Abstract
Background
Thoracentesis and thoracoscopy are used to diagnose malignant pleural effusions (MPE). Data on how sensitivity varies with tumour type is limited.
Methods
Systematic review using PubMed was performed through August 2020 to determine the sensitivity of thoracentesis and thoracoscopy for MPE secondary to malignancy, by cancer type, and complication rates. Tests to identify sources of heterogeneity were performed. Study quality was assessed using Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 and National Institutes of Health quality assessment tools. Publication bias was tested using funnel plots.
Results
Meta-analyses for sensitivity of thoracentesis for MPE secondary to malignancy, mesothelioma and lung and breast cancer included 29, eight, 12 and nine studies, respectively. Pooled sensitivities were 0.643 (95% CI 0.592–0.692), 0.451 (95% CI 0.249–0.661), 0.738 (95% CI 0.659–0.836) and 0.820 (95% CI 0.700–0.917), respectively. For sensitivity of thoracoscopy for MPE secondary to malignancy and mesothelioma, 41 and 15 studies were included, respectively. Pooled sensitivities were 0.929 (95% CI 0.905–0.95) and 0.915 (95% CI 0.871–0.952), respectively. Pooled complication rates of thoracentesis and thoracoscopy were 0.041 (95% CI 0.025–0.051) and 0.040 (95% CI 0.029–0.052), respectively. Heterogeneity was significant for all meta-analyses. Funnel plots were asymmetric.
Interpretation
Sensitivity of thoracentesis varied significantly per cancer type. Pooled complication rates were low. Awareness of how sensitivity of thoracentesis changes across cancers can improve decision-making when MPE is suspected.
Short abstract
Sensitivity of thoracentesis varies significantly per cancer type. Awareness of how sensitivity of thoracentesis changes across cancer types can improve decision-making in patients with suspected malignant pleural effusion. https://bit.ly/3bEiPYq
Introduction
Approximately 15% of patients with malignancy have malignant pleural effusion (MPE) [1, 2]. Currently, procedures to diagnose MPE include thoracentesis with pleural fluid cytology and thoracoscopy with pleural biopsy; the latter is often used as gold standard [3]. Accurate estimates of sensitivity of thoracoscopy and thoracentesis are clinically important because test sensitivity and complication rates drive decision-making on how to best manage MPEs [4].
We undertook this meta-analysis as part of a project to create a decision analysis model for diagnosis of MPE. Estimates of the sensitivity of thoracentesis and thoracoscopy for MPE by cancer type and complication rates were required. During the decision analysis process, we found that no meta-analysis had been published reporting the pooled sensitivity of thoracentesis for MPE [5]. Instead, the British Thoracic Society guidelines provide a mean sensitivity of thoracentesis for MPE for any malignancy of 0.6 (range 0.4–0.87) based on an analysis of four studies [6–9]. However, recent studies have shown that sensitivity of thoracentesis varies significantly between tumour types [10, 11]. For effective decision analysis, more rigorous estimates of the pooled sensitivity of thoracentesis are required, ideally stratified by cancer type.
Regarding thoracoscopy, a recent meta-analysis by Wei et al. [12] reported a pooled sensitivity of thoracoscopy for exudative pleural effusions, but did not report a pooled sensitivity for MPE and did not report sensitivity by cancer type. Instead, diagnostic accuracy of thoracoscopy for MPE and mesothelioma was reported [12]. Thus, we decided to perform meta-analyses on thoracentesis and thoracoscopy to provide updated estimates of their sensitivity for MPE stratified by cancer type when possible.
The primary objective of this study was to obtain a pooled sensitivity of thoracentesis and thoracoscopy for MPE due to any malignancy. Secondary analyses were performed to determine thoracentesis and thoracoscopy sensitivity for the following specific types of cancer: mesothelioma, lung cancer and breast cancer. Secondary objectives were to obtain the pooled complication rate of thoracentesis and thoracoscopy and the pooled pneumothorax rate of thoracentesis.
Methods
Literature search and study selection
We performed a systematic review and meta-analysis of thoracentesis and thoracoscopy following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis diagnostic test accuracy guidelines [13]. We pre-specified that a total of 161 studies (71 for thoracentesis and 90 for thoracoscopy; figure 1) included in the quantitative review have a sample size ≥10 patients with pleural effusions ultimately shown to be due to malignancy. We also pre-specified that only studies reporting results for ultrasound-guided thoracentesis with either diagnostic or both diagnostic and therapeutic purposes would be considered for pooled complication rate calculations. We specified that medical thoracoscopy, pleuroscopy and video-assisted thoracoscopic surgery would be grouped as one procedure type. Studies that used a substantially different technique (e.g. fibreoptic bronchoscope instead of a thoracoscope) were excluded. Only randomised controlled studies (RCTs) and cohort studies published in English were considered. Reviews, letters to the editor, abstracts, meta-analysis, case reports and studies without the full text available were excluded.
FIGURE 1.
Study selection algorithm. Results of search and study selection algorithm for sensitivity and complication rates of a) thoracentesis and b) thoracoscopy.
Searches were performed in July 2020 using the electronic MEDLINE database (PubMed) for sensitivity and complications of thoracentesis and thoracoscopy (supplementary table E1). A manual search of references cited in original investigations and review papers and cross-referencing was performed, to ensure that all articles were captured.
Two authors, S. Molina and G. Martinez-Zayas, independently and blindly performed the search strategies and screened the articles based on the inclusion and exclusion criteria described. The studies were first screened by title and then by abstract. Discordance was resolved by discussion and further review of the papers. Studies were selected for inclusion only after both reviewers assessed the full text.
Data extraction
S. Molina and G. Martinez-Zayas independently abstracted each study and then compared results of their data abstraction. In cases where there was discordance, further review was performed.
For studies reporting sensitivity, data was abstracted to record true positives, false positives, true negatives and false negatives. All thoracentesis cytology results that revealed cancer were considered true positives. Similarly, all thoracoscopy biopsies that revealed cancer were considered true positives. The reference standard of truth used to define false-negative results was recorded as well. For all studies, when patients were found to have MPE, the sensitivity stratified by cancer type was also recorded if information was available.
