Abstract
The architecture of the vertebrate eye is optimized for efficient delivery and transduction of photons and processing of signaling cascades downstream from phototransduction. The cornea, lens, retina, vasculature, ciliary body, ciliary muscle, iris and sclera have specialized functions in ocular protection, transparency, accommodation, fluid regulation, metabolism and inflammatory signaling, which are required to enable function of the retina - light sensitive tissue in the posterior eye that sends visual signals to visual centers in the midbrain. Non-visual stimuli such as mechanical (tension, compression, shear), thermal, nociceptive, immune and chemical stimuli target these eye regions to induce pain and precipitate vision loss in glaucoma, diabetic retinopathy, retinal dystrophies, retinal detachment, cataract, corneal dysfunction, ocular trauma and dry eye disease. The principal role for TRPV4, a polymodal nonselective cation channel, is to integrate these non-visual inputs with homeostatic functions of the eye. Under- and overactivation of TRPV4 may affect intraocular pressure, maintenance of blood-retina barriers, neuronal function and neuroinflammation. While most ocular cell types express the TRPV4 gene, its gating, modulation, oligomerization, participation in protein:protein and protein:lipid interactions, and regulation of downstream signaling mechanisms are highly divergent across cell types and ocular structures. Because its dysregulation precipitates many pathologies across anterior and posterior eye, TRPV4 targeting could be employed to mitigate vision loss.
Keywords: TRPV4, eye, retina, cornea, trabecular meshwork, ciliary body, glaucoma
Introduction
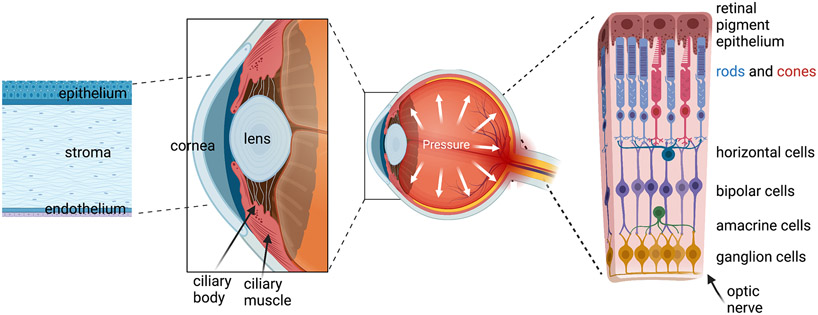
The vertebrate eye has remained remarkably similar over 500 million years, from our early lamprey-like ancestors to humans (Lamb et al., 2007). The eye is enclosed in bone and perforated to pass the optic nerve and vasculature, with photons entering across the cornea and refracted by a contractile crystalline lens onto the retina, a photosensitive tissue (Fig. 1) that transmits the visual signal to the brain. The anterior segment of the eye includes the ciliary body (which produces aqueous humor), trabecular meshwork (the primary drainage mechanism for the aqueous humor), ciliary muscle (secondary drainage mechanism, and regulator of lens contraction) and iris, which determines the color of the eye, and is anatomically divided into two compartments (anterior and posterior chamber) important for aqueous humor circulation. The cornea transitions into an opaque sclera (connective tissue) that protects the eye, is innervated by the trigeminal nerve, and consists of multiple layers with different functions (Fig. 2). The retina is supplied by two vascular beds (choroidal and posterior), functions as a feature extractor that distills 3D information about the shape, color, motion information of the visual stimulus from the 2D pattern of light refracted onto photoreceptors and transmits it via the optic nerve to visual centers in the midbrain.
Figure 1.
Schematic representation of the vertebrate eye (modified after Biorender.com). The multicellular structures in the anterior eye (cornea, lens, ciliary body & muscle, trabecular meshwork) control photon access to the eye, intraocular pressure and light refraction onto the light-sensitive tissue (retina) in the posterior eye. The retina is composed of many cell types; phototransduction takes place in rod and cone photoreceptors, which transmit signals down an excitatory bipolar-RGC pathway that is modulated by horizontal and amacrine cells; only the main neuronal classes and the RPE are shown; glia, endothelial cells, pericytes and exotic subtypes of neuron not depicted).
Figure 2.
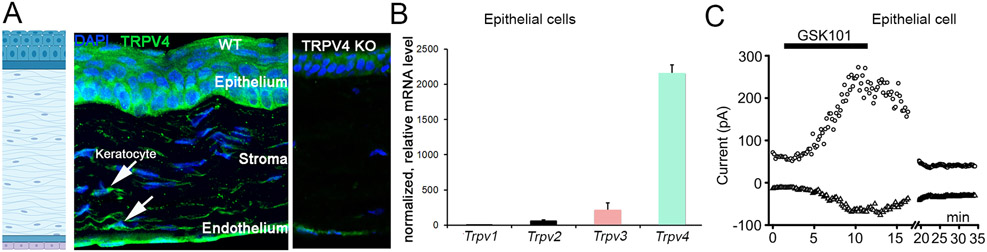
(A) Antibody labeling. TRPV4 expression is observed in all cellular layers of the cornea, including epithelial, stromal (keratocyte) and endothelial cells. The epithelial layer shows graded basal-to-apical expression. Expression specificity validated in corneas from TRPV4−/− mice developed by W. Liedtke. (B) Relative transcript levels, mouse corneal epithelial cells. TRPV4 expression is strong. (C) Corneal epithelial cell, voltage-clamped at Vm = −100 and +100 mV. At room temperature, the agonist GSK1016790A induces a slow, reversible current. Note that the amplitude of the current will be much smaller at the resting potential (~−30 mV).
The Transient Receptor Potential Vanilloid Isoform 4 (TRPV4) channel is expressed in most, if not all, ocular structures - as a plasma membrane channel and possible auxiliary functions in cilia and intracellular store release (Luo et al., 2014; Ryskamp et al., 2011, 2016). The channel pore is likely homotetrameric, with heteromerization with TRPV1 and TRPC1 subunits suggested for retinal endothelial and glial cells, respectively (Jo et al., 2022; O’Leary et al., 2019). Among its likely functions are: cell and tissue volume regulation (Jo et al., 2015; 2016), wound healing and cell proliferation (Lapajne et al., 2020; Okada et al., 2022), lens contractility and accommodation (Chen et al., 2019; Shahidullah & Delamere, 2022), production and secretion of aqueous humor (Jo et al., 2016; Ryskamp et al., 2016), corneal and retinal inflammation (Okada et al., 2022; Ryskamp et al., 2014), regulation of epithelial and endothelial barriers (Lapajne et al., 2020; Phuong et al., 2017; Ríos et al., 2019), neuronal excitability (Ryskamp et al., 2011; Gao et al., 2019) and apoptosis (Li et al., 2021; Ryskamp et al., 2011). Global TRPV4−/− mice don’t exhibit obvious morphological ocular abnormalities: the animals develop normal eye size and intraocular pressure, and their light-evoked neuronal responses and visual behavior are indistinguishable from wild type mice (Ryskamp et al., 2016; Yarishkin et al., 2019; Phuong et al., 2017, Lakk, et al., 2018). Absence of obvious ocular phenotypes is consistent with the normal development, appearance, fertility and behavior noted in original KO studies by Suzuki et al. (2003) and Liedtke et al. (2003), possibly indicating involvement of compensatory cognate vanilloid isoforms (TRPV2 and TRPV3). TRPV4-dependent phenotypes do emerge in pathophysiological states such as ocular hypertension, blood-retina barrier breakdown, cholesterolemia and ischemia/hyperoxia (Cappelli et al., 2021; Križaj, 2019; Križaj et al., 2014), and TRPV4 channelopathies perturb eye development and function (Thibodeau et al., 2017), and may be associated with juvenile glaucoma (Young et al., 2022). In addition to recent reviews of TRPV4 signaling within the eye (Guarino et al., 2020; Thébault, 2021), this review briefly summarizes the ocular landscape of TRPV4 expression and concludes with a reminder TRPV4 function is ultimately defined by its expressing cell type.
1. Cornea
The cornea maintains optical transparency and protects the eye from mechanical, chemical and microbial damage. All cellular layers of the cornea – the epithelium, stroma and endothelium (Fig. 2) prominently express TRPV4.
1. 1. Corneal epithelial cells.
The corneal epithelium consists of multiple cellular layers forming a barrier that resists the flow of tear fluid and protects the eye from mechanical and chemical injury, desiccation and temperature changes. The cells continually experience intraocular pressure, tear tonicity together with ~14 dyn/cm2 shear from osmotically driven transepithelial ion and water gradients. Chemical injury and mechanical damage from contact lens abrasion, keratoablative interventions, acute mechanical trauma and eyelid sliding over corneal surfaces with reduced moistness may result in loss of corneal transparency. Moreover, because epithelial cells function as surrogate Schwann cells to corneal afferents, epithelial injury can induce debilitating pain due to overexcitation of mechano-nociceptors in the trigeminal nerve.
