Abstract
Objectives: To better understand the clinical profile of patients attending a large Australian pediatric gender service. Retrospective clinical audit of patients seen at the Royal Children’s Hospital Gender Service (RCHGS) over 10 years (2007-16).
Setting: The RCHGS: Australia’s largest pediatric gender service.
Participants: Patients were eligible for inclusion if they had an appointment with the RCHGS between January 2007 - December 2016, and had either a self-reported gender which differed from what was presumed for them at birth or sought guidance regarding gender identity/expression.
Main outcome measures: Demographic/developmental history, clinical presentation including information about gender identity/dysphoria, comorbidities, self-harm, suicidal ideation, gender-affirming treatment, psychosocial functioning.
Results: 359 patients were first seen during the study period. Assigned females (54%) slightly outnumbered assigned males (46%), and presented at an older age (14.8 vs 12.4 years. Patients predominantly identified as transgender (87.2%) or non-binary (7.2%). Across the cohort, gender diversity was evident from a young age (median age 3), and symptoms of gender dysphoria were noted earlier in assigned males (median age 4) than assigned females (median age 11). Although 81% of patients met eligibility for GD, rates of hormonal treatment were much lower, with 29% of young people ≥10 years of age receiving puberty blocking treatment and 38% of adolescents ≥ 16 years of age receiving gender-affirming hormones (i.e. testosterone or estrogen). Many patients had mental health difficulties and/or neurodevelopment disorders, including major depressive disorder/low mood (51%), self-harm (25%), suicidal ideation (30%) and autism spectrum disorder (16%).
Conclusion: This audit illustrates the complex profile and needs of transgender and gender diverse children and adolescents presenting to specialist gender services.
Supplemental data for this article is available online at https://doi.org/10.1080/26895269.2021.1939221 .
Keywords: gender dysphoria, gender identity, health services research, pediatric medicine
Introduction
Increased numbers of individuals are identifying as transgender and gender diverse (TGD)(Meerwijk & Sevelius, 2017). TGD individuals have a gender identity which differs from what was presumed for them at birth (the latter of which will be referred to hereafter as ‘assigned gender/male/female’). In a survey of 6,327 Australian students in grades 10-12, 2.3% identified as TGD (Fisher et al., 2019). These rates are comparable to studies from around the world, with rates of 1.2% reported in students from New Zealand (Clark et al., 2014) and 2.7% in the USA (Gower et al., 2018) for example.
Many TGD children and adolescents experience gender dysphoria (GD), which is the distress that results from the incongruence between an individual’s assigned gender and their inner gender identity. In conjunction with GD, many TGD individuals experience mental health problems, including depression, anxiety, self-harm and attempted suicide (Strauss et al., 2017; Surace et al., 2020). Negative social experiences including bullying, assault, and discrimination are also common (Strauss et al., 2017; Wirtz et al., 2020) and these experiences are likely to drive poor mental health in this population.
Medical interventions for TGD children and adolescents were first initiated in the Netherlands in the late 1990s. Since then, referrals for medical care for TGD children and adolescents have dramatically risen in many countries, for example in the USA (Chen et al., 2016; Handler et al., 2019) and England (The Tavistock and Portman NHS Foundation Trust, 2018). The Royal Children’s Hospital Gender Service (RCHGS) in Melbourne, Australia, is Australia’s largest pediatric gender service and has seen a similar trend, from a single referral since its inception in 2003 to 336 in 2019. The RCHGS currently provides multidisciplinary, gender-affirming healthcare for TGD children and adolescents throughout the entire state of Victoria, Australia. All RCHGS patients receive psychosocial support, while medical interventions to help manage GD are available where appropriate, and can include menses suppression, anti-androgens, Gonadotropin releasing hormone analogues (GnRHa, “puberty blockers”), and gender affirming hormones (GAH; estrogen or testosterone). In a situation unique to Australia, those under the age of 18 years required approval from the Family Court of Australia for commencement of puberty blockers until July 2013 (Re: Jamie, 2013) and for GAH until November 2017 (Re: Kelvin, 2017). Although court authorization is now no longer required in the majority of cases, it is however still needed to access medical treatment in certain circumstances (for example, where there is dispute over the diagnosis/treatment of GD or the Gillick competence of the adolescent, or where a parent/guardian does not consent to treatment) (Re: Imogen, 2020).
With increasing clinical demand for this relatively new area of health care, it is important to better understand the clinical profile of TGD patients to provide optimal care. Chart reviews of pediatric gender services around the world have been undertaken to serve this purpose (e.g., Chen et al., 2016; de Graaf et al., 2018; Spack et al., 2012), but the only previous review of Australian data published in 2012 included just 39 patients at the RCHGS (Hewitt et al., 2012). Given the rapid increase in patient numbers since then as well as emerging international trends – such as an unexplained increase in the proportion of assigned females referred to pediatric gender clinics (de Graaf et al., 2018) – we felt it timely to reexamine the cohort of patients attending the RCHGS. This study therefore aimed to describe the clinical profile of TGD children and adolescents who presented to the RCHGS during the 10-year period leading up to the change in requirements for Family Court approval of GAH (2007-16), since access to GAH was relatively limited during this period. In doing so, we focused on not only gender presentation and subsequent hormonal interventions but also broader clinical features of patients attending the RCHGS.