For studies reporting complications of thoracentesis, the types of complications were recorded. We specified that inadequate sampling, transient cough and mild chest pain would not be considered as complications. All other complications were grouped together as the complication rate. When specified, the frequency of pneumothorax was recorded separately. Because pneumothorax can occur after thoracentesis, but does not tend to do so immediately after thoracoscopy, and patients usually have chest tube placement after thoracoscopy, we conducted an exploratory analysis of thoracentesis complications other than pneumothorax. The thoracentesis complication rate excluding pneumothorax was used to facilitate comparison of complication rates between thoracentesis and thoracoscopy.
For studies on thoracoscopy, we specified that peri-procedural hypotension and respiratory difficulty, pain, dislodged drains, inadequate sampling, post-procedure fever that resolved in <72 h, additional chest tube use and minor bleeding would not be included as complications. The frequency of all other complications were grouped together as the complication rate.
Risk of bias within studies and study quality assessment
For studies reporting sensitivity of thoracoscopy or thoracentesis, assessment of within study bias was done using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 instrument (supplementary material) [14]. Studies were independently assessed by G. Martinez-Zayas and S. Molina. Results were compared and consensus was reached after discussion with adjudication by a third reviewer as needed. Because the QUADAS-2 tool can only be used for diagnostic studies, the National Institutes of Health (NIH) quality assessment tool was used for studies reporting complications [14, 15]. This tool can be used both for RCTs and observational studies (supplementary material).
If a study reported only sensitivity, we used QUADAS-2. If only complication rates were reported, we only used the NIH quality assessment tool. Finally, if both sensitivity and complication rates were reported, we used both tools.
Summary measures
The primary objective was to determine the sensitivity of thoracentesis and thoracoscopy for MPE due to any malignancy, defined as true positives/(true positives+false negatives). Secondary objectives included determining the complication and pneumothorax rates of thoracentesis, and complication rate of thoracoscopy.
For meta-analyses on sensitivity, extracted data were pooled using weighted averages based on the inverse of the variance. The 95% confidence intervals were calculated using the exact binomial method and the Freeman–Tukey double arcsine transformation of proportions using the “metaprop” command in Stata [16]. We used the “midas” command in Stata to generate summary receiver operating characteristic curves (sROCs) [17, 18]. Sensitivities were calculated per patient.
Given that many studies had zero complications, for meta-analyses on complications or pneumothorax we calculated the pooled estimate of the complication rate and pneumothorax rate after Freeman–Tukey double arcsine transformation and used the score statistic for determining confidence intervals. Complication rates were calculated per procedure.
For all statistical tests, we chose an α=0.05.
Synthesis of results
For all pooled estimate calculations, we expected to find significant heterogeneity, so random-effects models were used. We assessed heterogeneity using the I2 statistic and the heterogeneity Chi-squared. The I2 statistic describes the percentage of total variation across studies due to heterogeneity between studies rather than chance. A value >75% is considered as major heterogeneity and a value <40% is considered insufficient to prove heterogeneity [19]. For heterogeneity Chi, the null hypothesis is that there is no heterogeneity, therefore p<0.05 rejects the null hypothesis of no heterogeneity.
Causes of heterogeneity between studies were evaluated using stratified analysis for categorical variables and meta-regression for continuous variables. Pre-specified subgroups included year of study publication and prevalence of MPE. For studies on thoracoscopy, additional subgroups were type of thoracoscope (rigid versus semi-rigid versus mixed) and type of anaesthesia (general versus local with moderate sedation).
Publication bias and sample-size effects
We used funnel plot asymmetry to identify publication bias. For our primary analysis, we used funnel plots of sample size using a logarithmic scale versus the logit of sensitivity, based on previous work that suggests that this is better than standard funnel plots for meta-analysis of proportions [20]. Secondary analysis was done using standard funnel plots, plotting the inverse of standard error versus the logit of sensitivity. We used both the Egger and Begg tests to assess for publication bias, acknowledging that both tests have limitations and can produce different results [21–23]. We used the same methods for analysis of complication rates.
In addition, for meta-analyses on sensitivity we adjusted for publication bias using the “metatrim” command in Stata. This command uses the nonparametric trim and fill method proposed by Duval and Tweedie [24]. This method provides a revised pooled sensitivity after incorporating theoretical missing studies in the meta-analysis.
Outlier analysis
We pre-specified that if significant heterogeneity in meta-analyses for sensitivity was identified, a secondary analysis would be performed after removing outliers. Outliers were identified using Cook's distance and a scatter plot of the standardised predicted random effects (supplementary material, methods section).
All statistical analysis was performed using Stata version 15.1 (StataCorp LLC, College Station, TX, USA).
Results
Thoracentesis: study selection and characteristics
After screening, 71 studies were included in the meta-analysis of sensitivity and complications of thoracentesis (figure 1a). Out of the 71 studies, 32 reported results for sensitivity, 38 for complications, and one for both sensitivity and complications. Two studies [25, 26] were RCTs (supplementary tables E2 and E3).
Thoracentesis: risk of bias within studies and study quality assessment
For sensitivity, included studies had a low risk of bias in terms of the index test and selection criteria and study applicability was good (supplementary figure E1, supplementary table E4). The most frequently used reference standards were thoracoscopy, closed pleural biopsy, thoracotomy and clinical-radiographic surveillance. Only negative results by thoracentesis were compared to the reference standard (i.e. specificity was assumed to be 100%). For complications, most included studies were considered high or fair quality (supplementary figure E2, supplementary table E5).
