Abstract
Aims:
To quantify the contributions of atopic disorders, sleep disturbance, and other health conditions to five common pain conditions.
Methods:
This cross-sectional analysis used data from 655 participants in the OPPERA study. The authors investigated the individual and collective associations of five chronic overlapping pain conditions (COPCs) with medically diagnosed atopic disorders and self-reported sleep disturbance, fatigue, and symptoms of obstructive sleep apnea. Atopic disorders were allergies, allergic rhinitis, atopic dermatitis, allergic asthma, urticaria, allergic conjunctivitis, and food allergy. Logistic regression models estimated odds ratios as measures of association with temporomandibular disorders, headache, irritable bowel syndrome, low back pain, and fibromyalgia. Measures of sleep and atopy disorders were standardized to z scores to determine the relative strength of their associations with each COPC. Sociodemographic characteristics and body mass index were covariates. Random forest regression analyzed all variables simultaneously, computing importance metrics to determine which variables best differentiated pain cases from controls.
Results:
Fatigue and sleep disturbance were strongly associated with each COPC and with the total number of COPCs. An increase of one standard deviation in fatigue or sleep disturbance score was associated with approximately two-fold greater odds of having a COPC. In random forest models, atopic disorders contributed more than other health measures to differentiating between cases and controls of headache, whereas other COPCs were best differentiated by measures of fatigue or sleep.
Conclusion:
Atopic disorders, previously recognized as predictors of poor sleep, are associated with COPCs after accounting for sleep problems.
Keywords: allergy, atopy, fatigue, sleep disturbance, symptom cluster
Sleep disturbance is a major complaint of people with pain.1-4 The relationship between sleep disturbance and pain is bidirectional, in that pain disrupts sleep maintenance5 while poor sleep increases sensitivity to pain.6 Longitudinal studies clarify that the pre-dominant effect is that sleep disturbance contributes to pain more than pain contributes to sleep disturbance.7 For example, most people with chronic tension-type headache (TTH) report insomnia. Not only does their insomnia predict new-onset TTH, but it also predicts the transition from episodic to chronic headache.8 In the authors’ previous prospective cohort study in the OPPERA project, poor subjective sleep quality at baseline predicted development of temporomandibular disorders (TMD) independently of other TMD risk factors, including somatic awareness, perceived stress, and comorbid illness.9,10
Atopy, a heightened immune response to allergens, is a major contributor to poor sleep. People with allergic rhinitis (ie, hay fever) suffer sleep-disordered breathing11,12 and excessive daytime sleepiness13 secondary to inflamed nasal mucosa, nocturnal congestion, and airway resistance. For people with atopic dermatitis (ie, eczema) or urticaria (ie, hives), circadian changes in neuropeptide-mediated vasodilation and skin temperature exacerbate nocturnal pruritus (ie, itch), severely disrupting their sleep.14 Polysomnogram assessment of sleep shows that individuals with atopic dermatitis have delayed sleep onset latency, greater sleep fragmentation, less nonrapid eye movement sleep, and reduced sleep efficiency.15 It is estimated that up to 80% of children and as many as 87% of adults with atopic dermatitis report sleep disturbance.16
Given that sleep disturbance coexists with pain and that atopy disturbs sleep, it is not surprising to find an excess burden of atopic disorders in people with unexplained pain conditions. In a pooled analysis of 401,002 U.S. children and adolescents from 19 studies of the population-based National Survey of Children’s Health and the National Health Interview Survey, Silverberg noted that participants with a history of asthma, allergic rhinitis, food allergy, or atopic dermatitis had greater covariate-adjusted odds of headache in 14 of these 19 studies.17 Notably, this analysis found stronger associations between atopic disorders and headache in the presence of sleep disturbance, but since these associations also occurred in the absence of sleep disturbance and fatigue,17 atopy was independent of these sleep problems in its association with pain.
Silverberg’s study17 of headache in the presence of atopic disorders and sleep disturbance is one of very few studies to examine the associations of both atopic disorders and sleep disturbance with a pain condition. To date, no study has jointly examined these health characteristics with overlapping pain conditions. The present study therefore explored the similarities and differences among five chronic overlapping pain conditions (COPCs) with respect to their associations with atopic disease, sleep disturbance, and other health measures. The first aim was to estimate univariate associations of the health measures with the five COPCs, individually and collectively. A second aim was to identify the multivariable contributions of health status measures in discriminating between cases and controls of each COPC. The third aim was to investigate the associations of these health measures with the total number of COPCs.
Materials and Methods
Ethical Considerations
Reporting of this observational study conforms with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.18 The primary data collection was from the National Institute of Dental and Craniofacial Research (NIDCR) Study Protocol 12-050-E, conducted in the second phase of the OPPERA project. Institutional review boards at each academic institution reviewed and approved the study.
Study Design, Setting, and Participants
The cross-sectional design used data from adults recruited into the first phase of OPPERA between May 2006 and May 2013. At that time, subjects aged 18 to 44 years were selected for two epidemiologic studies of TMD.
The baseline case-control study of chronic TMD enrolled 1,008 cases with examiner-verified painful TMD and 3,258 controls who had examiner-verified absence of TMD. During the next 5 years, a prospective cohort study examined the incidence of TMD among subjects who were originally controls in the case-control study. All subjects were recruited at US academic health centers located at: University of Maryland, Baltimore, Maryland; University at Buffalo, Buffalo, New York; University of North Carolina, Chapel Hill, North Carolina; and University of Florida, Gainesville, Florida. Previous papers have described the details of recruitment and baseline data collection for the case-control study19 and follow-up for the prospective cohort study.20
This cross-sectional analysis reports findings from the most recent wave of data collection, labeled OPPERA-2. Between December 2014 and May 2016, attempts were made to contact all subjects originally enrolled in the OPPERA-1 case-control study. For those who consented and attended the research clinics, data were then collected using clinical examinations, quantitative sensory testing (QST), cardiovascular measures of autonomic function, blood samples, and self-report questionnaires. Further details of recruitment and data collection methods are provided elsewhere in this issue (see Slade et al, current issue).
Classification of COPCs
The presence or absence of five COPCs was classified as described in detail elsewhere in this volume (see Ohrbach et al, current issue) and summarized below.
TMD was classified by examiners according to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).21 To be classified as a TMD case, participants had to have all four of the following findings: (1) history of orofacial pain in examiner-verified locations of the masseter, temporalis, submandibular, or temporomandibular joint (TMJ) area(s) that had occurred on 5 or more days of the 30 days preceding the examination; (2) evoked pain in the same muscles and/or TMJ(s) following palpation of these structures or jaw maneuvers; (3) reported familiarity of evoked pain, as judged by a positive response to the question “Was the pain you felt [during palpation or jaw maneuver] familiar to the pain that you reported during the last 30 days?”; and (4) pain that was modified by jaw function, as judged by a positive response to the question “During the last 30 days, was any of the pain modified by chewing hard food, opening the mouth, jaw habits such as clenching, or other jaw activities?”
