Abstract
After a detailed description of orexins and their roles in sleep and other medical disorders, we discuss here the current clinical evidence on the effects of dual (DORAs) or selective (SORAs) orexin receptor antagonists on insomnia with the aim to provide recommendations for their further assessment in a context of personalized and precision medicine. In the last decade, many trials have been conducted with orexin receptor antagonists, which represent an innovative and valid therapeutic option based on the multiple mechanisms of action of orexins on different biological circuits, both centrally and peripherally, and their role in a wide range of medical conditions which are often associated with insomnia. A very interesting aspect of this new category of drugs is that they have limited abuse liability and their discontinuation does not seem associated with significant rebound effects. Further studies on the efficacy of DORAs are required, especially on children and adolescents and in particular conditions, such as menopause. Which DORA is most suitable for each patient, based on comorbidities and/or concomitant treatments, should be the focus of further careful research. On the contrary, studies on SORAs, some of which seem to be appropriate also in insomnia in patients with psychiatric diseases, are still at an early stage and, therefore, do not allow to draw definite conclusions.
Keywords: insomnia, orexin, hypocretin, orexin receptor antagonist, dual orexin receptor antagonists, selective orexin receptor antagonists
Introduction
The discovery of the neuropeptides named orexins1 (orexin A and orexin B) or hypocretins2 (hypocretin 1 and hypocretin 2) in 19981 has triggered in the last decades an enormous research effort aimed at the definition of the roles of orexins in multiple physiological functions and as a druggable target in pathophysiology. Besides the involvement of orexins in narcolepsy type 1 (NT1, narcolepsy with cataplexy),3 which is the prototypal disorder in which a deficit in orexins is the main biological feature, the importance of orexins has gradually become evident in a large series of disorders, as also briefly summarized in this review.
Among the disorders in which orexins are involved, a particularly important position is held by insomnia because of its distinct epidemiology and health and socioeconomic impact.4 Despite the undoubtful efficacy of cognitive-behavioral therapy for insomnia (CBT-I), which currently remains the first-line treatment, there still is a considerable number of patients who do not remit with this approach.5 This implies that the use of drugs to treat insomnia is often needed and useful.4 In addition to the conventional drug classes used, the most recent class of drugs for the treatment of insomnia is represented by the dual (DORAs) or selective (SORAs) orexin receptor antagonists.6,7 After a detailed description of orexins and their roles in sleep and other medical disorders, we will discuss here the current clinical evidence on the effects of DORAs and SORAs on insomnia based on peer-reviewed published papers.
The Biology of Orexins
General Considerations
In 1998, two independent groups working on rat hypothalamic mRNA and proteins reported the discovery of two novel hypothalamic neuropeptides, which they named hypocretin 1 and 2,2 or orexin A and B,1 respectively. The double orexin/hypocretin nomenclature of these peptides persists to date. However, the International Union of Basic and Clinical Pharmacology recommended in 2012 to designate the peptides and their receptors after the term orexin, and to designate their respective genes and mRNA after the term hypocretin (abbreviated as HCRT).8 The HCRT gene codes for a precursor peptide, named pre-pro-orexin, which is cleaved into the orexin-A and orexin-B active peptides. Differences between the structures of orexin A and orexin B, which are highly conserved among mammals, are thought to confer relative resistance to proteolytic degradation to orexin A,1 which binds two G-protein coupled receptors named OX1R and OX2R with similar affinity. The affinity of orexin B for OX2R is similar to that of orexin A, whereas that for OX1R is 2–3 orders of magnitude lower.1
The orexins are released by neurons in the tuberal region of the hypothalamus9 that also release glutamate.10,11 Orexin neurons are inhibited by glucose and excited by non-essential amino acids and by acidification and increases in the CO2 concentration.12,13 Orexin neurons are also directly excited by the orexigenic hormone ghrelin and indirectly inhibited by the anorexigenic hormone leptin.14
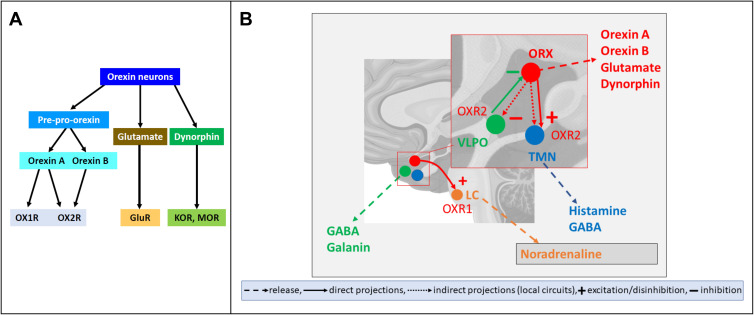
Orexin neurons receive projections from several brain areas that include the hypothalamus, the basal forebrain, the amygdala, and the brainstem.15,16 Projections from the hypothalamus involve the ventrolateral preoptic nucleus (VLPO), which is key to Sleep control, and the suprachiasmatic nucleus, which is the site of the master circadian clock, with the latter projections being largely indirect.16 In turn, orexin neurons project to multiple neuronal systems widely distributed in the brain. The targets of these projections include the VLPO and the tuberomammillary nucleus (TMN) of the hypothalamus and the locus coeruleus (LC) in the pons, which are involved in sleep control, as well as the arcuate nucleus (ARC) of the hypothalamus, which is involved in the control of feeding, and the midbrain ventral tegmental area (VTA), which is relevant to motivation and reward.17 In the pons and medulla, orexin fibers are distributed through the raphe nuclei and the reticular formation, which are relevant to sleep and motor control.17 The orexin neurons also widely project to structures of the central autonomic network involved in sympathetic and parasympathetic control, including the rostral ventrolateral medulla (RVLM) and the hypothalamic paraventricular nucleus (PVN).18 In general, these structures express OX1R and OX2R to different extents: for instance, VLPO, TMN, and LC neurons mostly express OX2R, OX2R, and OX1R, respectively (Figure 1).19
Figure 1.
Neurochemistry and neuroanatomy of orexin neuron signaling. (A) summarizes the neurochemistry of orexin neuron signaling. Orexin neurons synthetize pre-pro-orexin, which is cleaved into the orexin (A and B) peptides (OXA and OXB, respectively). Orexin A acts by binding both orexin receptor 1 and (OXR1 and OXR2, respectively), whereas orexin B binds with high potency only OXR2. Orexin neurons may co-release glutamate, which binds its receptors (GluR), and dynorphin, which binds kappa opioid receptors (KOR) and mu opioid receptors (MOR). (B) illustrates three of the main projections of orexin neurons that are of particular relevance to wake-sleep control. Orexin (ORX) neurons indirectly inhibit neurons of the ventrolateral preoptic nucleus (VLPO) of the hypothalamus, which promote sleep, express gamma-aminobutyric acid (GABA) and galanin, and inhibit ORX neurons. In addition, ORX neurons directly excite and indirectly disinhibit neurons of the hypothalamic tuberomammillary nucleus (TMN), which co-release histamine and GABA to promote wakefulness. Finally, ORX neurons excite neurons of the locus coeruleus in the pons, which release noradrenaline and promote wakefulness. The effects of ORX neurons on the VLPO and TMN are mainly mediated by OX2R, whereas those on the LC are mainly mediated by OX1R. Other projections of ORX neurons as well as of VLPO, TMN, and LC neurons were omitted for clarity.
Orexin Modulation of the Wake-Sleep States
Orexins are key to vigilance state control, as shown dramatically by the profound wake-sleep state instability in patients with NT1, who develop near-complete loss of orexin neurons,9 and in orexin knock-out (KO) mice.20,21 Moreover, early work at Stanford University22 revealed the occurrence of a NT1 phenotype in dogs with either congenital lack of functional OX2R or sporadic loss of orexin peptides. The vigilance state instability occurring due to impaired orexin signaling entails excessive daytime sleepiness (EDS) and sleep attacks fragmenting wakefulness, as well as a striking dysregulation of motor control that manifests both as lack of muscle tone during episodes of cataplexy and sleep paralysis20,21,23 and as excessive muscle tone during sleep.24,25 Intriguingly, inappropriately high and/or inappropriately timed orexin release also destabilizes wake-sleep states and muscle control during sleep.26 Excessive orexin neuron excitability underlies the increase of sleep fragmentation that occurs with aging, even despite the age-related decrease in orexin neuron number.27
Preclinical evidence suggests that orexins are critical in sustaining active waking behaviors that drive sleep need.28 Accordingly, orexin neurons discharge action potentials during active wakefulness and particularly during exploration behavior, whereas they are almost silent during sleep except for occasional burst discharge occurring during the phasic (rapid-eye-movement) REM sleep episodes, sometimes in association with spontaneous muscle twitches.29 During transitions from sleep to wakefulness, orexin neurons fire prior to the onset of electroencephalographic (EEG) activation and respond with a short latency to arousing sound stimuli during sleep.30 Accordingly, optogenetic activation of orexin neurons is sufficient to drive awakening.31 However, brain interstitial orexin concentration depends quite loosely on wake-sleep states.32–34 While the mechanisms of this apparent mismatch remain unclear, this mismatch may explain why orexin deficiency adversely affects vigilance state control well beyond active wakefulness.
The histaminergic and other aminergic neurons in the brainstem have been compared to an orchestra, with orexin neurons as a director and the histamine neurons as the first violin.35 An influential model of wake-sleep state regulation postulated the existence of mutually inhibitory neural circuits, or “flip-flop switches”, controlling transitions between wake-sleep states.36,37 One switch consists of sleep-on neurons, including in the VLPO, and of sleep-off neurons, noradrenergic neurons in the LC, histaminergic neurons in the TMN, and serotonergic neurons in the dorsal and median raphe nuclei.36 The other switch consists of REM-on neurons, such as in the murine pontine sublaterodorsal (SLD) nucleus (also named subcoeruleus nucleus in humans and peri-locus coeruleus alpha in cats38), and of REM-off neurons, including in the ventrolateral periaqueductal gray.37 The mutually inhibitory connections in flip-flop switches allow for rapid and complete transitions between vigilance states, although they are inherently prone to instability. Orexin neurons were thought to increase the activity of wake-on neurons of one switch and of REM-off neurons of the other switch without being part of either switch,36,37 thereby acting like a “finger” on the switches that might prevent unwanted state transitions.36,37 Nevertheless, recent findings challenged this aspect of the model, showing that orexin neurons may indeed be part of the wake on-off flip-flop switch. In particular, sleep-promoting VLPO neurons that are inhibited by noradrenaline project to and inhibit orexin neurons,39 and are themselves indirectly inhibited by orexin neurons through orexins and co-released glutamate.40 This pathway, which depends on OX2R,40 may be alternative to orexin-mediated excitation of LC neurons,41 which depends on OX1R,19,42 in driving arousal from sleep.40 There is also evidence that the wake-promoting effects of orexin-A, at least at supraphysiological concentrations, are mediated by excitation of histaminergic TMN neurons,43 which depends on OX2R.19 Other preclinical studies indicate that although OX2R play the more prominent role in the orexin modulation of wake-sleep states, OX1R are also relevant to the physiological control of REM sleep and vigilance-state related muscle tone and to the promotion of arousal.42,44,45 Nevertheless, recent work demonstrated that pharmacological OX2R agonism is sufficient to ameliorate cataplexy and sleep/wake fragmentation in orexin KO mice46 and in orexin-neuron deficient orexin-ataxin 3 mice.47 This discrepancy awaits clarification.
Orexin Modulation of Other Physiological Functions
Orexins modulate multiple physiological functions in addition to vigilance state control.48 Here, we will focus on four functions that have been relatively well explored and that may be particularly relevant to the large-scale treatment of insomnia with orexin receptor antagonists: cardiovascular and respiratory control, control of energy metabolism, reward and addiction, and stress.