Thoracentesis: results of individual studies and synthesis of results
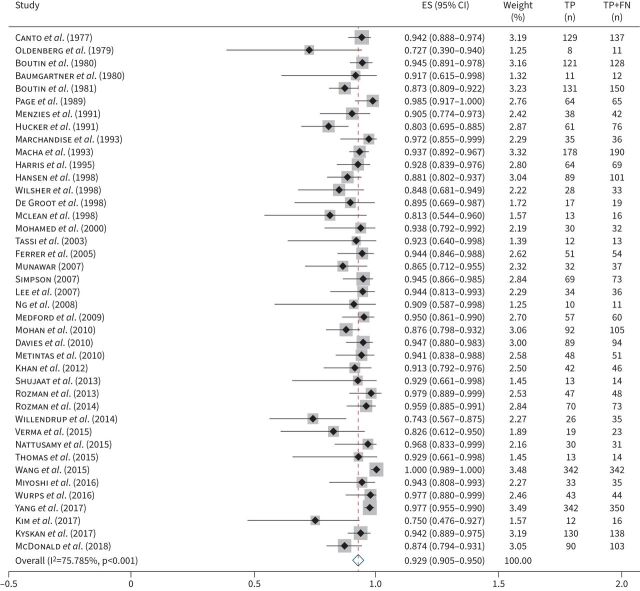
Pooled sensitivities are shown in table 1. 29 studies (5444 patients) were included in the meta-analysis for sensitivity of thoracentesis for MPE secondary to any malignancy (figure 2). The pooled sensitivity was 0.643 (95% CI 0.59–0.692).
TABLE 1.
Meta-analyses for sensitivity of thoracentesis and thoracoscopy
| Studies (n) | Patients in the studies (n) | Patients included in the sensitivity analysis (n) | Pooled sensitivity (95% CI) | I2 statistic# (%) | Within-strata heterogeneity p-value | sROC-AUC (95% CI) | Test of heterogeneity between groups¶ p-value | Meta-regression¶ p-value | |
| Thoracentesis | |||||||||
| Primary meta-analysis of sensitivity for MPE secondary to any malignancy | 29 | 11 952 | 5444 | 0.643 (0.592–0.692) | 92.302 | <0.001 | 0.85 (0.82–0.88) | ||
| Year of publication | 0.779 | ||||||||
| Prevalence of MPE | 0.820 | ||||||||
| Secondary meta-analysis of sensitivity for MPE secondary to mesothelioma | 8 | 8565 | 1133 | 0.451 (0.249–0.661) | 97.52 | <0.001 | 0.86 (0.82–0.89) | ||
| Secondary meta-analysis of sensitivity for MPE secondary to lung cancer | 12 | 6267 | 1184 | 0.738 (0.648–0.819) | 89.505 | <0.001 | 0.99 (0.97–0.99) | ||
| Secondary meta-analysis of sensitivity for MPE secondary to breast cancer | 9 | 5620 | 532 | 0.820 (0.700–0.917) | 88.668 | <0.001 | 1.0 (0.98–1.00) | ||
| Thoracoscopy | |||||||||
| Primary meta-analysis of sensitivity for MPE secondary to any malignancy | 41 | 5652 | 2963 | 0.929 (0.905–0.95) | 75.79 | <0.001 | 0.99 (0.98–1.00) | ||
| Year of publication | 0.510 | ||||||||
| Prevalence of MPE | 0.753 | ||||||||
| Thoracoscope used | 0.824 | ||||||||
| Rigid | 21 | 2666 | 0.929 (0.908–0.948) | 41.80 | 0.024 | ||||
| Semi-rigid | 15 | 2521 | 0.931 (0.88–0.971) | 84.88 | <0.001 | ||||
| Rigid and semi-rigid | 4 | 348 | 0.906 (0.819–0.969) | 63.90 | 0.040 | ||||
| Anaesthesia used | 0.928 | ||||||||
| General anaesthesia | 3 | 240 | 0.917(0.735–1.00) | <0.001 | |||||
| Local anaesthesia | 4 | 568 | 0.931 (0.905–0.954) | 76.34 | <0.001 | ||||
| General and local anaesthesia | 34 | 4844 | 0.920 (0.879–0.954) | 28.89 | <0.001 | ||||
| Secondary meta-analysis of sensitivity for MPE secondary to mesothelioma | 15 | 1400 | 453 | 0.915 (0.871–0.952) | 48.79 | 0.017 | 0.90 (0.94–0.97 | ||
| Secondary meta-analysis of sensitivity for MPE secondary to lung cancer+ | 1 | 48 | |||||||
| Secondary meta-analysis of sensitivity for MPE secondary to breast cancer+ |
Sensitivities are reported by patient. sROC: summary receiver operating characteristic; AUC: area under the curve; MPE: malignant pleural effusions. #: the I2 statistic describes the percentage of total variation across studies due to heterogeneity between studies rather than chance. A value >75% is considered as major heterogeneity and a value <40% is considered insufficient to prove heterogeneity [19]; ¶: causes of heterogeneity between groups and meta-regressions are only reported for the combined analysis given the very few studies included in the stratified analysis that limit the power of the tests; +: a pooled estimate could not be calculated given that only one study with >10 patients with MPE reported sensitivity of thoracoscopy for MPE secondary to lung cancer and breast cancer.
FIGURE 2.
Forest plot: sensitivity of thoracentesis. Pooled estimate for sensitivity (ES) of thoracentesis for malignant pleural effusion secondary to any malignancy. TP: true positives; FN: false negatives.
In stratified analyses, eight studies (1133 patients) were included in the meta-analysis for sensitivity for MPE secondary to mesothelioma (figure 3a). The pooled sensitivity was 0.451 (95% CI 0.249–0.661). Stratified analysis of sensitivity for MPE secondary to lung cancer included 12 studies (1184 patients). The pooled sensitivity was 0.752 (95% CI 0.659–0.836; figure 3b). Finally, stratified analysis of sensitivity for MPE secondary to breast cancer included eight studies (532 patients). The pooled sensitivity was 0.820 (95% CI 0.700–0.917; figure 3c). For all meta-analyses, heterogeneity was significant (table 1) and the sROCs are shown in supplementary figure E3. We failed to find an association between sensitivity and year of publication or cancer prevalence (table 1).
FIGURE 3.
