Abstract
Anti-androgen drugs are the standard pharmacological therapies for treatment of non-metastatic prostate cancer (PCa). However, the response of PCa cells may depend on the anti-androgen used and often patients become resistant to treatment. Thus, studying how the anti-androgen drugs affect oncogenes expression and action and the identification of the best strategy for combined therapies are essential to improve the efficacy of treatments. The Six Transmembrane Epithelial Antigen of the Prostate 1 (STEAP1) is an oncogene associated with PCa progression and aggressiveness, although its relationship with the androgen receptor signaling remains to be elucidated. The present study aimed to evaluate the effect of anti-androgens in regulating STEAP1 expression and investigate whether silencing STEAP1 can make PCa cells more sensitive to anti-androgen drugs. For this purpose, wild-type and STEAP1 knockdown LNCaP cells were exposed to bicalutamide, enzalutamide and apalutamide. Bicalutamide decreased the expression of STEAP1, but enzalutamide and apalutamide increased its expression. However, decreased cell proliferation and increased apoptosis was observed in response to all drugs. Overall, the cellular and molecular effects were similar between LNCaP wild-type and LNCaP-STEAP1 knockdown cells, except for c-myc expression levels, where a cumulative effect between anti-androgen treatment and STEAP1 knockdown was observed. The effect of STEAP1 knockdown alone or combined with anti-androgens in c-myc levels is required to be addressed in future studies.
Keywords: bicalutamide, enzalutamide, apalutamide, six transmembrane epithelial antigen of the prostate 1, prostate cancer
Introduction
Prostate cancer (PCa) is the most frequently diagnosed neoplasm in men worldwide, affecting 1,276,106 of new cases in 2018 (1). PCa incidence has been rising in the last decades due to the increase in population aging, obesity caused by dietary habits and lifestyle, among other causes (2,3). The development and progression of PCa is initially dependent on the stimulatory action of androgens, which validate the use of therapies reducing the biosynthesis of androgens and/or antagonizing the action of androgens through the androgen receptor (AR) (4). Bicalutamide (second generation) and enzalutamide and apalutamide (third generation) are anti-androgens that antagonize the AR activity, inhibiting gene expression associated with PCa progression and consequently having therapeutic benefits in PCa (5). However, several patients become resistant to androgen-deprivation therapy (ADT), giving rise to the so-called castrate-resistant PCa (6). Clinical trials have investigated the efficacy of ADT in combination with other drugs, as strategies for improved management of PCa and slowing the progression to castrate-resistant stage (7). The identification of new therapeutic targets, in combination with the ADT, remains a fundamental aspect to improve PCa treatment and to identify novel predictive biomarkers for response to ADT.
The human Six-Transmembrane Epithelial Antigen of the Prostate 1 (STEAP1) is highly expressed in several types of cancer, with special emphasis on PCa, where the STEAP1 protein expression levels are 5–10 fold higher compared with other cancer types (8). The mechanisms underlying the overexpression of STEAP1 in cancer remain poorly explored, but epigenetic changes associated with increased expression levels in PCa have been identified in the STEAP1 promoter region (9). Among non-tumoral tissues, STEAP1 is almost restricted to the epithelial cells of the prostate gland, which makes it a promising biomarker and/or therapeutic target for PCa (8,10). The potential role of STEAP1 in cancer progression has been studied extensively and its oncogenic role emphasized (11–13). However, the physiological role of STEAP1 and the specific actions driven the carcinogenic process remain to be elucidated. Nevertheless, the STEAP1 protein seems to act as a channel for small molecules, being involved in intercellular communication (14). In addition, there is evidence for formation of STEAP1-STEAP2 heterotrimers, which seem to be associated with the activity of metal reductase and superoxide synthase enzymes (15). This is in accordance with a previous study demonstrating that STEAP1 actions, favoring cancer progression, are associated with oxidative stress (16).
Several research groups have demonstrated the contribution of STEAP1 to tumor progression, namely, by its involvement in promoting cell proliferation, migration and invasion (17–21). Regarding PCa, it was shown that silencing the STEAP1 gene reduces androgen-dependent PCa cell viability and proliferation and increases the apoptosis rate. In addition, the anti-proliferative and pro-apoptotic effects triggered by STEAP1 knockdown are not abrogated by the treatment with dihydrotestosterone (DHT), suggesting that STEAP1 inhibition might be a good option of treatment to prevent the effects of DHT in PCa (17). The effect of androgens in regulating STEAP1 expression has also been studied, with contradictory results. Some studies found STEAP1 as androgen-stimulated, as androgen-inhibited, or as androgen-independent in cell lines of PCa (8,22–24). Concerning LNCaP cells, DHT downregulates STEAP1 expression, but this effect should not directly involve the AR because no androgen response elements are found in promoter region of the STEAP1 gene (23,25). This indirect action of AR in downregulating STEAP1 means that de novo protein synthesis should be required (23). In human PCa biopsies, an association between STEAP1 overexpression and metastasis was found, with the presence of more aggressive tumors and the majority of patients becoming resistant to treatment with anti-androgens (26). In addition, a marginally positive significant association between STEAP1 overexpression and presurgical prostate specific antigen (PSA) levels was detected, indicating a potential crosstalk between STEAP and AR (26). Considering the use of anti-androgens in PCa treatment and the potential of STEAP1 as therapeutic target, associated with the putative relationship between STEAP1 and AR, it was hypothesized that STEAP1 expression may be regulated by anti-androgens. Also, it was hypothesized that blocking the action of STEAP1 may sensitizes PCa cells to treatment with anti-androgens drugs. Therefore, the main goal of the present study was to investigate the effect of several types of anti-androgens, bicalutamide, enzalutamide and apalutamide, in LNCaP wild-type (LNCaP-WT) and LNCaP-STEAP1 knockdown cells.
Materials and methods
Cell culture
Human prostate adenocarcinoma cell line (LNCaP) was purchased from the European Collection of Authenticated Cell Cultures and maintained in Roswell Park Memorial Institute medium (RPMI)-1640 (MilliporeSigma) supplemented with 10% fetal bovine serum (FBS; Biochrom AG) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), in a humidified chamber at 37°C and a 5% CO2 atmosphere.
STEAP1 knockdown and experimental design
LNCaP cells at 50% confluence in T-flasks or multiwell plates were transfected with 20 nM of a small interfering RNA (siRNA) targeting the STEAP1 (cat. no. s226093; Ambion; Thermo Fisher Scientific, Inc.) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h at 37°C in Opti-Minimum Essential Medium (MEM) (Invitrogen; Thermo Fisher Scientific, Inc.) as recommended by the manufacturer. These cells are referred to as LNCaP-STEAP1 knockdown. As a control for STEAP1-knockdown, a scrambled siRNA sequence (cat. no. 4390846, Ambion; Thermo Fisher Scientific, Inc.) was used and these control cells are designated as LNCaP-wild type (WT) cells. The sequences of STEAP1 and scramble siRNA were not provided by the manufacturer. Then, 24 h following transfection, the cells were stimulated with anti-androgens, 100 µM bicalutamide (MilliporeSigma), 10 µM enzalutamide (MilliporeSigma) and 10 µM apalutamide (Alfa Aesar) for 24 h at 37°C. All drugs stock solutions were prepared in dimethyl sulfoxide (DMSO). Cells were harvested at 24 h after drugs treatment and the efficiency of STEAP1 knockdown expression was analyzed by reverse transcription-quantitative (RT-q) PCR and western blotting.
MTT assay
In order to determine the viability of LNCaP cells silenced for STEAP1 and exposed to the three anti-androgenic drugs, 3-(4,5-dimethylthiazol-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay (MilliporeSigma) was used according to the manufacturer's instructions. Briefly, 100 µl of MTT solution at 0.5 mg/ml concentration was added to cells. After 1 h of incubation at 37°C, the MTT solution was removed and 100 µl DMSO was added for solubilization of the formazan crystals. Next, the optical density was measured at 570 nm using the xMark Microplate Absorbance Spectrophotometer (Bio-Rad Laboratories, Inc.).
