Abstract
Osteosarcoma is a primary malignant tumor of the skeleton with the morbidity of 2.5 in 1 million. The regularly on-set is in the epiphysis of the extremities with a high possibility of early metastasis, rapid progression, and poor prognosis. The survival rate of patients with metastatic or recurrent osteosarcoma remains low, and novel diagnostic and therapeutic methods are urgently needed. Exosomes are extracellular vesicles 30–150 nm in diameter secreted by various cells that are widely present in various body fluids. Exosomes are abundant in biologically active components such as proteins, nucleic acids, and lipids. Exosomes participate in numerous physiological and pathological processes via intercellular substance exchange and signaling. This review presents the novel findings of exosomes in osteosarcoma in diagnosis, prognosis, and therapeutic aspects.
Keywords: Exosomes, Osteosarcoma, Biomarkers, Functions, therpeutic potential
Introduction
Osteosarcoma (OS) is a primary malignant bone tumor originating from primitive osteogenic mesenchymal in adolescents and young adults under 20[1]. Although the quality of life of patients affected by osteosarcoma has significantly improved over the last few decades, its etiology remains obscure. Studies aiming to determine the causes of osteosarcoma have classically focused on multiple factors, including genetics, epidemiology, and the environment[2]. Research has identified associations with secondary osteosarcoma in patients with Paget disease, electrical burns, trauma, exposure to beryllium, exposure to alkylating agents, FBJ virus, osteochondromatosis, enchondromatosis, fibrous dysplasia, orthopedic prosthetics as well as bone infarction and infection. Additionally, osteosarcoma reportedly correlates with exposure to ionizing radiation, radium, and archaic contrast agents such as thorotrast[3]. Besides, research has identified several genetic aberrations in cases of primary osteosarcoma, including Hereditary Retinoblastoma, Li-Fraumeni Syndrome, Rothmund-Thompson Syndrome, Bloom Syndrome, and Werner Syndrome[4]. Radiographs of osteosarcoma present osteogenic, osteolytic, or mixed bone destruction at the lesion. The “Codman’s triangle” and sun-exposed periosteal reaction[5] are typical radiographic features. MRI provides an accurate picture of osteosarcoma based on tumor cell differentiation and proliferation[6]. Radionuclide scans can determine whether bone metastases occur in osteosarcoma[7]. Frozen biopsies are used for rapid intraoperative diagnosis, and paraffin sections are used for obtaining accurate histological findings postoperatively[8]. High levels of serum alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) predict a poor prognosis[9]. Treatment for osteosarcoma includes neoadjuvant chemotherapy, surgical resection, chemotherapy, and interventional therapy[10]. In addition, cellular immunotherapy, gene therapy, and stem cell therapy have also made some progress in recent years[11]. However, these methods are still in the experimental stage. Approximately 18% of patients present micrometastases at the diagnosis, and the 5-year survival rate stays gloomy for patients with metastasis and recurrent[12]. Osteosarcoma treatment outcomes remain suboptimal due to the asymptom, early onset of metastasis, and high malignancy. The 5-year survival rate of patients with osteosarcoma is less than 30% without chemotherapy. The leading cause of death was lung metastasis[13]. The 2-year survival rate of patients with osteosarcoma with pulmonary metastases is less than 25%, and the survival period after treatment enters a plateau, making it challenging to obtain breakthrough efficacy with traditional treatment regimens[14]. Therefore, it is essential to reveal the underlying mechanisms of osteosarcoma development and metastasis and discover novel markers for clinical detection and effective therapeutic targets.
Currently, exosomes have been reported to be involved in regulating cellular behavior by transferring cargoes (proteins, DNA, RNA, and lipids) intercellularly. Increasing evidence shows that exosomes have significant potential in promoting osteosarcoma progression and development, the therapeutic potentials of exosomes in osteosarcoma is gaining attention. Exosomes are membranous vesicles 30–100 nm in diameter originating from endonuclease[15]. The first double-layered lipid structure containing no organelles was identified in blood erythrocytes and named exosomes[16]. Exosomes contain various nucleic acids and evolutionarily conserved proteins[17], which transmit biological information through cellular communication for biological processes and disease progression[18]. Exosomal LINC00273 transfer to lung adenocarcinoma (LUAD) in M2 macrophages, recruits NEDD4 to promote LATS2 ubiquitination, which inhibits the Hippo pathway and YAP-induced RBMX transcription, resulting in malignancy of LUAD[19]. Anlotinib-resistant NSCLC cells promote the proliferation of parental NSCLC cells by transferring functional miR-136–5p from anlotinib-resistant non-small-cell lung cancer (NSCLC) cells to parental NSCLC cells via exosomes. Exosomal miR-136–5p can lead to anlotinib resistance in NSCLC cells by targeting PPP2R2A and promoting activation of the AKT pathway[20]. Exosomes secreted by different cells in the osteosarcoma enable intercellular communication of ncRNAs and protein components, effectively regulating the tumor microenvironment to activate proliferation and metastasis. In addition, exosomes are stable in the circulatory systems with diagnostic and therapeutic potential. This article reviews the biological properties of exosomes and their role in the diagnosis and treatment of osteosarcoma.
1. Exosome Formation and Biological Characteristics
Extracellular vesicles (EVs) are universal in cells and carry proteins, genetics, and metabolites[21]. Based on the size and release mechanism, EVs are classified into exosomes (30–150 nm in diameter); microvesicles/extranuclear granulosomes (100–1000 nm in diameter); and apoptotic vesicles (50–1500 nm in diameter)[22]. Exosome formation involves dual invagination of the protoplasmic membrane and the formation of intracellular multivesicular bodies (MVBs), which contain intraluminal vesicles (ILVs)[23]. The endoplasmic reticulum also contributes to early endonucleosome formation[24]. The maturity of intranucleosomes eventually forming MVBs, which fuse with lysosomes or autophagosomes for degradation or fuse with the plasma membrane to release the contained ILVs as exosomes[25]. Exosomes are present in almost all body fluids, including plasma, urine, ascites, and breast milk[26].
