Abstract
The preconception period is a critical window for gametogenesis, therefore preconception exposure to air pollutants may have long-term effects on children. We systematically reviewed epidemiological evidence concerning the effects of preconception ambient air pollution exposure on children’s health outcomes and identified research gaps for future investigations. We searched PubMed and Web of Science from journal inception up to October 2022 based on an established protocol (PROSPERO: CRD42022277608). We then identified 162 articles based on searching strategy, 22 of which met the inclusion criteria. Studies covered a wide range of health outcomes including birth defects, preterm birth, birthweight, respiratory outcomes, and developmental outcomes. Findings suggested that exposure to outdoor air pollutants during maternal preconception period were associated with various health outcomes, of which birth defects has the most consistent findings. A meta-analysis revealed that during 3-month preconception period, a 10 μg/m3 increase in PM10 and PM2.5 was associated with relative risk (RR) of birth defects of 1.06 (95% confidence interval (CI): 1.00, 1.02) and 1.14 (95% CI: 0.82, 1.59), respectively. Preterm birth, low birthweight, and autism have also been associated with maternal preconception exposure to PM2.5, PM10, O3 and SO2. However, the significance of associations and effect sizes varied substantially across studies, partly due to the heterogeneity in exposure and outcome assessments. Future studies should use more accurate exposure assessment methods to obtain individual-level exposures with high temporal resolution. This will allow the exploration of which specific time window (weeks or months) during the preconception period has the strongest effect. In future epidemiologic studies, integrating pathophysiologic biomarkers relevant to clinical outcomes may help improve the causal inference of associations between preconception exposure and health outcomes suggested by the current limited literature. Additionally, potential effects of paternal preconception exposure need to be studied.
Keywords: Preconception, Air pollution, Birth defects, Birthweight, Preterm birth
1. Introduction
The Developmental Origins of Health Disease (DOHaD) hypothesis stems from interest regarding how fetal experiences can lead to adult disorders, and it suggests that early life environmental exposures may have a significant influence on short and long-term health outcomes (Wadhwa et al., 2009). Air pollution, a leading risk factor for morbidity and mortality across the world (Cohen et al., 2017), is known to contribute to early life risk factors. Prenatal air pollution exposure has been associated with an increased risk of preterm birth (Han et al., 2018), impairment in neurodevelopment (Volk et al., 2021), and childhood respiratory disease (Hehua et al., 2017). However, it is unknown whether the adverse effects of air pollution start earlier than the prenatal period during preconception. Preconception is a critical developmental window for gametogenesis. Air pollution exposure from both mothers and fathers during preconception or early stages of pregnancy may have adverse effects on gametogenesis of sperm and ova cells (Udagawa et al., 2018; Vecoli et al., 2016; Yauk et al., 2008). This may cause a diminishing ovarian reserve (Ogliari et al., 2013), disruption of first cell lineage segregation at the blastocyst stage (Perin et al., 2008) and consequently lead to long-term adverse outcomes of fetal and neonatal development (Klepac et al., 2018; Proietti et al., 2013).
Toxicological in-vitro and in-vivo studies have revealed that air pollution exposure during preconception and early pregnancy period could influence epigenetics and DNA methylations in gametes, increase maternal oxidative stress, act as an endocrine disruptor in gametogenesis, and alter placental mitochondrial DNA content (Januário et al., 2010; Li et al., 2019; Yauk et al., 2008). However, current epidemiological studies on preconception air pollution exposure on birth and developmental outcomes are sparse and inconsistent. For example, some studies showed adverse effects of preconception air pollution on small-for-gestational age (SGA) (Williams et al., 2021), while others did not find an association (Chen et al., 2021; Ha et al., 2017; Nobles et al., 2019). Due to the varied composition of air pollutants and different health outcomes investigated by epidemiological studies, there are gaps in both qualitative and quantitative knowledge of preconception air pollution effects. Gaps in quantitative pooling of current epidemiological evidence linking exposure during the preconception period and children’s outcomes include birthweight, child growth rates, and cardiovascular and respiratory health outcomes (Schwartz, 2004; Johnson et al., 2021; Stieb et al., 2012; Pun et al., 2021). In addition, to our knowledge, there have been no systematic reviews on this subject. Hence, we conducted this systematic review of published epidemiological studies reporting effects of preconception ambient air pollution exposures on birth outcomes and children’s health outcomes. This review will help provide evidence for the long-term health effects of preconception ambient air pollution exposure and identify knowledge gaps and limitations of previous studies to guide future studies.
2. Materials and Methods
2.1. Search Strategy and Screening Criteria
Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (Moher et al., 2009), we searched the databases PubMed and Web of Science for studies published before June of 2021 in English, with the combination of exposure and outcomes yet restricted to the preconception period. We used the following query to search in the two databases: (preconception) OR preconceived OR pre-conception OR pre-conceived OR “before pregnancy” AND ((air pollution) OR (particulate) OR (PM2.5)) AND ((health effect) OR (birthweight) OR (infant growth) OR (Intrauterine) OR (neural) OR (treatment outcome) OR (cardiovascular) OR (cardiac) OR (respiratory)). We only searched literature in English as we found that literature published in English covers all relevant studies. Both of PubMed and Web of Science also contain literatures with the full text in another language, but we had not screened any eligible literature published in languages other than English. This search query focused on ambient air pollutants, but PM2.5 was specifically included in the query because it is a prominent air pollutant among all criteria pollutants. In addition, this search query included both terms of generic outcomes and specific health outcomes that are commonly investigated with air pollution (Schwartz, 2004; Johnson et al., 2021; Stieb et al., 2012; Pun et al., 2021). This query yielded 81 results from PubMed and 49 results from Web of Science, 34 of which were duplicated studies that were then removed. An additional search conducted in October 2022 identified 32 studies created after June 2021 in the PubMed database, resulting in an adjusted number of 128 total studies (Figure 1). Based on eligibility criteria, two independent reviewers (JL and NB) screened the title and abstract of each study to determine whether to retrieve the full text. Then, the two independent reviewers (JL and NB) conducted the second screening on the full text of the retrieved studies. In each step of the screening process, the two reviewers compared the results. If their results were not in agreement, the two reviewers discussed and resolved the disagreement. Studies were included if they fit each of the following criteria: (1) written in English, (2) studies involving human subjects, (3) assessed maternal or paternal exposure to ambient air pollutants in the preconception period, (4) assessed some aspects of the children’s health outcomes such as cardiac function, neurodevelopment, birth weight, respiratory condition, birth defect, and preterm or full-term delivery. The preconception period in this review is defined as between 1 month to 1 year before conception, with 3 months before conception being most common period. Studies were excluded if they (1) were not original research articles, (2) were not human-based research, (3) did not investigate health effects of outdoor air pollution exposure, (4) only investigated exposure period in-utero or post-delivery but not in the preconception period, (5) did not measure health outcomes of children, and (6) had no results available. Out of 128 studies, 64 were found to meet the criteria and were downloaded for full-text review. From these 64 studies, 22 fit the criteria for data extraction. The flowchart below shows the screening process (Figure 1).
Figure 1.
Flowchart Showing the Study Selection and Screening Processing.
2.2. Data Extraction
From each of the 22 papers that fit the data-extraction criteria, data was organized by study author and year, location, number of participants, age of children at time of outcome assessment, study design, air pollution exposure type, exposure time window, adjusted covariates, main findings (including point estimates and 95% confidence intervals of preconception air pollution on child health outcomes shown as risk ratio, hazard ratio or odds ratio) and air pollution ranges. If studies reported multiple populations separately, we only extracted information from the general population in the paper. There are 16 cohort studies and 6 case-control studies from which the data was extracted. Two independent investigators (JL and NB) extracted the data and resolved disagreements through discussion. The quality of included studies was also independently assessed by two investigators (JL and NB), using the nine-point Newcastle-Ottawa Assessment Scale (NOS) guideline for cohort studies and case-control studies (Stang, 2010).
2.3. Synthesis methods
We summarized the effects of preconception air pollution exposure on children’s health outcomes and organized the data by different outcomes. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework with special considerations of studies in environmental health (Morgan et al., 2016) to assess the quality of findings for the health outcomes that two or more studies had investigated. We additionally considered the large effect, dose response, human and experimental animal data, and mechanistic data availability when assessing the quality of evidence for each pair of air pollutant and children’s health outcome. We anticipated there would be large heterogeneities between studies in regard to air pollutant types, definition of air pollution exposure window, effect size, and health outcome. Therefore, we applied the random-effect model for each disease outcome to account for both within-study and between-study heterogeneity to summarize effect estimates of the same air pollutant. No pooled estimates across different air pollutants were conducted. We conducted a meta-analysis and summarized the effect size as per 10 μg/m3 increase for PM2.5 and PM10, per 10 ppb increase for NO2, O3, and SO2, and per 100 ppb increase for CO. I2 statistics and associated p-value of testing for heterogeneity in the random effects analysis were also reported. Adjusted odds ratio (OR) was approximated to relative risk (RR) if the health outcomes are rare outcomes (less than 10%) and hazard ratio (HR) was approximated to relative risk (RR) if the overall population risk is low (less than 5%), provided that the study used proportional hazard model (Stare and Maucort-Boulch, 2016; Zhang and Yu, 1998). Additionally, we did not assess publication bias due to the small number of studies. The protocol of the systematic review is registered in PROSPERO (CRD42022277608).