Ki-67 fluorescence immunocytochemistry
Fluorescent immunocytochemistry of the proliferation marker Ki67 was used to estimate the proliferation index between LNCaP cells knocked down for STEAP1 and LNCaP-WT, both treated with the three drugs. LNCaP cells were fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature and permeabilized with 0.1% Triton X-100 for 5 min also at RT. A blocking step was performed by incubating cells with 20% FBS in phosphate buffer saline (PBS) containing 0.1% Tween-20 (PBST) for 1 h at RT and then cells were incubated with rabbit anti-Ki-67 (1:50; cat. no. 16667; Abcam) for 90 min at RT. The Alexa Fluor 546 goat anti-rabbit IgG (1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) was used as a secondary antibody. This incubation was performed at RT for 60 min. The specificity of the staining was assessed by omission of the primary antibody. Cell nuclei were stained with Hoechst 33342 (5 µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min at RT. Coverslips were washed and mounted onto microscope slides with Dako fluorescent mounting medium (Dako; Agilent Technologies, Inc.). Images were acquired using a AxioImager Z2 optical microscope (Carl Zeiss AG). Proliferation was determined by the percentage of Ki-67-positive cells out of the total number of Hoechst-stained nuclei in eight randomly selected fields per microscope cover glass.
Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) assay
Cells were fixed with 4% PFA for 10 min at RT, and then, permeabilized in 1% Triton X-100 with PBST for 5 min also at RT. TUNEL reaction mixture (40 µl; Roche Applied Science) was added to each sample for 1 h at room temperature in the dark. Cells were washed in PBS and incubated for 5 min at RT with Hoechst-33342 (5 µg/ml, Invitrogen, Thermo Fisher Scientific, Inc.). Coverslips were then mounted using Dako fluorescent mounting medium (Dako; Agilent Technologies, Inc.) and analyzed by fluorescence microscopy using the AxioImager Z2 Optical Microscopy (Carl Zeiss AG). The percentage of apoptotic cells was estimated by counting the number of TUNEL-positive cells and Hoechst-stained nuclei in eight randomly selected ×40 magnification fields in each coverslip. The ratio between the number of TUNEL-positive cells and total number was calculated.
RNA extraction and RT-qPCR
Total RNA from 3×106 LNCaP cells was obtained using TRI reagent (Grisp, Lda.) according to the manufacturer's instructions. The RNA pellet was dried, resuspended in 20 µl of diethylpyrocarbonate treated-water and maintained at −80°C. In order to assess the quantity of total RNA, its optical density was determined by measuring absorbance at 260 and 280 nm on a nanospectrometer (Ultrospec 3000; GE Healthcare). Total RNA integrity was verified by agarose gel electrophoresis. RT-qPCR was used to determine expression levels of STEAP1, p21 and kallikrein-related peptidase 3 (KLK3; encodes the PSA protein) genes, using Power SYBR Green RNA-to-CT, 1-Step kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) on the CFX connect real-time system (Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. RT-qPCR was performed with 0.2 µg of RNA in 10 µl of total reaction with specific primers for STEAP1 (sense: 5′GGCGATCCTACAGATACAAGTTGC3′ and anti-sense: 5′CCAATCCCACAATTCCCAGAGAC3′), p21 (sense: 5′TCCAGCGACCTTCCTCATC3′ and anti-sense: 5′AGCCTCTACTGCCACCATC3′), KLK3 (sense: 5′ACCAGAGGAGTTCTTGACCCC3′ and anti-sense 5′ CCCCAGAATCACCCGAGCAG3′) and β-2-microglobulin housekeeping (β2M, sense: 5′ATGAGTATGCCTGCCGTGTG3′ and anti-sense: 5′CAAACCTCCATGCTGCTTAC3′). All primers were synthesized by the services of STAB VIDA and were previously characterized with qPCRs optimized by our research group in previous papers (17,23). After cDNA synthesis at 48°C for 30 min, qPCR was performed with the following steps: Initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 sec, annealing temperature at 60°C for 30 sec and extension at 72°C for 20 sec. The amplified PCR fragments were analyzed by melting curves. β2M housekeeping was used as internal control to normalize gene expression. Fold differences were calculated following the mathematical model proposed by Pfaffl (27).
Protein extraction and western blotting
LNCaP cells were lysed in an appropriate volume of Radioimmunoprecipitation assay (150 mM NaCl, 1% Nonidet-P40 substitute, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and 50 mM Tris) supplemented with 10% phenylmethylsulfonyl fluoride and 1% protease cocktail (A7779, PanReac AppliChem, ITW Reagents, Ottoweg 2, Darmstadt, Germany). The total protein extract was obtained after centrifugation of the cell lysate for 20 min at 18 620 g at 4°C. Quantification of the total protein was measured using the Pierce 660 nm Protein assay reagent (Thermo Fisher Scientific, Inc.). Then, ~20 µg of total protein were resolved on 10% TGX Stain-Free polyacrylamide gels (Bio-Rad Laboratories, Inc.), scanned in the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.) with one minute of exposure time and then, transferred into a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.). After blocking with 5% milk solution for 1 h at room temperature, membranes were incubated overnight at 4°C with following antibodies: Rabbit anti-STEAP1 (1:1,000; cat. no. D8B2V; Lot 1; Cell Signaling Technology, Inc.), rabbit anti-p-AKT (1:500; cat. no. 9271S; Lot 14; Cell Signaling Technology, Inc.), rabbit anti-AKT (1:500; cat. no. 9272S; Lot 27; Cell Signaling Technology, Inc.), rabbit anti-p-ERK (1:500; cat. no. 9101S; Lot 12; Cell Signaling Technology, Inc.), rabbit anti-ERK (1:500; cat. no. 9102S; Lot 27; Cell Signaling Technology, Inc.), rabbit anti-p-c-myc (1:500; cat. no. 13748S; Lot 4; Cell Signaling Technology, Inc.), rabbit anti-c-myc (1:500; A-14; cat. no. sc-789; Santa Cruz Biotechnology, Inc.), rabbit anti-Bcl-2 (1:1,000; cat. no. 2876S; Lot 6; Cell Signaling Technology, Inc.), rabbit anti-Bax (1:1,000; cat. no. 2772S; Lot 11; Cell Signaling Technology, Inc.) and rabbit anti-p53 (1:1,000; FL-393; cat. no. sc-6243; Lot L2713, Santa Cruz Biotechnology, Inc.). Membranes were incubated for 1 h at room temperature with secondary anti-rabbit IgG-horseradish peroxidase (1:15,000; cat. no. 9003-99-0; MilliporeSigma). After this, immunoreactivity was visualized using the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.) after the incubation with enhanced chemiluminescence substrate (Bio-Rad Laboratories, Inc.). Total protein normalization was carried out using the Image Lab 5.1 software (Bio-Rad Laboratories, Inc.), by opening a multichannel image, configure two channels: channel 1, target protein blot and channel 2, stain-free gel image. Normalization icon from the Analysis Toolbox was used to detect bands and lanes and after were adjusted if needed. Stain-free image was selected as normalization channel and the normalized volumes are indicated in the Analysis Table on the tool bar. The target protein band intensity value is adjusted for variation in the protein total load on the gel, following other studies (28,29).
Caspase-3-like activity assay
The caspase-3-like activity was determined after the cleavage of the labeled substrate by the detection of the chromophore p-nitroaniline (pNA), measured spectrophotometrically at 405 nm. Total protein extract (25 µg) was incubated with a reaction buffer [25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 10% sucrose and supplemented with 10 mM dithiothreitol (pH 7.5)] and 2 mM of caspase-3 substrate (N-Acetyl-Asp-Glu-Val-Asp pNA and Ac-DEVD-pNA, Sigma-Aldrich) for 2 h at 37°C. The amount of generated pNA was calculated by extrapolation with a standard curve of free pNA.