As previously discussed, the orexin neurons are part of the central autonomic network,18 which underlies their prominent role in autonomic cardiovascular control.49 Intracerebroventricular injection of orexins increases arterial pressure and heart rate,50 an effect mediated by OX1R.51 Downregulation of OX1R in the PVN, another target of orexin neurons, reduces hypertension in the obese Zucker rat lacking leptin receptors.52 On the other hand, orexin microinjection in the RVLM, which is a key pre-sympathetic structure of the central autonomic network, increases heart rate, sympathetic nerve activity, and arterial pressure via both OX1R and OX2R,53,54 and OX2R blockade decreases arterial blood pressure in spontaneously hypertensive rats.55 Orexin-mediated cardiovascular activation may thus involve both OX1R and OX2R and be relevant to the pathophysiology of arterial hypertension. Patients with NT1, orexin KO mice lacking orexin peptides, and orexin-ataxin 3 mice lacking orexin neurons show an attenuated decrease in arterial blood pressure between daytime wakefulness and nighttime sleep (ie, a non-dipper blood pressure pattern), associated with smaller cardiovascular deactivation from wakefulness to non-REM sleep and with enhanced cardiovascular activation from non-REM sleep to REM sleep.21,56,57 This pattern was traced down to a dysregulation of sympathetic activity to the heart and blood vessels during sleep.58 On the other hand, even partial lack of orexins increases atherosclerosis burden in susceptible mice by increasing circulating inflammatory monocytes, an effect mediated by OX1R in the bone marrow.59
The role of orexins in respiratory control is worth addressing in light of the possible implications for the treatment with orexin receptor antagonists of insomnia comorbid with obstructive sleep apnea. Orexin neurons in mice are excited by hypercapnia.13,60 Early studies on mice convincingly reported a role of orexins in chemoreflex responses to hypercapnia, but not to hypoxia, during wakefulness, and suggested a role for both orexin receptor types, with OX2R being the more involved.61 However, later work pointed to a specific role of OX1R in the rostral medullary raphe and the retrotrapezoid nucleus in the chemoreflex response to hypercapnia in rats,62,63 and recent data indicate that orexins facilitate chemoreflex responses to both hypercapnia and hypoxia in these animals.64 On the other hand, impaired chemoreflex responsiveness during wakefulness and frequent central sleep apneas were reported in orexin KO mice.65 However, work on people with NT1 found that hypercapnic responsiveness was unaltered, whereas hypoxic responsiveness was reduced only in subjects carrying the HLA DQB1*0602 allele.66 Recent data did not confirm increased sleep apnea in orexin KO mice,67 whereas a mild increase in obstructive, rather than central, sleep apnea was reported in subjects with NT1.66,68 Taken together, these data point to a role of orexins in respiratory control, although this role is either not a major one or not well conserved among species.
The name orexins was given after the Greek word orexis, which stands for appetite, following the discovery that central orexin administration stimulated feeding, and that the hypocretin gene was upregulated with fasting.1 As discussed above, orexin neurons project to the ARC, which is key to feeding and metabolic control, and are sensitive to nutrient levels and hormones that control feeding and energy metabolism, such as ghrelin and leptin.14 Preclinical data indicate that orexin deficiency is causal to obesity, although this effect is also modulated by genetic background, orexin co-transmission, and histamine signaling.69,70 This effect has largely been ascribed to lack of OX2R signaling, as enhanced OX2R signaling prevents diet-induced obesity and improves leptin sensitivity in mice.71 However, recent data revealed that OX1R and OX2R both have unique roles in energy metabolism and modulate the interaction of diet and exercise on body weight gain, with OX2R being more relevant to the control of energy expenditure and OX1R being more relevant to reward-based feeding.72
The role of orexins in promoting reward and addiction73 is a burgeoning field of research of potentially critical relevance to public health.74–77 Addiction is defined by the American Society of Addiction Medicine as a chronic disease whereby people use substances or engage in behaviors that become compulsive and often continue despite harmful consequences (www.asam.org, last accessed on December 4, 2022). Orexis may also mean conation or desire; in hindsight, this term appears quite appropriate, as research revealed the orexin role in modulating motivational, rather than primary reinforcing aspects, of drug reward.76 These effects likely depend on orexin neuron projections to the VTA and to other brain structures involved in motivation and reward, such as the nucleus accumbens and the paraventricular nucleus of the thalamus.17,75,76 A significant shortcoming in the present literature is the greater focus on OX1R and a relative paucity of studies of OX2R signaling for addiction.75 Nevertheless, the available evidence indicates that the stimulation effects of orexins on reward and addiction are mediated by both OX1R and OX2R.75,76 The susceptibility of people with NT1 to drug addiction is still debated.76,78 However, the relevance of orexin signaling to opiate addiction is dramatically revealed by the striking increase in orexin neurons detected at immunohistochemistry with chronic opiate administration in human heroin addicts and in mouse models.77 The mechanisms underlying this increase in neuron number are incompletely understood and may concern a population of orexin neurons, whose orexin synthesis is so limited at baseline to remain below the immunohistochemical detection threshold, but may be boosted and brought above detection threshold by chronic opiate administration. Translationally, this finding has potentially wide implications not only for addiction to drugs and its interaction with insomnia, but also for the therapy of NT1.
Substantial evidence links orexin signaling with the acute stress response, the body’s physiological reaction to threats and unpredictable occurrences that increases arousal and helps coping and survival.79 In rats, intracerebroventricular orexin administration increases circulating adrenocorticotropic hormone (ACTH) levels, indicating activation of the hypothalamo-pituitary-adrenocortical axis,80 whereas removal of circulating glucocorticoids with adrenalectomy decreases orexin mRNA levels, with rescue by peripheral glucocorticoid treatment.81 There is also evidence that orexin signaling is required for the full manifestation of autonomic and respiratory correlates and stress-induced analgesia.82 On the other hand, the effects of chronic stress on the orexin system are variable.79 To reconcile the roles of orexins in stress and reward, it has been proposed that orexin neurons coactivate motivation and aversion to orchestrate stress-countering responses, with orexin neuron stimulation being perceived as rewarding or aversive depending on whether baseline orexin neuron activity is low or high, respectively.83
Figure 1 shows, schematically, the neurochemistry and neuroanatomy of orexin neuron signaling.
Insomnia
Epidemiology, Diagnosis, and Clinical Spectrum
A diagnosis of insomnia disorder does not simply imply sleep disturbance. According to the International Classification of Sleep Disorders 3rd edition criteria,84 chronic insomnia is characterized by difficulty in falling asleep or in maintaining sleep or by early awakening, with a negative impact on daily performance, which can manifest itself with fatigue, EDS, difficulty concentrating and paying attention. Nocturnal symptoms of sleep disturbance are present for at least 3 times a week and a duration of at least 3 months, not attributable to other sleep disorders or medical or pharmacological conditions. Insomnia at all ages can present variable phenotype characteristics. In particular, in early childhood, insomnia can present with motor restlessness, without difficulty falling asleep but with long-lasting morning awakenings, or with multiple nocturnal awakenings and difficulty falling asleep.85
Insomnia has a high prevalence in the general population, from 6% to 33%,86,87 with increased risk of comorbidity and health care costs.88 Its prevalence varies among the studies, depending on the definition used and the setting. For example, it is a very frequently encountered disorder in general practice, in which about 40% of patients report significant sleep disturbances.89 In adults, the prevalence is higher among women than among men,89 and is often related to psychiatric disorders90 or comorbidity with other motor or respiratory sleep disorders. In particular, an association has emerged between insomnia and sleep apnea syndrome (COMISA), with an estimated prevalence between 40% and 60%.91 The need to identify different disease phenotypes has been suggested for a personalized therapeutic approach.4,87 Insomnia is not a homogeneous disorder and its clinical characteristics can vary over time and within the same patient.
The family history of insomnia is an important factor to consider in the medical history because it can predispose to the onset of the disorder.92 A genetic predisposition to insomnia is recognized, with the involvement of several genes and the presence of pleiotropism. There is an overlap with the genetic factors common to anxiety and depression, consistent with links between insomnia, stress, and emotions.4 Higher arousability predisposition, poorer self-rated general health, and higher bodily pain have also been found as predisposing factors associated with a new onset of insomnia.93 Pregnancy may be associated with a significant prevalence of insomnia symptoms, particularly in the third trimester, which may not be explained by symptoms of depression.94
Current Therapeutic Options
Early diagnosis and treatment of insomnia disorder comorbidities, such as COMISA and psychiatric disorders, is key. Persistence of insomnia despite effective comorbidity treatment strengthens the rationale for treating insomnia per se. According to current guidelines, CBT-I is the first approach to consider in the management of the disorder.4,86,95 With or without pharmacotherapy, changes to lifestyle and diet are often essential.
Benzodiazepines, novel benzodiazepine receptor agonists (“Z compounds”), and sedating antidepressants are effective in the short-term treatment of insomnia (≤ 4 weeks; weak recommendation, moderate quality evidence); antihistamines, antipsychotics, melatonin and herbal medicines are not recommended for the treatment of insomnia (strong to weak recommendations, low to very low quality tests).86 Light therapy and exercise need to be further evaluated to judge their usefulness in the treatment of insomnia, and so do non-invasive brain stimulation techniques (eg, repetitive transcranial magnetic stimulation,96–98 transcranial direct current stimulation)99–101 and complementary and alternative treatments (eg homeopathy, acupuncture, acupressure).86,102 The 2017 European Sleep Research Society guidelines did not generally recommend long-term treatment of insomnia with benzodiazepines, benzodiazepine receptor agonists, or sedating antidepressants.86 On the other hand, the guidelines of the American Academy of Sleep Medicine, also published in 2017, issued weak recommendations to use a range of drugs for the treatment of chronic insomnia, including a double orexin receptor antagonist (suvorexant), benzodiazepines (triazolam, temazepam), Z compounds (eszopiclone, zaleplon, zolpidem), a melatonin receptor agonist (ramelteon), and a tricyclic antidepresSant (doxepin). Discontinuation of modern hypnotic agents, such as selective benzodiazepine receptor agonists, melatonin agonists, sleep-promoting antidepressants, or orexin receptor antagonists, does not show withdrawal symptoms similar to older types of hypnotics or rebound effects on insomnia (ie, an exacerbation of insomnia to a level worse than baseline), even after sudden interruption of treatment.86
The Role of Orexins in Disease
Narcolepsy and Other Sleep Disorders
NT1, formerly named narcolepsy with cataplexy, with low or undetectable cerebrospinal fluid (CSF) levels of orexin A, is characterized by EDS, cataplexy, sleep paralysis, hypnagogic/hypnopompic hallucinations, and disrupted nocturnal sleep.84 Narcolepsy type 2 (NT2), with normal CSF orexin-A levels, shares most clinical features with NT1, except cataplexy, but is more heterogeneous and currently less well defined.23 Post-mortem studies identified a selective loss of orexin cells in the hypothalamus in the brain of narcoleptic patients with cataplexy.17,103 Animal model studies indicate that a near-complete decrease in functional orexin neuron number and in CSF orexin-A levels is required to elicit cataplexy, whereas the extent of orexin neuron loss required to elicit other NT1 symptoms and signs is smaller but still largely undetermined.104 Although the exact pathogenic underlying process remains unclear, there is evidence that NT1 is an autoimmune disease:105 some environmental triggers, such as viral infections, in subjects with a genetic predisposition, may lead to immune activation resulting in orexin-A cell loss.23
Obstructive sleep apnea syndrome (OSAS) is a multifactorial disease with a complex pathophysiology. A role of orexins in the pathophysiology of OSAS has been hypothesized.106 Extracellular pH and CO2/H+-sensitive neurons in the brain are fundamental for regulating breathing and behavioral arousal. As previously discussed, studies have suggested that orexin neurons are highly CO2/H+-sensitive,13 and orexin neurons are also activated by CO2 in vivo.60 As a result, it is reasonable that repeated intermittent hypoxia or hypercapnia caused by OSAS might activate orexin neurons, eventually leading to arousal and sleep fragmentation.106
In restless legs syndrome (RLS), orexin-A levels were found to be increased in evening CSF samples, especially in early-onset disease,107 although a subsequent study did not confirm these results and showed no correlation with symptom severity and polysomnographic measures.108 Nevertheless, the circadian variation of orexin A may be dysregulated in early onset RLS, with elevated nocturnal levels.109
The complex interconnection between cancer, narcolepsy, and neurodegenerative diseases has recently been proposed as a possible case of orexin-dependent inverse comorbidity.110 Orexin signaling has been linked to neuroinflammation in mouse models and to cancer in cell lines.110,111 Moreover, the expression of the orexin-A receptor in various tumors, including colon, pancreas, and prostate cancers, and its ability to induce a proapoptotic activity in tumor cells,112 suggested that orexin signaling through OX1R might have a promising therapeutic role also in cancer-related sleep disorders.