3. Results
As shown in Figure 1, there were 22 studies included in the review. The key characteristics of these studies are presented in Table 1. Among these studies, 16 were cohort studies (5 prospective cohort and 11 retrospective cohort study) and 6 were case-control studies. Eleven of the included studies were conducted in China, while the other Eleven studies were conducted in the United States involving large population cohorts (N > 10,000) with diverse race and ethnicity backgrounds. Exposures to multiple air pollutants (O3, PM2.5, PM10, SO2, NO2, and CO) during the preconception period has been investigated for their associations with children’s health outcomes. Thirteen studies assessed the effect of more than one air pollutant, whereas nine studies assessed the effect of only one air pollutant (PM2.5 or PM10). In all studies, air pollution exposure was quantitatively assessed as ambient concentrations and used as a continuous exposure independent variable. Air pollution concentrations were generated by utilizing established air pollution models with ambient concentrations measured at a monitoring station close to the participants’ residence or birth hospital. For the preconception time window of exposure, despite some heterogeneities, most studies (N = 20) use three months (80–90 days or 12 weeks) as an exposure window (Chen et al., 2021a, 2021b, Ha et al., 2017; Jo et al., 2019; J. Li et al., 2021, 2021; Liu et al., 2020; Lu et al., 2021, 2017; Mao et al., 2017; Mendola et al., 2016; Nobles et al., 2019; Ren et al., 2018; Seeni et al., 2018; Williams et al., 2021; Yao et al., 2016; Zhang et al., 2020; Zhu et al., 2015, H. Li et al., 2021, Kalkbrenner et al., 2015; McGuinn et al., 2020). However, one study (Sun et al., 2021) assessed air pollution during 0 – 28 days before conception using a distributed lag model (DLM), and another study (Deng et al., 2016) assessed average air pollution exposure for one year before conception. The exposure levels of air pollutants vary across studies as well. For instance, studies conducted in China showed PM2.5 levels well above 50 μg/m3, while those conducted in the United States had a mean of 10 μg/m3 (Table 1). All studies examined the effect of maternal preconception exposure while omitting paternal exposures. Potential confounders that studies adjusted for include maternal age, gestational age, race, parity, smoking or alcohol use during pregnancy, and body-mass index (BMI). Lastly, all included studies showed high or moderate quality in the Newcastle-Ottawa Assessment Scale (NOS) Quality Assessment. The Newcastle-Ottawa assessment is scored out of 9 points based on three sections: selection (4 points), comparability (1 point), and exposure (3 points). All 22 studies scored with 7 or more points were considered of good quality thus were included in the review. Most of these studies used medical records for outcome assessment, but two studies (Deng et al., 2016; Lu et al., 2017) relied on self-reported disease information from participants, therefore they received a deduction of one point. Some studies have not adjusted for marital status (Li et al., 2021, 2021; Lu et al., 2021, 2017; Yao et al., 2016; Zhang et al., 2020), while some studies have not adjusted for the spatial and temporal trend of the air pollution levels and disease outcomes (Yao et al., 2016; Williams et al., 2021; Deng et al., 2016; Mendola et al., 2016). Therefore, we deducted one point due to lack of comparability of the cohort. Two case-control studies have not reported response rate (Chen et al., 2021a, McGuinn et al., 2020) so they received a one point deduction as well. Details of the NOS Quality Assessment of each study is presented in Table S2. We found all studies were funded by research grants from public sources (Table S3).
Table 1.
Characteristics of Included Studies (n = 15) and Newcastle-Ottawa Assessment Scale (NOS) Quality Assessment Score
| Author, Year | Location | Children Number | Age of children at time of assessment | Study Design | Air pollutant and-pollution exposure assessment method, exposure time window | Health outcome | Covariates Adjusted | Effect estimate | Air pollution levels | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Yao, 2016 | China | 16332 | At birth | Retrospective cohort study | SO2, NO2, and PM10 from fixed monitoring sites, 3 months before conception | Birth defects | Maternal age, infanťs sex, gestational age, first pregnancy, other two pollutants | Odds ratio (OR) per 10 μ/m3: SO2: 1.20 (1.09, 1.29); NO2: 1.02 (0.82 – 1.28); PM10: 1.01 (0.95, 1.09) | Mean (SD) in μg/m3 PM10: 86.26 (17.79), SO2: 47.36 (14.37), NO2: 33.28 (5.78) | 8/9 |
|
| ||||||||||
| Williams, 2021 | United States | 204951a | At birth for birth outcome s, others matched with clinical record | Retrospective cohort study | O3, PM2.5, SO2, CO, PM10, and NOx from CMAQ model, 3 months before conception | Preterm | Maternal age, race, preconception BMI, health insurance, marital status, smoking, alcohol use, chronic disease, census region | Relative risk (RR) per 1 IQR: O3: 1.00 (1.00, 1.01); PM2.5: 0.96 (0.96, 0.97); SO2: 0.94 (0.93, 0.95); | Mean (IQR): PM2.5 (μg/m3): 5.36 (4.6, 22.03); PM10 6.05 (10.25, 39.44); NOx (ppb) 27 (5.09, 92.97); SO2 (ppb) 3.46 (0.8, 21); CO (ppm) 260.23 (171.68, 1125.23); O3 (ppb) 12.16 (10.04, 49.36) | 8/9 |
| Small for gestational age | RR per 1 IQR: O3: 0.99 (0.99, 0.99); PM2.5: 1.00 (1.00,1.01); SO2: 1.03 (1.02,1.03) | |||||||||
| Neonatal respiratory distress syndrome | RR per 1 IQR: O3: 1.00 (1.00,1.00); PM2.5: 0.95 (0.94, 0.96); SO2: 0.92 (0.90, 0.94) | |||||||||
| NICU | RR per 1 IQR: O3: 1.00 (1.00,1.00); PM2.5: 0.99 (0.99,0.99); SO2: 0.99 (0.99,0.99) | |||||||||
| Perinatal mortality | RR per 1 IQR: O3: 0.99 (0.99,1.00); PM2.5: 0.96 (0.95, 0.98); SO2: 0.97 (0.94, 1.00) | |||||||||
|
| ||||||||||
