Abstract
Clomiphene citrate is the first-line treatment for women with abnormal or failed ovulation. Currently, it is available as oral tablets, and the parenteral formulation does not exist. In this study, we prepared clomiphene citrate-hydroxypropyl-β-cyclodextrin inclusion complex for its use in intravenous injection. The inclusion complex was characterized in the liquid state (phase solubility) and solid state by differential scanning calorimetry, Fourier transform infrared spectroscopy, and nuclear magnetic resonance spectroscopy analyses. The sterile intravenous injection containing 0.5% clomiphene citrate was prepared and characterized for its physical properties, assay, pH, and osmolality. A stability-indicating high-performance liquid chromatography (HPLC) method for the injection was developed. The HPLC method was validated for the assay, linearity, precision and repeatability, benchtop stability, and forced degradation to elute clomiphene isomers from the degradation products. The injection was packed in sterile 10-ml glass vials with butyl rubber stoppers and stored at 40°C, room temperature, and 4°C. The samples at 0, 0.5, 1, 2, 3, and 6 months were analyzed for clarity, pH, osmolality, and drug assay. The HPLC method was linear (R2 = 0.9999), precise (0.86% relative standard deviation), and stability indicating. The stability data at the accelerated (40°C) storage condition for 6 months showed satisfactory results: the drug assay in the injection was between 90 and 105%, the injection remained clear, pH was between 4.0 and 4.4, and osmolality was between 270 and 350 mOsm. The stability data suggests that the product is stable and meets the given analytical specifications.
Supplementary Information
The online version contains supplementary material available at 10.1208/s12249-023-02513-y.
Keywords: clomiphene citrate, hydroxypropyl-β-cyclodextrin, inclusion complex, injection, stability
Introduction
Clomiphene citrate [2-(4-(chloro-1,2-diphenylethenil)-phenoxyl)-N, N-diethylethanamine] is a stilbene-based synthetic antiestrogen compound with weak estrogenic properties. Clomiphene citrate as 50-mg tablets is often prescribed as a first-line treatment for women having difficulty with conception because of abnormal or failed ovulation. It is a member of class of drugs referred to as selective estrogen receptor modulators (SERMs) and binds to estrogen receptors in the hypothalamus, ovary, endometrium, and cervix. This binding of estrogen receptors interrupts the negative feedback normally in the presence of increasing estrogen concentrations, augments GnRH pulsatility, and increases the systemic concentration of follicle-stimulating and luteinizing hormones (FSH and LH) [1–4]. The results of this cascade can be an increase in the number of ovarian follicles and increased rates of follicular ovulation [5–8]. Clomiphene citrate overcomes one of the biggest challenges in the world of in vitro fertilization (IVF) and in vitro embryo production (IVP) in that, while it increases LH secretion, it simultaneously suppresses the LH surge. Unfortunately, this undesired and premature LH surge is responsible for the costly cancellation of oocyte collection procedures in a significant number of IVF treated cycles [7, 9, 10]. Reports of increased oocyte availability and fertilization rates following clomiphene citrate therapy provide promise for the improvement in the efficiency of IVP of the embryo [7, 8]. Overall, the use of clomiphene citrate for IVF appears to increase the chances that a couple will experience the successful outcome of receiving an IVP embryo(s) [6–8].
Pharmacological manipulation of reproductive hormones utilizing clomiphene citrate has also been documented in veterinary species [11–14], but a significant barrier to the applied research of clomiphene citrate still exists in that bioavailability and other pharmacokinetic data is nonexistent. Clomiphene citrate is only available in tablets, but if an effective effort at assessing the feasibility of clomiphene citrate use in veterinary species is to be put forth, it would be desirable for the development of an injection for intravenous administration. Utilization of an intravenous formulation would serve an important role in that it could act as a reference formulation for comparison to that of oral pharmacokinetics of clomiphene citrate in veterinary practice. Additionally, being that oral bioavailability can be compromised in veterinary species for various reasons (e.g., herbivorous diets, retention of fermentative bacteria within the stomach’s microbiome, the continuous presence of feed within the stomach, patient propensity for promptly spitting out orally administered medication), drugs that can be administered via alternative routes can be advantageous in veterinary medicine [15].
A parenteral liquid formulation can be a solution, a suspension, or an emulsion for injection/infusion. A clear drug solution is usually preferred, especially for intravenous administration. Many drugs are insoluble and require the use of pharmaceutical solvents and surfactants as solubilizers. The formulation also contains inactive ingredients such as buffers to stabilize the drug for enhanced shelf-life and to render the formulation isosmotic with biological fluids. Many drugs are poorly soluble in water and organic solvents, making it difficult to develop parenteral formulations. Among many solubility enhancement techniques, cyclodextrins (CD) have been widely utilized to enhance the aqueous solubility of hydrophobic drugs via the formation of inclusion complexes enabling the formulation of parenteral dosage forms [16, 17]. Hydroxypropyl-β-cyclodextrin (HPβCD) and sulfobutylether-β-cyclodextrin (SBEβCD) are approved by various regulatory agencies for use in intravenous injection formulations for human and veterinary use. As an example, HPβCD is used in the formulation of diclofenac sodium injection (Dyloject), itraconazole (Sporanox), and mitomycin (MitoExtra), and SBEβCD is used in the formulation of remdesivir (Veklury), aripiprazole (Abilify), and voriconazole (Vfend) for intravenous use.
Clomiphene citrate is poorly soluble in water and other pharmaceutical solvents, making it difficult to develop intravenous injection. Our preliminary studies attempting the use of parenteral organic solvents were not successful in solubilizing clomiphene citrate for intravenous injection formulation. Hence, we postulate that clomiphene citrate inclusion complex with HPβCD can provide an injection formulation with desired attributes such as increased aqueous solubility, pH, osmolality, and physical and chemical stability for intravenous administration. The objective of the study was to characterize the clomiphene citrate HPβCD complexation in the solid and liquid states and formulate an injection for intravenous use. We have conducted stability studies on the clomiphene injection using a validated, stability-indicating reversed-phase high-performance liquid chromatography (RP-HPLC) assay method.
Materials and Methods
Materials
Clomiphene citrate (C6272) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Endotoxin controlled hydroxypropyl-β-cyclodextrin (THPB-EC) was procured from CTD Inc. (Alachua, FL, USA), and Super Refined® propylene glycol was obtained from Croda Inc. (Mill Hall, PA, USA). Methanol (HPLC grade), sodium hydroxide (ACS grade), hydrochloric acid (ACS grade), and hydrogen peroxide (3% solution), Whatman® 25 mm, and 0.2-µm nylon membrane filters were procured from VWR International (Suwanee, GA, USA). Milli-Q water was obtained from an in-house Millipore water purification unit.
Methods
Saturation Solubility of Clomiphene Citrate
The saturation solubility of the drug was determined in various solvents at room temperature. An excess amount of the drug was added to each of the solvents in a glass vial, and then the resulting mixture was shaken reciprocally at 25°C for 24 h and further equilibrated for 24 h. The samples were then centrifuged at 10,000 rpm for 10 min and filtered through a 0.45-µm nylon membrane filter. Finally, the solution was diluted appropriately with the HPLC mobile phase (70% methanol acidified with trifluoroacetic acid, or TFA) and analyzed as described in the HPLC section.
Preparation of Clomiphene-HPβCD Complex
Dry Mixture
Clomiphene citrate and HPβCD were weighed and gently mixed in equimolar concentrations using mortar and pestle to achieve a homogenous mass. The mixture was then stored in a tightly sealed glass vial inside a vacuum desiccator with 15 ± 2% RH (relative humidity) until needed for quantification and further analysis.
