day |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | High risk | Not all were reported |
PREVENTIHS 2020.
| Study characteristics | ||
| Methods |
Design: parallel group RCT Setting: multicentre at hospitals in Italy Dates: 1 May 2016 to 30 March 2020 |
|
| Participants |
Sample size: 73 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age (years, mean (SD)): 70 +/‐14 (intervention) versus 72 +/‐ 12 (comparator) Sex: 22/38 (intervention) versus 18/35 (comparator) were male |
|
| Interventions |
Intervention: enoxaparin 0.4mL (4000 units) once daily for 10 days ± 1 day plus standard therapy (38 participants) Comparator: standard therapy alone (35 participants) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Duration: CT brain and venous eco‐color‐Doppler examination with a compression test performed bilaterally on the lower limbs 10 +/‐ 1 day following the start of treatment. Clinical follow‐up was done 90 days after randomisation |
|
| Notes |
Declarations of interest: Maurizio Paciaroni ‐ member of the speaker bureau of Sanofi‐Aventis, Boehringer Ingelheim, Bayer, Bristol Meyer Squibb, Daiichi Sankyo, and Pfizer. Giancarlo Agnelli ‐ member of the speaker bureau of Boehringer Ingelheim and Bayer. Cecilia Becattini ‐ member of the speaker bureau of Bristol Meyer Squibb and Bayer. Valeria Caso ‐ received honoraria as a member of the speaker bureau and as consultant or advisory board of Boehringer Ingelheim. Walter Ageno ‐ received speaker’s honoraria from, and participated in scientific advisory boards for, Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, and Daiichi Sankyo, and has received research support from Bayer and Boehringer Ingelheim Sources of funding: Ministero della Salute (Health Minister) of the Italian Government (n. FARM12L9JE) Other: this trial was stopped prematurely because of slow recruitment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were centrally randomised over the phone using a random list of numbers (even numbers – treatment A; odd numbers – treatment B) closed in an envelope." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were centrally randomised over the phone using a random list of numbers (even numbers – treatment A; odd numbers – treatment B) closed in an envelope." It is not clear if the envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label (participants and personnel were aware of allocated treatment) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessment was described as being blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data for randomised participants were reported |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | Premature termination due to slow recruitment |
Qian 2021.
| Study characteristics | ||
| Methods |
Design: randomised placebo‐controlled parallel group trial Setting: multicentre at 4 hospitals in Finland. Dates: 2008 to 2015 |
|
| Participants |
Sample size: 149 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age (years, mean (SD)): 66 ± 19 in the intervention group and 68 ± 6 in the comparator group Sex (male): 36/71 (51%) in the intervention group and 36/68 (53%) in the comparator group |
|
| Interventions |
Intervention: subcutaneous injections of enoxaparin were started 24 hours after the onset of ICH and repeated twice daily at 12‐hour intervals. Each injection contained 20 mg (2000 IU) of enoxaparin, implying a daily dose of 40 mg. Treatment was stopped once the patient was able to walk independently, or if a severe recurrence of bleeding was observed. Intermittent pneumatic compression devices were used until discharge to home or to another institute (68 participants) Comparator: subcutaneous injections of the placebo were started 24 hours after the onset of ICH and repeated twice daily at 12‐hour intervals. Each injection contained saline injections according to the same regime as the intervention, and this was replaced with enoxaparin 72 hours after the onset of the stroke. Enoxaparin treatment was stopped once the patient was able to walk independently, or if a severe recurrence of bleeding was observed. Intermittent pneumatic compression devices were used until discharge to home or to another institute (71 participants) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Duration of follow‐up: 3 months |
|
| Notes |
Declarations of interest: none Sources of funding: TT received academic grants for ICH research from Helsinki University Central Hospital, University of Gothenburg, Sahlgrenska University Hospital, and Sigrid Juselius Foundation. ST received academic grants from Finnish Medical Foundations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Subcutaneous injections were obtained from the hospital pharmacy. There were always 2–3 sets of injections ready for all eligible study patients i.e. enoxaparin or placebo. All the injection sets looked the same whether they included enoxaparin or saline. The investigator randomly chose one of the injection sets and the code for the injection set was placed in the sealed envelope together with the patient study number The same information was sealed for the emergency envelope. One emergency envelope was opened during the study due to severe rebleeding 36 hours after the onset, and in that case, the patient had received the placebo. The patient study code and ID were sealed in another envelope, which was opened only after the study |
| Allocation concealment (selection bias) | Low risk | Enoxaparin and saline placebo looked identical |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The envelopes containing patient study code and the injection set code were opened during a witnessed meeting only after the end of the study. It is not clear whether outcomes were identified and adjudicated before the end of the study, or afterwards |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up data existed for all 139 patients |
| Selective reporting (reporting bias) | High risk | Cardiovascular death was pre‐specified in the trial register, but was not reported |
RESTART 2019.
| Study characteristics | ||
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre at 122 hospitals in the UK Dates: 22 May 2013 to 31 May 2018 |
|
| Participants |
Sample size: 537 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
Age(years, median (IQR)): 77 (69–82) in the intervention group versus 76 (69–82) in the comparator group Sex (male): 173 (65%) in the intervention group versus 187 (70%) in the comparator group |
|
| Interventions |
Intervention: one or more of oral aspirin, dipyridamole, or clopidogrel, begun within 24 hours of randomisation with doses determined at the discretion of the consultant responsible for the participant Comparator: policy of avoiding antiplatelet medication |
|
| Outcomes |
Primary outcome
Secondary outcome measures
Duration of follow‐up: at least 6 months (1064 person‐years) |
|
| Notes |
Declarations of interest: RA‐SS and GDM report a grant from the British Heart Foundation (SP/12/2/29422) paid to the University of Edinburgh for the conduct of RESTART. RA‐SS reports grants from The Stroke Association, Chest Heart and Stroke Scotland, and GE Healthcare Limited, outside the submitted work. DEN reports grants and personal fees from AstraZeneca, Eli Lilly, Bristol Myers Squibb, and Jansen, during the conduct of the study. PAGS reports funding from Bayer outside the submitted work. NS reports a grant from National Institute for Health
Research (NIHR) Health Technology Assessment for the TICH‐2 trial, outside the submitted work. JMW reports grants from EU Framework 7, Medical Research Council, and the British Heart Foundation, outside the submitted work. DJW reports personal fees from Bayer and JFB consulting, outside the submitted work. PMW reports personal fees from Stryker Global Advisory Board on Haemorrhagic Stroke and MicroVention‐Terumo, and a grant from MicroVention‐Terumo outside the submitted work. WNW reports a Chief Scientist Office of the Scottish Government Health Department Senior Fellowship (SCAF_17_01) and a grant from the European Stroke Organisation, outside the submitted work. MSD, JS, and CLMS declare no competing interests. Sources of funding: British Heart Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation system with minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | The web interface displayed each participant’s unique study identification number and their allocation to either starting or avoiding antiplatelet therapy, which was also sent in an email to all investigators at the hospital site, having been concealed until that point |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment allocation was open to the clinicians caring for patients in primary and secondary care, and local investigators and to participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome adjudication was blinded to treatment allocation and receipt of antiplatelet therapy during follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 (0.2%) participant withdrew from follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
SoSTART 2021.
