Abstract
Introduction The associations of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL-C) with reduced saliva flow rates have not been previously reported.
Objective The present study aimed to assess the association of cholesterolemia with reduced saliva flow rates in community-dwelling elderly subjects.
Methods The present study analyzed 342 participants (170 males and 172 females aged between 78 and 79 years old). Unstimulated salivary flow rate (USFR) was assessed using a cotton roll method. Low-USFR was defined as 0.10 g/30 seconds. Stimulated salivary flow rate (SSFR) was assessed by having the participants chew tasteless gum for 3 minutes. Low-SSFR was defined as 1.0 mL/minute. Blood samples were collected for the measurement of LDL-C, HDL-C, rheumatoid factor, hemoglobin A1c, and creatinine. To assess depression, the General Health Questionnaire 30 was used. A standardized questionnaire was completed, covering the current and previous medications of the participants and smoking status. We stratified the serum LDL-C levels of the participants as normal, moderate or severe hyper-LDL cholesterolemia and serum HDL-C levels as normal or hypo-HDL cholesterolemia. Multivariate logistic regression models were established and low-USFR or low-SSFR were set as dependent variables in the aforementioned models.
Results After controlling for the effects of the other variables, the odds ratios (ORs) (95% confidence intervals [CIs]) for low-USFR were 2.25 (1.10–4.61) for moderate and 5.69 (1.55–20.8) for severe hyper-LDL cholesterolemia, while that of hypo-HDL cholesterolemia was 3.40 (1.33–8.69). Severe hyper-LDL cholesterolemia was also associated with low-SSFR with an OR of 3.89 (1.39–10.88).
Conclusion Elderly patients with cholesterolemia have a risk of reduced salivary flow rate.
Keywords: cholesterol, saliva, aged
Introduction
Saliva has important roles in preventing dental caries and gingival disease. 1 2 Decreased saliva flow can lead to certain oral problems such as candidosis, burning mouth, oral dryness, difficulties with mastication and swallowing, altered taste sensation, and halitosis. 1 3 4 5 In addition, hyposalivation was reported to be a possible risk factor of acute respiratory infection. 6 Thus, the prevention and treatment of hyposalivation are important for maintaining good oral health and quality of life.
Many researchers have reported on factors and indicators of hyposalivation; for example, the destruction of salivary glands after radiation therapy, 7 8 Sjögren syndrome, 9 and rheumatoid arthritis 10 11 are direct reasons for hyposalivation. In addition, patients with certain systemic diseases such as diabetes mellitus 12 and chronic kidney disease 13 showed reduced stimulated and unstimulated salivary flow rates. Female gender, 14 15 active smoking habit, 15 and the use of certain medications 15 16 were similarly reported as risk factors of hyposalivation, while patients with depression, anxiety, and stress showed hyposalivation and oral dryness. 5 17 18 19 Aging was also reported as a risk factor, 14 as the acini decrease in number and are replaced with adipose and fibrotic tissues as the salivary glands age. 20 Several studies have noted, however, that the stimulated saliva flow does not change with aging. 14 21 One review article that targeted studies with sample sizes of > 100 participants concluded that the unstimulated saliva flow decreased and stimulated saliva flow either remained stable or decreased with aging. 22
Saliva is produced from blood in the salivary glands. Katsura et al. 23 reported that changes in the blood flow velocity of the facial artery through stimulation with acid as observed on pulse Doppler sonography were associated with the saliva flow rate and any decrease in this velocity reduces the saliva flow rate. Meanwhile, the serum level of low-density lipoprotein cholesterol (LDL-C) positively correlated with brain blood flow velocity, while that of high-density lipoprotein cholesterol (HDL-C) showed a negative correlation. 24
Thus, the hypothesis of the present study was that patients with high serum LDL-C levels or low serum HDL-C levels would show low saliva flow rates. The aim of the present study was to assess the relationship between cholesterolemia and reduced saliva flow rates among community-dwelling elderly in Japan.