Freeze-Dried Complexation
Clomiphene citrate was solubilized entirely in methanol and added to an aqueous solution of HPβCD in an equimolar ratio of 1:1. The solution was mixed, vortexed for 5 min, followed by sonication under an ice bath for 20 min to ensure dissolution. The solution was filtered using a nylon syringe filter (0.22 µm) and kept open at 40°C in the oven for an hour to ensure the removal of methanol. The sample was stored in a deep freezer for 24 h at − 80°C before being subjected to freeze drying in a Labconco FreeZone Freeze Dryer (Welch 8917 vacuum pump) until a dry fluffy mixture was obtained. The freeze-dried complex was then stored in a tightly capped glass vial for further characterization inside a vacuum desiccator with 15 ± 2% RH.
Phase Solubility Studies
Phase solubility studies were conducted according to the method suggested by Higuchi and Connors (1965) [18]. An excess amount of clomiphene citrate, exceeding its solubility, was added to 2 ml of aqueous solutions of HPβCD at different concentrations ranging from 1 to 25 mM. The solutions were capped in a 5-ml vial to avoid changes due to evaporation. The mixtures were vortexed for 5 min, followed by sonication for 30 min, to confirm the presence of the insoluble drug. The tubes were kept in a shaker (150 rpm at 25°C) for 24 h, which was followed by 24 h of benchtop equilibration at room temperature. Following equilibration, the mixtures were filtered using a 0.45-µm nylon filter, diluted appropriately in a diluent, and analyzed for clomiphene citrate concentration by HPLC. The drug concentration was calculated from the standard curve of clomiphene citrate (R2 > 0.9999, concentration range = 2–200 µg/mL). All the samples in the experiments were conducted in triplicate. The data were used to obtain saturation solubility of clomiphene citrate, binding constant of inclusion complex (clomiphene citrate-HPβCD), and complexation efficiency according to Higuchi and Connors equation. The drug concentration remained constant, and no degradation was observed throughout the experiments.
The apparent stability rate constant (Ks) was calculated using the slope of the phase solubility plot and the saturation solubility (So) of the clomiphene citrate (Eq. 1) without the addition of cyclodextrins.
The complexation efficiency (CE), expressing the ratio of cyclodextrins to clomiphene citrate, was calculated using the following equation (Eq. 2):
The change in the Gibbs free energy was calculated using the following equation:
where R is the ideal gas constant (8.314 J/molK) and T is the temperature (295 K).
Thermal Analysis Using Differential Scanning Calorimetry (DSC)
Thermal analysis of clomiphene citrate, HPβCD, dry mixture, and freeze-dried complex with clomiphene citrate:HPβCD in a molar ratio of 1:1 were performed using DSC Q200 calorimeter (V24.10, TA Instruments). Samples were weighed (between 2 and 5 mg), placed on a non-hermetic aluminum pan, covered with a lid, and sealed with an encapsulating crimping press. The samples were heated under an inert atmosphere of nitrogen gas with a temperature range from 10 to 250°C at a heating rate of 10°C/min. An empty aluminum pan with a lid was used as a reference pan.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra for clomiphene citrate, HPβCD, dry mixture, and freeze-dried complex were recorded using a Perkin Elmer Spectrum 400 FT-IR/ FT-NIR spectrophotometer with a resolution of 4 cm−1, and the detector scanned a range from 4000 to 650 cm−1. A total of 64 scan accumulations were performed for each spectrum.
Nuclear Magnetic Resonance (NMR) Studies
Proton nuclear magnetic resonance spectroscopy studies (1H NMR) were performed using Varian (400 MHz) Premium Shield NMR Spectrometer. Clomiphene citrate was dissolved in deuterated dimethyl sulfoxide (DMSO), HPβCD in deuterated D2O, dry mixture, and freeze-dried mixture in D2O: DMSO (50:50 v/v).
Formulation of 0.5% Clomiphene Citrate Intravenous Injection
Based on the solubility and complexation studies, we find that use of HPβCD (20% w/v) was necessary to achieve the drug solution for injection. Clomiphene citrate solubility was inadequate in various organic solvents for their use in the parenteral preparation. Propylene glycol was added to achieve the iso-osmotic solution for injection. Propylene glycol concentration was chosen within the permissible limits of intravenous injection according to the inactive ingredients database of the US Food and Drug Administration (FDA).
HPβCD was added in portions to water for injection at 20% w/v concentration and dissolved under stirring. Super Refined® propylene glycol (1.0% w/v) was then added and mixed well. Finally, clomiphene citrate (0.5% w/v) was added in small portions under stirring until a clear solution was obtained. The final volume was made up to 100 ml with water for injection. The solution was filtered aseptically under a laminar hood using a sterile 0.22-µm nylon membrane filter. The filtered solution was collected directly into pre-sterilized glass vials and crimp sealed with butyl rubber stoppers and aluminum casing. For the stability study, three batches (batch 1, 2, 3) of 100 ml each were prepared. The following analytical stability parameters were evaluated.
Characterization of 0.5% Clomiphene Citrate Intravenous Injection
Visual Appearance
Three batches of the formulation stored at different temperatures were visually observed against a white background for 0, 0.5, 1, 2, 3, and 6 months. The formulations should be clear, transparent, and free of particles or precipitation.
pH
The pH of the injections was measured for three stability batches using a pH meter (Fisher Scientific Accumet® XL150) at room temperature. The pH meter was initially calibrated, and the measurements were conducted at 0, 0.5, 1, 2, 3, and 6 months for three different storage temperatures. The acceptable pH range of the formulations is between 4 and 8.
Osmolality
The osmolality of the formulations was determined using a vapor pressure osmometer (VAPRO®) at room temperature using the vapor pressure depression principle. The osmolality measurements were conducted for initial, 0.5, 1, 2, 3, and 6 months for three different storage temperatures. The acceptable range of the preparations is less than 450 mOsm.
HPLC Method Development and Validation
Chromatographic Conditions
Analytical method development and validation were performed using a Waters HPLC system equipped with Alliance e2695 Separation module plus autosampler and 2998 PDA detector and a thermostatically controlled column compartment. Chromatographic separation of two isomers (Fig. 1) and their degradation products was achieved on a Phenomenex, Luna® C18 column (5 µm, 250 × 4.6 mm, P/N: 00G-04252-E0). The mobile phase consisted of methanol:water with 0.05% TFA (70:30%v/v), which was run at a flow rate of 1 ml/min. The samples were injected in 10-µL volumes, and the run time was set to 10 min, the detection wavelength was set at 245 nm, and the column temperature was set at 40°C. Isocratic elution was applied for the separation of clomiphene citrate isomers and their degradation products [19]. The data was collected using Empower® 3 software (Waters Corp. Milford, MA, USA).
Fig. 1.
Chemical structures of Z- and E-clomiphene citrate
HPLC Method Validation
System Suitability
The clomiphene citrate intravenous solution (100 μg/ml) was injected six times and peak area responses were collected. The tailing factor, theoretical plate number, and relative standard deviation (RSD) values for Z-clomiphene and E-clomiphene peaks were determined.
Linearity
A calibration curve for clomiphene citrate was generated using the optimized chromatographic conditions, as mentioned earlier. Standard solutions of clomiphene citrate were prepared at different concentrations ranging from 0.5 to 200 µg/ml, and each concentration was analyzed in triplicate. Regression analysis for the results was processed using the least-square method. The correlation coefficient (r) value should be not less than 0.999.
Precision and Repeatability
Precision and repeatability were determined by analyzing six samples of clomiphene citrate injection at 100% target concentration. For the assessment of intraday and interday precision, samples were analyzed in triplicate on the same day and on different days, respectively. The RSD for the precision and repeatability samples was determined. The mean percentage recovery must be between 95 and 105% of the theoretical assay value, and the RSD should be no more than 2.0.