| Study characteristics | ||
| Methods |
Design: randomised, open‐label, assessor‐masked, parallel group, pilot‐phase, non‐inferiority trial Setting: multicentre at 67 hospitals in the UK Dates: 29 March 2018 to 27 February 2020 |
|
| Participants |
Sample size: 203 participants Diagnosis: spontaneous intracranial haemorrhage Inclusioncriteria
Exclusion criteria
Age (years), median (IQR): 79 (74–85) intervention versus 79 (74–84) comparator Sex (male): 62/101 (61%) intervention versus 65/102 (64%) comparator |
|
| Interventions |
Intervention: start oral anticoagulation, restricted to the use of either a DOAC (factor Xa inhibitor (apixaban, rivaroxaban, or edoxaban) or direct thrombin inhibitor (dabigatran etexilate)) or vitamin K antagonist (warfarin sodium, acenocoumarol, or phenindione) at full treatment dose (with adjustment if required by renal function, age, bodyweight, or concomitant medications), initiated within 24 hours of randomisation (101 participants) Comparator: standard clinical practice without oral anticoagulation (either an antiplatelet agent or no antithrombotic agents). Participants were permitted to start or discontinue anticoagulant or antiplatelet agents if clinically indicated by outcome events during follow‐up, regardless of treatment allocation (102 participants) Duration of follow‐up: median 1.2 years, 251 person‐years |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Declarations of interest: JMW reports grants from EU Horizon 2020, Medical Research Council, Fondation Leducq, The Stroke Association, Alzheimer’s Society, and the British Heart Foundation, outside the submitted work. PMW reports personal fees from Stryker Global Advisory Board on Haemorrhagic Stroke and MicroVention‐Terumo, and institutional grants from MicroVention‐Terumo, Penumbra, Stryker, and Medtronic, outside the submitted work. GYHL reports consultancy and speaker fees (not received personally) from Bristol Myers Squibb/Pfizer, Boehringer Ingelheim, and Daiichi‐Sankyo, outside the submitted work. A‐PJ declares personal fees from Alexion Pharmaceuticals, outside the submitted work. Sources of funding: British Heart Foundation, Medical Research Council, Chest Heart & Stroke Scotland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central, web‐based, computerised randomisation system incorporating a minimisation algorithm randomly assigned participants |
| Allocation concealment (selection bias) | Low risk | Concealed allocation on the web application until all baseline data were entered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome event adjudicator was masked to participant identity, treatment allocation, and drug use by redaction of this information from source documents |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrew. All others were followed‐up for at least 1 year or until death |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
AF: atrial fibrillation BP: blood pressure CHA2DS2‐VASc: a score for predicting the risk of stroke or thromboembolism in AF, based on congestive heart failure [1 point]; hypertension [1 point]; age ≥75 years [2 points]; diabetes [1 point]; previous stroke, transient ischaemic attack, or thromboembolism [2 points]; vascular disease [1 point]; age 65–74 years [1 point]; and sex category [1 point for female]). CT: computed tomography CTPA: CT pulmonary angiography DOAC: direct oral anticoagulant DVT: deep vein thrombosis ICH: intracerebral haemorrhage INR: international normalised ratio IQR: interquartile range IVH: intraventricular haemorrhage LAAO: left atrial appendage occlusion LMWH: low‐molecular‐weight heparin MOCA: Montreal Cognitive Assessment MRI: magnetic resonance imaging mRS: modfield Rankin Scale NIHSS: National Institutes of Health Stroke Scale NOAC: novel oral anticoagulants PE: pulmonary embolism RCT: randomised controlled trial SAH: subarachnoid haemorrhage SD: standard deviation SDH: subdural haemorrhage VKA: vitamin K antagonist VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boeer 1991 | This is an extension phase of Dickmann 1988 that introduces non‐randomised data to the previously published data |
| CAST 1997 | This trial included some people with ICH who were randomised prior to CT. These data were not available in the original manuscript. We had hoped that there would be useable data published in the Keir 2002 systematic review, but this did not separate spontaneous ICH from haemorrhagic transformations of acute ischaemic stroke and we were therefore unable to include data from CAST 1997 in this review |
| ChiCTR2000040166 | This is an ongoing randomised controlled study included patients with cerebral haemorrhage. The two intervention groups are traditional Chinese medicines, Musk huayu Xingnao and Vermiculus huoxue Tong yu granules, which are not proven to have an antithrombotic effect. We excluded the trial because the intervention was not an antithrombotic drug |
| Frontera 2014 | This is a controlled clinical trial that was not randomised |
| IST 1997 | This trial included several hundred participants with ICH who were randomised prior to CT. These data were not available in the original manuscript. We had hoped that data from the Keir 2002 systematic review or the published individual data forms (Sandercock 2011) would yield useable data for this review, but Keir 2002 did not report spontaneous ICH separately from haemorrhagic expansion of an acute ischaemic stroke, and after contacting the chief investigator of IST 1997 we discovered that it was not possible to extract data on spontaneous ICH separately |
| Kuramatsu 2018 | This study used data from an observational study, German‐wide multicenter analysis of oral anticoagulation associated intracerebral haemorrhage (RETRACE). The study was excluded because it was not randomised |
| Li 2013 | The intervention randomised in this study is transfusion of frozen apheresis platelets in participants on aspirin and with ICH, not an antithrombotic drug |
| RESTART extended follow‐up | This study reported extended follow‐up of the RESTART trial cohort, first reported in 2019. Patients were aware of their treatment allocation throughout, and were aware of the results of the main report of the trial when undergoing extended follow‐up. Therefore, there was more opportunity for bias in this open trial, arising from awareness of the main results during extended follow‐up, so we included RESTART 2019 in preference to this |
| Venturelli 2014 | This is a post‐hoc analysis of the INTERACT2 trial. The intervention that is the subject of this analysis, prophylactic subcutaneous heparin after ICH, was not randomly assigned in INTERACT2. Therefore, this analysis is observational in nature |
| Yan 2014 | The participant group did not have spontaneous ICH; it was a cohort of people with and without cerebral microbleeds and haemorrhagic transformation, with acute ischaemic stroke following rtPA treatment |
CT: computed tomography ICH: intracerebral haemorrhage rtPA: Recombinant tissue plasminogen activator
Characteristics of studies awaiting classification [ordered by study ID]
ELDERCARE‐AF 2020.
