Abstract
Background:
Risk assessment for breast cancer-related lymphedema has emphasized upper-limb symptoms and treatment-related risk factors. This article examined breast cancer-related lymphedema after surgery, overall and in association with broader demographic and clinical features.
Methods:
The Carolina Breast Cancer Study Phase 3 followed participants for breast cancer-related lymphedema from baseline (on average 5 months after breast cancer diagnosis) to 7 years post-diagnosis. Among 2645 participants, 552 self-reported lymphedema cases were identified. Time-to-lymphedema curves and inverse probability weighted conditional Cox proportional hazards model were used to evaluate whether demographics and clinical features were associated with breast cancer-related lymphedema.
Results:
Point prevalence of breast cancer-related lymphedema was 6.8% at baseline, and 19.9% and 23.8% at two- and seven-years post diagnosis, respectively. Most cases had lymphedema in the arm (88-93%), while 14-27% presented in the trunk and/or breast. Beginning approximately 10 months post diagnosis, younger Black women had the highest risk of breast cancer-related lymphedema and older non-Black women had the lowest risk. Positive lymph node status, larger tumor size (>5 cm), and estrogen receptor-negative breast cancer, as well as established risk factors such as higher body mass index, removal of more than 5 lymph nodes, mastectomy, chemotherapy, and radiation therapy, were significantly associated with increased hazard (1.5 to 3.5-fold) of lymphedema.
Conclusions:
Findings highlight that hazard of breast cancer-related lymphedema differs by demographic characteristics and clinical features. These factors could be used to identify those at greatest need of lymphedema prevention and early intervention.
Keywords: longitudinal study, breast cancer-related lymphedema, demographics, clinical correlates, USA
Lay summary:
In this study, we aimed to investigate breast cancer-related lymphedema (BCRL) burden, and found that risk of BCRL differs by race, age, and other characteristics.
Precis for use in the Table of Contents:
We used a population-based racially diverse cohort of women with breast cancer to assess burden of lymphedema as well as related demographic and clinical features. Our findings could be used to identify those at greatest need of lymphedema prevention and early intervention after breast cancer diagnosis.
BACKGROUND
Approximately 1 in 5 women treated for breast cancer (BC) are affected by breast cancer-related lymphedema (BCRL),1,2 a distressing side-effect distinguished by interstitial protein-rich fluid accumulation and/or regional swelling.3,4 Associated physical impairments, particularly of the upper-limb, are common and include reduction in range of motion, weakness, and paresthesia.5 BCRL is also associated with depression and anxiety, difficulty in social, domestic, vocational, and sexual domains,6 and financial and time burdens.7 Improved understanding of disease burden and risk factors for BCRL would aid in prevention, early detection, and management.
Increased risk of BCRL has consistently been observed among women who have more extensive lymph node dissection and chest wall surgery as treatment for breast cancer, as well as women who are overweight or obese.8 Historically, radiation therapy, but not chemotherapy, has been associated with increased risk,9 but more recent research suggests that adjuvant chemotherapy may also be associated with increased risk.2 Furthermore, lower levels of physical activity and a higher number of metastatic lymph nodes have been identified as risk factors.2 However, a paucity of studies have evaluated risk factors for BCRL in diverse cohorts. In the limited number of studies evaluating racial differences in BCRL risk,10–13 one study reported no association between race [Black versus non-Black (including Hispanic white and non-Hispanic white) and BCRL risk after multivariable adjustment.10 In contrast, Kwan and colleagues found that African American women bore two-fold BCRL risk as high compared with white women; however, the percentage of African American women included within the sample was relatively small (n=210; 7%).11 Incidence of BCRL by age is also underexplored and inconclusive,14 despite reasonable mechanisms for age-related differences.9,15 Larger, longitudinal studies are needed to advance understanding of prevalence and factors associated with BCRL in diverse populations.
The third phase of the Carolina Breast Cancer Study (CBCS3) is a population-based racially diverse cohort study of women that oversampled Black and younger women diagnosed with BC in North Carolina between 2008 and 2013. Participants were followed prospectively for BCRL diagnosis and other disease characteristics from 5 months (defined as baseline in this study) to 7 years following BC diagnosis. Using data from CBCS, we estimated prevalence of BCRL according to self-reported diagnosis of BCRL, by body location, according to type of diagnosis provider, and with respect to acute versus chronic symptoms. Because CBCS3 is designed to study younger and Black women, we assessed stratum-specific association with BCRL for race and age.
METHODS
Study Population
The CBCS3 is a prospective, population-based cohort study of women with invasive BC based in 44 counties in eastern and central North Carolina.16 This study was initiated to evaluate patterns of survivorship following diagnosis.17 Eligible participants were female, English-speaking, newly diagnosed with invasive BC, and aged 20 to 74 years. Younger (<50 years in age) and Black women with BC were oversampled to each represent approximately 50% of the study population.16 Through rapid case ascertainment, a total of 2998 incident, invasive, pathologically confirmed BC cases were identified from the North Carolina Central Cancer Registry between May 1, 2008 and October 21, 2013 and recruited within two months of diagnosis.17–19 This study was conducted following informed consent by all participants, under a protocol approved by the University of North Carolina School of Medicine Institutional Review Board. In this study, we interpret race as a social construct under a cells-to-society framework where molecular, tissue, individual, community-level, and structural factors act simultaneously to potentially alter lymphedema risk.20
Study participants were interviewed in-person by trained nurses about demographics, lifestyle factors, and diagnosis of BCRL within 9 months (range 2-9, median 5 months) of BC diagnosis.18 Of additional note, the surgery initiation occurred on average at 1.7 months post diagnosis in our study population, prior to baseline survey. At the initial interview, participants consented for researchers to extract their medical records by chart review to collect information on baseline comorbidities, BC treatment and type of surgery.21 Women also completed two follow-up surveys at approximately 2 years (range 20-36, median 25 months) and 7 years (range 60-110, median 84 months) post BC diagnosis. Tumor characteristics (e.g., stage, grade) were ascertained from pathology reports.22
The current analysis excluded CBCS3 participants who did not have their first course surgery within 18 months of BC diagnosis (n=58). Additional exclusions included women who already had self-reported lymphedema before BC surgery (n=113), women who were diagnosed with stage IV BC (n=63) due to different treatment strategies compared with women diagnosed with stage I-III BC,23 and women who had unknown date of BCRL diagnosis (n=119). After applying the study criteria, the final study population consisted of 2645 participants, with a weighted mean age of 55.9 years at diagnosis; 47.5% of the participants were Black women.
