Abstract
Background and study aims We assessed sessile serrated lesion detection rate (SSLDR) at a large academic medical center from 2008 to 2020 and modeled a local, aspirational target SSLDR. We also assessed SSLDRs among all gastroenterology fellows to better understand the relationship between SSLDRs and total colonoscopies performed.
Patients and methods SSL-positive pathology results were flagged from a dataset composed of all screening colonoscopies for average-risk patients from 2008 to 2020. Unadjusted SSLDRs were calculated for individual endoscopists by year. A mixed effects logistic regression was used to estimate the log odds of SSL detection, with one model estimating division-wide predictors of SSL detection and a second model focused exclusively on colonoscopies performed by fellows. Model-adjusted SSLDRs were estimated for all 13 years and across both categories of all endoscopists and fellows only.
Results Adjusted SSLDRs showed a consistent improvement in SSLDR from a low of 0.37 % (95 % confidence interval [CI]: 0.10–0.63) in 2008 to a high of 7.94 % (95 % CI: 6.34–9.54) in 2020. Among fellows only, the odds of SSL detection were significantly lower during their first year compared to their second year (OR: 0.80, 95 % CI: 0.66–0.98) but not significantly higher in their third year compared to their second year (OR: 1.09, 95 % CI: 0.85–1.4).
Conclusions SSLDR increased steadily and significantly throughout our study period but variance among endoscopists persists. The peak SSLDR from 2020 of 7.94 % should serve as the local aspirational target for this division’s attendings and fellows but should be continuously reevaluated.
Introduction
Despite a decades-long reduction in colorectal cancer (CRC) incidence and mortality rates, CRC is still the third most commonly diagnosed cancer and the third leading cause of cancer mortality in the US 1 2 . Population-wide improvement in CRC screening adherence is believed to be one of the major drivers of recent decreases in CRC incidence and mortality rates 3 . However, for this downward trajectory of CRC incidence and mortality rates to continue, the quality, and not just the quantity, of CRC screening must improve. A growing body of evidence suggests that the current recommended CRC screening modalities are not equally effective at detecting all types of precursor lesions. Of particular interest are serrated lesions, which account for approximately one-third of all CRC and an even greater percentage of post-colonoscopy interval cancers 4 5 6 . These lesions are more likely to be missed than conventional adenomas by high-sensitivity guaiac fecal occult blood tests (HS-gFOBT) 7 , fecal immunochemical tests (FIT) 7 8 9 , computed tomographic (CT) colonography when diminutive (≤ 5 mm) 10 , and, given serrated lesions’ preponderance for the proximal colon 4 11 , flexible sigmoidoscopies 12 .
This leaves colonoscopy as the best-suited screening modality for detecting serrated lesions 3 . However, even colonoscopy exhibits disadvantages for detection relative to conventional adenomas. Serrated lesions are generally flat—or sessile—and are often covered with a mucous cap. Combined with their common location in the proximal colon, these lesions can be easily missed by endoscopists. Even when detected, incomplete resection is more common with serrated lesions given the difficulty in accurately detecting borders 13 . Furthermore, even upon successful detection and resection, the pathological criteria for defining serrated lesions have evolved over the past 15 years 14 15 16 17 . These endoscopic and pathological detection characteristics, combined with the relatively nascent knowledge and training base related to the serrated pathway, have contributed to highly variable detection rates among endoscopists 18 19 . Two recent meta-analyses of serrated lesion detection included studies with sessile serrated lesion detection rates (SSLDRs) as low as 0.6 % and as high as 10.3 % 11 20 . Both meta-analyses were conducted to begin the process of approximating an aspirational SSLDR, which does not yet exist, similar to the adenoma detection rate (ADR) benchmark of at least 25 % (men and women combined) 3 .
While the field continues to move toward determining the true prevalence of serrated lesions across diverse patient populations, a call has been made to focus on local SSLDR and to use the highest rate from a unit as the local aspirational target 21 . Doing so incorporates nuances of the local patient population while continuing to call attention to this less understood cancer pathway. In this study, we retrospectively examined the SSLDR of the Gastroenterology Division at the University of New Mexico Health Sciences Center from 2008 to 2020 and model a local, aspirational target SSLDR per Kahi & Rex’s recommendation 21 . We also assessed the progression of SSLDR among gastroenterology fellows to better understand the relationship between SSLDR and total number of colonoscopies performed through the course of a multi-year fellowship.