1.1. Exosome Formation
Exosome formation is activated at the endosomal endocytosis, where the endosomal limiting membrane undergoes multiple deformation and outgrows inward to generate ILVs. The ILVs transform into MVBs with dynamic subcellular structures. MVBs are generated at the endosomal limiting membrane either by the endosomal sorting complex required for transport (ESCRT) or by a non-dependent ESCRT mechanism[27]. The ESCRT mechanism functions through the recognition of cytoplasmic protein complexes with ubiquitinylated modified membrane proteins. As the ubiquitin marker, ESCRT-0 is enriched in the endosomal membrane. The ESCRT-I complex recognizes and passes ESCRT-0 to ESCRT II. TSG101 in ESCRT I identifies disulfide bonds to induce endosomal membrane depression, which shears the bud neck via ESCRT III to form MVBs[28]. MVBs formation is initiated in the absence of ESCRT as the accessory protein ALG-2 interacting protein X (AIix), which binds directly to the intracellular bridging protein syntenin to participate in exosome formation[29]. The abundant tetratransmembrane protein can facilitate the production of these ESCRT-nondependent MVBs CD63-α on MVBs by ceramide-induced membrane outgrowth[30]. MVBs fusion with lysosomes will induce the degradation and recirculation of their contents. Cholesterol levels in MVBs play an essential role in regulating their sorting, with cholesterol-rich MVBs being targeted to the cell membrane for release as exosomes and low-cholesterol MVBs being targeted for transport to lysosomes[31].
1.2. Exosomes Mechanism in Biological Function
Exosome-mediated intercellular transmission relies on membrane receptors. Exosomes activate receptors on recipient cells to activate the take-up exosomes through cytokinesis[32]. The mechanism is related not only to the origin of exosomes and receptor cells but also to downstream responses. Current studies have focused on exploring the function of some cell-derived exosomes and the use of exosomes for disease treatment[33]. Target cell specificity may be determined by specific interactions between proteins enriched on the surface of exosomes and receptors on the membrane of recipient cells[34]. Known mediators include transmembrane tetraspanins, integrins, and extracellular matrix components[35].
1.3. Exosomes Potential in Tumor Diagnosis and Treatment
Exosomes primarily exclude redundant and nonfunctional cellular components[36]. Exosomes are intercellular linkers that transport proteins, lipids, and nucleic acids to target cells in various biological processes, such as angiogenesis, antigen presentation, apoptosis, and inflammation[37]. The specific component captured by the exosome reflects the cellular origin and physiological state, with significant disease specificity, making them ideal biomarkers. Exosomes are involved in various cancer-related processes, including proliferation, apoptosis, angiogenesis, and metastasis, suggesting noninvasive biomarkers for cancer diagnosis[38, 39]. The miR-21, miR-222, and miR-124–3p in serum exosomes are detectable early tumor progression during postsurgical treatment of patients with high-grade gliomas (HGG)[40]. The miR-21, miR-451, and miR-636 in urinary exosomes of prostate cancer patients were closely correlated with preoperative prostate-specific antigen (PSA) levels, the urinary exosomal miRNAs potentially function as noninvasive markers to predict prostate cancer metastasis and prognosis [41]. Plasma exosomal miR-363–5p had a high diagnostic performance in discriminating against LN (+) and LN (−) breast cancer patients. Increasing miR-363–5p expression levels were intensely indicating a lower overall survival.[42]. The therapeutic potentials of exosomes are concentrated on targeted drug delivery and biomedical regeneration. Exosomes have great potential in treating diseases due to their nontumorigenic, bactericidal, and lower immunogenicity characteristics[43]. Ligand enrichment on engineered exosomes can induce or inhibit signaling in receptor cells or target exosomes to specific cells[44]. The chemotherapeutic agents loaded exosomes are promising for antineoplastic drugs with low toxicity and high tolerance[45].
2. Exosomes in Osteosarcoma Progression
Exosomes can transmit intercellular signals to regulate proliferation and metastasis. Exosomes promote tumor proliferation and metastasis by inducing epithelial-mesenchymal transition (EMT) of related cells and accelerating tumor neovascularization and immunosuppression through regulating the microenvironment and transformation of cancer-associated fibroblasts[46, 47]. Exosomes are dominant in regulating proliferation, invasion metastasis, and osteosarcoma angiogenesis by participating in intercellular contacts and controlling cellular signaling.
2.1. Exosomes in Osteosarcoma Proliferation
The potential to proliferate indefinitely is the fundamental feature of cancer cells[48]. Osteosarcoma cells express growth factor receptors and achieve rarely negative feedback regulation, manifesting as continuous activation of signal stimulation and unlimited division and proliferation[49]. Exosomes participate in various processes in the proliferation of osteosarcoma (Table 1). The miR-208a from BMSC-derived exosomes promoted osteosarcoma cell proliferation and inhibited apoptosis by suppressing PDCD4 expression and activating the ERK1/2 and Hippo pathways. BMSC-derived exosomal miR-206 could inhibit cell proliferation by targeting TRA2B[50]. In addition, BMSC-derived exosomes could encapsulate PVT1 and translocate it into osteosarcoma cells. PVT1 could promote tumor growth and metastasis by binding to miR-183–5p to promote ERG expression[51]. The MALAT1/miR-143/NRSN2/Wnt/β-catenin axis is another vital target for BMSE-EVs to promote proliferation[52]. ADSC exosomes could deliver COLGALT2 to osteosarcoma cells, leading to the malignancy of osteosarcoma[53]. BMSC-derived exosomes promote OS proliferation and metastasis via the LCP1/JAK2/STAT3 pathway. Meanwhile, targeting the miR-135a-5p/LCP1 axis could inhibit osteosarcoma progression[54]. MG-63 cell-derived exosomes promoted the proliferation of osteosarcoma and inhibited apoptosis. The Hic-5 from MG-63 cell-derived exosomes interacts with smad4 and regulates Wnt/β-catenin signaling by decreasing TCF/LEF activity[55]. Osteosarcoma cell-derived exosomal miR-1307 could promote OS cell proliferation by inhibiting AGAP1 expression, indicating that the miR-1307-AGAP1 axis could be a potential therapeutic target for OS[56]. In osteosarcoma patients, exosomal miR-15a expression decreased in plasma exosomes. The exosomal miR-15a inhibited the GATA2/MDM2 axis via the p53 signaling pathway, thereby inhibiting the proliferation and invasion of OS cells in vitro[57].