Methods
The current investigation is a subset study of the Niigata study 25 conducted from 1998 to 2007. Briefly, the Niigata study was a prospective community-based study initiated in 1998 to evaluate relationships between systemic health status and history of dental diseases. In April 1998, all 4,542 Niigata citizens aged 70 years old (2,099 males and 2,443 females) were sent a written request to participate in the survey. The invitation was mailed again to nonrespondents 3 weeks later; 81.4% (3,695/4,542) responded positively, agreeing to participate in the survey. Considering the availability of resources, examination appointments were arranged for 600 individuals. The final study sample was randomly recruited from several areas of Niigata to include an approximately equal number of male ( n = 306) and female participants ( n = 294). The present study included 380 participants aged between 78 and 79 years old who joined the Niigata study in 2007 ( Fig. 1 ).
Fig. 1.
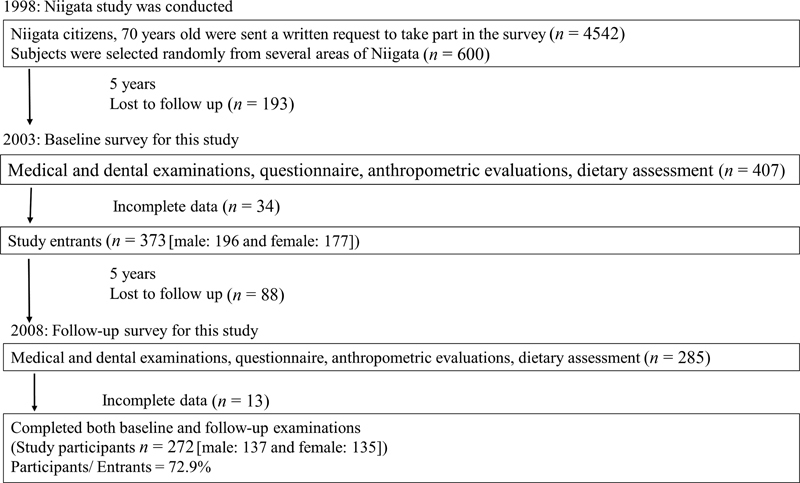
Overview of the recruitment of participants for the present study.
The unstimulated salivary flow rate (USFR) was measured using a cotton roll method. 26 27 We placed a preweighed cotton roll into the sublingual area for 30 seconds, then weighed the resultant saliva volume on an electronic scale. We defined a cutoff value for low-USFR as the median of the current study (corresponding to 0.10 g/30 seconds). The stimulated salivary flow rate (SSFR) was collected by directing participants to chew tasteless and odorless gum for 3 minutes and then measuring the flow of saliva that was expectorated into a test tube, calculated as mL/minute. The cutoff value for low-SSFR was set at 1.0 mL/minute. 28
Blood samples were drawn for the measurement of serum LDL-C, HDL-C, rheumatoid factor, creatinine, and hemoglobin A1c (HbA1c) levels. We defined serum LDL-C levels of 140 to 159 mg/dL and ≥ 160 mg/dL as moderate and severe hyper-LDL cholesterolemia, respectively, and serum HDL-C levels ≤ 40 mg/dL as hypo-HDL cholesterolemia according to guidelines of the Japan Atherosclerosis Society. 29 Rheumatoid arthritis was defined as a rheumatoid factor ≥ 15 30 and diabetes mellitus was defined as an HbA1c concentration ≥ 6.5%. 31 To evaluate kidney function, we considered the estimated glomerular filtration rate (eGFR), whose equation is described in our previous reports. 32 33 We defined an eGFR < 60 mL/minute/1.73 m 2 as indicating reduced function. 33
We conducted personal interviews with the study participants to obtain information regarding their smoking habits and gleaned data on current medication usage from their personal medication records. The General Health Questionnaire 30 (GHQ-30), containing 30 questions, was used to assess depression. The GHQ-30 was scored in the Goldberg 0–0-1–1 format, in which any response indicating deviation from the norm was scored as 1 point. The total possible score on the GHQ-30 ranges from zero to 30 points. For the Japanese version of the GHQ-30, a cutoff score of 7 points was used, in which those who scored ≥ 7 points were designated as the depressive symptoms group. 34
The dental examination for assessing the number of teeth (excluding 3 rd molars) was conducted under sufficient illumination using artificial light. Body height and weight were measured to calculate the body mass index (BMI).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human individuals were approved by the Ethics Committee of the Faculty of Dentistry, Niigata University (approval number: 12-R1–4-21). Written informed consent was obtained from all study participants prior to their inclusion in the study.