Statistical analysis
All experimental data are shown as mean ± standard error of the mean. Statistical significance of differences among experimental groups were evaluated by two-way analysis of variance followed by Tukey's multiple comparisons test, using GraphPad Prism v7.01 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of bicalutamide, enzalutamide and apalutamide on STEAP1 expression in LNCaP-WT and LNCaP-STEAP1 knockdown cells
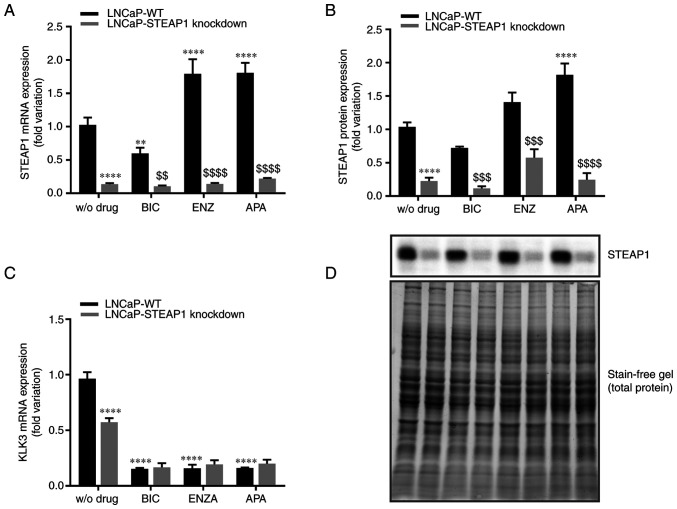
The effect of anti-androgens in regulating STEAP1 gene expression was evaluated in LNCaP-WT and LNCaP-STEAP1 knockdown cells. After stimulation with bicalutamide (100 µM), enzalutamide (10 µM) or apalutamide (10 µM) for 24 h, the STEAP1 mRNA and protein expression was evaluated by qPCR and western blotting, respectively. As can be seen in Fig. 1, using a siRNA targeting STEAP1 silenced STEAP1 gene was confirmed at mRNA (87±0.008% compared with scramble siRNA; Fig. 1A) and protein (80±0.053% compared with scramble siRNA; Fig. 1B) levels. The efficiency and validation of the anti-androgen treatment was shown by analysing the expression levels of an AR target gene, the KLK3 gene that encodes the PSA protein. Fig. 1C showed that suppressing androgen actions with bicalutamide, enzalutamide or apalutamide significantly reduced the KLK3 gene expression (0.15±0.01-, 0.16±0.03- and 0.16±0.01-fold variation, respectively). It was also verified that STEAP1 knockdown decreased the KLK3 gene levels (0.573±0.04- compared with 0.964±0.06-fold variation; Fig. 1C).
Figure 1.
Effect of bicalutamide, enzalutamide and apalutamide on the expression of STEAP1 and KLK3 in LNCaP-WT and LNCaP-STEAP1 knockdown prostate cancer cells. STEAP1 and KLK3 expression levels in LNCaP cells following transfection with siRNA for 24 h following treatment with 100 µM of BIC, or 10 µM of ENZA or 10 µM of APA for 24 h. Relative (A) STEAP1 mRNA, (B) STEAP1 protein and (C) KLK3 mRNA expression were determined by reverse transcription-quantitative PCR following normalization with the β2M housekeeping gene and western blot after normalization with total protein as represented in (D). Representative immunoblots are also showed in (D). Results are expressed as fold-variation relative to LNCaP-WT (control group). Error bars indicate mean ± standard error of the mean (n≥3). **P<0.01 and ****P<0.0001 vs. the LNCaP-WT condition; $$P<0.01, $$$P<0.001 and $$$$P<0.0001 vs. LNCaP-WT plus respective drug. STEAP1, six transmembrane epithelial antigen of the prostate 1; KLK3, kallikrein related peptidase 3; WT, wild type; siRNA, small interfering RNA; BIC, bicalutamide; ENZA, enzalutamide; APA, apalutamide; β2M, β-2-microglobulin.
When LNCaP-WT cells were treated with bicalutamide, there was a significant decrease in STEAP1 mRNA expression (0.59±0.23-fold variation; Fig. 1A), but no significant effect was observed at STEAP1 protein level (0.73±0.020-fold variation; P=0.108; Fig. 1B). On the other hand, enzalutamide and apalutamide increased the expression of STEAP1 mRNA (1.79±0.018 and 1.77±0.275-fold variation, respectively; Fig. 1A). Concerning the STEAP1 protein levels, the increase of STEAP1 in LNCaP-WT cells was observed with the treatment of apalutamide (1.89±0.177-fold variation; Fig. 1B), but not with enzalutamide (1.47±0.167-fold variation; P=0.108; Fig. 1B).
Regarding the effect of anti-androgens in LNCaP-STEAP1 knockdown, no significant differences in STEAP1 mRNA and protein expression were verified in comparison with LNCaP STEAP1-knockdown without treatment with anti-androgens.
Cell viability and proliferation of LNCaP-STEAP1 knockdown cells in response to anti-androgenic drugs
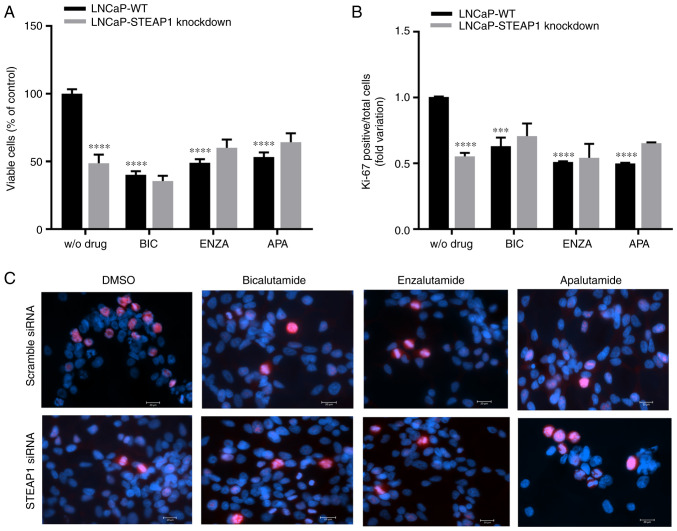
The effect of bicalutamide, enzalutamide and apalutamide anti-androgenic drugs on viability and proliferation of LNCaP-STEAP1 knockdown cells were determined by the MTT assay and immunofluorescent labelling of Ki-67, respectively. At 24 h after knockdown of STEAP1, LNCaP cells were exposed to bicalutamide, enzalutamide and apalutamide drugs. Viability of LNCaP cells markedly decreased upon STEAP1 silencing (47.2±11.8% reduction compared with scramble siRNA; Fig. 2A). Bicalutamide (100 µM), enzalutamide (10 µM) and apalutamide (10 µM) significantly decreased the viability of LNCaP-WT cells (59.55±5.5%, 50.68±3.7% and 46.1±6.7% of reduction, respectively; Fig. 2A). The silencing of STEAP1 in LNCaP cells did not significantly change cell viability when treated with anti-androgenic drugs (Fig. 2A). The results of Ki-67 fluorescent immunocytochemistry were similar to the results observed for the MTT assay, also showing that the number of Ki-67-positive cells relative to total cells (Hoechst-positive) was significantly decreased in the LNCaP-STEAP1 knockdown cells when compared with LNCaP-WT cells (0.553±0.03-compared with 1.01±0.003-fold variation; Fig. 2B). Administration of bicalutamide, enzalutamide and apalutamide drugs in LNCaP-WT cells significantly decreased the number of Ki-67 positive cells compared with LNCaP-WT without drug (0.630±0.06-, 0.511±0.01- and 0.500±0.01-fold variation, respectively; Fig. 2B). However, the STEAP1 gene silencing in LNCaP cells did not significantly change the Ki-67-positive cells number obtained when treated with anti-androgenic drugs (Fig. 2B). Representative fluorescent immunocytochemistry images of Ki-67-labelled LNCaP cells in all experimental conditions were represented in Fig. 2C.
Figure 2.
Effect of bicalutamide, enzalutamide and apalutamide in cell viability and proliferation of LNCaP-WT and LNCaP-STEAP1 knockdown cells. Cell viability and proliferation of LNCaP cells following transfection with siRNA for 24 h following treatment with BIC (100 µM), ENZA (10 µM) and APA (10 µM) for 24 h. (A) Percentage of viable cells was determined by the MTT assay. (B) Ki-67 positive cells relative to the total cell number after different conditions were obtained by the immunofluorescence analysis of Ki-67 assay; eight randomly selected fields per microscope cover glass were assessed. (C) Representative fluorescent immunocytochemistry images of Ki-67 labelled cells (red staining) and Hoechst 33342 stained nuclei (blue) were obtained with the AxioImager Z2 fluorescence microscope (magnification, ×400). Results are expressed as percentage of control and fold-variation relative to the LNCaP-WT condition. Error bars indicate mean ± standard error of the mean (n≥2). ***P<0.001 and ****P<0.0001 vs. the LNCaP-WT condition. WT, wild type; STEAP1, six transmembrane epithelial antigen of the prostate 1; siRNA, small interfering RNA; BIC, bicalutamide; ENZA, enzalutamide; APA, apalutamide.