Table 1.
The biological function of exosome in the proliferation and metastasis of osteosarcoma
| Exosomal target | Parent cell | Target cell | Mechanism | Biological function | Ref. |
|---|---|---|---|---|---|
|
| |||||
| Proliferation and Metastasis | |||||
|
| |||||
| miR-208 | BMSCs | Osteosarcoma cells | PDCD4/ERK1/2 | Increase the viability, migration, and clonogenicity of OS | [95] |
| miR-206 | BMSCs | Osteosarcoma cells | TRA2B | Promote OS cell proliferation, migration, invasion and induce cell apoptosis | [96] |
| MALAT1 | BMSCs | Osteosarcoma cells | MALAT1/miR-143/NRSN2/Wnt/β-catenin | Promote OS cell proliferation, migration, and invasion | [52] |
| PVT1 | BMSCs | Osteosarcoma cells | PVT1/miR-183–5p/ERG | Promote OS growth and metastasis | [51] |
| ATG5 | BMSCs | Osteosarcoma cells | / | Promote OS cell proliferation, migration, and invasion | [97] |
| COLGALT2 | ADSCs | Osteosarcoma cells | / | Promote OS cell proliferation, migration, and invasion | [98] |
| Linc00852 | high AXL expression Osteosarcoma cells | low AXL expression Osteosarcoma cells | Linc00852/miR-7–5p/AXL | Promote cell proliferation, migration and invasion | [99] |
| LCP1 | BMSCs | Osteosarcoma cells | miR-135a-5p/LCP1/JAK2/STAT3 | Induce the proliferation andmetastasis of OS cells | [54] |
| Hic-5 | MG-63 | MG-63 and HOS cells | Hic-5/smad4-TCF/LEF -Wnt/β-catenin | Promote cell proliferation and inhibit cell apoptosis | [55] |
| miR-1307 | Osteosarcoma cells | Osteosarcoma cells | AGAP1 | Promote OS cell proliferation, migration, and invasion | [56] |
| miR-15a | Serum-derived exosome | Osteosarcoma cells | miR-15a/p5/GATA2/MDM2 | Promote OS cell proliferation and invasion | [100] |
| miR-769–5p | BMSCs | Clinical specimens | DUSP16/JNK/p38 MAPK | promotes OS proliferation and metastasis | [101] |
| SHNG17 | CAFs\NFs | HOS cells | miR-2861 | promotes OS proliferation and metastasis | [102] |
| miR-143 | / | Osteosarcoma cells | / | Inhibit cell invasion | [59] |
| miR-675 | Osteosarcoma cells | hFOB1.19 | CALN1 | Promote cell migration and invasion | [25] |
| Rab22a-NeoF1 /PYK2 | PYK2-positive osteosarcoma cells | macrophages | RhoA | Facilitate the pre-metastatic niche formation | [62] |
| miR-1307 | Osteosarcoma cells | Osteosarcoma cells | AGAP1 | Promote cell proliferation, migration and invasion | [62] |
| LCP1 | BMSCs | Osteosarcoma cells | miR-135a-5p/Nrdp1/JAK2/STAT3 | Promotes OS proliferation and metastasis | [54] |
| LIFR-AS1 | Macrophages | Osteosarcoma cells | miR-29a/NFIA | Promote cell proliferation, invasion, and restrain cell apoptosis | [103] |
|
| |||||
| Angiogenesis | |||||
|
| |||||
| miR-25–3p | / | Osteosarcoma cells | DKK3 | promoted capillary formation and the invasion of vascular endothelial cells | [42] |
| EWSAT1 | / | Osteosarcoma cells | / | increase in sensitivity/ reactivity of vascular endothelial cells | [104] |
| OIP5-AS1 | Osteosarcoma cells | Osteosarcoma cells | miR-153/ATG5 | Increase in the angiogenesis level | [66] |
| miR-199a-5p | Osteosarcoma cells | HUVECs | VEGFA | Inhibiting the growth and angiogenesis of osteosarcoma | [105] |
| miR-148a-3p and miR-21–5p | Osteosarcoma cells | Raw264.7 and Huvec cells | / | Influence osteoclastogenesis, bone resorption and tumor angiogenesis | [69] |
|
| |||||
| Immunosuppressive | |||||
|
| |||||
| miR-148a-3p and miR-21–5p | Osteosarcoma cells | Raw264.7 and Huvec cells | / | Influence osteoclastogenesis, bone resorption and tumor angiogenesis | [69] |
| Tim-3 | MG63 | Macrophages | / | Induce M2 type differentiation of macrophages | [106] |
2.2. Exosomes in Osteosarcoma Metastasis
Epithelial-mesenchymal transition (EMT) is a biological phenomenon in which epithelial cells lose their epithelial properties and acquire a mesenchymal phenotype. In this process, epithelial features reduce, changing from polygonal to spindle-shaped fibroblast-like morphology, with loss of cell polarity and reduced adhesion, acquiring the ability to invade and metastasize[58]. Exosomes are essential in the invasive metastasis of osteosarcoma (Table 1). The miR-143 could transfer to osteosarcoma cells via exosomes and significantly inhibit tumor invasiveness[59]. Highly invasive OS cells could secret exosomal miR-675 into recipient cells and suppress CALN1 expression. The expression level of exosomal miR-675 in the serum of patients with osteosarcoma was strongly correlated with prognosis[60]. Mazumdar et al. found that both highly metastatic 143-B cells and low metastatic SAOS-2 cell-derived EVs could induce the recruitment of bone marrow cells to the lung, the components in exosomes may inhibit remote metastasis of osteosarcoma[61]. In osteosarcoma, the Rab22a-NeoF1 fusion protein could be assimilated into exosomes. The exosomal Rab22a-NeoF1 fusion protein promotes the formation of premetastatic lung niche by recruiting bone marrow-derived macrophages[62]. OS cell-derived exosomal miR-1307 promotes proliferation, migration, and invasion by regulating AGAP1 expression, indicating the inhibitive features of miR-1307 in the malignant progression of osteosarcoma[56].
