Abstract
The action of Shiga toxin (Stx) on the central nervous system was examined in rabbits. Intravenous Stx1 was 44 times more lethal than Stx2 and acted more rapidly than Stx2. However, Stx1 accumulated more slowly in the cerebrospinal fluid than did Stx2. Magnetic resonance imaging demonstrated a predominance of Stx1-dependent lesions in the spinal cord. Pretreatment of the animals with anti-Stx1 antiserum intravenously completely protected against both development of brain lesions and mortality.
In 1996, there were a total of 16 outbreaks of Shiga toxin (Stx)-producing Escherichia coli (STEC) infections in Japan. In Sakai City, the number of patients increased to 9,492, of which 7,889 were schoolchildren. Of these patients, 758 were hospitalized, 121 suffered from hemolytic uremic syndrome (HUS), and 11 children plus one adult died as a result of the infection (6a). Four of the 11 deaths were due to central nervous system (CNS) impairment. STEC produce one or more Stxs, such as Stx1, Stx2, and Stx2c, that are toxic to humans (4). Stx1 and Stx2 bind to a specific glycosphingolipid receptor, globotriaosylceramide (Gb3) (6), and are associated with hemorrhagic colitis (10), HUS (5), and neurological complications (11, 14). We previously reported that in mice Stx2c was toxic to both capillary endothelial cells and nerve fibers in the brain cortex and spinal cord following oral infection with STEC (3). Furthermore, using magnetic resonance imaging (MRI), we detected brain lesions of rabbits given purified Stx2 intravenously (1) and determined that the cause of death was a dysfunction of baroreflex (15). Impairment of the carotid sinus baroreflex control system in the brainstem leads to poor maintenance of blood pressure. In the present study, we have compared the action of Stx1 and Stx2 in the brain of rabbits exposed to the purified toxins and tested anti-Stx1 antibody as a protective agent in this model.
Determination of LD50 of intravenous Stx1 and Stx2 in rabbits.
Stx1 and Stx2 used in this study were purified to homogeneity as described previously (17) and determined to be free of detectable lipopolysaccharide (LPS) by toxicolor test and sodium dodecyl sulfate-polyacrylamide gel electrophoresis with silver staining. The cytotoxic potency of Stx1 and Stx2 was 105 and 106 50% cytotoxic doses (CD50)/μg of protein, respectively, as tested in Vero cells using the WST-1 cell proliferation assay (Chemicon International, Inc.) as described Yoh et al. (16). The titers of anti-Stx1 and anti-Stx2 antisera were examined by the Ouchterlony double gel diffusion test and in the cytotoxicity neutralization assay. In both assay systems, the Stx2 antiserum exhibited twice the potency of the Stx1 antiserum, 1:64 versus 1:32 and 1:6,400 versus 1:3,200, respectively.
The 50% lethal doses (LD50) of intravenous Stx1 and Stx2 were measured using male Japanese white rabbits weighing 2 kg each (Shizuoka Experimental Animals, Hamamatsu, Japan). To determine the LD50 of intravenous Stx1, 10 groups of five rabbits each were challenged intravenously with 2.4, 4.9, 9.8, 20, 39, 78, 156, or 313 ng of Stx1 per kg. Using the same protocol, rabbits were challenged with intravenous Stx2 at 313, 625, 1,250, or 2,500 ng/kg. These animals were monitored for 1 month. The LD50 of intravenous Stx1 and Stx2 for rabbits was calculated to be 20 and 884 ng/kg, respectively (Table 1). Thus, the LD50 of Stx2 was 43.8-fold higher than that of Stx1 (P = 0.0001 by analysis of variance).
TABLE 1.
Determination of LD50 of Stx1 and Stx2 for rabbits after an intravenous injection
| Stx | Dose (ng/kg) | No. of rabbits treated | No. of deaths | LD50 (ng/kg) |
|---|---|---|---|---|
| Stx1 | 313 | 5 | 5 | 20 |
| 156 | 5 | 4 | ||
| 78 | 5 | 4 | ||
| 39 | 5 | 3 | ||
| 19 | 5 | 2 | ||
| 9.8 | 5 | 0 | ||
| 4.9 | 5 | 2 | ||
| 2.4 | 5 | 0 | ||
| Stx2 | 2,500 | 5 | 5 | 884a |
| 1,250 | 5 | 3 | ||
| 625 | 5 | 2 | ||
| 313 | 5 | 0 |
Probit method. Potency ratio, 43.8.