Headache was classified using responses to a questionnaire designed for OPPERA that asked about symptoms of TTH and migraine during the preceding 12 months. Participants who experienced more than one type of headache recorded responses separately for up to three different types of headache. Questions about TTH were from the International Classification of Headache Disorders (ICHD) 3rd edition.22 Symptoms of migraine were based on questions used in the ID-Migraine questionnaire.23 Migraine was classified when participants reported headache(s) on 1 or more day per month and when at least two of three symptoms (nausea, sensitivity to light, or being kept from everyday activities) accompanied the headache. For this analysis, headache was classified for any subject who reported symptoms consistent with probable TTH, TTH, or migraine and who had experienced such headache(s) in the preceding 3 months.
Irritable bowel syndrome (IBS) was classified using responses to four questions about abdominal pain from the Rome III diagnostic criteria.24 Participants were classified with IBS if they met both of the following criteria: (1) abdominal pain on at least 1 day in the preceding 3 months that was not related to menstrual periods; and (2) pain that was associated with at least two symptoms of bowel function (ie, pain altered by bowel movements; greater frequency of bowel movements; less frequency of bowel movements; looser stools; harder stools).
Low back pain (LBP) was classified using responses to screening questions recommended for studies of back pain prevalence.25 Participants were classified with LBP if they reported pain that occurred in the lower back (as illustrated with a shaded manikin drawing) during the preceding 3 months that was not related to fever or menstruation and wthat restricted usual activities for at least 1 day.
Fibromyalgia was classified based on findings from an examination and questionnaire, consistent with the 1990 American College of Rheumatology (ACR) criteria.26 Participants were classified with fibromyalgia when ≥ 11 of 18 body sites were tender to digital palpation by the examiner and when tenderness occurred in the axial skeleton and in at least one set of opposing diagonal quadrants of the body. Also, fibromyalgia cases had to report a history of pain lasting for at least 1 day per month in the preceding 3 months.
Demographic Characteristics
Demographic characteristics included age (years), sex (male, female), and race/ethnicity (white, non-Hispanic; black/African American; Hispanic; other).
Assessment of Health Measures
This paper focuses on the relationship between COPCs and a set of health measures, assessed as follows.
Fatigue.
Fatigue was assessed using the 7-item Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Adult Short Form.27 The scale is generic rather than disease-specific, assessing both the experience of fatigue and the interference of fatigue on daily activities over the past week. It evaluates self-reported symptoms, ranging from mild subjective feelings of tiredness to an overwhelming, debilitating, and sustained sense of exhaustion. For example, one question asks “How often were you too tired to think clearly?” Response categories on a 5-point numeric rating scale (NRS) of never, rarely, sometimes, often, and always are scored 1 to 5, respectively. One question (“How often did you have enough energy to exercise strenuously?”) was reverse scored. A total score is obtained by summing the scores of all items, and higher scores indicate greater fatigue. Adequacy of the scale’s psychometric properties was established in diverse populations comprised of healthy controls and patients with fibromyalgia, sickle cell disease, cardiometabolic risk, and pregnancy.28
Subjective Sleep Disturbance.
The 19 items of the Pittsburgh Sleep Quality Index (PSQI) evaluate 7 self-reported dimensions of sleep quality and sleep disturbance over a 1-month reference period.29 These dimensions are subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Adequacy of the scale’s psychometric properties is established in clinical and nonclinical samples.29-32 Each item is scored from 0 to 3, and the sum of scores assigned to the equally weighted dimensions yields a global score ranging from 0 to 21. This global score is often dichotomized at a cut-off score of 5 to distinguish good sleep (< 5) from poor sleep (≥ 5). In this analysis, the continuous global score was used, and consistent with the scale’s directionality, referred to as a measure of sleep disturbance.
Obstructive Sleep Apnea Symptoms.
Obstructive sleep apnea (OSA) is characterized by recurrent periods of pharyngeal airway obstruction and hypoxemia during sleep and excessive sleepiness during wakefulness. In its medical history questionnaire, OPPERA included the STOP screening questionnaire items for OSA. This simple 4-item scale has been validated against overnight polysomnography for detecting OSA.33 Its scoring criteria classify people as being at high risk for OSA or not at high risk. Applying the same criteria as in OPPERA, participants at high risk for OSA were classified as those who reported any two or more of: loud snoring; daytime tiredness; witnessed apnea (breathing cessation) during sleep; or hypertension. In addition, anyone reporting a pre-existing diagnosis of OSA was classified as high risk. Adults with fewer than two of these conditions were classified as low risk for OSA. Hence, OSA symptoms was modeled as a binary variable.
Atopic Disorders.
OPPERA’s medical history questionnaire assessed self-reported medical diagnosis of atopic disorders. Each question was prefaced with the words, “Has a doctor or other health professional ever told you that you have … ?” The specific disorders were: allergies; hay fever (also known as allergic rhinitis); eczema (also known as atopic dermatitis); allergic asthma; hives (also known as urticaria); allergic conjunctivitis (itching or burning eyes); and food allergy. Response options were “yes,” “no,” and “don’t know.” The count of self-reported atopic disorders was summed to produce a potential range from 0 to 7, and this was modeled as a continuous variable. The count (ie, sum) of atopic diagnoses is more informative than a simple binary outcome; for example, it may be found that an increasing number of atopic diagnoses is associated with an increase in the number of COPCs.
Body Mass Index.
Height and weight were determined using standardized equipment during the clinical examination. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters and modeled as a continuous variable. BMI was included because of its positive associations with OSA and previous reports of association with chronic pain conditions.
Statistical Analyses
Raw values of each health measure were used to generate descriptive statistics for cases and controls of each COPC and according to the number of COPCs. All other analyses of continuous variables used z-transformed values of health measures, and the data were weighted during analysis. The goal of data transformation was to produce measures of association (eg, odds ratios [ORs], regression estimates) that could be readily compared between health measures that use different scales of measurement. The goal of weighting was to adjust for the way in which study participants were selected in OPPERA-2. This took into consideration the original sampling design for the OPPERA-1 case-control study (in which TMD cases were oversampled relative to their prevalence in the population) and to adjust for differential loss to follow-up of subjects between enrollment in OPPERA-1 and participation in OPPERA-2. Such weighting is important for this analysis in order to make valid estimates of association between any two variables (eg, health measures and headache) in a sample that was originally stratified according to a third variable (ie, presence or absence of chronic TMD in OPPERA-1).34 The analytic weights for OPPERA-2 were computed as the inverse of the sampling probability for OPPERA-1, multiplied by the inverse of loss to follow-up probability between OPPERA-1 and OPPERA-2. With the exception of univariate statistics describing the distribution of explanatory variables, all means, percentages, and measures of association were calculated using generalized estimating equations with the GENMOD procedure in SAS version 9.4 (IBM), with analytic weights and robust error variance calculation.35
The analysis first assessed the associations between each health measure and the presence or absence of each COPC using statistical methods for case-control analysis of cross-sectional data. Univariate ORs of associations between each COPC and each health measure were estimated in separate binary logistic regression models: the dependent variable was presence vs absence of the COPC, and the main explanatory variable was the standardized (using z-score transformation) value of a single health measure. The models also adjusted for study site (four categories) and subjects’ demographic characteristics: age (measured in years); gender (two categories), and race/ethnicity (five categories: white, black/African American, Asian, Hispanic, or other).