2.3. Exosomes in Osteosarcoma Angiogenesis
Proangiogenic and antiangiogenic factors are dominant in the formation of blood vessels[63]. Tumor cells require nutrient supply and metabolite excretion for survival and development[64]. Tumor-derived exosomes are critical mechanisms that promote angiogenesis (Table 1). The miR-25–3p increased in osteosarcoma tissues to promote tumor proliferation, metastasis, and drug resistance by inhibiting DKK3. EWSAT1 promoted OS angiogenesis by wrapping it into the exosome-driven vascular endothelial cell to increase the secretion and the sensitivity/responsiveness of angiogenic factors[65]. Osteosarcoma cells with high exosome abundance could regulate OS tumor angiogenesis and autophagy through miR-153 and ATG5 by secreting exosomal lnc-OIP5-AS1 into adjacent osteosarcoma cells[66].
2.4. Exosomes in Osteosarcoma Immunol Response
Exosomes participate in the immune response and regulate immunocompetence[67]. Tumor cell-derived exosomes carry tumor-associated antigens and stimulate the immune cells to generate antitumor immune responses. However, they can interfere with immune recognition, and inhibit T cells and immune-related cells, thereby accelerating tumor cells’ immune escape and metastasis [17]. Immune cells derived from the tumor microenvironment regulate proliferation and metastasis through exosomes[68]. Exosomes also have a critical role in the tumor immune microenvironment of osteosarcoma (Table 1). The exosomal miR-1228 secreted by cancer-associated fibroblasts (CAFs) could promote osteosarcoma invasion and migration by targeting SCAI. The mir-1228 functions as a potential therapeutic target for osteosarcoma[42]. Exosomes enhanced tube formation in endothelial cells and increased the expression of angiogenic markers. The second-generation sequencing reveals that specific miRNAs, such as miR-148a and miR-21–5p, have essential roles in the tumor microenvironment[69]. The exosomes of metastatic osteosarcoma cells secrete TGFβ2 into tumor-associated macrophages, promoting the M2 phenotype and contributing to immunosuppression and tumorigenesis[70]. Osteosarcoma cell-derived EVs promote myofibroblast/cancer-associated fibroblast differentiation, smooth muscle actin expression, and fibronectin production. In addition, they significantly promoted the invasiveness of human lung fibroblasts[71]. Osteosarcoma-derived exosomes induced M2 polarization of macrophages via Tim-3, promoting osteosarcoma invasion and metastasis[72]. Exosomal Col6a1 converts normal fibroblasts into CAFs by secreting proinflammatory cytokines. Activated CAFs promote OS cell invasion and migration by mediating the TGF-β/COL6A1 signaling pathway[73]. Macrophage-derived exosomal lncRNA LIFR-AS1 could promote the malignant progression of osteosarcoma by binding miR-29a to promote NFIA expression[74].
3. Exosomes Potentials in Osteosarcoma
Exosomes contain various biologically active molecules in circulation and mediate remote intercellular interaction[75]. Tumor-derived exosomes contain multiple proteins, genetics, lipids, and other molecules that reflect the physiological and pathological status of the tumor[76]. The specific lipid bilayer structure of exosomes protects their RNA molecules from degradation[77]. Therefore, detecting tumor exosomes has become a significant advantage of liquid biopsy. Exosomes show good application potential in the early diagnosis, efficacy, and prognosis monitoring of various diseases. They have become new and ideal biomarkers and possible targeted drug carriers in clinical diagnosis and treatment.
3.1. Exosomes Potientials for Osteosarcoma Diagnosis
Exosomes are essential in the early diagnosis and prognostic assessment of osteosarcoma. Eight novel miRNAs were identified by NGS in three distinct osteosarcoma cell lines, and five are present in circulating exosomes of osteosarcoma patients[57]. EV-miR-101 expression levels were significantly lower in osteosarcoma patients. In plasma from patients with osteosarcoma metastases, EV-miR-101 was even lower than those without metastases, indicating a potential diagnostic marker for osteosarcoma[78]. Ye et al. revealed that the expression levels of miR-92a-3p, miR-130a-3p, miR-195–3p, miR-335–5p, and let-7i-3p were significantly upregulated in exosomes of osteosarcoma patients, which may be potential diagnostic markers for osteosarcoma[79]. The HSATI, HSATII, LINE1-P1, and Charlie 3 were overexpressed at the DNA level but not at the RNA level in OS patients’ serum exosomes with potential use as biomarkers for OS[80]. Exosome-derived SENP1 in patients with osteosarcoma was closely correlated with tumor size, location, necrosis rate, lung metastasis, and surgical staging. The higher plasma exosome-derived SENP1 levels indicate poorer disease-free survival (DFS) and overall survival [81]. Seven exosomal proteins are identified as potential biomarkers of osteosarcoma lung metastasis[82]. In addition, SERS and MALDI-TOF MS exosomes have shown great potential for the rapid diagnosis of osteosarcoma[83].