Materials and methods
Setting
The RCHGS is currently the largest multidisciplinary pediatric gender service in Australia. The service provides TGD children and adolescents throughout Victoria with support, assessment and a range of gender affirming treatments to help manage GD. Young people attending the RCHGS are seen by a designated mental health clinician (either a clinical psychologist or psychiatrist) and a pediatrician, both of whom specialize in transgender health and are trained in the assessment of GD during childhood and adolescence.
Data collection
Assessments typically take place over a series of face-to-face appointments spanning several months, during which information is collected by clinicians from both the young person and their parents. Specifically, clinicians gather information about a young person’s gender identity, gender diversity, gender expression and GD – often with the aid of relevant assessment tools – as well as broader information regarding the young person’s mental and physical health, all of which is stored in the patient’s medical record.
Patients
A retrospective file audit of medical records of patients seen at the RCHGS from January 1, 2007, to December 31, 2016 was undertaken. To be included, patients needed to have attended their first appointment with RCHGS during this time, and either had a self-reported gender identity which differed from what was presumed for them at birth (as reported during any stage of their clinical assessment) or sought clinical guidance regarding their gender. The RCHGS registry revealed 359 patients meeting eligibility for inclusion in this study.
Data extraction
Electronic medical records were retrieved via each patient’s unique hospital record number and checked for eligibility against the inclusion criteria. During 2017, data were extracted from patients’ medical records using a standardized data extraction form in EpiData (Christiansen & Lauritsen, 2010). This form comprised predetermined fields and responses and was used in conjunction with a data dictionary to ensure integrity and consistency in the data extraction process. Data was extracted directly from clinician-recorded notes collected at the time of each clinic appointment with patients and/or their parents/caregivers, and included: patient demographics; postcode; developmental history; gender identity, gender diversity and GD symptoms; comorbidities; history of low mood, self-harm, and suicidal ideation; gender-affirming treatment received; psychosocial and school functioning. To classify each patient’s gender identity at time of initial presentation, their gender identity first mentioned in the file was compared with their assigned gender and categories of ‘transgender’, ‘non-binary’, ‘cisgender’ or ‘not sure’ were assigned.
In addition, confirmatory diagnostic information about GD was gathered from clinicians at the time of audit. For each patient, this involved either their mental health clinician or pediatrician providing the research team with information about whether the patient met DSM-5 criteria for a diagnosis for GD, and clinicians were encouraged to refer to their clinical notes in order to reduce the likelihood of recall bias.
The Index of Relative Socio-Economic Advantage and Disadvantage from the Australian Bureau of Statistics (Pink, 2013) was used to measure socio-economic advantage and disadvantage based on patient postcode, and RCHGS patients were classified into one of five possible reference quintiles, ranked from least to most advantaged.
Data analysis
Data from EpiData were exported and analyzed in Stata/IC (version 15.0). Descriptive statistics were used to characterize patients. Unless specified, presented proportions were based on N = 359. Proportion tests were used to examine differences in proportions by assigned gender. Quantile regressions were used to determine differences in medians according to assigned gender.
Ethics
This study was approved by the Royal Children’s Hospital Human Research Ethics Committee (#36323).
Results
Who was seen at RCHGS?
Demographics
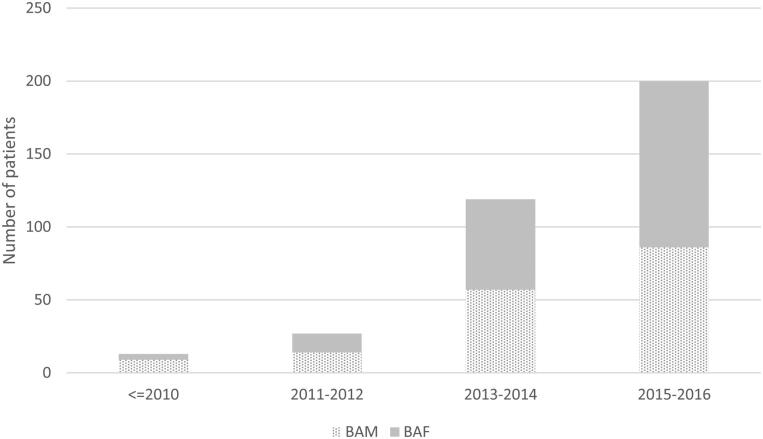
There were 359 patients first seen at the service over the review period. Referral numbers increased rapidly over time from 13 referrals received before 2011 to 200 received between 2015 − 2016 (Figure 1). Slightly more assigned females (n = 193, 53.8%) than assigned males (n = 166 46.2%) were seen.
Figure 1.
Time of referral for patients seen at RCHGS between 2007- 2016 (n = 359).
The median age at first presentation was 14.3 years (IQR 5.8, range 3.6-18.1). Assigned females had a median age of 14.8 years (IQR 3.1, range 3.6-18.1), while assigned males presented younger (M = 12.4 years, IQR 8.6, range 3.8-17.9) (diff 2.4 years; 95% CI 1.3-3.6; p < 0.001).