| Methods | Phase 3, multicentre, randomised, double‐blind, placebo‐controlled, event‐driven trial |
| Participants | Eligible patients were 80 years of age or older, had a history of nonvalvular atrial fibrillation documented on an electrocardiogram or on a monitor recording obtained within 1 year before consent was given, and had a CHADS2 score of 2 or higher. Eligible patients were also considered to be inappropriate candidates for oral anticoagulants (i.e, warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban) at the recommended therapeutic strength (in the case of warfarin) or at approved doses for one or more of the following reasons: a low creatinine clearance (15 to 30 mL per minute), a history of bleeding from a critical area or organ or gastrointestinal bleeding, low body weight (≤45 kg), continuous use of nonsteroidal antiinflammatory drugs (NSAIDs), or current use of an antiplatelet drug. |
| Interventions | 15 mg of edoxaban once daily versus placebo. |
| Outcomes | The primary efficacy end point was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding according to the definition of the International Society on Thrombosis and Haemostasis. Secondary efficacy end points included the composite of stroke, systemic embolism, or death from cardiovascular causes; major adverse cardiovascular events (the composite of nonfatal myocardial infarction, nonfatal stroke, nonfatal systemic embolism, or death from cardiovascular causes or bleeding); the composite of stroke, systemic embolism, or death from any cause; net clinical benefit (the composite of stroke, systemic embolism, major bleeding, or death from any cause); and death from any cause. Secondary safety end points included the composite of major bleeding or clinically relevant nonmajor bleeding; clinically relevant nonmajor bleeding; minor bleeding; and all bleeding. |
| Notes |
PRAGUE‐17 2020.
| Methods | Investigator‐initiated, multicentre, prospective, open‐label, randomised, noninferiority trial conducted at 10 cardiac centres in the Czech Republic. |
| Participants | Moderate‐ or high‐risk patients with nonvalvular AF were eligible if indicated for anticoagulation and had: 1) history of bleeding requiring intervention or hospitalization; 2) history of a cardioembolic event while taking anticoagulation agents; or 3) a moderate to high risk profile, defined as CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category [female]) of ≥3 plus HAS‐BLED (uncontrolled hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol) of ≥2. Key exclusion criteria included mechanical valve prosthesis, mitral stenosis, comorbidities other than AF mandating anticoagulation, patent foramen ovale with large atrial septal aneurysm, mobile aortic plaque, symptomatic carotid arterial atherosclerosis, clinically significant bleeding within 30 days, cardioembolic event within 30 days, and creatinine clearance of <30 mL/min. |
| Interventions | Left atrial appendage closure versus non‐vitamin K direct oral anticoagulant (DOAC). Patients randomised to the DOAC group could receive either rivaroxaban, apixaban, or dabigatran at the manufacturer‐recommended dose. |
| Outcomes | The primary outcome was a composite of safety and efficacy characteristics of both strategies: 1) stroke (ischaemic or haemorrhagic) or transient iachaemic attack (TIA)2) systemic embolism; 3) clinically significant bleeding; 4) cardiovascular death; or 5) significant peri‐procedural or device‐related complications. Clinically significant bleeding was a composite of major and nonmajor clinically relevant bleeding (NMCRB), according to the International Society on Thrombosis and Hemostasis (ISTH) criteria. Major bleeding includes either a decrease in haemoglobin of ≥2.0 g/dL during a 24‐h period, transfusion of ≥2 units of packed red cells, bleeding at a critical site (intracranial, intraspinal, intraocular, pericardial, intramuscular with compartment syndrome, or retroperitoneal), or fatal bleeding. NMCRB is defined as bleeding requiring hospitalization or an invasive procedure but not meeting ISTH major criteria. Complications included pericardial effusion requiring drainage/pericardiocentesis or surgery, cardioembolism, peri‐procedural bleeding requiring surgical revision or transfusion, device embolisation, device‐related thrombus with cardioembolism, or others as assessed by the operator and clinical endpoint committee (CEC). Secondary endpoints included the individual components of the primary endpoint. |
| Notes |
a Patients such as those not eligible for continued administration at the approved dosage for the drug due to concerns about bleeding risk, or those who have not been administered available oral anticoagulants at the approved dosage but are expected to have a high bleeding risk from available oral anticoagulants at the approved dosage.
b For warfarin, INR controlled between 1.6 and 2.6.
c Subcutaneous bleeding will be included if there is at least 1 hematoma with a maximal diameter of at least 5 cm, and urinary findings will be included if frank hematuria is observed.
d History of mitral valve repair is allowed in the trial. CHADS2, Congestive heart failure, Hypertension, Age, Diabetes, prior Stroke or transient ischaemic attack; INR, international normalized ratio.