3.2. Exosomes Potentials for Osteosarcoma Treatment
Exosomes have great potential in the treatment of osteosarcoma. Multidrug-resistant osteosarcoma cells secrete exosomes containing MDR-1 mRNA and P-glycoprotein to promote doxorubicin resistance in sensitive cells. Exosomes targeting drug-resistant osteosarcoma cells may inhibit the malignant progression of osteosarcoma[84]. Compared to normal osteoblasts, osteosarcoma-derived exosomes contain immunomodulatory substances that significantly reduce T cell proliferation rates and promote T regulatory phenotypes, thereby promoting osteosarcoma progression[10, 85]. The miR-135b, miR-148a, miR-27a, and miR-9 were highly expressed in serum exosomes of osteosarcoma patients and could potentially be reliable biomarkers of chemotherapy sensitivity[16, 86]. Exosome-loaded doxorubicin (Exo-Dox) enhanced cellular uptake efficiency and antitumor effects in the osteosarcoma MG63 cell line with low cytotoxicity, which may be a good targeting regimen for osteosarcoma[87]. Osteosarcoma cells could promote osteosarcoma lung metastasis by releasing exosomes containing PD-L1 and N-calcineurin. In addition, the expression levels of exosomal PD-L1 and N-calcineurin in the serum of OS patients could predict the progression of pulmonary metastasis in OS patients[88]. Exosomes from CDDP-resistant osteosarcoma cells decreased the expression of multidrug resistance-associated protein 1 and P-glycoprotein in MG63 and U2OS cells, increased cellular sensitivity to CDDP and inhibited apoptosis through exosomal-hsa_circ_103801[89]. Moreover, exosomes from drug-resistant HMPOS-2.5R cell lines transferred drug resistance to drug-sensitive HMPOS cells, thereby reducing the therapeutic sensitivity of osteosarcoma[90].
Conclusions
The dominant to promote the prognosis and survival of tumor patients lies in early diagnosis[91]. Exosomes are stable and widespread in all tissues, organs, and body fluids, and these nanosized vesicles can be released by all types of cells (Figure 1) [92]. Tumor exosomes can also regulate tumor progression, angiogenesis, metastasis, and immune escape by interacting with other cells in the tumor microenvironment[93]. We need a standard method for liquid biopsy to isolate exosomes quickly, easily, and specifically. Exosomes are a promising biomarker for the diagnosis of osteosarcoma, predicting prognosis, and monitoring treatment response in real-time, large multicenter studies are needed to develop the validity of liquid biopsies. For biological functions study, it is impossible to determine whether exosomes have similar regulatory functions in vivo as they do in vitro. For therapeutic purposes, exosome-derived cells should be carefully selected to ensure the safety of the treatment. Erythrocytes are the most promising exosome-producing cells because they are readily available in blood banks, do not contain a nucleus, and lack genetic material. In addition to their great potential as biomarkers, exosomes offer new research directions for the precision treatment of tumors[87]. To improve the effectiveness of antitumor drug therapy, a drug-loading system is a key challenge. As a natural therapeutic carrier, exosomes contain their bioactive molecules and avoid immune rejection[94], in addition to loading exogenous drugs to maintain drug stability in vivo. These advantages make exosomes an ideal loading system to break the traditional drug delivery model and will be an important tool for the development of precision medicine for tumors. Han et al. constructed fusion gene iRGD-Lamp2b-modified MSCs to isolate and purify exosomes and loaded anti-miRNA-221 oligonucleotides into exosomes. AMO-loaded exosomes effectively inhibited the proliferation and clonal formation of colon cancer cells in vitro[51].
Figure 1. The interaction of osteosarcoma and related cells through exosomes.
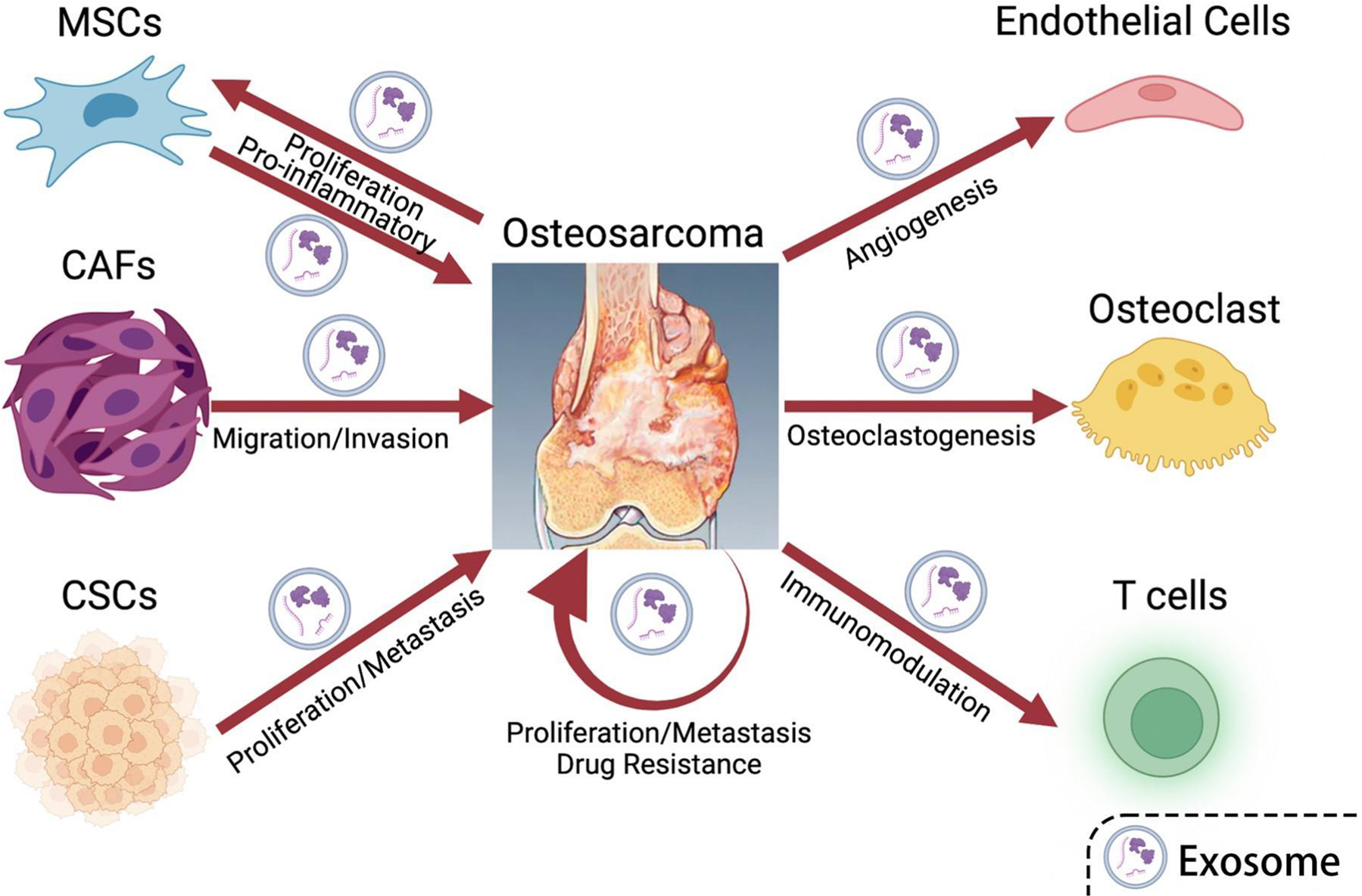
The MSCs, CAFs, and CSCs secrete exosomes containing specific proteins and genetic materials to promote the proliferation, metastasis, and invasion of osteosarcoma. Meanwhile, osteosarcoma cells generate exosomes targeting specific cells to promote angiogenesis, osteoclastogenesis, and immunomodulation of the supporting cells. Osteosarcoma promotes drug resistance, proliferation, and metastasis through exosome secretion. (Created in Biorender.com)
This review discusses the biological functions of exosomes in the progression of osteosarcoma and clinical applications. Exosomes from osteosarcoma promote malignant progression by regulating tumor metastasis, angiogenesis, tumor immunity, and drug resistance. Exosomes provide us with a new potential therapeutic target.
