Abstract
Vibrio cholerae hemagglutinin/protease (Hap) was induced upon nutrient limitation and strongly repressed by glucose. Hap was not produced in a mutant defective in the cyclic AMP (cAMP) receptor protein, suggesting that intracellular cAMP levels mediate Hap expression. No difference was found in Hap production between an rpoS deletion mutant and its isogenic wild-type precursor, indicating that the alternate ςs factor is not essential for Hap expression. Based on these and previous results, we discuss the role of Hap in the pathogenesis of cholera.
In order to cause disease, most enteric pathogens need to overcome the protective mucus barrier covering the gastrointestinal epithelium. Vibrio cholerae produces a soluble Zn-dependent metalloprotease (mucinase), which was originally identified as a hemagglutinin: hemagglutinin/protease (Hap) (3, 8). Hap can proteolytically activate cholera toxin (CT) A subunit (4) and the El Tor cytolysin and hemolysin (19) and hydrolyze several physiologically important proteins, such as mucin, fibronectin, and lactoferrin (6). Hap perturbs the paracellular barrier of cultured MDCK-1 cells (27, 28) and T84 intestinal epithelial cells (17). Hap has been associated with detachment of vibrios from cultured intestinal cells (7, 11). Recent results have suggested that Hap could play an important role in intestinal colonization and that it is also a potential reactogenic factor of genetically attenuated vaccine strains (1, 17; presented at the 35th U.S. Japan Cholera and Other Bacterial Enteric Infections Joint Panel Meeting, 3–15 December 1999).
We have investigated the role of Hap in adherence to HT29-18N2 cells (2). HT29-18N2 cells differentiate into columnar monolayers of mucus-secreting goblet cells when shifted to protein-free hybridoma medium (12, 22). A thick meshwork of heterogeneously glycosylated MUC2, MUC3, and MUC5AC mucins is observed covering the differentiated monolayers (21, 26). Examination of V. cholerae adherence to HT29-18N2 cells by transmission and scanning electron microscopy showed the presence of cholera vibrios throughout the mucin meshwork and close to the underlying microvilli (2). Inactivation of the hapA gene encoding Hap increased adherence to HT29-18N2 cells and diminished detachment of vibrios into the washings (2).
The newly developed CTXΦ-negative Hap-defective live cholera vaccine candidate 638 was well tolerated in volunteers (1, 23). In contrast, V. cholerae 81, the hap+ precursor of strain 638, was strongly reactogenic (presented at the 35th U.S. Japan Cholera and Other Bacterial Enteric Infections Joint Panel Meeting, 3–15 December 1999). In order to cause disease or reactogenicity, cholera vibrios must penetrate the mucus barrier (16). We postulate that the Hap mucinase activity that mediates detachment of vibrios from the mucin meshwork of HT29-18N2 cells is the same activity that facilitates penetration of the mucus barrier in vivo.
Very little is known about the environmental signals regulating production of Hap. The expression of hapA requires transcriptional activation by the product of hapR, an analog of Vibrio harveyi luxR (13). In the above-mentioned study, activation of hapA expression occurred in the early stationary phase (13). A similar time course was observed with a transduction assay based on the capacity of Hap to inactivate the CTXΦ phage (14). In the present work, we investigate the environmental signal(s) required for production of Hap, provide evidence that hapA is controlled by carbon catabolite repression, and discuss a model for the role of this protein in intestinal colonization.