Outcome Ascertainment
BCRL diagnosis was obtained by self-report. Among 2645 eligible women, 552 BCRL cases were identified over the 7-year follow-up period. The CBCS3 questionnaire included questions addressing a wide range of health outcomes. Participants were asked to self-report current or prior diagnosis of BCRL since their BC diagnosis, BCRL location [i.e., left/right arm, trunk, or breast, and dominant hand (left, right, or both)], time of diagnosis (in month/year), health professional who provided the diagnosis (i.e., medical doctor, nurse, physical therapist, other), and were asked to characterize episodic nature of BCRL (i.e., single, recurrent, or persistent). Because participants were asked to choose between “single”, “recurrent”, or “persistent”, we believe that some participants may have used these categories as indicators of severity rather than episodicity. To be maximally conservative, we included any report of BCRL as cases.
Time to BCRL was calculated in months from BC diagnosis to subsequent self-reported BCRL diagnosis. Diagnosis was provided as month/year, and therefore date was assigned as day 15 to calculate months between BC and BCRL diagnosis. For participants with incomplete time (n=8; missing month or year) of BCRL diagnosis, dates of their 2-year survey completion were used. For participants reporting different diagnosis dates across surveys (n=445), the date reported on the earliest complete follow-up questionnaire was selected. The average difference between most extreme dates reported by a given participant was 4.7 months. Additionally, multiple reports of diagnosis time were not interpreted as recurrence of BCRL, as BCRL is widely considered as a chronic condition.24
BCRL prevalence is thought to be a reasonable estimate of incidence as the proportion of women with BCRL before BC surgery has been reported to be very low (as low as 0%).25 Previous research has used incidence and prevalence interchangeably.2 In this specific study, women were asked to report whether they had been diagnosed with lymphedema since BC diagnosis. For precision of language, we used prevalence to represent the disease burden of BCRL; however results were compared against previous “incidence” studies.
Covariates
Covariates were identified based on a priori knowledge, associations in this particular study, and directed acyclic graphs (DAGs). Information collected at baseline included demographics (i.e., age at diagnosis, self-identified race) and physical characteristics [i.e., body mass index (BMI)]. Clinical features including baseline hypertension and diabetes, BC surgery type, number of lymph nodes removed, receipt of adjuvant therapy (chemotherapy and/or radiotherapy) were extracted from medical records. Pathology reports provided information on tumor characteristics such as tumor size, lymph node status, histologic grade, and estrogen-receptor (ER) status. Treatment on dominant side was defined using information from BC treatment and dominant hand of participants.
Statistical Analysis
Point prevalence (n, percent) of ever having BCRL since BC diagnosis was calculated at three time points, corresponding to responses from each of the three surveys. Participants were excluded for surveys to which they did not respond. Prevalence percentages were also calculated for location, types of diagnosis provider, and episodic nature.
Crude time-to-BCRL curves were estimated as a measure of the general burden of BCRL by age and race, and were used to calculate crude 5-year risk of BCRL for each potential or established risk factor. Inverse probability of exposure weighting was used to adjust for covariates in time-to-BCRL analyses. Inverse probability weighting (IPW) accounts for baseline characteristics similar to adjusted Cox models, but does not assume hazards to be proportional across strata.26 To assign weights to estimate the association of each factor with BCRL hazard, we used logistic regression to calculate the probability of belonging to each group (e.g., hypertension versus no hypertension) accounting for other characteristics. These probabilities were used to calculate stabilized inverse probability of exposure weights. Standardized hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated using inverse probability weighted Cox proportional hazards models. Specifically, weights were obtained separately for each variable.
Models for stratum-specific associations with age at diagnosis and race were not adjusted for other factors. Models for baseline comorbidities (i.e., hypertension, diabetes), tumor characteristics (i.e., tumor size, lymph node status, histologic grade, and ER status), and treatment on dominant side were adjusted for age and race. Models for established risk factors of BCRL [i.e., surgery type, number of lymph nodes removed (>5 versus ≤5, cutoff determined based on a previous study of optimal number for a sentinel lymph node dissection),27 receipt of chemotherapy, receipt of radiotherapy]2 were adjusted for demographic characteristics (i.e., age and race) and tumor characteristics. The proportional hazards assumption for each Cox model was assessed using log-log plots of time-to-BCRL and by Wald test of interaction term between lymphedema occurrence time and covariates of interest. For several covariates (e.g., age, tumor size, ER status), the log-log curves for different groups crossed at approximately 6.7 months, and the interaction terms with lymphedema occurrence time had p-values < 0.05, which indicated that the proportional hazards assumption was violated and suggested that factors associated with early BCRL after diagnosis may differ from those associated with later BCRL. Therefore, analyses were conducted conditional on follow-up length: data were truncated at 6.7 months and then hazard was assessed conditional upon free from lymphedema the first 6.7 months. As 6.7 months was a data-driven cut-off point, we interpreted the results nominally and separately for early BCRL occurring within first 7 months and later BCRL occurring after 7 months. We conducted a sensitivity analysis using weighted Cox proportional hazards model accounting for competing risk from death. Competing risk models assigned outcome status to deceased participants according to their last reported BCRL status, and these participants were censored at death. Additionally, to evaluate potential misclassification of BCRL, we also performed a sensitivity analysis restricting our analytic sample to doctor-diagnosed BCRL.
All statistical tests were two-sided and considered statistically significant at P<0.05; statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc, Cary, NC).
RESULTS
Prevalence of Breast Cancer-related Lymphedema
The point prevalence of BCRL increased monotonically from 6.8% (n=180) at baseline to 19.9% (n=452) at 2 years and 23.8% (n=393) at 7 years (Table 1). The number of cumulative BCRL cases was 180, 477, 552 at baseline, 2 years, and 7 years, respectively. The majority of cases had BCRL in the arm (88-93%), while 14-27% presented in the trunk and/or breast. There was a suggestion that trunk BCRL decreased in prevalence over time (24% at baseline and 14% at 2-year and 7-year follow-up). Less than 10% of cases reported BCRL in three body parts simultaneously (breast, arm, and trunk). BCRL presented on the dominant side and non-dominant side in similar proportions. Over 80% of cases reported having chronic BCRL, including 50% persistent and 40% recurrent. The vast majority (>70%) of women reported receiving their BCRL diagnosis from medical doctors, with physical therapist being the second most reported diagnostic method.