Acknowledgments
This work was supported by the Liaoning Cancer Hospital & Institute (Shenyang), St. John’s University and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
Funding
This work was supported by NCI grants R01 CA230916 and funds from the Sylvester Comprehensive Cancer Center/University of Miami. The authors Chen is supported by St. John’s University Department of Pharmaceutical Sciences.
ABBREVIATIONS
- OS
Osteosarcoma
- ALP
alkaline phosphatase
- LDH
lactate dehydrogenase
- miRNAs
microRNAs
- lncRNA
long noncoding RNA
- mRNA
messenger RNA
- LUAD
lung adenocarcinoma
- NSCLC
non-small-cell lung cancer
- EVs
extracellular vesicles
- MVBs
multivesicular bodies
- ILVs
luminal vesicles
- ESCRT
endosomal sorting complex required for transport
- Aiix
ALG-2 interacting protein X
- HGG
high-grade gliomas
- PSA
prostate-specific antigen
- MSCs
mesenchymal stem cell
- EMT
epithelial-mesenchymal transition
- BMSCs
bone marrow-derived mesenchymal stem cells
- CAFs
cancer-associated fibroblasts
- NGS
next-generation sequencing
- DFS
disease-free survival
- CDDP
cisplatin-resistant
Footnotes
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
None.
Consent for publication
None.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Fujiwara T, et al. , Clinical significance of circulating miR-25–3p as a novel diagnostic and prognostic biomarker in osteosarcoma. Oncotarget, 2017. 8(20): p. 33375–33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng C, et al. , PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim Biophys Acta Rev Cancer, 2020. 1874(2): p. 188405. [DOI] [PubMed] [Google Scholar]
- 3.Jerez S, et al. , Proteomic Analysis of Exosomes and Exosome-Free Conditioned Media From Human Osteosarcoma Cell Lines Reveals Secretion of Proteins Related to Tumor Progression. J Cell Biochem, 2017. 118(2): p. 351–360. [DOI] [PubMed] [Google Scholar]
- 4.Hameed M and Mandelker D, Tumor Syndromes Predisposing to Osteosarcoma. Adv Anat Pathol, 2018. 25(4): p. 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Permata TBM, et al. , High linear energy transfer carbon-ion irradiation upregulates PD-L1 expression more significantly than X-rays in human osteosarcoma U2OS cells. J Radiat Res, 2021. 62(5): p. 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, et al. , Pulmonary artery osteosarcoma masquerading as pulmonary thromboembolism: the role of multimodality imaging. ESC Heart Fail, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi J, et al. , Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell Physiol Biochem, 2017. 42(6): p. 2242–2254. [DOI] [PubMed] [Google Scholar]
- 8.Laskar S, et al. , Outcomes of osteosarcoma, chondrosarcoma and chordoma treated with image guided-intensity modulated radiation therapy. Radiother Oncol, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Mahyudin F, et al. , The Escalation of Osteosarcoma Stem Cells Apoptosis After the Co-Cultivation of Peripheral Blood Mononuclear Cells Sensitized with Mesenchymal Stem Cells Secretome and Colony Stimulating Factor-2 in vitro. J Blood Med, 2021. 12: p. 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyer RM, et al. , Exosomes from Osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res, 2017. 358(2): p. 369–376. [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, et al. , The role of tumor-associated macrophages in osteosarcoma progression - therapeutic implications. Cell Oncol (Dordr), 2021. 44(3): p. 525–539. [DOI] [PubMed] [Google Scholar]
- 12.Prudowsky ZD and Yustein JT, Recent Insights into Therapy Resistance in Osteosarcoma. Cancers (Basel), 2020. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie JD, et al. , Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol Ther Oncolytics, 2021. 22: p. 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, et al. , Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett, 2021. 500: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 15.Psaraki A, et al. , Extracellular vesicles derived from Mesenchymal Stem/Stromal Cells: the regenerative impact in liver diseases. Hepatology, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Xu JF, et al. , Exosomes containing differential expression of microRNA and mRNA in osteosarcoma that can predict response to chemotherapy. Oncotarget, 2017. 8(44): p. 75968–75978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, et al. , Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B, 2021. 11(8): p. 2136–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaac R, et al. , Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab, 2021. 33(9): p. 1744–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayer A, et al. , Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta, 2018. 61: p. 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu G, et al. , Exosomal miR-136–5p Derived from Anlotinib-Resistant NSCLC Cells Confers Anlotinib Resistance in Non-Small Cell Lung Cancer Through Targeting PPP2R2A. Int J Nanomedicine, 2021. 16: p. 6329–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hur JY and Lee KY, Characteristics and Clinical Application of Extracellular Vesicle-Derived DNA. Cancers (Basel), 2021. 13(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady JV, et al. , A Preliminary Proteomic Investigation of Circulating Exosomes and Discovery of Biomarkers Associated with the Progression of Osteosarcoma in a Clinical Model of Spontaneous Disease. Transl Oncol, 2018. 11(5): p. 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, et al. , Exosomes: Advances, development and potential therapeutic strategies in diabetic nephropathy. Metabolism, 2021. 122: p. 154834. [DOI] [PubMed] [Google Scholar]
- 24.Ruan S, et al. , Extracellular Vesicles as an Advanced Delivery Biomaterial for Precision Cancer Immunotherapy. Adv Healthc Mater, 2021: p. e2100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong L, et al. , Exosomal miR-675 from metastatic osteosarcoma promotes cell migration and invasion by targeting CALN1. Biochem Biophys Res Commun, 2018. 500(2): p. 170–176. [DOI] [PubMed] [Google Scholar]
- 26.Tang XH, et al. , Exosome-derived noncoding RNAs in gastric cancer: functions and clinical applications. Mol Cancer, 2021. 20(1): p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chivero ET, et al. , Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders. Cell Mol Life Sci, 2021. 78(11): p. 4849–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagerweij T, Perez-Lanzon M, and Baglio SR, A Preclinical Mouse Model of Osteosarcoma to Define the Extracellular Vesicle-mediated Communication Between Tumor and Mesenchymal Stem Cells. J Vis Exp, 2018(135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, et al. , Targeted delivery of extracellular vesicles in heart injury. Theranostics, 2021. 11(5): p. 2263–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shehzad A, et al. , Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol Ther, 2021. 223: p. 107806. [DOI] [PubMed] [Google Scholar]