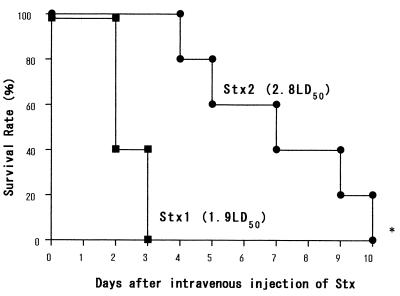
Survival curves of rabbits injected intravenously with either 1.9 LD50 (39 ng/kg) of Stx1 or 2.8 LD50 (2,500 ng/kg) of Stx2 are indicated in Fig. 1. The survival of the rabbits injected with Stx1 was significantly shorter than that of animals which received Stx2 (P = 0.023, log rank test) even though the LD50 of Stx1 administered was lower than that of Stx2. The mean survival days for Stx1 and Stx2 were 2.4 and 7.0, respectively.
FIG. 1.
Survival curves for rabbits injected with Stx1 or Stx2 intravenously. Two groups of rabbits were injected intravenously with 1.9 LD50 (39 ng/kg) of Stx1 or with 2.8 LD50 (2,500 ng/kg) of Stx2. The survival of the rabbits injected with Stx1 was significantly shorter than that of rabbits that received Stx2 (P = 0.023 by log rank test). The mean survival with Stx1 and Stx2 was 2.4 and 7.0 days, respectively.
To better understand why Stx1 is more toxic to rabbits than Stx2, we further examined the action of these toxins in vivo. The following data presented for our rabbit model help explain why Stx1 causes more CNS damage than Stx2 in rabbits. These results may also relate to the action of these toxins in humans as Stx2-producing STEC present a greater risk for development of acute renal failure and CNS abnormalities (8, 12, 13).
Measurement of Stx1 concentration in CSF of rabbits.
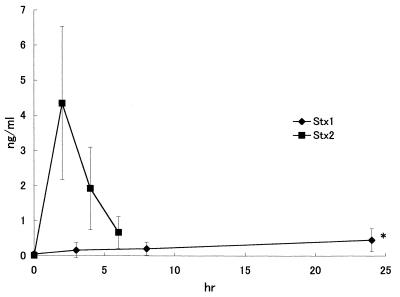
Ten rabbits were given Stx1 (2 μg/kg) intravenously. At 0, 3, 8, and 24 h after injection of Stx1, cerebrospinal fluid (CSF) was obtained from the cisterna magna. Stx1 concentration of the fluid was determined by enzyme immunoassay (EIA) using the Novapath Verotoxin EIA kit (Japan Bio-Rad Laboratories, Tokyo) with values derived from a linear standard curve prepared using purified Stx1. The concentrations of Stx1 in CSF of rabbits after an intravenous injection of Stx1 are shown in Fig. 2 and compared to our previous results obtained with Stx2 (2). The results show that Stx1 accumulates more slowly in the CSF than does Stx2, suggesting that Stx2 crossed the blood-brain barrier more readily than did Stx1 (3, 8). However, significantly higher levels of Stx1 were detected in CSF at 24 h compared with the time zero sample (P < 0.05 by t test). Whether this was due to more rapid endothelial transport of Stx2 or to selective destruction of the endothelial layer by Stx2 has not been determined. Stx2 not only entered the CSF more readily, it also appeared to be more potent than Stx1 within this site. Indeed, intrathecally injected Stx2 was more potent than intrathecal Stx1 based on the LD50s of intravenous versus intrathecal Stx2, 3.4 versus 0.36 (μg/kg) (2). In support of this concept, lethality data from the present study also revealed some important properties of Stx1, as it was clearly demonstrated that intravenously presented Stx1 was more than five times more potent than intrathecally administered Stx1 based on LD50 values of 20 and >100 ng/kg, respectively. Together, the data suggest that the primary damage caused by Stx1 takes place outside of the CNS, such as large intestine, because the Stx1 toxemic rabbits showed hemorrhagic diarrhea. Stx2 toxemic rabbits also developed hemorrhagic diarrhea, but in the Stx2 toxemic rabbits, Stx2 in CSF increased rapidly and reached a maximum 2 h after Stx2 injection (Fig. 2). This suggests that Stx2 may accumulate in rabbit CNS earlier than in large intestine because the hemorrhagic diarrhea was recognized 24 h after the injection of Stx2 (1).
FIG. 2.
Time course of Stx1 and Stx2 concentrations in CSF after intravenous injection of Stx1. Stx1 concentrations in CSF 8 and 24 h after intravenous Stx1 injection were significantly higher than those in the nontreated control (∗, P < 0.01 by t test).
MRI of brain lesions induced by Stx1.