Transcript expression of the vanilloid TRP subfamily in mouse corneal epithelial cells is dominated by Trpv4 (Lapajne et al., 2020) (Fig. 2). Immunohistochemistry and analysis of TRPV4eGFP corneas show robust expression in central and limbal regions, with the well-defined basal-to-squamous gradient of TRPV4-ir suggesting potential functions in differentiation/stemness and/or barrier permeability. The selective agonist TRPV4 GSK1016790A (GSK101) evokes outwardly rectifying whole cell currents and [Ca2+]i elevations in dissociated, cultured and wholemount corneal epithelial cell preparations (Fig. 2C). The channel is activated by moderate temperature and swelling, and may mediate regulatory volume decrease (RVD) in the presence of large hypotonic gradients (Lapajne et al., 2020; Pan et al., 2008). TRPV4 activation in intact corneal epithelial sheets manifests as massive propagation of transepithelial calcium waves that are attenuated by the selective antagonist HC067047, probenecid, suramin and BAPTA-AM. This suggests a model whereby TRPV4 is upstream from Pnx1 hemichannel-mediated ATP release and P2 purinergic receptors (Lapajne et al., 2020), as proposed previously for human pulmonary fibroblasts (Rahman et al., 2018), cholangiocyte cilia (Gradilone et al., 2007), renal epithelia (Silva & Garvin, 2008) and the urothelium (Roberts et al., 2020). We suggest that the TRPV4-Pnx1-P2 receptor axis modulates corneal sensitivity and nociception during mechanical injury, acid/alkaline pH exposure and wound healing.
1.2. Corneal stroma.
The stroma makes up to ~90 percent of the thickness of the cornea populated by keratocytes, myofibroblasts and stromal stem cells. Stromal keratocytes label strongly with TRPV4 antibodies and express the TRPV4eGFP marker (Lapajne et al., 2020) (Fig. 2). TRPV4 may contribute to scar formation resulting from mechanical/chemical injury by promoting TGFβ-dependent transdifferentiation of keratinocytes into myofibroblasts (Okada et al., 2016). Overactivation of keratocyte TRPV4 channels could contribute to cell swelling, calcium overloads and purinergic excitation that underlie corneal edema and transparency loss.
1.3. Corneal endothelial cells.
The corneal endothelium maintains corneal transparency by transporting fluid from the stroma into the anterior chamber. Human and mouse corneal endothelial cells express TRPV4 (Lapajne et al., 2020; Mergler et al., 2011) (Fig 2B). Immortalized cells respond to the selective agonist 4α-PDD, hypotonicity and moderate heat (<38 deg C) with inward currents and calcium signals (Mergler et al., 2011). Overexpression in an immortalized human corneal endothelial cell line suppressed metabolism and reduced viability but surprisingly did not impact monolayer permeability (Donau et al., 2022).
1.4. Corneal nerves.
As the most densely innervated surface epithelium in our body, the cornea is exquisitely sensitive to pain mediated by Aδ-mechanoreceptors and polymodal C-type nociceptors from the ophthalmic branch of the fifth (trigeminal) nerve. Subsets of afferents express TRPV1, Piezo2 and TRPM8 (Fernández-Trillo et al., 2020) but insertion of the TRPV4 gene into trigeminal neurons promotes NGF-mediated healing of the corneal epithelium (Okada et al., 2019; Okada et al., 2022).
2. Sclera
The sclera, a connective tissue that envelops much of the eye (Fig. 1), is composed of collagen-bound ECM with embedded scleral fibroblasts. Stretch is a major regulator of scleral ECM deposition and presumably regulates fibroblast responses to myopia and glaucoma. In contrast to a vast literature on TRPV4 expression in generic fibroblasts (Adapala et al., 2021), not much is known about TRPV4 expression in scleral cells. The tissue is TRPV4-ir, and it might be interesting to examine whether scleral deformation affects a TRPV4-dependent myopic loop with the retina.
3. Ciliary body
The ciliary body consists of nonpigmented epithelial cells (NPE), pigmented epithelial cells (PE) and the ciliary muscle, which together produce aqueous humor and stabilize the lens. TRPV4 expression within the pig and mouse CB is largely confined to NPE cells (Delamere et al., 2016; Jo et al., 2016), which respond to the agonist GSK101 and hypotonicity with [Ca2+]i elevations. Analogy with the choroid plexus (Bothwell et al., 2021; Preston et al., 2018) suggests TRPV4 regulates transepithelial ion fluxes and secretion of aqueous humor, partially through modulation of neurohormone secretion (Alkozi et al., 2017; Alkozi & Pintor, 2015). If, similar to retinal glia and overexpressing oocytes (Toft-Bertelsen et al., 2019), NPE TRPV4 channels are capable of sensing small osmotic gradients, they could play a role in steady-state release of the aqueous fluid. Another potential function is regulation of RVD, which was observed in NPE, but not PE, cells (Edelman et al., 1994).
4. Trabecular meshwork
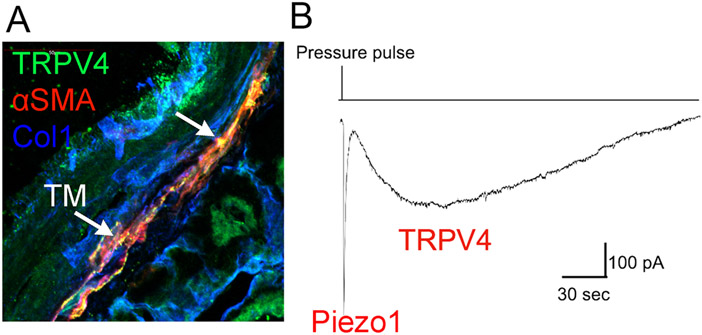
The trabecular meshwork (TM) is a key outflow pathway for the aqueous humor and as such a primary determinant of IOP and hypertensive glaucoma (Križaj, 2019), located just underneath the sclera (Fig. 1). TM cells are subjected to various forms of mechanical stress that include IOP-induced stretching of ECM beams, compression and aqueous humor shear. The pathways by which biomechanical stimuli impact homeostatic and pathological function of TM cells are not completely understood, but TRPV4 is suspected to play an important role due to strong expression and regulation of multiple signaling mechanisms in mouse and human TM. Both rodent and mammalian tissues express TRPV4 mRNA and protein; TRPV4 antibodies colocalize with the TM marker αSMA in situ (Fig 3A) and label the cell membrane, primary cilia and intracellular compartments in cultured cells (Luo et al., 2014; Ryskamp et al., 2016). The TM TRPV4 channel is activated by GSK101, moderate temperature, swelling, ECM strain, lipid stretch (Fig. 4B) and partially by low (0.5 dyn/cm2) levels of shear (Ryskamp et al., 2016; Yarishkin et al., 2021; Yarishkin et al., 2018). Its activation by pressure is delayed relative to the rapid onset of the mechanosensitive Piezo1 current (Fig. 3B), presumably due to obligatory activation of the PLA2-eicosanoid cascade (Ryskamp et al., 2016). Strain-induced TRPV4 activation regulates gene expression, promotes actin polymerization, increases the density and size of focal adhesions and facilitates the secretion of extracellular matrix (Ryskamp et al., 2016; Lakk & Križaj, 2021). Studies implicated ciliary and membrane TRPV4 in hypotension (Luo et al., 2014; Patel et al., 2021), hypertension (Ryskamp et al., 2016) and in myofibroblast differentiation that maintains elevated IOP (Lakk & Križaj, 2021). Topical application of the agonist GSK1016790A reduced IOP in a nocturnal model of ocular hypertension (Patel et al., 2021), yet intracameral injections and eye drop application of the antagonist HC067047 also lower IOP (Redmon et al., 2021b; Ryskamp et al., 2016). The hypotensive effect was ascribed to stimulation of TM-intrinsic eNOS synthase (Patel et al., 2021) but this is under debate (Ethier and Stamer, 2021). Indeed, the agonist GSK1016790A increased outflow resistance and the antagonist HC067047 increased outflow facility in the Glauconix model of conventional outflow (Ryskamp et al., 2016).
Figure 3.
Trabecular meshwork in a vertical section from a human eye, labeled for TRPV4, the tissue marker αSMA (alpha smooth muscle actin) and the ECM marker Collagen 1. (B) Pressure clamp, whole cell recording, cell held at −100 mV. 25 mm Hg pressure pulse induces a rapid inward current mediated by Piezo1, followed by a delayed TRPV4 activation.
Figure 4.
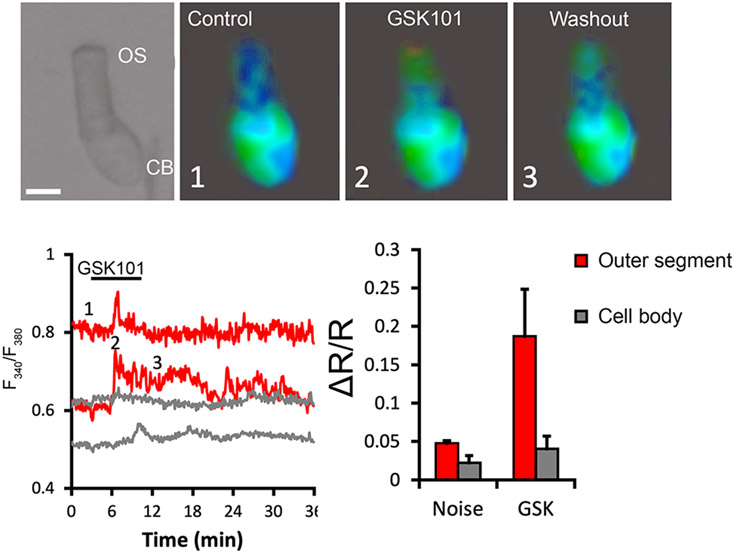
Tiger salamander (Ambystoma tigrinum) rods. The photoreceptor cells were loaded with the calcium indicator Fura-2 AM and exposed to GSK1016790A (50 nM). [Ca2+]i elevations are observed at the tip of the outer segment (OS) in contrast to the weak signals observed in the cell bodies. Scale bar = 10 μm.