Solution Stability
The benchtop solution stability of the clomiphene citrate intravenous injection and standard solution were analyzed by storing them at ambient room temperature for 96 h. The responses of the stored solutions against freshly prepared standards were measured, and any concentration changes were noted. The % assay for the samples should be within 95–105% of the initial results.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOD for clomiphene were estimated by injecting a series of dilute solutions with known concentrations. Solutions of clomiphene were prepared in the range of 0.1–10 µg/ml and injected in triplicate. The LOD and LOQ for Z- and E-clomiphene were calculated at signal-to-noise ratios of 3:1 and 10:1, respectively [20].
Stress Study (Forced Degradation Study)
The clomiphene citrate intravenous injection and placebo formulations in 0.5-g quantities were exposed to acidic, alkaline, and oxidative conditions, i.e., 1 ml of 1 N HCl at 70°C for 24 h, 1 ml of 1 N NaOH at 70°C for 1 h, and 1 ml of 3% H2O2 at room temperature for 24 h, respectively. An untreated standard with and without heating at 70°C for 24 h was used as a control. After exposures, the reaction was terminated by neutralizing, and the volume was made to 25 ml with diluent. The percentage degradation and the % drug recovery along with purity angle/threshold were noted for Z- and E-clomiphene citrate.
Accelerated Stability Study of 0.5% Clomiphene Citrate Injection
Three batches of 0.5% clomiphene citrate injection were packed into sterile 10-ml glass containers with butyl rubber stoppers. The samples were then stored at 40°C, ambient room temperature (RT) ~ 23°C, and refrigerated temperature ~ 4°C for 6 months. The stability parameters were analyzed for each storage condition at 0, 0.5, 1, 2, 3, and 6 months. The stability study was performed to verify that the integrity of the product is within the analytical specifications, i.e., physical stability (color changes, clarity, free from any visible particulates), pH in the range of 4.0–8.0, osmolality in the range of 260–340 mOsm, and the clomiphene citrate assay not less than 90% and not more than 110% of the label claim.
Results
Characterization of Inclusion Complex in the Liquid and Solid State
Saturation Solubility and Phase Solubility Studies of Clomiphene Citrate
Table I shows the saturation solubility of clomiphene citrate in various pharmaceutical solvents. Clomiphene citrate has poor solubility in water (0.015 ± 0.006 mg/ml) and in the pharmaceutical solvents tested. The inclusion complexation of clomiphene citrate with HPβCD in the liquid state is shown in the phase solubility diagram (Fig. 2). A linear increase in the solubility of the clomiphene citrate with increasing HPβCD concentration is classified as an AL-type phase solubility curve (Higuchi and Connors 1965). The overall aqueous solubility of clomiphene citrate increased from 0.043 ± 0.023 mM to 2.258 ± 0.031 mM (> 50-folds) at 25 mM of HPβCD. The stability constant (Ks) for the complexation was found to be very high at 3828 M-1 (So = 0.0153 mg/ml). The complexation efficiency was found to be 0.1, whereas the change in the Gibbs free energy was calculated as − 18.8 kJ/mol, indicating the rapid spontaneous formation of the complex between clomiphene citrate and HPβCD [21].
Table I.
Solubility of Clomiphene Citrate in Various Solvents at 25°C. Values Represent Mean ± SD, n = 3
| Solvent | Solubility of clomiphene citrate (mg/ml ± SD) |
|---|---|
| Water | 0.015 ± 0.006 |
| Ethanol | 1.12 ± 0.18 |
| Tween 80 | 3.81 ± 0.04 |
| PEG 400 | 1.95 ± 0.10 |
| Propylene glycol | 1.16 ± 0.50 |
Fig. 2.
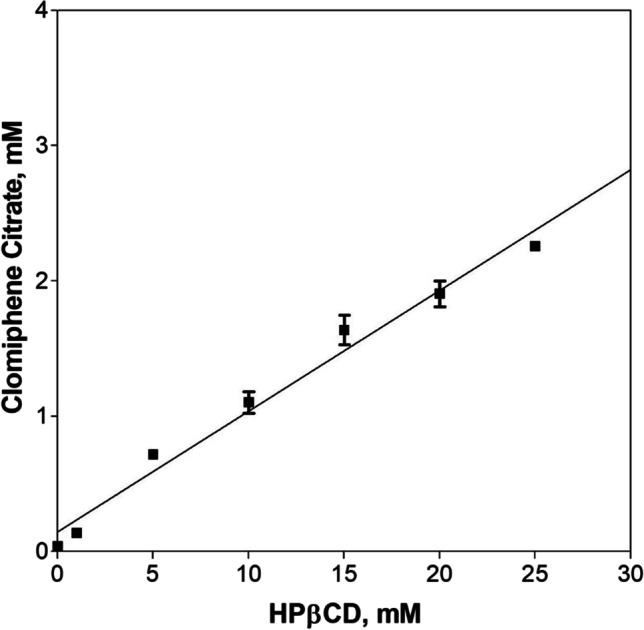
Phase solubility diagram of clomiphene citrate with the increasing concentrations of HPβCD
Differential Scanning Calorimetry (DSC)
Thermal analysis of the complex and pure drug was performed using differential scanning calorimetry, in which the successful complexation was shown by the absence of the shift of the drug peak. Figure 3 compares the thermograms of pure clomiphene citrate, HPβCD, dry mixture (drug:CD at 1:1 molar ratio), and freeze-dried complex (drug:CD at 1:1 molar ratio). The thermogram of clomiphene citrate demonstrated an endothermic peak at 114.94°C and 121.75°C, suggesting two melting points for clomiphene citrate isomers. HPβCD thermogram displayed a broad endothermic peak at 124.17°C, which is indicative of the loss of water molecules from the cyclodextrin core [22]. The thermogram of the dry mixture showed two peaks of drug superimposed into the thermogram of cyclodextrin, corresponding to endothermic shifts at 100.55°C and 118.51°C. The shift could indicate weak interactions between clomiphene citrate and HPβCD. The thermograms of the freeze-dried complex displayed a broad endothermic peak at 97.58°C, which is a shift from the peak of HPβCD in the presence of the drug. The intense two endothermic peaks of the drug remain completely hidden in the thermogram, suggesting the successful inclusion of the clomiphene citrate within the cyclodextrin cavity.
Fig. 3.

DSC thermograms of clomiphene citrate, HPβCD, dry mixture, and freeze-dried complex
Fourier Transform Infrared (FTIR) Spectroscopy Analysis
The FTIR spectra of clomiphene citrate, HPβCD, dry mixture (drug:CD at 1:1 molar ratio), and freeze-dried complex (drug:CD at 1:1 molar ratio) are illustrated in Fig. 4. The FTIR spectrum of clomiphene citrate showed characteristic peaks at 2992 (C-H stretching vibration), 1730 (a strong C-O stretching peak), 1575, 1508, 1471, 1215 (another strong indication of C–O–C stretch), and 1175 cm−1. The presence of three benzyl rings shows peaks in the range of 650–900 cm−1. The presence of chlorine also resulted in a strong peak in the range of 600–800 cm−1. The FTIR spectrum of HPβCD exhibited characteristic peaks at 3343 cm−1 (OH-stretching vibration), 2926 cm−1 (C-H stretching vibration),1331 cm−1, and a very sharp peak at 1021 cm−1 (C-O stretching vibration). The dry mixture retained the FTIR peaks of both clomiphene citrate (1732, 1579, 1509, and 1216 cm−1) and HPβCD ( 3349, 2929, 1323, and 1024 cm−1), but the intensity of the peaks of the drug was clearer with the spectrum. The FTIR spectrum of the clomiphene citrate-HPβCD complex showed the maximum decrease in the intensity of the strong peaks of the clomiphene citrate (1730 showed decreased intensity and shift to 1720 cm−1, 1215 showed decreased intensity and shift to 1229 cm−1). Other prominent peaks of the drug, like 2992, 1575, 1471, and 1175 were not observed in the freeze-dried complex, which suggests that some of the functional groups of clomiphene citrate are complexed within the hydrophobic pocket of the cyclodextrin.