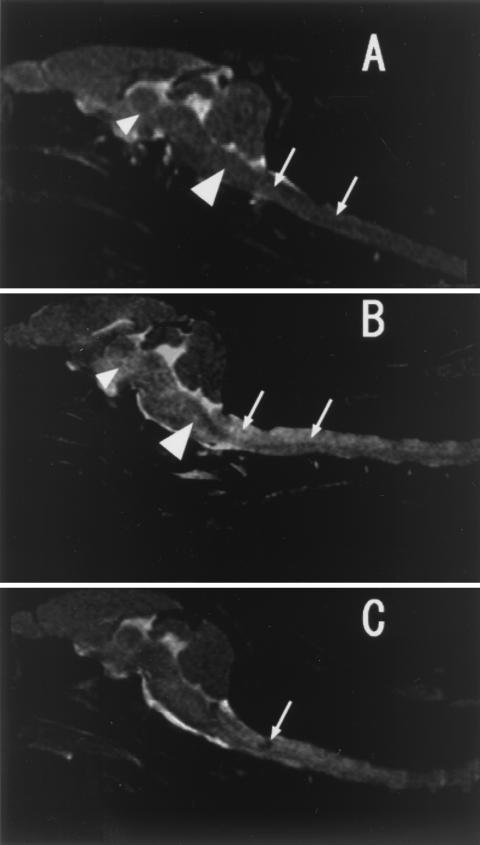
Analysis of Stx1-induced brain lesions in rabbits in the present study (Fig. 3) was performed using previously reported MRI methodology (1). The results revealed damage that was less severe than we had described for Stx2 (1). Rabbits given a 1.95 LD50 dose of Stx1 (39 ng/kg) developed a major spinal cord lesion, appearing as lighter areas indicated by arrows in Fig. 3B (48 h) and with an accompanying hematoma at 24 h (Fig. 3C). At 48 h, additional lesions also developed in the brain stem (Fig. 3B, large arrowhead) and midbrain (Fig. 3B, small arrowhead). In control untreated animals, all of these areas appeared normally dark (Fig. 3A). It is likely that the observed flaccid paresis in these Stx1-treated animals was a result of such localized damage. We could not find lesions in the cerebellum and cerebrum of Stx1-treated rabbits.
FIG. 3.
MRI of brains of normal and Stx1-challenged rabbits. (A) Midsagittal T2-weighted image of a nontreated control rabbit's brain. Two arrows show spinal cord areas, a small arrowhead shows midbrain, and a large arrowhead shows brain stem. (B) T2-weighted image at 48 h after Stx1 administration (39 ng/kg, 1.95 LD50). A high-intensity area of brain stem (large arrowhead) and midbrain (small arrowhead) with spinal cord lesion (two arrows) is seen. (C) T2-weighted image at 24 h after Stx1 administration (39 ng/kg, 1.95 LD50). A low-intensity area of spinal cord is seen as a hematoma (arrow).
In contrast, previous studies with intravenously Stx2-treated animals revealed lesions of the brain stem but not of the spinal cord (1). Lesions were first detected in the hypothalamic area of all experimental animals at 24 h. After 57 h, lesions appeared in the white matter of the cerebral hemisphere or the hippocampus (1). It is important to note that both Stx1 and Stx2 caused brain stem lesions that were the apparent cause of death.
Aside from the different mobilities of Stx1 and Stx2 in crossing the blood-brain barrier, receptor specificity for these toxins may contribute to their different lethal potencies in the rabbit model. Stx2 binds to the same receptor as Stx1, Gb3 (6). However, it has become increasing clear that Gb3 represents a heterogeneous group of glycolipids that differ in fatty acid carbon chain length and degree of fatty acid saturation and hydroxylation (9). It is well accepted that these structural differences in toxin receptor may determine toxin binding affinities. We propose that the structure of Gb3 may differ in the spinal cord and the cerebrum or cerebellum. In humans and mice, we guess that Stx2 may bind to the kidney and the target sites of brain rather than Stx1 because of the different structure of Gb3.
Effect of intrathecal versus intravenous injection of anti-Stx1 antiserum on brain damage induced by intravenous Stx1.
To determine the protective effects of an intrathecal or intravenous injection of rabbit anti-Stx1 antiserum, 15 rabbits were examined in three different treatment groups of five rabbits each. An intrathecal injection was used as described by Mizuguchi et al. (7). One group of rabbits were injected intrathecally with 0.5 ml of rabbit serum (the titer of anti-Stx1 antibody was 32) 1 h before an intravenous injection of Stx1 (78 ng/kg; 3.86 LD50). Another group of rabbits was injected with the same dose of rabbit serum intravenously 1 h before the intravenous injection of Stx1. A control group of rabbits were injected with Stx1 only. The lethality results recorded at day 10 showed 100% survival (five of five) of the rabbits injected intravenously with the rabbit serum containing anti-Stx1 antibody (32 titer) 1 h before the intravenous injection of Stx1. All five rabbits in the control group died. However, only 60% (three of five) survival was observed when the anti-Stx1 antiserum was injected directly into the CSF space 1 h before the intravenous injection of Stx1. This result differs from our previous success in therapy of an intrathecal injection of rabbit serum containing anti-Stx2 antibody for the brain lesion induced by intravenous Stx2 injection (2). It is concluded the lethal toxic effects of Stx1 in rabbits involve damage within as well as outside of the brain, whereas Stx2 acts primarily within the brain.