Patients and methods
After receiving approval from the institutional review board of the University of New Mexico Health Sciences Center (Ref. 18–355), we conducted a retrospective, longitudinal analysis of all average-risk colonoscopies conducted by the Division of Gastroenterology and Hepatology at the University of New Mexico Health Sciences Center from January of 2008 through December of 2020. The Albuquerque, New Mexico-based Division of Gastroenterology and Hepatology is an academic research center that cares for more than 10,000 patients annually across numerous clinics. The program offers a three-year fellowship program with an optional fourth year for advanced endoscopy specialization. At the time of this analysis, the Division used white light with high-definition (HD) endoscopes with the ability to switch to narrow band imaging on selected cases. The Division switched to HD endoscopes in 2008. As such, no cases included in this study were conducted using standard definition colonoscopes. No ancillary techniques such as underwater immersion colonoscopies were performed in the Division during the study period. Similarly, no distal attachments or caps were used. For withdrawal technique besides standard withdrawal, second forward view was used in most cases. The practice of retroflexion in the right colon to examine proximal folds has increased during the past five years but is still not widely adopted by gastroenterologists across the country and was used only in a minority of our study cases. Documentation of these technologies and techniques were sporadic or were not machine-readable in colonoscopy reports and were therefore excluded from this analysis.
Study sample and variable selection
Average-risk patient colonoscopies were identified through billing data and included codes: G0121 (Average risk screening); 45378-33 (colonoscopy with modifier 33 indicating a preventive service); ICD-9 code V76.51; or ICD-10 code Z12.11. Billing data were joined to electronic health record (EHR) and pathology record data using a clinical encounter number. Pathology records for the Division are created by attending pathologists, most of whom, but not all, are gastrointestinal pathologists. Pathology records were queried for any observations of SSL to capture the evolution of the pathological nomenclature used over the 13-year study period. This search included any mention in a pathology report for: sessile serrated lesion, sessile serrated adenoma, sessile serrated polyp, SSA, SSL, or sessile serrated polyp (SSP). Proximal hyperplastic polyps were not included in the query. Although the evidence base now suggests that many SSLs were historically misclassified as hyperplastic polyps, histopathological distinction did not emerge until later in our study period. Reviewing and reclassifying all previously diagnosed hyperplastic polyps in our data warehouse from 2008 onward as either SSL or confirmed hyperplastic polyps was outside of the scope of this study. SSL-positive pathology records were then manually reviewed to confirm positive SSL identification and to rule out instances of negation (e. g. “not SSL”). EHR data were used to obtain patient demographics including sex, age, race, and ethnicity. Only patients aged 50 years and older were included in the analysis. Division administrative data were used to identify if the performing endoscopist was an attending alone or a fellow. (All fellows perform colonoscopies under the supervision of an attending.) Due to a change in billing record vendors, fellows could not be identified prior to 2014 and were excluded from the analysis from 2008 through 2013, apart from a single fellow who became an attending in 2013. Any endoscopist with fewer than 50 qualified screening colonoscopies over the course of 1 calendar year was excluded from that year’s data. Due to numerous changes in software and EHR vendors during the 13-year study period, withdrawal times were not available or were not machine-readable for all eligible colonoscopies and were, therefore, excluded from this analysis.
Statistical analysis
We began with unadjusted, descriptive analyses of SSLDR at the endoscopist and Division levels over the 13-year study period. Unadjusted SSLDRs were calculated for individual endoscopists by year. Next, given the binary outcome variable of SSL detection and the longitudinal and hierarchical nature of the dataset (i. e., colonoscopies could be performed by a particular attending or a fellow or an attending who had been a fellow during the 13-year study period), a mixed effects logistic regression was used. The model estimated the log odds of SSL detection, exponentiated to odds ratios, as a linear combination of fixed and random predictor variables. One key fixed variable was year, and the included random effect term estimated a per-physician effect in order to account for their performing multiple colonoscopies over the study period. Two models were utilized. The first model used all colonoscopies and endoscopists to estimate Division-wide predictors of SSL detection. The second model focused exclusively on colonoscopies performed by fellows to determine if and to what degree SSLDR improved during their multi-year fellowships. Model-adjusted SSLDRs were then estimated for all years and across both categories of all endoscopists and fellows. All analyses were performed using Stata Statistical Software: Release 17 (College Station, TX).