Characteristics of ongoing studies [ordered by study ID]
NCT02966119.
| Study name | REstart or STop Antithrombotic Randomised Trial in France (RESTART‐Fr) |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre in the North of France Dates: December 2016 to December 2022 |
| Participants |
Sample size: 292 Diagnosis: ICH Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: antiplatelet agent (aspirin or clopidogrel or dypyridamole), chosen by the patient's physician before the randomisation Comparator: avoid antiplatelet drugs |
| Outcomes |
Primary outcome: symptomatic ICH (fatal or non fatal) proven radiologically Secondary outcomes
Duration of follow‐up: 2 years |
| Starting date | 7 December 2016 |
| Contact information | Contact information: Professor Charlotte Cordonnier charlotte.cordonnier@chru-lille.fr |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT03186729.
| Study name | STudy of Antithrombotic Treatment after IntraCerebral Haemorrhage (STATICH) |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre in Nordic countries Dates: July 2018 to June 2023 |
| Participants |
Sample size: 500 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
For MRI substudy: contraindication for MRI |
| Interventions |
Intervention: antithrombotic treatment (for patients with vascular disease and indication for antiplatelet drugs: antiplatelet drugs; for patients with AF and indication for anticoagulant drugs: anticoagulant drugs) Comparator: no antithrombotic treatment |
| Outcomes |
Primary outcome:
Secondary outcomes
Duration of follow‐up: 2 years |
| Starting date | 1 July 2018 |
| Contact information | Torgeir Bruun Wyller t.b.wyller@medisin.uio.no |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT03243175.
| Study name | Avoiding Anticoagulation After IntraCerebral Haemorrhage (A3ICH) |
| Methods | Design: randomised controlled PROBE 3‐arm trial (1:1:1) Setting: multicentre, France Dates: January 2019 to December 2023 |
| Participants |
Sample size: 300 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria for all treatment groups
Exclusion criteria related to LAAO only
Exclusion criteria related to DOAC only
|
| Interventions |
Intervention: apixaban 5mg twice daily Intervention: LAAO Comparator: no OAC or LAAO. May include antiplatelet agents (in the setting of co‐morbidities) or no antithrombotic drugs at all |
| Outcomes |
Primary outcome
Secondary outcomes
Duration of follow‐up: 2 years |
| Starting date | 24 January 2019 |
| Contact information | Prof Charlotte Cordonnier, MD, PhD e‐mail: charlotte.coprdonnier@chru‐lille.fr |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT03907046.
| Study name | Anticoagulation in ICH Survivors for Stroke Prevention and Recovery (ASPIRE) |
| Methods |
Design: randomised controlled parallel group quadruple blind trial Setting: multicentre at NIH/NINDS StrokeNet sites in the USA Dates: January 2020‐April 2024 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: apixaban 5mg twice daily, or 2.5mg twice daily in the setting of ≥ 2 of the following: age ≥ 80 years, body weight ≤6 0 kg, or serum creatinine 1.5‐2.4 mg/dL, or patient is taking a strong CYP3A4/pGP inhibitor (e.g. ketoconazole, itraconazole, ritonavir, or clarithromycin) Comparator: aspirin 81mg once daily |
| Outcomes |
Primary outcome
Secondary outcome
Duration: 12‐36 months. |
| Starting date | 28 January 2020 |
| Contact information | Kevin N Sheth (kevin.sheth@yale.edu), Hooman Kamel (hok9010@med.cornell.edu) |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT03950076.
| Study name | EdoxabaN foR IntraCranial Hemorrhage survivors with Atrial Fibrillation (ENRICH‐AF) |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre, international Dates: September 2019 to July 2023 |
| Participants |
Sample size: 1200 participants Diagnosis: symptomatic, spontaneous and non‐traumatic ICH, IVH, and/or convexity SAH, and symptomatic spontaneous or non‐penetrating traumatic SDH Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: edoxaban 60 mg (or 30 mg as determined by clinical criteria) Comparator: non‐anticoagulant medical therapy as determined by the local investigator includes 1) no antithrombotic therapy, 2) antiplatelet monotherapy, including de novo indication for antiplatelet monotherapy during course of the study |
| Outcomes |
Primary outcomes
Secondary outcomes
Duration of follow‐up: median 2 years |
| Starting date | 20 September 2019 |
| Contact information | Kevin Reeh ENRICH-AF@phri.ca |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT04522102.
| Study name | Antiplatelet Secondary Prevention International Randomised trial after INtracerebral haemorrhaGe (ASPIRING) ‐ pilot phase |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre hospital sites in China and Australia Dates: September 2021 to June 2023 |
| Participants |
Sample size: 120 participants Diagnosis: ICH Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: start antiplatelet monotherapy (one antiplatelet drug available in local standard clinical practice, chosen by patient's physician pre‐randomisation) Comparator: avoid antiplatelet therapy |
| Outcomes |
Primary outcome
Secondary outcomes
Duration of follow‐up: up to 3 years |
| Starting date | 3 September 2021 |
| Contact information | Rustam AI‐Shahi Salman, +44 131 242 7014, Rustam.Al-Shahi@ed.ac.uk Lily Song, +86 13916466400, lsong@georgeinstitute.org.cn |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
NCT04820972.
| Study name | Early‐start antiplatelet treatment after neurosurgery in patients with spontaneous intracerebral haemorrhage |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre in China Dates: 1 May 2021 to May 2023 |
| Participants |
Sample size: 250 participants Diagnosis: post‐operative ICH Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: aspirin 100 mg once daily antiplatelet therapy starting from the third day after surgery Comparator: 'traditional' |
| Outcomes |
Primary outcomes
Secondary outcomes: none specified Duration of follow‐up: 90 days |
| Starting date | 1 May 2021 |
| Contact information | JUN WU, MD wujunslf@126.com Shuo Wang, MD captain9858@126.com |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: not specified in trials register |
PRESTIGE‐AF.