Other Disorders
Headache
A role of orexins in pain conditions, such as primary headache113 and cluster headache114 has been proposed.113,115,116 This is in agreement with the fact that the hypothalamus is involved in the regulation of homeostatic mechanisms and migraine-related trigeminal nociception. Nevertheless, further work is required to better understand the underlying pathophysiological mechanisms and develop more targeted and effective therapies.117
Alcohol Withdrawal
The orexin system also plays a role in the emotional dysregulation that occurs during withdrawal from alcohol use and in alcohol-seeking behaviors. Indeed, symptoms of post-acute alcohol withdrawal syndrome typically include sleep disturbances. A recent systematic review associated these symptoms also with orexin levels, along with neuroadaptation changes in the nucleus accumbens and in the prefrontal cortex.118 Therefore, the use of orexin receptor antagonists has been proposed to treat insomnia in alcohol-dependent individuals during and after alcohol withdrawal.119
Epilepsy
Sleep disorders are common in epilepsy, and their early recognition and treatment can improve seizure frequency. Additionally, epilepsy is associated with cyclic patterns, which has led to new treatment approaches, including orexin receptor antagonists.120 Specifically, the loss of orexinergic activity has been associated with REM sleep onset,29,32 and REM sleep is generally protective against seizures. As such, a dynamic modulation of seizure threshold by orexin (through a so-called “orexi-cortical axis”) has been postulated, in which orexinergic activity regulates sleep-wake states to modify ascending subcortical influences on cortical synchronization, with subsequent effects on seizure threshold.121
Pain
Very recently, among potential therapeutic targets for the treatment of opioid abuse and pain,122 orexin-A receptor antagonists and mixed nociceptin/μ-opioid peptide partial agonists have shown promising results in animal models.123 A contribution of the orexin system has also been found in the sleep-wake and arousal dysfunctions of post-traumatic stress disorder (PTSD).124 Orexin receptors have a prominent role in the neural circuit underlying PTSD, and orexin activation of an infralimbic-amygdala circuit impedes fear extinction, while treatment with orexin receptor antagonists enhances it.125 In PTSD with sleep disturbances (eg, increased sleep latency and more transitions to wakefulness), increased orexinergic activity impairs sleep by promoting wakefulness and reducing total sleep time (TST), whereas treatment with orexin receptor antagonists improves sleep onset and maintenance.124
Neurodegenerative Disorders
Orexin is a key modulator of the sleep-wake cycle, which is commonly dysregulated in AD. Increased orexin in the CSF is associated with decreased sleep efficiency and REM sleep, as well as cognitive impairment in patients with Alzheimer’s disease (AD). The orexin system has profuse projections to brain regions that are implicated in arousal and cognition and has been found to participate in the progression of AD pathology.126 Conversely, orexin receptor antagonists are able to consolidate sleep and might even slow down the progression of AD pathology.127 Several lines of evidence suggest that the orexin system is involved also in Parkinson’s disease (PD), especially in its non-motor symptoms (mostly, sleep disorders). PD has been associated with pathological changes in the lateral hypothalamus, loss of orexin neurons, and fluctuations of orexin CSF level.128 Recently, some studies have revealed the protective actions and potential therapeutic applications of orexin receptor agonists in both cellular and animal models of PD,129 whereas orexin receptor antagonists may improve the abnormal sleep pattern observed in PD.130
The orexin system is also involved in the pathogenesis of Huntington’s disease (HD). A significant reduction of orexin neurons in the hypothalamus was observed in patients with HD, compared to controls.131,132 Interestingly, the administration of a OX1R or OX2R antagonist significantly reduced sleep/wake rhythm deficiency and efficiently improved the behavioral performance in HD mice.133 Additionally, orexin A increased the surface levels of a-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor in the dorsal striatum (the main affected brain structure in HD, in which both OX1R and OX2R have been detected134), leading to functional changes in the striatal circuits.135 This suggests that the orexin system may be involved in HD pathology by regulating the activity of striatal circuits, and that the modulation of the orexin system in striatal circuits may be a potential therapeutic target for HD.136
Sleep disturbance is a trigger of relapse in multiple sclerosis (MS),137 indicating a potential role of the orexin system.138 Given the complexity and variety of the pathological courses of MS, further investigation is necessary.139 Nevertheless, preclinical studies indicate that the orexin system may exert neuroprotection in MS by inhibiting neuroinflammation.140
Binge Eating Disorder
The orexin system has recently been proposed as a potential link between compulsive eating and sleep dysregulation in binge eating disorder (BED).141 Based on the evidence that the orexin system exhibits significant plasticity in response to drugs of abuse, it has been hypothesized that chronic palatable food consumption may likewise increase orexin system activity, eventually resulting in dysregulated sleep/wake patterns.142 Poor sleep, in turn, exacerbates BED, contributing to a vicious cycle of uncontrolled food consumption.141 Translationally, pharmacotherapies normalizing orexin signaling, which are currently being trialed for the treatment of substance use disorders, might also have utility in the management of eating disorders.
Prader-Willi Syndrome
Prader-Willi syndrome (PWS) is a complex genetic disorder with multiple cognitive, behavioral, and endocrine dysfunctions and with different sleep disorders, such as sleep-disordered breathing and central disorders of hypersomnolence, frequently occurring either isolated or in comorbidity.143 A recent meta-analysis of CSF orexin levels showed significantly lower levels in PWS compared to controls, although the levels in PWS were significantly higher than those in NT1. This may support, at least in part, the role of hypothalamic dysfunction in the metabolic, respiratory, and sleep/wake characteristics of patients with PWS.144
Clinical Trials with Orexin Receptor Antagonists for Insomnia
Rationale
The discovery of the anatomical interconnection of the orexin system with sleep/wake regulatory circuits and with wider motivation and reward circuits has played a crucial role, over the past decade, in the development of antagonists of the orexin receptors for the treatment of insomnia.76 As previously discussed, the orexin system seems to be a promising therapeutic target given its regulatory action on sleep, on the mechanisms of drug addiction, and on the regulation of stress and emotions, energy balance and the arousal system.145 Drug addiction might also lead to an increase in the production of orexins, with a feedback mechanism or “vicious cycle” that eventually leads to a worsening of insomnia.
The findings on the orexin system and the mechanisms of action of DORAs and SORAs in the last decade have triggered multiple trials on these innovative drugs, with the aim to provide further therapeutic options for insomnia short-term and long-term treatment with fewer side effects than other hypnotic drugs (hangover, dependence and tolerance, rebound insomnia, muscle atonia, inhibition of the respiratory system, cognitive dysfunctions, and increased anxiety).6 Studies conducted on the assessment of sleep architecture in patients treated with DORAs and SORAs have shown that DORAs increase the total sleep time mainly by promoting REM sleep, without affecting, or even decreasing, non-REM sleep.7 Conversely, there are still too few studies on SORAs to be able to draw definite conclusions on them.7 Regarding their efficacy and safety profile, DORAs showed an excellent profile, although there are only a few systematic comparisons among these drugs.146
Suvorexant
Suvorexant belongs to the DORA class.147 It was the first of this class of drugs to be approved in 2014148 for the treatment of insomnia due to difficulty in initiating and/or maintaining sleep149 in the USA and Japan.95,150
A systematic review published in 2017 suggested that suvorexant was associated with significant improvements in subjective time to sleep onset, subjective TST, and subjective quality of sleep at 1 month and 3 months in patients with primary insomnia, with somnolence, fatigue, and abnormal dreams as the most common adverse effects.151 According to a recent suvorexant trial in both young and older patients with insomnia, dosing for three months of 20/15 mg and 40/30 mg improved sleep to a greater extent than placebo, as shown by the Insomnia Severity Index.152 A good therapeutic efficacy was also demonstrated with objective sleep measures, such as polysomnography (PSG)-derived indexes, with improvement of sleep latency, sleep efficiency, and sleep maintenance, both after the first night of administration and after 4 weeks, with a dose dependent effect,153,154 in a 50-to-100 mg study.155 Relative to placebo, suvorexant was found to decrease substantially the time spent in long wake bouts (> 2 minutes) and to increase slightly the time spent in short wake bouts (≤ 2 minutes) in patients with insomnia.154 This fits well with preclinical evidence that in mice, progressive loss of the orexin neurons dramatically reduced the ability to maintain long wake bouts, but it also favored brief wake bouts, increasing the risk of awakening at sleep onset and reducing the likelihood of falling back asleep.156
An EEG spectral analysis study showed that suvorexant, at clinically effective doses, had limited effects on EEG power spectral density compared to placebo in healthy subjects and patients with insomnia, suggesting that suvorexant induces both subjective and objective sleep quality improvement, without major changes in cortico-thalamic neurophysiology.157 A different work showed that the EEG power spectral density profile after suvorexant administration in healthy subjects is closer to the physiological profile after placebo than to that obtained with zolpidem treatment.158 Suvorexant may improve sleep while preserving the ability to awaken to auditory stimuli during the night.159
A neuroimaging study by magnetic resonance spectroscopy was recently conducted to evaluate the relationship between functional changes in the VTA, which also plays an important role in regulating the sleep-wake cycle,160 and suvorexant treatment. The authors concluded that the changes in choline/creatine and phosphorylcreatine ratios in VTA might be used to distinguish suvorexant responders from non-responders before starting treatment, allowing a more appropriate selection of patients in clinical practice.161
Since suvorexant is primarily metabolized by cytochrome P450 3A (CYP3A) and its pharmacokinetics may be affected by CYP3A modulators, caution should be used when administering CYP3A activators or inhibitors.162 Several studies have shown that suvorexant is well tolerated and effective in both men and women.163,164 Suvorexant may be an excellent therapeutic option in menopause, a period in which insomnia, especially associated with vasomotor symptoms, is frequent.165,166 Suvorexant 20 mg also improved sleep time in women with fibromyalgia, reducing next-day pain sensitivity.167
Suvorexant has also been evaluated in older people showing good efficacy, also in subjects with probable Alzheimer’s disease dementia with insomnia,168 and fair tolerance profile with few side effects, among which the most common was somnolence,169 and with unremarkable residual effects.170 On the other hand, although a study on younger healthy volunteers found no clinically meaningful residual effect of 20 mg or 40 mg suvorexant on next-morning driving, some individuals did experience next-day effects.171 Suvorexant was evaluated also in adolescence, with the occurrence of abnormal dreams being the most commonly reported adverse event.172 However, recent evidence suggests that initiation of suvorexant treatment after switching from other insomnia medications requires careful monitoring for insomnia-related adverse reactions, which could be due to the abrupt discontinuation of previous drugs.173 Although it has been shown that this agent is safe and well tolerated for up to one year, a sudden discontinuation of the treatment is associated with an increased likelihood of symptom return.174
Suvorexant improved sleep quality and obesity-associated parameters, including glycemic control, in patients with type 2 diabetes,175,176 which fits well with preclinical data obtained with suvorexant on db/db diabetic mice.177 In treated patients with hypertension and insomnia, suvorexant 20 mg had no overall effect on daytime, nighttime, or morning blood pressure.178 In light of the well-known association that often occurs between insomnia and sleep apnea (COMISA),91 it is very interesting to note that suvorexant does not have clinically important respiratory effects during sleep at doses above the maximum recommended dose for the treatment of insomnia in the USA and Japan, corresponding to 20 mg.179 Similar results were obtained in patients with OSAS of mild-to-moderate degree180 or with chronic obstructive pulmonary disorders,181 at a dosage of 40 mg.