A second set of analyses examined the associations between the number of COPCs and each health measure. Separate linear regression models, one for each health measure, used the health measure z-score as the dependent variable. The number of COPCs was modeled three ways to test for the different effects of the combined number of COPCs: (1) as a categorical variable to evaluate potential nonlinear relationships with the explanatory variable, with pairwise comparisons used to test for differences between subjects with no COPCs (reference group) vs the other five possibilities (1, 2, 3, 4 or 5 COPCs); (2) as a continuous variable to reveal a potential linear relationship with the dependent variable, with a test of the null hypothesis of no linear relationship (β = 0); and (3) with all five COPCs modeled as binary predictor variables in a multivariable model, with parameter estimates tested for independent contributions of each COPC to the health status measure.
Multivariable contributions of all health measures to single COPCs were investigated using random forest models, one for each COPC. As described in detail in a previous OPPERA paper,36 random forest model methodology uses all potential explanatory variables (in this paper, all health status measures) to create decision trees predicting the dependent variable (each pain condition). The goals were to identify individual measures germane to TMD that make the greatest statistical contributions to the occurrence of each pain condition and to quantify the collective accuracy of all explanatory variables in predicting each pain condition. Random forests are nonparametric statistical models that can handle interactions and nonlinear associations without the need to pre-specify the interactions or the form of the nonlinear relationships. Due to this flexibility, random forests demonstrate excellent classification performance across a broad range of tasks. For this paper, a separate random forest model was created for each pain condition, and predictor variables for each model were all health status measures. Five steps are used to create each model36: (1) a random sample of study participants is selected for replacement; (2) a random sample of predictor variables is selected, and each one is used to partition the data and create a decision tree; (3, 4) steps 1 and 2 are repeated 1,000 times each; and (5) the estimated probability of the dependent variable is then calculated as the average of all 1,000 probabilities.
Missing values of explanatory variables were imputed using on-the-fly imputation, which is the decision tree analog of multiple imputation.37 Because random forests are nonparametric statistical models that can handle interactions and nonlinear associations without the need to pre-specify the interactions or the form of the nonlinear relationships, they demonstrate excellent classification performance across a broad range of tasks. Through a combination of the bootstrap aggregating and random subspace methods used in the construction of random forests, they achieve this classification performance without overfitting to the training dataset, thus maintaining good out-of-sample performance.38 Contributions of individual variables in the random forest models were quantified using variable importance scores, which estimate the relative contribution of each predictor to the model’s classification of true positives and true negatives. Overall classification performance of the models was quantified with area under the receiver operating characteristic curve (AUROC) and area under the precision recall curve (AUPR). In datasets with unequal numbers of cases and controls, AUPR is a better measure of classification performance than AUROC, though no single metric can adequately capture classification performance.39 However, both measures accord equal weight to false positives and false negatives, whereas the relative importance of those errors may vary according to COPC. Therefore, the Brier score was also computed,40 which provides an analog to mean squared error, as well as the proportion of variance explained for the binary prediction models. Mutual information provides sensible rankings of classifiers in scenarios (such as class imbalance) that break more commonly used measures such as precision, recall, and AUROC.41
Results
With the exception of LBP, for which cases were older than controls, cases had a younger mean age than controls for each COPC (Table 1). The percentage of women was greater among cases than controls for headache and for fibromyalgia. However, race was not appreciably associated with any COPC, and none of the demographic characteristics were significantly associated with the number of COPCs. Overall, 51.4% of participants had been told by a doctor or other health professional that they had allergies. The percentage reporting specific atopic disorders were: allergic rhinitis = 19.8%; eczema = 13.5%; allergic asthma = 11.0%; urticaria = 13.9%; conjunctivitis = 11.8%; and food allergy = 10.5% (not tabulated).
Table 1.
Descriptive Univariate Analyses of OPPERA Study Participants
| Classification | Group | Unweighted n | Weighted n | Mean age (SE), y |
P | % female (SE) |
P | % white (SE) | P |
|---|---|---|---|---|---|---|---|---|---|
| TMD | Case | 182 | 108 | 33.0 (0.6) | .027 | 61.2 (3.6) | 0.574 | 50.7 (3.7) | .844 |
| Control | 473 | 547 | 35.4 (0.4) | 57.0 (2.3) | 52.2 (2.3) | ||||
| Headache | Case | 270 | 201 | 34.6 (0.5) | .412 | 71.1 (2.8) | 0.001 | 55.5 (3.0) | .392 |
| Control | 385 | 454 | 35.3 (0.4) | 51.7 (2.6) | 50.4 (2.6) | ||||
| IBS | Case | 158 | 134 | 34.6 (0.7) | .629 | 53.7 (4.0) | 0.470 | 60.1 (3.9) | .140 |
| Control | 497 | 521 | 35.2 (0.3) | 58.7 (2.2) | 49.8 (2.2) | ||||
| LBP | Case | 139 | 99 | 37.6 (0.7) | .009 | 56.7 (4.2) | 0.872 | 62.9 (4.1) | .078 |
| Control | 516 | 556 | 34.6 (0.3) | 57.9 (2.2) | 50.0 (2.2) | ||||
| Fibromyalgia | Case | 52 | 24 | 34.3 (1.1) | .673 | 77.2 (5.9) | 0.062 | 52.7 (7.0) | .943 |
| Control | 603 | 631 | 35.1 (0.3) | 56.9 (2.0) | 51.9 (2.0) | ||||
| No. of COPCs | 0 | 252 | 307 | 35.6 (0.5) | .149 | 54.0 (3.1) | 0.147 | 46.5 (3.1) | .277 |
| 1 | 178 | 209 | 34.4 (0.5) | 60.0 (3.7) | 55.5 (3.7) | ||||
| 2 | 109 | 83 | 33.6 (0.8) | 57.5 (4.8) | 58.7 (4.7) | ||||
| 3 | 71 | 33 | 36.5 (1.1) | 63.6 (5.8) | 68.4 (5.6) | ||||
| 4 | 33 | 15 | 38.7 (1.4) | 92.2 (4.7) | 39.7 (8.6) | ||||
| 5 | 12 | 6 | 30.9 (1.7) | 48.8 (15.1) | 52.8 (15.1) |
SE = standard error.
For descriptive purposes, Table 2 reports the mean values of health measures for the cases and controls of each COPC. Standardized ORs, signifying the univariate association of each health measure with each COPC, provide the basis for direct comparison of the strength of association. For example, an increase of one standard deviation (SD) in fatigue score was associated with approximately two-fold greater odds of TMD (OR = 1.94), while the association was less pronounced for headache (OR = 1.64) and IBS (OR = 1.58), but more pronounced for LBP (OR = 2.51) and fibromyalgia (OR = 2.50). The association with sleep disturbance was of a similar magnitude, ranging from 1.72 for headache to 2.14 for IBS and LBP. Associations were generally stronger for OSA symptoms, but weaker for atopic disorder, and they were nonsignificant for BMI.
Table 2.