Fig. 4.
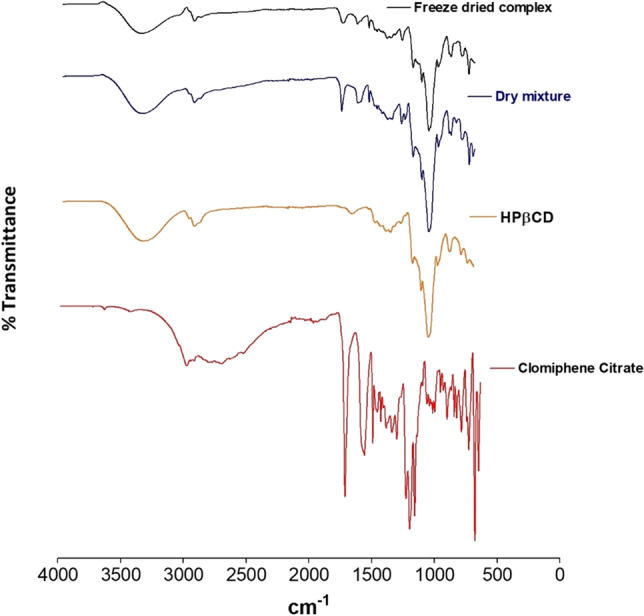
FTIR spectra of clomiphene citrate, HPβCD, dry mixture, and freeze-dried complex to confirm the inclusion complexation in the solid state
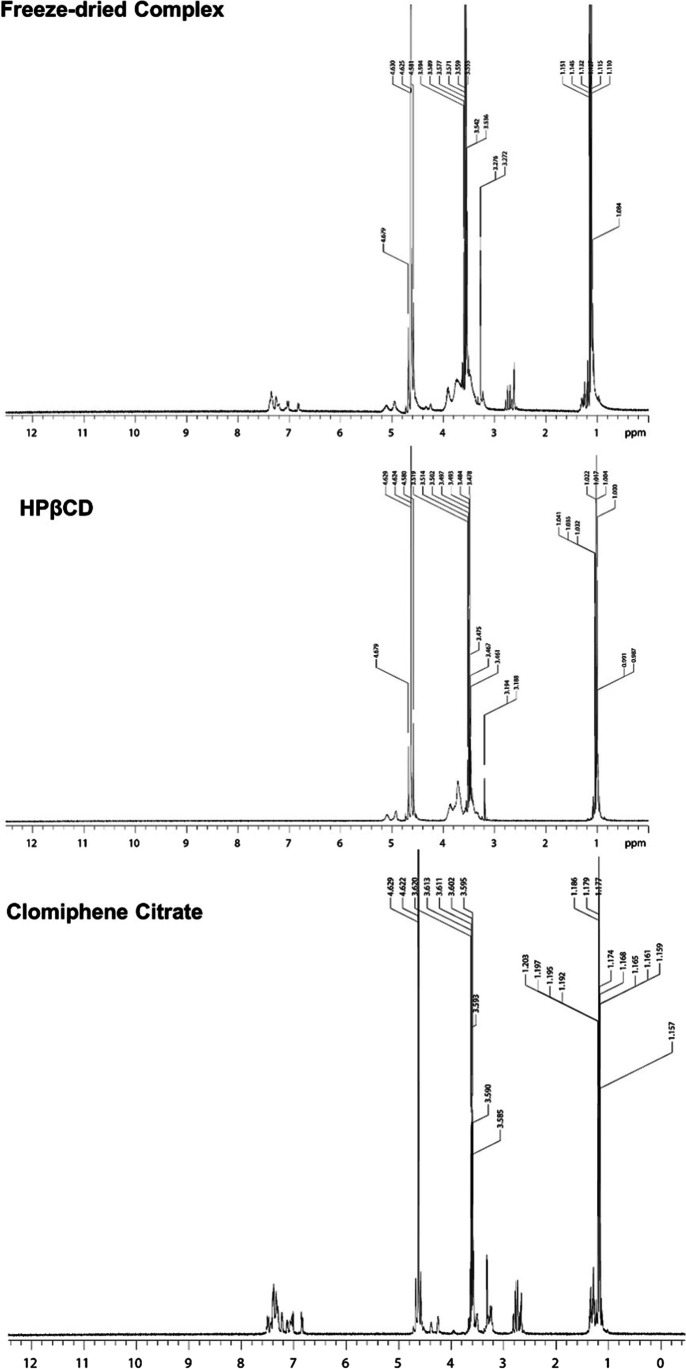
Nuclear Magnetic Resonance (NMR) Studies
NMR spectroscopy could be a powerful tool to indicate the change in the electronic and chemical environment around protons involved in the complexation, which is represented through the change in the chemical shifts. Figure 5 shows the comparison in the observed chemical shifts for clomiphene citrate, HPβCD, and the freeze-dried mixture. A comparison of the spectra of freeze-dried mixture and dry mixture is shown in Figure S1. From the data, it is evident that the chemical shifts of the protons in clomiphene citrate is also observed in the freeze-dried mixture. The aromatic protons of the clomiphene citrate showed relatively smaller upfield shift than expected (between 0.1 and 0.2 ppm) in the freeze-dried mixture, suggesting that the protons are involved in weaker intermolecular interactions with HPβCD. Most aromatic protons showed upfield shift implying that most of the molecular structure could be enclosed within the cyclodextrin structure. The protons of the cyclodextrins located within or near the cavity exhibited large downfield shifts affirming the interaction between the drug and hydrophobic pocket of cyclodextrin [23]. These chemical shifts modification partially give evidence of formation of inclusion complex. The dry mixture showed more intense chemical shifts than the freeze-dried mixture. The downfield shifts of the cyclodextrins in the dry mixture were also not prominent as in freeze-dried mixture. It indicates that dry mixture did not exhibit the similar intermolecular interaction between drug and the cyclodextrin, compared to freeze-dried mixture.
Fig. 5.
Comparison of NMR spectra of clomiphene citrate, HPβCD, and freeze-dried complex
Formulation and Characterization of 0.5% Clomiphene Citrate Intravenous Injection
Clomiphene citrate 0.5% intravenous injection with desirable attributes (solubility, pH, osmolality, physical and chemical stability) was achieved by using HPβCD as a solubilizer and propylene glycol as a tonicity adjuster. The drug content was close to the theoretical value, and all the characterization parameters, such as pH, osmolality, and visual appearance, were within acceptable limits (Table II). The formulation was prepared using materials that are endotoxin controlled. The final product was sterile-filtered using a 0.22-µm nylon membrane filter under a vertical laminar airflow workbench. Risk factors associated in sterile operations including personnel and environmental contamination, container, and closure sterility have been assessed as per the Sterility Assurance Level (SAL) guidelines.
Table II.