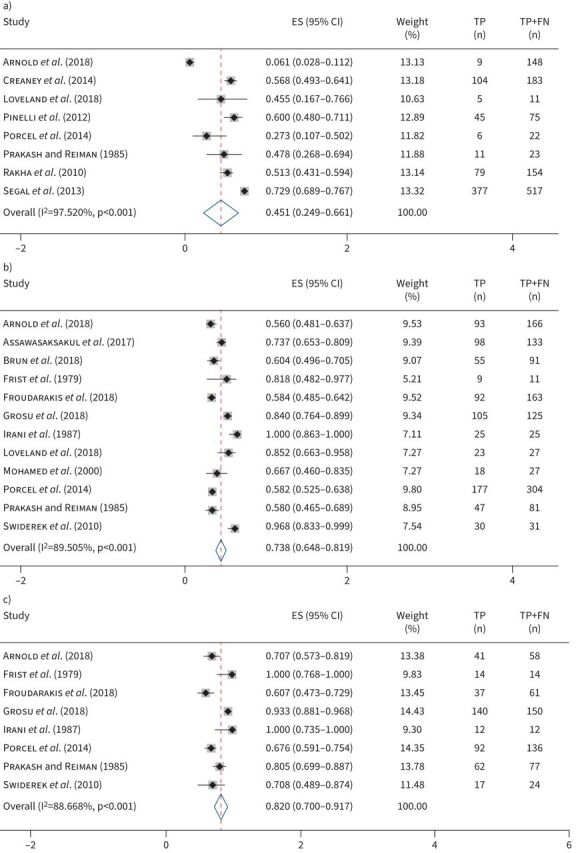
Forest plots: sensitivity of thoracentesis by cancer type. Sensitivity of thoracentesis for malignant pleural effusion secondary to a) mesothelioma, b) lung cancer and c) breast cancer. ES: estimated sensitivity; TP: true positives; FN: false negatives.
Complications included pneumothorax, re-expansion pulmonary oedema and haemothorax, among others (supplementary table E6). 23 studies were included in the meta-analyses for complication rate. The pooled complication rate was 0.041 (95% CI 0.025–0.061; supplementary figure E4). Similarly, 36 studies were included in the meta-analyses for pneumothorax rate. The pooled estimate was 0.025 (95% CI 0.017–0.034; supplementary figure E5A). Finally, the pooled estimate of complications other than pneumothorax (supplementary table E6) was 0.015 (95% CI 0.009–0.023; supplementary figure E5B). Heterogeneity was significant in all meta-analyses (table 1). We failed to find an association between complication rate and year of publication or cancer prevalence (table 1).
Thoracentesis: publication bias and sample size effects
Funnel plots demonstrated asymmetry across studies that reported the sensitivity of thoracentesis for MPE secondary to any malignancy (supplementary figure E6). Interestingly, funnel plots seemed to suggest that small studies reporting high sensitivity were less likely to be published than small studies with low sensitivity. After trim and fill, the bias-adjusted pooled sensitivity of thoracentesis was 0.63 (95% CI 0.582–0.676; supplementary figure E7, supplementary table E7). Funnel plots for complication and pneumothorax rates are discussed in the supplementary material, results section.
The Begg and Egger tests for publication bias were nonsignificant (p=0.561 and p=0.374, respectively; supplementary table E8). There were too few studies in the stratified analyses to perform meaningful funnel plot analysis and the Begg and Egger tests. Begg and Egger test for complication and pneumothorax rates are discussed in the supplementary material, results section.
Thoracentesis: outlier analysis
The outlier analysis identified six [9, 27–31] outliers. Removing them did not significantly change the pooled sensitivity for any malignancy, within strata heterogeneity p-value or I2 (supplementary figure E8, supplementary table E9). Funnel plot asymmetry and significance of the Begg and Egger tests did not change either (supplementary figure E8 and supplementary table E8). For the stratified analysis, removing one [11] identified outlier did not significantly change the pooled sensitivity for mesothelioma, heterogeneity p-value or I2 (supplementary figure E10, supplementary table E9). No outliers were identified for lung or breast cancer.
Thoracoscopy: study selection and characteristics
90 studies were included in the meta-analysis for complications and sensitivity of thoracoscopy (figure 1b). Out of the 90 studies, 11 reported results for sensitivity, 47 for complications and 32 for both sensitivity and complications. There was variation on the type of thoracoscope used across studies (supplementary table E10 and E11). Seven studies [32–38] were RCTs.
Thoracoscopy: risk of bias within studies and study quality assessment
For sensitivity, included studies had a low risk of bias in terms of the index test and selection criteria and study applicability was good (supplementary figure E1, supplementary table E12). The most frequently used reference standards were thoracotomy, computed tomography guided biopsy, autopsy, repeat thoracoscopy and clinical-radiographic follow-up. Except for two studies [39, 40], only negative results were compared to the reference standard (i.e. specificity was assumed to be 100%). For complications, most included studies were considered high or fair quality (supplementary figure E2, supplementary table E13).
Thoracoscopy: results of individual studies and synthesis of results
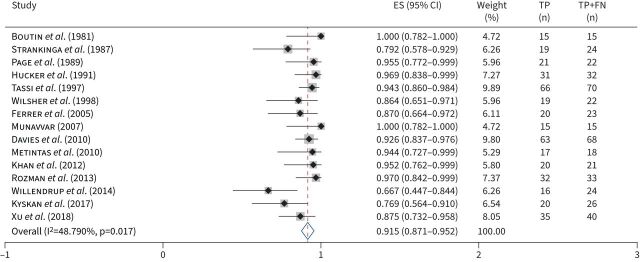
41 studies (2963 patients) were included in the meta-analysis for sensitivity for MPE due to any malignancy. The pooled sensitivity was 0.929 (95% CI 0.905–0.95; figure 4). In the stratified analysis, 15 studies (453 patients) were included in the meta-analysis for sensitivity for MPE secondary to mesothelioma. The pooled sensitivity was 0.915 (95% CI 0.871–0.952; figure 5). Heterogeneity was significant in both meta-analyses (table 2) and the sROCs are shown in supplementary figure E11. Only one study provided stratified results for lung cancer and no studies reported stratified results for breast cancer, so meta-analyses were not performed. We failed to find an association between potential causes of heterogeneity and the sensitivity of thoracoscopy (table 2).
FIGURE 4.
Forest plot: sensitivity of thoracoscopy for malignant pleural effusion secondary to any malignancy. ES: estimated sensitivity; TP: true positives; FN: false negatives.
FIGURE 5.
Forest plot: sensitivity of thoracoscopy by cancer type. Sensitivity of thoracoscopy for malignant pleural effusion secondary to mesothelioma. ES: estimated sensitivity; TP: true positives; FN: false negatives.
TABLE 2.