| Seeni, 2018 | United States | 223385 | At birth | Retrospective cohort study | CO, NOx, O3, PM2.5, SO2 from CMAQ model, 90 days before conception | Transient tachypnea | Region, marital status, parity, maternal age, cesarean delivery, insurance, maternal BMI, smoking, alcohol, comorbidities, season, asthma | RR per 1 IQR: CO: 0.96 (0.91, 1.02); NOx: 1.06 (0.97, 1.16); O3: 0.97 (0.91, 1.04); PM10: 1.10 (1.04, 1.17); PM2.5: 1.01 (0.94, 1.10); SO2: 0.84 (0.79, 0.90) | Median (IQR): CO (ppm) 0.26 (0.17,1.13); NOx (ppb) 28.51(5.09,92.97); O3 (ppb) 12.30 (7.55,49.37); PM10 (μg/m3) 6.29 (10.26,39.44); PM2.5 (μg/m3) 5.52 (4.6,22.04); SO2 (ppb) 3.29 (0.80,21) | 9/9 |
| Asphyxia | RR per 1 IQR: CO: 1.15 (0.93, 1.58); NOx: 1.22 (0.74, 1.99); O3: 1.76 (1.25, 2.48); PM10: 0.77 (0.67, 0.89); PM2.5: 1.80 (0.96, 3.35); SO2: 0.47 (0.30, 0.74) | |||||||||
| Respiratory distress syndrome | RR per 1 IQR: CO: 0.73 (0.68, 0.78); NOx: 1.39 (1.25, 1.54), O3: 1.09 (1.01, 1.18); PM10: 1.02 (0.96, 1.09); PM2.5: 0.83 (0.76, 0.91); SO2: 0.76 (0.71, 0.82) | |||||||||
|
| ||||||||||
| Nobles,2019 | United States | 112203 | At birth | Retrospective cohort study | SO2, NOx, NO2, O3, CO, PM10, PM2.5 from CMAQ model, 3 months preconception | Small for gestational age (SGA) | Maternal age, race/ethnicity, pre-pregnancy BMI, smoking, alcohol use, parity, insurance type, marital status, history of asthma, ambient temperature | RR per 1 IQR: SO2: 1.02 (0.99, 1.05); O3: 0.96 (0.93, 0.98); NOx: 1.02 (0.96, 1.08); NO2: 1.02 (0.98, 1.07); CO: 1.00 (0.97, 1.06); PM2.5: 1.00 (0.97, 1.02); PM10: 1.01 (0.98, 1.05) | First pregnancy, Median (IQR): SO2 (ppb) 1.92 (1.52,2.26); O3 (ppb) 42.5 (39.4, 44.5); NOx (ppb) 30.4 (19.7, 49.5); NO2 (ppb) 16.6 (13.5, 20.9); CO (ppb) 573 (433,750); PM2.5 (μg/m3) 7.52 (6.51, 9.84); PM10 (μg/m3) 22.9 (20.1, 26.4); Second pregnancy Median (IQR): SO2 1.93 (1.63, 2.20); O3 39.1 (31.3, 42.9); NOx 26.1 (16.7, 43); NO2 15.0 (12.3,19.4); CO 543 (341, 621); PM2.5 7.54 (6.57,9.35); PM10 21.9 (19.3,24.7); Third pregnancy Median (IQR): SO2 1.91 (1.59, 2.19); O3 36.2 (29.3, 41.4); NOx 23.8 (15.0, 40.5); NO2 13.9 (10.7, 18.7); CO 402 (307, 562); PM2.5 7.39 (6.39, 9.18); PM10 21.9 (19.4, 24.3) | 9/9 |
| RR per IQR: SO2: 1.15 (1.09, 1.23); O3: 0.88 (0.83, 0.94); NOx: 1.23 (1.10, 1.38); NO2: 1.19 (1.08, 1.31); CO: 1.15 (1.05, 1.26); PM2.5: 1.07 (1.02, 1.13); PM10: 1.13 (1.06, 1.21) | ||||||||||
| Fetal growth restriction (FGR) | ||||||||||
|
| ||||||||||
| Ha, 2017 | United States | 220572 | At birth | Retrospective Cohort study | CO, NOx, O3, PM2.5, PM10, and SO2 from CMAQ model, 3 months preconception | Small for gestational age (SGA) | Infant sex, maternal race/ethnicity, maternal age, marital status, parity, pre-pregnancy BMI, smoking, alcohol use, gestational complications, chronic comorbidity, insurance, season of conception, study site, temperature, humidity | RR per IQR: CO: 0.98 (0.95, 1.01); NOx: 1.02 (0.98, 1.06); O3: 0.97 (0.94, 1.00); PM10: 0.98 (0.96, 1.00); PM2.5: 0.96 (0.92, 0.99); SO2: 0.98 (0.95, 1.01); EC: 1.04 (1.01, 1.07); Ammonium ions: 0.98 (0.95, 1.01); NOx: 1.00 (0.97, 1.03); OC: 0.99 (0.96, 1.02); Sulfate: 0.96 (0.93, 0.99); Dust: 1.01 (0.98, 1.04) | Mean (SD): CO (ppb) 555.2 (174.9), NOx (ppb) 29.7(19.5), O3 (ppb) 28.9 (8.5), PM10 (μg/m3) 22.1 (5.2), PM2.5 (μg/m3) 12.0(3.5), SO2 (ppb) 4.1(2.7) | 9/9 |
| Term low birthweight (tLBW) | RR per IQR: CO: 0.96 (0.90, 1.03); NOx:1.05 (0.95, 1.16); O3: 1.00 (0.92, 1.07); PM10: 1.02 (0.96, 1.08); PM2.5: 0.97 (0.89, 1.05); SO2: 0.98 (0.90, 1.06); EC: 1.03 (0.97, 1.10); Ammonium ions: 0.98 (0.91, 1.06); NOx:0.98 (0.91, 1.05); OC: 0.97 (0.91, 1.04); Sulfate: 0.96 (0.88, 1.03); Dust: 1.10 (1.03, 1.17) | |||||||||
|
| ||||||||||
| Zhang, 2020 | China | 7950 | At birth | Case-control study | PM10 from Environment Protection Bureau daily readings, 3 months preconception | Neural tube defects | Maternal age, and sulfur dioxide and nitrogen dioxide exposure, gravidity, parity, maternal education, season of conception, infanťs gender | RR for PM10 compared to tertile 1 (T1): first month before pregnancy: T2: 1.41 (1.24–1.61); T3: 1.74 (1.48–2.05); second month before pregnancy: T2: 1.36 (1.2–1.55); T3: 1.47 (1.26–1.73); third month before pregnancy: T2: 1.28 (1.12–1.45), T3: 1.55 (1.32–1.81); 3 months total: T2: 1.55 (1.36–1.76); T3: 1.61 (1.37–1.91 ), per 10 μg/m3: 1.10 (1.07, 1.13) | PM10 (μg/m3): mean (SD), case 87 (23); control 93 (27) | 9/9 |
|
| ||||||||||
| Sun, 2021 | China | 1449 | In gestation | Retrospective cohort study | PM2.5, PM10, O3 from the environmental monitoring center, 28 days before conception | Termination of pregnancy (TOP) | Maternal age, season, year, temperature, air pollution, wind speed, precipitation, air humidity | RR per IQR, cumulative effects: PM2.5:1.176 (0.898, 1.541); PM10: 1.216 (0.959, 1.542; O3: 1.244 (0.888, 1.743) | PM2.5 (μg/m3): mean (SD) 41.91 (28.4); PM10 (μg/m3): mean (SD) 74.88 (40.85); O3 (μg/m3) 98.90 (39.18) | 7/9 |
| Pregnancy loss (PL) | RR per IQR, cumulative effects: PM2.5:1.176 (0.898, 1.541); PM10: 1.216 (0.959,; O3: 1.244 (0.888, 1.743) | |||||||||
| Congenital malformatio ns (CM) | RR per IQR, cumulative effects: PM2.5: 0.987 (0.663, 1.471); PM10: 1.119 (0.790, 1.584); O3: 1.205 (0.727, 1.996) | |||||||||
|
| ||||||||||
| Jo, 2019 | United States | 246420 | Followed from birth to age 5 | Retrospective cohort study | O3, PM2.5, PM10, NO2 from exposure model, 12 weeks before last menstrual period | Autism spectrum disorder (ASD) | Birth year, KPSC center, maternal age, parity, race, education, census tract median household income, maternal history of comorbidities, child sex, family specified as a random effect | Hazard ratios (HR) per IQR: O3: 0.98 (0.93–1.04); PM2.5: 1.11 (1.03–1.20); PM10: 1.05 (0.96–1.14); NO2: 1.07 (0.99–1.17) | Mean (SD) for air pollution, no diabetes: O3 (ppb) 41.6 (8.1), PM2.5 (μg/m3) 17.9 (4.7), PM10 (μg/m3), 38.1 (9.1) NO2 (ppb) 25.2 (7.0) | 9/9 |
|
| ||||||||||