Acceptance Criteria for 0.5% Clomiphene Citrate Intravenous Injection (Batch 3). Assay Values Represent Mean ± SD, n = 3
| Test | Acceptance criteria | Actual value |
|---|---|---|
| Appearance | Clear solution, free from any visible particulates | Clear, without any visible particulates |
| Assay | 90–110% | 98.31 ± 2.40 |
| pH | 4.0–8.0 | 4.07 |
| Osmolality, mOsm | 280–340 | 321 |
The prepared 0.5% clomiphene citrate intravenous solution was clear and free from particulate matter. The preparation was sterile and endotoxin controlled. The pH value was within 4–8 limit, osmolality was < 340 mOsm, and the content of clomiphene citrate was close to the theoretical addition at 100%.
Optimization of Stability-Indicating HPLC Method
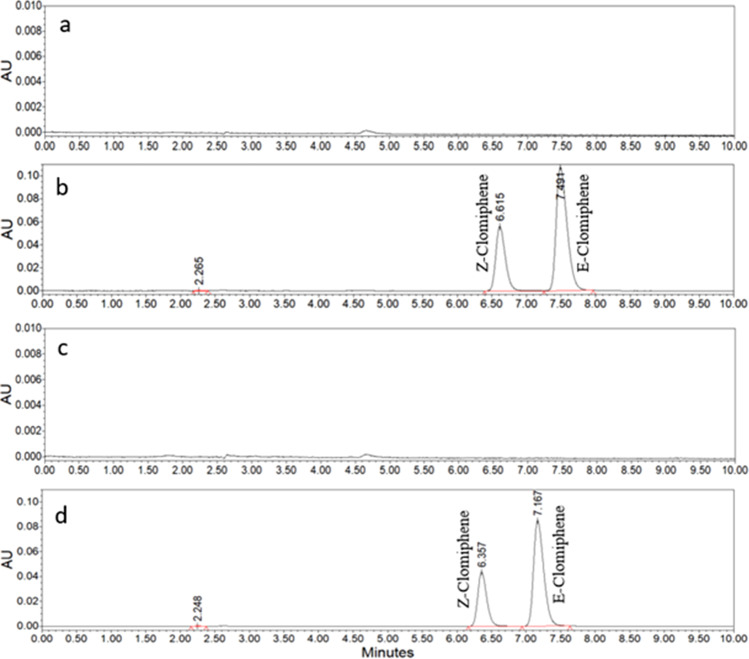
The HPLC method developed in the present study showed the separation of two isomers, cis (Z) and trans (E) clomiphene citrate, with trans isomer being the major product (50–60%) compared to cis isomer, which was about 30–50%. The HPLC method provided excellent resolution of the peaks at the optimum ratio of methanol and water (0.5%TFA) 70:30 (%v/v). A comparison of the chromatograms obtained from the working standard and sample showed a good selectivity of the method and a good baseline, as shown by the placebo injection (Fig. 6). Therefore, the developed method is convenient for routine quantification in pharmaceutical preparations.
Fig. 6.
HPLC chromatograms of a diluent/blank vehicle; b clomiphene citrate standard, 100 µg/ml; c placebo clomiphene citrate injection (vehicle); d 0.5% clomiphene citrate injection
Validation of Stability-Indicating HPLC Method
The HPLC method was validated with respect to system suitability, linearity, precision, and solution stability. The linearity of the standard solution of clomiphene citrate was examined at various concentration ranges of 1–200 µg/ml. According to USP, the correlation coefficient (R2) for a calibration curve must be > 0.999. The correlation coefficient was found to be more than 0.9999, indicating excellent linearity. For precision and repeatability, the RSD values were around 0.86%, indicating that the proposed method provides acceptable intraday variation for clomiphene citrate intravenous injection. The results summarized in Table III prove that inter and intraday precision and repeatability of the proposed method were acceptable. All the system suitability parameters, including tailing factor, theoretical plates, and retention time, met the compendium acceptance limits. The tailing factors of Z- and E-clomiphene citrate were 1.29 and 1.35, respectively, which is within the USP limit of ≤ 2%. Similarly, the theoretical plates were 8005 and 7217 for Z- and E-clomiphene, respectively, which met USP acceptance limits of ≥ 2000 (USP 2011). The analytical stability under laboratory conditions showed that the samples prepared were fairly stable in the dilution media for up to 4 days. The RSD of areas was below 3%; the LOD and LOQ for z-clomiphene were 0.5 and 1 µg/ml, respectively, and for E-clomiphene were 0.2 and 0.5 µg/ml, respectively. The calculated LOD and LOQ concentrations for both Z- and E-clomiphene confirmed that the HPLC method was sufficiently sensitive.
Table III.
HPLC Method Validation Data for 0.5% Clomiphene Citrate 0.5% Intravenous Injection
| System suitability | ||||||
|---|---|---|---|---|---|---|
| Tailing factor (USP) | Theoretical plates (USP) | Peak area (mean ± SD) | RSD (%, n = 6) | |||
| Z-clomiphene | E-clomiphene | Z-clomiphene | E-clomiphene | |||
| Clomiphene citrate | 1.29 | 1.35 | 8005.5 | 7217.35 | 101.74 ± 0.17 | 0.17 |
| Precision and repeatability | ||||||
|
Theoretical concentration (μg/ml) |
Experimental concentration (μg/ml) ± SD | Recovery (%) | RSD (%) | |||
| Intraday precision | 100 | 94.58 ± 0.82 | 94.58 ± 0.82 | 0.86 (n = 5) | ||
| Interday precision | 100 | 95.19 ± 1.56 | 95.19 ± 1.56 | 1.64 (n = 10) | ||
| Solution stability | ||||||
| Theoretical concentration (μg/ml) | Experimental concentration (μg/ml ± SD) | Recovery (%) | RSD (%, n = 5) | |||
| Day 0 | 100 | 97.60 ± 2.72 | 97.60 ± 2.72 | 2.78 | ||
| Day 1 | 100 | 94.58 ± 0.82 | 94.58 ± 0.82 | 0.86 | ||
| Day 4 | 100 | 95.80 ± 1.97 | 95.80 ± 1.97 | 2.06 | ||
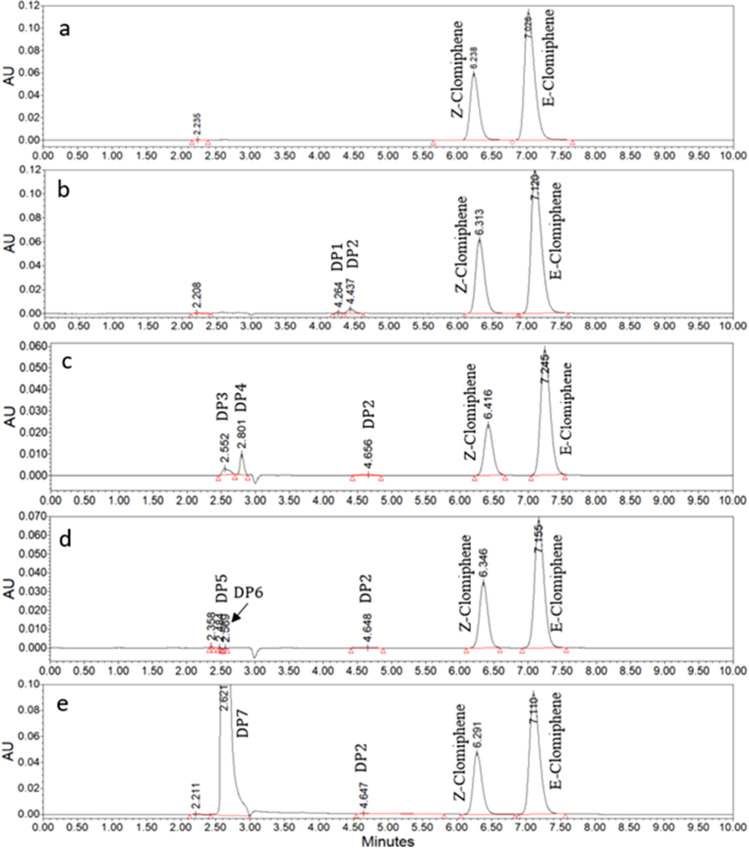
For the forced degradation study, the mobile phase consisting of water with 0.05% trifluoroacetic acid:methanol in the ratio of 40:60 was first optimized to analyze the standard and sample clomiphene citrate formulations. It was then applied to a mixture of stressed samples. The drug, upon reacting with 1 N HCl at 70°C resulted in the formation of two major and one minor degradation peaks. Within 24 h, around 64% and 50% fall in Z- and E-clomiphene peak areas was observed, with 36% and 50% drug recovery, respectively. In contrast to acidic conditions, the drug was found to be highly unstable in alkali conditions. On reacting the drug with 1 N NaOH at 70°C for 1 h, around 26% and 19% of the Z- and E-clomiphene were degraded, leaving only 74% and 81% of the drug recovered, respectively. In the case of oxidative conditions (3% H2O2, RT, 24 h), the drug was highly stable, with only 36% and 43% of Z- and E-clomiphene degradation, respectively. From the results summarized in Table IV, it was found that both isomers of clomiphene citrate were degraded significantly under alkaline conditions. However, no