| Study name | PREvention of STroke in Intracerebral haemorrhaGE survivors with Atrial Fibrillation (PRESTIGE‐AF) |
| Methods |
Design: randomised controlled PROBE parallel group trial Setting: multicentre international in Europe Dates: 3 June 2019 to 30 November 2022 |
| Participants |
Sample size: 654 participants Diagnosis: ICH Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention: DOAC (dabigatran, apixaban, rivaroxaban, or edoxaban) Comparator: no OAC. If the patient is randomized in this arm investigators will use their best judgment to decide upon the prescription of an antiplatelet drug of their choice or no such therapy |
| Outcomes |
Primary outcomes
Secondary outcomes
Duration of follow‐up: 3 years |
| Starting date | 3 June 2019 |
| Contact information | Kirsten H Harvey hello@prestige-af.org; kirsten.harvey@imperial.ac.uk |
| Notes |
Declarations of interest: not specified in trials register Sources of funding: EU Horizon 2020 |
AF: atrial fibrillation AVM: arteriovenous malformation CCA: common carotid artery cSAH: convexity subarachnoid hemorrhage CT: computed tomography DBP: diastolic blood pressure DOAC: direct oral anticoagulant ICH: intracerebral haemorrhage IVH: intraventricular haemorrhage LAAO: left atrial appendage occlusion MRI: magnetic resonance imaging mRS: modified Rankin Scale NOAC: novel oral anticoagulant NSAID: non‐steroidal anti‐inflammatory drug OAC: oral anticoagulation SAH: subarachnoid haemorrhage SBP: systolic blood pressure SDH: subdural haemorrhage TIA: transient ischaemic attack VKA: vitamin K antagonist
Differences between protocol and review
Pooling of included RCTs: we grouped RCTs investigating short‐term and long‐term treatment separately, instead of performing subgroup analyses.
Types of intracranial haemorrhage: most of the RCTs were restricted to participants with intracerebral haemorrhage (ICH), but some included a small number of participants with other forms of intracranial haemorrhage; similarly, some of the RCTs reported an outcome of intracranial haemorrhage, not just ICH.
Outcome nomenclature: we retained the primary outcome pre‐specified in the protocol, but renamed it major adverse cardiovascular events (MACE), to reflect the commonest terminology for this composite outcome.
Outcome inclusion: we retained the secondary outcomes, including individual components of the MACE composite primary outcome, and added outcomes that had not been mentioned specifically in the protocol, but which are most appropriate for the comparison of start versus avoid short‐term prophylactic dose anticoagulation: deep vein thrombosis, pulmonary embolism, venous thromboembolism and ICH growth. We added three composite outcomes reported by the included RCTs that had not been pre‐specified in our protocol, because there is no standardised definition of MACE and these variations on the MACE composite provide clinically informative assessments of safety, efficacy, and effectiveness: 1) any stroke (ischaemic or haemorrhagic); 2) any stroke (ischaemic or haemorrhagic) or vascular death; and 3) major vascular events as defined by the Antithrombotic Trialists’ Collaboration.
Outcome importance: given the number of pre‐specified outcomes and comparisons in this review, we prioritised the five following clinically important outcomes for reporting in the summary of findings tables, Abstract, and Plain Language Summary (according to the MECIR R12 standard): 1) MACE, 2) Death, 3) major occlusive vascular events, 4) ICH, and 5) functional outcome.
Contributions of authors
RASS, GJH, OMR and other co‐authors of the protocol conceived the review; RASS, OMR, and GJH designed the review; RASS co‐ordinated the review; AC and CC reviewed the results of the search and selected studies for inclusion in the review; AC and CC collected data for the review; AC and CC assessed the risk of bias in the included studies; AC and CC assessed the certainty in the body of evidence; AC and CC interpreted data; RASS, AC and CC wrote the review, which all authors reviewed and approved.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Alexia Cochrane: none
Chen Chen: none
Jacqueline Stephen: none
Ole Morten Rønning: Trial Steering committee member for NCT03186729.
Craig S Anderson: Trial steering committee member for NCT04522102. He has received grant funding as follows:
(1) Credit pharma, China: Grant / Contract Contract Start Date: Jan 1, 2020 Contract End Date: Ongoing / No Known End Date Additional Information: grant support paid to my institution
(2) Genesis Pharma: Grant / Contract Contract Start Date: Jan 1, 2020 Contract End Date: Ongoing / No Known End Date Additional Information: grant support paid to my institution
(3) National Health and Medical Research Council: Grant / Contract Contract Start Date: Jan 1, 2020 Contract End Date: Ongoing / No Known End Date Additional Information: grant support
(4) Penumbra, Inc: Grant / Contract Contract Start Date: Jun 30, 2021 Contract End Date: Ongoing / No Known End Date Additional Information: grant support paid to my institution
Graeme J Hankey: in the past three years, GJH has received honoraria from AC Immune for chairing the data safety monitoring committee of two clinical trials of vaccines for Alzheimer’s disease, from Bayer for lecturing about stroke prevention in atrial fibrillation at sponsored scientific symposia, and from Medscape, Web MD for participating in a discussion about stroke prevention in atrial fibrillation for theheart.org. NCT04522102 Trial Steering Committee member.