Distribution of Measures of Sleep Disturbance, Atopic Disorders, and Body Mass Index for the Five COPCs, Stratified by Case and Control Status
| Case |
Control |
||||
|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | SOR (95% CL), P | |
| PROMIS fatigue score | |||||
| TMD | 160 | 1.90 (0.05) | 437 | 1.32 (0.03) | 1.94 (1.43, 2.62), < .001 |
| Headache | 249 | 1.67 (0.04) | 348 | 1.34 (0.03) | 1.64 (1.28, 2.09) < .001 |
| IBS | 144 | 1.69 (0.05) | 453 | 1.41 (0.03) | 1.58 (1.22, 2.06), < .001 |
| LBP | 127 | 1.95 (0.06) | 470 | 1.35 (0.03) | 2.51 (1.78, 3.55), < .001 |
| Fibromyalgia | 44 | 2.08 (0.10) | 553 | 1.43 (0.03) | 2.50 (1.52, 4.10), < .001 |
| PSQI | |||||
| TMD | 178 | 7.33 (0.30) | 453 | 4.99 (0.16) | 1.84 (1.44, 2.35), < .001 |
| Headache | 265 | 6.59 (0.23) | 366 | 4.97 (0.18) | 1.72 (1.35, 2.19), < .001 |
| IBS | 153 | 7.18 (0.31) | 478 | 5.16 (0.16) | 2.14 (1.68, 2.74), < .001 |
| LBP | 132 | 7.97 (0.36) | 499 | 5.04 (0.15) | 2.14 (1.65, 2.78), < .001 |
| Fibromyalgia | 51 | 8.18 (0.52) | 580 | 5.43 (0.15) | 1.82 (1.31, 2.54), < .001 |
| Obstructive sleep apnea symptoms | |||||
| TMD | 180 | 32.78 (3.51) | 465 | 18.92 (1.82) | 2.49 (1.20, 5.07), .014 |
| Headache | 269 | 28.62 (2.76) | 374 | 1.00 (0.07) | 1.42 (1.10, 1.84), .007 |
| IBS | 155 | 27.10 (3.58) | 490 | 21.43 (1.86) | |
| LBP | 137 | 45.26 (4.27) | 508 | 16.73 (1.66) | 4.47 (2.33, 8.60), < .001 |
| Fibromyalgia | 52 | 44.23 (6.95) | 593 | 20.91 (1.67) | 3.99 (1.84, 8.64), < .001 |
| Sum of atopic disorders | |||||
| TMD | 180 | 1.89 (0.12) | 463 | 1.06 (0.06) | 1.49 (1.08, 2.07), .016 |
| Headache | 269 | 1.70 (0.10) | 374 | 1.00 (0.07) | 1.42 (1.10, 184), .007 |
| IBS | 155 | 1.74 (0.13) | 488 | 1.15 (0.06) | 1.25 (0.97, 1.61), .079 |
| LBP | 137 | 1.59 (0.13) | 506 | 1.21 (0.06) | 1.23 (0.92, 1.65), .155 |
| Fibromyalgia | 52 | 1.83 (0.21) | 591 | 1.25 (0.06) | 1.08 (0.72, 1.64), .702 |
| Body mass index (kg/m2) | |||||
| TMD | 178 | 28.77 (0.52) | 467 | 28.71 (0.34) | 1.01 (0.76, 1.33), .969 |
| Headache | 265 | 28.75 (0.46) | 380 | 28.72 (0.36) | 1.24 (0.95, 1.61), .115 |
| IBS | 154 | 28.34 (0.56) | 491 | 28.85 (0.33) | 0.88 (0.63, 1.24), .473 |
| LBP | 138 | 30.69 (0.62) | 507 | 28.20 (0.31) | 1.26 (0.97, 1.63), .080 |
| Fibromyalgia | 50 | 30.17 (1.08) | 595 | 28.61 (0.29) | 1.10 (0.67, 1.82), .699 |
Note that frequencies, means, and standard errors (SE) are unweighted. Standardized odds ratios (SORs) with 95% confidence intervals (CIs) are weighted and adjusted for study site and the demographic characteristics age, sex (male, female), and race/ethnicity (white, non-Hispanic; black/African American; Hispanic; other). Estimates are interpreted as the increase in odds of having the COPC associated with an increase of one SD in the health measure.
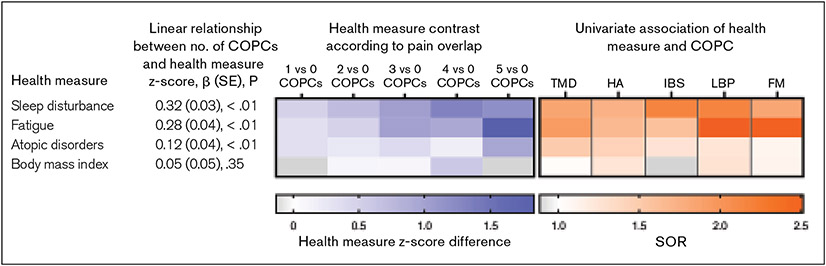
With the exception of BMI, mean values for each health measure increased monotonically as the number of COPCs increased (Table 3). In linear regression models that each used a z-transformed health measure as the dependent variable, there were significant linear associations with the number of COPCs (see Fig 1 and the underlying data for the figures tabulated in Appendices 1 and 2; see all appendices in the online version of this article at www.quintpub.com/journals). The relationship was most pronounced for sleep disturbance, which increased by 0.32 SDs for each additional COPC. In the multivariable analysis that modeled all five COPCs simultaneously as separate binary predictor variables, sleep disturbance was associated with each of the COPCs except for fibromyalgia (see tabulated regression coefficients in Fig 1b). Also striking were the relationships seen between fatigue and pain conditions. The relationship for atopy was less pronounced, although there was a statistically significant linear relationship (β = 0.12; Fig 1d).
Table 3.
Distribution of Health Measures According to Number of COPCs
| Health measure | No. of COPCs | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| PROMIS fatigue score | 1.20 (0.04), 228 | 1.42 (0.04), 167 | 1.63 (0.07), 98 | 1.87 (0.08), 65 | 2.24 (0.12), 29 | 2.43 (0.17), 10 |
| PSQI sleep disturbancea | 4.19 (0.19), 239 | 5.45 (0.25), 172 | 6.57 (0.36), 107 | 7.54 (0.50), 70 | 9.19 (0.67), 32 | 9.36 (0.75), 11 |
| OSA symptomsa | 16.33 (2.37), 245 | 17.51 (2.87), 177 | 26.61 (4.25), 109 | 31.43 (5.59), 70 | 53.13 (8.96), 32 | 66.67 (14.21), 12 |
| Sum of atopic disorders | 0.88 (0.08), 243 | 1.27 (0.10), 177 | 1.45 (0.15), 109 | 1.93 (0.21), 70 | 2.31 (0.31), 32 | 2.33 (0.36), 12 |
| Body mass index (kg/m2) | 29.05 (0.46), 250 | 27.79 (0.54), 174 | 28.00 (0.63), 107 | 29.39 (0.89), 70 | 31.63 (1.44), 33 | 30.36 (1.92), 11 |
Data are reported as mean (standard error), number of participants (unweighted).
PROMIS = Patient-Reported Outcomes Measurement Information System Fatigue Short Form; PSQI = Pittsburgh Sleep Quality Index; OSA = obstructive sleep apnea.
Values are percent (SE).
Fig 1.