Lastly, suvorexant was recently found to ameliorate sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper, suggesting that it might be a promising treatment for sleep and opioid withdrawal.182 On the other hand, drug abuse, which encompasses the use of prescription drugs for purposes other than those for which they are meant to be used or in excessive amounts (www.cancer.gov, last accessed on December 4, 2022), may be an issue with suvorexant. In particular, a study in healthy recreational polydrug users revealed abuse potential for suvorexant, although the overall abuse liability of suvorexant was similar to, and possibly lower than, that of zolpidem.183
Lemborexant
Lemborexant184 is a DORA approved in December 2019 in the USA and in January 2020 also in Japan for the treatment of adult insomnia characterized by difficulties in falling asleep or maintaining sleep.185 Lemborexant represents a promising therapeutic option for the treatment of insomnia,186 although better efficacy than suvorexant or other hypnotics has not been demonstrated.187
Studies on PSG parameters have shown an improvement in sleep efficiency and latency, as well as a reduction in wakefulness after sleep onset (WASO), even in the second part of the night, with a minimal tolerance at 5–10 mg, both after one month of treatment188 and in the long term after one year of therapy.189 No rebound effect was observed in case of discontinuous intake or discontinuation of treatment, and adverse effects such as headache and drowsiness and upper respiratory airway infections were rare.190–193 A good safety profile of lemborexant has also been demonstrated on respiratory parameters, such as the apnea-hypopnea index and the nocturnal oxygen saturation,194 and on cardiac parameters, such as the QT interval.195 Several trials with lemborexant have also shown little or no impairment of daily activities, such as cognitive performance, driving vehicles, and subjective sleepiness.196 Overall, it has been suggested that lemborexant at doses up to 25 mg provides a pharmacokinetic, pharmacodynamic, and overall safety profile suitable for both obtaining the target pharmacological effect on insomnia and minimizing the residual effects during wakefulness.197,198 Lemborexant has been found effective also in older adults on insomnia disorder188 and in subjects with Alzheimer’s disease dementia.199 On the other hand, a study on healthy nondependent recreational sedative users revealed that lemborexant has abuse potential relative to placebo to an extent similar to that of suvorexant and zolpidem.200
Daridorexant
Daridorexant201 is a DORA that was approved in March 2022 in the USA for the treatment of insomnia in adults due to difficulty in falling asleep or maintaining sleep. The expected effect duration is approximately 8 hours, at a dose of 25 mg, with a half-life intended to minimize residual effects that could impair daytime functioning. Daridorexant is mainly metabolized by CYP3A4 and excreted mostly via feces (~57%) and urine (~28%).202 Daridorexant can be safely co-administered with citalopram, a widely used antidepressant medication203 and in patients with liver cirrhosis.204
Potential central nervous system (CNS) depressive effects and diurnal impairment have been described, especially with concomitant intake of other CNS depressant drugs, including ethanol.205 These effects include depression or suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, symptoms similar to cataplexy, and sleepwalking. The effects on respiratory function are also worthy of investigation.202 In this respect, daridorexant was found not to impair nighttime respiratory function in patients with moderate chronic obstructive pulmonary disease206 or with mild or moderate OSAS.207 Moreover, daridorexant does not alter cardiac indexes, such as the QT interval.208
Some studies have also evaluated objective PSG parameters with daridorexant. One study has shown that at a dosage of 5, 10, 25, or 50 mg for a month, it induces a reduction in the time spent awake, with minimal tolerance.209 Another trial evaluated the effects at three months, showing that daridorexant 25 mg and 50 mg improved sleep parameters (ie, sleep latency and WASO) and, even after a single month of treatment at a dosage of 50 mg, also subjective daytime performance, with a favorable safety profile.210 Daridorexant was well tolerated and effective also in elderly subjects with insomnia disorder.211 However, daridorexant showed potential for drug abuse, with dose-related drug-liking among recreational sedative drug users with lower effects at the highest phase-3 dose, and similar effects at higher doses compared to supratherapeutic doses of suvorexant and zolpidem.212
Other Trials on DORAs and SORAs
Preclinical results have been published for other DORAs, such as ORN0829213 and YNT-185.214 The DORA TS-142, at the dosage of 5, 10, and 30 mg, administered in a single dose, demonstrated an objective improvement of PSG and clinical parameters, as well as minimal tolerance.215 Among SORAs antagonizing OX2R, JNJ-48816274, at a dosage of 20 and 50 mg, was associated with increases in TST, REM sleep duration, and sleep efficiency, with a reduction in sleep latency, and with improvement in the perceived sleep quality. Conversely, the spectral power density of the EEG in non-REM sleep and REM sleep was not affected by either dosage.216 The OX2R SORA JNJ-42847922 (seltorexant) has been shown to exert clinically meaningful reductions in depressive symptoms217 and to improve sleep218 in patients with major depressive disorder, with improved sleep efficiency, TST, and latency to persistent sleep. However, seltorexant did not change sleep architecture and particularly did not increase the percentage of time spent in N3,218 which is commonly reduced in these patients.90 This may represent an unmet therapeutic issue with seltorexant and possibly other SORAs in patients with major depression. The OX2R-selective SORA danavorexton (TAK-925) was recently shown to elicit marked improvements in sleep latency in individuals with NT1 or with NT2 after intravenous administration.47 Other OX2R-selective SORAs have been subjected to preclinical development.214,219,220 Recently, OX1R-selective SORAs221–225 have been developed, but their effects on sleep are minor225 or still unclear.221–224
A recent review compared the effects of orexin receptor antagonists to those of benzodiazepines and Z-drugs, in the short and long term.226 The authors concluded that zopiclone caused more dropouts than eszopiclone, daridorexant, and suvorexant; moreover, benzodiazepines, eszopiclone, zolpidem and zopiclone caused more side effects than placebo, doxepin, seltorexant and zaleplon. For long-term treatment, eszopiclone and lemborexant were more effective than placebo, while lemborexant and eszopiclone were more effective than ramelteon and zolpidem.226 Overall, this review did not clarify the benefits of only targeting OX2R with SORAs compared with targeting both OX2R and OX1R with DORAs. This critical question thus awaits clarification.
Table 1 summarizes the profiles of the main orexin receptor antagonists discussed in this review.
Table 1.
Brief Profiles of the Main Orexin Receptor Antagonists Reported Above
| Drug | Type | Half-Life, Hours | Side Effects* | Approved | WASO | TST | Sleep Latency |
|---|---|---|---|---|---|---|---|
| Suvorexant | DORA | 12 | Somnolence, headache | USA, Japan | ↓ | ↑ | ↓ |
| Lemborexant | DORA | 17–19 | Somnolence, fatigue, headache, abnormal dreams | USA, Canada, Australia, Japan | ↓ | ↑ | ↓ |
| Daridorexant | DORA | 8 | Headache, somnolence, fatigue | USA, EU | ↓ | ↑ | ↓ |
| Vornorexant (TS-142) | DORA | 1.3–3.3 | Under development/ evaluation | ↓ | ↑ | ↓ | |
| JNJ-48816274 | OX2R SORA | 1 | Somnolence, abnormal dreams | Under development/ evaluation | ↓ | ↑ | ↓ |
| Seltorexant (JNJ-42847922) | OX2R SORA | 2–3 | Somnolence, fatigue, dizziness, headache, abdominal discomfort, and nightmares | Under development/ evaluation | ↓ | ↑ | ↓ |
Note: *Possible narcoleptic-like symptoms, potentially for all agents, at high doses.
Abbreviations: SORA, selective orexin receptor antagonist; DORA, dual orexin receptor antagonist; WASO, wakefulness after sleep onset; TST, total sleep time; OX2R, orexin receptor 2; ↓, decreased; ↑, increased.
Discussion
Insomnia has a high prevalence in the general population and is an important cause of disability, very often associated with other medical diseases.87,88 It is a chronic disease which, therefore, frequently requires long-lasting CBT-I and drug therapy, often with occurrence of habituation and side effects, especially when the therapeutic approaches and the drug dosage used are not optimal. In this scenario, in the last decade, many trials have been conducted with orexin receptor antagonists, which represent an innovative and valid therapeutic option based on the multiple mechanisms of action of orexins on different biological circuits, both centrally and peripherally, and on their role in a wide range of medical conditions often associated with insomnia. A very interesting aspect of this new category of drugs is that they have limited abuse liability and their discontinuation may not be associated with significant rebound effects.4
Orexin receptor antagonists currently marketed in various countries, mainly USA and Japan, are DORAs with action on OXR1 and OXR2, while SORAs with a selective action on OXR2 are under development. DORAs have shown good efficacy and tolerance profile, even in the long term. Most of the studies have been conducted on suvorexant, also with objective methods (PSG), and on large series of patients, showing a remarkable efficacy of this drug in the treatment of insomnia. This effect was seen in both sexes, and suvorexant is currently the only DORA for which the efficacy has also been evaluated in menopause,166 in older people,169 and in adolescence.172 Another very important property of suvorexant is the apparent absence of side effects on respiratory parameters during sleep.179–181 Finally, suvorexant is the only DORA for which neuroimaging has been performed, reporting changes in glial function in VTA, which might be used to distinguish suvorexant responders from non-responders before starting the treatment, thus allowing a more appropriate selection of patients in clinical practice.161
Studies on lemborexant are less numerous than those on suvorexant. Although lemborexant represents a promising therapeutic option for the treatment of insomnia, better efficacy than that of suvorexant or other hypnotics has not been conclusively demonstrated.187 Nevertheless, an improvement in both subjective and objective (PSG) sleep parameters has been reported also for lemborexant, with a good tolerance profile even in long-term treatment, absence of rebound effects in case of suspension,190–193 and lack of changes of sleep cardio-respiratory parameters.194,195
Finally, there are even fewer studies on daridorexant, which agree in demonstrating good efficacy on both subjective and objective sleep parameters. However, further studies are needed to evaluate the impact of daridorexant on cardiorespiratory parameters. Potential CNS depressant effects of daridorexant have also been described, as well as diurnal impairment, especially when associated with other CNS depressant drugs. Therefore, these aspects should also be further investigated.202
Taken together, the multiple biological roles of orexins indicate that orexin receptor antagonists can exert effects on different functions and ameliorate or worsen comorbid pathologies. Insomnia is often associated with other medical conditions and shows a considerable individual variability4 in terms of clinical characteristics, sleep architecture, and comorbidities.33,227,228 The orexin receptor antagonists have also been shown to afford an improvement in PSG parameters, sleepiness, and sleep quality, with differences related to the patient characteristics: some DORAs seem to have a good efficacy in the older subject or adolescent population169,172 or in postmenopausal women,166 whereas SORAs have been found effective in psychiatric patients.218,229,230 Therefore, these drugs offer interesting perspectives for a targeted and personalized approach.
As mentioned before, a close association of insomnia with OSAS has recently emerged (COMISA),91 even in children.231 The use of orexin receptor antagonists provides interesting implications for the treatment of this clinical association, also because of lack of effects on respiratory parameters, as reported with some DORAs, although further clinical trials are desirable to evaluate this aspect systematically. Furthermore, it would be interesting to evaluate the possible effects of DORAs on somnolence in patients with COMISA.
There is a close interconnection between insomnia and headache.232 In light of the recently proposed role of orexins in pain modulation,113 DORAs might be very useful in the treatment of patients with insomnia and headache, who may be prone to drug abuse or abrupt suspension of drug therapies or who may require drug detoxification. The same goes for psychiatric patients suffering from insomnia, in whom the abuse of drug therapies for insomnia and the consequent need for detoxification sometimes is a crucial problem in clinical practice. The use of SORAs seems to be promising in this type of patients.218,229,230
Preserving good sleep quality and adequate sleep architecture is very important also in patients with epilepsy,120 as this may ensure a good control of epileptic seizures as well. Thus, the use of drugs such as orexin receptor antagonists can be helpful in these patients, also considering the role of orexins in the control of REM sleep, which is relevant to patients with epilepsy.121
Another intriguing aspect to consider is the evaluation of the use of DORAs and SORAs in the treatment of insomnia in patients with neurodegenerative diseases, in whom alterations in circadian rhythm and alertness are often associated and sometimes difficult to manage with traditional drugs because of patient age, cardio-pulmonary comorbidities and drug interactions. Orexins seem to affect also neurodegeneration,111 and most DORAs have shown good efficacy and tolerance profiles in these patients, mostly older subjects, along with the absence of QT interval alterations.169,195,208
Lastly, insomnia is very common in patients with cancer, including children.233,234 As expected, the treatment of comorbid insomnia in patients with cancer is often very difficult, due to concomitant depressive complaints, risk of interactions due to polytherapy, radio- and chemotherapy, and disease complications and/or comorbidities. In this complex scenario, DORAs and SORAs have shown a good tolerance profile and few side effects, even at low doses, with limited drug interactions. Therefore, they could represent an innovative and promising therapeutic option in these patients, also based on evidence that orexin signaling may be of therapeutic relevance for some types of cancer.111
Box 1 * summarizes recommendations for the clinical practice based on the findings discussed above.
Box 1.
Clinical Practice Recommendations
|
|---|
|
|
|
|
| CBT-I: Cognitive and Behavioral Therapy for Insomnia. DORA: dual orexin receptor antagonist. COMISA: comorbid insomnia and sleep apnea. |
Future Perspectives and Recommendations
Insomnia is not a homogeneous disorder, and its clinical characteristics can vary over time and within the same patient. There is an ongoing discussion about different insomnia phenotypes, considering for example insomnia with vs without objective short sleep or sleep-onset vs sleep-maintenance insomnia. However, robustly subtyping insomnia disorder remains challenging at present.5 In perspective, insomnia subtyping, together with careful evaluation of insomnia comorbidity, may be key to therapeutic success with a personalized precision medicine approach and to prediction of treatment responses with single or sequential treatment approaches.
Further studies on the efficacy of DORAs, and particularly of newer molecules such as lemborexant and daridorexant, are required, especially on children and adolescents and in particular conditions, such as menopause. The effects of daridorexant on sleep cardiorespiratory parameters also need to be more precisely assessed. Finally, which DORAs are most suitable for each patient based on comorbidities and/or concomitant treatments should be the focus of further careful analyses, in order to use this class of drugs in accordance with the principles of personalized and precision medicine.