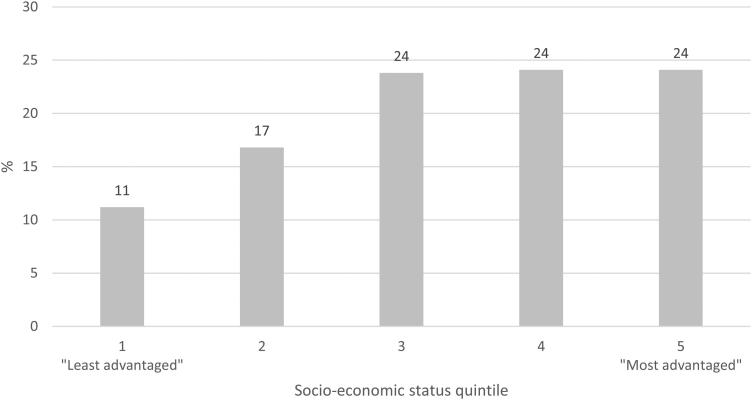
Figure 2 shows the proportion of patients according to socioeconomic status, with an under-representation of those from the least advantaged neighborhoods observed.
Figure 2.
Socio-economic profile of patients seen at RCHGS between 2007- 2016 (n = 359). Using postcode and SEIFA data, information on the overall socioeconomic status of each patient’s suburb was extracted and categorized into quintile-based categories.
Gender identity
The vast majority of patients initially presented as transgender (87.2%). A small proportion were non-binary (7.2%) which describes someone who does not identify exclusively as male or female. Some were unsure of their gender identity (3.9%) and a minority identified with the gender presumed for them at birth (1.7%) (Table 1).
Table 1.
Gender identity of patients at RCHGS.
|
Assigned Male
(N = 166) |
Assigned Female
(N = 193) |
Total
(N = 359) |
||||
|---|---|---|---|---|---|---|
| n | (%) | N | (%) | N | (%) | |
| Gender identity a | ||||||
| Transgender | 139 | (83.7) | 174 | (90.2) | 313 | (87.2) |
| Non-binary | 14 | (8.4) | 12 | (6.2) | 26 | (7.2) |
| Cisgender | 4 | (2.4) | 2 | (1.0) | 6 | (1.7) |
| Not sure | 9 | (5.4) | 5 | (2.6) | 14 | (3.9) |
aTransgender and cisgender gender identity were determined by comparing gender identity first mentioned in patients’ medical record against birth-assigned gender. Gender identities labeled as ‘non-binary’ and ‘not sure’ were based on gender identity first mentioned in patients’ medical records.
For 11.5% of patients (n = 41), the gender identity recorded in their files changed at least once (Table 2). Table 3 details the nature of these changes. Of those who were transgender at presentation, 3.5% and 2.6% later identified as cisgender and non-binary respectively. Of the 26 patients who identified as non-binary at presentation, 31% later identified as transgender and 11.5% cisgender.
Table 2.
Consistency of gender identity of patients at RCHGS.
|
Assigned Male
N = 165 |
Assigned Female
N = 191 |
Total
N = 356 |
||||
|---|---|---|---|---|---|---|
| n | (%) | N | (%) | N | (%) | |
| Consistency of gender identity over time | ||||||
| Gender identity did not change | 144 | (87.3) | 171 | (89.5) | 315 | (88.5) |
| Gender identity changed | 21 | (12.7) | 20 | (10.5) | 41 | (11.5) |
In Table 2, Total was based on N = 356 not N = 359 due to missing data: n = 3 patients did not have information about gender identity over time in their file.
Table 3.
Gender identity change over time.
| Changed to…… |
||||
|---|---|---|---|---|
| Transgender | Cisgender | Non-binary | Not sure | |
| n (%) | n (%) | n (%) | n (%) | |
| Transgender (n = 313) to | n/a | 11 (3.5) | 8 (2.6) | 1 (0.3) |
| Cisgender (n = 6) to | 2 (33.3) | n/a | 1 (16.7) | 1 (16.7) |
| Non-binary (n = 26) to | 8 (30.8) | 3 (11.5) | n/a | 0 (0) |
| Not sure (n = 14) to | 2 (14.3) | 0 (0) | 4 (28.6) | n/a |
Note. Table 3 reflects first identity and last identity recorded in patients’ files.
Gender diversity and dysphoria
The median age at which gender diversity (identified as variation in gender identity and/or expression from that traditionally associated with their assigned gender) was first expressed was significantly higher for assigned females (4.8 years) than assigned males (3 years) (quantile regression: diff 2; 95% CI 0.71-3.29; p = 0.003) (Table 4).
Table 4.