degradation was seen for the standard mixture of clomiphene citrate solution either exposed or unexposed to heat (70°C, 24 h). The percentage recovery of control exposed and unexposed to heat (70°C) for 24 h was around 97% and 95%, respectively. In addition, photodiode array (PDA) analyses showed that the purity angle/threshold value for the drug peaks (Z- and E-clomiphene) in a mixture of stressed samples was not significantly different from the peak purity/threshold values of the pure drug (controls) as evident from the Table IV, indicating that the drug peak was free from any co-eluting peaks, and thus the method was found to be specific for the drug. The optimized HPLC method was able to separate the drug peaks from major and minor degradation products, and the resulting chromatograms are shown in Fig. 7. The drug, upon reaction with 1 N HCl at 70°C for 24 h, resulted in the formation of two major and one minor degradation peaks. In the same way, clomiphene, when subjected to alkaline conditions, resulted in three minor degradation peaks. In contrast, under oxidative conditions, the clomiphene was degraded into one major and one minor degradation product. Surprisingly, two degradation peaks were observed when the control (clomiphene injection) was exposed to heat (70°C for 24 h), without any reduction in the percentage of drug recovery.
Table IV.
Stress Study/Forced Degradation Study of Z- and E-Clomiphene Citrate Injection. Values Represented as Mean ± SD, n = 3
| Purity angle/threshold, Z-clomiphene | Purity angle/threshold , E-clomiphene | Recovery of clomiphene citrate (%) | Degradation of clomiphene citrate (%) | |
|---|---|---|---|---|
| Control (untreated) | 0.141/0.326 | 0.125/0.295 | 95.02 ± 1.29 | - |
| Control, 70°C, 24 h | 0.106/0.260 | 0.082/0.239 | 98.26 ± 0.08 | - |
| 1 N HCl, 70°C, 24 h | 0.226/0.378 | 0.130/0.276 | 44.95 ± 0.82 | 55.05 ± 0.82 |
| 1 N NaOH, 70°C, 1 h | 0.181/0.349 | 0.122/0.271 | 59.26 ± 2.64 | 40.74 ± 2.65 |
| 3% H2O2, RT, 24 h | 0.173/0.324 | 0.124/0.267 | 78.62 ± 0.61 | 21.38 ± 0.61 |
Fig. 7.
HPLC chromatograms of 0.5% clomiphene citrate intravenous injection subjected to a forced degradation study under various stress conditions such as a control, untreated (0.5% clomiphene citrate injection); b control, 70°C, 24 h; c 1 N HCl, 70°C, 24 h; d 1 N NaOH, 70°C, 1 h; e 3%H2O2, RT, 24 h. Degradation products were labeled as DP1-DP7
Stability of Clomiphene Citrate Intravenous Injection
The physical appearance, clarity, pH, and osmolality data collected on the injection for 6 months is shown in Table V. The % clomiphene citrate assay of the injection is shown in Table VI. The % assay of individual isomers in the formulation in the stability samples is shown in Table S1. All three batches of sterile formulation stored at 4°C, RT, and 40°C showed clarity and no color change, and there was negligible change in pH and osmolality. There was no significant change in the content of clomiphene, with no degradation peaks observed after 6 months of storage at 4°C, RT, and 40°C. At accelerated conditions (40°C), the formulation exhibited insignificant variation in the clomiphene citrate content (% drug in the solution). Current Good Manufacturing Practice (cGMP) for finished pharmaceuticals established requirements regarding the expiration date on a drug product and stability testing to assure the appropriateness of that date since each drug product may be unique because of differences in physical and chemical properties of the active ingredients, the excipients, and formulations. The FDA guideline suggested that combining data compiled at room and accelerated temperatures can justify an expiration date of over two years. The stability data showed that the formulation remained above 90%, suggesting that the product met the requirement to be stable for at least 2 years of shelf life.
Table V.
pH and Osmolality Measurement of 0.5% Clomiphene Citrate IV injection (Three Different Batches) Subjected to Stability Studies at Different Temperatures (4°C, RT, and 40°C) and Various Time Points (0, 0.5, 1, 2, 3, and 6 Months)
| Test | Conditions | Analysis | |||
|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | |||
| Appearance | 0–6 months, 40°C, RT, and 4°C | Clear | Clear | Clear | |
| pH | Initial | 4.43 | 4.15 | 4.07 | |
| 0.5 months | 40°C | 4.19 | 4.26 | 4.2 | |
| RT | 4.18 | 4.12 | 4.09 | ||
| 4°C | 4.19 | 4.21 | 4.11 | ||
| 1 month | 40°C | 4.23 | 4.05 | 4.07 | |
| RT | 4.23 | 4.19 | 4.21 | ||
| 4°C | 4.19 | 4.15 | 4.19 | ||
| 2 months | 40°C | 4.22 | 4.16 | 4.18 | |
| RT | 4.15 | 4.16 | 4.09 | ||
| 4°C | 4.11 | 4.09 | 4.27 | ||
| 3 months | 40°C | 4.31 | 4.13 | 4.02 | |
| RT | 4.32 | 4.14 | 4.12 | ||
| 4°C | 4.35 | 4.12 | 4.04 | ||
| 6 months | 40°C | 4.01 | 4.00 | 4.24 | |
| RT | 4.17 | 4.06 | 4.28 | ||
| 4°C | 4.28 | 4.13 | 4.08 | ||
| Osmolality, mOsm | Initial | 327 | 310 | 321 | |
| 0.5 months | 40°C | 323 | 355 | 330 | |
| RT | 328 | 330 | 326 | ||
| 4°C | 338 | 332 | 350 | ||
| 1 month | 40°C | 330 | 350 | 349 | |
| RT | 316 | 349 | 279 | ||
| 4°C | 315 | 355 | 271 | ||
| 2 months | 40°C | 373 | 333 | 325 | |
| RT | 306 | 334 | 321 | ||
| 4°C | 307 | 327 | 322 | ||
| 3 months | 40°C | 309 | 334 | 322 | |
| RT | 314 | 330 | 318 | ||
| 4°C | 318 | 329 | 315 | ||
| 6 months | 40°C | 300 | 317 | 318 | |
| RT | 301 | 320 | 327 | ||
| 4°C | 306 | 316 | 322 | ||
Table VI.