Among the 380 participants, 7 participants who refused blood collection; 29 participants who did not complete the questionnaires including the GHQ-30 and smoking status; and 2 participants who had a history of head and neck cancer were excluded. Thus, the present study included 342 participants (170 males and 172 females) in the analysis. The measured variables were categorized as follows: USFR (normal or low); SSFR (normal or low); LDL-C (normal, moderate, or severe); HDL-C (normal or low); presence or absence of rheumatoid arthritis, diabetes mellitus, reduced function of the kidneys, and depressive symptoms; current smoker or nonsmoker; and taking antihypertensive, diuretic, antidepressant, or anticholinergic medication (yes or no). The groups were compared using the chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables. Multivariate logistic regression models were performed and low-USFR or low-SSFR were set as dependent variables in regression models. All independent variables with p < 0.25 in the univariate analysis and those with clinical epidemiological relevance, according to data in the literature, were included in the multivariable model as potential confounders. 35 Data analyses were performed using the IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). The value for the rejection of the null hypothesis was set at p < 0.05.
Results
Table 1 shows selected characteristics related to hyposalivation in previous reports. Almost half of our participants were female. Furthermore, there were 175 participants (51.2%) with low-USFR saliva flow and 118 participants (34.5%) with low-SSFR. The means ( ± standard deviations [ SDs] ) of USFR and SSFR were 0.139 ( ± 0.130) g/30 seconds and 1.45 (±0.86) mL/minute, respectively. Forty-one (12.0%) and 19 (5.6%) of the patients had moderate and severe hyper-LDL cholesterolemia, respectively, while 25 patients (7.3%) had hypo-HDL cholesterolemia.
Table 1. Selected characteristics of the study participants ( n = 342) .
| Variable | n (%) or mean ± SD |
|---|---|
| Gender: female | 172 (50.3) |
| Unstimulated flow rate (g/30 seconds) | 0.139 ± 0.130 |
| Low-USFR (≤ 0.10 g/30 seconds) | 175 (51.2) |
| Stimulated flow Rate (mL/minute) | 1.45 ± 0.86 |
| Low-SSFR (≤ 1.0mL/minute) | 118 (34.5) |
| Serum LDL level (mg/dL) | 115.6 ± 26.2 |
| Moderate hyper-LDL (140–159 mg/dL) | 41 (12.0) |
| Severe hyper-LDL (≥ 160 mg/dL) | 19 (5.6) |
| Serum HDL level (mg/dL) | 59.3 ± 14.5 |
| Hypo-HDL-C (≤ 40 mg/dL) | 25 (7.3) |
| Serum rheumatoid ator (IU/mL) | 9.81 ± 63.6 |
| Rheumatoid arthritis (≥ 15) | 21 (6.1) |
| HbA1C (%) | 5.36 ± 0.69 |
| Diabetes mellitus (HbA1c ≥ 6.5) | 21 (6.1) |
| eGFR (mL/minute/1.73 m 2 ) | 68.4 ± 16.4 |
| Reduced kidney function (< 60) | 102 (29.8) |
| GHQ score | 4.95 ± 5.28 |
| Depression (GHQ score ≥ 7) | 103 (30.1) |
| Antihypertensive agent intake | 157 (45.9) |
| Diuretic agent intake | 17 (5.0) |
| Antidepressant agent intake | 58 (17.0) |
| Anticholinergic agent intake | 18 (5.3) |
| Number of medications | 3.06 ± 3.01 |
| Number of remaining teeth | 15.7 ± 9.2 |
| Body mass index | 21.9 ± 3.07 |
| Current smoker | 30 (8.8) |
Abbreviations: eGFR, estimated glomerular filtration rate; GHQ, General Health Questionnaire; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation; SSFR, stimulated salivary flow rate; USFR, unstimulated salivary flow rate.
Tables 2 and 3 show associations of measured variables with USFR and SSFR. Hyper-LDL and hypo-HDL cholesterolemia were significantly associated with low-USFR. Hyper-LDL cholesterolemia was also significantly associated with low-SSFR. Out of 19 participants with severe hyper-LDL cholesterolemia, 16 (84.2%) and 13 (68.4%) had low-USFR and low-SSFR, respectively. Gender was significantly associated with low-USFR and low-SSFR. The frequency of females with reduced saliva flow rates was higher than that of males. Depression symptoms were significantly associated with low-SSFR only. There was no significant difference in the proportions of participants with reduced saliva flow rates between those with and those without current medication use, regardless of drug efficacy.