| Mao, 2017 | United States | 1446 | 2–9 years | Prospective cohort study | PM2.5 based on the distance to the monitoring station, 90 days before pregnancy | Childhood overweight/obesity (COWO) | Maternal age, race, education, smoking, diabetes, marriage status, income, the season of delivery, preterm birth, breastfeeding, birth weight | RR of each quartile of PM2.5 compared to Q1. Q2: 1.1 (0.9, 1.3); Q3: 1.2 (1.0,1.4); Q4: 1.2 (1.0, 1.4); Per IQR increase RR: 1.2 (1.0,1.4) | Preconception PM25 (μg/m3): Quartile 1(Q1): 4.48 – 8.85; Q2: 8.86 – 10.59; Q3: 10.59 – 12.28, Q4: > 12.28 | 9/9 |
|
| ||||||||||
| Zhu, 2015 | United States | 188102 | At birth | Retrospective cohort study | CO, NOx, O3, PM10, PM2.5, SO2 from the model at the delivery hospital, 3 months before conception) | Orofacial defect | Site, maternal age, race, marital status, insurance, pre-pregnancy BMI, nulliparity, season of conception, smoking, alcohol use, multiple birth, gestational diabetes | OR per IQR: CO: 2.24 (1.21, 4.16), NOx: 1.58 (0.70, 3.55), O3: 0.53 (0.23, 1.23), PM10: 1.72 (1.12, 2.66), PM2.5: 1.14 (0.61, 2.13), SO2: 1.64 (0.52, 5.15) | Isolated cleft palate patient IQR (Q3 - Q1): CO 0.30 ppm; NOx 22.97 ppb; O3 13.19 ppb; PM10 7.40 μg/m3; PM2.5 5.74 μg/m3; SO2 1.97 ppb | 9/9 |
|
| ||||||||||
| Liu, 2020 | China | 11036 | After age of 1 | Case control study | PM10 from the air monitoring station, 3 months before pregnancy | Oral cleft | Maternal age, education, the season of conception, SO2 exposure, NO2 exposure | OR per 10 μg/m3 of PM10 exposure: first month before pregnancy: 1.02 (1.00 to 1.04); second month before pregnancy: 1.02 (1.00 to 1.04); third month before pregnancy: 1.06 (1.04 to 1.09); 3 months before pregnancy: 1.04 (1.01 to 1.07) | Annual mean of PM2.5 range from 74 – 99 μg/m3 from 2010 – 2015. | 8/9 |
|
| ||||||||||
| Ren, 2017 | United States | 548863 | At birth | Retrospective cohort study | PM2.5 using EPA standard models, 1–3 months before conception | Congenital malformations | Maternal age, race, diabetes, smoking, marital status, education level, the season of conception, infant sex | OR per 10 μg/m3 of PM2.5: 1.13 (0.801.59), average 3 month before conception | 10 km: mean (SD) (in μg/m3) 2 months before 13.93 (3.97), 1 month before 13.97 (4.00), month of conception 13.85 (3.87); 7 km: 2 months before 13.92 (3.95), 1 month before 14.13 (4.01), month of conception 13.92 (4.00); 5 km: 2 months before 14.07 (3.94), 1 month before 14.23 (3.89), month of conception 13.89 (3.97) | 9/9 |
|
| ||||||||||
| Lu, 2017 | China | 2598 | 3–6 years old | Prospective cohort study | PM10, SO2, NO2 daily concentrations average from 7 municipal air stations; 4–6th and 1–3rd months before pregnancy | Eczema | Infanťs sex, infanťs age, birth season, breastfeeding, parental atopy, house size, environmental Tabaco smoke, furniture, redecoration, mold, water condensation, pets, cleaning everyday | OR per IQR: 4–6 months before pregnancy: PM10: 0.89 (0,76, 1.04); SO2: 1.05 (0.94, 1.18); NO2: 1.03 (0.90, 1.17); 1–3 months before pregnancy: PM10: 1.05 (0.90,1.23); SO2: 1.12 (0.97, 1.29); NO2: 1.19 (1.04, 1.37) | Mean (SD) μg/m3: PM10 115 (19), SO2 88 (42), NO2 44 (10) | 7/9 |
|
| ||||||||||
| Lu, 2021 | China | 1510 | 0–14 years | Case control study | PM10, SO2, NO2 from 10 monitoring stations, 1 year and 3 months before conception | Childhood pneumonia | Infanťs sex, infanťs age, birth season, parity, gestational age, birthweight, mode of delivery, parental atopy | OR per 10 μg/m3: 3 months before conception: PM10: 1.30 (1.17, 1.45); SO2: 2.65 (2.27, 3.10); NO2: 0.92 (0.74, 1.15); 1 year before conception: PM10: 1.72 (1.49, 1.98); SO2: 2.96 (2.50, 3.51); NO2: 1.26 (0.96, 1.65) | 3 months preconception mean (SD) in μg/m3: PM10 89(20), SO2 37(22), NO2 45(11) | 9/9 |
|
| ||||||||||
| J. Li, 2021 | China | 551 | 24 months | Prospective cohort study | PM2.5 from land-use regression models, 3 months preconception to gestational 12th week of gestation | psychomotor developme nt index (PDI) | child (sex, breastfeeding, offspring neurodevelopment) mother (age at delivery, passive smoke, household income, gestational age, outdoor temperature, season of delivery) | Beta for log-transformed PM2.5: all participants: −8.23 (−10.01, −6.44) | Median (IQR) PM2.5 (μg/m3): 83.3 (73.0, 142.5) | 8/9 |
| Mental developme nt index (MDI) | Beta for log transformed PM25: all participants: 0.35 (−1.80, 2.49) | |||||||||
|
| ||||||||||
| Deng, 2016 | China | 2598 | 3–6 years old | Retrospective cohort study | PM10, SO2, NO2 from air pollution station, 1 year before conception | Asthma | Child's sex, age, breastfeeding, parental atopy, ETS at home, and household pets | OR per 2 SD: PM10: 0.91 (0.51, 1.62); SO2: 1.44 (1.01, 2.05); NO2: 1.43 (1.00, 2.05) | Mean (μg/m3): PM10 119 (13); SO2 91(25); NO2 43(7) | 7/9 |
| Allergicrhinitis | OR per 2 SD: PM10: 0.87 (0.49, 1.53); SO2: 1.42 (1.01, 2.00); NO2: 1.27 (0.90, 1.80) | |||||||||
| Eczema | OR per 2 SD: PM10: 1.78 (1.00, 3.19); SO2: 1.44 (0.90, 2.31); NO2: 1.51 (1.01, 2.27) | |||||||||
|
| ||||||||||
| Mendola, 2016 | United States | 223502 | At birth | Retrospective cohort study | CO, NOx, O3, PM10, PM2.5, SO2 from CMAQ model, 3 months preconception | Preterm birth | Maternal age, race, pre-pregnancy BMI, smoking and alcohol use, study site, parity, insurance status, marital status, comorbidities | OR per IQR: CO: 1.01 (0.97, 1.04), NOx: 1.00 (0.97, 1.02); O3: 0.95 (0.91, 0.98); PM10: 0.93 (0.89, 0.96); PM2.5: 0.90 (0.85, 0.95); SO2: 0.92 (0.87, 0.96) | Mean(Q1-Q3): CO(ppb) 556.19 (171.69–1125.23); NOx(ppb): 30.16, (5.09–92.97); O3(ppb) 29.67 (7.55–49.37); PM10(μg/m3): 22.25 (10.26–39.44), PM2.5(μg/m3) 11.84 (4.60–22.04); SO2(ppb) 4.06 (0.8–21) | 8/9 |
| Early preterm birth | OR per IQR: CO: 1.04 (0.98, 1.11), NOx: 1.09 (0.99, 1.20); O3: 0.90 (0.85, 0.95); PM10: 0.81 (0.76, 0.87); PM2.5: 0.77 (0.70, 0.85); SO2: 0.85 (0.78, 0.92) | |||||||||
|
| ||||||||||