Features of gender diversity and dysphoria among RCHGS patients.
| Assigned Male |
Assigned Female |
Total |
||||
|---|---|---|---|---|---|---|
| Median | (IQR, range) | Median | (IQR, range) | Median | (IQR, range) | |
| N = 163 | N = 190 | N = 353 | ||||
| Age first expressed gender diversity | 3 | (4, 1.5-17.0) | 4.8 | (9, 2-16.8) | 3 | (8, 1.5-17.0) |
| N = 159 | N = 191 | N = 350 | ||||
| Age first experienced GD symptoms | 4 | (10, 1.5-17.0) | 11 | (10, 2-16.8) | 8 | (9, 1.5-17.0) |
| n | (%) | n | (%) | n | (%) | |
| N = 166 | N = 193 | N = 359 | ||||
| GD diagnosis | ||||||
| Met criteria | 126 | (75.9) | 165 | (85.5) | 291 | (81.1) |
| N = 145 | N = 172 | N = 317 | ||||
| Parental support of gender diversity | ||||||
| Both parents supportive | 103 | (71.1) | 121 | (70.4) | 224 | (70.7) |
| Mother (only) or father (only) supportive | 37 | (25.5) | 46 | (26.7) | 83 | (26.2) |
| Neither parent/caregiver supportive | 5 | (3.5) | 5 | (2.9) | 10 | (3.2) |
Note. Median age first expressed gender diversity was based on N = 353, n = 6 had missing data on this variable. Median age first experienced GD symptoms was based on N = 350, n = 9 had missing data on this variable.
The median age at which young people began experiencing GD symptoms was significantly lower in assigned males (age 4 years) than assigned females (age 11 years) (quantile median regression: diff 7; 95% CI 4.9-9.1; p < 0.001). Consistent with this, a minority of assigned males (n = 47; 29.6%) had GD symptoms commence at or after the typical age of pubertal onset (∼11 years in assigned males, ∼10 years in assigned females) (Brix et al., 2019), while this was the case for a majority of assigned females (n = 104; 54.5%). Nevertheless, among these individuals, 42.6% of assigned males and 40.8% of assigned females had previously expressed gender diversity prior to this.
Based on the diagnostic information about GD gathered from clinicians at time of audit, 81.1% of patients met DSM-5 criteria for GD. Of note, the vast majority of RCHGS patients (96.9%) had at least one parent who supported their gender diversity.
Medical treatment
The proportion of patients who received medical intervention for GD - including GnRHa, menses suppression, anti-androgens, and/or GAH - was determined in a subcohort of 234 patients (112 assigned females, 122 assigned males). This subcohort comprised only those patients within the main cohort who were first seen before 1 July, 2016. We created this subcohort so that the proportion of young people who had commenced medical treatment was based on only those patients who were seen at the service at least 6 months before the end of the review period; in this way, we were able to account for the up to six month assessment period usually required before a clinical decision is made regarding the commencement of such treatment (Supplementary table 1). Among these patients, 23% (54/234) were treated with GnRHa, with assigned males (39/122, 32%) more likely to receive puberty blockers than assigned females (15/112, 13.4%), and just a single patient stopping this treatment without progressing to GAH. 20.5% received GAH, with assigned females (29/112; 25.9%) more likely to receive testosterone than assigned males (19/122; 15.6%) were to receive estrogen, and just a single patient stopping GAH unexpectedly. Considering GnRHa and GAH together, 23 patients (9.8%) received both GnRHa and GAH, 31 (13.2%) received GnRHa alone, and 25 (10.7%) received GAH alone. In this way, 79 patients (33.8%) received either GnRHa and/or GAH. 57 assigned females (50.9%) received menses suppression, and a small number of assigned males (n = 7, 5.7%) were treated with anti-androgens. Overall, 73/112 assigned females (65.2%) had at least one form of medical treatment compared with 45/122 of assigned males (36.9%).
Since different forms of medical treatment are only appropriate at certain developmental stages, patients were classified according to age at end of review period and the proportion of individuals receiving each type of treatment was re-calculated. Three groups were formed by the research team to guide this analysis. The groups consisted of “children” (aged <10 years) for whom medical treatment is not usually relevant, “younger adolescents” (aged 10-15.99 years) for whom puberty suppression, menses suppression and anti-androgens may be appropriate, and “adolescents” (aged 16 years and older) for whom GAH may be an additional option (N.B. given the legal requirement to access GAHs via approval of the Family Court of Australia, for practical reasons 16 years was generally the minimum age at which GAH were started at the RCHGS up until the end of our study period, consistent with international clinical guidelines at the time ((Coleman et al., 2012; Hembree et al., 2017)). As seen in Table 5, no children received any medical treatment, while similar rates of GnRHa treatment were observed for assigned males in the young adolescent group (45.5%) and the adolescent group (43.6%), with much lower rates among assigned females (17.2% younger adolescent, 14.7% adolescent). Over half of assigned females in the young adolescent group (55.2%) and the adolescent group (60.3%) received medication to suppress their menses. GAH were predominately observed in adolescents, with 34.6% assigned males treated with estrogen and 41.2% assigned females treated with testosterone, and only 1 assigned female <16 years accessing GAH.
Table 5.