An important aspect of TRPV4 signaling pertains to its role in lipid metabolism. Radial stretch significantly increases the membrane phosphatidycholine:cholesterol ratio (Lakk et al., 2021). Because TRPV4 functions as a primary sensor of TM strain (Lakk & Križaj, 2021; Ryskamp et al., 2016), it could regulate the membrane through its lipid composition. Conversely, TRPV4 activity is itself impacted by membrane cholesterol, a planar polycyclic lipid that promotes membrane stiffness while keeping the membrane fluid. Cholesterol depletion facilitates agonist- and mechanically induced TRPV4 activation in trabecular meshwork cells (Lakk et al., 2021), suppresses it in glia (Lakk et al., 2017) and does not affect RGC TRPV4 activation (Lakk and Križaj, unpublished). Unlike endothelial cells (Saliez et al., 2008), TRPV4 in trabecular cells does not partition into lipid rafts or caveolar domains, with the exception of ~75kDa variant of the protein, which co-immunoprecipitates with Caveolin-1. Lakk et al., (2021) suggested that TRPV4 variants might be differentially susceptible to caveolar interactions, with loss of cholesterol interactions outside of lipid rafts resulting in gain-of-function for the channel.
5. Ciliary muscle
The ciliary muscle connects the ciliary body the lens through the zonules of Zinn (Fig. 1). Its contraction and relaxation modulate hydrostatic pressure in the lens by altering water content. Mouse and human ciliary muscle immunolabels with TRPV4 antibodies (Ryskamp et al., 2016).
6. Schlemm’s canal
This lymphatic-vascular structure is in series with juxtacanalicular TM and functions as a drain for aqueous humor outflow. Immunohistochemistry shows strong TRPV4 expression in the canal of Schlemm (Ryskamp et al., 2016).
7. Lens
The crystalline lens consists of lens capsule, epithelial cells, and cortical and nuclear fiber cells that comprise lens cortex and nucleus, respectively. TRPV4 was implicated in maintaining lens transparency, survival and accommodation by regulating force-dependent ion fluxes between lens epithelial and lens fiber cells. In mice, TRPV4 was localized to epithelial and lens fiber cells whereas pig TRPV4 is mainly epithelial (Nakazawa et al., 2019; Shahidullah & Delamere, 2022). Delamere’s group showed that swelling-induced Ca2+ influx opens connexin and possibly pannexin hemichannels to mediate ATP release, which in turn that stimulates Src family kinases (SFKs) and downstream Na/K-ATPase activity (Shahidullah et al., 2012). TRPV4 channels in lens epithelial cells may be able to detect remote damage in lens nucleus (Shahidullah et al., 2015; Shahidullah & Delamere, 2022). Lens fiber cells in adults may lose virtually all organelles but appear to retain functional TRPV4 channels (Nakazawa et al., 2019). TRPV4 activity seems to have little impact on visual quality (Chen et al., 2022) and the retinal light response (Yarishkin et al., 2018). Nonetheless, TRPV4 function as a transducer of hydrostatic pressure within the lens was suggested to operate in a negative feedback loop with TRPV1-dependent signaling, with TRPV4 sensing positive change in hydrostatic pressure (i.e., tracking cell swelling), while TRPV1 is activated by cell shrinkage (Gao et al., 2015).
8. Retina
The vertebrate retina is a complex tissue composed of more than 100 cell types that include many types of neurons and glia, endothelial, smooth muscle-like and immune cells. The flow of visual signal consists of a vertical glutamatergic pathway mediated by excitatory photoreceptor, bipolar and ganglion cell synapses and inhibitory/modulatory contributions from horizontal networks mediated by horizontal and amacrine cells, respectively. Retinal glia consist of Müller cells which provide critical ionic, metabolic and modulatory of support to neurons, astrocytes that line the inner limiting membrane (ILM) and (in vascularized retinas) control permeability of the blood-retina barrier, and microglia as the resident immune cells. In mammals, TRPV4 channels are largely expressed in the inner retina, with by far the strongest expression in RGCs and Müller cells (Lakk et al., 2018). TRPV4 expression has been localized to rat but not mouse, bipolar cells (Gao et al., 2019; Ryskamp et al., 2011). The absence of light-evoked photoreceptor, bipolar and amacrine phenotypes in mammalian retinas (Yarishkin et al., 2018) suggests its functions may be confined to excitatory signaling in the inner plexiform layer. Accordingly, TRPV4−/− mice do not exhibit changes in retinal b-wave (outer retinal function) or amacrine cell-mediated oscillatory potentials (Yarishkin et al., 2018) while TRPV4 agonists promote RGC excitability and reactive gliosis (Ryskamp et al., 2014). TRPV4 overactivation induces RGC apoptosis (Ryskamp et al., 2011) whereas inhibition improves neuronal survival following retinal detachment (Matsumoto et al., 2018; Taylor et al., 2017).
8.1. RPE
The RPE recycles visual photopigment and acts as a restraining barrier to between the choroid, a non-fenestrated blood vessel, and the retina. TRPV4 inhibition may help maintain the integrity of the outer retina-blood barrier in diabetic retinas (Arredondo Zamarripa et al., 2017).
8.2. Photoreceptors
Mammalian photoreceptors do not express TRPV4 channels and photoreceptor signaling is unaffected by deletion of the TRPV4 gene (Yarishkin et al., 2018). In contrast, pilot experiments in rod photoreceptors from the tiger salamander retina show that the agonist GSK1016790A evokes Ca2+ signals. Remarkably, these responses were detected in the outer segment, the modified ciliary compartment that hosts the phototransduction machinery (Fig. 4) rather than the cell body/inner segment region, which hosts most non-cyclic nucleotide-gated channels in photoreceptors (Križaj and Copenhagen, 2002). A more detailed recent study reports that application of pressure steps to the outer plexiform layer evokes cationic currents within the cell body that are sensitive to TRPV antagonists and may include a TRPV4 component (Pang et al., 2021). In contrast to mammals, many amphibian photoreceptors undergo large diurnal changes in shape and photosensitivity (Križaj et al., 1998). It is possible that TRPV4, potentially in conjunction with other mechanotransducers (Pang et al., 2021) functions to control such ‘retinomotor’ movements. Consistent with this conjecture, amphibian and teleost retinomotor movements require calcium and cytoskeletal signaling (Nagle and Burnside, 1984) and we and others showed that TRPV4-mediated Ca2+ influx can be upstream from actomyosin and microtubular remodeling (Goswami et al., 2010; Lakk and Križaj, 2021).
8.2. Retinal ganglion cells
Retinal ganglion cells (RGCs) are projection neurons that transfer visual information from the retina to ~ 50 midbrain areas (Martersteck et al., 2017). The >30 types of RGCs have distinct patterns of dendritic stratification, excitatory/inhibitory input, rod/cone input, and axonal projections. Comparison of Trpv4 gene expression between purified retinal cell populations revealed it to be the strongest in RGCs, with the following vanilloid gene expression: Trpv4>Trpv2>Trpv1=Trpv3 (Lakk et al., 2018) (Fig. 4B). TRPV4-immunoractivity has been reported in teleost (salmon; Nisembaum et al., 2022), tiger salamander (Ryskamp & Križaj, unpublished observations), rat (Li et al., 2021; Sappington et al., 2015), pig (Taylor et al., 2017), non-human primate (Gao et al., 2019) and human (Ryskamp et al., 2011) RGCs, with expression generally the strongest in cells with large somata. GSK1016790A and phorbol esters evoke a nonselective inward cationic current and elevate [Ca2+]i in rodent and primate RGCs (Gao et al., 2019; Lakk et al., 2018; Ryskamp et al., 2011, 2014) (Fig 6A). TRPV4 agonists induce RGC apoptosis (Ryskamp et al., 2011) whereas TRPV4 inhibition and gene deletion improve neuronal survival in pig (Taylor et al., 2017) and mouse (Matsumoto et al., 2018) models of retinal detachment.
Figure 6.
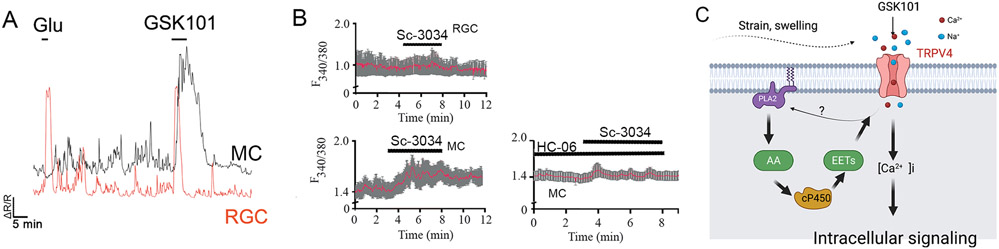
The PLA2-dependence of TRPV4 signaling is cell type-specific. (A) Calcium imaging. Simultaneous recording from a mouse RGC and Müller cell stimulated with GSK1016790A shows a rapid inactivating response in the neuron and a slow-onset sustained response in the glial cell. Compare the amplitude of TRPV4-mediated response to the calcium signal evoked by saturating concentration of glutamate (100 μM). (B) Calcium imaging. Müller cells (MC) but not RGCs respond to the PLA2 activator Sc-3034. The TRPV4 antagonist HC067047 blocks the Sc-3034-induced [Ca2+]i signal in glia. (C) Model of PLA2-dependent TRPV4 signaling in retinal glia, endothelial and epithelial cells.