| Chen, 2021a | China | 10916 | At birth | Prospective cohort study | PM2.5 from Chinese Air Quality Reanalysis data set (CAQRA) model, 12 weeks before pregnancy | Small for gestational age (SGA) | Maternal age, ethnicity, education, pre-pregnancy BMI, employment status, residence, gravidity, parity, smoking, husband smoking, alcohol use, the season of conception, the season of conception, temperature, dew point | HR per 10 μg/m3 of PM2.5: 1.08 (0.85, 1.37) | Mean (SD) in μ/m3: PM2.5 71.4(6.8); PM10 112.2 (10.4); SO2 21.8 (6.8); NO2 40.6 (4.7); O3 108.4 (16.0); CO (mg/m3) 1.2 (0.1) | 8/9 |
| Large for gestational age (LGA) | HR per 10 μg/m3 of PM2.5: 1.47 (1.28, 1.69) | |||||||||
| Chen, 2021b | China | 10960 | At birth | Prospective cohort study | PM2.5 from Chinese Air Quality Reanalysis data set (CAQRA) model, 12 weeks before pregnancy | Preterm birth | Maternal age, ethnicity, education, pre-pregnancy BMI, employment status, residence, gravidity, parity, smoking, husband smoking, alcohol use, the season of conception, the season of conception, temperature, dew point | HR per 5 jg/m3 PM2.5: 1.33 (1.19, 1.49); PM10: 1.34 (1.24, 1.45); 3 μg/m3 increase of SO2: 1.31 (1.24, 1.39); NO2: 1.24 (1.13, 1.37); O3: 0.66 (0.60, 0.72); 0.1 mg/m3 increase in CO: 1.64 (1.45, 1.85) HR per 5 μg/m3: PM2.5 1.13 (0.88, 1.46); PM10 1.30 (1.09, 1.54); SO2 1.42 (1.22. 1.66); NO2 1.16 (0.95, 1.43); O3 0.68 (0.57, 0.83); CO 1.59 (1.20, 2.10) | Mean (SD) in μ/m3: PM2.5 77.1 (11.0); PM10 120 (14.4); SO2 27.0 (10.1); NO2 43.4 (29.2); O3 104.6 (29.2); CO (mg/m3) 1.3 (0.2) | 9/9 |
| Low birthweight | ||||||||||
| McGuinn, 2020 | United States | 1529 | 30 – 68 months old | Case control study | PM2.5, O3 used satellite-based models linked to address at birth, 3 months before pregnancy | Autism spectrum disorder (ASD) | maternal age, maternal education, maternal race/ethnicity, maternal smoking, study site, month of birth, and year of birth. | 1 IQR PM2.5: OR 1.0 (0.92, 1.10); O3: OR 1.00 (0.87, 1.20) | Mean (IQR): O3 (ppb)): 37.3 (18.8); PM2.5 (μg/m3): 12.7 (5.0) | 8/9 |
| H. Li, 2021 | China | 8699 | At birth | Case control study | Monthly PM10 air pollution levels from 75 air monitoring stations in 14 cities, 3 months before pregnancy | Spina bifida | Maternal SO2 and NO2 exposures, maternal age, sex, season of conception, parity, maternal education | Q4 compared to Q1 PM10: OR 2.01 (1.43, 2.81); Per 10 jg/m3 OR 1.07 (1.02, 1.11) | Quartile of PM10 (μg/m3): Q1 :<73; Q2: 73 – 88; Q3: 89 – 105; Q4: >105 | 9/9 |
| Kalkb renner, 2015 | United States | 15645 | Under the age of 8 years | Case control study | Modelled daily PM10 assigned at address of birth certificate, 80 days before estimated date of conception | Autism spectrum disorder (ASD) | Race, maternal education, maternal age, census block group median household income, urbanization rate, calendar week of child's birth | Per 10 μg/m3 of PM10 OR: 0.94 (0.82, 1.08) | Mean of PM10: 23.3 – 23.9 μg/m3, SD of PM10: 4.8 – 5.5 μg/m3 | 9/9 |
only included children born to women without autoimmune diseases.
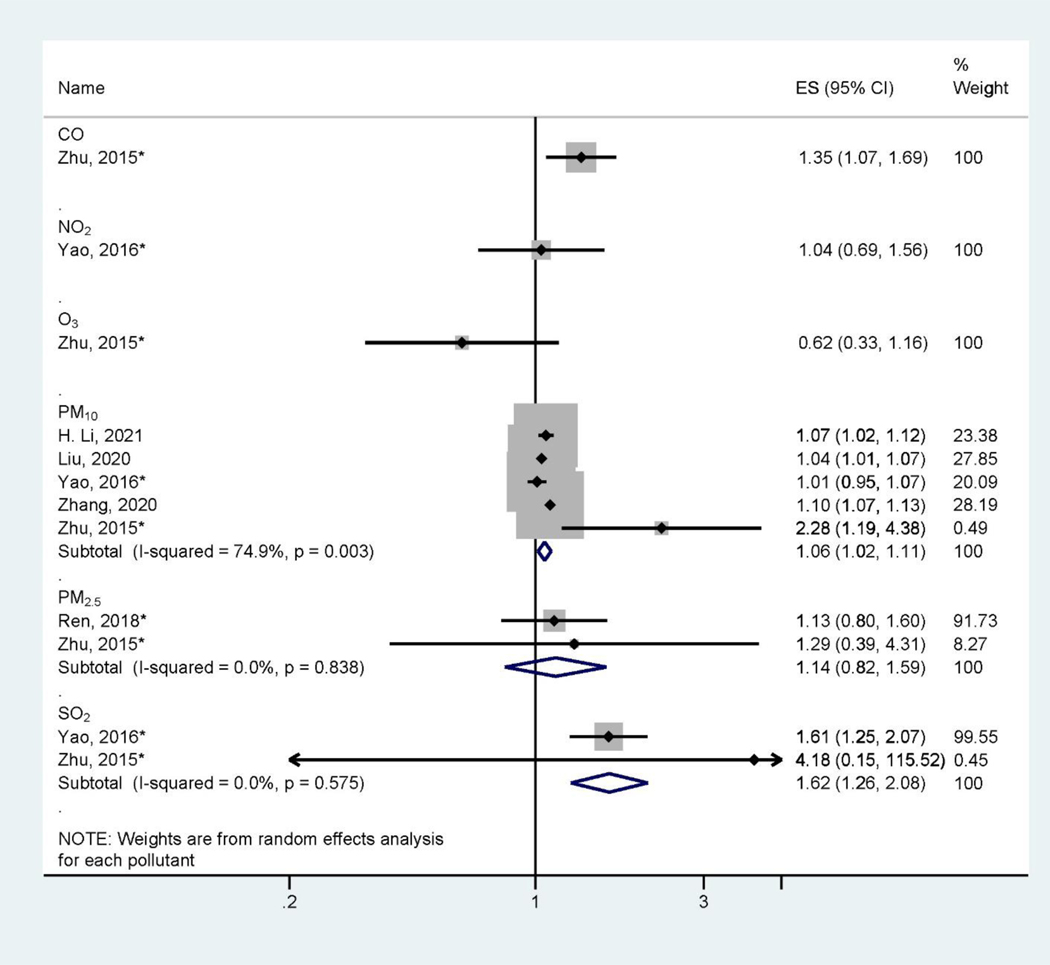
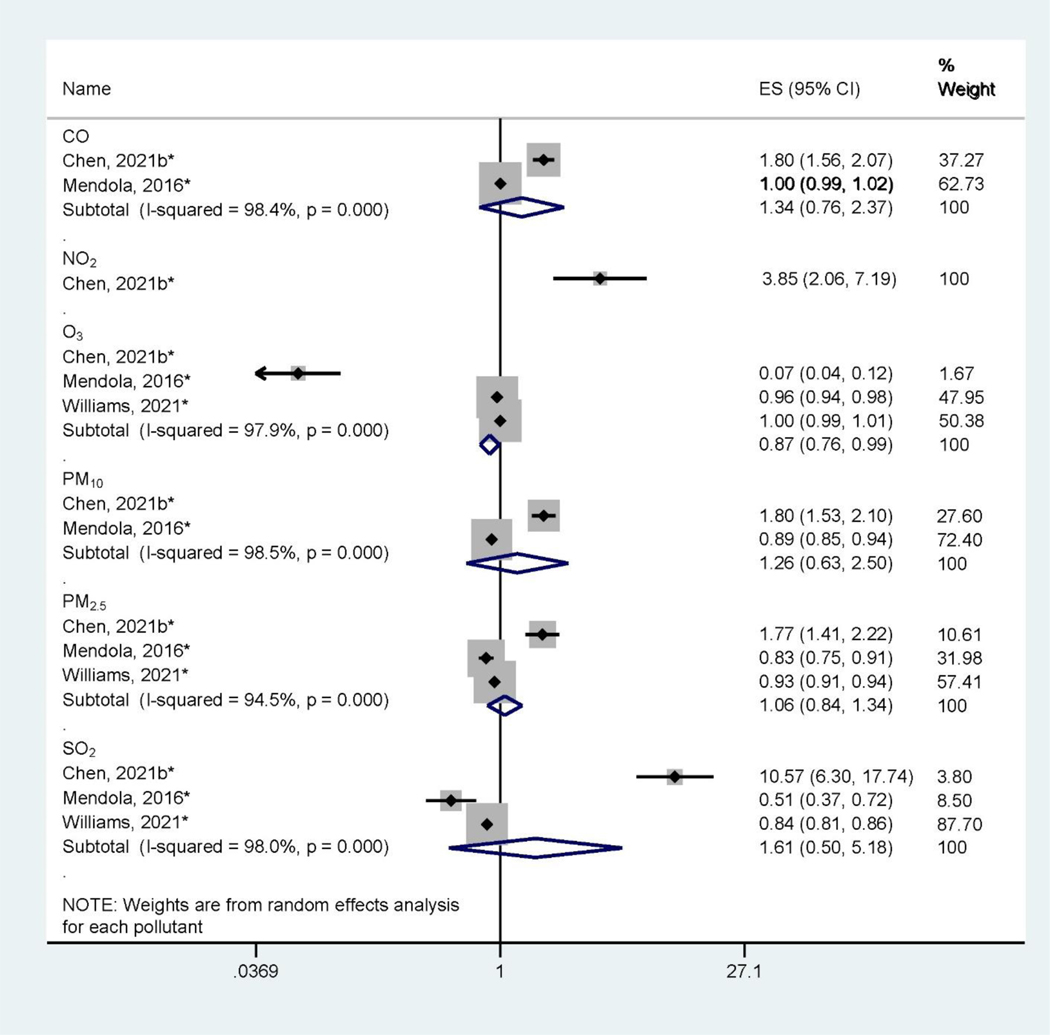
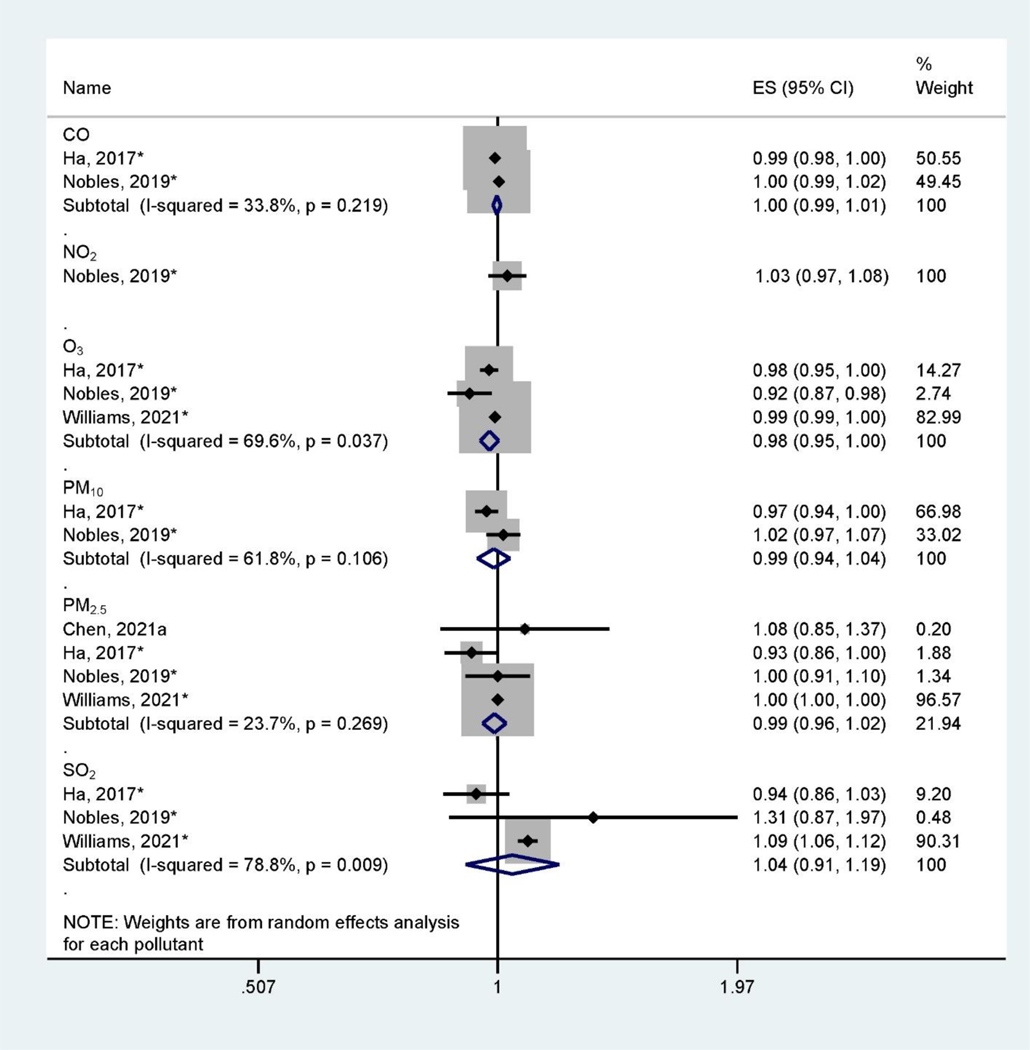
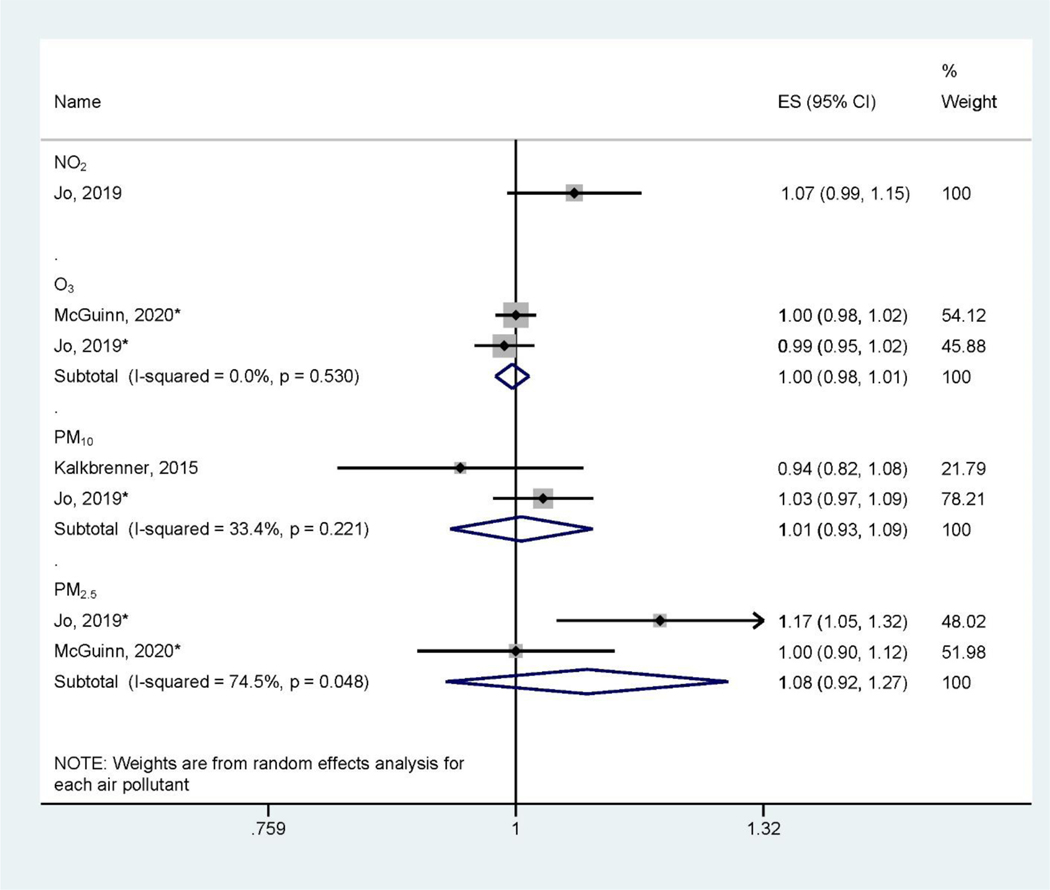
There are large heterogeneities of health outcomes assessed by the included studies. Pregnancy and birth outcomes are most studied, specifically birth defects (Figure 2), low birthweight and small for gestational age (Figure 3), and preterm birth (Figure 4). These outcomes were mainly assessed at the time of birth (Table 1). Three studies (Jo et al., 2019; Kalkbrenner et al., 2015; McGuinn et al., 2020) (Figure 5) assessed autism spectrum disorder (ASD) between ages 0 – 8 (Table 1). Other health outcomes that have been investigated include perinatal mortality (Williams et al., 2021), transient tachypnea, asphyxia (Seeni et al., 2018), fetal growth restriction (Nobles et al., 2019), termination of pregnancy, pregnancy loss (Sun et al., 2021), overweight in childhood (Mao et al., 2017), eczema (Deng et al., 2016; Lu et al., 2017), psychomotor development index (PDI), and mental development index (MDI) (Li et al., 2021). Across studies, consistent associations were found between preconception exposure to air pollution (O3, PM2.5, PM10, SO2, NO2, and CO) and a higher risk for birth defects, low birthweight, or preterm birth, although heterogeneities and inconsistencies exist. The GRADE assessment of pregnancy outcomes, birth outcomes, and ASD is presented in Supplemental Materials (Table S3–Table S6). We found the overall sample size is large for birth defects, low birthweight, preterm birth outcomes, and ASD, however, there are also inconsistencies across studies and heterogeneities in air pollutant levels and associations. We found moderate quality of evidence for PM10 and PM2.5 preconception exposure on birth defects and PM2.5 preconception exposure on birthweight (Table S3 – Table S6), while other air pollution – outcome associations were of low quality.
Figure 2.
Forest Plot Showing the Associations of Preconception Air Pollution Exposure with Birth Defect per 10 μg/m3 (for PM2.5 and PM10) or 10 ppb (for NO2, SO2, and O3) or 100 ppb (for CO) increase in 3-month Preconception Average Concentration. (ES: effect size; CI: confidence interval). *Indicating converted effect size from the original article
Figure 3.
Forest Plot Showing the Associations of Preconception Air Pollution Exposure with Preterm Birth per 10 μg/m3 (for PM2.5 and PM10) or 10 ppb (for NO2, SO2, and O3) or 100 ppb (for CO) increase in 3-month Preconception Average Concentration. (ES: effect size; CI: confidence interval). *Indicating converted effect size from the original article
Figure 4.
Forest Plot Showing the Associations of Preconception Air Pollution Exposure with Small for Gestational Age per 10 μg/m3 (for PM2.5 and PM10) or 10 ppb (for NO2, SO2, and O3) or 100 ppb (for CO) increase in 3-month Preconception Average Concentration. (ES: effect size; CI: confidence interval). *Indicating converted effect size from the original article
Figure 5.