Table 1.
Distribution of breast cancer-related lymphedema (BCRL) following surgery among women with breast cancer (stage I-III) in the Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013)
| Time of Assessment | ||||
|---|---|---|---|---|
| Characteristics | Baseline N=2645 |
Two-year follow-up N=2273 |
Seven-year follow-up N=1654 |
|
| Cumulative cases of BCRL | 180 | 477 | 552 | |
| Point prevalence of ever having BCRL, n (% of total population) | 180 (6.8%) | 452 (19.9%) | 393 (23.8%) | |
| Location; n (%)a |
Arm | 159 (88.3%) | 375 (90.1%) | 298 (93.1%) |
| Trunk | 43 (23.9%) | 57 (13.7%) | 44 (13.7%) | |
| Breast | 28 (15.6%) | 112 (26.9%) | 64 (20.0%) | |
| Arm, trunk AND breast | 7 (3.9%) | 34 (8.2%) | 16 (5.0%) | |
| Dominant side | 82 (45.6%) | 209 (50.2%) | 149 (46.6%) | |
| Non-dominant side | 100 (55.6%) | 220 (52.9%) | 187 (58.4%) | |
| Diagnosis by provider; n (%)b |
Medical doctor | 127 (70.6%) | 303 (73.2%) | 247 (77.2%) |
| Nurse | 7 (3.9%) | 27 (6.5%) | 20 (6.2%) | |
| Physical therapist | 30 (16.7%) | 145 (35.0%) | 92 (28.7%) | |
| Other | 20 (11.1%) | 21 (5.1%) | 11 (3.4%) | |
| Episodic nature; n (%)c |
Single episode | 12 (6.7%) | 70 (17.0%) | 62 (19.6%) |
| Recurrent (comes and goes) | 77 (42.8%) | 155 (37.7%) | 126 (39.7%) | |
| Persistent | 91 (50.6%) | 186 (45.3%) | 129 (40.7%) | |
Participants could report BCRL in multiple body parts, therefore the percentages do not add up to 100%.
Participants could respond yes to multiple diagnosis providers; therefore the percentages do not sum to 100%. 2 cases at the two-year follow-up did not respond to questions for diagnosis provider.
5 cases at the two-year follow-up and 3 cases at the seven-year follow-up did not respond to questions for episodic nature.
Association of Race and Age with Breast Cancer-related Lymphedema
26% (n=322) of Black women and 17% (n=230) of non-Black (including white, American Indian, Asian, and other races) women reported BCRL following breast cancer. Younger patients also had a higher burden of BCRL compared to older patients (23% versus 18%). We compared BCRL risk by race and age, and observed differences between Black versus non-Black BCRL curves as early as 5 months post BC diagnosis (Figure 1a). Given our emphasis on burden of BCRL, models for age and race were not adjusted for other factors. Similarly, BCRL curves for older versus younger women diverged, this time at roughly 10 months post BC diagnosis (Figure 1b). We also observed that after approximately 10 months post BC diagnosis, younger Black women were at the highest risk of BCRL and older non-Black women had lowest risk (Figure 1c). For early BCRL, the hazard ratios for Black versus non-Black women were 1.42 (95% CI= 1.09 – 1.83) overall, 1.21 (95% CI= 0.85 – 1.74) among women aged 50 years or older, and 1.68 (95% CI= 1.15 – 2.44) among women younger than 50 years of age (Table 2a). The Black versus non-Black hazard ratios were greater for later BCRL, particularly among older women (HR=1.86; 95% CI=1.29 – 2.66). Age was not significantly associated with early BCRL; however for later BCRL, younger age was significantly associated with increased hazard of BCRL with hazard ratios of 1.80 (95% CI= 1.43 – 2.25) overall, 1.69 (95% CI= 1.19 – 2.42) among non-Black women, and 1.94 (95% CI= 1.45 – 2.59) among Black women. The HRs from the competing risk models were slightly attenuated but significance remained the same (Supplementary Table 1). Additionally, the HRs did not differ substantially in sensitivity analyses restricting to women with doctor-diagnosed BCRL only (Supplementary Table 2).
Figure 1a.
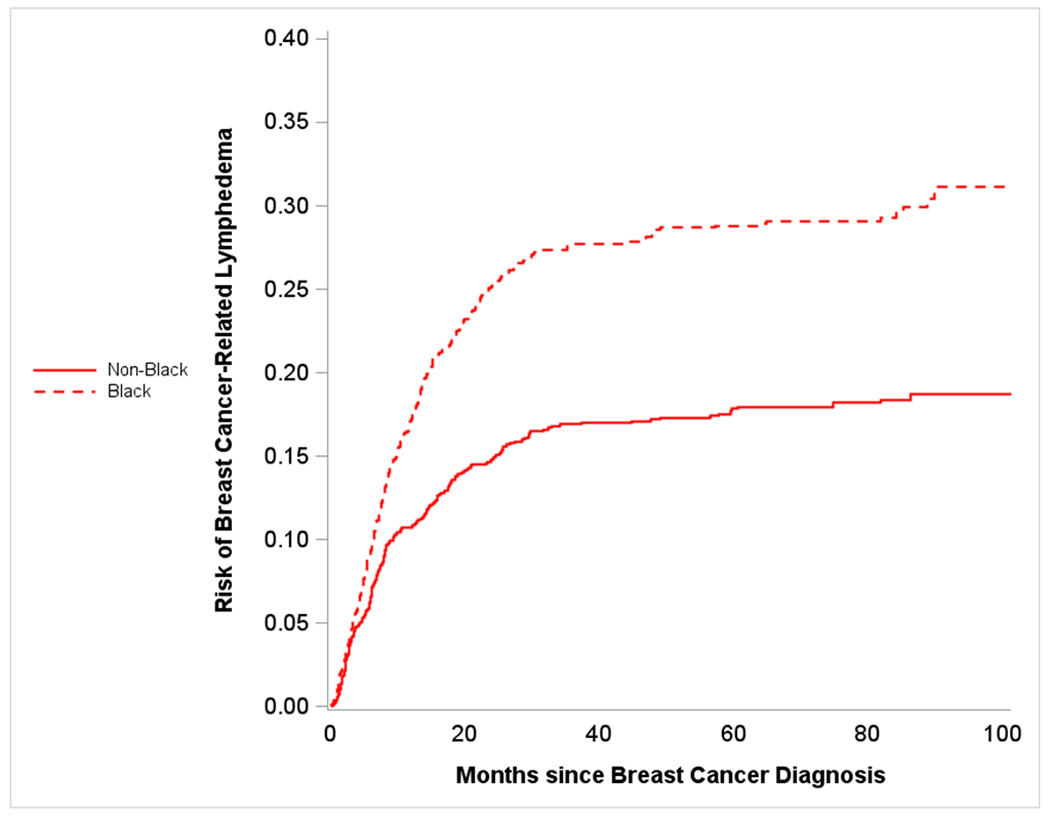
Plot shows crude risk of breast cancer-related lymphedema following surgery among women with breast cancer (stage I-III) stratified by race - Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013). Dashed line represents risk over time for Black participants, and solid line represents risk over time of Non-Black participants.