- 31.Schubert A and Boutros M, Extracellular vesicles and oncogenic signaling. Mol Oncol, 2021. 15(1): p. 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadi M, et al. , Emerging technologies and commercial products in exosome-based cancer diagnosis and prognosis. Biosens Bioelectron, 2021. 183: p. 113176. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A and Deep G, Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Lett, 2020. 479: p. 23–30. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, et al. , Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target. Cancers (Basel), 2019. 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, et al. , Exosome-based Tumor Therapy: Opportunities and Challenges . Curr Drug Metab, 2020. 21(5): p. 339–351. [DOI] [PubMed] [Google Scholar]
- 36.Shao J, Zaro J, and Shen Y, Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int J Nanomedicine, 2020. 15: p. 9355–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, et al. , Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer, 2020. 19(1): p. 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng W, et al. , Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer, 2019. 18(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Namee NM and O’Driscoll L, Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer, 2018. 1870(2): p. 123–136. [DOI] [PubMed] [Google Scholar]
- 40.Olioso D, et al. , Serum Exosomal microRNA-21, 222 and 124–3p as Noninvasive Predictive Biomarkers in Newly Diagnosed High-Grade Gliomas: A Prospective Study. Cancers (Basel), 2021. 13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin S, et al. , Urinary exosome microRNA signatures as a noninvasive prognostic biomarker for prostate cancer. NPJ Genom Med, 2021. 6(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida A, et al. , Clinical and Functional Significance of Intracellular and Extracellular microRNA-25–3p in Osteosarcoma. Acta Med Okayama, 2018. 72(2): p. 165–174. [DOI] [PubMed] [Google Scholar]
- 43.Yang E, et al. , Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther, 2020. 5(1): p. 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos P and Almeida F, Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front Immunol, 2021. 12: p. 711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez MJ, et al. , Evaluation of exosome derivatives as bio-informational reprogramming therapy for cancer. J Transl Med, 2021. 19(1): p. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashouri L, et al. , Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer, 2019. 18(1): p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, et al. , Molecular assessment of circulating exosomes toward liquid biopsy diagnosis of Ewing sarcoma family of tumors. Transl Res, 2018. 201: p. 136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D and Weinberg RA, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646–74. [DOI] [PubMed] [Google Scholar]
- 49.Huang T, et al. , Autophagy and Hallmarks of Cancer. Crit Rev Oncog, 2018. 23(5–6): p. 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, et al. , Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett, 2020. [DOI] [PubMed] [Google Scholar]
- 51.Zhao W, et al. , Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183–5p. Aging (Albany NY), 2019. 11(21): p. 9581–9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F, et al. , Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles Promote Proliferation, Invasion and Migration of Osteosarcoma Cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/beta-Catenin Axis. Onco Targets Ther, 2021. 14: p. 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abello J, et al. , Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics, 2019. 9(8): p. 2325–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge X, et al. , Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol Ther Nucleic Acids, 2020. 21: p. 900–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sha L, Ma D, and Chen C, Exosome-mediated Hic-5 regulates proliferation and apoptosis of osteosarcoma via Wnt/beta-catenin signal pathway. Aging (Albany NY), 2020. 12(23): p. 23598–23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han F, et al. , Osteosarcoma Cell-Derived Exosomal miR-1307 Promotes Tumorgenesis via Targeting AGAP1. Biomed Res Int, 2021. 2021: p. 7358153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuscino N, et al. , Gathering Novel Circulating Exosomal microRNA in Osteosarcoma Cell Lines and Possible Implications for the Disease. Cancers (Basel), 2019. 11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pal A, et al. , Partial EMT in head and neck cancer biology: a spectrum instead of a switch. Oncogene, 2021. 40(32): p. 5049–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimbo K, et al. , Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun, 2014. 445(2): p. 381–7. [DOI] [PubMed] [Google Scholar]
- 60.De Feo A, et al. , Exosomes from CD99-deprived Ewing sarcoma cells reverse tumor malignancy by inhibiting cell migration and promoting neural differentiation. Cell Death Dis, 2019. 10(7): p. 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazumdar A, et al. , Exploring the Role of Osteosarcoma-Derived Extracellular Vesicles in Pre-Metastatic Niche Formation and Metastasis in the 143-B Xenograft Mouse Osteosarcoma Model. Cancers (Basel), 2020. 12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong L, et al. , Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther, 2021. 6(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin P and Gurevich DB, Macrophage regulation of angiogenesis in health and disease. Semin Cell Dev Biol, 2021. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L, et al. , Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: opportunities and challenges. Oncogenesis, 2021. 10(7): p. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu CY, et al. , Fisetin activates Hippo pathway and JNK/ERK/AP-1 signaling to inhibit proliferation and induce apoptosis of human osteosarcoma cells via ZAK overexpression. Environ Toxicol, 2019. 34(8): p. 902–911. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, et al. , Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am J Transl Res, 2021. 13(5): p. 4211–4223. [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, et al. , The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B, 2021. 11(9): p. 2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng H, et al. , Tumor Microenvironment: Key Players in Triple Negative Breast Cancer Immunomodulation. Cancers (Basel), 2021. 13(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raimondi L, et al. , Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis, 2020. 41(5): p. 666–677. [DOI] [PubMed] [Google Scholar]