Table 2. Associations of measured variables with unstimulated saliva flow rate.
| Variable | Normal | Low | p-value | |||
|---|---|---|---|---|---|---|
| n = 167 | (48.8%) | n = 175 | (51.2%) | |||
| LDL-C | ||||||
| Moderate hyper | 15 | (36.6) | 26 | (63.4) | 0.002 | a |
| Severe hyper | 3 | (15.8) | 16 | (84.2) | ||
| HDL-C | ||||||
| Hypo | 7 | (28.0) | 18 | (72.0) | 0.030 | a |
| Gender | ||||||
| Female | 74 | (43.0) | 98 | (57.0) | 0.031 | a |
| Rheumatoid arthritis | ||||||
| Yes | 13 | (61.9) | 8 | (31.8) | 0.216 | a |
| Diabetes mellitus | ||||||
| Yes | 8 | (38.1) | 13 | (61.9) | 0.310 | a |
| Reduced kidney function | ||||||
| Yes | 55 | (53.9) | 47 | (46.1) | 0.219 | a |
| Depressive symptoms | ||||||
| Yes | 41 | (39.8) | 62 | (60.2) | 0.018 | a |
| Antihypertensive agent | ||||||
| Yes | 70 | (44.6) | 87 | (55.4) | 0.148 | a |
| Diuretic agent | ||||||
| Yes | 6 | (35.3) | 11 | (64.7) | 0.257 | a |
| Antidepressant agent | ||||||
| Yes | 28 | (48.3) | 30 | (51.7) | 0.834 | a |
| Anticholinergic agent | ||||||
| Yes | 11 | (61.1) | 7 | (38.9) | 0.284 | a |
| Number of medications | ||||||
| Mean ± SD | 2.86 ± 3.1 | 3.26 ± 3.0 | 0.083 | b | ||
| Number of remaining teeth | ||||||
| Mean ± SD | 15.2 ± 9.2 | 16.9 ± 9.0 | 0.134 | b | ||
| Body mass Index | ||||||
| Mean ± SD | 21.9 ± 3.0 | 22.1 ± 3.1 | 0.800 | b | ||
| Smoking | ||||||
| Current smoker | 17 | (56.7) | 13 | (43.3) | 0.369 | a |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
n (%) or Mean ± SD.
: Chi-squared test, b: Mann-Whitney U test.
Table 3. Associations of measured variables with stimulated saliva flow rate.
| Variables | Normal | Low | p-value | |||
|---|---|---|---|---|---|---|
| n = 224 | (65.5%) | n = 118 | (34.5%) | |||
| LDL-C | ||||||
| Moderate hyper | 27 | (65.9) | 14 | (34.1) | 0.006 | a |
| Severe hyper | 6 | (31.6) | 13 | (68.4) | ||
| HDL-C | ||||||
| Hypo | 17 | (68.0) | 8 | (32.0) | 0.785 | a |
| Gender | ||||||
| Female | 99 | (57.6) | 73 | (42.4) | 0.002 | a |
| Rheumatoid arthritis | ||||||
| Yes | 13 | (61.9) | 8 | (38.1) | 0.721 | a |
| Diabetes mellitus | ||||||
| Yes | 12 | (57.1) | 9 | (42.9) | 0.406 | a |
| Reduced kidney function | ||||||
| Yes | 72 | (70.6) | 30 | (29.4) | 0.197 | a |
| Depressive symptoms | ||||||
| Yes | 66 | (64.1) | 37 | (35.9) | 0.717 | a |
| Antihypertensive agent | ||||||
| Yes | 96 | (61.1) | 61 | (38.9) | 0.119 | a |
| Diuretic agent | ||||||
| Yes | 8 | (47.1) | 9 | (52.9) | 0.101 | a |
| Antidepressant agent | ||||||
| Yes | 39 | (67.2) | 19 | (32.7) | 0.759 | a |
| Anticholinergic agent | ||||||
| Yes | 11 | (61.1) | 7 | (38.9) | 0.688 | a |
| Number of medications | ||||||
| Mean ± SD | 2.88 ± 2.92 | 3.40 ± 3.17 | 0.131 | b | ||
| Number of remaining teeth | ||||||
| Mean ± SD | 16.2 ± 9.4 | 15.9 ± 8.6 | 0.431 | b | ||
| Body mass index | ||||||
| Mean ± SD | 21.85 ± 3.03 | 22.2 ± 3.13 | 0.312 | b | ||
| Smoking | ||||||
| Current smoker | 22 | (73.3) | 8 | (26.4) | 0.345 | a |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
n (%) or mean ± SD.