Figure 1b.
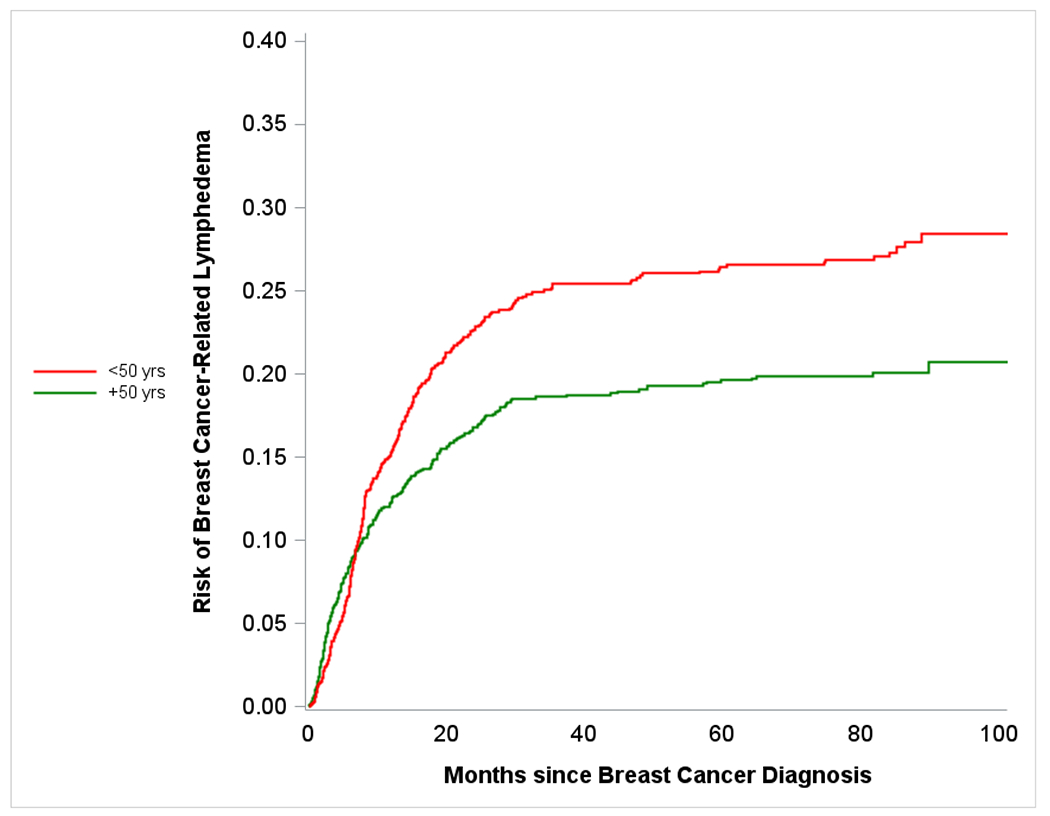
Plot shows crude risk of breast cancer-related lymphedema following surgery among women with breast cancer (stage I-III) stratified by age - Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013). Red line represents risk over time for younger (<50 years in age) participants, and green line represents risk over time of older (≥50 years in age) participants.
Figure 1c.
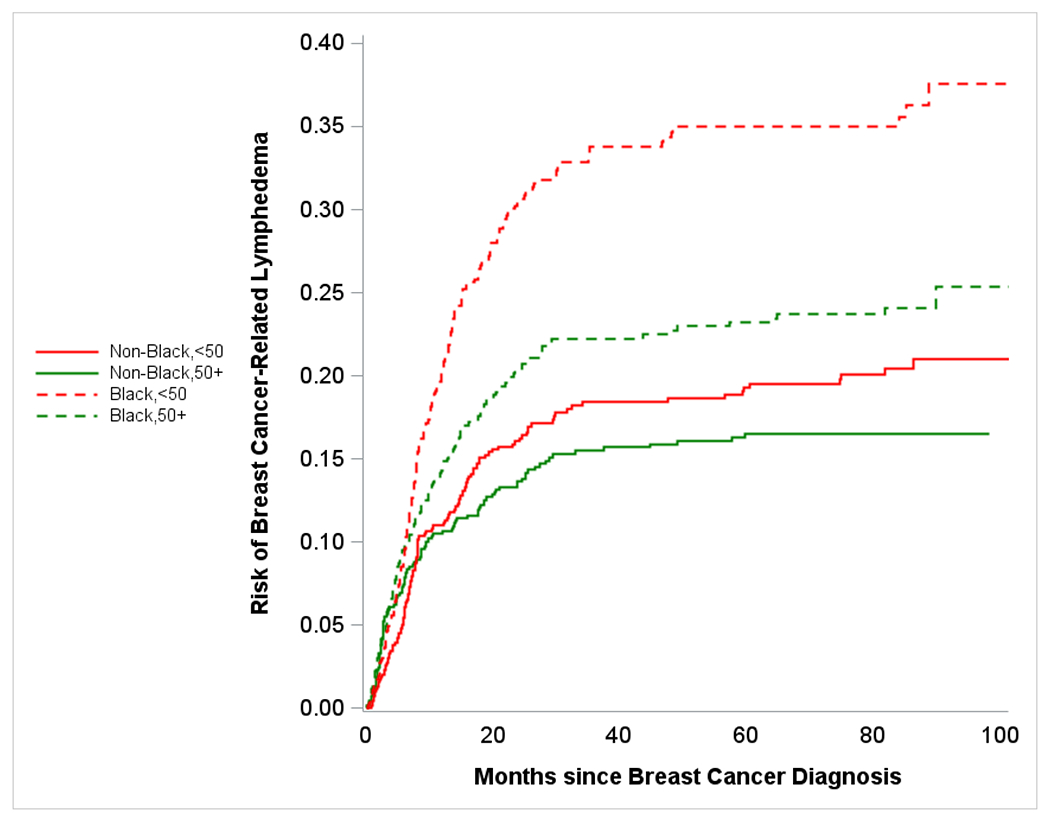
Plot shows crude risk of breast cancer-related lymphedema following surgery among women with breast cancer (stage I-III) stratified by race and age - Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013). Red dashed line represents risk over time for younger (<50 years in age) Black participants, green dashed line represents risk over time of older (≥50 years in age) Black participants, red solid line represents risk over time for younger non-Black participants, green solid line represents risk over time of older non-Black participants.