- 70.Wolf-Dennen K, Gordon N, and Kleinerman ES, Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology, 2020. 9(1): p. 1747677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazumdar A, et al. , Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int J Mol Sci, 2020. 21(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng Z, et al. , Tumor-Derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch Med Res, 2020. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, et al. , H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics, 2021. 11(3): p. 1473–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galardi A, et al. , Exosomal MiRNAs in Pediatric Cancers. Int J Mol Sci, 2019. 20(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang S and Yang YM, Exosomal microRNAs as diagnostic and therapeutic biomarkers in non-malignant liver diseases. Arch Pharm Res, 2021. 44(6): p. 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hosseini R, et al. , The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer, 2021. 20(1): p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bai K, et al. , Placenta-Derived Exosomes as a Modulator in Maternal Immune Tolerance During Pregnancy. Front Immunol, 2021. 12: p. 671093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang K, et al. , Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics, 2020. 10(1): p. 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jerez S, et al. , Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene, 2019. 710: p. 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cambier L, et al. , Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci Rep, 2021. 11(1): p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, et al. , Plasma Exosome-Derived Sentrin SUMO-Specific Protease 1: A Prognostic Biomarker in Patients With Osteosarcoma. Front Oncol, 2021. 11: p. 625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han Z, et al. , Matrix-assisted laser desorption ionization mass spectrometry profiling of plasma exosomes evaluates osteosarcoma metastasis. iScience, 2021. 24(8): p. 102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han Z, et al. , SERS and MALDI-TOF MS based plasma exosome profiling for rapid detection of osteosarcoma. Analyst, 2021. [DOI] [PubMed] [Google Scholar]
- 84.Torreggiani E, et al. , Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol, 2016. 49(1): p. 189–96. [DOI] [PubMed] [Google Scholar]
- 85.Ryskalin L, et al. , Prion Protein in Glioblastoma Multiforme. Int J Mol Sci, 2019. 20(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu C, et al. , The Emerging Role of Exosomal Non-coding RNAs in Musculoskeletal Diseases. Curr Pharm Des, 2019. 25(42): p. 4523–4535. [DOI] [PubMed] [Google Scholar]
- 87.Wei H, et al. , A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int J Nanomedicine, 2019. 14: p. 8603–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J, et al. , Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnology, 2020. 18(1): p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan Y, Lin Y, and Mi C, Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int, 2021. 45(4): p. 858–868. [DOI] [PubMed] [Google Scholar]
- 90.Weinman MA, et al. , Exosomal proteomic signatures correlate with drug resistance and carboplatin treatment outcome in a spontaneous model of canine osteosarcoma. Cancer Cell Int, 2021. 21(1): p. 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M and Zhang B, The Immunomodulation Potential of Exosomes in Tumor Microenvironment. J Immunol Res, 2021. 2021: p. 3710372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark RA, et al. , Functional intersections between extracellular vesicles and oncolytic therapies. Trends Pharmacol Sci, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang JW, et al. , Exosomal miR-1228 From Cancer-Associated Fibroblasts Promotes Cell Migration and Invasion of Osteosarcoma by Directly Targeting SCAI. Oncol Res, 2019. 27(9): p. 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mkhobongo B, Chandran R, and Abrahamse H, The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. Int J Mol Sci, 2021. 22(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin F, et al. , Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol, 2020. 235(5): p. 4734–4745. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, et al. , Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett, 2020. 490: p. 54–65. [DOI] [PubMed] [Google Scholar]
- 97.Huang Y, et al. , Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J Bone Oncol, 2020. 21: p. 100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, et al. , Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front Cell Dev Biol, 2020. 8: p. 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Q, et al. , Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med, 2020. 9(17): p. 6354–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu C, et al. , Tumor suppressing role of serum-derived exosomal microRNA-15a in osteosarcoma cells through the GATA binding protein 2/murine double minute 2 axis and the p53 signaling pathway. Bioengineered, 2021. 12(1): p. 8378–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu W, et al. , Exosomal transfer of miR-769–5p promotes osteosarcoma proliferation and metastasis by targeting DUSP16. Cancer Cell Int, 2021. 21(1): p. 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao A, et al. , Carcinoma-associated fibroblasts promote the proliferation and metastasis of osteosarcoma by transferring exosomal LncRNA SNHG17. Am J Transl Res, 2021. 13(9): p. 10094–10111. [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, et al. , Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int, 2021. 21(1): p. 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao SC, et al. , EWSAT1 Acts in Concert with Exosomes in Osteosarcoma Progression and Tumor-Induced Angiogenesis: The “Double Stacking Effect”. Adv Biosyst, 2020. 4(9): p. e2000152. [DOI] [PubMed] [Google Scholar]
- 105.Zhang L, et al. , Exosomal MiR-199a-5p Inhibits Tumorigenesis and Angiogenesis by Targeting VEGFA in Osteosarcoma. Front Oncol, 2022. 12: p. 884559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Z, et al. , Tumor-derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch Med Res, 2021. 52(2): p. 200–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