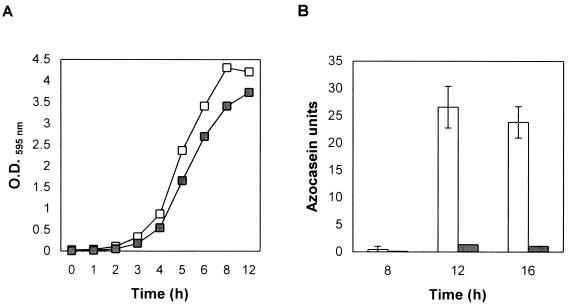
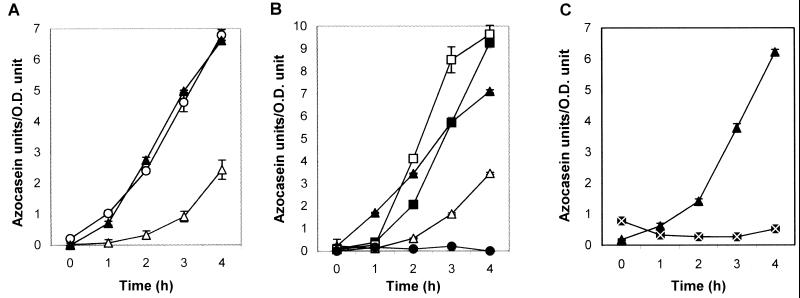
We studied the time course of protease secretion in V. cholerae strain C7258 (El Tor, Ogawa) and its hapA-defective derivative 638 (1, 23) grown in tryptic soy broth (TSB) (Fig. 1). Protease activity was measured in an azocasein assay (17). Briefly, 100 μl of azocasein (5 mg/ml) in 100 mM Tris (pH 8.0) was incubated with 100 μl of cell culture supernatants for 1 h at 37°C. The reaction was stopped by the addition of 400 μl of 10% trichloroacetic acid. After centrifugation, the trichloroacetic acid supernatant was transferred to 700 μl of 525 mM NaOH, and the optical density was determined at 442 nm (OD442). One azocasein unit was defined as the amount of enzyme producing an increase of 0.01 OD units per h. The strains studied reached the stationary phase in 8 h (Fig. 1A). No protease activity could be detected in the culture medium prior to 8 h (Fig. 1B). Very little proteolytic activity was detected in the supernatant of the insertionally inactivated hapA mutant 638, suggesting that Hap is the major protease secreted in strain C7258. The appearance of proteolytic activity at the early stationary phase could be explained by the accumulation of an autoinducer substance mediating quorum sensing (i.e., as luminescence in V. harveyi [9]) or by depletion of a repressor substance in the medium. To test the above interpretations, we grew V. cholerae strain C7258 for 8 h in TSB medium and subsequently tested the ability of these cells to produce Hap under different conditions. When cells grown in TSB for 8 h are resuspended in fresh medium, secretion of proteolytic activity is reduced compared to cells kept growing in the same 8-h spent medium (Fig. 2A). Boiling of the 8-h spent medium for 20 min—a treatment that inactivates the N-acyl-homoserine lactone family of autoinducer substances (9) or other heat-labile factors—had no effect on the capacity of the medium to sustain Hap production (Fig. 2A). Supplementing fresh medium with 8- and 12-h filter-sterilized spent medium (10 to 50% [vol/vol]) had no effect on protease secretion (data not shown). The above findings rule out a quorum-sensing mechanism for the regulation of Hap production. Next, we reconstituted cells from an 8-h TSB culture in media of reduced strength (Fig. 2B). Again, reconstitution of cells in 1× fresh TSB medium decreased the rate of protease secretion. On the other hand, reconstitution of cells in 0.5× and 0.25× TSB medium significantly enhanced protease production (Fig. 2B). No enhancing effect was observed in mutant 638, suggesting that the protease induced under these conditions is the product of the hapA gene. These results indicate that protease production is activated by nutrient limitation. To test the possibility that such a stimulus might be carbon source starvation, an 8-h TSB culture of strain C7258 was divided in halves; one part was kept growing in the same medium for 4 h, while the other was supplemented with d-glucose and similarly incubated. As shown in Fig. 2C, glucose strongly repressed protease secretion. Glucose did not have any effect on enzyme activity.
FIG. 1.
Time course of Hap production. (A) Strains C7258 (□) and 638 (▪) were grown from a single colony in 5 ml of TSB medium at 30°C. Overnight cultures were diluted 1:1,000 in 100 ml of fresh TBS in a 500-ml flask and incubated at 30°C with shaking (200 rpm). Samples were withdrawn periodically for OD595 readings. (B) Samples were taken at 4-h intervals from the cultures described above for determination of azocasein activity. Open bar, strain C7258; shaded bar, strain 638. Enzyme activities are the averages of at least three independent cultures. Standard deviations are indicated with error bars.