Results
Our pooled data for all 13 years of the study included 36,467 average-risk screening colonoscopies. Table 1 presents the patient and endoscopist characteristics for these colonoscopies. The number of colonoscopies performed annually were relatively stable from 2010 through 2019 but notably decreased by nearly one-third in 2020, likely a result of patients seeking fewer elective procedures during the SARS-CoV-2 pandemic. The average age and percentage of patients who were male were relatively stable throughout the study period, hovering around 60 years and 45 %, respectively. Finally, the percentage of colonoscopies performed by fellows versus attendings varied significantly during the period where data on fellows were available (2014–2020), from a low of 47.1 % in 2017 to a high of 67.6 % in 2019, with no apparent trend ( Table 1 ).
Table 1. Colonoscopy, patient, and endoscopist characteristics, 2008–2020.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total | |
| Total screening colonoscopies | 2,077 | 1,960 | 3,207 | 3,340 | 2,673 | 2,992 | 3,173 | 2,724 | 3,088 | 3,288 | 2,838 | 3,072 | 2,035 | 36,467 |
| Colonoscopies with SSLs detected | 8 | 20 | 80 | 50 | 77 | 140 | 157 | 159 | 186 | 255 | 204 | 215 | 150 | 1,701 |
| SSL detection rate | 0.4 % | 1.0 % | 2.5 % | 1.5 % | 2.9 % | 4.7 % | 4.9 % | 5.8 % | 6.0 % | 7.8 % | 7.2 % | 7.0 % | 7.4 % | 4.7 % |
| Patient characteristics | ||||||||||||||
|
60.0 (8.0) | 59.7 (7.8) | 58.8 (7.6) | 59.3 (7.7) | 59.6 (7.6) | 59.5 (7.5) | 59.4 (7.5) | 60.0 (7.7) | 60.1 (7.6) | 61.1 (7.7) | 61.7 (8.0) | 61.5 (7.8) | 62.1 (8.1) | 60.2 (7.8) |
|
41.4 | 42.6 | 41.0 | 44.6 | 42.8 | 44.6 | 45.4 | 45.7 | 44.0 | 45.6 | 46.2 | 47.6 | 46.0 | 44.5 |
| Race [count (column %)] | ||||||||||||||
|
131 (6.3) | 115 (5.9) | 185 (5.8) | 231 (6.9) | 167 (6.2) | 184 (6.1) | 200 (6.3) | 231 (8.5) | 232 (7.5) | 229 (7.0) | 208 (7.3) | 219 (7.1) | 164 (8.1) | 2,496 (6.8) |
|
46 (2.2) | 59 (3.0) | 87 (2.7) | 110 (3.3) | 71 (2.7) | 109 (3.6) | 98 (3.1) | 83 (3.0) | 123 (4.0) | 125 (3.8) | 95 (3.3) | 111 (3.6) | 57 (2.8) | 1,174 (3.2) |
|
40 (1.9) | 51 (2.6) | 86 (2.7) | 99 (3.0) | 68 (2.5) | 91 (3.0) | 102 (3.2) | 74 (2.7) | 90 (2.9) | 93 (2.8) | 73 (2.6) | 74 (2.4) | 56 (2.8) | 997 (2.7) |
|
324 (15.6) | 321 (16.4) | 630 (19.6) | 606 (18.1) | 487 (18.2) | 540 (18.0) | 493 (15.5) | 377 (13.8) | 524 (17.0) | 517 (15.7) | 415 (14.6) | 480 (15.6) | 336 (16.5) | 6,050 (16.6) |
|
1,536 (74.0) | 1,414 (72.1) | 2,219 (69.2) | 2,294 (68.7) | 1,880 (70.3) | 2,068 (69.1) | 2,280 (71.9) | 1,959 (71.9) | 2,119 (68.6) | 2,324 (70.7) | 2,047 (72.1) | 2,188 (71.2) | 1,422 (69.9) | 25,750 (70.6) |
| Ethnicity [count (column %)] | ||||||||||||||
|
634 (30.5) | 693 (35.4) | 1,315 (41.0) | 1,332 (39.9) | 1,128 (42.2) | 1,265 (42.3) | 1,325 (41.8) | 1,109 (40.7) | 1,341 (43.4) | 1,352 (41.1) | 1,145 (40.3) | 1,215 (39.6) | 838 (41.2) | 14,692 (40.3) |
|
877 (42.2) | 829 (42.3) | 1,329 (41.4) | 1,548 (46.3) | 1,391 (52.0) | 1,563 (52.2) | 1,645 (51.8) | 1,443 (53.0) | 1,556 (50.4) | 1,741 (53.0) | 1,509 (53.2) | 1,622 (52.8) | 1,030 (50.6) | 18,083 (49.6) |
|
566 (27.3) | 438 (22.3) | 563 (17.6) | 460 (13.8) | 154 (5.8) | 164 (5.5) | 203 (6.4) | 172 (6.3) | 191 (6.2) | 195 (5.9) | 184 (6.5) | 235 (7.6) | 167 (8.2) | 3,692 (10.1) |
| Endoscopist rank [count (column %)] | ||||||||||||||
|
2,077 (100) | 1,960 (100) | 3,207 (100) | 3,240 (100) | 2,618 (97.9) | 2,992 (100) | 1,659 (52.3) | 1,147 (42.1) | 1,439 (46.6) | 1,740 (52.9) | 1,111 (39.2) | 996 (32.4) | 858 (42.2) | 25,144 (69.0) |
|
0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 55 (2.1) | 0 (0.0) | 1,515 (47.7) | 1,577 (57.9) | 1,649 (53.4) | 1,548 (47.1) | 1,727 (60.9) | 2,077 (67.6) | 1,177 (57.8) | 11,323 (31.1) |
SD, standard deviation.