Acknowledgments
This study was supported by a grant for International Health Cooperation Research (9A-1) from the Ministry of Health and Welfare.
REFERENCES
- 1.Fujii J, Kinoshita Y, Kita T, Higure A, Takeda T, Tanaka N, Yoshida S. Magnetic resonance imaging and histopathological study of brain lesions in rabbits given intravenous verotoxin 2. Infect Immun. 1996;64:5053–5060. doi: 10.1128/iai.64.12.5053-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii J, Kinoshita Y, Yamada Y, Yutsudo T, Kita T, Takeda T, Yoshida S. Neurotoxicity of intrathecal Shiga toxin 2 and protection by intrathecal injection of anti-Shiga toxin 2 antiserum in rabbits. Microb Pathog. 1998;25:139–146. doi: 10.1006/mpat.1998.0220. [DOI] [PubMed] [Google Scholar]
- 3.Fujii J, Kita T, Yoshida S, Takeda T, Kobayashi H, Tanaka N, Ohsato K, Mizuguchi Y. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H− in mitomycin-treated mice. Infect Immun. 1994;62:3447–3453. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmali M A, Steele B T, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;i:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 6.Lingwood C A, Law H, Richardson S, Petric M, Brunton J L, De G S, Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–8839. [PubMed] [Google Scholar]
- 6a.Michino H, Araki K, Minami S, Takaya S, Sakai N, Miyazaki M, Ono A, Yanagawa H. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am J Epidemiol. 1999;150:787–796. doi: 10.1093/oxfordjournals.aje.a010082. [DOI] [PubMed] [Google Scholar]
- 7.Mizuguchi M, Tanaka S, Fujii I, Tanizawa H, Suzuki Y, Igarashi T, Yamanaka T, Takeda T, Miwa M. Neuronal and vascular pathology produced by verocytotoxin 2 in the rabbit central nervous system. Acta Neuropathol Berl. 1996;91:254–262. doi: 10.1007/s004010050423. [DOI] [PubMed] [Google Scholar]
- 8.Ostroff S M, Kobayashi J M, Lewis J H. Infections with Escherichia coli O157:H7 in Washington State. The first year of statewide disease surveillance. JAMA. 1989;262:355–359. [PubMed] [Google Scholar]
- 9.Pellizzari A, Pang H, Lingwood C A. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry. 1992;31:1363–1370. doi: 10.1021/bi00120a011. [DOI] [PubMed] [Google Scholar]
- 10.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 11.Rooney J C, Anderson R M, Hopkins I J. Clinical and pathological aspects of central nervous system involvement in the haemolytic uraemic syndrome. Aust Paediatr J. 1971;7:28–33. doi: 10.1111/j.1440-1754.1971.tb02465.x. [DOI] [PubMed] [Google Scholar]
- 12.Scotland S M, Willshaw G A, Smith H R, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O157 with special reference to production of Vero cytotoxins VT1 and VT2. Epidemiol Infect. 1987;99:613–624. doi: 10.1017/s0950268800066462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas A, Chart H, Cheasty T, Smith H R, Frost J A, Rowe B. Vero cytotoxin-producing Escherichia coli, particularly serogroup O 157, associated with human infections in the United Kingdom: 1989–91. Epidemiol Infect. 1993;110:591–600. doi: 10.1017/s0950268800051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upadhyaya K, Barwick K, Fishaut M, Kashgarian M, Siegel N J. The importance of nonrenal involvement in hemolytic-uremic syndrome. Pediatrics. 1980;65:115–120. [PubMed] [Google Scholar]
- 15.Yamada Y, Fujii J, Murasato Y, Nakamura T, Hayashida Y, Kinoshita Y, Yutsudo T, Matsumoto T, Yoshida S. Brainstem mechanisms of autonomic dysfunction in encephalopathy-associated Shiga toxin 2 intoxication. Ann Neurol. 1999;45:716–723. [PubMed] [Google Scholar]
- 16.Yoh M, Frimpong E K, Honda T. Effect of antimicrobial agents, especially fosfomycin, on the production and release of Vero toxin by enterohaemorrhagic Escherichia coli O157:H7. FEMS Immunol Med Microbiol. 1997;19:57–64. doi: 10.1111/j.1574-695X.1997.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 17.Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. Purification and some properties of a Vero toxin from Escherichia coli O157:H7 that is immunologically unrelated to Shiga toxin. Microb Pathog. 1987;3:21–30. doi: 10.1016/0882-4010(87)90034-9. [DOI] [PubMed] [Google Scholar]