Analysis of survival pathways in LNCaP-STEAP1 knockdown cells in response to anti-androgenic drugs
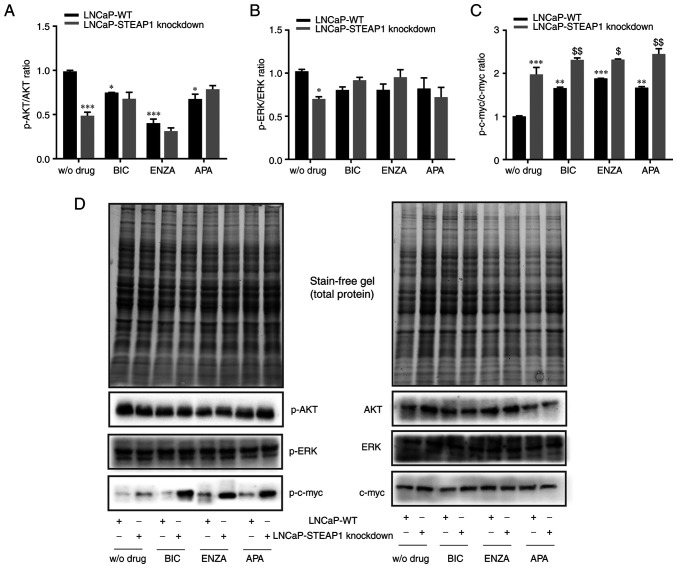
To understand the decreased viability and proliferative activity of LNCaP cells in response STEAP1 knockdown associated with anti-androgenic action, the expression of proteins related with cell survival pathways was evaluated. Fig. 3 shows the western blot analysis for the expression of the active phosphorylated (p-)AKT, ERK and c-myc isoforms, respectively to the expression of total proteins. The results showed that p-AKT/AKT and p-ERK/ERK ratio decreased in LNCaP-STEAP1 knockdown cells when compared with LNCaP-WT (0.487±0.04-compared with 0.989±0.01-fold variation and 0.701±0.02-compared with 1.02±0.024-fold variation, respectively; Fig. 3A and B). Treatment of LNCaP-WT cells with 100 µM of bicalutamide, 10 µM of enzalutamide and 10 µM of apalutamide also decreased the p-AKT/AKT ratio relatively to LNCaP-WT group without drug (0.748±0.003-compared with 0.989±0.01-, 0.402±0.058-compared with 0.989±0.01- and 0.676±0.05-compared with 0.989±0.01-fold variation, respectively; Fig. 3A). The silencing of STEAP1 did not alter the p-AKT/AKT ratio of LNCaP cells treated with anti-androgens (Fig. 3A). No statistically significant differences were found in the p-ERK/ERK ratio in LNCaP-WT or LNCaP-STEAP1 knockdown cells, both treated with bicalutamide, enzalutamide or apalutamide (Fig. 3B).
Figure 3.
Effect of BIC, ENZA and APA on the expression of p-AKT, AKT, p-ERK, ERK, p-c-myc and c-myc in LNCaP-WT and LNCaP-STEAP1 knockdown cells. LNCaP cells transfected with siRNA targeting STEAP1 or scramble siRNA. 24 h after tranfection, LNCaP cells were treated with 100 µM of BIC, or 10 µM of ENZA or 10 µM of APA for 24 h. Ratio of phospohorylated forms and total protein of (A) AKT, (B) ERK and (C) c-myc were determined by western blotting after independent normalization with total protein load on gel as represented in (D) together with representative immunoblots. Results are expressed as fold-variation relative to LNCaP-WT (control group). Error bars indicate mean ± standard error of the mean (n≥2). *P<0.05, **P<0.01 and ***P<0.001 vs. the LNCaP-WT condition; $P<0.05 and $$P<0.01 vs. LNCaP-WT plus respective drug. BIC, bicalutamide; ENZA, enzalutamide; APA, apalutamide; p-, phosphorylated; WT, wild type; STEAP1, six transmembrane epithelial antigen of the prostate 1; siRNA, small interfering RNA.
Regarding the levels of p-c-myc and c-myc, an increased of p-c-myc/c-myc ratio was observed in LNCaP-STEAP1 knockdown when compared with the LNCaP-WT cells (1.978±0.16-compared with 1.002±0.01-fold variation; Fig. 3C). Bicalutamide-, enzalutamide- and apalutamide-treated group in LNCaP-WT cells, high levels of p-c-myc/c-myc ratio was found in comparison with scramble siRNA group (1.659±0.02-compared with 1.002±0.01-, 1.883±0.003-compared with 1.002±0.01- and 1.668±0.03-compared with 1.002±0.01-fold variation, respectively; Fig. 3C). The p-c-myc/c-myc ratio in response to bicalutamide, enzalutamide and apalutamide drugs was higher in LNCaP-STEAP1 knockdown cells than in LNCaP-WT cells (2.315±0.04-compared with 1.659±0.02-fold variation to bicalutamide, 2.320±0.01-compared with 1.883±0.003-fold variation to enzalutamide, 2.451±0.12-compared with 1.668±0.03-fold variation to apalutamide; Fig. 3C).
In brief, anti-androgen treatment significantly decreased p-AKT/AKT and increased p-c-myc/c-myc ratio expression levels in LNCaP-WT cells. Moreover, a slight additive effect was observed in p-c-myc/c-myc ratio in LNCaP cells knocked-down for STEAP1 treated with bicalutamide and apalutamide.
Analysis of apoptotic pathways in LNCaP-WT and LNCaP-STEAP1 knockdown cells treated with anti-androgenic drugs
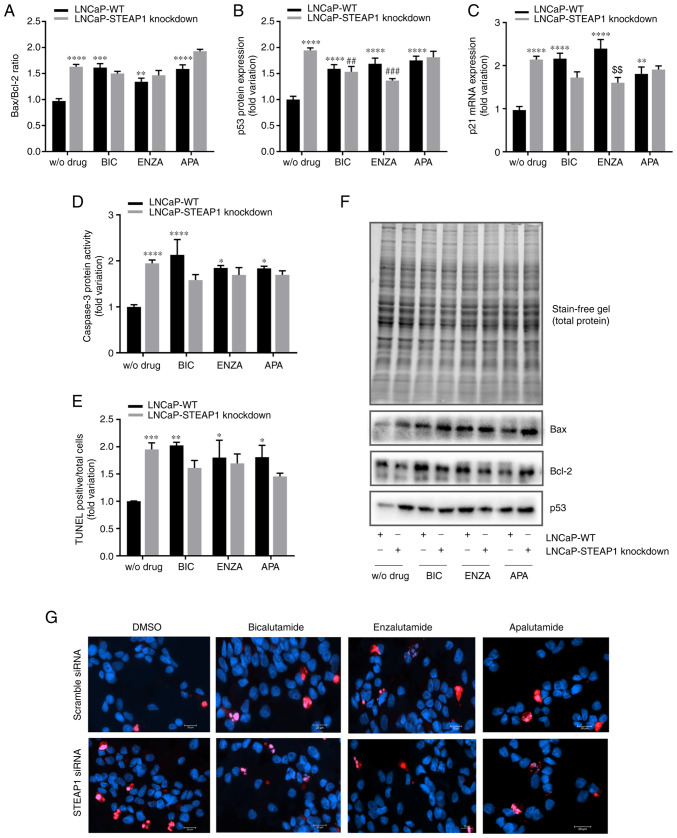
To determine whether the diminished viability/proliferation of LNCaP-STEAP1 knockdown cells in response to anti-androgens treatment was a consequence of increased apoptosis, the expression levels and activity of several apoptotic markers were evaluated. The knockdown of STEAP1 significantly increased the expression of several regulators involved in the apoptosis pathway, Bax/Bcl-2 ratio was increased 1.7-fold (Fig. 4A) and the expression of p53 protein and p21 mRNA were also increased (2.004±0.08- and 2.161±0.16-fold variation, respectively; Fig. 4B and C). A notable end-point of apoptosis is the activation of caspase-3 and the results showed an increased activity of caspase-3 in LNCaP-STEAP1 knockdown (1.944±0.27-fold variation; Fig. 4D) when compared with LNCaP-WT cells. Also, the number of TUNEL-positive cells relative to total cells was significantly increased in the LNCaP-STEAP1 knockdown cells when compared with the scramble siRNA group (1.951±0.12- compared with 1.002±0.004-fold variation; Fig. 4E). Treatment with 100 µM of bicalutamide, 10 µM of enzalutamide and 10 µM of apalutamide triggered an increased expression of apoptotic regulators (Fig. 4). The expression levels of pro- and anti-apoptotic members (Bax and Bcl-2 respectively) led to enhanced Bax/Bcl-2 ratio in response to anti-androgen drugs in LNCaP-WT cells (1.6±0.08- to bicalutamide, 1.3±0.07- to enzalutamide and 1.6±0.08-fold variation to apalutamide; Fig. 4A). However, in LNCaP-STEAP1 knockdown cells, there appeared to be a potentiating effect of Bax/Bcl-2 ratio with apalutamide treatment (1.9±0.04-compared with 1.6±0.08-fold variation; Fig. 4A), but not with bicalutamide and enzalutamide.