Meta-analysis results for complication rate of thoracentesis and thoracoscopy, and pneumothorax rate of thoracentesis
| Studies (n) | Procedures (n) | Pool-complication rate (95% CI) | I2 statistic (%)# | Within-study heterogeneity p-value | Test of heterogeneity within groups (p-value) | Meta-regression p-value | |
| Thoracentesis | |||||||
| Complication rate | 23 | 32863 | 0.041 (0.025–0.061) | 97.318 | <0.001 | ||
| Year of publication | 0.174 | ||||||
| Pneumothorax rate | 36 | 66359 | 0.025 (0.017–0.034) | 96.070 | <0.001 | ||
| Year of publication | 0.855 | ||||||
| Complications other than pneumothorax | 22 | 32578 | 0.015 (0.009–0.023) | 89.55 | <0.001 | ||
| Year of publication | 0.137 | ||||||
| Thoracoscopy | |||||||
| Complication rate per procedure | 79 | 15139 | 0.040 (0.029–0.052) | 85.910 | <0.001 | ||
| Year of publication | 0.116 | ||||||
| Thoracoscope used | 0.992 | ||||||
| Rigid | 33 | 2862 | 0.042 (0.023–0.066) | 83.86 | <0.001 | ||
| Semi-rigid | 30 | 3231 | 0.040 (0.020–0.064) | 84.79 | <0.001 | ||
| Rigid and semi-rigid | 9 | 1831 | 0.043 (0.01–0.091) | 92.29 | <0.001 | ||
| Anaesthesia used | 0.150 | ||||||
| General anaesthesia | 12 | 969 | 0.016 (0.001–0.042) | 73.87 | <0.001 | ||
| Local anaesthesia | 63 | 7258 | 0.045 (0.031–0.061) | 86.00 | <0.001 | ||
| General and local anaesthesia | 2 | 211 | 0.054 (0.025–0.090) | <0.001 | |||
All complications are reported by procedure. Meta-regression on prevalence was not made given that most studies reporting complications did not report results on sensitivity and prevalence of malignant pleural effusion. #: the I2 statistic describes the percentage of total variation across studies due to heterogeneity between studies rather than chance. A value >75% is considered as major heterogeneity and a value <40% is considered insufficient to prove heterogeneity [19].
Examples of complications of thoracoscopy that were included are persistent fluid leakage after 24 h, trapped lung and re-expansion pulmonary oedema among others (supplementary table E6). 79 studies were included in the meta-analysis for complication rate of thoracoscopy. The pooled complication rate was 0.040 (95% CI 0.029–0.052; supplementary figure E12). Heterogeneity was significant (table 2). We failed to find an association between potential causes of heterogeneity and the complication rate of thoracoscopy (table 2).
Thoracoscopy: publication bias and sample size effects
We observed funnel plot asymmetry for sensitivity for any malignancy, with plots suggesting that smaller studies with lower sensitivities were less likely to be published (supplementary figure E13). The Begg and Egger tests results were nonsignificant (p=0.545 and p=0.061, respectively; supplementary table E8). After trim and fill, the bias adjusted pooled sensitivity of thoracoscopy for MPE secondary to any malignancy decreased to 0.889 (95% CI 0.86–0.91; supplementary figure E14). There were too few studies in the stratified analyses to perform meaningful funnel plot analysis or for the Begg and Egger tests. Funnel plots, Begg and Egger tests for complication rates of thoracoscopy are discussed in the supplementary material results section.
Thoracoscopy: outlier analysis
Outlier analysis identified two [41, 42] outliers. Removing them did not significantly modify the pooled sensitivity for any malignancy, within strata heterogeneity p-value or the I2 (supplementary figure E15, supplementary table E9). Funnel plot asymmetry and significance of the Begg test did not change either (supplementary figure E16 and supplementary table E8, respectively). However, the Egger test became significant (p=0.016), suggesting that removing the outlier introduced publication bias.
For the stratified analysis, removing one [43] identified outlier slightly modified the pooled sensitivity for mesothelioma but changed significance of the heterogeneity p-value, and I2 (supplementary figure E17, supplementary table E9). The Begg and Egger tests were not performed for the stratified analysis, so modification after removing outliers was not assessed.
Discussion
In this meta-analysis, we report the pooled sensitivities of thoracentesis and thoracoscopy for MPE secondary to any type of malignancy and per cancer type, as well as the pooled complication rates of thoracentesis and thoracoscopy, and pneumothorax rate of thoracentesis. The pooled sensitivities of thoracentesis for MPE secondary to any malignancy, mesothelioma, lung cancer and breast cancer were 0.643 (95% CI 0.592–0.692), 0.451 (95% CI 0.249–0.661), 0.738 (95% CI 0.659–0.836) and 0.820 (95% CI 0.700–0.917), respectively. The pooled sensitivities of thoracoscopy for MPE secondary to any malignancy and mesothelioma were 0.929 (95% CI 0.905–0.95) and 0.915 (95% CI 0.871–0.952), respectively. The pooled complication rate of thoracentesis was 0.041 (95% CI 0.025–0.051). The pooled pneumothorax rate was 0.025 (95% CI 0.017–0.034). Finally, the pooled complication rate of thoracoscopy was 0.040 (95% CI 0.029–0.052).
To our knowledge, this is the largest meta-analysis on sensitivity of thoracentesis. Various studies have reported the combined sensitivity of thoracentesis for MPE [6–8, 44, 45], but a meta-analysis reporting the pooled sensitivity of thoracentesis for MPE stratified per cancer type has not been reported. More recently, studies have reported the sensitivity of thoracentesis for MPE varies according to tumour type [10, 11]. For instance, Grosu et al. [10] reported that the sensitivity of thoracentesis for MPE secondary to mesothelioma and breast cancer were 0.0 (95% CI 0.0–0.7) and 0.93 (95% CI 0.88–0.97), respectively. Similarly, the sensitivity of thoracentesis for lung cancer was stratified per lung cancer histology, with sensitivities ranging from 0.69 (95% CI 0.39–0.90) to 0.92 (95% CI 0.78–0.98), depending on lung cancer histology [10]. Arnold et al. [11] reported sensitivities of 0.06 (95% CI 0.028–0.11), 0.56 (95% CI 0.48–0.637) and 0.707 (95% CI 0.57–0.82) for mesothelioma, lung cancer and breast cancer, respectively. However, both studies were single-centre studies with relatively modest sample sizes (mesothelioma n=5 and n=148, lung cancer n=158 and n=166 and breast cancer n=165 and n=58, respectively). This meta-analysis builds on and extends their observations by pooling data from many studies. The resulting sample size is much larger (mesothelioma n=8565, n=1133 included in sensitivity analysis; lung cancer n=6267, n=1184 included in the sensitivity analysis; and breast cancer n=5620, n=532 included in the sensitivity analysis) and is drawn from a wide range of institutions.