: Chi-squared test, b: Mann-Whitney U test.
Table 4 shows the results of logistic regression analysis. After controlling for the impact of the other variables, hyper-LDL cholesterolemia was found to be associated with low-USFR. The odds ratios (ORs) (95% confidence intervals [CIs]) for low-USFR were 2.25 (1.10–4.61) and 5.69 (1.55–20.8) for moderate and severe hyper-LDL cholesterolemia, while the OR for hypo-HDL cholesterolemia was 3.41 (1.34–8.71). Low-SSFR was significantly associated with severe hyper-LDL cholesterolemia and female gender, with ORs (95%CIs) of 3.89 (1.39–10.8) and 1.89 (1.18–3.05), respectively. The coefficient of determination was 0.13 in the low-USFR model and 0.10 in the low-SSFR model.
Table 4. Odds ratios from the multiple logistic regression analysis with low-USFR and low-SSFR as independent variables.
| Independent variables | Dependent variable | ||||||
|---|---|---|---|---|---|---|---|
| Low-USFR | Low-SSFR | ||||||
| Odds ratio | 95%CI | Odds ratio | 95%CI | ||||
| LDL-C (ref: normal) | |||||||
| Moderate hyper (140–159 mg/dL) | 2.25 | 1.10–4.61 | * | 0.97 | 0.47–1.98 | ||
| Severe hyper (≥ 160 mg/dL) | 5.69 | 1.55–20.8 | ** | 3.89 | 1.39–10.88 | ** | |
| HDL-C (ref: normal) | |||||||
| Hypo (< 40 mg/dL) | 3.40 | 1.33–8.69 | * | ||||
| Gender (ref: male) | |||||||
| Female | 1.32 | 0.83–2.08 | 1.88 | 1.17–3.03 | ** | ||
| Rheumatoid Arthritis (ref: no) | |||||||
| Yes | 0.48 | 0.18–1.28 | |||||
| Reduced kidney function (ref: no) | |||||||
| Yes | 0.62 | 0.38–1.03 | 0.61 | 0.35–1.04 | |||
| Depressive symptoms ( ref: no ) | |||||||
| Yes | 1.64 | 0.98–2.71 | |||||
| Antihypertensive agent (ref: no) | |||||||
| Yes | 1.48 | 0.88–2.48 | 1.31 | 0.77–2.25 | |||
| Diuretic agent (ref: no) | |||||||
| Yes | 2.53 | 0.88–7.26 | |||||
| Number of medications (continuous) | 1.00 | 0.92–1.10 | 1.02 | 0.93–1.12 | |||
| Number of remainingteeth (continuous) | 1.02 | 1.00–1.05 | |||||
| Constant | 0.31 | 0.17 | |||||
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SSFR, stimulated salivary flow rate; USFR, unstimulated salivary flow rate.
Discussion
The present cross-sectional study was conducted to analyze the association of cholesterolemia with reduced saliva flow rates in community-dwelling same-age elders. In the present study, we used the cotton roll method to measure unstimulated saliva flow. Some standardized methods, such as draining, spitting, suction, and swab collection, were suggested for collecting unstimulated saliva. 36 Navazesh et al. 37 reported that unstimulated salivary flow values were roughly equivalent for these four methods. However, we selected the cotton roll method that positively correlated (rS = 0.48) with the spitting method for collecting unstimulated saliva, 26 as it is easy to use for elderly subjects and reduces the time of examination and physical load. In addition, the original literature that described this method suggested a cutoff value for reduced saliva flow of 0.14 mg/30 seconds. 26 However, the authors mentioned that a different cutoff value for elders should be considered because this finding was obtained from among relatively young participants, and new findings for the elderly have not been reported. Thus, the median of the observed value in the current study (corresponding to 0.10 g/30 seconds) was adopted for low-USFR. Meanwhile, the cutoff value for low-SSFR was set according to the criterion for Sjögren syndrome. 28 Our results from these criteria show that cholesterolemia, hyper-LDL-C, and hypo-HDL-C could all be risk indicators for reduced stimulated saliva flow rates even after controlling for prescribed confounders, such as gender 15 and depressive symptoms. 17 18 19 This is, at least to our knowledge, the first evidence that serum cholesterol levels are associated with saliva flow rate in elders.