Figure 4.
Effect of BIC, ENZA and APA on the expression of several apoptotic regulators in LNCaP-WT and LNCaP-STEAP1 knockdown cells. LNCaP cells were transfected with siRNA targeting STEAP1 or scramble siRNA. 24 h following transfection, LNCaP cells were treated with 100 µM of BIC, or 10 µM of ENZA or 10 µM of APA for 24 h. (A) Bax/Bcl-2 protein ratio, (B) p53 protein expression and (C) p21 mRNA expression were determined by western blotting and reverse transcription-quantitative PCR, respectively. (D) Caspase-3 activity was measured spectrophotometrically by the release of the product pNA and (E) immunofluorescence analysis of TUNEL-positive cells was determined by the TUNEL assay being the results expressed as the mean of TUNEL-positive cells (red staining) relatively to the total cell number [Hoechst 33342 (blue) staining]. (F) Relative protein expression was normalized with total protein load on gel as represented in and relative mRNA expression was normalized with the β2M housekeeping gene. Representative immunoblots are also shown. (G) Representative microscopy images showing TUNEL and Hoechst staining in the different groups were obtained in the AxioImager Z2 fluorescence microscope (magnification, ×400). Results are expressed as fold-variation relative to LNCaP-WT (control group). Error bars indicate mean ± standard error of the mean (n≥2). *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the LNCaP-WT condition; ##P<0.01 and ###P<0.001 vs. the LNCaP-STEAP1 knockdown condition; $$P<0.01 vs. LNCaP-WT plus respective drug. BIC, bicalutamide; ENZA, enzalutamide; APA, apalutamide; WT, wild type; siRNA, small interfering RNA; STEAP1, six transmembrane epithelial antigen of the prostate 1; β2M, β-2-microglobulin; WT, wild type.
The treatment of LNCaP-WT cells with anti-androgenic drugs significantly increased the expression of p53 protein (1.615±0.05- to bicalutamide, 1.689±0.16- to enzalutamide and 1.720±0.12-fold variation to apalutamide) and p21 mRNA (2.119±0.12- to bicalutamide, 2.396±0.001- to enzalutamide and 1.810±0.09-fold variation to apalutamide), as observed in Fig. 4B and C, respectively. The knockdown of STEAP1 did not alter the effect of bicalutamide and enzalutamide in expression of p53 protein (1.559±0.14-compared with 1.615±0.05- and 1.813±0.16 compared with 1.720±0.12-fold variation, respectively) and p21 mRNA (1.738±0.23-compared with 2.119±0.12- and 1.902±0.05 compared with 1.810±0.09-fold variation, respectively). However, the enzalutamide treatment seems to have less effect in LNCaP-STEAP1 knockdown compared with LNCaP-WT cells (1.366±0.02-compared with 1.689±0.16-fold variation to p53 levels and 1.605±0.23 compared with 2.396±0.001-fold variation to p21 levels; Fig. 4B and C).
The activity of caspase-3 significantly increased in LNCaP-STEAP1 knockdown cells treated with bicalutamide, enzalutamide and apalutamide (2.11±0.27-, 1.849±0.24- and 1.836±0.07-fold variation, respectively, compared with 0.999±0.002-fold variation; Fig. 4D); this effect did not significantly alter with silencing of STEAP1 (Fig. 4D).
The results of TUNEL fluorescent immunocytochemistry assay showed that the number of TUNEL-positive LNCaP-STEAP1 knockdown cells were significantly increased with the treatment of bicalutamide, enzalutamide and apalutamide when compared with LNCaP-WT cells (2.154±0.13-, 1.801±0.32- and 1.809±0.22-fold variation, respectively, compared with1.002±0.004-fold variation; Fig. 4E). No significant effect was observed in response to anti-androgen drugs in LNCaP-STEAP1 knockdown cells in comparison with LNCaP-WT cells (Fig. 4E).
In summary, anti-androgen treatment in LNCaP-WT cells increased the expression and activity of several apoptosis regulators. Silencing of STEAP1 did not significantly change the effect observed by bicalutamide, enzalutamide and apalutamide treatment.
Discussion
In the recent decades, the use of ADT to treat PCa patients has notably increased (30,31). However, ADT treatment alone becomes insufficient for the management of PCa, since most patients with this pathology progress to the castration-resistant disease within a few years (32,33). A way of improving PCa treatment is to evaluate combined action with other putative therapeutic targets. There are several proteins that are dysregulated in PCa, including STEAP1 (8). This transmembrane protein has been implicated in several forms of cancer due to its overexpression in malignant tissue compared with their non-malignant counterparts (11,13,34). Considering the oncogenic role of STEAP1 in PCa, associated with a lack of studies focusing on impact of ADT treatment in PCa cells overexpressing STEAP1, the main goals of the present study were to evaluate the effect of anti-androgens on expression of STEAP1 and to investigate if the sensitivity of PCa cells to anti-androgen drugs can be improved in response to STEAP1 knockdown. Thus, the effect of bicalutamide, enzalutamide and apalutamide was evaluated in LNCaP-WT and LNCaP-STEAP1 knockdown cells.
Deregulated cell proliferation and apoptosis are a well-established cancer hallmarks and of the first deregulated mechanisms underlying cancer progression (35). The silencing of STEAP1 was confirmed 24 h following transfection (Fig. 1), decreasing the viability and proliferation of LNCaP cells (Fig. 2 and 3) and accompanied by an increasing of apoptosis (Fig. 4). These results are in accordance with those previously described by our research group (17). Treatment of PCa cells with an antibody against the STEAP1 protein was associated with inhibition of cell growth (36), supporting the present results herein described and the role of STEAP1 as an oncoprotein. The high activity of caspase-3, an effector caspase activated by intrinsic and extrinsic pathway (37) and the high number of TUNEL-positive cells, an established marker of apoptosis by detection of free 3′-OH termini in single-stranded breaks in high-molecular-weight nuclear DNA fragments (38), highlighted the enhanced apoptosis of LNCaP cells in response to STEAP1 knockdown. The intrinsic apoptotic pathway should be involved considering the up- and downregulation of pro- and anti-apoptotic Bax and Bcl-2 proteins, respectively (Fig. 4). Furthermore, the inhibition of cell proliferation and the apoptotic effect triggered by the knockdown of STEAP1 was also supported by the upregulation of p53 and p21 levels (Fig. 4), which are involved in cell cycle arrest at G1 and S phase (39); p53 is also an important inducer of the apoptosis intrinsic pathway (40). The diminished viability and proliferation of LNCaP cells in response to STEAP1 knockdown was corroborated by the downregulation of p-AKT/total AKT and p-ERK/total ERK ratios, two oncogenic survival pathways associated with cancer progression (41,42). These results also supported the hypothesis that reduced activity of AKT may induce the expression of p53. In fact, AKT interacts with the ubiquitin E3 ligase Mdm2, which controls the expression levels and activity of p53 (43,44). AKT enhances Mdm2-mediated p53 ubiquitination and degradation, leading to cell survival (45). Therefore, it is likely to assume that silencing of STEAP1 in LNCaP cells decreased AKT activity, with increased levels of p53, which is associated with the suppression of cell proliferation and activation of apoptosis (40). The precise mechanism underlying the role of STEAP1 in the activation of AKT and ERK pathways remains to be elucidated, though it is possible that AKT and ERK activation may be mediated by increased levels of oxidative stress induced by STEAP1 overexpression (16). This hypothesis is supported by reports demonstrating AKT and ERK activation in response to the increased levels of reactive oxygen species in LNCaP cells (46). However, and in order to overcome the limitation of this study, additional studies should be carried out to clarify the relationship between STEAP1 and AKT/ERK using specific inhibitors, as well as its association with oxidative stress.