On the other hand, studies on SORAs are still at an early stage and, therefore, do not allow to draw definite conclusions. Data on seltorexant suggest that OX2R-selective SORAs may be appropriate also in patients with major depression,218 although limited effect on slow-wave sleep may be a therapeutic issue in these patients. In perspective, it is important to focus attention on the role of DORAs and SORAs in patients with psychiatric comorbidity and especially in patients with depression. The potential application of these drugs to treat insomnia during pregnancy also deserves investigation. More generally, the question of the benefits of only targeting OX2R with SORAs compared with targeting both OX2R and OX1R with DORAs is a critical one that awaits clarification.
Box 2 * reports a series of topics and suggestions for future research aimed at clarifying some of the most important areas that need further insight and emerged in the analysis of the literature reported in this review.
Box 2.
Future Research Agenda
|
|---|
|
|
|
|
|
|
| DORA and SORA: dual and selective orexin receptor antagonists, respectively. OX1R and OX2R: orexin receptor 1 and 2, respectively. |
Acknowledgments
This was not an industry-supported study. This study was partially supported by a fund from the Italian Ministry of Health “Ricerca Corrente” (RC n. 2773803) (G.L., R.F.). Maria P Mogavero and Alessandro Silvani are co-first authors for this study.
Disclosure
Prof. Luigi Ferini-Strambi reports personal fees for lectures and advisory board work from Idorsia. Prof. Alessandro Silvani is an inventor in Italian patent application n. 102022000013894 related to a novel dual orexin receptor agonist. The other authors report no potential conflicts of interest in this work.
References
- 1.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 2.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel JM. Narcolepsy: a key role for hypocretins (orexins). Cell. 1999;98(4):409–412. doi: 10.1016/S0092-8674(00)81969-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferini-Strambi L, Auer R, Bjorvatn B, et al. Insomnia disorder: clinical and research challenges for the 21st century. Eur j Neurol. 2021;28(7):2156–2167. doi: 10.1111/ene.14784 [DOI] [PubMed] [Google Scholar]
- 5.Riemann D, Benz F, Dressle RJ, et al. Insomnia disorder: state of the science and challenges for the future. J Sleep Res. 2022;31(4):e13604. doi: 10.1111/jsr.13604 [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Chanana P, Choudhary S. Emerging role of orexin antagonists in insomnia therapeutics: an update on SORAs and DORAs. Pharmacol Rep. 2016;68(2):231–242. doi: 10.1016/j.pharep.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 7.Clark JW, Brian ML, Drummond SPA, Hoyer D, Jacobson LH. Effects of orexin receptor antagonism on human sleep architecture: a systematic review. Sleep Med Rev. 2020;53:101332. doi: 10.1016/j.smrv.2020.101332 [DOI] [PubMed] [Google Scholar]
- 8.Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64(3):389–420. doi: 10.1124/pr.111.005546 [DOI] [PubMed] [Google Scholar]
- 9.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains 124. Nat.Med. 2000;6(9):991–997. doi: 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 10.Mickelsen LE, Kolling F, Chimileski BR, et al. Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis. eNeuro. 2017;4(5). doi: 10.1523/ENEURO.0013-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schone C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32(36):12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viskaitis P, Arnold M, Garau C, et al. Ingested non-essential amino acids recruit brain orexin cells to suppress eating in mice. Curr Biol. 2022;32(8):1812–1821 e1814. doi: 10.1016/j.cub.2022.02.067 [DOI] [PubMed] [Google Scholar]
- 13.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104(25):10685–10690. doi: 10.1073/pnas.0702676104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrigoni E, Chee MJS, Fuller PM. To eat or to sleep: that is a lateral hypothalamic question. Neuropharmacology. 2019;154:34–49. doi: 10.1016/j.neuropharm.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Nagata R, Yamanaka A, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain 172. J Comp Neurol. 2006;494(5):845–861. doi: 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastianini S, Silvani A. Clinical implications of basic research: the role of hypocretin/orexin neurons in the central autonomic network. Clin Translational Neurosci. 2018;2(2):2514183X18789327. doi: 10.1177/2514183X18789327 [DOI] [Google Scholar]
- 19.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- 20.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/S0092-8674(00)81973-X [DOI] [PubMed] [Google Scholar]
- 21.Bastianini S, Silvani A, Berteotti C, et al. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep. 2011;34(2):213–218. doi: 10.1093/sleep/34.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mignot EJ. History of narcolepsy at Stanford University. Immunol Res. 2014;58(2–3):315–339. doi: 10.1007/s12026-014-8513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barateau L, Pizza F, Plazzi G, Dauvilliers Y. Narcolepsy. J Sleep Res. 2022;31(4):e13631. doi: 10.1111/jsr.13631 [DOI] [PubMed] [Google Scholar]
- 24.Vandi S, Rodolfi S, Pizza F, et al. Cardiovascular autonomic dysfunction, altered sleep architecture, and muscle overactivity during nocturnal sleep in pediatric patients with narcolepsy type 1. Sleep. 2019;42(12). doi: 10.1093/sleep/zsz169 [DOI] [PubMed] [Google Scholar]
- 25.Silvani A, Ferri R, Lo Martire V, et al. Muscle Activity During Sleep in Human Subjects, Rats, and Mice: towards Translational Models of REM Sleep Without Atonia. Sleep. 2017;40(4). doi: 10.1093/sleep/zsx029 [DOI] [PubMed] [Google Scholar]
- 26.Willie JT, Takahira H, Shibahara M, et al. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during REM sleep in mice. J Mol Neurosci. 2011;43(2):155–161. doi: 10.1007/s12031-010-9437-7 [DOI] [PubMed] [Google Scholar]
- 27.Li SB, Damonte VM, Chen C, et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science. 2022;375(6583):eabh3021. doi: 10.1126/science.abh3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassalli A, Franken P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc Natl Acad Sci U S A. 2017;114(27):E5464–E5473. doi: 10.1073/pnas.1700983114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. doi: 10.1016/j.neuron.2005.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–870. doi: 10.1016/j.neuroscience.2008.02.058 [DOI] [PubMed] [Google Scholar]
- 31.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffet L, Kosar S, Panniello M, et al. A genetically encoded sensor for in vivo imaging of orexin neuropeptides. Nat Methods. 2022;19(2):231–241. doi: 10.1038/s41592-021-01390-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22(13):5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14(7):1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x [DOI] [PubMed] [Google Scholar]
- 35.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007 [DOI] [PubMed] [Google Scholar]
- 36.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- 37.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valencia Garcia S, Libourel PA, Lazarus M, Grassi D, Luppi PH, Fort P. Genetic inactivation of glutamate neurons in the rat sublaterodorsal tegmental nucleus recapitulates REM sleep behaviour disorder. Brain. 2017;140(2):414–428. doi: 10.1093/brain/aww310 [DOI] [PubMed] [Google Scholar]
- 39.Saito YC, Maejima T, Nishitani M, et al. Monoamines Inhibit GABAergic Neurons in Ventrolateral Preoptic Area That Make Direct Synaptic Connections to Hypothalamic Arousal Neurons. J Neurosci. 2018;38(28):6366–6378. doi: 10.1523/JNEUROSCI.2835-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Luca R, Nardone S, Grace KP, et al. Orexin neurons inhibit sleep to promote arousal. Nat Commun. 2022;13(1):4163. doi: 10.1038/s41467-022-31591-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109(39):E2635–2644. doi: 10.1073/pnas.1202526109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124(2):604–616. doi: 10.1172/JCI71017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang ZL, Qu WM, Li WD, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98(17):9965–9970. doi: 10.1073/pnas.181330998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31(17):6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38(5):715–730. doi: 10.1016/S0896-6273(03)00330-1 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Nagumo Y, Ishikawa Y, et al. OX2R-selective orexin agonism is sufficient to ameliorate cataplexy and sleep/wake fragmentation without inducing drug-seeking behavior in mouse model of narcolepsy. PLoS One. 2022;17(7):e0271901. doi: 10.1371/journal.pone.0271901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans R, Kimura H, Alexander R, et al. Orexin 2 receptor-selective agonist danavorexton improves narcolepsy phenotype in a mouse model and in human patients. Proc Natl Acad Sci U S A. 2022;119(35):e2207531119. doi: 10.1073/pnas.2207531119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- 49.Grimaldi D, Silvani A, Benarroch EE, Cortelli P. Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology. 2014;82(3):271–278. doi: 10.1212/WNL.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 50.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277(6):R1780–1785. doi: 10.1152/ajpregu.1999.277.6.R1780 [DOI] [PubMed] [Google Scholar]
- 51.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R382–387. doi: 10.1152/ajpregu.00496.2006 [DOI] [PubMed] [Google Scholar]
- 52.Zhou JJ, Ma HJ, Shao J, et al. Downregulation of Orexin Receptor in Hypothalamic Paraventricular Nucleus Decreases Blood Pressure in Obese Zucker Rats. J Am Heart Assoc. 2019;8(13):e011434. doi: 10.1161/JAHA.118.011434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334(2):522–529. doi: 10.1124/jpet.110.167791 [DOI] [PubMed] [Google Scholar]
- 54.Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165(7):2292–2303. doi: 10.1111/j.1476-5381.2011.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YH, Dai YW, Huang SC, Li TL, Hwang LL. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol. 2013;98(7):1145–1155. doi: 10.1113/expphysiol.2013.072298 [DOI] [PubMed] [Google Scholar]
- 56.Grimaldi D, Calandra-Buonaura G, Provini F, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35(4):519–528. doi: 10.5665/sleep.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berteotti C, Silvani A. The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clin Auton Res. 2018;28(6):545–555. doi: 10.1007/s10286-017-0473-z [DOI] [PubMed] [Google Scholar]
- 58.Alvente S, Berteotti C, Bastianini S, et al. Autonomic mechanisms of blood pressure alterations during sleep in orexin/hypocretin-deficient narcoleptic mice. Sleep. 2021;44(7). doi: 10.1093/sleep/zsab022 [DOI] [PubMed] [Google Scholar]
- 59.McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387. doi: 10.1038/s41586-019-0948-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166(3):184–186. doi: 10.1016/j.resp.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 61.Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103(5):1772–1779. doi: 10.1152/japplphysiol.00075.2007 [DOI] [PubMed] [Google Scholar]
- 62.Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587(Pt 9):2059–2067. doi: 10.1113/jphysiol.2008.168260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias MB, Li A, Nattie E. The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol. 2010;170(1):96–102. doi: 10.1016/j.resp.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spinieli RL, Ben Musa R, Cornelius-Green J, Hasser EM, Cummings KJ. Orexin facilitates the ventilatory and behavioral responses of rats to hypoxia. Am J Physiol Regul Integr Comp Physiol. 2022;322(6):R581–R596. doi: 10.1152/ajpregu.00334.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102(1):241–248. doi: 10.1152/japplphysiol.00679.2006 [DOI] [PubMed] [Google Scholar]
- 66.Han F, Mignot E, Wei YC, et al. Ventilatory chemoresponsiveness, narcolepsy-cataplexy and human leukocyte antigen DQB1*0602 status. Eur Respir J. 2010;36(3):577–583. doi: 10.1183/09031936.00174609 [DOI] [PubMed] [Google Scholar]
- 67.Berteotti C, Lo Martire V, Alvente S, et al. Effect of ambient temperature on sleep breathing phenotype in mice: the role of orexins. J Exp Biol. 2020;223(Pt 13). doi: 10.1242/jeb.219485 [DOI] [PubMed] [Google Scholar]