Medical treatment access by age.
| Child (age 0-9.99 years) |
Young adolescent (age 10-15.99) |
Adolescent (age 16+ years) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Assigned Male
N = 34 |
Assigned Female
N = 15 |
Total
N = 49 |
Assigned Male
N = 33 |
Assigned Female
N = 29 |
Total
N = 62 |
Assigned Male
N = 55 |
Assigned Female
N = 68 |
Total
N = 123 |
|||||||||||
| n | % | n | % | n | % | n | % | N | % | n | % | N | % | n | % | n | % | ||
| Puberty blocking treatment | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 45.5 | 5 | 17.2 | 20 | 32.3 | 24 | 43.6 | 10 | 14.7 | 34 | 27.6 | |
| Menses suppression | n/a | n/a | 0 | 0 | 0 | 0 | n/a | n/a | 16 | 55.2 | n/a | n/a | n/a | n/a | 41 | 60.3 | n/a | n/a | |
| Anti-androgens | 0 | 0 | n/a | n/a | 0 | 0 | 2 | 6.1 | n/a | n/a | n/a | n/a | 5 | 9.1 | n/a | n/a | n/a | n/a | |
| Gender affirming hormones | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3.5 | 1 | 1.6 | 19 | 34.6 | 28 | 41.2 | 47 | 38.2 | |
Note. Interventions are not mutually exclusive, therefore individuals who received more than one intervention were counted more than once across the intervention categories. Age calculated as at 31/12/16.
Psychosocial difficulties and neurodevelopmental disorders
Many patients presented with neurodevelopmental, school and/or mental health difficulties (Table 6). ASD was common (16.2%), but intellectual disability (0.3%) and ADHD (2.5%) were not. A third of patients (32.3%) experienced bullying, and school refusal (16.4%) and early school dropout (10%) were relatively common. 50.7% had a history of low mood and/or major depressive disorder, with higher rates noted in assigned females (56.0%) than assigned males (44.6%) (diff −0.11; 95% CI −0.22, −0.01; p = 0.03). Rates of suicidal ideation (29.5%) and self-harm (24.8%) were also high, with both more common in assigned females (36.3% and 33.2% respectively) than assigned males (21.7% and 15.1% respectively). Psychotic illness was very rare (<1%) as were eating disorders and post-traumatic stress disorder (both 1.4%).
Table 6.
Neurodevelopmental, school and mental health difficulties experienced by TGD young people attending RCHGS.
| Assigned Male |
Assigned Female |
Total |
|||||
|---|---|---|---|---|---|---|---|
| N = 166 | N = 193 | N = 359 | |||||
| n | (%) | N | (%) | N | (%) | ||
| Neurodevelopmental disorder | |||||||
| Intellectual disability | 1 | (0.6) | 0 | – | 1 | (0.3) | |
| ADHD | 6 | (3.6) | 3 | (1.6) | 9 | (2.5) | |
| Autism spectrum disorder (ASD) | 37 | (22.3) | 21 | (10.9)* | 58 | (16.2) | |
| School-related difficulties | |||||||
| Peer bullying (ever) | 60 | (36.1) | 56 | (29.0) | 116 | (32.3) | |
| History of school refusal | 20 | (12.1) | 39 | (20.2)* | 59 | (16.4) | |
| Dropped out of school | 17 | (10.2) | 19 | (9.8) | 36 | (10.0) | |
| Mental health | |||||||
| Major depressive disorder/history of low mood | 74 | (44.6) | 108 | (56.0)* | 182 | (50.7) | |
| Psychotic disorders | |||||||
| Dissociative identity disorder | 0 | – | 0 | – | 0 | – | |
| Schizophrenia | 0 | – | 1 | (0.5) | 1 | (0.3) | |
| Bipolar disorder | 0 | – | 0 | – | 0 | – | |
| Eating disorder | 1 | (.6) | 4 | (2.1) | 5 | (1.4) | |
| Post-traumatic stress disorder | 1 | (.6) | 4 | (2.1) | 5 | (1.4) | |
| History of self-harm | 25 | (15.1) | 64 | (33.2)* | 89 | (24.8) | |
| History of suicidal ideation | 36 | (21.7) | 70 | (36.3)* | 106 | (29.5) | |
Note: % calculated based on total N as no mention of disorder in patient file assumed that patient did not have a diagnosis. * p < 0.05.
Discussion
This study profiled 359 consecutively referred patients seeking care for gender-related concerns at the RCHGS over a 10-year period (2007–2016). The findings demonstrate the complex profile of children and adolescents accessing specialist gender-related health care.
Taken together, our results are broadly consistent with previous studies in this field. Firstly, in keeping with a recent trend observed at clinics overseas (Kaltiala-Heino et al., 2015; Spack et al., 2012), more assigned females than assigned males presented to RCHGS seeking gender-related health care, which contrasts with older historical data showing greater numbers of assigned males (Aitken et al., 2015; de Graaf et al., 2018). Secondly, our study revealed an important subpopulation of patients who were non-binary and made up 7% of our cohort, a rate similar to the 11-14.7% reported by other clinics (Thorne et al., 2018; Twist & de Graaf, 2018). Thirdly, our observation that 16.2% of patients had a diagnosis of ASD is much higher than expected compared to the ∼3% prevalence of ASD among Australian children (May et al., 2017), and is in keeping with previous studies of TGD children and adolescents reporting co-occurrence rates of 5.4-14.5% (Van Der Miesen et al., 2016). Fourthly, like previous studies (Strauss et al., 2017; Surace et al., 2020), patients attending RCHGS had high rates of suicidal ideation, self-harm and depression, although these were considerably lower than those reported in community-based samples of TGD youth from Australia (e.g. Trans Pathways (Strauss et al., 2017)). Reasons for this discrepancy may be due to differences in the data collection methods used in the two studies (i.e., self-report used in Trans Pathways vs clinician-collected in the current study) as well as differences in sample characteristics including age of the samples (age range in Trans Pathways was 14-25 years vs 3-18 years in the current study) and the sociodemographic features of the samples. For instance, research has long documented the links between socioeconomic inequalities and mental health (Reiss, 2013), and there was an underrepresentation of patients from disadvantaged backgrounds attending the RCHGS. Furthermore, family support is known to be positively associated with better mental health for TGD individuals (Johns et al., 2018), and the vast majority of RCHGS patients reported at least one parent who supported their gender diversity, whereas the community-based Trans Pathways study reported most of their participants (65.8%) lacked family support (Strauss et al., 2017).