The transcription factor c-myc is a master regulator of the transcriptional program that controls cell survival and proliferation (47). Increasing evidence demonstrates that c-myc signaling has a tumor-promoting role and is able to significantly increase proliferation and metastasis of tumors (47–49). Iijima et al (21) showed that the knockdown of STEAP1 leads to cell-growth inhibition in liver cancer by targeting the suppression of c-myc. Unexpectedly, it was observed that silencing of STEAP1 increased c-myc expression in LNCaP PCa cells. Although c-myc is associated with PCa progression, there are several studies showing that it is among the most robust inducers of apoptosis in hematologic diseases, as well as in solid tumors such as breast and lung cancer (50–54). Murphy et al (55) showed that activation of the p53-mediated apoptotic intrinsic pathway requires high levels of c-myc. Thus, the knockdown of STEAP1 in LNCaP cells increased the p-c-myc/total c-myc ratio, which may activate mechanisms of surveillance, such as p53 induction. These changes ultimately culminate in apoptosis, as a way to eliminate cancer cells. On the other hand, the increased levels of c-myc may also be a strategy of cancer cells to overcome the inhibitory effect triggered by STEAP1 knockdown. This possibility cannot be ignored, and more studies should be addressed in the future to clarify the relationship between STEAP1 and c-myc.
It is well documented that the treatment of PCa with anti-androgens, such as bicalutamide, enzalutamide and apalutamide, result in blockage of PCa cell growth due to antagonistic effects on AR transactivation (30,56). At present, it is being evaluated the use of anti-androgens in combination with other therapeutic targets. To date, no studies have focused on effect of anti-androgens on expression of STEAP1 or the effect of combined action between anti-androgens and STEAP1 inhibition in PCa treatment. Therefore, the present study intended to determine the effect of anti-androgens in STEAP1 expression, as well as to evaluate the hypothesis that silencing STEAP1 may improve the effectiveness of anti-androgen therapy.
The present study observed that AR inhibition in LNCaP-WT cells affected STEAP1 expression (Fig. 1). Overall, bicalutamide decreased STEAP1 expression, but enzalutamide and apalutamide increased the levels of STEAP1. Using microarray analysis, Carter et al (57) also showed that STEAP1 is downregulated in LNCaP cells treated with bicalutamide. In contrast to the findings of the present study, Doran et al (25) showed that treatment of CWR22 PCa cells with enzalutamide and apalutamide represses STEAP1 mRNA and protein expression. Beyond the slightly different concentrations used in that Doran study and the present study, the effect may differ between cell lines. Although the precise explanation for different effects between bicalutamide and enzalutamide/apalutamide in STEAP1 expression is unclear, the differences observed might be due to different affinities of these drugs to AR. In fact, it the conformational dynamics of AR with bicalutamide, enzalutamide and apalutamide was evaluated and it was shown that enzalutamide and apalutamide induce different conformational changes in AR compared with bicalutamide (58). In addition, point mutations in AR, namely F877L and T878A, are associated with resistance to enzalutamide and apalutamide, but not to bicalutamide (59–61). Considering that T878A mutation in AR is found in LNCaP cells, one could hypothesize that upregulation of STEAP1 in response to enzalutamide and apalutamide may occur as a mechanism of resistance (62). However, further studies should be performed to clarify the role of STEAP1 in resistance to these drugs.
As expected, anti-androgen drugs efficiently reduced the expression of the KLK3 gene, which is an AR target gene. It suggested that the effect of anti-androgen on STEAP1 expression was dependent on other factors besides the expression of AR. Nevertheless, it should be highlighted that in LNCaP cells, the knockdown of STEAP1 inhibited the expression of the KLK3 gene. This result is in accordance with the results previously described by our research group, demonstrating that the proliferative effect of DHT is abrogated in LNCaP cells knocked down for STEAP1 (17). Moreover, these findings are also supported by Ihlaseh-Catalano et al (26), who describe a positive strong trend between STEAP1 and PSA levels. The present study explored the cellular pathways of proliferation and apoptosis of anti-androgen treatment in LNCaP cells. Data obtained from LNCaP-WT cells treated with bicalutamide, enzalutamide and apalutamide showed a reduction on cell viability, determined by MTT assay, and cell proliferation, as indicated by the estimated cell proliferation index assessed by the Ki-67 fluorescent immunocytochemistry (Fig. 2). Anti-androgen effects modulating PCa cells behavior has been underpinned by alterations on key protein targets associated with cell proliferation, survival and oncogenic pathways, namely the AKT and ERK signaling pathway (41,42,63). The results of the present study showed a significant decreased of p-AKT/AKT ratio in response to bicalutamide, enzalutamide and apalutamide treatment in LNCaP-WT cells (Fig. 3), but no significant differences in p-ERK/ERK ratio were observed. Altogether, these results suggested that treatment of LNCaP cells with anti-androgen drugs inhibited the signaling pathways associated with cell proliferation and survival in an independent manner of their effect in expression of STEAP1, at least in early treatment phase.
Considering the coordinated action of c-myc and AR in PCa development (64,65), the effect of anti-androgens was evaluated. The results showed an increased expression of p-c-myc with bicalutamide, enzalutamide and apalutamide exposure of LNCaP-WT cells (Fig. 3). These findings are in line with a recent study showing that androgen deprivation in vitro and castration in vivo leads to rapid and persistent increases in c-myc expression (66). This observation suggests that decreased AR activity can be compensated by increased levels of c-myc, contributing to progression to castrate-resistant PCa following ADT.
Anti-androgen treatment in LNCaP-WT cells was also characterized by the increased expression and activity of several apoptosis regulators (Fig. 4). These results are in agreement with other studies describing the apoptotic effect of anti-androgens in PCa cells (67–69). Furthermore, a few reports have shown that administration of anti-androgens in combination with other anti-cancer drugs trigger cytotoxic effects in PCa (70–72). The co-administration of enzalutamide and abiraterone (an inhibitor of the steroidal enzyme CYP17A1) inhibits the proliferation and promotes the apoptosis of LNCaP cells (70) and enzalutamide combined with AS602801 (an inhibitor of c-Jun N-terminal kinase) synergistically kills PCa cells, decreasing their migration and invasion capacity (71). In addition, apalutamide, in combination with autophagy inhibitors, provides a significantly elevated anti-tumor effect in LNCaP cells (72). However, the present study is the first, to the best of the authors' knowledge, to evaluate the combined effect of STEAP1 knockdown with anti-androgen therapy on PCa cells. LNCaP-STEAP1 knockdown cells treated with bicalutamide, enzalutamide and apalutamide exhibited a decrease on cell viability and proliferation, but no significant differences were observed in comparison with the effect of these anti-androgens in LNCaP-WT cells (Fig. 2). Similar data was observed regarding the effect of bicalutamide and enzalutamide in p-AKT/AKT and p-ERK/ERK ratios (Fig. 3). These observations are in accordance with increased expression and activity of regulators/effectors of apoptosis in response to anti-androgen treatment and no significant differences between LNCaP-WT and LNCaP-STEAP1 knockdown cells were detected (Fig. 4). Thus, the results suggested that there is no synergistic effect between the silencing of STEAP1 and anti-androgens treatment, at least in LNCaP cells treated for 24 h with anti-androgen drugs.
Unexpectedly, an additive effect of c-myc expression levels in LNCaP cells knocked-down for STEAP1 and treated with bicalutamide, enzalutamide and apalutamide was observed (Fig. 3). To the best of the authors' knowledge, there are no studies corroborating these discoveries and further studies are needed to improve understanding of the role of c-myc in response to combined action between anti-androgen and STEAP1 knockdown in PCa cells.