- 68.Sansa G, Iranzo A, Santamaria J. Obstructive sleep apnea in narcolepsy. Sleep Med. 2010;11(1):93–95. doi: 10.1016/j.sleep.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 69.Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380(3):239–242. doi: 10.1016/j.neulet.2005.01.046 [DOI] [PubMed] [Google Scholar]
- 70.Bastianini S, Silvani A, Berteotti C, et al. Histamine Transmission Modulates the Phenotype of Murine Narcolepsy Caused by Orexin Neuron Deficiency. PLoS One. 2015;10(10):e0140520. doi: 10.1371/journal.pone.0140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Funato H, Tsai AL, Willie JT, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kakizaki M, Tsuneoka Y, Takase K, et al. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience. 2019;20:1–13. doi: 10.1016/j.isci.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kourosh-Arami M, Gholami M, Alavi-Kakhki SS, Komaki A. Neural correlates and potential targets for the contribution of orexin to addiction in cortical and subcortical areas. Neuropeptides. 2022;95:102259. doi: 10.1016/j.npep.2022.102259 [DOI] [PubMed] [Google Scholar]
- 74.McGregor R, Thannickal TC, Siegel JM. Pleasure, addiction, and hypocretin (orexin). Handb Clin Neurol. 2021;180:359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hopf FW. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology. 2020;168:108013. doi: 10.1016/j.neuropharm.2020.108013 [DOI] [PubMed] [Google Scholar]
- 76.James MH, Mahler SV, Moorman DE, Aston-Jones G. A Decade of Orexin/Hypocretin and Addiction: where Are We Now? Curr Top Behav Neurosci. 2017;33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thannickal TC, John J, Shan L, et al. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med. 2018;10(447). doi: 10.1126/scitranslmed.aao4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barateau L, Jaussent I, Lopez R, et al. Smoking, Alcohol, Drug Use, Abuse and Dependence in Narcolepsy and Idiopathic Hypersomnia: a Case-Control Study. Sleep. 2016;39(3):573–580. doi: 10.5665/sleep.5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sargin D. The role of the orexin system in stress response. Neuropharmacology. 2019;154:68–78. doi: 10.1016/j.neuropharm.2018.09.034 [DOI] [PubMed] [Google Scholar]
- 80.Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104(1–3):97–103. doi: 10.1016/S0167-0115(01)00353-6 [DOI] [PubMed] [Google Scholar]
- 81.Stricker-Krongrad A, Beck B. Modulation of hypothalamic hypocretin/orexin mRNA expression by glucocorticoids. Biochem Biophys Res Commun. 2002;296(1):129–133. doi: 10.1016/S0006-291X(02)00848-3 [DOI] [PubMed] [Google Scholar]
- 82.Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161(1–2):20–27. doi: 10.1016/j.autneu.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 83.Peleg-Raibstein D, Burdakov D. Do orexin/hypocretin neurons signal stress or reward? Peptides. Nov. 2021;145:170629. [DOI] [PubMed] [Google Scholar]
- 84.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 85.Bruni O, DelRosso LM, Mogavero MP, Angriman M, Ferri R. Chronic insomnia of early childhood: phenotypes and pathophysiology. Neurosci Biobehav Rev. 2022;137:104653. doi: 10.1016/j.neubiorev.2022.104653 [DOI] [PubMed] [Google Scholar]
- 86.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 87.Benjamins JS, Migliorati F, Dekker K, et al. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med Rev. 2017;36:71–81. doi: 10.1016/j.smrv.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 88.Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020;26(4 Suppl):S76–S84. [DOI] [PubMed] [Google Scholar]
- 89.Morin CM, Jarrin DC. Epidemiology of Insomnia: prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med Clin. 2022;17(2):173–191. doi: 10.1016/j.jsmc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 90.Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45(1):74–89. doi: 10.1038/s41386-019-0411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ragnoli B, Pochetti P, Raie A, Malerba M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): current Concepts of Patient Management. Int J Environ Res Public Health. 2021;18(17):9248. doi: 10.3390/ijerph18179248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jarrin DC, Morin CM, Rochefort A, et al. Familial Aggregation of Insomnia. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw053 [DOI] [PubMed] [Google Scholar]
- 93.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. doi: 10.1093/sleep/32.8.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sedov ID, Anderson NJ, Dhillon AK, Tomfohr-Madsen LM. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30(1):e13207. doi: 10.1111/jsr.13207 [DOI] [PubMed] [Google Scholar]
- 95.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lanza G. Repetitive TMS for sleep disorders: are we ready? Sleep Med. 2020;71:111–112. doi: 10.1016/j.sleep.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 97.Cantone M, Lanza G, Ranieri F, Opie GM, Terranova C. Editorial: non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front Neurol. 2021;12:721906. doi: 10.3389/fneur.2021.721906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fisicaro F, Lanza G, Bella R, Pennisi M. ”Self-Neuroenhancement”: the Last Frontier of Noninvasive Brain Stimulation? J clin neurol. 2020;16(1):158–159. doi: 10.3988/jcn.2020.16.1.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner X, Steinberg H. Electric neurostimulation in sleep disorders - yesterday and today. A comparative analysis of historical and contemporary case reports and clinical studies. Sleep Med. 2021;86:1–6. doi: 10.1016/j.sleep.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 100.Saebipour MR, Joghataei MT, Yoonessi A, Sadeghniiat-Haghighi K, Khalighinejad N, Khademi S. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: a pilot study. J Sleep Res. 2015;24(5):518–525. doi: 10.1111/jsr.12301 [DOI] [PubMed] [Google Scholar]
- 101.Zhou Q, Yu C, Yu H, et al. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020;70:17–26. doi: 10.1016/j.sleep.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 102.Lanza G, Centonze SS, Destro G, et al. Shiatsu as an adjuvant therapy for depression in patients with Alzheimer’s disease: a pilot study. Complement Ther Med. 2018;38:74–78. doi: 10.1016/j.ctim.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 103.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy 107. Neuron. 2000;27(3):469–474. doi: 10.1016/S0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nature Rev Dis Primers. 2017;3:16100. doi: 10.1038/nrdp.2016.100 [DOI] [PubMed] [Google Scholar]
- 106.Wang W, Pan Y, Li Q, Wang L. Orexin: a potential role in the process of obstructive sleep apnea. Peptides. 2013;42:48–54. doi: 10.1016/j.peptides.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 107.Allen RP, Mignot E, Ripley B, Nishino S, Earley CJ. Increased CSF hypocretin-1 (orexin-A) in restless legs syndrome. Neurology. 2002;59(4):639–641. doi: 10.1212/WNL.59.4.639 [DOI] [PubMed] [Google Scholar]
- 108.Stiasny-Kolster K, Mignot E, Ling L, Moller JC, Cassel W, Oertel WH. CSF hypocretin-1 levels in restless legs syndrome. Neurology. 2003;61(10):1426–1429. doi: 10.1212/01.WNL.0000094196.50155.38 [DOI] [PubMed] [Google Scholar]
- 109.Baier PC, Goder R, Hallschmid M. Circadian variation of hypocretin-1 (orexin A) in restless legs syndrome. Sleep Med. 2009;10(2):271. doi: 10.1016/j.sleep.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 110.Salemi M, Mogavero MP, Lanza G, Mongioi LM, Calogero AE, Ferri R. Examples of Inverse Comorbidity between Cancer and Neurodegenerative Diseases: a Possible Role for Noncoding RNA. Cells. 2022;11(12):1930. doi: 10.3390/cells11121930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mogavero MP, Silvani A, DelRosso LM, Salemi M, Ferri R. Focus on the Complex Interconnection between Cancer, Narcolepsy and Other Neurodegenerative Diseases: a Possible Case of Orexin-Dependent Inverse Comorbidity. Cancers. 2021;13(11):2612. doi: 10.3390/cancers13112612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alain C, Pascal N, Valerie G, Thierry V. Orexins/Hypocretins and Cancer: a Neuropeptide as Emerging Target. Molecules. 2021;26(16):4849. doi: 10.3390/molecules26164849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Razavi BM, Hosseinzadeh H. A review of the role of orexin system in pain modulation. Biomed Pharmacother. 2017;90:187–193. doi: 10.1016/j.biopha.2017.03.053 [DOI] [PubMed] [Google Scholar]
- 114.Moreno-Ajona D, Hoffmann J. From basic mechanisms to therapeutic perspectives in cluster headache. Curr Opin Neurol. 2022;35(3):336–342. doi: 10.1097/WCO.0000000000001055 [DOI] [PubMed] [Google Scholar]
- 115.Strother LC, Srikiatkhachorn A, Supronsinchai W. Targeted Orexin and Hypothalamic Neuropeptides for Migraine. Neurotherapeutics. 2018;15(2):377–390. doi: 10.1007/s13311-017-0602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaneko T, Kuwaki T, Kashiwadani H. Hypothalamic orexinergic neurons modulate pain and itch in an opposite way: pain relief and itch exacerbation. J Physiol Sci. 2022;72(1):21. doi: 10.1186/s12576-022-00846-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bentivegna E, Luciani M, Ferrari V, et al. Recently approved and emerging drug options for migraine prophylaxis. Expert Opin Pharmacother. 2022;23(11):1325–1335. doi: 10.1080/14656566.2022.2102420 [DOI] [PubMed] [Google Scholar]
- 118.Bahji A, Crockford D, El-Guebaly N. Neurobiology and Symptomatology of Post-Acute Alcohol Withdrawal: a Mixed-Studies Systematic Review. J Stud Alcohol Drugs. 2022;83(4):461–469. doi: 10.15288/jsad.2022.83.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campbell EJ, Norman A, Bonomo Y, Lawrence AJ. Suvorexant to treat alcohol use disorder and comorbid insomnia: plan for a Phase II trial. Brain Res. 2020;1728:146597. doi: 10.1016/j.brainres.2019.146597 [DOI] [PubMed] [Google Scholar]
- 120.Roliz AH, Kothare S. The Interaction Between Sleep and Epilepsy. Curr Neurol Neurosci Rep. 2022;22(9):551–563. doi: 10.1007/s11910-022-01219-1 [DOI] [PubMed] [Google Scholar]
- 121.Ng MC. Orexin and Epilepsy: potential Role of REM Sleep. Sleep. 2017;40(3). doi: 10.1093/sleep/zsw061 [DOI] [PubMed] [Google Scholar]
- 122.Chavan P, Chikahisa S, Shiuchi T, et al. Dual orexin receptor antagonist drug suvorexant can help in amelioration of predictable chronic mild stress-induced hyperalgesia. Brain Res Bull. 2022;188:39–46. doi: 10.1016/j.brainresbull.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 123.Kiguchi N, Ko MC. Potential therapeutic targets for the treatment of opioid abuse and pain. Adv Pharmacol. 2022;93:335–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaplan GB, Lakis GA, Zhoba H. Sleep-wake and arousal dysfunctions in post-traumatic stress disorder: role of orexin systems. Brain Res Bull. 2022;186:106–122. doi: 10.1016/j.brainresbull.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 125.Richards A, Kanady JC, Neylan TC. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology. 2020;45(1):55–73. doi: 10.1038/s41386-019-0486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dauvilliers Y. Hypocretin/Orexin, Sleep and Alzheimer’s Disease. Front Neurol Neurosci. 2021;45:139–149. [DOI] [PubMed] [Google Scholar]
- 127.Gao F, Liu T, Tuo M, Chi S. The role of orexin in Alzheimer disease: from sleep-wake disturbance to therapeutic target. Neurosci Lett. 2021;765:136247. doi: 10.1016/j.neulet.2021.136247 [DOI] [PubMed] [Google Scholar]
- 128.Liu C, Xue Y, Liu MF, Wang Y, Chen L. Orexin and Parkinson’s disease: a protective neuropeptide with therapeutic potential. Neurochem Int. 2020;138:104754. doi: 10.1016/j.neuint.2020.104754 [DOI] [PubMed] [Google Scholar]
- 129.Liu MF, Xue Y, Liu C, et al. Orexin-A Exerts Neuroprotective Effects via OX1R in Parkinson’s Disease. Front Neurosci. 2018;12:835. doi: 10.3389/fnins.2018.00835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar S, Behl T, Sehgal A, et al. Exploring the Role of Orexinergic Neurons in Parkinson’s Disease. Neurotox Res. 2021;39(6):2141–2153. doi: 10.1007/s12640-021-00411-4 [DOI] [PubMed] [Google Scholar]
- 131.Petersen A, Gil J, Maat-Schieman ML, et al. Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14(1):39–47. doi: 10.1093/hmg/ddi004 [DOI] [PubMed] [Google Scholar]