An interesting observation from our study is that, although a large majority of patients met criteria for a diagnosis of GD, only a minority received medical intervention, with 29% of young people ≥10 years of age receiving puberty blocking treatment and 38% of adolescents ≥16 years of age being treated with GAH. Reasons for these relatively low rates are likely to be manifold and could include age at presentation, barriers to care, personal desires, and family factors. For example, many of our patients were still children at the time of analysis, and thus not eligible for medical intervention. Another likely reason is that approval from the Family Court of Australia was required for TGD individuals under 18 years to access GAH throughout the study period (while approval for GnRHa was required up until 2013), and it is highly probable that these legal requirements acted as a significant barrier to treatment. With court authorization now no longer required in the vast majority of cases, it will be interesting to see if uptake of these hormonal treatments increases. Finally, it is important to remember that this audit provides a cross-sectional snapshot of medication use, and it is possible that some of the young people in the review may have gone onto receive hormonal treatment after the review period.
This study also revealed much lower rates of puberty blocking treatment in assigned females than assigned males. This was likely to be related to both the more advanced age and pubertal status of assigned females at the time of presentation. For instance, the median age at presentation for assigned females was 14.8 years; given the average age of menarche in Australia is around 12 years (Le-Ha et al., 2018), the vast majority of assigned females thus present to the RCHGS after menarche (i.e. at least Tanner stage 4), by which time the benefits of providing puberty blockers are likely to be outweighed by the risks and can be achieved through simpler means (e.g. progestogens to suppress menses). The implications of so few assigned females accessing puberty blockers at an earlier stage of development are likely to be significant. After all, the development of unwanted secondary sex characteristics that do not align with their gender identity can be expected to worsen gender dysphoria, exacerbate other mental health concerns, increase stigma and discrimination, and necessitate chest masculinization surgery.
Our study provides important information about the timing and related experiences of GD. For instance, although most young people first receive care at the RCHGS in adolescence, a significant proportion had previously displayed gender diversity in childhood. These findings suggest that gender questioning processes may be occurring many years before young people present to specialist gender services. Such processes, in combination with gender diversity, are known to have both positive and negative impacts on families, including parents and siblings (Westwater et al., 2019). Supporting young people and their families to navigate and manage these processes at an early stage is therefore likely to be helpful in ensuring that they receive timely and appropriate care. We also observed a marked difference in age of onset of GD symptoms between assigned females and assigned males, with a much later onset for assigned females. On the one hand, this may be due to the development of feminine secondary sex characteristics during puberty acting as a potent trigger for distress among assigned females. On the other hand, this may also reflect differences in sociocultural norms and attitudes around gender that are known to intensify with the onset of puberty (Chandra-Mouli et al., 2017). For example, different expectations of boys and girls – in relation to appropriate behavior, permitted activities and available opportunities – become further entrenched in early adolescence (Chandra-Mouli et al., 2017), and may act to strengthen symptoms of GD, such as the desire to be of the other gender and to be treated as such. This may especially be true among assigned females, for whom adolescence brings increased restrictions in contrast to their male counterparts who typically enjoy greater freedom and autonomy at this time (Chandra-Mouli et al., 2017).
Our study also provides relevant data in relation to concerns about widespread ‘desistence’ among TGD young people (i.e., TGD young people who later identify as cisgender). Previous studies report 45-88% of children (≤12 years) with gender concerns later identify with the gender presumed for them at birth (see Temple Newhook et al. (2018) for a review of these studies). In contrast, we observed that only 4% of our TGD cohort later reported a cisgender identity, although such comparisons should be undertaken with caution given differences in participants’ age (median age at presentation was 14.3 years in the current study whereas participants in the studies cited in Temple Newhook et al. (2018) were all ≤12 years at initial assessment) and potential differences in follow-up periods across these studies.
Findings from our study should be considered in light of its retrospective nature, which is a clear limitation. Limitations of retrospective chart reviews, including information not being screened for or explicitly recorded in medical files by clinicians, and reporting of inconsistent information, may have resulted in missing data and conservative estimates as well as missed opportunities to examine particular research questions of interest. More specifically, an important limitation of the current study was that pubertal status was not always uniformly documented in the clinical notes, and was therefore not extracted or analyzed in the current study. Although we used age as a proxy for pubertal status in this study, having information about pubertal status would have allowed for better estimations of those eligible for certain medical interventions. Although many of our findings are broadly consistent with other clinical cohorts overseas, another limitation is that they may not be representative of broader community-based samples of TGD young people, given our observations around socio-economic status and parental support. Finally, we are conscious that this study did not provide details about the length of care and number of visits each patient received, which would have been an interesting parameter to assess. Despite these limitations, this study has notable strengths, including providing a comprehensive account of the characteristics of consecutively referred patients seen at the largest pediatric gender service in Australia over a 10-year period.