Table 2a.
5-year risks (%), and hazard ratios (HRs) of breast cancer-related lymphedema following surgery, by follow-up length (FU), for age and race among women with breast cancer (stage I-III) in the Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013)
| 5-year risk (%) | Hazard ratio (95% confidence interval)a | ||||
|---|---|---|---|---|---|
| Age (yr) | Non-Black | Black | Non-Black | Black FU ≤ 7 months |
Black FU > 7 months |
| 50+ | 16.5 (13.6, 19.4) | 23.2 (19.7, 26.7) | 1 | 1.21 (0.85, 1.74) | 1.86 (1.29, 2.66) |
| <50 | 19.3 (16.1, 22.5) | 35.0 (30.8, 39.2) | 1 | 1.68 (1.15, 2.44) | 2.12 (1.59, 2.82) |
| Overall | 17.9 (15.7, 20.0) | 28.8 (26.1, 31.6) | 1 | 1.42 (1.09, 1.83) | 1.98 (1.58, 2.47) |
| Race | Age 50+ yr | Age <50 yr | Age 50+ yr | Age <50 yr FU ≤ 7 months |
Age <50 yr FU > 7 months |
| Non-Black | 16.5 (13.6, 19.4) | 19.3 (16.1, 22.5) | 1 | 0.80 (0.54, 1.17) | 1.69 (1.19, 2.42) |
| Black | 23.2 (19.7, 26.7) | 35.0 (30.8, 39.2) | 1 | 1.09 (0.77, 1.54) | 1.94 (1.45, 2.59) |
| Overall | 19.6 (17.4, 21.9) | 26.3 (23.7, 28.9) | 1 | 0.94 (0.73, 1.22) | 1.80 (1.43, 2.25) |
Cox proportional hazards model for race was only adjusted for age; Cox proportional hazards model for age was only adjusted for race.
Tumor Characteristics and Established Risk Factors
Positive lymph node status, higher BMI, removal of more than 5 lymph nodes, and chemotherapy were significantly associated with both increased hazard of early- and later-onset BCRL (Table 2b). BMI and number of lymph nodes removed had greater hazard ratios in association with early BCRL (1.7- to 2.7-fold versus 1.2- to 1.9-fold in association with later BCRL); whereas lymph node status and chemotherapy had greater hazard ratios in association with later BCRL (2.2- to 3.5-fold versus 1.8- to 2.2-fold in association with early BCRL). Larger tumor size (> 5 cm), ER- subtype, and radiation therapy were only significantly associated with increased hazard of later-onset BCRL (1.5- to 2.3-fold). More extensive surgery (i.e., mastectomy) was only significantly associated with increased hazard of early-onset BCRL (HR=1.46; 95% CI= 1.20 – 1.91). Additionally, histologic grade, side of treatment (dominant versus non-dominant), and baseline comorbidities such as hypertension and diabetes, were not significantly associated with BCRL hazard in either period. Specifically for hypertension and diabetes, we did not observe different BCRL hazards across race groups. Sensitivity analysis adjusting for death as a competing event did not impact significant associations, except for association with histologic grade, which was of similar magnitude but became significant for later-onset BCRL (Supplementary Table 1). Results from the sensitivity analysis restricting to women with doctor-diagnosis only were also similar to those from the primary analysis, with similar magnitude and significance (Supplementary Table 2). We also performed a sensitivity analysis wherein we excluded single episodes of lymphedema, and the results are shown in Supplementary Table 3. Briefly, hazard ratios for demographic and clinical factors were even more pronounced among those with chronic BCRL.
Table 2b.