FIG. 2.
Kinetics of Hap production. Strain C7258 was grown from a single colony in 5 ml of TSB medium at 30°C. An overnight culture was diluted 1:1,000 in fresh TSB and incubated for 8 h with shaking (200 rpm) at 30°C. Aliquots of this culture were centrifuged, the cells were resuspended in 1 volume of each of the different media described below, and incubation continued for 4 h. Samples were taken at 1-h intervals for OD595 readings and determination of azocasein activity. (A) Cells were resuspended in original 8-h spent medium (▴), original 8-h spent medium boiled for 20 min (○), or 1× fresh TBS (▵). (B) Cells were resuspended in original 8-h spent medium (▴), 1× fresh TBS (▵), 0.5× fresh TBS (▪), or 0.25× fresh TBS (□). Mutant strain 638 (●) was cultivated as described for C7258, and the 8-h cell pellet was resuspended in 1 volume of 0.25× fresh TBS. (C) Cells were resuspended in original 8-h spent medium (▴) and original 8-h spent medium supplemented with 0.4% (22 mM) d-glucose (⊠). Azocasein activities are the averages of at least three independent cultures. Standard deviations are indicated with error bars.
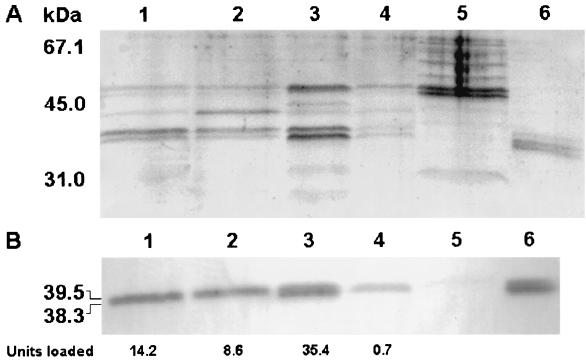
In order to confirm that the protease induced upon nutrient limitation and repressed by glucose is the product of hapA, culture supernatants of strain C7258 grown under different conditions were concentrated by passing them through Centricon-10 centrifugal filters (Amicon Bioseparations) and separated in a 12% polyacrylamide gel (Fig. 3A). Hap was observed as a 39.5- and 38.3-kDa doublet which comigrated with pure Hap (8) (Fig. 3A). These bands were absent in the hapA mutant 638. To further confirm that these bands were Hap, identical samples were loaded in a second gel and transferred to a polyvinylidene difluoride membrane. The membrane was treated with a rabbit Hap antiserum and developed using a peroxidase-conjugated goat anti-rabbit immunoglobulin G. The Western blot confirmed that the 39.5- and 38.3-kDa protein bands were Hap. A good correlation was observed between the total azocasein units loaded in each lane (Fig. 3, bottom) and the amount of protein observed in the Coomassie stain and the Western blot. For instance, more protein and immunoreactive material was observed in cells reconstituted in 0.25× TSB and very little was observed in the cells reconstituted in 0.25× TSB supplemented with glucose (Fig. 3A and B, lanes 3 and 4). We also tested the abilities of culture supernatants to hemagglutinate chicken red blood cells (RBC). Four samples of defibrinated chicken blood drawn from chickens belonging to different lots were obtained from Hema Resources & Supply, Inc. (Aurora, Oreg.). The RBC were washed twice with saline and KRT buffer and resuspended in Krebbs-Ringer-Tris (KRT) buffer (128 mM NaCl, 5.1 mM KCl, 1.34 mM MgSO4, 2.7 mM CaCl2, 10 mM Tris [pH 7.5]) at 1.5% (vol/vol). Hemagglutination was performed in microtiter plates, and the titer was defined as the highest dilution showing complete hemagglutination. Only two RBC suspensions were agglutinated by supernatants of strain C7258. A distinguishing feature of Hap is its capacity to agglutinate a percentage (approximately 50%) of RBC from different chickens: “responder RBC” (8). The difference in Hap protein observed in Fig. 3A and B between lanes 3 and 4 translated into a reduction in titer from 64 (lane 3) to 2 (lane 4). The addition of glucose to the RBC had no effect on hemagglutination. These results demonstrate that V. cholerae Hap is induced upon nutrient limitation and repressed by glucose.