Knowledge of strata-specific sensitivities of thoracentesis for MPE by cancer type adds to the existing body of knowledge because it can be used to tailor the diagnostic approach to individual patients. For example, older guidelines suggested repeating thoracentesis up to three times before progressing to thoracoscopy in patients with suspected MPE [46, 47]. Recent guidelines suggest performing one thoracentesis, and if nondiagnostic to progress directly to thoracoscopy [6, 48, 49]. However, both old and new guidelines did not consider that the sensitivity of thoracentesis varies by cancer type. Here, we confirmed the findings of prior studies that the sensitivity of thoracentesis for MPE is indeed very low for mesothelioma. In addition, by pooling the results from many studies, we were able to confirm the preliminary findings of Grosu et al. [10] and Arnold et al. [11] that sensitivity of thoracentesis varies significantly for other cancer types, being intermediate for lung cancer and high for breast cancer. Our study suggests that rather than a one-size-fits-all approach to patients with suspected MPE, a more nuanced approach is warranted. For instance, in patients with high pre-test probability of mesothelioma (e.g. asbestos exposure, chest pain, pleural plaques and thickening) presenting with a pleural effusion, proceeding directly to thoracoscopy is reasonable given the low sensitivity of thoracentesis for mesothelioma and relatively low complication rate of thoracoscopy [50]. Conversely, in a patient with known breast cancer presenting with a large pleural effusion without evidence of infection or heart failure, chances are that the effusion is secondary to breast cancer. In this case, given the high sensitivity of thoracentesis for breast cancer MPE and because thoracentesis is less invasive than thoracoscopy, two or more thoracenteses might be warranted prior to proceeding to thoracoscopy. For patients with known lung cancer presenting with a large pleural effusion, moderate sensitivity of thoracentesis suggests that consistent with current guidelines, a single thoracentesis may be worthwhile, and if that is nondiagnostic proceeding to thoracoscopy would be an appropriate next step. Decision-making could be further guided if the histology of the lung cancer is known [10].
Regarding complications of thoracentesis, the most recent meta-analysis in 2010 by Gordon et al. [51] reported a pooled pneumothorax rate slightly higher than ours (0.04, 95% CI 0.029–0.056), but had fewer studies (n=16) and included studies that did not use ultrasound guidance. Ultrasound guidance for thoracentesis is now standard, so we limited our meta-analysis to studies using ultrasound so that our estimate of pneumothorax rates and complications would be more applicable to current practice.
In addition, our study is the first meta-analysis to report the pooled sensitivity of thoracoscopy. A recent meta-analysis by Wei et al. [12] calculated the pooled sensitivity of thoracoscopy for exudative pleural effusions in 1783 patients. However, all aetiologies of exudative effusions (i.e. cancer and infection) were grouped together and not stratified by cancer type. Their study did report the diagnostic accuracy of thoracoscopy for MPE secondary to any malignancy (0.92, 95% CI 0.88–0.95) and mesothelioma (0.42, 95% CI 0.22–0.62%), but did not report sensitivities. Another meta-analysis evaluating the sensitivity of thoracoscopy for exudative effusions identified 17 studies in the literature through 2013 [52]. It reported a pooled sensitivity of 0.91 (95% CI 0.89–0.93) [52]. This meta-analysis was also for exudative pleural effusions and grouped all aetiologies together without stratifying by cancer type.
Finally, regarding complications of thoracoscopy, Wei et al. [12] reported a complication rate higher than ours (0.08, 95% CI, 0.06–0.11), probably because they included all complications reported in all studies when calculating their pooled estimate. But different studies used different definitions of what constituted a complication, including some that are not broadly recognised as complications (e.g. inadequate sample) and some that are generally considered as unavoidable (e.g. pain). A list of the types of complications they counted was not provided. To address the issue of varying definitions, we compared the complications listed in different studies at a granular level, eliminating those complications that were not consistently reported and those that were nonstandard (supplementary table E6).
There are multiple causes of funnel plot asymmetry such as publication bias, under-publication of negative studies, conflicts of interest, inadequate design and analysis, among others [53–55]. When publication bias is related to size effects, the small studies reporting lower sensitivities are missing from the funnel plots [56–58], as observed in our funnel plots for sensitivity of thoracoscopy. However, our funnel plots for thoracentesis showed that small studies reporting larger sensitivities seemed more likely to be missing, suggesting the presence of sample size effects unrelated to publication bias. In other words, the asymmetry observed in our funnel plots could be related to other unassessed causes [53–55]. Note that we failed to find significance in the Begg and Egger tests. However, for small meta-analyses such as those used in typical medical research (and in our study, publication), bias should not be ruled out when the tests are not significant [22, 23, 55].
It is important to be cognisant of the limitations of our study. First, we failed to obtain sufficient studies reporting sensitivity of thoracoscopy for MPE secondary to lung or breast cancer and therefore could not obtain cancer-specific sensitivities for thoracoscopy. Another limitation is that very few studies [9–11, 59] reported sensitivities of thoracentesis for MPE secondary to malignancies other than mesothelioma, lung or breast cancer. While our approach highlights the potential for a more individualised approach to the evaluation of pleural effusion, additional data on thoracentesis sensitivity for many other cancer types are required. It is also important to acknowledge that most of the studies included in the meta-analyses for sensitivity for mesothelioma were published before studies reporting that BAP1 immunostaining and CDKN2A increased sensitivity for mesothelioma were published [60, 61]. Therefore, in future with more studies using BAP1 and CDKN2A, the pooled sensitivities for mesothelioma may vary. Similarly, we did not perform a subgroup analysis for sensitivity of thoracentesis for mesothelioma using cell block techniques, which have been reported to increase the pooled sensitivity for mesothelioma [62] and other metastatic cancers [63]. Unfortunately, much of the literature does not specify sensitivity according to whether cell blocks were used in individual cases, so a more refined subgroup analysis is not possible at this time. This type of subgroup analysis can be addressed in future studies. Finally, there was significant heterogeneity between studies; however, the relatively low number of studies limited our ability to identify factors that might be explain the observed heterogeneity. Therefore, the stratified results and meta-regression analysis should be viewed as exploratory. It is possible differences in the frequency of various cancer types between studies (e.g. Grosu et al. [10] versus Arnold et al. [11]) could account for the observed heterogeneity, but the data in the original reports do not permit a more detailed analysis.