8.3. Müller glia and retinal homeostasis
Müller cells constitute ~90% of the retinal glial population with crucial functions in water transport, recycling of neurotransmitters, synaptogenesis, immune signaling, vascular function, and K+ siphoning (Musada et al., 2020; Reichenbach & Bringmann, 2010; Vecino et al., 2016). TRPV4 expression was reported for mouse (Jo et al., 2015; Ryskamp et al., 2014), rat (Li et al., 2021; Sappington et al., 2015), pig (Taylor et al., 2017), non-human primate (Gao et al., 2019) and human (Ryskamp et al., 2011) Müller cells, and an immortalized human cell line (Netti et al., 2017). In contrast to sparse TRPV4 expression in brain astrocytes (20-30%; Pivonkova et al., 2018; Shibasaki et al., 2014), every Müller cells manifests robust TRPV4-immunoreactivity and functional responsiveness (Jo et al., 2015; Ryskamp et al., 2014). Retinal detachment, intraocular pressure, swelling and indentation induce inward currents and [Ca2+]i increase that are associated with TRPV4 activity (Jo et al., 2022; Ryskamp et al., 2014). Consistent with this, TRPV4 agonists mimic, and antagonists inhibit, mechanically evoked calcium signals, and TRPV4−/− cells exhibit reduced sensitivity to stretch, swelling, and retinal detachment (Jo et al., 2022; Ryskamp et al., 2014). The additivity of polymodal signaling by the TRPV4 channel can be unmasked by combing pressure and moderate temperature stimuli (Matsumoto et al., 2018) or swelling with GSK101 (Toft-Bertelsen et al., 2017). The increase in cytosolic [Ca2+] evoked by swelling of native Müller cells is suppressed by TRPV4 antagonists and Trpv4 gene deletion whereas hypotonicity-induced Ca2+ increases in immortalized Müller cells do not appear to involve TRPV4 (Netti et al., 2017). TRPV4 inhibition obliterates calcium signals induced by weak (~5-15 mOsm) stimuli and suppresses 40-70% of the calcium response evoked by large (120 - 190 mOsm) hypotonic gradients (Ryskamp et al., 2014; Toft-Bertelsen et al., 2019). The activating stimulus is the rate of water influx (“swelling”), controlled by functional coupling with the AQP4 water channel that constitutes the limiting step for channel activation (Jo et al., 2015; Toft-Bertelsen et al., 2017). Interestingly, TRPV4 activation itself promotes calcium-dependent increase in cell volume through mechanisms that remain to be studied (Jo et al., 2015). While we find Müller cell volume regulation to be Ca2+-dependent (Jo et al., 2015), the osmosensitivity of RVD may be too low for it to play significant physiological roles.
Both overactivation or underactivation of Müller glial TRPV4 signaling induce reactive gliosis, suggesting that the channel participates in homeostatic maintenance and inflammatory activation (Ryskamp et al., 2014). Activation of the channel does not increase NLRP3 and caspase-1 signaling, arguing against the involvement of the NLRP3 inflammasome (Li et al., 2021) whereas conditional ablation of the channel protects the retina from detachment-induced inflammation (Matsumoto et al., 2018). Analogy with CNS astroglia (Diaz-Otero et al., 2019; Turovsky et al., 2020) implicates TRPV4-dependent signaling in Müller glial endfeet in neurovascular coupling, functional hyperemia, intravascular pressure, and barrier permeability. Another important secondary mechanism may be modulation of RGC viability via TRPV4-mediated release of cytokines MCP-1 and TNFα from Müller cells (Li et al., 2021; Matsumoto et al., 2018).
8.4. Microglia and immune signaling
In contrast to cortical microglia in which Trpv4 dominates thermoTRP expression, its expression in mouse retinal microglia is weak (Fig. 4A) and was therefore missed in an early immunohistochemical study (Redmon et al., 2021; Ryskamp et al., 2011). GSK1016790A and hypotonicity evoke small (10-20 pA) currents but substantial [Ca2+]i increases and hyperpolarizations (~10 mV) that are presumably mediated by a Ca2+-activated K+ conductance. TRPV4 activation in intact retinas is associated with Ca2+-dependent retraction of higher-order branches. Perfusion of intact retinas with the TRPV4 antagonist HC06047 did not affect microglial [Ca2+]i, resting membrane potential or branching, indicating that, at least at room temperature, TRPV4 does not mediate a constitutive depolarizing signal. However, this does not preclude TRPV4 involvement in dendritic surveillance in ‘resting’ cells under in vivo conditions. Indeed, Tominaga’s group showed that TRPV4 mediates microglial motility in response to moderate temperature (Nishimoto et al., 2021).
8.5. Astrocytes
Astrocytes in intact retina are immuno-negative for TRPV4 (Ryskamp et al., 2011) and analysis of Trpv4 mRNA in astrocytes purified from mouse retinas shows it to be low relative to RGCs and Müller cells. Astroglial thermoTRP expression may be region-, mouse strain- and context-specific, with modest Trpv4 expression reported in astrocytes from the optic nerve head of wild type and DBA/2J mice (Choi et al., 2015).
8.6. Endothelial cells and blood-retina barrier regulation
The retina is associated with two different classes of endothelial cell (EC): choroidal in the outer retina and microvascular (capillary) in the inner retina. TRPV4 antibodies weakly label capillaries in the intact mouse retina (Phuong et al., 2017) whereas transgenic retinas that express GFP downstream from the TRPV4 promotor show strong fluorescence (Redmon et al., 2021). Among the many physiological processes in retinal microvascular ECs that are sensitive TRPV4 are Ca2+ homeostasis, angiogenesis, cytoskeletal remodeling and junctional permeability (Phuong et al., 2016; Arredondo-Zamarippa et al., 2019; Capelli et al., 2021). TRPV4 agonists increased in vitro and in vivo permeability of endothelial barriers, increased [Ca2+]i, and promoted calcium-dependent disassembly of VE-cadherin:β-catenin- containing adherens/occludens junctions and reorganization of the actin cytoskeleton. The agonist GSK101 evokes the ‘canonical’ response signature typical PLA2-dependent TRPV4 responses (White et al., 2016): outward rectification, reversal at ~0 mV and delayed onset-to-peak and a sustained plateau (Ryskamp et al., 2014). The potency of GSK101 in elevating [Ca2+]i and modulating barrier permeability in human ECs (EC50 ~ 1.6 nM) is similar to affinities reported for mouse and human TRPV4 channels (EC50 3 – 20 nM; Thorneloe et al., 2008) and ~10-fold higher compared to other TRPV4-expressing retinal glia, epithelial and trabecular meshwork cells. TRPV4 activation is coupled to Calcium-Induced Calcium release, with SERCA blockers reducing the amplitude of GSK101-evoked response by ~31% in human (Phuong et al., 2017) and ~75% in bovine ECs (Monaghan et al., 2015). The large reversible decrease in the microvascular transendothelial resistance induced by GSK101 correlates with remodeling of cortical F-actin, redistribution of VE-cadherin from interdigitated processes between adjacent cells to the cell membrane, dislocation of β-catenin and decreased expression of the tight junction protein occludin and is mirrored in vivo by ~30% increase in capillary Evans Blue extravasation (Phuong et al., 2017). The time course of recovery from increased transendothelial permeability is slower (~hours) compared to the relatively rapid response to the agonist (~minutes), presumably due to the compensatory activation of downstream signaling pathways (MAPKs/Src/Raf/Ras, RhoA and myosin-light chain kinase pathways). We hypothesize that Ca2+ influx controls local actin remodeling and bonding between VE-cadherin and β-catenin, with relocation of TRPV4 channels from adherens junctions possibly controlled by the focal adhesion kinase, integrins and the Rho/Rho kinase pathway (Bagnell et al., 2022). Consistent with this, TRPV4-mediated calcium influx impaired filopodial ruffling and stress fiber organization in cells transfected with F-actin:mApple constructs (Phuong et al., 2017). Another important process may be the vascular tone, with TRPV4 as a coordinator of signaling between endothelial cells, pericytes, and glia that maintains the balance between vasodilation and vasoconstriction (Sonkusare et al., 2012; White et al., 2016).
Shear flow, endothelial stretch and eicosanoids could maintain vascular autoregulation and regulate redistribution of blood flow from superficial to deep vascular plexi by stimulating TRPV4 channels through local elevations of EETs and 20-HETE. This process is almost certainly disturbed under pathological circumstances such as edema, blast trauma, transendothelial invasion of monocytes and ocular hypertension (Križaj, 2019). Studies in cultured cells implicated TRPV4 in regulation of retinal endothelial cell migration, sprouting and tube formation. TRPV4 inhibition promotes developmental angiogenesis (O’Leary et al., 2019) yet deletion of the channel does not appear to affect retinal blood vessel development in mice (Cappelli et al., 2021). Cultured human retinal endothelial cells respond to unidirectional strain by reorienting their long axis orthogonally in a TRPV4-dependent manner (Cappelli et al., 2021). Its sensitivity to swelling and cholesterol implicates TRPV4 in diabetic retinal vascular phenotypes. Consistent with this, TPRV4 blockers suppress BRB breakdown in a rodent model of diabetes (Arredondo Zamarripa et al., 2017) and TRPV4 knockdown may reduce oxygen-induced neovascularization and VEGF-VEGFR2 signaling (Cappelli et al., 2021; O’Leary et al., 2019) yet hyperglycemia also downregulates TRPV4 expression (Monaghan et al., 2015). An extensive body of work has documented a central role for TRPV4 in retina, brain and body edema formation, often in partnership with aquaporins (Ríos et al., 2019; Kitchen et al., 2020; Weber et al., 2020; Michinaga et al., 2021). Studies on the way are exploring whether cytotoxic and vascular edema in the retina might be controlled with therapeutic targeting of TRPV4 and/or AQP4 proteins (Iuso and Križaj, 2016). Given that suppression of TRPV4 channels promotes physiological revascularization of ischemic retinas (O’Leary et al., 2019), pharmacological interventions should carefully consider the biological context.