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of Hap production. An 8-h culture of strain C7258 was divided into aliquots, the cells were resuspended in 1 volume of the different media described below, and incubation continued at 30°C with shaking (200 rpm) for 4 h. The cultures were centrifuged, and 2 ml of each supernatant was concentrated 10-fold by centrifuging it through Centricon-10 centrifugal filters. The retentate was adjusted to 0.2 ml, and 0.02-ml aliquots were analyzed in an SDS–12% PAGE gel. (A) SDS-PAGE. Lane 1, cells resuspended in the original 8-h spent medium; lane 2, cells resuspended in 1× fresh TBS; lane 3, cells resuspended in 0.25× fresh TBS; lane 4, cells resuspended in 0.25× fresh TBS supplemented with 0.4% d-glucose; lane 5, concentrated supernatant of mutant 638; lane 6, pure Hap (2.5 μg). (B) Western blot. Identical samples were loaded in a second gel and transferred to a polyvinylidene difluoride membrane. Hap was detected by using a rabbit anti-Hap serum and peroxidase-conjugated goat anti-rabbit immunoglobulin G. The total azocasein units loaded per lane are indicated below the blot. Molecular masses were calculated with reference to SDS molecular mass standards (low range) from Bio-Rad laboratories.
Other sugars were poorer repressors at the same molar concentration. The percentages of activity detected after the addition of the different carbon sources (tested as shown in Fig. 2C) were as follows: d-glucose, 0.9%; d-mannose, 22.7%; d-galactose, 30.7%; maltose, 19.9%; sucrose, 4.3%; glycerol, 36.8%; and lactose, 90.9%. Lactose, which is not taken up by V. cholerae, did not cause significant repression. Such variation in repression activity could be due to differences in the intracellular concentration of cyclic AMP (cAMP) among cells utilizing different carbon sources (10). Growth in glucose-containing medium is known to lead to low cAMP levels, preventing transcriptional activation of responsive promoters by the cAMP-cAMP receptor protein (CRP) complex (15).
We hypothesized that regulation of Hap production could be mediated by the cAMP-CRP global regulator. To test this hypothesis, we measured production of Hap—as determined by proteolytic and hemagglutination activity—in isogenic crp mutant and crp+ strains (25). Hap was not produced in the crp mutant KSK394. This result strongly suggests that CRP is required for hapA expression (Table 1).
TABLE 1.
Role of CRP and ςs in the expression of Hap
| Straina | Allele | Azocasein units/OD unit | Hemagglutination titer |
|---|---|---|---|
| C6706 | crp+ | 9.36 ± 0.20 | 64 |
| KSK394 | crp | 0.28 ± 0.07 | >2 |
| C6709-1 | rpoS+ | 7.45 ± 0.30 | 128 |
| DSM-V491 | ΔrpoS | 2.07 ± 0.27 | 64–128 |
Three independent single-colony cultures of each strains were diluted 1,000-fold in 50 ml of TSB and incubated with shaking (200 rpm) at 30°C to an OD595 of >4 to ensure complete derepression of Hap in each wild-type strain.
It has been reported that inactivation of the rpoS gene encoding the stationary phase ςs factor diminished production of Hap (29) and that rpoS is required for efficient intestinal colonization (18). We compared Hap production in isogenic rpoS+ and rpoS mutant strains. The rpoS deletion mutant DSM-V491 (18) secreted less proteolytic activity, but very little effect was observed on the hemagglutination titer determined as described above (Table 1). Since Hap is a protease and a hemagglutinin, it can be distinguished from other proteases produced by V. cholerae by simultaneously measuring its two activities. Our results do not support a role for ςs in the production of Hap in the strains studied. An active rpoS gene could be required for the expression or secretion of other proteases different from Hap.