Hazard ratios (HRs) of breast cancer-related lymphedema (BCRL) following surgery in association with clinical features, by follow-up length, among women with breast cancer (stage I-III) in the Carolina Breast Cancer Study (Phase 3, diagnosis years 2008-2013)
| Clinical features | Total participants (n with BCRL) | Crude 5-y risk of BCRL, % (95% confidence interval) | HR (95% confidence interval)a | ||
|---|---|---|---|---|---|
| FU ≤ 7 months | FU > 7 months | ||||
| Hypertension | No | 1508 (300) | 21.9 (19.6, 24.1) | 1 | 1 |
| Yes | 1137 (252) | 24.3 (21.6, 26.9) | 1.06 (0.82, 1.37) | 1.19 (0.96, 1.48) | |
| Diabetes | No | 2268 (472) | 22.9 (21.0, 24.7) | 1 | 1 |
| Yes | 377 (80) | 23.1 (18.5, 27.7) | 1.30 (0.93, 1.83) | 0.83 (0.59, 1.16) | |
| Lymph node status | Negative | 1657 (224) | 14.3 (12.6, 16.1) | 1 | 1 |
| Positive | 986 (328) | 38.6 (35.2, 42.0) | 2.17 (1.67, 2.81) | 3.50 (2.80, 4.38) | |
| Tumor size | ≤5cm | 2368 (472) | 21.6 (19.9, 23.4) | 1 | 1 |
| >5 cm | 271 (77) | 36.5 (29.6, 43.4) | 0.97 (0.64, 1.48) | 2.28 (1.69, 3.07) | |
| Histologic grade (differentiation) | Well/Moderate | 671 (125) | 20.1 (16.9, 23.4) | 1 | 1 |
| Poor | 1826 (401) | 24.3 (22.2, 26.4) | 0.95 (0.71, 1.27) | 1.23 (0.94, 1.60) | |
| ER status | Positive/Borderline | 1954 (378) | 21.1 (19.2, 23.1) | 1 | 1 |
| Negative | 682 (171) | 28.2 (24.5, 31.9) | 0.90 (0.67, 1.21) | 1.56 (1.24, 1.97) | |
| BMI, kg/m2 | ≤25 | 657 (89) | 14.8 (11.9, 17.8) | 1 | 1 |
| 25-29 | 736 (135) | 20.4 (17.2, 23.5) | 1.71 (1.11, 2.63) | 1.17 (0.83, 1.66) | |
| 30+ | 1241 (327) | 29.0 (26.3, 31.7) | 2.17 (1.47, 3.20) | 1.82 (1.35, 2.45) | |
| Number of lymph nodes removed | ≤5 | 1510 (184) | 12.9 (11.1, 14.7) | 1 | 1 |
| >5 | 1129(366) | 37.1 (34.0, 40.3) | 2.65 (1.99, 3.53) | 1.92 (1.53, 2.41) | |
| Surgery type | Local excision | 1413 (251) | 18.8 (16.7, 21.0) | 1 | 1 |
| Mastectomy | 1232 (301) | 28.0 (25.2, 30.7) | 1.46 (1.20, 1.91) | 1.17 (0.93, 1.47) | |
| Chemotherapy | No | 967 (97) | 10.7 (8.6, 12.7) | 1 | 1 |
| Yes | 1678 (455) | 30.3 (27.9, 32.7) | 1.79 (1.29, 2.47) | 2.24 (1.68, 2.99) | |
| Radiation therapy | No | 725 (117) | 18.2 (15.2, 21.2) | 1 | 1 |
| Yes | 1920 (435) | 24.7 (22.6, 26.7) | 0.88 (0.65, 1.17) | 1.48 (1.10, 2.00) | |
| Treated on dominant side | No | 1318 (286) | 23.4 (20.1, 25.8) | 1 | 1 |
| Yes | 1325 (266) | 22.4 (20.0, 24.9) | 0.84 (0.65, 1.09) | 0.96 (0.77, 1.19) | |
All models were adjusted for age and race. Models for surgery type, number of lymph nodes removed, chemotherapy, radiotherapy were additionally adjusted for tumor characteristics (i.e., tumor size, lymph node status, histologic grade, and ER status).
DISCUSSION
The current study found that the prevalence of BCRL increased over 7 years of follow-up to include at least 20% of BC survivors. Arm was by far the most common site, and most (>80%) were chronic cases. Aggressive tumor features, aggressive clinical treatment, and obesity or overweight status, were associated with increased BCRL hazard in our study. Few prior studies have specifically evaluated the burden of BCRL by race, and in this study we found that younger Black participants had substantially higher 5-year risk of BCRL. Additionally, compared to non-Black women, a larger proportion of Black women reported BCRL following breast cancer surgery, underscoring the high burden of BCRL experienced by these patients. Lastly, younger patients also had a higher burden of BCRL compared to older patients. We also considered BCRL during two windows: early onset (i.e., occurring within 7 months post BC diagnosis) and later onset (i.e., occurring >7 months post BC diagnosis), and found that risk factors differed slightly between these windows, with surgery type associated with only early-onset BCRL; tumor size, ER status, and radiation therapy associated with only later onset; and lymph node status, BMI, number of lymph nodes removed, and chemotherapy associated with both early and later onset.
Prevalence of BCRL in our study are similar to previous studies.2,28 Our study reported slightly higher BCRL prevalence at the 7-year follow-up compared to the pooled estimate (21.0%; 95% CI=15.1 – 28.5%) in North America from a meta-analysis by DiSipio et al.2 That study also included a study of long-term (up to 20 years) BCRL, but only focused on unilateral arm BCRL and no other sites (i.e., breast, trunk).2,28 Our result is consistent with the pooled estimate for prospective cohort studies (21.4%; 95% CI=14.9 – 29.8%) from that same meta-analysis, and it is higher than the estimate for randomized clinical trials (10.4%) and retrospective studies (8.4%), of which more than 80% used objective measurements such as arm circumferences to define BCRL.2 Different methods of outcome classification for BCRL (e.g., physical measurement, medical record abstraction, self-report) have different levels of sensitivity and specificity.29 In our study, we used self-reported questionnaires asking participants to report their BCRL diagnosis by health care providers (not to report BCRL-related symptoms such as arm swelling), including medical doctor, nurse, and physical therapist. Clinical practice suggests that any postoperative swelling could be considered as BCRL by untrained eyes, and even by some less experienced therapists.30,31 Therefore, the prevalence of BCRL from our study is likely more sensitive, but also potentially less specific. While self-report is over sensitive, physical measurement and medical record extraction tend to under-report BCRL presentation.32
Mitigating some concern about outcome classification, we did find associations with risk factors that were consistent with previous estimates from both cohort studies and medically-validated clinical trials.2 We note that in our study, we emphasized burden, unadjusted for all possible clinical variables for each risk factor. While it is well-known that cancer aggressiveness varies by demographic factors,17 the attendant complications of aggressive tumors (in this case BCRL) are not well documented. Therefore, we presented burden of BCRL by demographics without adjusting for clinical factors. Previous literature2 has identified the influence of clinical factors on BCRL and we have also included these associations in our work. For example, both our study and previous literature2 offered accumulating evidence that more extensive treatment (in particular mastectomy and axillary lymph node dissection), as well as adjuvant therapy such as chemotherapy and radiation therapy, increase the risk of BCRL, although mastectomy only influenced the hazard of early BCRL and radiation therapy only influenced the hazard of later BCRL. Our study also demonstrates that being overweight or obese was associated with increased hazard of BCRL (1.2- to 2.2-fold), consistent with the estimate from previous literature.2
It is concerning that some demographic groups experience higher burden of BCRL. Consistent with Kwan et al.,11 we observed higher hazard in Black patients compared to non-Black patients. In our analysis, we interpret race as a social construct, and we note that many factors including tumor subtype and access to care differ by race in the CBCS cohort, which could further affect women’s risk of BCRL.17 Two previous studies did not find racial differences in BCRL risk after multivariable adjustment.10,12 However, it is difficult to compare our results to those studies because they sought to estimate effect of race after clinical factor adjustment, while we sought to estimate burden, and therefore did not adjust for clinical risk factors. One previous study has looked at race as a modifier of BCRL risk. Namely, the Togawa study reported that hypertension was a BCRL risk factor only for Black women.10 In the larger CBCS population, we found that hypertension did not significantly increase BCRL hazard either overall or within race groups.