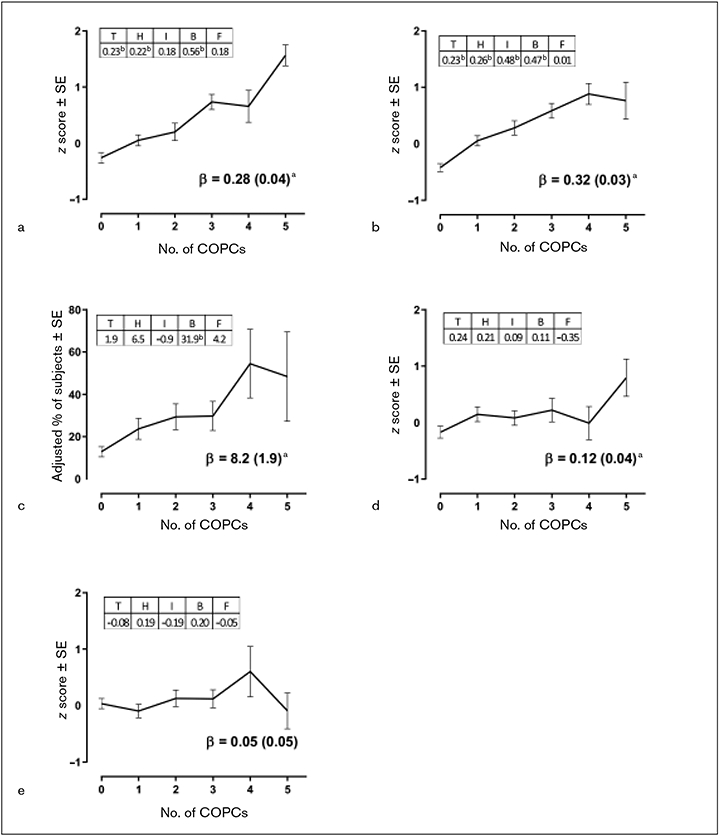
Relationships between number of COPCs and health measures in OPPERA-2 (n = 655 participants). (a) PROMIS Fatigue Score. (b) PSQI Sleep Disturbance. (c) Obstructive Sleep Apnea. (d) Number of atopic disorders. (e) Body mass index. Each health measure was the dependent variable in separate linear regression models that used weighted estimates from generalized estimating equations with robust error variance calculation and with adjustment for study site, age, gender, and race. Each plot summarizes the results from the three linear regression models: (1) Plotted values are adjusted means of the z-transformed health measure ± standard error (SE) from models in which the number of COPCs was the categorical predictor variable. (2) Beta (β) estimate (SE) represents amount of change in the dependent variable associated with a unit increase in number of COPCs, modeled as a continuous variable. aP < .05 for the null hypothesis that β = 0. (3) All five COPCs were modeled as binary predictor variables in a multivariable linear regression model that adjusted for covariates to show independent contributions of the COPCs to each clinical measure. The tabulated numbers, denoted as T = temporomandibular disorder, H = headache, I = irritable bowel syndrome, B = low back pain, and F = fibromyalgia, are parameter estimates for COPCs. bP < .05 for the null hypothesis that the parameter estimate for the dummy variable = 0.
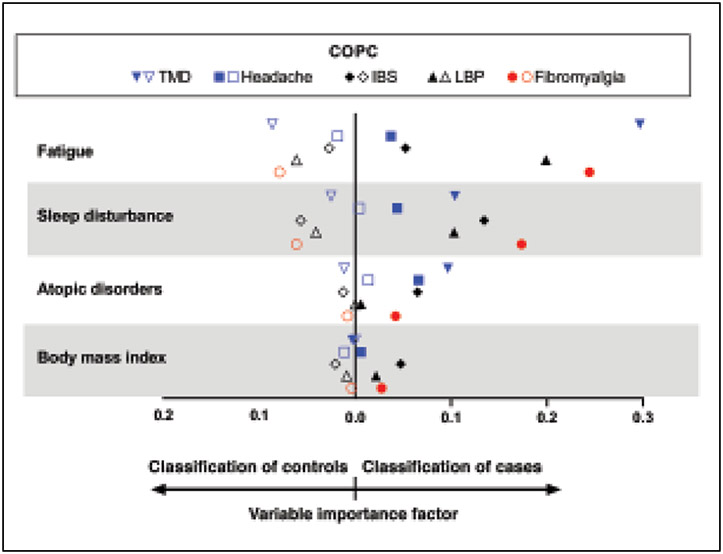
To understand which health measures best differentiated cases from controls, random forest algorithms simultaneously assessed the contributions of all health measures—including all possible interactions of health measures—to a single COPC. An important feature of random forests is the production of variable importance scores. These scores are used to identify the most important predictor variables from among all health status measures considered simultaneously. TMD cases and controls were best differentiated by fatigue, followed by sleep disturbance and atopic conditions. Headache cases and controls were best differentiated by atopic disorders (Fig 2). IBS cases and controls were best differentiated by sleep disturbance. Fatigue and sleep disturbance were of the greatest importance to fibromyalgia. BMI was unable to differentiate between cases and controls of any COPC. Of note, no single health measure had good discriminative ability for all five COPCs. The data underlying Fig 2 are reported in Appendix 3. A correlation matrix showing the strength of association between health measures is reported in Appendix 4. As reported in an accompanying paper in this issue, (see Slade et al, current issue) there were statistically signficant univariate associations between pairs of COPCs, with ORs ranging from 1.9 for headache and IBS to 19.7 for fibromyalgia and TMD.
Fig 2.
Multivariable contributions of health measures to COPCs in OPPERA-2 (n = 655 participants). Random forest modeling explored the multivariable contributions of all health measures to each binary COPC case classification, with study site, age, gender, and race also included as covariates. Contributions of individual variables in the random forest models were quantified using variable importance scores, which estimate the relative contribution of each predictor to the model’s classification of true positives and true negatives. Other health measures were included in the models, but are excluded from the figure due to negligible variable importance scores. The threshold for exclusion from the figure was set to 0.0004 to ensure a clear, concise plot. A variable importance score < 0.0004 means that in the presence of all the other measures included in the random forest model, these health measures improved the misclassification error rate by less than 0.04 percentage points. Filled symbols = COPC cases; open symbols = controls.
The preceding results are summarized visually using heat maps (Fig 3) in which the color intensity conveys the quantitative results that are reported numerically in Appendices 1 and 2 (blue heat map) and in Table 2 (orange heat map). In the blue heat map, the deepening color gradient seen from left to right across the rows as the number of COPCs increases above 0 is indicative of higher scores for the health measures. The figure shows a qualitative contrast in the way that fatigue and sleep are associated with COPCs: Fatigue was strongly associated with both fibromyalgia and LBP, and it was elevated conspicuously for people with all five COPCs. In contrast, sleep disturbance was associated with a greater number of COPCs, and there was a clear pattern of increase with each additional COPC up to four.
Fig 3.
The blue portion is a heat map depicting health measure z-score differences according to number of COPCs, based on data presented in Appendix 1. For example, the first cell in the top row depicts the mean sleep disturbance z-score difference between groups with 1 COPC vs 0 COPCs. Rows are ordered in descending strength of association, as determined by beta coefficients, reported in Appendix 2. The orange portion is a heat map depicting standardized odds ratios (SORs), reported in Table 2, that quantify the strength of the association between the health measure and each individual COPC. HA = headache. FM = fibromyalgia.