DISCUSSION
We demonstrated that Hap is produced by V. cholerae in response to nutrient, and specifically glucose, limitation. Glucose has been identified as a signal that could modulate the expression of virulence factors (24). The concentration of luminal glucose has been estimated to range from 0.2 to 48 mM, depending on diet, with the higher concentrations found in the proximal small intestine (5). Repression of Hap was observed at glucose concentrations as low as 1 mM (data not shown). Inactivation of the crp gene in V. cholerae results in derepression of CT and toxin-coregulated pili (TCP) (25), suggesting that glucose concentration could modulate the expression of these virulence factors in vivo by lowering the intracellular cAMP concentration. Conditions that would make the presence of CRP superfluous, such as high glucose concentrations leading to low cAMP levels, would favor production of CT and TCP and repress Hap. Conversely, low glucose levels would favor production of Hap and down regulate CT and TCP. A direct effect of glucose on CT production was not observed in a previous study, although more CT was made in glucose-containing medium, leading to cAMP levels lower than those in media containing other sugars (10). This study was conducted with the classical biotype strain 569B and should be repeated with the El Tor biotype strains, culture conditions, and glucose concentrations used in our study. Our results suggest the existence of a previously unrecognized coordination between expression of CT and TCP and protease production. It is conceivable that other environmental cues conducive to low intracellular cAMP concentrations would mimic the glucose repression of Hap observed in this study. The mechanism by which glucose specifically diminishes production of Hap is unknown, but the involvement of CRP suggests transcriptional control of hapA or a gene required for hapA expression. A conserved cAMP-CRP binding TGTGA pentamer separated by 10 bp from the less conserved TCANA pentamer is located upstream from the presumptive hapA −35 sequence. The positioning of the cAMP-CRP binding site in hapA is similar to that of the Escherichia coli arabinose operon and suggests the participation of an additional transcription factor (i.e., HapR).
Our data do not support an essential role for the alternative ςs factor in the transcription of hapA or of any other gene(s) required for Hap production, as previously reported (29). In this study, which involved different strains and growth conditions, the active rpoS gene on a plasmid did not fully complement the protease production defect of an rpoS insertion mutant (29). Therefore, the occurrence of an unintentional mutation affecting Hap production cannot be excluded.
The findings that hapA− mutants adhere more (because they do not detach as much) to the mucin meshwork of HT29-18N2 cells (2) and that the Hap-defective strain 638 was well tolerated in volunteers while its Hap+ precursor was reactogenic (1; presented at the 35th U.S. Japan Cholera and Other Bacterial Enteric Infections Joint Panel Meeting, 3–15 December 1999) suggests that hapA encodes an activity that simultaneously mediates detachment and penetration of the mucus barrier. Lack of this activity renders V. cholerae less capable of overcoming the mucus barrier, very much like the previously nonreactogenic nonmotile strain Peru-15 (16). We propose a “mucinase-detachase” role for Hap in the pathogenesis of cholera. At the onset of intestinal colonization (low bacterial cell density and rich medium), Hap is not expressed, and binding to mucus prevails over detachment. As adherent bacteria multiply, nutrient limitation allows derepression of Hap. Hap then facilitates penetration of the mucus gel at the cost of increased detachment. Detached vibrios could bind again along the gastrointestinal tract to establish new infection foci and be excreted in the stools. Detachment of adherent vibrios in vivo was initially observed in a rabbit model (20). Derepression of Hap, in synergy with chemotaxis and motility, allows V. cholerae to overcome the mucus barrier.
Acknowledgments
We are grateful to Karen Skorupski (Dartmouth Medical School, Hanover, N.H.) and Andrew Camilli (Tufts University School of Medicine) for kindly providing isogenic crp and rpoS mutant strains, respectively.