Conclusion
The results of this study demonstrate the complex profile of children and adolescents accessing specialist gender-related health care. Characteristics of the RCHGS patient population are similar to those described by other gender services internationally. Information from this study helps better understand the healthcare needs of TGD children and adolescents presenting to pediatric gender services in Australia and internationally and, in doing so, can be used to maximize quality care provision by ensuring clinical management addresses the unique and current needs of these patients.
Supplementary Material
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, KP, upon reasonable request.
References
- Aitken, M., Steensma, T. D., Blanchard, R., VanderLaan, D. P., Wood, H., Fuentes, A., Spegg, C., Wasserman, L., Ames, M., Fitzsimmons, C. L., Leef, J. H., Lishak, V., Reim, E., Takagi, A., Vinik, J., Wreford, J., Cohen‐Kettenis, P. T., de Vries, A. L. C., Kreukels, B. P. C., & Zucker, K. J. (2015). Evidence for an altered sex ratio in clinic‐referred adolescents with gender dysphoria. The Journal of Sexual Medicine, 12(3), 756–763. 10.1111/jsm.12817 [DOI] [PubMed] [Google Scholar]
- Brix, N., Ernst, A., Lauridsen, L. L. B., Parner, E., Støvring, H., Olsen, J., Henriksen, T. B., & Ramlau‐Hansen, C. H. (2019). Timing of puberty in boys and girls: A population‐based study. Paediatric and Perinatal Epidemiology, 33(1), 70–78. 10.1111/ppe.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra-Mouli, V., Plesons, M., Adebayo, E., Amin, A., Avni, M., Kraft, J. M., Lane, C., Brundage, C. L., Kreinin, T., Bosworth, E., Garcia-Moreno, C., & Malarcher, S. (2017). Implications of the global early adolescent Study’s formative research findings for action and for research. Journal of Adolescent Health, 61(4), S5–S9. 10.1016/j.jadohealth.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Fuqua, J., & Eugster, E. A. (2016). Characteristics of referrals for gender dysphoria over a 13-year period. Journal of Adolescent Health, 58(3), 369–371. 10.1016/j.jadohealth.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen, T., Lauritsen, J. (2010). EpiData - Comprehensive Data Management and Basic Statistical Analysis System. EpiData Association. http://www.epidata.dk
- Clark, T., Lucassen, M., Bullen, P., Denny, S. J., Fleming, T., Robinson, E., & Rossen, F. (2014). The health and well-being of transgender high school students: Results from the New Zealand adolescent health survey (Youth’12). Journal of Adolescent Health, 55(1), 93–99. 10.1016/j.jadohealth.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Coleman, E., Bockting, W., Botzer, M., Cohen-Kettenis, P., DeCuypere, G., Feldman, J., Fraser, L., Green, J., Knudson, G., Meyer, W. J., Monstrey, S., Adler, R. K., Brown, G. R., Devor, A. H., Ehrbar, R., Ettner, R., Eyler, E., Garofalo, R., Karasic, D. H., … Zucker, K. (2012). Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. International Journal of Transgender Health, 13(4), 165–232. 10.1080/15532739.2011.700873 [DOI] [Google Scholar]
- de Graaf, N. M., Carmichael, P., Steensma, T. D., & Zucker, K. J. (2018). Evidence for a change in the sex ratio of children referred for gender dysphoria: Data from the Gender Identity Development Service in London (2000-2017). The Journal of Sexual Medicine, 15(10), 1381–1383. 10.1016/j.jsxm.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Fisher, C. M., Waling, A., Kerr, L., Bellamy, R., Ezer, P., Mikolajczak, G., Brown, G., Carman, M., & Lucke, J. (2019). National Survey of Australian Secondary Students and Sexual Health 2018, (ARCSHS Monograph Series No. 113). Australian Research Centre in Sex, Health & Society, La Trobe University. [Google Scholar]
- Gower, A. L., Rider, G. N., Coleman, E., Brown, C., McMorris, B. J., & Eisenberg, M. E. (2018). Perceived gender presentation among transgender and gender diverse youth: Approaches to analysis and associations with bullying victimization and emotional distress. LGBT Health, 5(5), 312–319. 10.1089/lgbt.2017.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler, T., Hojilla, J. C., Varghese, R., Wellenstein, W., Satre, D. D., & Zaritsky, E. (2019). Trends in referrals to a pediatric transgender clinic. Pediatrics, 144(5), e20191368. 10.1542/peds.2019-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., Rosenthal, S. M., Safer, J. D., Tangpricha, V., & T’Sjoen, G. G. (2017). Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 102(11), 3869–3903. 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- Hewitt, J. K., Paul, C., Kasiannan, P., Grover, S. R., Newman, L. K., & Warne, G. L. (2012). Hormone treatment of gender identity disorder in a cohort of children and adolescents. Medical Journal of Australia, 196(9), 578–581. 10.5694/mja12.10222 [DOI] [PubMed] [Google Scholar]