Summary of interests: Company or Organization
(1) AC Immune Data And Safety Monitoring Category: Data And Safety Monitoring Contract Description: DSMB member of ACI‐35‐1802 Phase Ib/IIa trial of tau‐targeted vaccines in early Alzheimers Disease End Date: Ongoing / No Known End Date
(2) Bayer Category: Consultant Contract Description: Stroke Prevention Initiative End Date: Oct 20, 2020
(3) Bristol‐Myers Squibb Category: Other Contract Description: Member of the Steering Committee of the AXIOMATIC‐SSP phase 2 trial of milvexian End Date: Ongoing / No Known End Date
(4) Janssen Research and Development Category: Consultant Contract Description: Review of clinical trial protocol End Date: Ongoing / No Known End Date
Intellectual Property Description: Antiplatelet Secondary Prevention International Randomised Trial After Intracerebral Haemorrhage
Rustam Al‐Shahi Salman: UK chief investigator of RESTART 2019, SoSTART 2021, and NCT03950076. Trial Steering Committee member for NCT03186729, NCT04522102, and NCT02966119. He has received grant funding as follows:
(1) British Heart Foundation: Grant / Contract Contract Start Date: Aug 1, 2018 Contract End Date: Jul 31, 2021 Additional Information: Clinical study grant for the SoSTART trial
(2) British Heart Foundation: Grant / Contract Contract Start Date: Mar 1, 2013 Contract End Date: Jul 31, 2021 Additional Information: Special project grant for RESTART
(3) Population Health Research Institute: Grant / Contract Contract Start Date: Apr 22, 2019 Contract End Date: Ongoing / No Known End Date Additional Information: Grant for the ENRICH‐AF trial
Edited (no change to conclusions)
References
References to studies included in this review
APACHE‐AF 2021 {published data only}NL4395
- Schreuder FH, Nieuwenhuizen KM, Hofmeijer J, Vermeer SE, Kerkhoff H, Zock E, APACHE-AF Trial Investigators. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurology 2021;20(11):907-16. [DOI: 10.1016/S1474-4422(21)00298-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhuizen KM, Van der Worp HB, Algra A, Kappelle LJ, Rinkel GJ, Van Gelder IC, et al. Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials 2015;16:393. [DOI: 10.1186/s13063-015-0898-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickmann 1988 {published data only}
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral haemorrhage. Klinische Wochenschrift 1988;66(23):1182-3. [DOI: 10.1007/BF01727666] [PMID: ] [DOI] [PubMed] [Google Scholar]
NASPAF‐ICH 2020 {published data only}
- Shoamanesh A, Sharma M, Dowlatshahi D, Pikula A, Smith EE, Gladstone DJ, et al. Non-vitamin K oral anticoagulants in intracerebral hemorrhage survivors with atrial fibrillation: primary results of the NASPAF-ICH randomized trial. In: International Stroke Conference, Session A28 - Late-Breaking Science Oral Abstracts II. Los Angeles, 2020. [CLINICALTRIALS.GOV: NCT02998905]
Orken 2009 {published data only}
- Orken DN, Kenangil G, Ozkurt H, Guner C, Gundogdu L, Basak M, et al. Prevention of deep venous thrombosis and pulmonary embolism in patients with acute intracerebral hemorrhage. Neurologist 2009;15(6):329-31. [DOI: 10.1097/NRL.0b013e3181a93bac] [PMID: ] [DOI] [PubMed] [Google Scholar]
PICASSO sub‐group 2020 {published data only}
- Kim BJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LK, PICASSO Investigators. Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO Trial. Stroke 2020;51(3):931-7. [CLINICALTRIALS.GOV: NCT01013532] [DOI: 10.1161/STROKEAHA.119.023855] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, PICASSO investigators. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurology 2018;17(6):509-18. [DOI] [PubMed] [Google Scholar]
PREVENTIHS 2020 {published data only}
- Paciaroni M, Agnelli G, Alberti A, Becattini C, Guercini F, Martini G, et al. PREvention of VENous Thromboembolism In Hemorrhagic Stroke patients - PREVENTIHS Study: a randomized controlled trial and a systematic review and meta-analysis. European Neurology 2020;83(6):566-75. [DOI: 10.1159/000511574] [PMID: ] [DOI] [PubMed] [Google Scholar]
Qian 2021 {published data only}
- Qian C, Huhtakangas J, Juvela S, Bode MK, Tatlisumak T, Savolainen M, et al. Early vs. late enoxaparin for the prevention of venous thromboembolism in patients with ICH: a double blind placebo controlled multicenter study. Clinical Neurology and Neurosurgery 2021;202:106534. [DOI: 10.1016/j.clineuro.2021.106534] [PMID: ] [DOI] [PubMed] [Google Scholar]
RESTART 2019 {published data only}ISRCTN71907627
- Al-Shahi Salman R, Dennis M, Murray G, Innes K, Drever J, Dinsmore L, et al. The REstart or STop Antithrombotics Randomised Trial (RESTART) after stroke due to intracerebral haemorrhage: study protocol for a randomised controlled trial. Trials 2018;19:162. [DOI: 10.1186/s13063-018-2542-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shahi Salman R, Minks DP, Mitra D, Rodrigues MA, Bhatnagar P, du Plessis JC, RESTART Collaboration. Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: subgroup analyses of the RESTART randomised, open-label trial. Lancet Neurology 2019;18(7):643-52. [DOI: 10.1016/S1474-4422(19)30184-X] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet 2019;393(10191):2613-23. [DOI: 10.1016/S0140-6736(19)30840-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SoSTART 2021 {published data only}
- SoSTART Collaboration. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurology 2021;20(10):842-53. [DOI: 10.1016/S1474-4422(21)00264-7.] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boeer 1991 {published data only}
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 1991;54(5):466-7. [DOI: 10.1136/jnnp.54.5.466] [DOI] [PMC free article] [PubMed] [Google Scholar]
CAST 1997 {published data only}
- CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke; CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349(9066):1641-9. [DOI: 10.1016/S0140-6736(97)04010-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
ChiCTR2000040166 {published data only}
- ChiCTR2000040166. A multi-center, randomized, double-blind, placebo-controlled clinical study of SXHYXN combined with ZLHXTY in the treatment of cerebral hemorrhage. http://www.chictr.org.cn/showprojen.aspx?proj=63798 (first received 23 November 2020). [CHICTR: 2000040166]
Frontera 2014 {published data only}
- Frontera JA, Jovine M, Zach V, Gordon E. Safety of venous thromboembolism prophylaxis in intracranial hemorrhage patients with external ventricular drains. Stroke 2014;45:236. [Google Scholar]
IST 1997 {published data only}
- International Stroke Trial Collaborative Group. The International Stoke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet 1997;349(9065):1569-81. [DOI: 10.1016/S0140-6736(97)04011-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuramatsu 2018 {published data only}
- Kuramatsu JB, Huttner HB. Restarting therapeutic anticoagulation in patients with intracerebral hemorrhage and mechanical heart valves. Stroke 2018;49 Suppl 1:Abstract 50. [DOI: 10.1161/str.49.suppl_1.50] [DOI] [Google Scholar]
Li 2013 {published data only}
- Li X, Zhaosheng S, Zhao W, Zhang J, Chen J, Li Y, et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of Neurosurgery 2013;118:94-103. [DOI: 10.3171/2012.9.JNS112286] [DOI] [PubMed] [Google Scholar]
RESTART extended follow‐up {published data only}
- Al-Shahi Salman R, Dennis MS, Sandercock PA, Sudlow CLM, Wardlaw JM, Whiteley WN, RESTART Collaboration. Effects of antiplatelet therapy after stroke caused by intracerebral hemorrhage: extended follow-up of the RESTART randomized clinical trial. JAMA Neurology 2021;78(10):1179-86. [DOI: 10.1001/jamaneurol.2021.2956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venturelli 2014 {published data only}