Total SSLs detected on an annual basis increased steadily throughout the study period, from a low of eight in 2008 to 255 in 2017. SSLs detected appear to drop off to 150 in 2020, but taking into account the significantly lower denominator of total colonoscopies performed that year due to SARS-CoV-2, the unadjusted SSLDR is the second highest in 2020 at 7.37 %. Fig. 1 , which presents unadjusted SSLDR at the physician level, shows significant SSLDR variance across providers throughout the study period but also illustrates general improvement in SSLDR at both the physician and division levels. Most fellows, generally identifiable in Fig. 1 as those with only 2 or 3 years of data, show consistent year-over-year improvement in SSLDR.
Fig. 1.
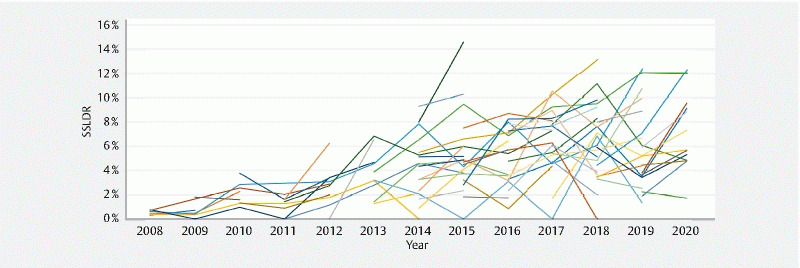
Unadjusted physician-level sessile serrated lesion detection rate, 2008–2020. Each line represents the sessile serrated lesion detection rate of an individual physician.
Table 2 presents the odds ratios (ORs) for SSL detection using all colonoscopies and endoscopists from the mixed effects logistic regression model. The odds of SSL detection were lower for first-year fellows compared to non-first-year fellows and all attendings, with first-year fellows having a 19 % reduction in the odds of detecting SSL (OR: 0.81, 95 % confidence interval [CI]: 0.67–0.96). Male patients had a 14 % reduction in the odds of having SSL detected than females (OR: 0.86, 95 % CI: 0.78–0.96). Each additional year of age for a patient yielded a moderately higher likelihood of SSL detection (OR: 1.01, 95 % CI: 1.001–1.01). Compared to white patients, American Indian patients had a 61 % reduction in the odds of having SSL detected, Asian patients, a 43 % reduction, and Black patients, a 60 % reduction. Non-Hispanics/Non-Latinos had 69 % higher odds of an SSL detected than Hispanics/Latinos (OR: 1.69, 95 % CI: 1.51–1.89). Finally, the probability of SSL detection increased significantly in every year compared to 2008. Most notably, the odds of having an SSL detected in 2020 were nearly 24 times higher than in 2008, controlling for all other variables (OR: 23.81, 95 % CI: 11.17–50.75). The non-zero random effect supports our utilization of a mixed model and suggests heterogeneity among endoscopists in detecting SSL. The reported random effects variance of 0.099 suggests that the best endoscopist had a roughly 2.6 higher odds of detection than the average endoscopist (e (3 × √0.099) = 2.6) when controlling for all other predictors in the model.