Discussion
This cross-sectional study of middle-aged adults found markedly elevated odds of TMD, headache, IBS, LBP, and fibromyalgia among adults with various sleep-related problems. Drawing on three types of analysis, sleep disturbance and fatigue were associated with each of these interrelated but distinct pain conditions, as well as with a higher propensity for multiple COPCs. Symptoms of OSA were also associated with all COPCs except for IBS. A novel finding was that atopy disorders, which have been previously shown to predict sleep disorders, were likewise associated with TMD and headache in univariate analyses, while in the random forest models, atopy disorders made a sizable contribution to TMD even after accounting for other health measures.
Sleep disturbance is an umbrella term broadly covering difficulties initiating or maintaining sleep, excessive daytime sleepiness, disorders of the sleep-wake schedule, and dysfunction with sleep stages.42 The global score of the PSQI provides a summary of these dimensions.29 It has been previously shown that poor sleep quality10 and symptoms of OSA43 were associated with chronic TMD and were predictive of first-onset TMD.9,10 The present study extends these findings, showing that sleep disturbance and OSA symptoms were also more likely to occur with four other COPCs and that these sleep measures were associated with a greater number of COPCs. While some degree in commonality of associations with COPCs is to be expected given that the COPCs themselves overlap (see Slade et al, current issue) sleep disturbance was associated with four COPCs in the model that assessed its association with all five COPCs simultaneously.
Fatigue differentiated between TMD cases and controls better than any other assessed heath measure. Indeed, fatigue was the single best discriminator for any pain condition. Although the definition of fatigue is debated,44 it generally refers to a “lessened capacity or motivation for work and reduced efficiency of accomplishment, usually accompanied by a feeling of weariness”45 that interferes with daily activities and is variable in response to rest. In this study, the intensity of fatigue was related in a linear fashion to the extent of pain overlap. Fatigue was associated with all COPCs—but most strongly with LBP and fibromyalgia—in the univariate analysis and had the highest variable importance scores for predicting TMD and fibromyalgia in the presence of all health measures in the random forest models.
A relatively novel finding was that a history of diagnosed atopic disorders was associated with greater occurrence of headache, even after accounting for sleep problems and other health measures. These findings build on Silverberg’s findings that, in children, an association of atopic dermatitis and headache was independent of the effect of sleep disturbance.17
Among large observational studies of adults, a UK study of the electronic medical records of primary care patients found elevated odds of asthma, atopic dermatitis, allergic rhinitis, and allergic conjunctivitis in patients with IBS compared to patients without functional gastrointestinal disorders.46 In the population-based German National Health Survey, investigators studied 41 physical disorders to identify those comorbid with LBP, and atopic conditions were prominent. Compared to adults without LBP, those with the pain disorder had greater odds of allergic asthma, urticaria, atopic dermatitis, and food allergy.47
Mast cell activation is a possible mediator for the relationship between atopic disorders and pain. Several small clinical studies provide evidence of mast cell abundance and activity in pain disorders, including migraine,48 interstitial cystitis and pelvic pain,49,50 fibromyalgia,51 and vulvodynia.52,53 Apart from their obvious role in regulating allergy responses, mast cells degranulate upon activation, releasing a wide range of molecular signals engaged in diverse physiologic activities, including inflammatory processes and the activation and sensitization of nociceptors. The close anatomical proximity of peripheral mast cells to nociceptive neurons54 facilitates molecular crosstalk at the neuroimmune interface, and these nociceptor-sensory neuroimmune interactions are critical regulators in pain processing.55,56 For reviews of the clinical and preclinical evidence of mast cell mediators in nociception and pain pathologies, see Aich et al,57 Chatterjea and Martinov,58 and Gupta and Harvima.59
LBP was the only COPC for which BMI approached a statistically significant association. Given that overweight and obesity are risk factors for so many chronic illnesses, the strikingly null associations between BMI and COPCs was somewhat unexpected. In a meta-analysis of 33 observational studies, overweight and obesity were associated with chronic back pain in the cross-sectional studies, and among the cohort studies, obesity predicted incident LBP.60 However, the magnitude of effects was fairly modest: odds of LBP were elevated 33% (95% confidence limits [CL]: 1.14, 1.54) among obese adults relative to those with weight within the normal range. Observational studies also report a positive association between BMI and fibromyalgia,61 although the effect may be partly attributable to physical inactivity.62 There is little evidence that BMI is involved in the other pain conditions. For example, a meta-analysis of 11 studies examining migraine and BMI found only weak evidence of increased risk of migraine in underweight subjects and obese women compared to normal-weight participants and no increased risk among pre-obese participants.63 Published evidence of a relationship between BMI and either TMD or IBS was not found. The null findings for BMI in the present study imply that weight management may have limited value for managing these pain conditions.
There were several limitations to this study beyond its cross-sectional design that preclude the determination of temporal sequence or causality. The novelty of assessing the relationship between a history of atopic disorders and overlapping pain is important work, but the crude assessment of atopic disorders is acknowledged. The severity of the atopic disorders was not assessed, and self-reports were not validated against skin tests or measured allergen-specific immunoglobulin E or G levels in blood.
Unlike TMD and fibromyalgia, which were classified according to examiner assessment, classification of the other pain conditions relied on self-report. Nonetheless, each pain condition was assessed the same way for all subjects using widely accepted standardized self-report measures. For example, the widely accepted Rome III diagnostic criteria for IBS have a sensitivity of 69% and a specificity of 80%,64 and in the absence of a gold standard diagnostic test are the only realistic methods of assessment in a population-based study.
The PSQI yields valid measures of subjective sleep disturbance. Its global score cut-off point of ≥ 5 yields good reliability and validity of sleep disturbance in different patient groups. For primary insomnia, it has a sensitivity of 98.7 and a specificity of 84.4.30 Correlations of the PSQI with polysomnogram-assessed sleep parameters, such as sleep efficiency, sleep onset latency, and total sleep time without Stage 1, are weaker, but the scale is a useful first-line questionnaire in population settings. Likewise, the STOP questions are intended to screen for OSA prior to surgery.
The implication of the small number of fibromyalgia cases is worth noting. For example, the finding reported in Fig 1 that fibromyalgia made no additional contribution to sleep disturbance independent of the other COPCs is likely a false negative due to lower statistical power; in contrast, the random forest models signify an independent contribution of sleep disturbance to fibromyalgia after considering all other health measures.
Conclusions
These cross-sectional associations emphasize the importance of sleep disorders in multiple COPCs and highlight for the first time the possible role of atopic disorders in overlapping pain. When considered together with results from the other papers in this volume, these findings emphasize the importance of health status when considering these COPCs within a biopsychosocial model.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Dental and Craniofacial Research (U01-DE017018). The authors acknowledge the contribution of Dr Bill Maixner in the acquisition of funding and for co-directing the study along with Dr Slade. The authors also thank Dr Eric Bair, who advised the statistical analysis of OPPERA datasets. Dr Fillingim has equity ownership in Algynomics Inc. The authors have nothing else to disclose.