A principal strength of this study is a diverse population based on a large population-based prospective cohort composed of nearly 50% Black women and 50% younger women. This yielded novel insights. For example, most previous studies did not identify age as a risk factor, and had few younger patients.14 Additionally, more than 50% of the evidence contributing to BCRL clinical associations is drawn from cross-sectional or retrospective cohort studies, predominantly among white and older women.2,11,14,33–39 Of the prospective cohort studies, 90% involve samples sizes <1000, limiting statistical power.2,10,11,40–47 Our cohort also has a long-term follow-up (i.e., up to 7 years post BC diagnosis), with detailed questionnaires and medical record abstraction. While duration of follow-up has varied (6 months to 20 years post BC diagnosis) for previous research,2 the largest study (n>5000) had short follow-up (6 months post diagnosis).48 BCRL incidence tends to peak between 12-18 months post-diagnosis,2,23 but our data showed that some cases do continue to accrue in the two- to seven-year interval.
There were also some limitations for our study, including self-reported outcome assessment. We note that episodic nature (classification as single, recurrent, and persistent) in our study is not typically how BCRL is described. BCRL usually progresses through a series of stages from 0 to 3, which represent symptom severity and could impact normal functioning. However, conventional staging is not available for our study. Future studies should continue to examine how BCRL stage affects quality of life. On the other hand, by looking at self-reported data, our study revealed that nearly 20% of the cases reported their BCRL as a single episode rather than a chronic condition. This conflicts with the notion that presence of BCRL at any point in time reflects a compromised lymphatic system, and that even if swelling reduces, it continues to be a “poor” performing system which could be overloaded easily.24 Resolution of self-reported BCRL suggests that either some single episodes reflect normal post-surgical or post-radiation swelling and move rapidly into a subclinical or remission-like state, or some people experience less bothersome symptoms over time, because the number of single episodes has been accumulating during the follow-up even after the maximum time (i.e., 14 months post BC diagnosis) of first course surgery completion. Patients treated with taxane-based chemotherapy may also experience mild swelling (23%) that could be misinterpreted as lymphedema in self-reported data.42 However, although some patients may experience a single BCRL episode, it does not preclude future onset and they remain even at enhanced risk due to the compromised lymphatic system. Additionally, we did not assess whether lymphedema presenting in different sites (i.e., breast, trunk, or arm) might have disparate risk factors. As local excision as BC treatment and breast radiation can cause puffiness in the breast, which typically resolves over time,49 whereas lymphedema of the arm is more likely to become a chronic problem,23 factors associated with trunk/breast lymphedema might differ from those associated with arm lymphedema. Therefore, a future study is needed to take lymphedema sites into account. Nonetheless, by exploring risk factors by timing of presentation identified a more comprehensive list of risk factors that may have otherwise not been identified. Understanding risk factors provides the necessary platform for identifying who may benefit most from risk reduction strategies.
In summary, our study presents prevalence and cumulative burden of BCRL among BC survivors after surgery. The results highlight that groups who already suffer BC disparities (i.e. younger Black women, and women with more aggressive cancer and more extensive treatment) are at increased hazard of developing BCRL. BCRL can reduce quality of life and inhibit ability to return to work, a feedback loop that has multiple consequences. Continued research on pathways of age and race to BCRL is critical for developing preventive strategies for women after BC surgery.
Supplementary Material
Acknowledgments:
The authors would like to acknowledge the University of North Carolina BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (http://bsp.web.unc.edu/). We are grateful to CBCS participants and study staff.
Funding statement:
This research was supported by a grant from UNC Lineberger Comprehensive Cancer Center, which is funded by the University Cancer Research Fund of North Carolina, the Susan G Komen Foundation (OGUNC1202), the National Cancer Institute of the National Institutes of Health (P01CA151135), and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA058223). This research recruited participants &/or obtained data with the assistance of Rapid Case Ascertainment (RCA), a collaboration between the North Carolina Central Cancer Registry and UNC Lineberger. RCA is supported by a grant from the National Cancer Institute of the National Institutes of Health (P30CA016086).
Footnotes
Conflict interest statement: We have no conflicts of interest to disclose.