Forest Plot Showing the Associations of Preconception Air Pollution Exposure with Autism Spectrum Disorder per 10 μg/m3 (for PM2.5 and PM10) or 10 ppb (for NO2, SO2, and O3) increase during 3-month Preconception Period. (ES: effect size; CI: confidence interval). *Indicating converted effect size from the original article
Five studies assessed the association between preconception air pollution exposure on birth defects (H. Li et al., 2021; Liu et al., 2020; Ren et al., 2018; Yao et al., 2016; Zhang et al., 2020; Zhu et al., 2015) (Figure 2). Although all or some of the criteria pollutants (O3, PM2.5, SO2, NO2, CO, and PM10) regulated by the US Environmental Protection Agency were examined in some studies, most studies only assessed the effects of particulate matter (PM2.5 and PM10) on birth defects. Of 4 studies on PM10 and 2 studies on PM2.5, a consistent positive association was observed between PM10 or PM2.5 exposure and the risk of birth defects or congenital anomalies (Ren et al., 2018), including oral cleft (Liu et al., 2020; Yao et al., 2016; Zhu et al., 2015) and neural tube defects (H. Li et al., 2021; Zhang et al., 2020). A 10 μg/m3 increase in PM10 exposure during the 3-month preconception period was associated with a 6% (95% confidence interval (CI): 2 – 11%) increase in the risk of birth defects, and PM2.5 exposure was associated with a 14% (95% CI: −18% - 59%) increased risk of birth defects. The analysis of PM10 on birth defects showed heterogeneity (I2 = 74.9%, P < 0.05) while less heterogeneity was found with PM2.5 (I2 = 0.0, P = 0.84). Furthermore, two studies found that SO2 were positively and significantly associated with the risk of birth defects or congenital anomalies (Yao et al., 2016; Zhu et al., 2015). These two studies showed relatively large significant associations between SO2 on the risk of birth defects (RR = 1.62, 95% CI: 1.26, 2.08) per 10 ppb increase of SO2 (I2 = 0.0, P = 0.58). Only one study assessed the association of NO2 (Yao et al., 2016), CO, and O3 (Zhu et al., 2015) with birth defects, and they did not find statistically significant associations. In the GRADE assessment, we found moderate quality evidence for associations between PM10 and PM2.5 with birth defect outcomes and low-quality evidence of SO2 (Table S4). We upgraded the evidence quality for PM10 and PM2.5 because 1) there are many studies from areas with low and high pollution levels and 2) the meta-analysis shown some larger effect, 3) existence of animal studies and mechanistic evidence (Tanwar et al., 2018).
Preterm birth was investigated in two studies (Mendola et al., 2016; Williams et al., 2021) (Figure 3), which assessed the associations of preterm birth with exposure to CO, NO2, O3, PM10, PM2.5, and SO2. The pooled results from these studies indicated some inconsistencies across air pollutants (I2 > 80%, P < 0.05). The meta-analysis found a protective effect of O3 for preterm birth (Chen et al., 2021b, Mendola et al., 2016; Williams et al., 2021), showing the relative risk (RR) of 0.87 (95% CI: 0.76 – 0.99) of preterm birth per 10 ppb increase of 3-month preconception O3 exposure. However, there are large inconsistencies of these associations (I2 = 97.9%, P < 0.001).
For CO, PM10, PM2.5, and SO2, the associations with preterm birth were not statistically significant in the meta-analysis. We also found two studies assessing outcomes related to preterm birth: pregnancy loss, termination of pregnancy (Sun et al., 2021), and perinatal mortality (Williams et al., 2021). However, both studies did not report statistically significant associations between 3-month preconception air pollution (PM10, PM2.5, O3, and SO2) exposures and the risk of these outcomes. We characterized the evidence linking air pollution exposure during the preconception period and preterm birth as low quality due to the inconsistencies between the relatively small number of observational studies available (Table S5).
Low birthweight (birth weight less than 2,500g) and small for gestational age (SGA, less than 10th percentile in the distribution of birthweight for a given gestational age and sex at birth) were investigated in four studies (Chen et al., 2021a; Ha et al., 2017; Nobles et al., 2019; Williams et al., 2021) (Figure 4). These studies assessed the associations of exposures to CO, NO2, O3, PM10, PM2.5, and SO2 during the preconception period with the risk of SGA. For most of these air pollutants, there were non-statistically significant associations. The pooled RR estimates for a 100 ppb increase in 3-month preconception CO exposure was 1.00 (95%CI: 0.99, 1.01), and the pooled RR estimate for a 10 μg/m3 increase in 3-month preconception exposure to PM10 and PM2.5 were 0.99 (95% CI: 0.94, 1.04) and 0.99 (95% CI: 0.96, 1.02), respectively. However, three studies found that O3 was negatively associated with low birthweight, with a pooled RR of 0.98 (95%CI: 0.95, 1.00) per 10 ppb increase of 3-month preconception O3 exposure (Ha et al., 2017; Nobles et al., 2019; Williams et al., 2021). The pooled results of O3 on the low birthweight also showed heterogeneity across three studies (I2 = 69.6%, P = 0.037). Two studies investigated fetal growth restriction (Nobles et al., 2019) and childhood obesity (Mao et al., 2017). Nobles et al. found that preconception exposures to SO2, NOx, NO2, CO, PM2.5, and PM10 were positively associated with fetal growth restriction, while O3 showed a negative association (Nobles et al., 2019). Mao et al. found that increasing 3-month preconception exposure to PM2.5 was associated with an increased risk of childhood obesity (Mao et al., 2017). We characterized the quality of evidence linking air pollution exposure and low birthweight as generally low. However, we upgraded the quality for PM2.5 to moderate (Table S6) because we found four studies that assessed this association in both China and United States with consistent findings (I2 = 23.7%, P = 0.269) despite not achieving statistical significance. Additionally, there are several animal experimental studies providing mechanistic evidence linking preconception PM2.5 exposure and birthweight, which further increased the quality of this evidence (Xu et al., 2019; Veras et al., 2008).
We found three studies that assessed effects of preconception NO2, O3, PM10 and PM2.5 exposure (Jo et al., 2019; Kalkbrenner et al., 2015; McGuinn et al., 2020) on autism spectrum disorder (ASD). The pooled associations between preconception air pollutant exposure and ASD did not reach statistical significance (Figure 5), therefore we characterized the quality evidence as low in GRADE assessment (Table S7). Besides ASD, other neural developmental outcomes including psychomotor development index (PDI) and mental development index (MDI) were assessed (J. Li et al., 2021). It was found that average 3-month preconception exposure to PM2.5 was significantly associated with a lower risk of PDI and not significantly associated with MDI by age 2 (J. Li et al., 2021).
Lastly, we found five studies that analyzed associations of preconception air pollution exposure with children’s respiratory and allergic outcomes, including respiratory disease syndrome, transient tachypnea, asphyxia, childhood pneumonia, asthma, and eczema (Deng et al., 2016; Lu et al., 2021, 2017; Seeni et al., 2018; Williams et al., 2021). For respiratory distress syndrome (RDS), one study found an inverse association between 3-month CO preconception exposure and the risk of RDS (Seeni et al., 2018); while two studies found inverse associations between preconception PM2.5 exposure and RDS (Seeni et al., 2018; Williams et al., 2021). No significant association was found with other air pollutants (NO2, O3, PM10, or SO2) and RDS (Lu et al., 2021; Seeni et al., 2018; Williams et al., 2021). For childhood pneumonia, only one study found a positive association of 3-month preconception PM10 and SO2 exposure (Lu et al., 2021). Two studies found positive associations of 3-month preconception exposure to NO2 with asthma, allergic rhinitis, and eczema (RR = 1.19 (95% CI: 1.04, 1.37) per 1 IQR increase), but not consistently for PM10 and SO2 exposures (PM10: RR = 1.05 (95% CI: 0.90, 1.23) per 1 IQR increase; SO2: RR = 1.12 (95% CI: 0.97, 1.29) per 1 IQR increase).
4. Discussion
In this systematic review, we found a large heterogeneity for studies in terms of air pollutant types, exposure assessment methods, and health outcomes. We found that birth defects, birth weight (low birth weight and small for gestational age), preterm birth, and autism spectrum disorder have been investigated in more than two studies, while other health outcomes were only investigated in one or two studies. Other health outcomes included perinatal mortality (Williams et al., 2021), transient tachypnea, asphyxia (Seeni et al., 2018), fetal growth restriction (Nobles et al., 2019), termination of pregnancy, pregnancy loss (Sun et al., 2021), childhood obesity (Mao et al., 2017), eczema (Deng et al., 2016; Lu et al., 2017), psychomotor development index (PDI), mental development index (MDI) (J. Li et al., 2021), asthma, eczema (Deng et al., 2016; Lu et al., 2017) and childhood pneumonia (Lu et al., 2021). In addition to heterogeneity of health outcomes assessed in these studies, we also found heterogeneities in levels of air pollutants across studies. Due to the small number of studies and large heterogeneity among studies, we have not conducted an analysis to assess biases of the studies. We found moderate quality of evidence for PM10 and PM2.5 preconception exposure on birth defect and PM2.5 preconception exposure on birthweight, while other air pollution – outcome associations were of low quality. There is an evidence base supporting associations of maternal preconception air pollution exposure and children’s health outcomes, specifically preconception particulate matters (PM10 and PM2.5) exposure on birth defects and birth weight. However, we are not able to conclude direction of associations between preconception exposure and health outcomes because the reviewed studies were observational in design and there were large heterogeneities in exposure assessment methods and effect estimates across the studies.
Previous systematic reviews and meta-analyses have been conducted for the associations between maternal air pollution exposure during pregnancy and birth outcomes and children’s health outcomes (Li et al., 2017; Stieb et al., 2012). Compared to a high number of studies of prenatal gestational air pollution exposure, fewer studies have examined the effects of preconception exposure. Therefore, we were not able to provide reliable pooled estimates of associations between preconception air pollution and children’s health outcomes.
Most of the studies in this review used air pollution exposure data from fixed air pollution stations that were closest to residential addresses of participants (Liu et al., 2020; Lu et al., 2021; Sun et al., 2021; Yao et al., 2016; Zhang et al., 2020) or the hospital where children were born (Zhu et al., 2015) to represent individual air pollution exposure. Other studies applied exposure models to extrapolate monitoring station measurements to participants’ residential address (Chen et al., 2021a, 2021b; Ha et al., 2017; Jo et al., 2019; J. Li et al., 2021; Mendola et al., 2016; Nobles et al., 2019; Ren et al., 2018; Seeni et al., 2018; Williams et al., 2021). These exposure assessment methods based on outdoor air pollution monitoring stations could be biased from individual air pollution exposure (Steinle et al., 2013; Xie et al., 2017). As a result, future studies should consider more precise exposure assessment methods, such as personal exposure assessment, spatial-temporal model with activity patterns, and better time-solved exposure assessment. Additionally, future studies should treat air pollution as a mixture of various air pollutants and use exposure mixture analysis to assess the preconception effects of multiple air pollution exposures. There may be a synergistic effect from co-pollutants or interactions between co-pollutants in terms of exerting adverse health effects, so it is important to research this in the future.