The results of the present study should be interpreted with some caution given the lack of significant relationships between factors reported in previous studies and reduced saliva flow rates. The number of medications also was not associated with both saliva flow volumes, although antihypertensive medication and diuretic agent intake tended to be correlated ( p < 0.25) with low saliva flow in the univariate analysis. Moreover, there were also no significant differences in the amount of unstimulated and stimulated saliva flow volumes between the use of certain medications (data not shown). In addition, we did not find significant relationships between reduced saliva flow rates and certain systemic diseases such as rheumatoid arthritis, diabetes mellitus, and chronic kidney disease. These findings might be considered as simply due to the fact that none of the participants had severe cases of these diseases in the population of the present study. In addition, we did not find relationships between oral status and saliva flow. Furthermore, the number of remaining teeth was not associated with reduced saliva flow rate, and differences in the proportion of low-USFR or low-SSFR between participants with or without dentures were not noted (data not shown).
Previous research revealed that blood flow velocity upon stimulation with acid using pulse Doppler sonography was associated with saliva flow rate and that a decrease in velocity would reduce the saliva flow rate. 23 Seki et al. 24 reported relationships between whole-blood passage time and serum cholesterol concentration; the LDL-C level was positively correlated (r = 0.221) and the HDL-C level was negatively correlated (r = − 0.227). We assumed, from these findings, that hyper–LDL-C and hypo–HDL-C had approximately equal effects on salivary reduction. However, our results showed that the LDL-C level was significantly associated with reduced saliva flow rates. The ORs were also found to increase along with the grade of severity of hyper-LDL cholesterolemia. We speculated from these findings that a high LDL-C concentration leads to low blood velocity, resulting in reduced saliva flow rate. The serum HDL-C level was, however, associated only with USFR in our analysis. Of note, arteries plaques are caused by increased serum LDL-C levels, and HDL-C absorbs LDL-C and carries it back to the liver. 38 We considered, therefore, that LDL-C would assume the role to change the blood flow directly and that HDL-C would play a supportive role, while LDL-C would also play an important role in the reduction of salivary volume. In addition, Izumi et al 39 reported that the parotid gland in patients with hyperlipidemia was associated with extensive lipid infiltrations from magnetic resonance imaging (MRI) features, elevated plasma triglyceride levels correlated with parotid gland swelling, and increased total cholesterol levels significantly affected salivary flow impairment in patients with hyperlipidemia. These previous findings might support our result, which is reduction in not only stimulated salivary flow but also unstimulated salivary flow that does not require as great an increase in salivary gland blood flow.
Psychological factors such as depression, stress, and anxiety, as well as female gender, were indicated as risk factors for low saliva flow. Some studies reported that psychological factors were associated with unstimulated saliva flow. 17 18 40 Elsewhere, Hugo et al. 19 reported that caregiving, a proxy of chronic stress, was associated with low stimulated saliva flow, however, and not associated with low unstimulated saliva flow. The results of our univariate analysis are in agreement with the former. Female gender is a well-known risk factor for reduced saliva flow rates, and our findings from the univariate analysis were also in accordance with this finding.
Our study showed that the adjusted ORs of the impact of cholesterolemia for reduced saliva flow rates were higher in females, which might be an important finding when considering the cause of hyposalivation.
Our results showed a stronger relationship between factors reported in previous studies, including gender, with salivary reduction. However, these findings were sourced from a cross-sectional study, and the relationship between changes in LDL-C and HDL-C levels and saliva flow rate must be further investigated in the future.