Percentage Drug Content of 0.5% Clomiphene Citrate IV Injection (Three Different Batches) Subjected to Stability Studies at Different Temperatures (4°C, RT, and 40°C) and Various Time Points (0, 0.5, 1, 2, 3, and 6 Months)
| Time (after months) | Storage condition | Assay (average% ± SD) (n = 3) | ||
|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | ||
| Initial | 95.26 ± 1.22 | 92.49 ± 0.25 | 98.31 ± 2.40 | |
| 0.5 | 40°C | 92.83 ± 0.80 | 92.68 ± 0.21 | 95.01 ± 0.05 |
| RT | 97.76 ± 3.22 | 94.85 ± 0.20 | 92.13 ± 0.08 | |
| 4°C | 95.15 ± 1.95 | 92.51 ± 0.13 | 92.60 ± 0.10 | |
| 1 | 40°C | 99.46 ± 2.61 | 100.10 ± 0.19 | 101.92 ± 0.22 |
| RT | 98.42 ± 0.33 | 101.42 ± 0.22 | 104.08 ± 0.14 | |
| 4°C | 102.35 ± 0.73 | 102.24 ± 0.32 | 102.45 ± 0.06 | |
| 2 | 40°C | 92.86 ± 0.09 | 103.42 ± 0.48 | 99.82 ± 0.41 |
| RT | 90.59 ± 0.10 | 101.44 ± 1.03 | 100.84 ± 0.18 | |
| 4°C | 92.76 ± 0.10 | 104.95 ± 0.78 | 104.72 ± 0.61 | |
| 3 | 40°C | 94.92 ± 0.09 | 98.49 ± 0.24 | 99.84 ± 0.47 |
| RT | 96.87 ± 0.50 | 97.81 ± 0.09 | 97.30 ± 0.19 | |
| 4°C | 97.52 ± 0.14 | 96.18 ± 0.24 | 98.04 ± 0.26 | |
| 6 | 40°C | 100.68 ± 0.92 | 94.39 ± 0.71 | 103.75 ± 0.56 |
| RT | 99.86 ± 0.52 | 98.58 ± 0.34 | 95.60 ± 0.36 | |
| 4°C | 100.62 ± 0.09 | 100.88 ± 1.75 | 99.45 ± 1.42 | |
Discussion
Clomiphene citrate is currently available as a tablet dosage form. Its liquid dosage forms or the drug stability in the aqueous state is not reported. Due to the unknown stability profile (in the aqueous state) and due to poor aqueous solubility, formulation of clomiphene citrate injection can be challenging. HPβCD has been extensively investigated with hydrophobic molecules to improve their apparent aqueous solubility. In this study, clomiphene citrate was solubilized using HPβCD to enable making a parenteral solution for intravenous administration. The phase solubility diagram reveals an AL type of phase solubility curve assuming 1:1 inclusion complex is formed. The very high stability constant suggests a strong inclusion of clomiphene citrate with HPβCD [24]. The linearity also suggests the 1:1 stoichiometry between clomiphene citrate and HPβCD, implying one molecule of cyclodextrin complexing with a molecule of a drug.
Thermal analysis shows the complete disappearance of the melting endotherm of the clomiphene citrate for the freeze-dried mixture suggesting the formation of an inclusion complex and the existence of a new solid phase [25]. The FTIR analysis provides more direct evidence of the inclusion complex formation as shown by the decrease in the intensity of the strong peaks of the clomiphene citrate (1730 showed decreased intensity or absence of several peaks in the freeze-dried complex, which suggests that HPβCD complexation of clomiphene citrate through its hydrophobic zone of the molecular structure [26]. The NMR data showed stronger intermolecular interactions for the freeze-dried mixture versus dry mixture. The NMR results corroborate with the DSC and FTIR data to indicate the existence of HPβCD/clomiphene citrate inclusion complex in the solid state.
HPβCD concentration up to 20% w/v has been approved for its use in the formulation of intravenous injections [27–29]. The HPβCD 20% w/v and clomiphene citrate 0.5% w/v in the solution were hypotonic and needed a tonicity adjuster. Hence, we added propylene glycol to achieve isotonic solution. The sterile operations were conducted in a vertical laminar airflow workbench, and the final formulation was sterile-filtered. The 0.5% clomiphene citrate injection complied the requirements of the parenteral solution intended for intravenous injection [30].
For quantitative analysis, the HPLC method was optimized using working standards of clomiphene citrate using a reported HPLC method [19]. Actual chromatographic conditions for the determination of clomiphene citrate were established after many preliminary experiments involving the column type, size, the effect of the composition of mobile phase and flow rate, and column temperature. The major criteria for developing the RP-HPLC method for the determination of clomiphene citrate using a UV detector was to develop an accurate, reproducible stability-indicating method that is free of interfering peaks from other formulation excipients and can resolve the degradation products. The degradation peaks were sufficiently resolved from the clomiphene isomer peaks. The peaks were pure, and the assay method was stability-indicating, as known from the data from the forced degradation studies.
The stability of a drug in pharmaceutical formulations is a function of the storage conditions and chemical properties of the drug. Conditions used in stability studies should likely be the conditions encountered during sample handling and analysis. Stability data is required to show that the concentration and purity angle of the analyte at the time of analysis should be the same as the concentration and purity angle at the time of preparation. Moreover, the stability data should reflect that the formulation parameters are within acceptable limits during the entire shelf life [31]. As evidenced by the resulting chromatograms, measurement of intact drug isoforms recovered following forced stress, and analysis at various durations of storage, our objective of developing a stable intravenous formulation of clomiphene citrate was achieved. We have also formulated the injection by escalating the clomiphene citrate concentration to 2% w/v, and the formulation stability data shows the product is physically and chemically stable for more than six months (data not shown). This will allow us to initiate clinical trials following clomiphene citrate administration in veterinary species for the purposes of reproductive hormone manipulation to treat various pathologies of the reproductive system. Currently, clinical trials on horses (mares) using clomiphene citrate injection are in progress. The current injection formulation has been well tolerated by the animals with no adverse effects.
In summary, we have successfully formulated a 0.5% clomiphene citrate injection with desirable quality attributes. We also have established a stability-indicating HPLC method for clomiphene injection. Furthermore, the stability data at the accelerated (40°C) and room temperature conditions after 6 months of storage showed satisfactory stability of the clomiphene citrate in the injection formulation. The stability data suggest that the product will be stable for at least 2 years and meets the given analytical specifications.
Author Contribution
Conceptualization, C. Lyman, RJ Babu. Research methodology, M. Annaji, N Mita, I Poudel, Q. Wang. Formal analysis, C. Lyman, RJ Babu, M. Annaji, N. Mita, I Poudel. Resources, C. Lyman, RJ Babu. Supervision, C. Lyman, RJ Babu. Writing—original draft, M. Annaji, C. Lyman, RJ Babu, I Poudel. Writing—review and editing, C. Lyman, RJ Babu, M. Annaji, N. Mita, I Poudel, B. Tipton, Q Wang. All authors have read and agreed to the published version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Auburn University Presidential Awards for Interdisciplinary Research (PAIR) grants for financial support for graduate students (M. Annaji and I Poudel). The authors are grateful to Dr. Brian Via and Dr. Erramuspe, College of Forestry, Wildlife and Environment, and Ian Steinke, Harrison College of Pharmacy, for help with FTIR and NMR spectroscopy studies, respectively.
Data Availability
All data generated or analysed during this study are included in this published article.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manjusha Annaji and Nur Mita have equally contributed to this study.