In conclusion, the present findings showed that anti-androgen drugs affected the regulation of STEAP1 expression, but inhibition of STEAP1 did not alter the response of LNCaP cells to anti-androgen treatment. Although the levels of STEAP1 in LNCaP cells did not seem to change the effect of anti-androgens in cell proliferation and apoptosis, the synergic effect in p-c-myc levels deserves attention in future studies. Despite the limitations concerning the use of only one PCa cell line and the unique concentration and time of exposure tested for the anti-androgen drugs, the present study strengthened the potential use of STEAP1 knockdown in PCa therapy, as well as opening new avenues of research aimed at exploring the mechanisms underlying the role of STEAP1 in human PCa. Further studies deepening the role of STEAP1 in response to anti-androgen drugs and investigating its actions in the development of PCa resistance to treatments would be fundamental for improved management of the disease.
Acknowledgments
Not applicable.
Funding Statement
The authors acknowledge the Sandra M Rocha's individual PhD Fellowship (grant nos. SFRH/BD/115693/2016 and COVID/BD/151732/2021) from FCT-Fundação para a Ciência e Tecnologia. The present study was funded by FEDER funds through the POCI-COMPETE 2020-Operational Program Competitiveness and Internationalization in Axis I-Strengthening research, technological development and innovation (Project No. 029114) and developed within the scope of the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES. This work was also supported by the European Regional Development Fund through the Programa Operacional Regional do Centro (Centro 2020)-Sistema de Apoio à Investigação Científica e Tecnológica-Programas Integrados de IC&DT (Project Centro-01-0145-FEDER-000019-C4-Centro de Competências em Cloud Computing). The microscopy facility used in the development of this work is part of the PPBI-Portuguese Platform of BioImaging and is partially supported by the Project POCI-01-0145-FEDER-022122.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
SMR and CJM conceived and designed the study and wrote the manuscript. SMR, DN and AMC performed experiments. LP analyzed and interpreted data. SS participated in the study design, data analysis and revision of the manuscript. SMR and CJM confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223–238. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, Iversen P, Eisenberger MA, Labrie F. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J Urol. 2018;200:956–966. doi: 10.1016/j.juro.2018.04.083. [DOI] [PubMed] [Google Scholar]
- 6.Murray TBJ. The pathogenesis of prostate cancer. In: Bott SRJ, Ng KL, editors. Prostate Cancer [Internet] Exon Publications; Brisbane, AU: 2021. pp. 29–42. [DOI] [PubMed] [Google Scholar]
- 7.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, Madraswala R, Zhou Y, Kuo J, Raitano AB, et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–14528. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha SM, Sousa I, Gomes IM, Arinto P, Costa-Pinheiro P, Coutinho E, Santos CR, Jerónimo C, Lemos MC, Passarinha LA, et al. Promoter demethylation upregulates STEAP1 gene expression in human prostate cancer: In vitro and in silico analysis. Life (Basel) 2021;11:1251. doi: 10.3390/life11111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maitland NJ, Frame FM, Polson ES, Lewis JL, Collins AT. Prostate cancer stem cells: Do they have a basal or luminal phenotype? Horm Cancer. 2011;2:47–61. doi: 10.1007/s12672-010-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WJ, Wu HT, Li CL, Lin YK, Fang ZX, Lin WT, Liu J. Regulatory roles of six-transmembrane epithelial antigen of the prostate family members in the occurrence and development of malignant tumors. Front Cell Dev Biol. 2021;9:752426. doi: 10.3389/fcell.2021.752426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barroca-Ferreira J, Pais JP, Santos MM, Goncalves AM, Gomes IM, Sousa I, Rocha SM, Passarinha LA, Maia CJ. Targeting STEAP1 protein in human cancer: Current trends and future challenges. Curr Cancer Drug Targets. 2018;18:222–230. doi: 10.2174/1568009617666170427103732. [DOI] [PubMed] [Google Scholar]
- 13.Rocha SM, Socorro S, Passarinha LA, Maia CJ. Comprehensive landscape of STEAP family members expression in human cancers: Unraveling the potential usefulness in clinical practice using integrated bioinformatics analysis. Data. 2022;7:64. doi: 10.3390/data7050064. [DOI] [Google Scholar]
- 14.Challita-Eid PM, Morrison K, Etessami S, An Z, Morrison KJ, Perez-Villar JJ, Raitano AB, Jia XC, Gudas JM, Kanner SB, Jakobovits A. Monoclonal antibodies to six-transmembrane epithelial antigen of the prostate-1 inhibit intercellular communication in vitro and growth of human tumor xenografts in vivo. Cancer Res. 2007;67:5798–5805. doi: 10.1158/0008-5472.CAN-06-3849. [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Mitra S, Wu G, Berka V, Song J, Yu Y, Poget S, Wang DN, Tsai AL, Zhou M. Six-transmembrane epithelial antigen of prostate 1 (STEAP1) has a single b heme and is capable of reducing metal ion complexes and oxygen. Biochemistry. 2006;55:6673–6684. doi: 10.1021/acs.biochem.6b00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunewald TGP, Diebold I, Esposito I, Plehm S, Hauer K, Thiel U, da Silva-Buttkus P, Neff F, Unland R, Müller-Tidow C, et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol Cancer Res. 2012;10:52–65. doi: 10.1158/1541-7786.MCR-11-0524. [DOI] [PubMed] [Google Scholar]
- 17.Gomes IM, Rocha SM, Gaspar C, Alvelos MI, Santos CR, Socorro S, Maia CJ. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol. 2018;35:40. doi: 10.1007/s12032-018-1100-0. [DOI] [PubMed] [Google Scholar]
- 18.Huo SF, Shang WL, Yu M, Ren XP, Wen HX, Chai CY, Sun L, Hui K, Liu LH, Wei SH, et al. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci Rep. 2020;40:BSR20193169. doi: 10.1042/BSR20193169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao Z, Huang L, Sun J, Xie J, Wang T, Yin X, Zhang H, Chen J. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem Cell Biol. 2020;154:215–230. doi: 10.1007/s00418-020-01877-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Hou WB, Zhang C, Tan YE, Zhang DD, An W, Pan SW, Wu WD, Chen QC, Xu HM. A research of STEAP1 regulated gastric cancer cell proliferation, migration and invasion in vitro and in vivos. J Cell Mol Med. 2020;24:14217–14230. doi: 10.1111/jcmm.16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iijima K, Nakamura H, Takada K, Hayasaka N, Kubo T, Umeyama Y, Iyama S, Miyanishi K, Kobune M, Kato J. Six-transmembrane epithelial antigen of the prostate 1 accelerates cell proliferation by targeting c-Myc in liver cancer cells. Oncol Lett. 2021;22:546. doi: 10.3892/ol.2021.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques RB, Dits NF, Erkens-Schulze S, van Weerden WM, Jenster G. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PLoS One. 2010;5:e13500. doi: 10.1371/journal.pone.0013500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes IM, Santos CR, Socorro S, Maia CJ. Six transmembrane epithelial antigen of the prostate 1 is down-regulated by sex hormones in prostate cells. Prostate. 2013;73:605–613. doi: 10.1002/pros.22601. [DOI] [PubMed] [Google Scholar]
- 24.Marques RB, Dits NF, Erkens-Schulze S, van IJcken WFJ, van Weerden WM, Jenster G. Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines. PLoS One. 2011;6:e23144. doi: 10.1371/journal.pone.0023144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doran MG, Watson PA, Cheal SM, Spratt DE, Wongvipat J, Steckler JM, Carrasquillo JA, Evans MJ, Lewis JS. Annotating STEAP1 regulation in prostate cancer with 89Zr Immuno-PET. J Nucl Med. 2014;55:2045–2049. doi: 10.2967/jnumed.114.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ihlaseh-Catalano SM, Drigo SA, de Jesus CMN, Domingues MAC, Aparecida C, Filho JCS, de Camargo JLV, Rogatto SR. STEAP1 protein overexpression is an independent marker for biochemical recurrence in prostate carcinoma. Histopathology. 2013;63:678–685. doi: 10.1111/his.12226. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. Quantification strategies in real-time PCR. In: Bustin SA, editor. A-Z of Quantitative PCR. International University Line (IUL); La Jolla: 2004. pp. 89–113. [Google Scholar]