One protein to confound them all
The ubiquitous expression of the TRPV4 channels across ocular tissues mirrors its expression across the CNS, PNS and body (White et al., 2016). The uniquely divergent pattern of TRPV4 expression, activation and modulation within the eye, however, belies simplistic evaluations of its functional importance.
A. TRPV4 protein.
Different ocular cells express different versions of the protein. Western blots precipitate two molecular weight bands from protein lysates of human retinal endothelial cells and mouse RGCs (Phuong et al., 2017; Ryskamp et al., 2011) but show four bands in TM cells (Lakk et al., 2021), thus mirroring kidney, ventricular myocyte and choroid epithelial expression (Zhao et al., 2012; Hochstetler et al., 2020; Lakk et al., 2021). The bands could reflect presence of an unglycosylated ~75 kDa splice variant and multiple glycosylated variants, membrane vs. intracellular pools, splice variants, long vs. short forms of the protein or differential association with lipid rafts (Lakk et al., 2021).
B. Same cell type in retina vs other parts of CNS.
TRPV4 expression in retinal microglia is lower compared to their cortical counterparts, which furthermore show a different sequence of relative thermoTRP expression (Redmon et al., 2021). This indicates that microglia express region-specific sensomes that equip them for differential responsiveness to the external milieu.
C. Same cell type in different regions of the eye.
The choroid is a large blood vessel that perfuses the anterior part of the retina. TRPV4 expression in choroidal ECs is weaker relative to microvascular (inner retinal) ECs (Phuong et al., 2017), indicating heterogeneity of TRPV4 signaling across dissimilar vascular beds.
D. Expression across different cell types.
Relative expression of TRPV4 differs across retinal neurons vs. glial and endothelial cells (Phuong et al., 2017; Redmon et al., 2021). The same stimulus may therefore produce different response magnitudes across cell types.
E. Biophysical properties of the TRPV4 current.
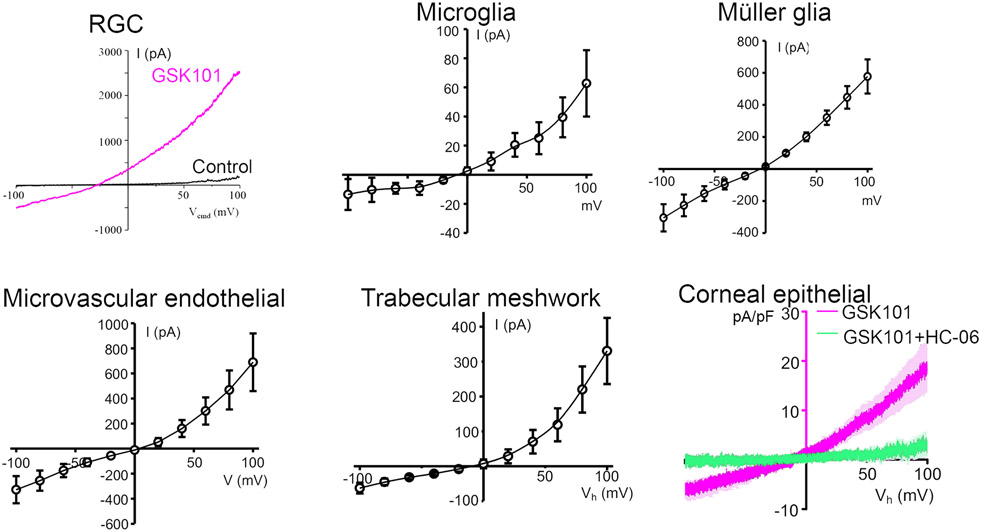
Fig. 5 shows the I-V relationships for cell types from different ocular tissues. The currents were acquired under voltage clamp by the same investigator on the same experimental setup under identical conditions (except for the Müller glia, which were recorded under K+-free conditions). The voltage-dependence of the GSK1016790A-evoked current ranges from minimally to moderately strong, showing outward rectification at positive membrane potentials in RGCs and microglia but a more linear relationship in Müller glia and epithelial cells.
Figure 5.
Whole cell voltage clamp in ocular cells stimulated with 25 nM GSK1016790A (with exception of the endothelial cell, exposed to 1 nM of the agonist). The agonist-evoked response varies in terms of the voltage-dependence of the evoked current, as in the shape of the I-V relationship, and current amplitudes/densities. All responses were blocked by blocker HC067047 (2-5 μM).
F. Time course and mechanism of activation.
Fig. 6 shows the time course of GSK1016790A-activated current simultaneously recorded in representative RGC and Müller cells. The former shows an inactivating Ca response with a rapid onset, in contrast to the slow onset and sustained elevation in the glia. The responses in trabecular meshwork cells are nested in-between the extremes shown by neurons and glia by show a peak [Ca2+]i response that relaxes to a steady plateau that can be maintained for hours (Yarishkin et al., 2022). Sustained activation in Müller cells, microglia, TM cells, corneal epithelial cells, nonpigmentary epithelial cells of the ciliary body and microvascular endothelial cells requires an intermediary PLA2-eicosanoid step while RGC signals appear to be independent of eicosanoid signaling (Jo et al., 2016; Lapajne et al., 2020; Phuong et al., 2017; Ryskamp et al., 2011, 2014). The difference can be unmasked with the PLA2 activator Sc-3034, which has no effect on RGC [Ca2+]i, but elevates [Ca2+]i in glia in a TRPV4-dependent manner (Fig 6B). Interestingly, overexpression of TRPV4 in an oocyte system leads to the expression of TRPV4-mediated PLA2-insensitive currents (Toft-Bertelsen et al., 2019) and TRPV4 remains functional in yeast, which lacks PLA2 and polyunsaturated fatty acids (Loukin and Kung, 2009). PLA2 might be downstream from a GPCR (Mamenko et al., 2011) or activated by an initial Ca2+ influx across the TRP channel (Fig. 6C). Another intriguing feature of TRPV4 signaling are Ca2+ oscillations that emerge during sustained TRPV4 activation in some cell types. In trabecular meshwork cells, TRPV4-dependent oscillations require release of Ca2+ from intracellular compartments together with Ca2+-dependent activation of TRPM4 channels (Yarishkin et al., 2022).
G. Heteromerization.
TRPV4 was suggested to heteromerize with TRPV1 in microvascular endothelial cells (O’leary et al., 2019) and with TRPC1 in Müller glia (Jo et al., 2022). Heteromerization significantly alters the properties of channel activation (Ma et al., 2011). Heteromerization is not obligatory in cells that express the channels. Thus, TRPV1 and TRPV4 coexpress in RGCs but do not functionally interact (Lakk et al., 2018).
H. Stimulus-response coupling.
Retinal glia, endothelial and trabecular meshwork cells respond to ECM strain with TRPV4-dependent calcium influx (Jo et al., 2022; Phuong et al., 2017; Ryskamp et al., 2016). Although corneal epithelial cells strongly express TRPV4 (Fig. 2) and respond to 1 – 12% ECM strain with [Ca2+]i elevations (Lapajne et al., 2020), neither the calcium signal nor the fraction of responders to strain are affected in Trpv4−/− and HC06047-treated cells. Similarly, membrane stretching in response to positive pressure activates TRPV4 in glia, trabecular meshwork cells and oocytes but not chondrocytes (Matsumoto et al., 2018; Ryskamp et al., 2016; Servin-Vences et al., 2017).
A universal stimulus to investigate TRPV4 activity and indeed the stimulus used by Strotmann et al. (2000) to originally characterize the channel is sensitivity to osmotic stimuli. The majority of studies tested TRPV4 activation with nonphysiological gradients (>100 mOsm) that are not directly relevant to physiology or pathology – it is possible that not many cell types are capable of utilizing TRPV4 for transduction of physiological gradients (5-15 mOsm). We find the exceptions to be retinal Müller cells and probably microglia (Toft-Bertelsen et al., 2019; Redmon et al., 2021), while RGCs respond to 190 mOsm but cannot sense 15 mOsm gradients (Ryskamp et al., 2011; Toft-Bertelsen et al., 2019). The differential responsiveness may reflect accessibility of the N-terminal TYR110, PLA2 activation and density of AQP channels (Iuso and Križaj, 2016; White et al., 2016; Toft-Bertelsen et al., 2019).