- 132.Gabery S, Murphy K, Schultz K, et al. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010;120(6):777–788. doi: 10.1007/s00401-010-0742-6 [DOI] [PubMed] [Google Scholar]
- 133.Cabanas M, Pistono C, Puygrenier L, et al. Neurophysiological and Behavioral Effects of Anti-Orexinergic Treatments in a Mouse Model of Huntington’s Disease. Neurotherapeutics. 2019;16(3):784–796. doi: 10.1007/s13311-019-00726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103(1):400–407. doi: 10.1111/j.1471-4159.2007.04748.x [DOI] [PubMed] [Google Scholar]
- 135.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378(3):409–413. doi: 10.1016/j.bbrc.2008.11.051 [DOI] [PubMed] [Google Scholar]
- 136.Wang Q, Cao F, Wu Y. Orexinergic System in Neurodegenerative Diseases. Front Aging Neurosci. 2021;13:713201. doi: 10.3389/fnagi.2021.713201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lanza G, Ferri R, Bella R, Ferini-Strambi L. The impact of drugs for multiple sclerosis on sleep. Multiple Sclerosis. 2017;23(1):5–13. doi: 10.1177/1352458516664034 [DOI] [PubMed] [Google Scholar]
- 138.Sahraian MA, Rezaali S, Hosseiny M, Doosti R, Tajik A, Naser Moghadasi A. Sleep Disorder as a Triggering Factor for Relapse in Multiple Sclerosis. Eur Neurol. 2017;77(5–6):258–261. doi: 10.1159/000470904 [DOI] [PubMed] [Google Scholar]
- 139.Berhe DF, Gebre AK, Assefa BT. Orexins role in neurodegenerative diseases: from pathogenesis to treatment. Pharmacol Biochem Behav. 2020;194:172929. doi: 10.1016/j.pbb.2020.172929 [DOI] [PubMed] [Google Scholar]
- 140.Becquet L, Abad C, Leclercq M, et al. Systemic administration of orexin A ameliorates established experimental autoimmune encephalomyelitis by diminishing neuroinflammation. J Neuroinflammation. 2019;16(1):64. doi: 10.1186/s12974-019-1447-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mehr JB, Mitchison D, Bowrey HE, James MH. Sleep dysregulation in binge eating disorder and “food addiction”: the orexin (hypocretin) system as a potential neurobiological link. Neuropsychopharmacology. 2021;46(12):2051–2061. doi: 10.1038/s41386-021-01052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lanza G, DelRosso LM, Ferri R. Sleep and homeostatic control of plasticity. Handb Clin Neurol. 2022;184:53–72. [DOI] [PubMed] [Google Scholar]
- 143.Duis J, Pullen LC, Picone M, et al. Diagnosis and management of sleep disorders in Prader-Willi syndrome. J Clin Sleep Med. 2022;18(6):1687–1696. doi: 10.5664/jcsm.9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cataldi M, Arnaldi D, Tucci V, et al. Sleep disorders in Prader-Willi syndrome, evidence from animal models and humans. Sleep Med Rev. 2021;57:101432. doi: 10.1016/j.smrv.2021.101432 [DOI] [PubMed] [Google Scholar]
- 145.Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32(8):451–462. doi: 10.1016/j.tips.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 146.Xue T, Wu X, Chen S, et al. The efficacy and safety of dual orexin receptor antagonists in primary insomnia: a systematic review and network meta-analysis. Sleep Med Rev. 2022;61:101573. doi: 10.1016/j.smrv.2021.101573 [DOI] [PubMed] [Google Scholar]
- 147.Coleman PJ, Gotter AL, Herring WJ, Winrow CJ, Renger JJ. The Discovery of Suvorexant, the First Orexin Receptor Drug for Insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509–533. doi: 10.1146/annurev-pharmtox-010716-104837 [DOI] [PubMed] [Google Scholar]
- 148.Yang LP. Suvorexant: first global approval. Drugs. 2014;74(15):1817–1822. doi: 10.1007/s40265-014-0294-5 [DOI] [PubMed] [Google Scholar]
- 149.Herring WJ, Connor KM, Snyder E, et al. Suvorexant in Patients with Insomnia: pooled Analyses of Three-Month Data from Phase-3 Randomized Controlled Clinical Trials. J Clin Sleep Med. 2016;12(9):1215–1225. doi: 10.5664/jcsm.6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–352. doi: 10.1002/wps.20674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2017;35:1–7. doi: 10.1016/j.smrv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 152.Herring WJ, Connor KM, Snyder E, et al. Effects of suvorexant on the Insomnia Severity Index in patients with insomnia: analysis of pooled Phase 3 data. Sleep Med. 2019;56:219–223. doi: 10.1016/j.sleep.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 153.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. doi: 10.1212/WNL.0b013e31827688ee [DOI] [PubMed] [Google Scholar]
- 154.Svetnik V, Snyder ES, Tao P, et al. Insight Into Reduction of Wakefulness by Suvorexant in Patients With Insomnia: analysis of Wake Bouts. Sleep. 2018;41(1). doi: 10.1093/sleep/zsx178 [DOI] [PubMed] [Google Scholar]
- 155.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267. doi: 10.5665/sleep.2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Branch AF, Navidi W, Tabuchi S, et al. Progressive Loss of the Orexin Neurons Reveals Dual Effects on Wakefulness. Sleep. 2016;39(2):369–377. doi: 10.5665/sleep.5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma J, Svetnik V, Snyder E, Lines C, Roth T, Herring WJ. Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects. Sleep. 2014;37(10):1609–1619. doi: 10.5665/sleep.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Struyk A, Gargano C, Drexel M, et al. Pharmacodynamic effects of suvorexant and zolpidem on EEG during sleep in healthy subjects. Eur neuropsychopharmacol. 2016;26(10):1649–1656. doi: 10.1016/j.euroneuro.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 159.Drake CL, Kalmbach DA, Cheng P, et al. Can the Orexin Antagonist Suvorexant Preserve the Ability to Awaken to Auditory Stimuli While Improving Sleep? Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. Sep. 2019;15(9):1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rahaman SM, Chowdhury S, Mukai Y, Ono D, Yamaguchi H, Yamanaka A. Functional Interaction Between GABAergic Neurons in the Ventral Tegmental Area and Serotonergic Neurons in the Dorsal Raphe Nucleus. Front Neurosci. 2022;16:877054. doi: 10.3389/fnins.2022.877054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Izuhara M, Miura S, Otsuki K, et al. Magnetic Resonance Spectroscopy in the Ventral Tegmental Area Distinguishes Responders to Suvorexant Prior to Treatment: a 4-Week Prospective Cohort Study. Front Psychiatry. 2021;12:714376. doi: 10.3389/fpsyt.2021.714376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wrishko RE, McCrea JB, Yee KL, et al. Effect of CYP3A Inhibition and Induction on the Pharmacokinetics of Suvorexant: two Phase I, Open-Label, Fixed-Sequence Trials in Healthy Subjects. Clin Drug Investig. 2019;39(5):441–451. doi: 10.1007/s40261-019-00764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yee KL, McCrea J, Panebianco D, et al. Safety, Tolerability, and Pharmacokinetics of Suvorexant: a Randomized Rising-Dose Trial in Healthy Men. Clin Drug Investig. 2018;38(7):631–638. doi: 10.1007/s40261-018-0650-4 [DOI] [PubMed] [Google Scholar]
- 164.Herring WJ, Connor KM, Snyder E, et al. Clinical profile of suvorexant for the treatment of insomnia over 3 months in women and men: subgroup analysis of pooled phase-3 data. Psychopharmacology. 2017;234(11):1703–1711. doi: 10.1007/s00213-017-4573-1 [DOI] [PubMed] [Google Scholar]
- 165.Proserpio P, Marra S, Campana C, et al. Insomnia and menopause: a narrative review on mechanisms and treatments. Climacteric. 2020;23(6):539–549. doi: 10.1080/13697137.2020.1799973 [DOI] [PubMed] [Google Scholar]
- 166.Rahman SA, Nathan MD, Wiley A, et al. A double-blind, randomized, placebo-controlled trial of suvorexant for the treatment of vasomotor symptom-associated insomnia disorder in midlife women. Sleep. 2022;45(3). doi: 10.1093/sleep/zsac007 [DOI] [PubMed] [Google Scholar]
- 167.Roehrs T, Withrow D, Koshorek G, Verkler J, Bazan L, Roth T. Sleep and pain in humans with fibromyalgia and comorbid insomnia: double-blind, crossover study of suvorexant 20 mg versus placebo. J Clin Sleep Med. 2020;16(3):415–421. doi: 10.5664/jcsm.8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimer’s Dementia. 2020;16(3):541–551. doi: 10.1002/alz.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Herring WJ, Connor KM, Snyder E, et al. Suvorexant in Elderly Patients with Insomnia: pooled Analyses of Data from Phase III Randomized Controlled Clinical Trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. doi: 10.1016/j.jagp.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 170.Uemura SI, Imanishi A, Terui Y, et al. Residual effects of low dose of suvorexant, zolpidem, and ramelteon in healthy elderly subjects: a randomized double-blind study. Neuropsychopharmacol rep. 2022;42(3):288–298. doi: 10.1002/npr2.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vermeeren A, Sun H, Vuurman EF, et al. On-the-Road Driving Performance the Morning after Bedtime Use of Suvorexant 20 and 40 mg: a Study in Non-Elderly Healthy Volunteers. Sleep. 2015;38(11):1803–1813. doi: 10.5665/sleep.5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kawabe K, Horiuchi F, Ochi M, Nishimoto K, Ueno SI, Oka Y. Suvorexant for the Treatment of Insomnia in Adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):792–795. doi: 10.1089/cap.2016.0206 [DOI] [PubMed] [Google Scholar]
- 173.Sano H, Asai Y, Miyazaki M, Iwakura M, Maeda Y, Hara M. Safety profile and clinical course of patients with insomnia administered suvorexant by initial treatment status in a post-marketing survey. Expert Opin Drug Saf. 2019;18(11):1109–1118. doi: 10.1080/14740338.2019.1657091 [DOI] [PubMed] [Google Scholar]
- 174.Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–471. doi: 10.1016/S1474-4422(14)70053-5 [DOI] [PubMed] [Google Scholar]
- 175.Yoshikawa F, Shigiyama F, Ando Y, et al. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabetes Res Clin Pract. 2020;169:108412. doi: 10.1016/j.diabres.2020.108412 [DOI] [PubMed] [Google Scholar]
- 176.Toi N, Inaba M, Kurajoh M, et al. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Translational Endocrinol. 2019;15:37–44. doi: 10.1016/j.jcte.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tsuneki H, Kon K, Ito H, et al. Timed Inhibition of Orexin System by Suvorexant Improved Sleep and Glucose Metabolism in Type 2 Diabetic db/db Mice. Endocrinology. 2016;157(11):4146–4157. doi: 10.1210/en.2016-1404 [DOI] [PubMed] [Google Scholar]
- 178.Kario K, Yamasaki K, Yagi K, et al. Effect of suvorexant on nighttime blood pressure in hypertensive patients with insomnia: the SUPER-1 study. J Clin Hypertens. 2019;21(7):896–903. doi: 10.1111/jch.13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uemura N, McCrea J, Sun H, et al. Effects of the orexin receptor antagonist suvorexant on respiration during sleep in healthy subjects. J Clin Pharmacol. 2015;55(10):1093–1100. doi: 10.1002/jcph.523 [DOI] [PubMed] [Google Scholar]
- 180.Sun H, Palcza J, Card D, et al. Effects of Suvorexant, an Orexin Receptor Antagonist, on Respiration during Sleep In Patients with Obstructive Sleep Apnea. J Clin Sleep Med. 2016;12(1):9–17. doi: 10.5664/jcsm.5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun H, Palcza J, Rosenberg R, et al. Effects of suvorexant, an orexin receptor antagonist, on breathing during sleep in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(3):416–426. doi: 10.1016/j.rmed.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 182.Huhn AS, Finan PH, Gamaldo CE, et al. Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Sci Transl Med. 2022;14(650):eabn8238. doi: 10.1126/scitranslmed.abn8238 [DOI] [PubMed] [Google Scholar]
- 183.Schoedel KA, Sun H, Sellers EM, et al. Assessment of the Abuse Potential of the Orexin Receptor Antagonist, Suvorexant, Compared With Zolpidem in a Randomized Crossover Study. J Clin Psychopharmacol. 2016;36(4):314–323. doi: 10.1097/JCP.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 184.Beuckmann CT, Suzuki M, Ueno T, Nagaoka K, Arai T, Higashiyama H. In Vitro and In Silico Characterization of Lemborexant (E2006), a Novel Dual Orexin Receptor Antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295. doi: 10.1124/jpet.117.241422 [DOI] [PubMed] [Google Scholar]
- 185.Scott LJ. Lemborexant: first Approval. Drugs. 2020;80(4):425–432. doi: 10.1007/s40265-020-01276-1 [DOI] [PubMed] [Google Scholar]
- 186.Waters K. Review of the Efficacy and Safety of Lemborexant, a Dual Receptor Orexin Antagonist (DORA), in the Treatment of Adults With Insomnia Disorder. Ann Pharmacother. 2022;56(2):213–221. doi: 10.1177/10600280211008492 [DOI] [PubMed] [Google Scholar]