REFERENCES
- 1.Benitez J A, Garcia L, Silva A, Garcia H, Fando R, Cedré B, Pérez A, Campos J, Rodriguez B L, Pérez J L, Valmaseda T, Pérez O, Pérez A, Ramírez M, Ledón T, Díaz Jidy M, Lastre M, Bravo L, Sierra G. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect Immun. 1999;67:539–545. doi: 10.1128/iai.67.2.539-545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benitez J A, Spelbrink R G, Silva A, Phillips T E, Stanley C M, Boesman-Finkelstein M, Finkelstein R A. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun. 1997;65:3474–3477. doi: 10.1128/iai.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth B A, Boesman-Finkelstein M, Finkelstein R A. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect Immun. 1983;42:639–644. doi: 10.1128/iai.42.2.639-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth B A, Boesman-Finkelstein M, Finkelstein R A. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984;45:558–560. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferraris R P, Yasharpour S, Lloyd K C, Mirzayan R, Diamond J M. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein R A, Boesman-Finkelstein M, Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci USA. 1983;80:1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Häse C C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein R A, Hanne L F. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect Immun. 1982;36:1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg E P, Hastings J W, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 10.Ganguly U, Greennough W B., III Adenosine 3′5′-cyclic monophosphate in Vibrio cholerae. Infect Immun. 1975;11:343–349. doi: 10.1128/iai.11.2.343-349.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Häse C C, Finkelstein R A. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol. 1991;173:3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huet C L, Sahuquillo-Merino C, Coudrier E, Louvard S. Absorptive and mucus secreting subclones isolated from a muiltipotent intestinal cell line (HT29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987;105:345–358. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jobling M G, Holmes R K. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimsey H H, Waldor M K. Vibrio cholerae hemagglutinin/protease inactivates CTXΦ. Infect Immun. 1998;66:4025–4029. doi: 10.1128/iai.66.9.4025-4029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 16.Mekalanos J J, Waldor M K, Gardel CL, Coster T S, Kenner J, Killeen K P, Beattie D T, Trofa A, Taylor D N, Sadoff J C. Live cholera vaccines: perspectives on their construction and safety. Bull Inst Pasteur. 1995;93:255–262. [Google Scholar]
- 17.Mel S F, Fullner K J, Wimer-Mackin S, Lencer W I, Mekalanos J J. Association of protease activity in Vibrio cholerae vaccine strains with decrease in transcellular epithelial resistance of polarized T84 intestinal cells. Infect Immun. 2000;68:6487–6492. doi: 10.1128/iai.68.11.6487-6492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrell D S, Tischler A D, Lee S H, Camilli A. Vibrio cholerae requires rpoS for efficient intestinal colonization. Infect Immun. 2000;68:6691–6696. doi: 10.1128/iai.68.12.6691-6696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagamune K, Yamamoto K, Naka A, Matsuyama J, Miwatani T, Honda T. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (Pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect Immun. 1996;64:4655–4658. doi: 10.1128/iai.64.11.4655-4658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson E T, Clements J D, Finkelstein R A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976;14:527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips T E, Frisch E B. Secretory glycoconjugates of a mucin-synthesizing human colonic adenocarcinoma cell line. Analysis using double labeling with lectins. Histochemistry. 1990;93:311–317. doi: 10.1007/BF00266394. [DOI] [PubMed] [Google Scholar]
- 22.Phillips T E, Huet C, Bilbo P R, Podolsky D K, Louvard D, Neutra M R. Human intestinal goblet cell in monolayer culture: characterization of a mucus-secreting subclone derived from HT29 colon adenocarcinoma cell line. Gastroenterology. 1988;94:1390–1403. doi: 10.1016/0016-5085(88)90678-6. [DOI] [PubMed] [Google Scholar]
- 23.Robert A, Silva A, Benitez J A, Rodriguez B L, Fando R, Campos J, Sengupta D K, Boesman-Finkelstein M, Finkelstein R A. Tagging a Vibrio cholerae El Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme: Clostridium thermocellum endoglucanase A. Vaccine. 1996;14:1517–1522. doi: 10.1016/s0264-410x(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 24.Skorupski K, Taylor R K. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25:1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 25.Skorupski K, Taylor R K. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin co-regulated pilus in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley C M, Phillips T E. Selective secretion and replenishment of discrete mucin glycoforms from intestinal goblet cells. Am J Physiol. 1999;227:G191–G200. doi: 10.1152/ajpgi.1999.277.1.G191. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Milton D, Nybon P, Sjo A, Magnusson K-E. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Nybom P, Magnusson K E. Distinct effects of Vibrio cholerae hemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 29.Yildiz F H, Schoolnik G K. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;180:773–784. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]