- Venturelli PA, Wang X, Arima H, Heeley E, Delcourt C, Lavados P, et al. Safety of prophylactic heparin in acute intracerebral haemorrhage: post-hoc analysis of the INTERACT2 study. International Journal of Stroke 2014;9 Suppl 1:38. [Google Scholar]
Yan 2014 {published data only}
- Yan L, Li YD, Li YH, Li MH, Zhao JG, Chen SW. Outcomes of antiplatelet therapy for haemorrhage patients after thrombolysis: a prospective study based on susceptibility-weighted imaging. La Radiologica Medica 2014;119(3):175-82. [DOI: 10.1007/s11547-013-0328-1] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ELDERCARE‐AF 2020 {published data only (unpublished sought but not used)}
- Okumura K, Akao M, Yoshida T, Kawata M, Yamashita T, et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. New England Journal of Medicine 2020;383:1735-45. [DOI: 10.1056/NEJMoa2012883] [DOI] [PubMed] [Google Scholar]
- Okumura K, Lip G, Akao M, Tanizawa K, Fukuzawa M, Abe K, et al. Edoxaban for the management of elderly Japanese patients with atrial fibrillation ineligible for standard oral anticoagulant therapies: rationale and design of the ELDERCARE-AF study. American Heart Journal 2017;194:99-106. [DOI: 10.1016/j.ahj.2017.08.017] [DOI] [PubMed] [Google Scholar]
PRAGUE‐17 2020 {published data only (unpublished sought but not used)}
- Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. Journal of the American College of Cardiology 2020;75(25):3122‐35. [DOI: 10.1016/j.jacc.2020.04.067] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02966119 {published data only}
- NCT02966119. REstart or STop Antithrombotic Randomised Trial in France (RESTART-Fr). https://clinicaltrials.gov/ct2/show/NCT02966119 (first received 17 November 2016). [NCT02966119]
NCT03186729 {published data only}
- NCT03186729. STudy of Antithrombotic Treatment after IntraCerebral Haemorrhage (STATICH). https://clinicaltrials.gov/ct2/show/NCT03186729 (first received 14 June 2017). [NCT03186729]
NCT03243175 {published data only}
- NCT03243175. Avoiding Anticoagulation After IntraCerebral Haemorrhage (A3ICH). https://clinicaltrials.gov/ct2/show/NCT03243175 (first received 8 August 2017). [NCT03243175]
NCT03907046 {published data only}
- NCT03907046. Anticoagulation for stroke prevention and recovery after ICH (ASPIRE). https://clinicaltrials.gov/ct2/show/NCT03907046 (first received 8 April 2019). [NCT03907046]
NCT03950076 {published data only}
- NCT03950076. EdoxabaN foR IntraCranial Hemorrhage survivors with Atrial Fibrillation (ENRICH-AF). Cochrane Database of Systematic Reviews (first received 15 May 2019).
NCT04522102 {published data only}
- NCT04522102. Antiplatelet Secondary Prevention International Randomised trial after INtracerebral haemorrhaGe (ASPIRING) - pilot phase. https://clinicaltrials.gov/ct2/show/NCT04522102 (first received 21 August 2020).
NCT04820972 {unpublished data only}
- NCT04820972. Early-start antiplatelet treatment after neurosurgery in patients with spontaneous intracerebral hemorrhage. https://clinicaltrials.gov/ct2/show/NCT04820972 (first received 29 March 2021).
PRESTIGE‐AF {published data only}
- Haas K, Korompoki E, Hugen K, Malzahn U, Rucker V, Harvey K, et al. Prevention of stroke in intracerebral haemorrhage survivors with atrial fibrillation (PRESTIGE-AF): objectives and design of a randomised clinical trial and an observational study. European Stroke Journal 2018;3 Suppl 1:233. [DOI: 10.1177/2396987318770127] [DOI] [Google Scholar]
Additional references
AHA ICH guideline 2015
- Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032-60. [DOI: 10.1161/STR.0000000000000069] [DOI] [PubMed] [Google Scholar]
Antithrombotic Trialists' Collaboration 2009
- Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. [DOI: 10.1016/S0140-6736(09)60503-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2020
- Banerjee G, Wilson D, Ambler G, Hostettler IC, Shakeshaft C, Cohen H, et al. Longer term stroke risk in intracerebral haemorrhage survivors. Journal of Neurology, Neurosurgery and Psychiatry 2020;91:840-5. [DOI: 10.1136/jnnp-2020-323079] [DOI] [PubMed] [Google Scholar]
Benz 2021
- Benz AP, Johansson I, Dewilde WJ, Lopes RD, Mehran R, Sartori S, et al. Antiplatelet therapy in patients with atrial fibrillation: a systematic review and meta-analysis of randomized trials. European Heart Journal - Cardiovascular Pharmacotherapy 2021 Jun 17 [Epub ahead of print]. [DOI: 10.1093/ehjcvp/pvab044] [DOI] [PubMed]
Canadian ICH best practice recommendation 2020
- Shoamanesh A, Lindsay MP, Castellucci LA, Cayley A, Crowther M, Wit K, et al. Canadian stroke best practice recommendations: nanagement of spontaneous intracerebral hemorrhage, 7th edition update 2020. International Journal of Stroke 2021;16:321-41. [DOI: 10.1177/1747493020968424] [DOI] [PubMed] [Google Scholar]
Casolla 2019
- Casolla B, Moulin S, Kyheng M, Hénon H, Labreuche J, Leys D, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke 2019;50:1100-7. [DOI: 10.1161/STROKEAHA.118.024449] [DOI] [PubMed] [Google Scholar]
Chapman 2004
- Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al . Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 2004;35:116-21. [DOI: 10.1161/01.STR.0000106480.76217.6F] [DOI] [PubMed] [Google Scholar]
Chen 2020
- Chen Y, Wright N, Guo Y, Turnbull I, Kartsonaki C, Yang L, et al. Mortality and recurrent vascular events after first incident stroke: a 9-year community-based study of 0.5 million Chinese adults. Lancet Global Health 2020;8:e580-e590. [DOI: 10.1016/S2214-109X(20)30069-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2021
- Cheng B, Li J, Peng L, Wang Y, Sun L, He S, et al. Efficacy and safety of restarting antiplatelet therapy for patients with spontaneous intracranial haemorrhage: a systematic review and meta-analysis. Journal of Clinical Pharmacy and Therapeutics 2021;46:957-65. [DOI: 10.1111/jcpt.13377] [DOI] [PubMed] [Google Scholar]
Chinese Stroke Association guidelines 2019
- Cao Y, Yu S, Zhang Q, Yu T, Liu Y, Sun Z, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of intracerebral haemorrhage. Stroke and Vascular Neurology 2020;5:396-402. [DOI: 10.1136/svn-2020-000433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ding 2018
- Ding X, Liu X, Tan C, Yin M, Wang T, Liu Y, et al. Resumption of antiplatelet therapy in patients with primary intracranial hemorrhage-benefits and risks: a meta-analysis of cohort studies. Journal of the Neurological Sciences 2018;384:133-8. [DOI: 10.1016/j.jns.2017.11.009] [DOI] [PubMed] [Google Scholar]
ESO guideline on antithrombotic treatment 2019
- Klijn CJ, Paciaroni M, Berge E, Korompoki E, Kõrv J, Lal A, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. European Stroke Journal 2019;4:198-223. [DOI: 10.1177/2396987319841187] [DOI] [PMC free article] [PubMed] [Google Scholar]
ESO ICH Guideline 2014
- Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International Journal of Stroke 2014;9:840-55. [DOI: 10.1111/ijs.12309] [DOI] [PubMed] [Google Scholar]
Gaist 2022
- Gaist D, Gonzalez-Perez A, Hald SM, Garcia Rodriguez LA. Higher risk of ischemic stroke after an intracerebral hemorrhage than in general population: a cohort study from the United Kingdom. Stroke 2022;53(2):e50-e52. [DOI: 10.1161/STROKEAHA.121.037633] [DOI] [PubMed] [Google Scholar]
GBD 2019 Stroke Collaborators 2021
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurology 2021;20:795-820. [DOI: 10.1016/S1474-4422(21)00252-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro Guideline Development Tool. McMaster University, 2015. Available from www.gradepro.org.