Appendices
Appendix 1.
Tests for Absolute Differences in Health Measures Between Successive Numbers of COPCs
| Health measures z score |
Linear association |
EST (SE), P for phenotype z score contrast of: |
||||
|---|---|---|---|---|---|---|
| β (SE), P | 1 vs 0 | 2 vs 0 | 3 vs 0 | 4 vs 0 | 5 vs 0 | |
| PROMIS fatigue score | 0.28 (0.04), < .01 | 0.31 (0.11), < .01 | 0.46 (0.14), < .01 | 1.00 (0.13), < .01 | 0.92 (0.30), < .01 | 1.83 (0.19), < .01 |
| PSQI | 0.32 (0.03), < .01 | 0.47 (0.10), < .01 | 0.70 (0.12), < .01 | 1.01 (0.13), < .01 | 1.31 (0.19), < .01 | 1.19 (0.33), < .01 |
| OSA symptomsa | 8.2 ( 1.9), < .01 | 10.9 (5.5), .04 | 16.5 (6.4), < .01 | 17.0 (7.0), .01 | 41.6 (16.3), .01 | 35.5 (21.2), .09 |
| Sum of atopic disorders | 0.12 (0.04), < .01 | 0.32 (0.11), < .01 | 0.25 (0.11), .02 | 0.39 (0.20), .04 | 0.16 (0.27), .54 | 0.96 (0.33), < .01 |
| BMI (kg/m2) | 0.05 (0.05), .35 | −0.13 (0.13), .32 | 0.09 (0.15), .53 | 0.08 (0.17), 0.61 | 0.57 (0.46), .21 | −0.13 (0.32), .68 |
Est = estimated mean difference; SE = standard error; PROMIS = Patient-Reported Outcomes Measurement Information System Fatigue Short Form; PSQI = Pittsburgh Sleep Quality Index; OSA = obstructive sleep apnea; BMI = body mass index.
Health measure is binary (not z score); lower 95% confidence limit is for the ratio difference (%) of health measure between successive numbers of COPCs.
Appendix 2.
Independent Contributions of Each COPC to Mean Values of Health Measures
| Health measure | TMD | Headache | IBS | LBP | Fibromyalgia |
|---|---|---|---|---|---|
| PROMIS fatigue score | 0.23 (0.12), .05 | 0.22 (0.10), .02 | 0.18 (0.10), .05 | 0.56 (0.14), < .01 | 0.18 (0.20), .36 |
| PSQI | 0.24 (0.10), .02 | 0.26 (0.10), .01 | 0.48 (0.10), < .01 | 0.47 (0.13), < .01 | 0.01 (0.17), .97 |
| OSA symptomsa | 1.90 (5.88), .75 | 6.50 (4.71), .17 | −0.90 (4.28), .84 | 31.90 (8.03), < .01 | 4.20 (8.97), .64 |
| Sum of atopic disorders | 0.24 (0.18), .19 | 0.21 (0.12), .08 | 0.09 (0.10), .36 | 0.11 (0.15), .45 | −0.35 (0.21), .10 |
| BMI (kg/m2) | −0.08 (0.13), .54 | 0.19 (0.15), .20 | −0.19 (0.13), .14 | 0.20 (0.13), .13 | −0.05 (0.29), .86 |
Values are z-score transformations of health measure, presented as estimated mean difference (standard error), P.
PROMIS = Patient-Reported Outcomes Measurement Information System Fatigue Short Form; PSQI = Pittsburgh Sleep Quality Index; OSA = obstructive sleep apnea; BMI = body mass index.
Values are percent (standard error), P.
Appendix 3.
Summary Measures of Model Fit for Random Forest Models
| Metric | Model fit for prediction of COPCs | ||||
|---|---|---|---|---|---|
| TMD | Headache | IBS | LBP | Fibromyalgia | |
| Observed cases, % | 0.278 | 0.412 | 0.241 | 0.212 | 0.079 |
| Area under precision-recall curve | 0.599 | 0.624 | 0.404 | 0.512 | 0.329 |
| Area under receiver operator characteristic curve | 0.783 | 0.701 | 0.704 | 0.777 | 0.786 |
| Brier score | 0.173 | 0.215 | 0.193 | 0.163 | 0.134 |
| Mutual information index | 0.064 | 0.036 | 0.023 | 0.071 | 0.046 |
| Proportion of variance explained | 0.202 | 0.137 | 0.129 | 0.198 | 0.158 |
| Maximum variable importance factor: Predicting cases | 0.297 | 0.089 | 0.134 | 0.236 | 0.269 |
| Maximum variable importance factor: Predicting controls | 0.006 | 0.014 | 0.005 | 0.005 | 0.008 |
| Maximum variable importance factor: All | 0.007 | 0.016 | 0.003 | 0.015 | 0.003 |
Appendix 4.
Pearson Correlation Coefficients Showing Strength of Associations Among Health Measures
| PSQI | PROMIS fatigue score |
OSA symptoms | Sum of atopic disorders |
BMI (kg/m2) | |
|---|---|---|---|---|---|
| PSQI | r = 1.000 | r = 0.529 | r = 0.356 | r = 0.115 | r = 0.152 |
| P < .001 | P < .001 | P = .004 | P < .001 | ||
| n = 631 | n = 578 | n = 626 | n = 624 | n = 621 | |
| PROMIS fatigue score | r = 1.000 | r = 0.294 | r = 0.163 | r = 0.044 | |
| P < .001 | P < .001 | P = .290 | |||
| n = 597 | n = 591 | n = 589 | n = 587 | ||
| Obstructive sleep apnea symptoms | r = 1.000 | r = 0.117 | r = 0.339 | ||
| P = .003 | P < .001 | ||||
| n = 645 | n = 643 | n = 635 | |||
| Sum of atopic disorders | r = 1.000 | r = 0.024 | |||
| P = .543 | |||||
| n = 643 | n = 633 | ||||
| Body mass index (kg/m2) | r = 1.000 | ||||
| n = 645 |
PSQI = Pittsburgh Sleep Quality Index; PROMIS = Patient-Reported Outcomes Measurement Information System Fatigue Short Form; OSA = obstructive sleep apnea; BMI = body mass index.
Contributor Information
Anne E. Sanders, Division of Pediatric and Public Health, Adams School of Dentistry, University of North Carolina, Chapel Hill, North Carolina, USA.
Joel D. Greenspan, Department of Neural and Pain Sciences, Brotman Facial Pain Clinic, School of Dentistry, University of Maryland, Baltimore, Maryland, USA.
Roger B. Fillingim, Department of Community Dentistry & Behavioral Science, Pain Research and Intervention Center of Excellence, College of Dentistry, University of Florida, Gainesville, Florida, USA.
Nuvan Rathnayaka, Department of Biostatistics, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, USA.
Richard Ohrbach, Department of Oral Diagnostic Sciences, University at Buffalo School of Dental Medicine, Buffalo, New York, USA; Department of Orofacial Pain and Jaw Function, Faculty of Odontology, Malmö University, Malmö, Sweden.
Gary D. Slade, Division of Pediatric and Public Health, Adams School of Dentistry, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, USA.