Conclusions
Our findings showed that elderly patients with cholesterolemia could be at risk of reduced salivary flow rate. It was suggested that educating the elderly to control their LDL-C and HDL-C levels is a viable means to help prevent arteriosclerotic and oral diseases. As hyposalivation might also be a risk factor of acute respiratory infection, 6 it is important, especially among elders, to maintain an adequate salivary flow volume.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol. 2001;46(05):413–423. doi: 10.1016/s0003-9969(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 2.Mizutani S, Ekuni D, Tomofuji T. Relationship between xerostomia and gingival condition in young adults. J Periodontal Res. 2015;50(01):74–79. doi: 10.1111/jre.12183. [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Arnold A M, Marek C A. The impact of saliva on patient care: A literature review. J Prosthet Dent. 2002;88(03):337–343. doi: 10.1067/mpr.2002.128176. [DOI] [PubMed] [Google Scholar]
- 4.Sreebny L. Saliva–salivary gland hypofunction (SGH). FDI Working Group 10. J Dent Assoc S Afr. 1992;47(11):498–501. [PubMed] [Google Scholar]
- 5.Bergdahl J, Bergdahl M. Environmental illness: evaluation of salivary flow, symptoms, diseases, medications, and psychological factors. Acta Odontol Scand. 2001;59(02):104–110. doi: 10.1080/000163501750157270. [DOI] [PubMed] [Google Scholar]
- 6.Iwabuchi H, Fujibayashi T, Yamane G Y, Imai H, Nakao H. Relationship between hyposalivation and acute respiratory infection in dental outpatients. Gerontology. 2012;58(03):205–211. doi: 10.1159/000333147. [DOI] [PubMed] [Google Scholar]
- 7.Kakoei S, Haghdoost A A, Rad M. Xerostomia after radiotherapy and its effect on quality of life in head and neck cancer patients. Arch Iran Med. 2012;15(04):214–218. [PubMed] [Google Scholar]
- 8.Tribius S, Sommer J, Prosch C. Xerostomia after radiotherapy. What matters–mean total dose or dose to each parotid gland? Strahlenther Onkol. 2013;189(03):216–222. doi: 10.1007/s00066-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 9.Kojima I, Sakamoto M, Iikubo M, Shimada Y, Nishioka T, Sasano T. Relationship of MR imaging of submandibular glands to hyposalivation in Sjögren's syndrome. Oral Dis. 2019;25(01):117–125. doi: 10.1111/odi.12941. [DOI] [PubMed] [Google Scholar]
- 10.Kobak S, Oksel F, Aksu K, Kabasakal Y. The frequency of sicca symptoms and Sjögren's syndrome in patients with systemic sclerosis. Int J Rheum Dis. 2013;16(01):88–92. doi: 10.1111/j.1756-185X.2012.01810.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagler R M, Salameh F, Reznick A Z, Livshits V, Nahir A M. Salivary gland involvement in rheumatoid arthritis and its relationship to induced oxidative stress. Rheumatology (Oxford) 2003;42(10):1234–1241. doi: 10.1093/rheumatology/keg362. [DOI] [PubMed] [Google Scholar]
- 12.López-Pintor R M, Casañas E, González-Serrano J. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. 2016;2016:4.372852E6. doi: 10.1155/2016/4372852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyetola E O, Owotade F J, Agbelusi G A, Fatusi O, Sanusi A, Adesina O M. Salivary flow rates of Nigerian patients with chronic kidney disease: A case-control study. J Contemp Dent Pract. 2015;16(04):264–269. doi: 10.5005/jp-journals-10024-1673. [DOI] [PubMed] [Google Scholar]
- 14.Percival R S, Challacombe S J, Marsh P D. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994;73(08):1416–1420. doi: 10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]
- 15.Thomson W M, Chalmers J M, Spencer A J, Slade G D. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(01):12–20. doi: 10.1111/j.1752-7325.2000.tb03286.x. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Wolff A, Narayana N. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction. Oral Dis. 2016;22(05):365–382. doi: 10.1111/odi.12402. [DOI] [PubMed] [Google Scholar]
- 17.Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(09):1652–1658. doi: 10.1177/00220345000790090301. [DOI] [PubMed] [Google Scholar]
- 18.Queiroz C S, Hayacibara M F, Tabchoury C P, Marcondes F K, Cury J A. Relationship between stressful situations, salivary flow rate and oral volatile sulfur-containing compounds. Eur J Oral Sci. 2002;110(05):337–340. doi: 10.1034/j.1600-0722.2002.21320.x. [DOI] [PubMed] [Google Scholar]