Hart 2007
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine 2007;146:857-67. [DOI: 10.7326/0003-4819-146-12-200706190-00007] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors) . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Keir 2002
- Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovascular Diseases 2002;14(3-4):197-206. [DOI: 10.1159/000065661] [DOI] [PubMed] [Google Scholar]
Korompoki 2017
- Korompoki E, Filippidis FT, Nielsen PB, Del Giudice A, Lip GY, Kuramatsu JB, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology 2017;89:687-96. [DOI: 10.1212/WNL.0000000000004235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021
- Li L, Poon MT, Samarasekera NE, Perry LA, Moullaali TJ, Rodrigues MA, et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral haemorrhage: pooled analyses of two population-based studies. Lancet Neurology 2021;20:437-47. [DOI: 10.1016/S1474-4422(21)00075-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murthy 2021
- Murthy SB, Zhang C, Diaz I, Levitan EB, Koton S, Bartz TM, et al. Association between intracerebral hemorrhage and subsequent arterial ischemic events in participants from 4 population-based cohort studies. JAMA Neurology 2021;78:809-16. [DOI: 10.1001/jamaneurol.2021.0925] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Clinical Guideline for Stroke 2016
- Intercollegiate Stroke Working Party. Royal College of Physicians. 5th edition. London, UK: Royal College of Physicians, 2016. [Google Scholar]
Nielsen 2022
- Nielsen PB, Melgaard L, Overvad TF, Jensen M, Larsen TB, Lip GY, et al. Risk of cerebrovascular events in intracerebral hemorrhage survivors with atrial fibrillation: a nationwide cohort study. Stroke 2022;53:2559-68. [DOI: 10.1161/STROKEAHA.121.038331] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paciaroni 2011
- Paciaroni M, Agnelli G, Venti A, Alberti A, Acciaressi M, Caso V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: a meta-analysis of controlled studies. Journal of Thrombosis and Haemostasis 2011;9:893-8. [DOI: 10.1111/j.1538-7836.2011.04241.x] [DOI] [PubMed] [Google Scholar]
Pasquini 2014
- Pasquini M, Charidimou A, Van Asch CJ, Baharoglu MI, Samarasekera N, Werring DJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after ICH. Stroke 2014;45(9):2643-8. [DOI: 10.1161/STROKEAHA.114.006202] [DOI] [PubMed] [Google Scholar]
PICASSO 2018
- Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, PICASSO investigators. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurology 2018;17:509-18. [DOI: 10.1016/S1474-4422(18)30128-5] [DOI] [PubMed] [Google Scholar]
Poon 2014
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and metaanalysis. Journal of Neurology, Neurosurgery and Psychiatry 2014;85(6):660-7. [DOI: 10.1136/jnnp-2013-306476] [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Samarasekera 2015
- Samarasekera N, Fonville A, Lerpiniere C, Farrall AJ, Wardlaw JM, White PM, et al. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke 2015;46:361-8. [DOI: 10.1161/STROKEAHA.114.007953] [DOI] [PubMed] [Google Scholar]
Sandercock 2011
- Sandercock PA, Niewada M, Czlonkowska A, the International Stroke Trial Collaborative Group. The International Stroke Trial database. Trials 2011;12:101. [DOI: 10.1186/1745-6215-12-101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, on behalf of the Cochrane GRADEing Methods Group. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Wang 2021
- Wang X, Ouyang M, Yang J, Song L, Yang M, Anderson CS. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD000024. [CENTRAL: CD000024] [DOI: 10.1002/14651858.CD000024.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yogendrakumar 2020
- YogendrakumarI V, Lun R, Khan F, Salottolo K, Lacut K, Graham C, et al. Venous thromboembolism prevention in intracerebral hemorrhage: a systematic review and network meta-analysis. PLOS One 2020;15:e0234957. [DOI: 10.1371/journal.pone.0234957] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Perry 2016
- Perry LA, Berge E, Bowditch J, Forfang E, Rønning OM, Hankey GJ, et al. Antithrombotic treatment after intracerebral haemorrhage. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD012144. [DOI: 10.1002/14651858.CD012144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2017
- Perry LA, Berge E, Bowditch J, Forfang E, Rønning OM, Hankey GJ, Villanueva E, Al-Shahi Salman R. Antithrombotic treatment after stroke due to intracerebral haemorrhage. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012144. [CENTRAL: CD012144] [DOI: 10.1002/14651858.CD012144.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