Table 2. Mixed effects logistic regression results: odds ratios for sessile serrated lesion detection.
| Fixed effects | Odds ratio | Standard error | P > z | 95 % CI | |
| Attending vs. fellow | |||||
|
REF | REF | REF | REF | REF |
|
0.934 | 0.094 | 0.497 | 0.768 | 1.137 |
| First-year fellow | 0.805 | 0.074 | 0.018 | 0.673 | 0.964 |
| Patient sex | |||||
|
REF | REF | REF | REF | REF |
|
0.859 | 0.044 | 0.003 | 0.777 | 0.949 |
| Patient age | 1.008 | 0.003 | 0.017 | 1.001 | 1.014 |
| Patient race | |||||
|
REF | REF | REF | REF | REF |
|
0.385 | 0.051 | 0.000 | 0.296 | 0.500 |
|
0.568 | 0.090 | 0.000 | 0.416 | 0.775 |
|
0.399 | 0.082 | 0.000 | 0.266 | 0.597 |
|
0.918 | 0.074 | 0.287 | 0.785 | 1.074 |
| Patient ethnicity | |||||
|
REF | REF | REF | REF | REF |
|
1.687 | 0.098 | 0.000 | 1.505 | 1.890 |
|
1.256 | 0.135 | 0.033 | 1.018 | 1.550 |
| Year | |||||
|
REF | REF | REF | REF | REF |
|
2.495 | 1.060 | 0.031 | 1.085 | 5.737 |
|
6.262 | 2.410 | 0.000 | 2.945 | 13.315 |
|
3.626 | 1.436 | 0.001 | 1.668 | 7.882 |
|
7.523 | 2.917 | 0.000 | 3.518 | 16.087 |
|
13.030 | 4.959 | 0.000 | 6.180 | 27.473 |
|
15.299 | 5.867 | 0.000 | 7.215 | 32.442 |
|
16.961 | 6.504 | 0.000 | 7.999 | 35.963 |
|
16.256 | 6.209 | 0.000 | 7.690 | 34.366 |
|
20.573 | 7.797 | 0.000 | 9.788 | 43.242 |
|
20.765 | 7.915 | 0.000 | 9.837 | 43.833 |
|
22.371 | 8.552 | 0.000 | 10.575 | 47.326 |
|
23.813 | 9.193 | 0.000 | 11.174 | 50.749 |
| Constant | 0.002 | 0.001 | 0.000 | 0.001 | 0.005 |
| Physician random effect (Variance of random intercepts) |
0.099 | 0.032 | 0.052 | 0.186 | |
CI, confidence interval.
Using the model’s fixed and physician random effects, we created an adjusted, unit-level SSLDR by year with 95 % confidence intervals ( Fig. 2 ). The adjusted SSLDR shows a consistent improvement in SSLDR from a low of 0.37 % (95 % CI: 0.10–0.63) in 2008 to a high of 7.94 % (95 % CI: 6.34–9.54) in 2020.
Fig. 2.
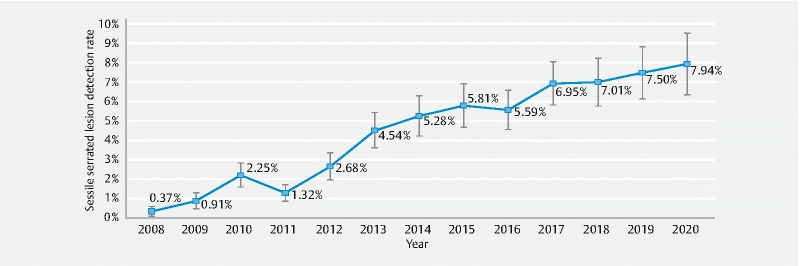
Adjusted sessile serrated lesion detection rate by year with 95 % confidence intervals. Each marker represents the model-adjusted, division-level sessile serrated lesion detection rate including its 95 % confidence interval.
Fig. 3 presents changes in SSLDR for each fellow between their first and second year and between their second and third year. Fellows with teal plots improved their SSLDR from the lower bound in the preceding year to the upper bound in the following year. Fellows with blue plots decreased their SSLDR from the upper bound in the preceding year to the lower bound in the following year. These unadjusted results indicate more year-over-year improvement between the first and second year of the gastroenterology fellowship than the second and third year. In comparing all eligible fellows between their first and second years, 28 of 34 fellows (82.4 %) had higher SSLDRs in their second year. Average unadjusted SSLDR for eligible fellows grew from 4.34 % in the first year of their fellowship to 5.79 % in their second year, an absolute improvement of 1.45 %. In comparing all eligible fellows between their second and third years, 13 out of 19 fellows (68.4 %) had higher SSLDRs in their third year. Average unadjusted SSLDRs for all eligible fellows grew from 5.24 % in the second year of their fellowship to 6.04 % in their third year, an absolute improvement of only 0.80%. Fig. 3 also illustrates the relatively high variance of SSLDRs across fellows at all points of time.