In conclusion, we found a pooled sensitivity of thoracentesis for malignancy of 0.643 and a pooled sensitivity of thoracoscopy for malignancy of 0.929, although there was significant heterogeneity between studies. Sensitivity of thoracentesis was lowest for mesothelioma, intermediate for lung cancer and highest for breast cancer. Pooled complication rates for both procedures were low. Awareness of how sensitivity of thoracentesis varies across different cancer types can inform decision making in patients with suspected MPE, allowing physicians to individualise diagnostic strategy in a more effective manner.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0053-2022.SUPPLEMENT (3.2MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflicts of interest: G. Martinez-Zayas, S. Molina and D.E. Ost have no conflicts of interest to disclose and take full responsibility for this paper.
References
- 1.Clive AO, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016; 2016: CD010529. doi: 10.1002/14651858.CD010529.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018; 52: 1800349. doi: 10.1183/13993003.00349-2018 [DOI] [PubMed] [Google Scholar]
- 3.Desai NR, Lee HJ. Diagnosis and management of malignant pleural effusions: state of the art in 2017. J Thorac Dis 2017; 9: Suppl. 10, S1111–S1122. doi: 10.21037/jtd.2017.07.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vetter TR, Schober P, Mascha EJ. Diagnostic testing and decision-making: beauty is not just in the eye of the beholder. Anesth Analg 2018; 127: 1085–1091. doi: 10.1213/ANE.0000000000003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han SB, Kim DK. Cytogenetic study in suspicious cases of malignant pleural effusion. Cancer Res Treat 2002; 34: 234–238. doi: 10.4143/crt.2002.34.3.234 [DOI] [PubMed] [Google Scholar]
- 6.Hooper C, Lee YCG, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65: Suppl. 2, ii4–ii17. [DOI] [PubMed] [Google Scholar]
- 7.Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest 1975; 67: 536–539. doi: 10.1378/chest.67.5.536 [DOI] [PubMed] [Google Scholar]
- 8.Nance KV, Shermer RW, Askin FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 1991; 4: 320–324. [PubMed] [Google Scholar]
- 9.Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985; 60: 158–164. doi: 10.1016/S0025-6196(12)60212-2 [DOI] [PubMed] [Google Scholar]
- 10.Grosu HB, Kazzaz F, Vakil E, et al. Sensitivity of initial thoracentesis for malignant pleural effusion stratified by tumor type in patients with strong evidence of metastatic disease. Respiration 2018; 96: 363–369. doi: 10.1159/000490732 [DOI] [PubMed] [Google Scholar]
- 11.Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 2018; 52: 1801254. doi: 10.1183/13993003.01254-2018 [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Shen K, Lv T, et al. Comparison between closed pleural biopsy and medical thoracoscopy for the diagnosis of undiagnosed exudative pleural effusions: a systematic review and meta-analysis. Transl Lung Cancer Res 2020; 9: 446–458. doi: 10.21037/tlcr.2020.03.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15.National Heart, Lung, and Blood Institute . Study Quality Assessment Tools: Quality Assessment of Controlled Intervention Studies. www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Date last updated: July 2021.
- 16.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbord RM, Whiting P. Metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009; 9: 211–229. doi: 10.1177/1536867X0900900203 [DOI] [Google Scholar]
- 18.Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. 2007. https://EconPapers.repec.org/RePEc:boc:bocode:s456880 Date last updated: 5 February 2009.
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014: 897–903. doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med 2001; 20: 641–654. doi: 10.1002/sim.698 [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 25.Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 1990; 150: 873–877. doi: 10.1001/archinte.1990.00390160119023 [DOI] [PubMed] [Google Scholar]
- 26.Perazzo A, Gatto P, Barlascini C, et al. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol 2014; 40: 6–12. doi: 10.1590/S1806-37132014000100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frist B, Kahan AV, Koss LG. Comparison of the diagnostic values of biopsies of the pleura and cytologic evaluation of pleural fluids. Am J Clin Pathol 1979; 72: 48–51. doi: 10.1093/ajcp/72.1.48 [DOI] [PubMed] [Google Scholar]
- 28.Irani DR, Underwood RD, Johnson EH, et al. Malignant pleural effusions. A clinical cytopathologic study. Arch Intern Med 1987; 147: 1133–1136. doi: 10.1001/archinte.1987.00370060129021 [DOI] [PubMed] [Google Scholar]
- 29.Froudarakis ME, Plojoux J, Kaspi E, et al. Positive pleural cytology is an indicator for visceral pleural invasion in metastatic pleural effusions. Clin Respir J 2018; 12: 1011–1016. doi: 10.1111/crj.12619 [DOI] [PubMed] [Google Scholar]
- 30.Aksoy E, Ataç G, Sevim T, et al. Diagnostic yield of closed pleural brushing. Tuberk Toraks 2005; 53: 238–244. [PubMed] [Google Scholar]
- 31.Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015; 70: 995–997. doi: 10.1136/thoraxjnl-2014-206567 [DOI] [PubMed] [Google Scholar]
- 32.Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010; 137: 1362–1368. doi: 10.1378/chest.09-0884 [DOI] [PubMed] [Google Scholar]
- 33.Khan MA, Ambalavanan S, Thomson D, et al. A comparison of the diagnostic yield of rigid and semirigid thoracoscopes. J Bronchology Interv Pulmonol 2012; 19: 98–101. doi: 10.1097/LBR.0b013e31824ee45b [DOI] [PubMed] [Google Scholar]
- 34.Khan FY. Ascites in the state of Qatar: aetiology and diagnostic value of ascitic fluid analysis. Singapore Med J 2007; 48: 434–439. [PubMed] [Google Scholar]