REFERENCES
- 1.Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland surgery. 2018;7(4):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The lancet oncology. 2013;14(6):500–515. [DOI] [PubMed] [Google Scholar]
- 3.Rockson SG. Lymphedema. The American journal of medicine. 2001;110(4):288–295. [DOI] [PubMed] [Google Scholar]
- 4.Thomas-MacLean R, Miedema B, Tatemichi SR. Breast cancer-related lymphedema: women’s experiences with an underestimated condition. Canadian Family Physician. 2005;51(2):246–247. [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto M, Gimigliano F, Tatangelo F, et al. Upper limb function and quality of life in breast cancer related lymphedema: a cross-sectional study. Eur J Phys Rehabil Med. 2013;49(5):665–73. [PubMed] [Google Scholar]
- 6.Eaton L, Narkthong N, Hulett J. Psychosocial issues associated with breast cancer-related lymphedema: a literature review. Current Breast Cancer Reports. 2020;12(4):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean LT, Moss SL, Ransome Y, et al. “It still affects our economic situation”: long-term economic burden of breast cancer and lymphedema. Supportive Care in Cancer. 2019;27(5):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson KY, Wallenius I, Nilsson-Wikmar LB, Lindman H, Johansson BB. Lymphoedema and health-related quality of life by early treatment in long-term survivors of breast cancer. A comparative retrospective study up to 15 years after diagnosis. Supportive Care in Cancer. 2015;23(10):2965–2972. [DOI] [PubMed] [Google Scholar]
- 9.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Annals of surgical oncology. 2009;16(7):1959–1972. [DOI] [PubMed] [Google Scholar]
- 10.Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Research. 2014;16(4):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan ML, Yao S, Lee VS, et al. Race/ethnicity, genetic ancestry, and breast cancer-related lymphedema in the Pathways Study. Breast cancer research and treatment. 2016;159(1):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast cancer research and treatment. 2009;113(2):383–391. [DOI] [PubMed] [Google Scholar]
- 13.Kwan ML, Darbinian J, Schmitz KH, et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Archives of surgery. 2010;145(11):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guliyeva G, Huayllani MT, Boczar D, Avila FR, Lu X, Forte AJ. Age as a risk factor for breast cancer-related lymphedema: a systematic review. Journal of Cancer Survivorship. 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Shang T, Liang J, Kapron CM, Liu J. Pathophysiology of aged lymphatic vessels. Aging (Albany NY). 2019;11(16):6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee SA, Durham DD, Tse C-K, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiology and Prevention Biomarkers. 2013;22(7):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerson MA, Golightly YM, Tan X, et al. Integrating access to care and tumor patterns by race and age in the Carolina Breast Cancer Study, 2008–2013. Cancer Causes & Control. 2020;31(3):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinheiro LC, Tan X, Olshan AF, et al. Examining health-related quality of life patterns in women with breast cancer. Quality of Life Research. 2017;26(7):1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer JC, Reeve BB, Troester MA, Wheeler SB. Factors associated with endocrine therapy non‐adherence in breast cancer survivors. Psycho‐Oncology. 2020;29(4):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American journal of public health. 2008;98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allott EH, Cohen SM, Geradts J, et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER ConsortiumAccuracy of Protein and RNA-Based Breast Cancer Subtyping. Cancer Epidemiology, Biomarkers & Prevention. 2016;25(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. The oncologist. 2013;18(9):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borman P Lymphedema diagnosis, treatment, and follow-up from the view point of physical medicine and rehabilitation specialists. Turkish journal of physical medicine and rehabilitation. 2018;64(3):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacomba MT, Sánchez MJY, Goñi ÁZ, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. Bmj. 2010;340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Computer methods and programs in biomedicine. 2004;75(1):45–49. [DOI] [PubMed] [Google Scholar]
- 27.Yi M, Meric‐Bernstam F, Ross MI, et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer. 2008;113(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. [DOI] [PubMed] [Google Scholar]
- 29.Hayes S, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema secondary to breast cancer: how choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008;41(1):18–28. [PubMed] [Google Scholar]
- 30.Gebruers N, Verbelen H, De Vrieze T, et al. Current and future perspectives on the evaluation, prevention and conservative management of breast cancer related lymphoedema: A best practice guideline. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2017;216:245–253. [DOI] [PubMed] [Google Scholar]
- 31.Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A. Cancer-associated secondary lymphoedema. Nature reviews Disease primers. 2019;5(1):1–16. [DOI] [PubMed] [Google Scholar]
- 32.Czerniec S, Ward L, Refshauge K, et al. Assessment of breast cancer-related arm lymphedema—comparison of physical measurement methods and self-report. Cancer investigation. 2010;28(1):54–62. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer-related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Annals of surgical oncology. 2017;24(10):2972–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoishi Y, Oura S, Nishiguchi H, et al. Risk factors for breast cancer-related lymphedema: correlation with docetaxel administration. Breast Cancer. 2020;27(5):929–937. [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Park IH, Lee KS, et al. Breast cancer–related lymphedema after neoadjuvant chemotherapy. Cancer research and treatment: official journal of Korean Cancer Association. 2015;47(3):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung S-Y, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast cancer research and treatment. 2014;148(1):91–98. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y-f, Liu J-E, Mak YW, et al. Prevalence and predictors of breast cancer-related arm lymphedema over a 10-year period in postoperative breast cancer patients: A cross-sectional study. European Journal of Oncology Nursing. 2021;51:101909. [DOI] [PubMed] [Google Scholar]
- 38.Park S, Lee JE, Yu J, et al. Risk factors affecting breast cancer-related lymphedema: serial body weight change during neoadjuvant anthracycline plus cyclophosphamide followed by taxane. Clinical breast cancer. 2018;18(1):e49–e54. [DOI] [PubMed] [Google Scholar]
- 39.da Costa Vieira RA, Da Costa AM, De Souza JL, et al. Risk factors for arm lymphedema in a cohort of breast cancer patients followed up for 10 years. Breast Care. 2016;11(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armer JM, Ballman KV, McCall L, et al. Factors associated with lymphedema in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection. JAMA surgery. 2019;154(9):800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boughey JC, Hoskin TL, Cheville AL, et al. Risk factors associated with breast lymphedema. Annals of surgical oncology. 2014;21(4):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swaroop MN, Ferguson CM, Horick NK, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast cancer research and treatment. 2015;151(2):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts SA, Gillespie TC, Shui AM, et al. Weight loss does not decrease risk of breast cancer–related arm lymphedema. Cancer. 2021;127(21):3939–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilbreath SL, Refshauge KM, Beith J, et al. Risk factors for lymphoedema in women with breast cancer: a large prospective cohort. The Breast. 2016;28:29–36. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. Journal of Clinical Oncology. 2016;34(7):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira ACPR, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. The Breast. 2017;36:67–73. [DOI] [PubMed] [Google Scholar]
- 47.Asdourian MS, Swaroop MN, Sayegh HE, et al. Association between precautionary behaviors and breast cancer–related lymphedema in patients undergoing bilateral surgery. Journal of Clinical Oncology. 2017;35(35):3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Annals of surgical oncology. 2006;13(4):491–500. [DOI] [PubMed] [Google Scholar]
- 49.Verbelen H, Tjalma W, Dombrecht D, Gebruers N. Breast edema, from diagnosis to treatment: state of the art. Archives of physiotherapy. 2021;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