- 19.Hugo F N, Hilgert J B, Corso S. Association of chronic stress, depression symptoms and cortisol with low saliva flow in a sample of south-Brazilians aged 50 years and older. Gerodontology. 2008;25(01):18–25. doi: 10.1111/j.1741-2358.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson J C, Fox P C. Salivary gland dysfunction. Clin Geriatr Med. 1992;8(03):499–511. [PubMed] [Google Scholar]
- 21.Heft M W, Baum B J. Unstimulated and stimulated parotid salivary flow rate in individuals of different ages. J Dent Res. 1984;63(10):1182–1185. doi: 10.1177/00220345840630100101. [DOI] [PubMed] [Google Scholar]
- 22.Sreebny L M. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(03):140–161. doi: 10.1111/j.1875-595x.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 23.Katsura K, Ito K, Nohno K, Funayama S, Saito M, Hayashi T. Evaluation of the relationship between salivation ability and blood flow velocity in the submandibular gland using pulsed Doppler ultrasonography. Oral Radiol. 2013;29:13–18. [Google Scholar]
- 24.Seki K, Sumino H, Murakami M.[Study on blood rheology measured by MC-FAN] Rinsho Byori 20035108770–775.. In Japanese. [PubMed] [Google Scholar]
- 25.Hirotomi T, Yoshihara A, Yano M, Ando Y, Miyazaki H. Longitudinal study on periodontal conditions in healthy elderly people in Japan. Community Dent Oral Epidemiol. 2002;30(06):409–417. doi: 10.1034/j.1600-0528.2002.00005.x. [DOI] [PubMed] [Google Scholar]
- 26.Funayama S, Ito K, Nohno K.Comparative study of salivary secretion in cotton roll method and spitting method Niigata Dent J 20083895–101.(in Japanese) [Google Scholar]
- 27.Ichikawa K, Sakuma S, Yoshihara A. Relationships between the amount of saliva and medications in elderly individuals. Gerodontology. 2011;28(02):116–120. doi: 10.1111/j.1741-2358.2009.00358.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004;14(06):425–434. doi: 10.3109/s10165-004-0338-x. [DOI] [PubMed] [Google Scholar]
- 29.Japan Atherosclerosis Society Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2007http://www.j-athero.org/publications/pdf/guideline_summary. Accessed Dec 31, 2019. [PubMed]
- 30.Thammanichanond D, Kunakorn M, Kitiwanwanich S, Attamasirikul K, Nantiruj K.Raising rheumatoid factor cutoff helps distinguish rheumatoid arthritis Asian Pac J Allergy Immunol 200523(2-3):165–168. [PubMed] [Google Scholar]
- 31.Cahill L E, Jensen M K, Chiuve S E. The risk of coronary heart disease associated with glycosylated hemoglobin of 6.5% or greater is pronounced in the haptoglobin 2–2 genotype. J Am Coll Cardiol. 2015;66(16):1791–1799. doi: 10.1016/j.jacc.2015.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshihara A, Kaneko N, Iwasaki M, Nohno K, Miyazaki H. Relationship between vitamin D receptor gene polymorphism and susceptibility to chronic kidney disease and periodontal disease in community-dwelling elderly. J Clin Periodontol. 2018;45(06):672–679. doi: 10.1111/jcpe.12896. [DOI] [PubMed] [Google Scholar]
- 33.Japanese Society of Nephrology Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease 2012https://cdn.jsn.or.jp/guideline/pdf/CKDguide2012.pdf. Accessed Dec 31, 2019.
- 34.Nakagawa Y, Daibou I. Tokyo, Japan: 1985. The Japanese Version of the GHQ. [Google Scholar]
- 35.Hosmer D W, Lemeshow S. New York: Wiley; 1989. Applied Logistic Regression. [Google Scholar]
- 36.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 37.Navazesh M, Christensen C M. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61(10):1158–1162. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- 38.Hao W, Friedman A. The LDL-HDL profile determines the risk of atherosclerosis: a mathematical model. PLoS One. 2014;9(03):e90497. doi: 10.1371/journal.pone.0090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izumi M, Hida A, Takagi Y, Kawabe Y, Eguchi K, Nakamura T. MR imaging of the salivary glands in sicca syndrome: comparison of lipid profiles and imaging in patients with hyperlipidemia and patients with Sjögren's syndrome. AJR Am J Roentgenol. 2000;175(03):829–834. doi: 10.2214/ajr.175.3.1750829. [DOI] [PubMed] [Google Scholar]
- 40.Gholami N, Hosseini Sabzvari B, Razzaghi A, Salah S. Effect of stress, anxiety and depression on unstimulated salivary flow rate and xerostomia. J Dent Res Dent Clin Dent Prospect. 2017;11(04):247–252. doi: 10.15171/joddd.2017.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