- 28.Neris RLS, Dobles AMC, Gomes AV. Western blotting using in-gel protein labeling as a normalization control: Advantages of stain-free technology. Methods Mol Biol. 2021;2261:443–456. doi: 10.1007/978-1-0716-1186-9_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posch A, Kohn J, Oh K, Hammond M, Liu N. V3 stain-free workflow for a practical, convenient, and reliable total protein loading control in western blotting. J Vis Exp. 2013;30:50948. doi: 10.3791/50948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Lanz C, Bennamoun M, Macek P, Cathelineau X, Sanchez-Salas R. The importance of antiandrogen in prostate cancer treatment. Ann Transl Med. 2019;7:S362–S362. doi: 10.21037/atm.2019.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, et al. Management of patients with advanced prostate cancer: The report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178–211. doi: 10.1016/j.eururo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Morgans AK, Beltran H. Isn't androgen deprivation enough? Optimal treatment for newly diagnosed metastatic prostate cancer. J Clin Oncol. 2022;40:818–824. doi: 10.1200/JCO.21.02530. [DOI] [PubMed] [Google Scholar]
- 34.Gomes IM, Arinto P, Lopes C, Santos CR, Maia CJ. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason score. Urol Oncol Semin Orig Investig. 2014;32:53.e23–53.e29. doi: 10.1016/j.urolonc.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Tamura Y, Kobayashi JI, Kamiguchi K, Hirohashi Y, Miyazaki A, Torigoe T, Asanuma H, Hiratsuka H, Sato N. Six-transmembrane epithelial antigen of the prostate-1 plays a role for in vivo tumor growth via intercellular communication. Exp Cell Res. 2013;319:2617–2626. doi: 10.1016/j.yexcr.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Green DR. Caspases and their substrates. Cold Spring Harb Perspect Biol. 2022;14:a041012. doi: 10.1101/cshperspect.a041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–47. doi: 10.1007/978-1-61779-860-3_5. [DOI] [PubMed] [Google Scholar]
- 39.He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, Khokhar AR, Kuang J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene. 2005;24:2929–2943. doi: 10.1038/sj.onc.1208474. [DOI] [PubMed] [Google Scholar]
- 40.Demir Ö, Barros EP, Offutt TL, Rosenfeld M, Amaro RE. An integrated view of p53 dynamics, function, and reactivation. Curr Opin Struct Biol. 2021;67:187–194. doi: 10.1016/j.sbi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: An updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 42.Cao Z, Liao Q, Su M, Huang K, Jin J, Cao D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019;459:30–40. doi: 10.1016/j.canlet.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb TM, Leal JFM, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- 44.Momand J, Wu HH, Dasgupta G. MDM2-master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/S0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 45.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 46.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labbé DP, Brown M. Transcriptional regulation in prostate cancer. Cold Spring Harb Perspect Med. 2018;8:a030437. doi: 10.1101/cshperspect.a030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faskhoudi MA, Molaei P, Sadrkhanloo M, Orouei S, Hashemi M, Bokaie S, Rashidi M, Entezari M, Zarrabi A, Hushmandi K, et al. Molecular landscape of c-Myc signaling in prostate cancer: A roadmap to clinical translation. Pathol Res Pract. 2022;233:153851. doi: 10.1016/j.prp.2022.153851. [DOI] [PubMed] [Google Scholar]
- 50.Uribesalgo I, Benitah SA, Croce LD. From oncogene to tumor suppressor: The dual role of Myc in leukemia. Cell Cycle. 2012;11:1757–1764. doi: 10.4161/cc.19883. [DOI] [PubMed] [Google Scholar]
- 51.McMahon SB. MYC and the control of apoptosis. Cold Spring Harb Perspect Med. 2014;4:a014407. doi: 10.1101/cshperspect.a014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams CM, Eischen CM. Histone deacetylase inhibition reveals a tumor-suppressive function of MYC-regulated miRNA in breast and lung carcinoma. Cell Death Differ. 2016;23:1312–1321. doi: 10.1038/cdd.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muthalagu N, Junttila MR, Wiese KE, Wolf E, Morton J, Bauer B, Evan GI, Eilers M, Murphy DJ. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell Rep. 2014;8:1347–1353. doi: 10.1016/j.celrep.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 55.Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Student S, Hejmo T, Poterała-Hejmo A, Leśniak A, Bułdak R. Anti-androgen hormonal therapy for cancer and other diseases. Eur J Pharmacol. 2020;866:172783. doi: 10.1016/j.ejphar.2019.172783. [DOI] [PubMed] [Google Scholar]
- 57.Carter SL, Centenera MM, Tilley WD, Selth LA, Butler LM. IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor. BMC Cancer. 2016;16:141. doi: 10.1186/s12885-016-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gim HJ, Park J, Jung ME, Houk KN. Conformational dynamics of androgen receptors bound to agonists and antagonists. Sci Rep. 2021;11:15887. doi: 10.1038/s41598-021-94707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rathkopf DE, Smith MR, Ryan CJ, Berry WR, Shore ND, Liu G, Higano CS, Alumkal JJ, Hauke R, Tutrone RF, et al. Androgen receptor mutations in patients with castration-resistant prostate cancer treated with apalutamide. Ann Oncol. 2017;28:2264–2271. doi: 10.1093/annonc/mdx283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, Chen Y, Greene GL, Shen Y, Sawyers CL. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B, Hager JH. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 62.Sun C, Shi Y, Xu LL, Nageswararao C, Davis LD, Segawa T, Dobi A, McLeod DG, Srivastava S. Androgen receptor mutation (T877A) promotes prostate cancer cell growth and cell survival. Oncogene. 2006;25:3905–3913. doi: 10.1038/sj.onc.1209424. [DOI] [PubMed] [Google Scholar]
- 63.Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci. 2020;21:4507. doi: 10.3390/ijms21124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu X, Boufaied N, Hallal T, Feit A, de Polo A, Luoma AM, Alahmadi W, Larocque J, Zadra G, Xie Y, et al. MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat Commun. 2022;13:2559. doi: 10.1038/s41467-022-30257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barfeld SJ, Urbanucci A, Itkonen HM, Fazli L, Hicks JL, Thiede B, Rennie PS, Yegnasubramanian S, DeMarzo AM, Mills IG. c-myc antagonises the transcriptional activity of the androgen receptor in prostate cancer affecting key gene networks. EBioMedicine. 2017;18:83–93. doi: 10.1016/j.ebiom.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo H, Wu Y, Nouri M, Spisak S, Russo JW, Sowalsky AG, Pomerantz MM, Wei Z, Korthauer K, Seo JH, et al. Androgen receptor and MYC equilibration centralizes on developmental super-enhancer. Nat Commun. 2021;12:7308. doi: 10.1038/s41467-021-27077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee ECY, Zhan P, Schallhom R, Packman K, Tenniswood M. Antiandrogen-induced cell death in LNCaP human prostate cancer cells. Cell Death Differ. 2003;10:761–771. doi: 10.1038/sj.cdd.4401228. [DOI] [PubMed] [Google Scholar]
- 68.Guerrero J, Alfaro IE, Gómez F, Protter AA, Bernales S. Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate. 2013;73:1291–1305. doi: 10.1002/pros.22674. [DOI] [PubMed] [Google Scholar]
- 69.Koukourakis MI, Kakouratos C, Kalamida D, Mitrakas A, Pouliliou S, Xanthopoulou E, Papadopoulou E, Fasoulaki V, Giatromanolaki A. Comparison of the effect of the antiandrogen apalutamide (ARN-509) versus bicalutamide on the androgen receptor pathway in prostate cancer cell lines. Anticancer Drugs. 2018;29:323–333. doi: 10.1097/CAD.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 70.Han J, Zhang J, Zhang W, Zhang D, Li Y, Zhang J, Zhang Y, Diao T, Cui L, Li W, et al. Abiraterone and MDV3100 inhibits the proliferation and promotes the apoptosis of prostate cancer cells through mitophagy. Cancer Cell Int. 2019;19:332. doi: 10.1186/s12935-019-1021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Sun C, Tao S, Osunkoya AO, Arnold RS, Petros JA, Zu X, Moreno CS. The JNK inhibitor AS602801 synergizes with enzalutamide to kill prostate cancer cells in vitro and in vivo and inhibit androgen receptor expression. Transl Oncol. 2020;13:100751. doi: 10.1016/j.tranon.2020.100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eberli D, Kranzbühler B, Mortezavi A, Sulser T, Salemi S. Apalutamide in combination with autophagy inhibitors improves treatment effects in prostate cancer cells. Urol Oncol. 2020;38:683.e19–683.e26. doi: 10.1016/j.urolonc.2020.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.