- 35.Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014; 59: 756–764. doi: 10.4187/respcare.02738 [DOI] [PubMed] [Google Scholar]
- 36.Dhooria S, Bal A, Sehgal IS, et al. Pleural cryobiopsy versus flexible forceps biopsy in subjects with undiagnosed exudative pleural effusions undergoing semirigid thoracoscopy: a crossover randomized trial (COFFEE trial). Respiration 2019; 98: 133–141. doi: 10.1159/000497212 [DOI] [PubMed] [Google Scholar]
- 37.Haridas N, Suraj KP, Rajagopal TP, et al. Medical thoracoscopy vs closed pleural biopsy in pleural effusions: a randomized controlled study. J Clin Diagn Res 2014; 8: MC01–MC04. doi: 10.1111/crj.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beheshtirouy S, Kakaei F, Mirzaaghazadeh M. Video assisted rigid thoracoscopy in the diagnosis of unexplained exudative pleural effusion. J Cardiovasc Thorac Res 2013; 5: 87–90. doi: 10.5681/jcvtr.2013.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen M, Faurschou P, Clementsen P. Medical thoracoscopy, results and complications in 146 patients: a retrospective study. Respir Med 1998; 92: 228–232. doi: 10.1016/S0954-6111(98)90100-7 [DOI] [PubMed] [Google Scholar]
- 40.Page RD, Jeffrey RR, Donnelly RJ. Thoracoscopy: a review of 121 consecutive surgical procedures. Ann Thorac Surg 1989; 48: 66–68. doi: 10.1016/0003-4975(89)90179-3 [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Wu YB, Wang Z, et al. Long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. Respir Med 2017; 124: 1–5. doi: 10.1016/j.rmed.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 42.Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration 2015; 90: 251–255. doi: 10.1159/000435962 [DOI] [PubMed] [Google Scholar]
- 43.Willendrup F, Bodtger U, Colella S, et al. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions in Denmark. J Bronchology Interv Pulmonol 2014; 21: 215–219. doi: 10.1097/LBR.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 44.Bielsa S, Panadés MJ, Egido R, et al. Rentabilidad del estudio citológico del líquido pleural en el derrame malign. [Accuracy of pleural fluid cytology in malignant effusions]. An Med Interna 2008; 25: 173–177. doi: 10.4321/s0212-71992008000400005 [DOI] [PubMed] [Google Scholar]
- 45.Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2018; 142: 89–108. doi: 10.5858/arpa.2017-0124-RA [DOI] [PubMed] [Google Scholar]
- 46.Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003; 58: Suppl. 2. ii8–ii17. doi: 10.1136/thx.58.suppl_2.ii8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolowski JW Jr, Burgher LW, Jones FL Jr, et al. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis 1989; 140: 257–258. doi: 10.1164/ajrccm/140.1.257 [DOI] [PubMed] [Google Scholar]
- 48.American Thoracic Society . Management of malignant pleural effusions. Am J Respir Crit Care Med 2000; 162: 1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00 [DOI] [PubMed] [Google Scholar]
- 49.Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018; 198: 839–849. doi: 10.1164/rccm.201807-1415ST [DOI] [PubMed] [Google Scholar]
- 50.Tsim S, Paterson S, Cartwright D, et al. Baseline predictors of negative and incomplete pleural cytology in patients with suspected pleural malignancy – data supporting ‘direct to LAT' in selected groups. Lung Cancer 2019; 133: 123–129. doi: 10.1016/j.lungcan.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 51.Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170: 332–339. doi: 10.1001/archinternmed.2009.548 [DOI] [PubMed] [Google Scholar]
- 52.Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013; 144: 1857–1867. doi: 10.1378/chest.13-1187 [DOI] [PubMed] [Google Scholar]
- 53.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 2000; 53: 207–216. doi: 10.1016/S0895-4356(99)00161-4 [DOI] [PubMed] [Google Scholar]
- 54.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999; 282: 1061–1066. doi: 10.1001/jama.282.11.1061 [DOI] [PubMed] [Google Scholar]
- 55.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1.0. 2020. https://training.cochrane.org/handbook/archive/v6.1 Date last updated: September 2020.
- 56.Moreno SG, Sutton AJ, Ades AE, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol 2009; 9: 2. doi: 10.1186/1471-2288-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–1129. doi: 10.1016/S0895-4356(00)00242-0 [DOI] [PubMed] [Google Scholar]
- 58.Sainz Zuñiga PV, Vakil E, Molina S, et al. Sensitivity of radial endobronchial ultrasound-guided bronchoscopy for lung cancer in patients with peripheral pulmonary lesions: an updated meta-analysis. Chest 2020; 157: 994–1011. doi: 10.1016/j.chest.2019.10.042 [DOI] [PubMed] [Google Scholar]
- 59.Assawasaksakul T, Boonsarngsuk V, Incharoen P. A comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion. J Thorac Dis 2017; 9: 3161–3167. doi: 10.21037/jtd.2017.08.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walts AE, Hiroshima K, McGregor SM, et al. BAP1 immunostain and CDKN2A (p16) FISH analysis: clinical applicability for the diagnosis of malignant mesothelioma in effusions. Diagn Cytopathol 2016; 44: 599–606. doi: 10.1002/dc.23491 [DOI] [PubMed] [Google Scholar]
- 61.Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015; 28: 1043–1057. doi: 10.1038/modpathol.2015.65 [DOI] [PubMed] [Google Scholar]
- 62.Segal A, Sterrett GF, Frost FA, et al. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20 year audit. Pathology 2013; 45: 44–48. doi: 10.1097/PAT.0b013e32835bc848 [DOI] [PubMed] [Google Scholar]
- 63.Porcel JM, Quirós M, Gatius S, et al. Examination of cytological smears and cell blocks of pleural fluid: complementary diagnostic value for malignant effusions. Rev Clin Esp 2017; 217: 144–148. doi: 10.1016/j.rce.2016.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0053-2022.SUPPLEMENT (3.2MB, pdf)