The majority of the studies used pollutant concentrations averaged over 3 months prior to conception date to represent preconception air pollution exposure. Only one study (Sun et al., 2021) applied a distributed lag model (DLM) to assess the daily critical time window of exposure to PM2.5, PM10, and O3 during the preconception period on termination of pregnancy, pregnancy loss, and birth defect. Three months before conception, however, may not represent the most susceptible time window for air pollution exposure, considering the different maturing process of gametes in father and mother and how air pollution may uniquely affect each (Sharma and Agarwal, 2011). Female gametes are susceptible to environmental pollution in the critical window of 2 weeks before conception (Sharma and Agarwal, 2011), while male gametes (sperm cells) have a critical window of 3 months before fertilization of the zygotes. Therefore, future studies of air pollution preconception exposure should consider these critical windows of exposure and provide separate maternal and parental exposure assessments that align with the biological process of gametes that may be affected by epigenetics leading long-term effects on fetus development and children’s health. Considering air pollution during preconception can have varying effects depending on the gender of the parent and in those with preexisting disease such as asthma (Tiotiu et al., 2020), future studies are needed to separately investigate both maternal and paternal effects and populations with comorbidities.
Currently, a few cohort studies have been designed and developed to study various risk factors during the preconception period (Harville et al., 2019; Wang et al., 2019). They are enrolling both maternal and paternal participants during the pre-pregnancy period to investigate health effects of nutritional factors, tobacco use, and substance use. Exploring the role of air pollution and other environmental factors in these prospective preconception cohort studies will help identify potential independent or synergistic effects of air pollution exposures in both parents. Therefore, more long-term follow-up of the outcomes such as trajectories of phenotypes and biomarkers are needed to ascertain the effect of preconception exposure to air pollution on disease development and progression.
Based on the Developmental Origins of Health and Disease (DOHaD) hypothesis (Mandy and Nyirenda, 2018), exposure to environmental risk factors such as air pollution during critical prenatal periods could affect the plasticity of development, leading to the development of diseases in children and adults. Several animal experimental and toxicological studies have provided evidence showing critical windows of preconception air pollution exposure. One study found that exposure to concentrated ambient particles (CAP) for 6 weeks decreased male offspring birth weight, yet increased growth trajectory and adipose mass in adulthood (Xu et al., 2019). A second study found that ambient levels of PM generated by urban traffic affected the maternal side of the placental interface between the mother and fetus causing reduced fetal weights (Veras et al., 2008). Another study found that preconception exposure to un-filtered ambient air, compared with filter air exposure, resulted in morphological changes in the placenta and uterine environment, suggesting the decreases in fetal weight correlated to decreased maternal blood space and volume due to air pollution exposure (Veras et al., 2008). In addition, an animal study suggested that preconception exposure to PM2.5 resulted in global cardiac dysfunction in adult offspring (Tanwar et al., 2018). These toxicological studies implied biological mechanisms of preconception air pollution exposure and children’s health.
Although the animal studies provided mechanistic insights, human studies incorporating pathophysiological pathway assessments are needed to confirm whether these findings can be translated to humans and to improve the causal evidence to support the observational associations between exposure and outcomes. For example, urine and blood sample collections can be incorporated into future human health studies (Harville et al., 2019), although it’s challenging due to these samples needing to be collected during the preconception period. Leveraging cohort resources with banked biospecimens can be a workable strategy. In addition, epidemiological study designs such as randomized intervention trials (e.g., indoor air purification or wearing N95 facemask) can be used to support causal inference. We acknowledge that it would be difficult for interventions to target the population during the preconception period, however, marriage-based intervention study could provide an example to causally study health outcomes of air pollution exposure during preconception (Pillarisetti et al., 2020).
Since we found that existing studies that have been conducted in cohorts and populations in China or the United States, there is minimal geographical diversity. Different air pollution levels between China and United States provide evidence for high and low air pollution levels, yet this difference may also result in heterogeneity of findings. In the future, studies on geographical areas such as South Asia, Europe, and Africa are needed to provide more globally relevant evidence with larger generalizability on the adverse effects of preconception air pollution exposure.
5. Conclusions
This systematic review identified 22 studies that have examined the association between preconception maternal air pollution exposure and children’s health outcomes. Air pollutants examined included PM2.5, PM10, NO2, SO2, O3 and CO. Health outcomes assessed included birth defects, preterm birth, autism, respiratory and allergic outcomes. We concluded that while there is an evidence base supporting associations of maternal preconception air pollution exposure and children’s health outcomes, variations in effects and sources of heterogeneity across studies should be explored further. Future studies should consider the effect of paternal preconception exposure in relation to the effect of maternal preconception exposure. To support the causality of the epidemiologic associations, future studies should investigate biological mechanisms using pathophysiologic biomarkers and control for potential confounding factors using novel study designs such as randomized intervention trials.
Supplementary Material
Table S1 lists the reasons of exclusion, if applicable, for the 64 eligible studies that met the criteria for full-text assessment.
Highlight.
22 studies from 2 countries assessed preconception air pollution on children’s health
Birth defects show most significant association with preconception air pollution
Preterm birth, birthweight and respiratory outcomes show large heterogeneities
Studies of critical preconception window with improved exposure method are needed
Funding:
This study was supported by the National Institute of Environmental Health Sciences (R01ES029945), the National Heart, Lung, and Blood Institute (R01HL118455), the Southern California Environmental Health Sciences Center (P30ES007048) funded by the National Institute of Environmental Health Sciences, the Children’s Environmental Health Center (P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency, the National Institute of Environmental Health Sciences (R00ES027870), and Hasting foundation.
Footnotes
Disclosures:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author statement
Natalie Blanc: Methodology, Formal analysis, Investigation, Data curation, Writing – Original Draft. Jiawen Liao: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – Original Draft, Visualization. Frank Gilliland: Conceptualization, Supervision, Funding acquisition. Junfeng (Jim) Zhang: Project administration, Funding acquisition, Writing – Review & Editing. Kiros Berhane: Writing – Review & Editing. Guoying Huang: Writing Editing. Weili Yan: Writing – Review & Editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- Chen J, Li P-H, Fan H, Li C, Zhang Y, Ju D, Deng F, Guo X, Guo L, Wu S, 2021a. Weekly-specific ambient fine particular matter exposures before and during pregnancy were associated with risks of small for gestational age and large for gestational age: results from Project ELEFANT. Int. J. Epidemiol 10.1093/ije/dyab166 [DOI] [PubMed] [Google Scholar]
- Chen J, Fang J, Zhang Y, Xu Z, Byun H-M, Li P-H, Deng F, Guo X, Guo L, Wu S, 2021b. Associations of adverse pregnancy outcomes with high ambient air pollution exposure: Results from the Project ELEFANT. Sci Total Environ 761, 143218. 10.1016/j.scitotenv.2020.143218 [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH, 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet Lond. Engl 389, 1907–1918. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Lu C, Ou C, Chen L, Yuan H, 2016. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere 152, 459–467. 10.1016/j.chemosphere.2016.03.032 [DOI] [PubMed] [Google Scholar]
- Ha S, Zhu Y, Liu D, Sherman S, Mendola P, 2017. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ. Res 155, 394–400. 10.1016/j.envres.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Jiang P, Dong T, Ding X, Chen T, Villanger GD, Aase H, Huang L, Xia Y, 2018. Maternal air pollution exposure and preterm birth in Wuxi, China: Effect modification by maternal age. Ecotoxicol. Environ. Saf 157, 457–462. 10.1016/j.ecoenv.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Harville EW, Mishra GD, Yeung E, Mumford SL, Schisterman EF, Jukic AM, Hatch EE, Mikkelsen EM, Jiang H, Ehrenthal DB, Porucznik CA, Stanford JB, Wen S-W, Harvey A, Symons Downs D, Yajnik C, Santillan D, Santillan M, McElrath TF., Woo JG., Urbina EM., Chavarro JE., Sotres-Alvarez D., Bazzano L., Zhang J., Steiner A., Gunderson EP., Wise LA., 2019. The Preconception Period analysis of Risks and Exposures Influencing health and Development (PrePARED) consortium. Paediatr. Perinat. Epidemiol 33, 490–502. 10.1111/ppe.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z, 2017. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ. Res 159, 519–530. 10.1016/j.envres.2017.08.038 [DOI] [PubMed] [Google Scholar]
- Januário DANF, Perin PM, Maluf M, Lichtenfels AJ, Nascimento Saldiva PH, 2010. Biological Effects and Dose-Response Assessment of Diesel Exhaust Particles on In Vitro Early Embryo Development in Mice. Toxicol. Sci 117, 200–208. 10.1093/toxsci/kfq165 [DOI] [PubMed] [Google Scholar]
- Johnson NM, Hoffmann AR, Behlen JC, Lau C, Pendleton D, Harvey N, Shore R, Li Y, Chen J, Tian Y, Zhang R, 2021. Air pollution and children’s health—a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ Health Prev Med 26, 72. 10.1186/s12199-021-00995-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Chen J-C, Cockburn M, Martinez MP, Chow T, Lurmann FW, Funk WE, Xiang AH, McConnell R, 2019. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environ. Int 133, 105110. 10.1016/j.envint.2019.105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL, 2015. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 26, 30–42. 10.1097/EDE.0000000000000173 [DOI] [PubMed] [Google Scholar]
- Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A, 2018. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res 167, 144–159. 10.1016/j.envres.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Li H, Huang Y-H, Li J, Liu S, Chen Y-L, Li L-L, Jiang C-Z, Chen Z-J, Li N, 2021. Maternal PM10 Exposure Increases Risk for Spina Bifida: A Population-Based Case-Control Study. Front Public Health 9, 695192. 10.3389/fpubh.2021.695192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liao J, Hu C, Bao S, Mahai G, Cao Z, Lin C, Xia W, Xu S, Li Y, 2021. Preconceptional and the first trimester exposure to PM2.5 and offspring neurodevelopment at 24 months of age: Examining mediation by maternal thyroid hormones in a birth cohort study. Environ. Pollut 284, 117133. 10.1016/j.envpol.2021.117133 [DOI] [PubMed] [Google Scholar]
- Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, Xue X, Chu Y, Liu F, Liu Y, Ren M, Chen X, Li N, Lu Y, Mao Z, Tian L, Xiang H, 2017. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ. Pollut 227, 596–605. 10.1016/j.envpol.2017.03.055 [DOI] [PubMed] [Google Scholar]
- Li Zhou, Tang Y, Song X, Lazar L, Li Zhen, Zhao J, 2019. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf 169, 248–254. 10.1016/j.ecoenv.2018.10.109 [DOI] [PubMed] [Google Scholar]
- Liu F-H, Dai H-X, Gong T-T, Zhang J-Y, Li J, Chen Z-J, Li L-L, Chen Y-L, Liu S, Jiang C-Z, Huang Y-H, Zhao Y-H, Wu Q-J, 2020. Maternal preconception and first trimester exposure to PM10 and the risk of oral clefts in offspring: a population-based, case–control study. Occup. Environ. Med 77, 721–727. 10.1136/oemed-2020-106434 [DOI] [PubMed] [Google Scholar]
- Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q, 2017. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J. Dermatol. Sci 85, 85–95. 10.1016/j.jdermsci.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Lu C, Peng W, Kuang J, Wu M, Wu H, Murithi RG, Johnson MB, Zheng X, 2021. Preconceptional and prenatal exposure to air pollution increases incidence of childhood pneumonia: A hypothesis of the (pre-)fetal origin of childhood pneumonia. Ecotoxicol. Environ. Saf 210, 111860. 10.1016/j.ecoenv.2020.111860 [DOI] [PubMed] [Google Scholar]
- Mandy M, Nyirenda M, 2018. Developmental Origins of Health and Disease: the relevance to developing nations. Int. Health 10, 66–70. 10.1093/inthealth/ihy006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Nachman RM., Sun Q., Zhang X., Koehler K., Chen Z., Hong X., Wang G., Caruso D., Zong G., Pearson C., J H., Biswal S., Zuckerman B., Wills Karp Marsha, Wang X, 2017. Individual and Joint Effects of Early-Life Ambient PM2.5 Exposure and Maternal Prepregnancy Obesity on Childhood Overweight or Obesity. Environ. Health Perspect 125, 067005. 10.1289/EHP261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Windham GC, Kalkbrenner AE, Bradley C, Di Q, Croen LA, Fallin MD, Hoffman K, Ladd-Acosta C, Schwartz J, Rappold AG, Richardson DB, Neas LM, Gammon MD, Schieve LA, Daniels JL, 2020. Early Life Exposure to Air Pollution and Autism Spectrum Disorder: Findings from a Multisite Case-Control Study. Epidemiology 31, 103–114. 10.1097/EDE.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola P, Wallace M, Hwang BS, Liu D, Robledo C, Männistö T, Sundaram R, Sherman S, Ying Q, Grantz KL, 2016. Preterm birth and air pollution: Critical windows of exposure for women with asthma. J. Allergy Clin. Immunol 138, 432–440.e5. 10.1016/j.jaci.2015.12.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP, 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 6, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, Guyatt G, Hooijmans C, Langendam M, Mandrioli D, Mustafa RA, Rehfuess EA, Rooney AA, Shea B, Silbergeld EK, Sutton P, Wolfe M, Woodruff TJ, Verbeek JH, Holloway AC, Santesso N, Schünemann HJ, 2016. GRADE: Assessing the quality of evidence in environmental and occupational health. Environ Int 92–93, 611–616. 10.1016/j.envint.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles CJ, Grantz KL, Liu D, Williams A, Ouidir M, Seeni I, Sherman S, Mendola P, 2019. Ambient air pollution and fetal growth restriction: Physician diagnosis of fetal growth restriction versus population-based small-for-gestational age. Sci. Total Environ 650, 2641–2647. 10.1016/j.scitotenv.2018.09.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogliari KS, Lichtenfels AJ de FC, Marchi MRR, Ferreira AT, Dolhnikoff M, Saldiva PHN, 2013. Intrauterine exposure to diesel exhaust diminishes adult ovarian reserve. Fertil. Steril 99, 1681–1688.e2. 10.1016/j.fertnstert.2013.01.103 [DOI] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Januário DN, Saldiva PH, 2008. Effects of short-term exposure of female mice to diesel exhaust particles on in vitro fertilization and embryo development. Fertil. Steril 90, S206. 10.1016/j.fertnstert.2008.07.48819007632 [DOI] [Google Scholar]
- Pillarisetti A, Roy S, Diamond-Smith N, Ghorpade M, Dhongade A, Balakrishnan K, Sambandam S, Patil R, Levine DI, Juvekar S, Smith KR, 2020. Marriage-based pilot clean household fuel intervention in India for improved pregnancy outcomes. BMJ Open 10, e044127. 10.1136/bmjopen-2020-044127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Dowling R, Mehta S, 2021. Ambient and household air pollution on early-life determinants of stunting—a systematic review and meta-analysis. Environ Sci Pollut Res 28, 26404–26412. 10.1007/s11356-021-13719-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti E, Röösli M, Frey U, Latzin P, 2013. Air pollution during pregnancy and neonatal outcome: a review. J. Aerosol Med. Pulm. Drug Deliv 26, 9–23. 10.1089/jamp.2011.0932 [DOI] [PubMed] [Google Scholar]
- Ren S, Haynes E, Hall E, Hossain M, Chen A, Muglia L, Lu L, DeFranco E, 2018. Periconception Exposure to Air Pollution and Risk of Congenital Malformations. J. Pediatr 193, 76–84.e6. 10.1016/j.jpeds.2017.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, 2004. Air Pollution and Children’s Health. Pediatrics 113, 1037–1043. 10.1542/peds.113.S3.1037 [DOI] [PubMed] [Google Scholar]
- Seeni I, Ha S, Nobles C, Liu D, Sherman S, Mendola P, 2018. Air pollution exposure during pregnancy: maternal asthma and neonatal respiratory outcomes. Ann. Epidemiol 28, 612–618.e4. 10.1016/j.annepidem.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Agarwal A, 2011. Spermatogenesis: An Overview, in: Zini A, Agarwal A. (Eds.), Sperm Chromatin. Springer; New York, New York, NY, pp. 19–44. 10.1007/978-1-4419-6857-9_2 [DOI] [Google Scholar]
- Stang A, 2010. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol 25, 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Stare J, Maucort-Boulch D, 2016. Odds ratio, hazard ratio and relative risk. Advances in Methodology and Statistics 13, 59–67. 10.51936/uwah2960 [DOI] [Google Scholar]
- Steinle S., Reis S., Sabel CE., 2013. Quantifying human exposure to air pollution—Moving from static monitoring to spatio-temporally resolved personal exposure assessment. Sci. Total Environ 443, 184–193. 10.1016/j.scitotenv.2012.10.098 [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S, 2012. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res 117, 100–111. 10.1016/j.envres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Sun S, Wang X, Ding L, Zhang Q, Li N, Sui X, Li C, Ju L, Zhao Q, Chen H, Ding R, Cao J, 2021. Association between preconceptional air pollution exposure and medical purposes for selective termination of pregnancy. Environ. Res 202, 111743. 10.1016/j.envres.2021.111743 [DOI] [PubMed] [Google Scholar]
- Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Katapadi A, Sugar BP, Falvo MJ, Baer LA, Stanford KI, Wold LE, 2018. Preconception Exposure to Fine Particulate Matter Leads to Cardiac Dysfunction in Adult Male Offspring. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis 7, e010797. 10.1161/JAHA.118.010797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, Kowal K, 2020. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public. Health 17, 6212. 10.3390/ijerph17176212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa O, Furuyama A, Imai K, Fujitani Y, Hirano S, 2018. Effects of diesel exhaustderived secondary organic aerosol (SOA) on oocytes: Potential risks to meiotic maturation. Reprod. Toxicol 75, 56–64. 10.1016/j.reprotox.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Vecoli C, Montano L, Andreassi MG, 2016. Environmental pollutants: genetic damage and epigenetic changes in male germ cells. Environ. Sci. Pollut. Res 23, 23339–23348. 10.1007/s11356-016-7728-4 [DOI] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Caldini EG, Ribeiro AACM, Mayhew TM, Saldiva PHN, Dolhnikoff M, 2008. Particulate Urban Air Pollution Affects the Functional Morphology of Mouse Placenta1. Biol. Reprod 79, 578–584. 10.1095/biolreprod.108.069591 [DOI] [PubMed] [Google Scholar]
- Volk HE, Perera F, Braun JM, Kingsley SL, Gray K, Buckley J, Clougherty JE, Croen LA, Eskenazi B, Herting M, Just AC, Kloog I, Margolis A, McClure LA, Miller R, Levine S, Wright R, 2021. Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environ. Res 196, 110320. 10.1016/j.envres.2020.110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM, 2009. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med 27, 358–368. 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang Y., Jiang Y., Ye Y., Ji M., Dou Y., Chen X., Li M., Ma X., Sheng W., Huang G., Yan W., 2019. Shanghai Preconception Cohort (SPCC) for the association of periconceptional parental key nutritional factors with health outcomes of children with congenital heart disease: a cohort profile. BMJ Open 9, e031076. 10.1136/bmjopen-2019-031076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Kanner J, Grantz KL, Ouidir M, Sheehy S, Sherman S, Robledo C, Mendola P, 2021. Air pollution exposure and risk of adverse obstetric and neonatal outcomes among women with type 1 diabetes. Environ. Res 197, 111152. 10.1016/j.envres.2021.111152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Semanjski I, Gautama S, Tsiligianni E, Deligiannis N, Rajan RT, Pasveer F, Philips W, 2017. A Review of Urban Air Pollution Monitoring and Exposure Assessment Methods. ISPRS Int. J. Geo-Inf 6, 389. 10.3390/ijgi6120389 [DOI] [Google Scholar]
- Xu Y, Wang W, Chen M, Zhou J, Huang X, Tao S, Pan B, Li Z, Xie X, Li W, Kan H, Ying Z, 2019. Developmental programming of obesity by maternal exposure to concentrated ambient PM2.5 is maternally transmitted into the third generation in a mouse model. Part. Fibre Toxicol 16, 27. 10.1186/s12989-019-0312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Chen Y, Zhu X, Liu Y, Zhang J, Hou L, Xu Y, Zhang C, Cao J, 2016. Air Pollution and the Risk of Birth Defects in Anqing City, China. J. Occup. Environ. Med 58, e124. 10.1097/JOM.0000000000000676 [DOI] [PubMed] [Google Scholar]
- Yauk C, Polyzos A, Rowan-Carroll A, Somers CM, Godschalk RW, Van Schooten FJ, Berndt ML, Pogribny IP, Koturbash I, Williams A, Douglas GR, Kovalchuk O, 2008. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc. Natl. Acad. Sci 105, 605–610. 10.1073/pnas.0705896105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu KF, 1998. What’s the Relative Risk?A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA 280, 1690–1691. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- Zhang J-Y, Wu Q-J, Huang Y-H, Li J, Liu S, Chen Y-L, Li L-L, Jiang C-Z, Chen Z-J, 2020. Association between maternal exposure to ambient PM10 and neural tube defects: A case-control study in Liaoning Province, China. Int. J. Hyg. Environ. Health 225, 113453. 10.1016/j.ijheh.2020.113453 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang C, Liu D, Grantz KL, Wallace M, Mendola P, 2015. Maternal ambient air pollution exposure preconception and during early gestation and offspring congenital orofacial defects. Environ. Res 140, 714–720. 10.1016/j.envres.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 lists the reasons of exclusion, if applicable, for the 64 eligible studies that met the criteria for full-text assessment.