I. Downstream coupling to interacting proteins.
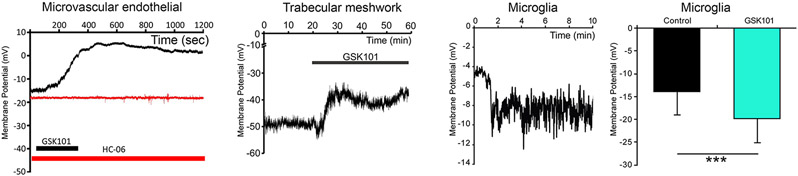
TRPV4-mediated calcium influx reflects channel’s coupling to a wide array of cell membrane, ECM, and cytosolic proteins/lipids. Examples in the eye include pannexin 1 (Lapajne et al., 2020), nitric oxide synthase (Patel et al., 2021), phospholipase A2 (Ryskamp et al., 2014), the Rho-ROCK pathway (Lakk & Križaj, 2021), caveolin-1 (Lakk et al., 2021), focal adhesions (Lakk & Križaj, 2021) and the JAK/NFkB pathway (Li et al., 2021). The agonist GSK101 depolarizes the membrane potential in endothelial and trabecular meshwork cells but hyperpolarizes microglia, presumably due to coupling with eicosanoid metabolites, nitric oxide synthase or K+ conductances (White et al., 2016; Phuong et al., 2017; Redmon et al., 2021; Yarishkin et al., 2022) (Fig. 7).
Figure 7.
Context-dependence of membrane potential regulation by TRPV4. The TRPV4 agonist GSK1016790A depolarizes trabecular meshwork and endothelial cells but hyperpolarizes microglia.
J. Lipid modulation.
Membrane stiffness and curvature are regulated by free membrane cholesterol. β-methyl cyclodextrin, a cholesterol-sequestering drug, inhibits TRPV4 signaling in Müller cells, enhances it in trabecular meshwork cells but has no effect on TRPV4 activity in RGCs (Lakk et al., 2017, 2021). TRPV4 associates with caveolin scaffolds that organize membrane signaling in a microvascular endothelial cell line (Goedicke-Fritz et al., 2015) but not in trabecular meshwork cells (Lakk et al., 2021).
Summary
TRPV4 is widely expressed across the eye, with likely functions in paradigmatic aspects of visual signaling and pathology such as corneal permeability and pain, lens accommodation and cataract, aqueous fluid production, secretion and glaucoma, regulation of retinal water content and edema, angiogenesis and barrier dysregulation, inflammation, neurodegeneration and glaucoma. While the responses to GSK1016890A and 4a-PDD are consistent with the universality of TRPV4 protein structure and function, agonists and physiological activation produce a remarkably diverse spectrum of activation phenotypes, sensitization, inactivation, coupling to modulatory pathways and roles in signal integration across the eye. Pharmacological manipulations and global knockdown may thus produce difficult-to-interpret phenotypes. For example, TRPV4-dependent loss of retina-blood barrier integrity (Arredondo Zamarripa et al., 2017; Phuong et al., 2017) that mirrors EC retraction, vascular leakage and circulatory collapse in pulmonary epithelia (Villalta et al., 2014; Willette et al., 2008) is likely to reflect parallel effects on endothelial cells, pericytes, Müller cell endfeet, astrocyte processes and ganglion cells. Similar complexity may pertain to TRPV4 signaling at the corneal epithelial-stromal-afferent interface, and its functions in the ciliary body, ciliary muscle, trabecular that may contribute to multi-level regulation of intraocular pressure, pressure-dependent neuronal excitation and neurodegeneration.
Acknowledgements
Supported by: NIH (R01EY027920, R01EY031817, P30EY014800, T32EY024234), Rankin-Stauss Foundation, USAMRAA and unrestricted support from Research to Prevent Blindness to the Moran Eye Center at the University of Utah. We thank Prof. Wolfgang Liedtke (Duke University) for the global TRPV4−/− mice.
References
- Adapala RK, Katari V, Teegala LR, Thodeti S, Paruchuri S, & Thodeti CK (2021). Trpv4 mechanotransduction in fibrosis. Cells, 10(11), 3053. 10.3390/cells10113053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkozi HA, de Lara MJP, Sánchez-Naves J, & Pintor J (2017). TRPV4 stimulation induced melatonin secretion by increasing arylalkymine N-acetyltransferase (AANAT) protein level. International Journal of Molecular Sciences, 18(4). 10.3390/ijms18040746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkozi HA, & Pintor J (2015). TRPV4 activation triggers the release of melatonin from human non-pigmented ciliary epithelial cells. Experimental Eye Research, 136, 34–37. 10.1016/j.exer.2015.04.019 [DOI] [PubMed] [Google Scholar]
- Arredondo Zamarripa D, Noguez Imm R, Bautista Cortés AM, Vázquez Ruíz O, Bernardini M, Fiorio Pla A, Gkika D, Prevarskaya N, López-Casillas F, Liedtke W, Clapp C, & Thébault S (2017). Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: Diabetic milieu makes a difference. Scientific Reports, 7(1), 1–16. 10.1038/s41598-017-13621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnell AM, Sumner CJ, & McCray BA (2022). TRPV4: A trigger of pathological RhoA activation in neurological disease. BioEssays, 44(6), 2100288. 10.1002/bies.202100288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell SW, Omileke D, Patabendige A, & Spratt NJ (2021). CSF secretion is not altered by NKCC1 nor TRPV4 antagonism in healthy rats. Brain Sciences, 11(9). 10.3390/brainsci11091117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli HC, Guarino BD, Kanugula AK, Adapala RK, Perera V, Smith MA, Paruchuri S, & Thodeti CK (2021). Transient receptor potential vanilloid 4 channel deletion regulates pathological but not developmental retinal angiogenesis. Journal of Cellular Physiology, 236(5), 3770–3779. 10.1002/jcp.30116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gao J, Li L, Sellitto C, Mathias RT, Donaldson PJ, & White TW (2019). The ciliary muscle and zonules of ZinN modulate lens intracellular hydrostatic pressure through transient receptor potential vanilloid channels. Investigative Ophthalmology and Visual Science, 60(13), 4416–4424. 10.1167/iovs.19-27794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Petrova RS, Qiu C, & Donaldson PJ (2022). Intracellular hydrostatic pressure regulation in the bovine lens: A role in the regulation of lens optics? American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 322(3), R263–R279. 10.1152/ajpregu.00309.2021 [DOI] [PubMed] [Google Scholar]
- Delamere NA, Mandal A, & Shahidullah M (2016). The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium. Journal of Ocular Pharmacology and Therapeutics, 32(8), 504–508. 10.1089/jop.2016.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Otero JM, Yen TC, Ahmad A, Laimon-Thomson E, Abolibdeh B, Kelly K, Lewis MT, Wiseman RW, Jackson WF, & Dorrance AM (2019). Transient Receptor Potential Vanilloid 4 Channels are Important Regulators of Parenchymal Arteriole Dilation and Cognitive Function. Microcirculation (New York, N.Y. : 1994), 26(6), e12535. 10.1111/MICC.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donau J, Luo H, Virta I, Skupin A, Pushina M, Loeffler J, Haertel FV, Das A, Kurth T, Gerlach M, Lindemann D, Reinach PS, Mergler S, & Valtink M (2022). TRPV4 Stimulation Level Regulates Ca2+-Dependent Control of Human Corneal Endothelial Cell Viability and Survival. Membranes, 12(3). 10.3390/membranes12030281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JL, Sachs G, & Adorante JS (1994). Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. American Journal of Physiology - Cell Physiology, 266(5 35-5). 10.1152/ajpcell.1994.266.5.c1210 [DOI] [PubMed] [Google Scholar]
- Ethier CR, Sigal IA, Flanagan JG (2005). What does the Sclera Have to Do With Glaucoma? International Glaucoma Review, 6(3) Retrieved From: https://www.e-igr.com/MR/index.php?issue=63&MRid=144 [Google Scholar]
- Fernández-Trillo J, Florez-Paz D, Íñigo-Portugués A, González-González O, del Campo AG, González A, Viana F, Belmonte C, & Gomis A (2020). Piezo2 mediates low-threshold mechanically evoked pain in the cornea. Journal of Neuroscience, 40(47), 8976–8993. 10.1523/JNEUROSCI.0247-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yang Z, Jacoby RA, Wu SM, & Pang JJ (2019). The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell Death and Disease, 10(5), 1–12. 10.1038/s41419-019-1576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, White TW, Delamere NA, & Mathias RT (2015). Feedback Regulation of Intracellular Hydrostatic Pressure in Surface Cells of the Lens. Biophysical Journal, 109(9), 1830–1839. 10.1016/j.bpj.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedicke-Fritz S, Kaistha A, Kacik M, Markert S, Hofmeister A, Busch C, Bänfer S, Jacob R, Grgic I, Hoyer J. Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur J Cell Biol. 2015. Jul-Sep;94(7-9):391–400. doi: 10.1016/j.ejcb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One. 2010. Jul 19;5(7):e11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, & LaRusso NF (2007). Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proceedings of the National Academy of Sciences of the United States of America, 104(48), 19138–19143. 10.1073/pnas.0705964104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino BD, Paruchuri S, & Thodeti CK (2020). The role of TRPV4 channels in ocular function and pathologies. Experimental Eye Research, 201, 108257. 10.1016/j.exer.2020.108257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstetler AE, Smith HM, Preston DC, Reed MM, Territo PR, Shim JW, Fulkerson D, & Blazer-Yost BL (2020). TRPV4 antagonists ameliorate ventriculomegaly in a rat model of hydrocephalus. JCI Insight, 5(18). 10.1172/JCI.INSIGHT.137646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuso A, & Križaj D (2016). TRPV4-AQP4 interactions ‘turbocharge’ astroglial sensitivity to small osmotic gradients. Channels, 10(3), 172–174. 10.1080/19336950.2016.1140956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Frye AM, Phuong TTT, Redmon SN, Roberts R, Berkowitz BA, Yarishkin O, & Križaj D (2016). Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proceedings of the National Academy of Sciences of the United States of America, 113(14), 3885–3890. 10.1073/pnas.1515895113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Rudzitis CN, & Križaj D (2022). TRPV4 and TRPC1 channels mediate the response to tensile strain in mouse Müller cells. Cell Calcium, 104, 102588. 10.1016/j.ceca.2022.102588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Ryskamp DA, Phuong TTT, Verkman AS, Yarishkin O, Macaulay N, & Križaj D (2015). TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal müller glia. Journal of Neuroscience, 35(39), 13525–13537. 10.1523/JNEUROSCI.1987-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen P, Salman MM, Halsey AM, Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan A, Kreida S, Al-Jubair T, Winkel Missel J, Gourdon P, Törnroth-Horsefield S, Conner MT, Ahmed Z, Conner AC, Bill RM. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell. 2020. May 14;181(4):784–799.e19. doi: 10.1016/j.cell.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D (2019). What is glaucoma? In Kolb H, Fernandez E, & Nelson R (Eds.), Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center; 1995-. [PubMed] [Google Scholar]
- Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, & Shestopalov VI (2014). From Mechanosensitivity to Inflammatory Responses: New Players in the Pathology of Glaucoma. Current Eye Research, 39(2), 105–119. 10.3109/02713683.2013.836541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Hoffmann GF, Gorusupudi A, Enyong E, Lin A, Bernstein PS, Toft-Bertelsen T, MacAulay N, Elliott MH, & Križaj D (2021). Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. Journal of Lipid Research, 62. 10.1016/J.JLR.2021.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, & Križaj D (2021). TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells. American Journal of Physiology - Cell Physiology, 320(6), C1013–C1030. 10.1152/ajpcell.00599.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Yarishkin O, Baumann JM, Iuso A, & Križaj D (2017). Cholesterol regulates polymodal sensory transduction in Müller glia. Glia, 65(12), 2038–2050. 10.1002/glia.23213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Young D, Baumann JM, Jo AO, Hu H, & Križaj D (2018). Polymodal TRPV1 and TRPV4 sensors colocalize but do not functionally interact in a subpopulation of mouse retinal ganglion cells. Frontiers in Cellular Neuroscience, 12, 353. 10.3389/fncel.2018.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, & Pugh EN (2007). Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. In Nature Reviews Neuroscience (Vol. 8, Issue 12, pp. 960–976). Nat Rev Neurosci. 10.1038/nrn2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapajne L, Lakk M, Yarishkin O, Gubeljak L, Hawlina M, & Križaj D (2020). Polymodal sensory transduction in mouse corneal epithelial cells. Investigative Ophthalmology and Visual Science, 61(4), 2–2. 10.1167/iovs.61.4.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheng Y, Zhang S, Sun X, & Wu J (2021). TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. Journal of Neuroinflammation, 18(1), 271. 10.1186/s12974-021-02315-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, & Friedman JM (2003). Abnormal osmotic regulation in trpv4−/− mice. Proceedings of the National Academy of Sciences of the United States of America, 100(23), 13698–13703. 10.1073/pnas.1735416100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009. Feb 18;583(4):754–8. doi: 10.1016/j.febslet.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, & Sun Y (2014). Primary cilia signaling mediates intraocular pressure sensation. Proceedings of the National Academy of Sciences of the United States of America, 111(35), 12871–12876. 10.1073/pnas.1323292111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cheng KT, Wong CO, O’Neil RG, Birnbaumer L, Ambudkar IS, & Yao X (2011). Heteromeric TRPV4-C1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium, 50(6), 502–509. 10.1016/j.ceca.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martersteck EM, Hirokawa KE, Evarts M, Bernard A, Duan X, Li Y, Ng L, Oh SW, Ouellette B, Royall JJ, Stoecklin M, Wang Q, Zeng H, Sanes JR, & Harris JA (2017). Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Reports, 18(8), 2058–2072. 10.1016/j.celrep.2017.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Sugio S, Seghers F, Križaj D, Akiyama H, Ishizaki Y, Gailly P, & Shibasaki K (2018). Retinal detachment-induced müller glial cell swelling activates TRPV4 ion channels and triggers photoreceptor death at body temperature. Journal of Neuroscience, 38(41), 8745–8758. 10.1523/JNEUROSCI.0897-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michinaga S, Onishi K, Shimizu K, Mizuguchi H, Hishinuma S. Pharmacological Inhibition of Transient Receptor Potential Vanilloid 4 Reduces Vasogenic Edema after Traumatic Brain Injury in Mice. Biol Pharm Bull. 2021;44(11):1759–1766. doi: 10.1248/bpb.b21-00512. [DOI] [PubMed] [Google Scholar]
- Mergler S, Valtink M, Taetz K, Sahlmüller M, Fels G, Reinach PS, Engelmann K, & Pleyer U (2011). Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Experimental Eye Research, 93(5), 710–719. 10.1016/j.exer.2011.09.021 [DOI] [PubMed] [Google Scholar]
- Minns MS, Teicher G, Rich CB, & Trinkaus-Randall V (2016). Purinoreceptor P2X7 regulation of Ca2+ mobilization and cytoskeletal rearrangement is required for corneal reepithelialization after injury. American Journal of Pathology, 186(2), 285–296. 10.1016/j.ajpath.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, McGeown JG, & Curtis TM (2015). Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS ONE, 10(6), e0128359. 10.1371/journal.pone.0128359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musada GR, Dvoriantchikova G, Myer C, Ivanov D, Bhattacharya SK, & Hackam AS (2020). The effect of extrinsic Wnt/β-catenin signaling in Muller glia on retinal ganglion cell neurite growth. Developmental Neurobiology, 80(3–4), 98–110. 10.1002/dneu.22741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle BW, Burnside B. Calmodulin-binding proteins in teleost retina, rod inner and outer segments, and rod cytoskeletons. Eur J Cell Biol. 1984. Mar;33(2):248–57. [PubMed] [Google Scholar]
- Nakazawa Y, Donaldson PJ, & Petrova RS (2019). Verification and spatial mapping of TRPV1 and TRPV4 expression in the embryonic and adult mouse lens. Experimental Eye Research, 186, 107707. 10.1016/j.exer.2019.107707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netti V, Fernández J, Kalstein M, Pizzoni A, Di Giusto G, Rivarola V, Ford P, & Capurro C (2017). TRPV4 Contributes to Resting Membrane Potential in Retinal Müller Cells: Implications in Cell Volume Regulation. Journal of Cellular Biochemistry, 118(8), 2302–2313. 10.1002/jcb.25884 [DOI] [PubMed] [Google Scholar]
- Nisembaum LG, Loentgen G, L’Honoré T, Martin P, Paulin CH, Fuentès M, Escoubeyrou K, Delgado MJ, Besseau L, & Falcón J (2022). Transient Receptor Potential-Vanilloid (TRPV1-TRPV4) Channels in the Atlantic Salmon, Salmo salar. A Focus on the Pineal Gland and Melatonin Production. Frontiers in Physiology, 12. 10.3389/fphys.2021.784416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary C, McGahon MK, Ashraf S, McNaughten J, Friedel T, Cincolà P, Barabas P, Fernandez JA, Stitt AW, McGeown JG, & Curtis TM (2019). Involvement of TRPV1 and TRPV4 channels in retinal angiogenesis. Investigative Ophthalmology and Visual Science, 60(10), 3297–3309. 10.1167/iovs.18-26344 [DOI] [PubMed] [Google Scholar]
- Okada Y, Shirai K, Miyajima M, Reinach PS, Yamanaka O, Sumioka T, Kokado M, Tomoyose K, & Saika S (2016). Loss of TRPV4 function suppresses inflammatory fibrosis induced by alkali-burning mouse corneas. PLoS ONE, 11(12). 10.1371/journal.pone.0167200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sumioka T, Ichikawa K, Sano H, Nambu A, Kobayashi K, Uchida K, Suzuki Y, Tominaga M, Reinach PS, Hirai S. ichi, Jester JV, Miyajima M, Shirai K, Iwanishi H, Kao WWY, Liu CY, & Saika S (2019). Sensory nerve supports epithelial stem cell function in healing of corneal epithelium in mice: the role of trigeminal nerve transient receptor potential vanilloid 4. Laboratory Investigation, 99(2), 210–230. 10.1038/s41374-018-0118-4 [DOI] [PubMed] [Google Scholar]
- Okada Y, Sumioka T, Reinach PS, Miyajima M, & Saika S (2022). Roles of Epithelial and Mesenchymal TRP Channels in Mediating Inflammatory Fibrosis. Frontiers in Immunology, 12. 10.3389/fimmu.2021.731674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby D, Stamer D (2022) “Glaucoma Dialogue: Comment by Darryl Overby, London UK and Daniel Stamer, Durham, NC, USA”. International Glaucoma Review, 22(1) 12–13 ISSN 1566-1040 [Google Scholar]
- Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, Kao WWY, Koziel H, Pleyer U, & Reinach PS (2008). Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium, 44(4), 374–385. 10.1016/j.ceca.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Generators of Pressure-Evoked Currents in Vertebrate Outer Retinal Neurons. Cells. 2021. May 22;10(6):1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Chen YL, Kasetti RB, Maddineni P, Mayhew W, Millar JC, Ellis DZ, Sonkusare SK, & Zode GS (2021). Impaired TRPV4-eNOS signaling in trabecular meshwork elevates intraocular pressure in glaucoma. Proceedings of the National Academy of Sciences of the United States of America, 118(16). 10.1073/pnas.2022461118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TTT, Redmon SN, Yarishkin O, Winter JM, Li DY, & Križaj D (2017). Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. Journal of Physiology, 595(22), 6869–6885. 10.1113/JP275052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivonkova H, Hermanova Z, Kirdajova D, Awadova T, Malinsky J, Valihrach L, Zucha D, Kubista M, Galisova A, Jirak D, & Anderova M (2018). The Contribution of TRPV4 Channels to Astrocyte Volume Regulation and Brain Edema Formation. Neuroscience, 394, 127–143. 10.1016/j.neuroscience.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Preston D, Simpson S, Halm D, Hochstetler A, Schwerk C, Schroten H, & Blazer-Yost BL (2018). Activation of TRPV4 stimulates transepithelial ion flux in a porcine choroid plexus cell line. American Journal of Physiology - Cell Physiology, 315(3), C357–C366. 10.1152/ajpcell.00312.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Sun R, Mukherjee S, Nilius B, & Janssen LJ (2018). TRPV4 stimulation releases ATP via pannexin channels in human pulmonary fibroblasts. American Journal of Respiratory Cell and Molecular Biology, 59(1), 87–95. 10.1165/rcmb.2017-0413OC [DOI] [PubMed] [Google Scholar]