Contributor Information
R. Jayachandra Babu, Email: ramapjb@auburn.edu.
Candace C. Lyman, Email: ccl0036@auburn.edu
References
- 1.Adashi EY, Hsueh AJ, Bambino TH, Yen SS. Disparate effect of clomiphene and tamoxifen on pituitary gonadotropin release in vitro. American Journal of Physiology-Endocrinology and Metabolism. 1981;240(2):E125–30. doi: 10.1152/ajpendo.1981.240.2.E125. [DOI] [PubMed] [Google Scholar]
- 2.Okia NO. The effect of clomiphene citrate on LH release by dispersed rat anterior pituitary cells. Life Sci. 1983;33(13):1261–1268. doi: 10.1016/0024-3205(83)90007-3. [DOI] [PubMed] [Google Scholar]
- 3.Wallach EE, Adashi EY. Clomiphene citrate: mechanism (s) and site (s) of action—a hypothesis revisited. Fertil Steril. 1984;42(3):331–344. doi: 10.1016/S0015-0282(16)48069-6. [DOI] [PubMed] [Google Scholar]
- 4.Kerin JF, Liu JH, Phillipou G, Yen SSC. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61(2):265–268. doi: 10.1210/jcem-61-2-265. [DOI] [PubMed] [Google Scholar]
- 5.Homburg R. Clomiphene citrate—end of an era? A mini-review Hum Reprod. 2005;20(8):2043–2051. doi: 10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- 6.Tavaniotou A, Albano C, Smitz J, Devroey P. Effect of clomiphene citrate on follicular and luteal phase luteinizing hormone concentrations in in vitro fertilization cycles stimulated with gonadotropins and gonadotropin-releasing hormone antagonist. Fertil Steril. 2002;77(4):733–737. doi: 10.1016/S0015-0282(01)03265-4. [DOI] [PubMed] [Google Scholar]
- 7.MacDougall MJ, Tan S-L, Hall V, Balen A, Mason BA, Jacobs HS. Comparison of natural with clomiphene citrate-stimulated cycles in in vitro fertilization: a prospective, randomized trial. Fertil Steril. 1994;61(6):1052–1057. doi: 10.1016/S0015-0282(16)56755-7. [DOI] [PubMed] [Google Scholar]
- 8.Ochin H, Ma X, Wang L, Li X, Song J, Meng Y, et al. Low dose clomiphene citrate as a mild stimulation protocol in women with unsuspected poor in vitro fertilization result can generate more oocytes with optimal cumulative pregnancy rate. Journal of Ovarian Research. 2018;11(1):1–6. doi: 10.1186/s13048-018-0408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branigan EF, Estes MA. Minimal stimulation IVF using clomiphene citrate and oral contraceptive pill pretreatment for LH suppression. Fertil Steril. 2000;73(3):587–590. doi: 10.1016/S0015-0282(99)00584-1. [DOI] [PubMed] [Google Scholar]
- 10.Al-Inany H, Azab H, El-Khayat W, Nada A, El-Khattan E, Abou-Setta AM. The effectiveness of clomiphene citrate in LH surge suppression in women undergoing IUI: a randomized controlled trial. Fertil Steril. 2010;94(6):2167–2171. doi: 10.1016/j.fertnstert.2010.01.069. [DOI] [PubMed] [Google Scholar]
- 11.El Sherry TM, Derar D, Hussein HA, Shahin AY, Fahmy S. Effect of clomiphene citrate on follicular recruitment, development, and superovulation during the first follicular wave in Rahmani ewes. Int J Endocrinol Metab. 2011;9(3):403–408.
- 12.Bukhari SAA, Ali S, Zubair M, Ahmad I, Rehman UU. Effect of clomiphene citrate and human chorionic gonadotropin (hCG) on ovulation induction in prepubertal Sahiwal heifers. Asian Pacific Journal of Reproduction. 2016;5(3):232–235. doi: 10.1016/j.apjr.2016.04.010. [DOI] [Google Scholar]
- 13.Wankar MS, Ingawale MV, Hajare SW, Ingole RS. Efficacy of clomiphene citrate and ovsynch protocol treatment on fertility during summer in buffalo heifers. 2017;2(5):21–23.
- 14.Robinson JR. Use of clomiphene citrate to induce estrus in anestrous mares. 1977;72(4):605–607. [PubMed]
- 15.Maxwell L. Horse of a different color: peculiarities of equine pharmacology. Equine Pharmacol. 2014;1–15.
- 16.Loftsson T. Cyclodextrins in parenteral formulations. J Pharm Sci. 2021;110(2):654–664. doi: 10.1016/j.xphs.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira L, Campos J, Veiga F, Cardoso C, Paiva-Santos AC. Cyclodextrin-based delivery systems in parenteral formulations: a critical update review. Eur J Pharm Biopharm. 2022;178:35–52. [DOI] [PubMed]
- 18.Higuchi T, Connors KA. Advances in analytical chemistry and instrumentation. Phase Sol Stud. 1965; 4:117–212.
- 19.Crewe HK, Ghobadi C, Gregory A, Rostami-Hodjegan A, Lennard MS. Determination by liquid chromatography–mass spectrometry of clomiphene isomers in the plasma of patients undergoing treatment for the induction of ovulation. J Chromatogr B. 2007;847(2):296–299. doi: 10.1016/j.jchromb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Mulabagal V, Annaji M, Kurapati S, Dash RP, Srinivas NR, Tiwari AK, et al. Stability-indicating HPLC method for acyclovir and lidocaine in topical formulations. Biomed Chromatogr. 2020;34(3):e4751. doi: 10.1002/bmc.4751. [DOI] [PubMed] [Google Scholar]
- 21.Shelley H, Grant M, Smith FT, Abarca EM, Jayachandra BR. Improved ocular delivery of Nepafenac by Cyclodextrin complexation. AAPS PharmSciTech. 2018;19(6):2554–2563. doi: 10.1208/s12249-018-1094-0. [DOI] [PubMed] [Google Scholar]
- 22.Oprean C, Mioc M, Csányi E, Ambrus R, Bojin F, Tatu C, et al. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. 2016;83:1095–1104. doi: 10.1016/j.biopha.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Dandawate PR, Vyas A, Ahmad A, Banerjee S, Deshpande J, Swamy KV, et al. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1: 2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012;29(7):1775–1786. doi: 10.1007/s11095-012-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuelsen L, Holm R, Lathuile A, Schönbeck C. Correlation between the stability constant and pH for β-cyclodextrin complexes. Int J Pharm. 2019;568:118523. doi: 10.1016/j.ijpharm.2019.118523. [DOI] [PubMed] [Google Scholar]
- 25.Moyano JR, Arias-Blanco MJ, Gines JM, Giordano F. Solid-state characterization and dissolution characteristics of gliclazide-β-cyclodextrin inclusion complexes. Int J Pharm. 1997;148(2):211–217. doi: 10.1016/S0378-5173(96)04848-X. [DOI] [Google Scholar]
- 26.Bilensoy E, Hincal AA. Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opin Drug Deliv. 2009;6(11):1161–1173. doi: 10.1517/17425240903222218. [DOI] [PubMed] [Google Scholar]
- 27.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62(11):1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 28.Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle-based drug delivery systems. J Pharm Sci. 2018;107(7):1741–1753. doi: 10.1016/j.xphs.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Shelley H, Annaji M, Smith FT, Babu RJ. Difluprednate-hydroxypropyl-β-cyclodextrin-based ophthalmic solution for improved delivery in a porcine eye model. J Ocul Pharmacol Ther. 2022;38(1):92–101. doi: 10.1089/jop.2021.0073. [DOI] [PubMed] [Google Scholar]
- 30.Allen L, Ansel HC. Ansel's pharmaceutical dosage forms and drug delivery systems, Tenth edition. Lippincott Williams & Wilkins. 2013. p. 781.
- 31.Huynh-Ba K, Dong MW. Stability studies and testing of pharmaceuticals: an overview. LCGC North Am. 2020;38(6):325–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.