- 187.Citrome L, Juday T, Frech F, Atkins N. Lemborexant for the Treatment of Insomnia: direct and Indirect Comparisons With Other Hypnotics Using Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed. J Clin Psychiatry. 2021;1:82. [DOI] [PubMed] [Google Scholar]
- 188.Rosenberg R, Murphy P, Zammit G, et al. Comparison of Lemborexant With Placebo and Zolpidem Tartrate Extended Release for the Treatment of Older Adults With Insomnia Disorder: a Phase 3 Randomized Clinical Trial. JAMA network open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roth T, Rosenberg R, Morin CM, et al. Impact of lemborexant treatment on insomnia severity: analyses from a 12-month study of adults with insomnia disorder. Sleep Med. 2022;90:249–257. doi: 10.1016/j.sleep.2022.01.024 [DOI] [PubMed] [Google Scholar]
- 190.Dash A, Pinner K, Inoue Y, et al. Efficacy and safety of lemborexant over 12 months in Asian adults with insomnia disorder. Sleep Med. 2022;4:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yardley J, Karppa M, Inoue Y, et al. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med. 2021;80:333–342. doi: 10.1016/j.sleep.2021.01.048 [DOI] [PubMed] [Google Scholar]
- 192.Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9). doi: 10.1093/sleep/zsaa123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lalovic B, Majid O, Aluri J, Landry I, Moline M, Hussein Z. Population Pharmacokinetics and Exposure-Response Analyses for the Most Frequent Adverse Events Following Treatment With Lemborexant, an Orexin Receptor Antagonist, in Subjects With Insomnia Disorder. J Clin Pharmacol. 2020;60(12):1642–1654. doi: 10.1002/jcph.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheng JY, Moline M, Zammit GK, Filippov G, Bsharat M, Hall N. Respiratory Safety of Lemborexant in Healthy Subjects: a Single-Dose, Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Clin Drug Investig. 2021;41(5):449–457. doi: 10.1007/s40261-021-01018-5 [DOI] [PubMed] [Google Scholar]
- 195.Murphy PJ, Yasuda S, Nakai K, et al. Concentration-Response Modeling of ECG Data From Early-Phase Clinical Studies as an Alternative Clinical and Regulatory Approach to Assessing QT Risk - Experience From the Development Program of Lemborexant. J Clin Pharmacol. 2017;57(1):96–104. doi: 10.1002/jcph.785 [DOI] [PubMed] [Google Scholar]
- 196.Moline M, Zammit G, Yardley J, et al. Lack of residual morning effects of lemborexant treatment for insomnia: summary of findings across 9 clinical trials. Postgrad Med. 2021;133(1):71–81. doi: 10.1080/00325481.2020.1823724 [DOI] [PubMed] [Google Scholar]
- 197.Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, Pharmacodynamics, and Safety of the Dual Orexin Receptor Antagonist Lemborexant: findings From Single-Dose and Multiple-Ascending-Dose Phase 1 Studies in Healthy Adults. Clin Pharmacol Drug Dev. 2021;10(2):153–165. doi: 10.1002/cpdd.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murphy P, Moline M, Mayleben D, et al. Lemborexant, A Dual Orexin Receptor Antagonist (DORA) for the Treatment of Insomnia Disorder: results From a Bayesian, Adaptive, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Sleep Med. 2017;13(11):1289–1299. doi: 10.5664/jcsm.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moline M, Thein S, Bsharat M, et al. Safety and Efficacy of Lemborexant in Patients With Irregular Sleep-Wake Rhythm Disorder and Alzheimer’s Disease Dementia: results From a Phase 2 Randomized Clinical Trial. j Prevention Alzheimer’s Dis. 2021;8(1):7–18. doi: 10.14283/jpad.2020.69 [DOI] [PubMed] [Google Scholar]
- 200.Landry I, Hall N, Aluri J, et al. Abuse Potential of Lemborexant, a Dual Orexin Receptor Antagonist, Compared With Zolpidem and Suvorexant in Recreational Sedative Users. J Clin Psychopharmacol. 2022;42(4):365–373. doi: 10.1097/JCP.0000000000001561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roch C, Bergamini G, Steiner MA, Clozel M. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology. 2021;238(10):2693–2708. doi: 10.1007/s00213-021-05954-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Markham A. Daridorexant: first Approval. Drugs. 2022;82(5):601–607. doi: 10.1007/s40265-022-01699-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Berger B, Kornberger R, Dingemanse J. Pharmacokinetic and pharmacodynamic interactions between daridorexant, a dual orexin receptor antagonist, and citalopram in healthy subjects. Eur neuropsychopharmacol. 2021;51:90–104. doi: 10.1016/j.euroneuro.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 204.Berger B, Dingemanse J, Sabattini G, et al. Effect of Liver Cirrhosis on the Pharmacokinetics, Metabolism, and Tolerability of Daridorexant, A Novel Dual Orexin Receptor Antagonist. Clin Pharmacokinet. 2021;60(10):1349–1360. doi: 10.1007/s40262-021-01028-8 [DOI] [PubMed] [Google Scholar]
- 205.Berger B, Brooks S, Zuiker R, Richard M, Muehlan C, Dingemanse J. Pharmacological Interactions between the Dual Orexin Receptor Antagonist Daridorexant and Ethanol in a Double-Blind, Randomized, Placebo-Controlled, Double-Dummy, Four-Way Crossover Phase I Study in Healthy Subjects. CNS drugs. 2020;34(12):1253–1266. doi: 10.1007/s40263-020-00768-8 [DOI] [PubMed] [Google Scholar]
- 206.Boof ML, Dingemanse J, Brunke M, et al. Effect of the novel dual orexin receptor antagonist daridorexant on night-time respiratory function and sleep in patients with moderate chronic obstructive pulmonary disease. J Sleep Res. 2021;30(4):e13248. doi: 10.1111/jsr.13248 [DOI] [PubMed] [Google Scholar]
- 207.Boof ML, Dingemanse J, Lederer K, Fietze I, Ufer M. Effect of the new dual orexin receptor antagonist daridorexant on nighttime respiratory function and sleep in patients with mild and moderate obstructive sleep apnea. Sleep. 2021;44(6). doi: 10.1093/sleep/zsaa275 [DOI] [PubMed] [Google Scholar]
- 208.Schilling U, Henrich A, Muehlan C, Krause A, Dingemanse J, Ufer M. Impact of Daridorexant, a Dual Orexin Receptor Antagonist, on Cardiac Repolarization Following Bedtime Dosing: results from a Thorough QT Study Using Concentration-QT Analysis. Clin Drug Investig. 2021;41(8):711–721. doi: 10.1007/s40261-021-01062-1 [DOI] [PubMed] [Google Scholar]
- 209.Dauvilliers Y, Zammit G, Fietze I, et al. Daridorexant, a New Dual Orexin Receptor Antagonist to Treat Insomnia Disorder. Ann Neurol. 2020;87(3):347–356. doi: 10.1002/ana.25680 [DOI] [PubMed] [Google Scholar]
- 210.Mignot E, Mayleben D, Fietze I, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multiCentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–139. doi: 10.1016/S1474-4422(21)00436-1 [DOI] [PubMed] [Google Scholar]
- 211.Zammit G, Dauvilliers Y, Pain S, Sebok Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–e2232. doi: 10.1212/WNL.0000000000009475 [DOI] [PubMed] [Google Scholar]
- 212.Ufer M, Kelsh D, Schoedel KA, Dingemanse J. Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem. Sleep. 2022;45(3). doi: 10.1093/sleep/zsab224 [DOI] [PubMed] [Google Scholar]
- 213.Futamura A, Suzuki R, Tamura Y, et al. Discovery of ORN0829, a potent dual orexin 1/2 receptor antagonist for the treatment of insomnia. Bioorg Med Chem. 2020;28(13):115489. doi: 10.1016/j.bmc.2020.115489 [DOI] [PubMed] [Google Scholar]
- 214.Mezeiova E, Janockova J, Konecny J, et al. From orexin receptor agonist YNT-185 to novel antagonists with drug-like properties for the treatment of insomnia. Bioorg Chem. 2020;103:104179. doi: 10.1016/j.bioorg.2020.104179 [DOI] [PubMed] [Google Scholar]
- 215.Uchiyama M, Kambe D, Imadera Y, Kajiyama Y, Ogo H, Uchimura N. Effects of TS-142, a novel dual orexin receptor antagonist, on sleep in patients with insomnia: a randomized, double-blind, placebo-controlled phase 2 study. Psychopharmacology. 2022;239(7):2143–2154. doi: 10.1007/s00213-022-06089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Revell VL, Della Monica C, Mendis J, et al. Effects of the selective orexin-2 receptor antagonist JNJ-48816274 on sleep initiated in the circadian wake maintenance zone: a randomised trial. Neuropsychopharmacology. 2022;47(3):719–727. doi: 10.1038/s41386-021-01175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Savitz A, Wajs E, Zhang Y, et al. Efficacy and Safety of Seltorexant as Adjunctive Therapy in Major Depressive Disorder: a Phase 2b, Randomized, Placebo-Controlled, Adaptive Dose-Finding Study. int j neuropsychopharmacol. 2021;24(12):965–976. doi: 10.1093/ijnp/pyab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brooks S, Jacobs GE, de Boer P, et al. The selective orexin-2 receptor antagonist seltorexant improves sleep: an exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J psychopharmacol. 2019;33(2):202–209. doi: 10.1177/0269881118822258 [DOI] [PubMed] [Google Scholar]
- 219.Maehara S, Yuge N, Higashi C, Ota T, Furukawa J, Takeuchi T. Pharmacological characterization of a novel potent, selective, and orally active orexin 2 receptor antagonist, SDM-878. Neuropsychopharmacol rep. 2020;40(2):182–189. doi: 10.1002/npr2.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van der Ark PD, Golor G, van Nueten L, Nandy P, de Boer P. Multiple daytime administration of the selective orexin-2 receptor antagonist JNJ-42847922 induces somnolence in healthy subjects without residual central effects. J psychopharmacol. 2018;32(12):1330–1340. doi: 10.1177/0269881118791521 [DOI] [PubMed] [Google Scholar]
- 221.Lessel U, Ferrara M, Heine N, et al. Identification of Highly Selective Orexin 1 Receptor Antagonists Driven by Structure-Based Design. J Chem Inf Model. 2021;61(12):5893–5905. doi: 10.1021/acs.jcim.1c01055 [DOI] [PubMed] [Google Scholar]
- 222.Hellmann J, Drabek M, Yin J, et al. Structure-based development of a subtype-selective orexin 1 receptor antagonist. Proc Natl Acad Sci U S A. 2020;117(30):18059–18067. doi: 10.1073/pnas.2002704117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Katoh K, Kutsumura N, Yamamoto N, et al. Essential structure of orexin 1 receptor antagonist YNT-707: conversion of the 16-cyclopropylmethyl group to the 16-sulfonamide group in d-nor-nalfurafine derivatives. Bioorg Med Chem Lett. 2022;59:128550. doi: 10.1016/j.bmcl.2022.128550 [DOI] [PubMed] [Google Scholar]
- 224.Preville C, Bonaventure P, Koudriakova T, et al. Substituted Azabicyclo[2.2.1]heptanes as Selective Orexin-1 Antagonists: discovery of JNJ-54717793. ACS Med Chem Lett. 2020;11(10):2002–2009. doi: 10.1021/acsmedchemlett.0c00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Salvadore G, Bonaventure P, Shekhar A, et al. Translational evaluation of novel selective orexin-1 receptor antagonist JNJ-61393215 in an experimental model for panic in rodents and humans. Transl Psychiatry. 2020;10(1):308. doi: 10.1038/s41398-020-00937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.De Crescenzo F, D’Alo GL, Ostinelli EG, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400(10347):170–184. doi: 10.1016/S0140-6736(22)00878-9 [DOI] [PubMed] [Google Scholar]
- 227.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Blasiak A, Gundlach AL, Hess G, Lewandowski MH. Interactions of Circadian Rhythmicity, Stress and Orexigenic Neuropeptide Systems: implications for Food Intake Control. Front Neurosci. 2017;11:127. doi: 10.3389/fnins.2017.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.De Boer P, Drevets WC, Rofael H, et al. A randomized Phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J psychopharmacol. 2018;32(6):668–677. doi: 10.1177/0269881118773745 [DOI] [PubMed] [Google Scholar]
- 230.Recourt K, de Boer P, Zuiker R, et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry. 2019;9(1):216. doi: 10.1038/s41398-019-0553-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Meira ECM, Salles C, Seixas L, Rocha I, Gozal D. Comorbid insomnia and sleep apnea in children: a preliminary explorative study. J Sleep Res. 2022;e13705. doi: 10.1111/jsr.13705 [DOI] [PubMed] [Google Scholar]
- 232.Tiseo C, Vacca A, Felbush A, et al. Migraine and sleep disorders: a systematic review. J Headache Pain. 2020;21(1):126. doi: 10.1186/s10194-020-01192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. 2020;56:101409. doi: 10.1016/j.smrv.2020.101409 [DOI] [PubMed] [Google Scholar]
- 234.Mogavero MP, Bruni O, DelRosso LM, Ferri R. Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: the Role of Sleep. Brain Sci. 2020;10(7):411. doi: 10.3390/brainsci10070411 [DOI] [PMC free article] [PubMed] [Google Scholar]