- Redmon SN, Yarishkin O, Lakk M, Jo A, Mustafić E, Tvrdik P, & Križaj D (2021). TRPV4 channels mediate the mechanoresponse in retinal microglia. Glia, 69(6), 1563–1582. 10.1002/glia.23979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, & Bringmann A (2010). Müller cells in the healthy and diseased retina. Müller Cells in the Healthy and Diseased Retina, 1–417. 10.1007/978-1-4419-1672-3 [DOI] [PubMed] [Google Scholar]
- Ríos MO, Imm RN, Godínez NMH, Cortes AMB, Escalante DDL, Liedtke W, Torres AM, Concha L, & Thébault S (2019). TRPV4 inhibition prevents increased water diffusion and blood-retina barrier breakdown in the retina of streptozotocin-induced diabetic mice. PLoS ONE, 14(5), e0212158. 10.1371/journal.pone.0212158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MWG, Sui G, Wu R, Rong W, Wildman S, Montgomery B, Ali A, Langley S, Ruggieri MR, & Wu C (2020). TRPV4 receptor as a functional sensory molecule in bladder urothelium: Stretch-independent, tissue-specific actions and pathological implications. FASEB Journal, 34(1), 263–286. 10.1096/fj.201900961RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Frye AM, Phuong TTT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY, & Križaj D (2016). TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Scientific Reports, 6(1), 1–15. 10.1038/srep30583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MaCaulay N, Thoreson WB, & Križaj D (2014). Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. Journal of Neuroscience, 34(47), 15689–15700. 10.1523/JNEUROSCI.2540-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Rentería RC, Liedtke W, & Križaj D (2011). The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. Journal of Neuroscience, 31(19), 7089–7101. 10.1523/JNEUROSCI.0359-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, & Dessy C (2008). Role of Caveolar Compartmentation in Endothelium-Derived Hyperpolarizing Factor–Mediated Relaxation. Circulation, 117(8), 1065–1074. 10.1161/CIRCULATIONAHA.107.731679 [DOI] [PubMed] [Google Scholar]
- Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, & Calkins DJ (2015). Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels, 9(2), 102–113. 10.1080/19336950.2015.1009272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin-Vences MR, Moroni M, Lewin GR, & Poole K (2017). Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. ELife, 6. 10.7554/eLife.21074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, & Delamere N (2022). Nitric Oxide Inhibits TRPV4-mediated Hemichannel Opening in Porcine Nonpigmented Ciliary Epithelium. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 36. 10.1096/fasebj.2022.36.S1.L7418 [DOI] [Google Scholar]
- Shahidullah M, Mandal A, & Delamere NA (2012). TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American Journal of Physiology - Cell Physiology, 302(12), C1751. 10.1152/ajpcell.00010.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, & Delamere NA (2015). Damage to lens fiber cells causes TRPV4-dependent Src family kinase activation in the epithelium. Experimental Eye Research, 140, 85–93. 10.1016/j.exer.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, & Ishizaki Y (2014). A novel subtype of astrocytes expressing TRPV4 (Transient Receptor Potential Vanilloid 4) Regulates neuronal excitability via release of gliotransmitters. Journal of Biological Chemistry, 289(21), 14470–14480. 10.1074/jbc.M114.557132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GB, & Garvin JL (2008). TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. American Journal of Physiology - Renal Physiology, 295(4). 10.1152/ajprenal.90365.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012. May 4;336(6081):597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, & Imai M (2003). Impaired pressure sensation in mice lacking TRPV4. Journal of Biological Chemistry, 278(25), 22664–22668. 10.1074/jbc.M302561200 [DOI] [PubMed] [Google Scholar]
- Taylor L, Arnér K, & Ghosh F (2017). Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Experimental Eye Research, 154, 10–21. 10.1016/j.exer.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Thébault S (2021). Minireview: Insights into the role of TRP channels in the retinal circulation and function. Neuroscience Letters, 765, 136285. 10.1016/j.neulet.2021.136285 [DOI] [PubMed] [Google Scholar]
- Thibodeau ML, Peters CH, Townsend KN, Shen Y, Hendson G, Adam S, Selby K, Macleod PM, Gershome C, Ruben P, Jones SJM, Friedman JM, Gibson WT, & Horvath GA (2017). Compound heterozygous TRPV4 mutations in two siblings with a complex phenotype including severe intellectual disability and neuropathy. American Journal of Medical Genetics, Part A, 173(11), 3087–3092. 10.1002/ajmg.a.38400 [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, DeMarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, … Westfall TD (2008). N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino} -3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2- carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urin. Journal of Pharmacology and Experimental Therapeutics, 326(2), 432–442. 10.1124/jpet.108.139295 [DOI] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, Križaj D, & MacAulay N (2017). When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. Journal of Physiology, 595(11), 3287–3302. 10.1113/JP274135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, Yarishkin O, Redmon S, Phuong TTT, Križaj D, & MacAulay N (2019). Volume sensing in the transient receptor potential vanilloid 4 ion channel is cell type-specific and mediated by an N-terminal volume-sensing domain. Journal of Biological Chemistry, 294(48), 18421–18434. 10.1074/jbc.RA119.011187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovsky EA, Braga A, Yu Y, Esteras N, Korsak A, Theparambil SM, Hadjihambi A, Hosford PS, Teschemacher AG, Marina N, Lythgoe MF, Haydon PG, & Gourine AV (2020). Mechanosensory signaling in astrocytes. Journal of Neuroscience, 40(49), 9364–9371. 10.1523/JNEUROSCI.1249-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, & Sharma SC (2016). Glia-neuron interactions in the mammalian retina. Progress in Retinal and Eye Research, 51, 1–40. 10.1016/j.preteyeres.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Villalta PC, Rocic P, & Townsley MI (2014). Role of MMP2 and MMP9 in TRPV4-induced lung injury. American Journal of Physiology - Lung Cellular and Molecular Physiology, 307(8), L652–L659. 10.1152/ajplung.00113.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J, Rajan S, Schremmer C, Chao YK, Krasteva-Christ G, Kannler M, Yildirim AÖ, Brosien M, Schredelseker J, Weissmann N, Grimm C, Gudermann T, Dietrich A. TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight. 2020. Oct 15;5(20):e134464. doi: 10.1172/jci.insight.134464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TI, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout REL, Votta BJ, … Xu X (2008). Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. Journal of Pharmacology and Experimental Therapeutics, 326(2), 443–452. 10.1124/jpet.107.134551 [DOI] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev. 2016. Jul;96(3):911–73. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Baumann JM, De Ieso ML, Vazquez-Chona F, Rudzitis CN, Sundberg C, Lakk M, Stamer WD, & Križaj D (2021). Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. Journal of Physiology, 599(2), 571–592. 10.1113/JP281011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M, Crandall A, Heurteaux C, Hartnett ME, & Križaj D (2018). TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. Journal of General Physiology, 150(12), 1660–1675. 10.1085/jgp.201812179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Lakk M, & Križaj D (2018). TRPV4 does not regulate the distal retinal light response. Advances in Experimental Medicine and Biology, 1074, 553–560. 10.1007/978-3-319-75402-4_67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Vazquez-Chona F, Bertrand J, van Battenburg-Sherwood J, Redmon SN, Rudzitis CN, Lakk M, Baumann JM, Freichel M, Hwang EM, Overby D, & Križaj D (2022). Emergent Temporal Signaling in Human Trabecular Meshwork Cells: Role of TRPV4-TRPM4 Interactions. Frontiers in Immunology, 13, 805076. 10.3389/fimmu.2022.805076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TL, Whisenhunt K, Martin S, & Tompson S (2022). PIEZO1 and PIEZO2 pathogenic variants identified in primary congenital glaucoma. Investigative Ophthalmology & Visual Science, 63(7), 1127–1127 [Google Scholar]
- Zhao Y, Huang H, Jiang Y, Wei H, Liu P, Wang W, Niu W. Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat. Eur J Histochem. 2012. Jul 24;56(3):e32. doi: 10.4081/ejh.2012.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]