References
- 1.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221–227. [DOI] [PubMed] [Google Scholar]
- 2.Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J 2011;20:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci 2000;45:952–959. [DOI] [PubMed] [Google Scholar]
- 4.Ødegård SS, Engstrøm M, Sand T, Stovner LJ, Zwart JA, Hagen K. Associations between sleep disturbance and primary headaches: The third Nord-Trøndelag Health Study. J Headache Pain 2010;11:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavigne G, Zucconi M, Castronovo C, Manzini C, Marchettini P, Smirne S. Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain 2000;84:283–290. [DOI] [PubMed] [Google Scholar]
- 6.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev 2006;10:357–369. [DOI] [PubMed] [Google Scholar]
- 7.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain 2013;14:1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rains JC, Davis RE, Smitherman TA. Tension-type headache and sleep. Curr Neurol Neurosci Rep 2015;15:520. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AE, Akinkugbe AA, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, et al. Subjective sleep quality deteriorates before development of painful temporomandibular disorder. J Pain 2016;17:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders AE, Slade GD, Bair E, et al. General health status and incidence of first-onset temporomandibular disorder: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T51–T62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis 1982;126:625–628. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol 1997;99:s757–s762. [DOI] [PubMed] [Google Scholar]
- 13.Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: A controlled clinical trial. J Allergy Clin Immunol 2004;113:663–668. [DOI] [PubMed] [Google Scholar]
- 14.Beltrani VS. Managing atopic dermatitis. Dermatol Nurs 1999;11:171–176,179–185. [PubMed] [Google Scholar]
- 15.Chang YS, Chou YT, Lee JH, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 2014;134:e397–e405. [DOI] [PubMed] [Google Scholar]
- 16.Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: A 2-way street? J Allergy Clin Immunol 2018;142:1033–1040. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg JI. Association between childhood eczema and headaches: An analysis of 19 US population-based studies. J Allergy Clin Immunol 2016;137:492.e5–499.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 19.Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativenessin the OPPERA study. J Pain 2011;12(11 suppl):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, et al. Study protocol, sample characteristics, and loss to follow-up: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003;61:375–382. [DOI] [PubMed] [Google Scholar]
- 24.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 25.Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 27.Christodoulou C, Schneider S, Junghaenel DU, Broderick JE, Stone AA. Measuring daily fatigue using a brief scale adapted from the Patient-Reported Outcomes Measurement Information System (PROMIS). Qual Life Res 2014;23:1245–1253. [DOI] [PubMed] [Google Scholar]
- 28.Ameringer S, Elswick RK Jr, Menzies V, et al. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res 2016;65:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 30.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002;53:737–740. [DOI] [PubMed] [Google Scholar]
- 31.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms 2006;4:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 33.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812–821. [DOI] [PubMed] [Google Scholar]
- 34.Richardson DB, Rzehak P, Klenk J, Weiland SK. Analyses of case-control data for additional outcomes. Epidemiology 2007;18:441–445. [DOI] [PubMed] [Google Scholar]
- 35.Monsees GM, Tamimi RM, Kraft P. Genome-wide association scans for secondary traits using case-control samples. Genet Epidemiol 2009;33:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bair E, Ohrbach R, Fillingim RB, et al. Multivariable modeling of phenotypic risk factors for first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T102–T115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang F, Ishwaran H. Random Forest Missing Data Algorithms. Stat Anal Data Min 2017;10:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Delgado M, Cernadas E, Barro S, Amorim D. Do we need hundreds of classifiers to solve real world classification problems? J Machine Learning Res 2014;15:3133–3181. [Google Scholar]
- 39.Lever J, Krzywinski M, Altman N. Points of significance: Classification evaluation. Nature Methods 2016;13:603–604. [Google Scholar]
- 40.Rufibach K. Use of Brier score to assess binary predictions. J Clin Epidemiol 2010;63:938–939. [DOI] [PubMed] [Google Scholar]
- 41.Wallach H. Evaluation metrics for hard classifiers [technical report]. Cambridge: Cavendish Laboratory, University of Cambridge, 2006. [Google Scholar]
- 42.Cormier RE. Sleep disturbances. In: Walker HK, Hall WD, Hurst JW (eds). Clinical Methods: The History, Physical, and Laboratory Examinations, ed 3. Boston: Butterworths, 1990. [PubMed] [Google Scholar]
- 43.Sanders AE, Essick GK, Fillingim R, et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res 2013;92(7 suppl):70S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013;80:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stedman TL. Stedman’s Medical Dictionary. Philadelphia: Lippincott Williams & Wilkins, 2000. [Google Scholar]
- 46.Jones MP, Walker MM, Ford AC, Talley NJ. The overlap of atopy and functional gastrointestinal disorders among 23,471 patients in primary care. Aliment Pharmacol Ther 2014;40:382–391. [DOI] [PubMed] [Google Scholar]
- 47.Schneider S, Mohnen SM, Schiltenwolf M, Rau C. Comorbidity of low back pain: Representative outcomes of a national health study in the Federal Republic of Germany. Eur J Pain 2007;11:387–397. [DOI] [PubMed] [Google Scholar]
- 48.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev 2005;49:65–76. [DOI] [PubMed] [Google Scholar]
- 49.Logadottir Y, Delbro D, Fall M, al. Cytokine expression in patients with bladder pain syndrome/interstitial cystitis ESSIC type 3C. J Urol 2014;192:1564–1568. [DOI] [PubMed] [Google Scholar]
- 50.Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol 2012;187:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanco I, Béritze N, Argüelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol 2010;29:1403–1412. [DOI] [PubMed] [Google Scholar]
- 52.Harlow BL, He W, Nguyen RH. Allergic reactions and risk of vulvodynia. Ann Epidemiol 2009;19:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: A prospective study. Am J Obstet Gynecol 2010;202:614.e1–e8. [DOI] [PubMed] [Google Scholar]
- 54.Forsythe P, Bienenstock J. The mast cell-nerve functional unit: A key component of physiologic and pathophysiologic responses. Chem Immunol Allergy 2012;98:196–221. [DOI] [PubMed] [Google Scholar]
- 55.Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 2017;38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010;16:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Mol Sci 2015;16:29069–29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chatterjea D, Martinov T. Mast cells: Versatile gatekeepers of pain. Mol Immunol 2015;63:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev 2018;282:168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between obesity and low back pain: A meta-analysis. Am J Epidemiol 2010;171:135–154. [DOI] [PubMed] [Google Scholar]
- 61.Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: Longitudinal data from the Norwegian Nord-Trøndelag Health Study. Arthritis Care Res (Hoboken) 2010;62:611–617. [DOI] [PubMed] [Google Scholar]
- 62.Yunus MB, Arslan S, Aldag JC. Relationship between body mass index and fibromyalgia features. Scand J Rheumatol 2002;31:27–31. [DOI] [PubMed] [Google Scholar]
- 63.Ornello R, Ripa P, Pistoia F, et al. Migraine and body mass index categories: A systematic review and meta-analysis of observational studies. J Headache Pain 2015;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lacy BE, Patel NK. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J Clin Med 2017;6(11). pii: E99. [DOI] [PMC free article] [PubMed] [Google Scholar]