- Johns, M. M., Beltran, O., Armstrong, H. L., Jayne, P. E., & Barrios, L. C. (2018). Protective factors among transgender and gender variant youth: A systematic review by socioecological level. The Journal of Primary Prevention, 39(3), 263–301. 10.1007/s10935-018-0508-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino, R., Sumia, M., Tyolajarvi, M., & Lindberg, N. (2015). Two years of gender identity service for minors: overrepresentation of natal girls with severe problems in adolescent development. Child and Adolescent Psychiatry and Mental Health, 9(1), 9. 10.1186/s13034-015-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Ha, C., Beilin, L. J., Burrows, S., Huang, R.-C., Hickey, M., Mori, T. A., & Hart, R. J. (2018). Age at menarche and childhood body mass index as predictors of cardio-metabolic risk in young adulthood: A prospective cohort study. PLOS One, 13(12), e0209355. 10.1371/journal.pone.0209355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, T., Sciberras, E., Brignell, A., & Williams, K. (2017). Autism spectrum disorder: updated prevalence and comparison of two birth cohorts in a nationally representative Australian sample. BMJ Open, 7(5), e015549. 10.1136/bmjopen-2016-015549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerwijk, E. L., & Sevelius, J. M. (2017). Transgender population size in the United States: a meta-regression of population-based probability samples. American Journal of Public Health, 107(2), e1–e8. 10.2105/AJPH.2016.303578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink, B. (2013). Socio-economic indexes for areas (SEIFA) 2011: Technical paper. Commonwealth of Australia: Canberra. https://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/22CEDA8038AF7A0DCA257B3B00116E34/$File/2033.0.55.001%20seifa%202011%20technical%20paper.pdf [Google Scholar]
- Re: Imogen . (2020). FamCA 761. 10 September 2020. http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/cth/FamCA/2020/761.html
- Re: Jamie . (2013). FamCAFC 110. 31 July 2013. https://www.aph.gov.au/DocumentStore.ashx?id=2c1d4622-95c7-4f6e-b9c7-88b66261d1c5
- Re: Kelvin . (2017). FamCAFC 258. 30 November 2017. http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/cth/FamCAFC/2017/258.html?context=1;query=re:kelvin;mask_path=
- Reiss, F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Social Science & Medicine, 90, 24–31. 10.1016/j.socscimed.2013.04.026 [DOI] [PubMed] [Google Scholar]
- Spack, N. P., Edwards-Leeper, L., Feldman, H. A., Leibowitz, S., Mandel, F., Diamond, D. A., & Vance, S. R. (2012). Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics, 129(3), 418–425. 10.1542/peds.2011-0907 [DOI] [PubMed] [Google Scholar]
- Strauss, P., Cook, A., Winter, S., Watson, V., Wright Toussaint, D., & Lin, A. (2017). Trans Pathways: The mental health experiences and care pathways of trans young people. Summary of results. Telethon Kids Institute. [Google Scholar]
- Surace, T., Fusar-Poli, L., Vozza, L., Cavone, V., Arcidiacono, C., Mammano, R., Basile, L., Rodolico, A., Bisicchia, P., Caponnetto, P., Signorelli, M. S., & Aguglia, E. (2020). Lifetime prevalence of suicidal ideation and suicidal behaviors in gender non-conforming youths: a meta-analysis. European Child & Adolescent Psychiatry, 10.1542/peds.2011-0907. [DOI] [PubMed] [Google Scholar]
- Temple Newhook, J., Pyne, J., Winters, K., Feder, S., Holmes, C., Tosh, J., Sinnott, M.-L., Jamieson, A., & Pickett, S. (2018). A critical commentary on follow-up studies and “desistance” theories about transgender and gender-nonconforming children. International Journal of Transgender Health, 19(2), 212–224. [Google Scholar]
- The Tavistock and Portman NHS Foundation Trust . (2018). GIDS referrals increase in 2017/18 Retrieved January 16, 2019, from http://gids.nhs.uk/news-events/2018-05-16/gids-referrals-increase-201718
- Thorne, N., Witcomb, G. L., Nieder, T., Nixon, E., Yip, A., & Arcelus, J. (2019). A comparison of mental health symptomatology and levels of social support in young treatment seeking transgender individuals who identify as binary and non-binary. International Journal of Transgender Health, 20(2-3), 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twist, J., & de Graaf, N. M. (2018). Gender diversity and non-binary presentations in young people attending the United Kingdom’s National Gender Identity Development Service. Clinical Child Psychology and Psychiatry, 24(2), 277–290. [DOI] [PubMed] [Google Scholar]
- Van Der Miesen, A. I., Hurley, H., & De Vries, A. L. (2016). Gender dysphoria and autism spectrum disorder: A narrative review. International Review of Psychiatry, 28(1), 70–80. 10.3109/09540261.2015.1111199 [DOI] [PubMed] [Google Scholar]
- Westwater, J. J., Riley, E. A., & Peterson, G. M. (2019). What about the family in youth gender diversity? A literature review. International Journal of Transgender Health, 20(4), 351–370. 10.1080/15532739.2019.1652130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz, A. L., Poteat, T. C., Malik, M., & Glass, N. (2020). Gender-based violence against transgender people in the United States: a call for research and programming. Trauma, Violence, & Abuse 21(2), 227–241. 10.1177/1524838018757749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, KP, upon reasonable request.