Fig. 3.
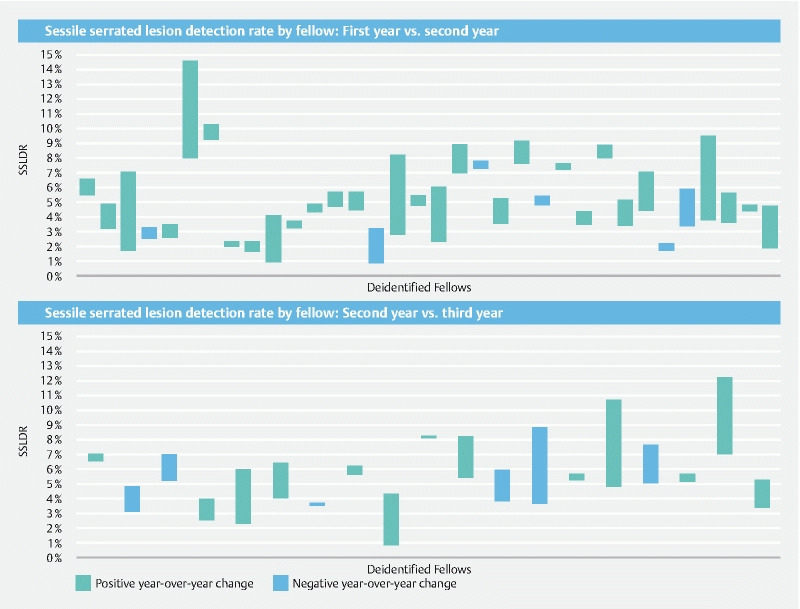
Sessile serrated lesions detection rate by fellow: first year vs. second year (top) and second year vs. third year (bottom). Each column represents the year-over-year percentage change in sessile serrated lesion detection rate (SSLDR) of an individual fellow. Striped columns represent positive year-over-year change (improvement) is SSLDR while checkered columns represent negative year-over-year change (diminishment).
Table 3 presents the ORs for SSL detection using only fellow-performed colonoscopies from a second mixed effects logistic regression model. Results confirmed the analysis of the unadjusted trends across years of fellowship ( Fig. 3 ), with the odds of SSL detection being significantly lower during a fellow’s first year compared to their second year (OR: 0.80, 95 % CI: 0.66–0.98) but not significantly higher in their third year compared to their second year (OR: 1.09, 95 % CI: 0.85–1.4). With fellows only, male patients had a 16 % reduction in the odds of having an SSL detected (OR: 0.84, 95 % CI: 0.71–0.99), American Indians and Black patients were less likely than Whites to have SSL detected (OR: 0.43, 95 % CI: 0.30–0.62, and OR: 0.48, 95 % CI: 0.27–0.85, respectively), and Non-Hispanics/Non-Latinos had 94 % higher odds to have SSL detected than Hispanics/Latinos (OR: 1.94, 95 % CI: 1.61–2.33). Using 2014 as a referent, fellows were no more or less likely to detect SSLs during any of the subsequent years.
Table 3. Mixed Effects Logistic Regression Results: Odds Ratios for Sessile Serrated Lesion Detection among Fellows Only.
| Fixed effects | Odds ratio | Standard error | P > z | 95 % CI | |
| Fellowship year | |||||
|
0.800 | 0.082 | 0.030 | 0.654 | 0.979 |
|
REF | REF | REF | REF | REF |
|
1.089 | 0.141 | 0.509 | 0.845 | 1.403 |
| Patient sex | |||||
|
REF | REF | REF | REF | REF |
|
0.838 | 0.069 | 0.032 | 0.714 | 0.985 |
| Patient Age | 1.002 | 0.005 | 0.705 | 0.992 | 1.013 |
| Patient Race | |||||
|
REF | REF | REF | REF | REF |
|
0.428 | 0.080 | 0.000 | 0.297 | 0.617 |
|
0.700 | 0.153 | 0.103 | 0.456 | 1.074 |
|
0.480 | 0.139 | 0.011 | 0.272 | 0.846 |
|
1.038 | 0.139 | 0.782 | 0.799 | 1.348 |
| Patient ethnicity | |||||
|
REF | REF | REF | REF | REF |
|
1.937 | 0.181 | 0.000 | 1.613 | 2.327 |
|
1.125 | 0.226 | 0.558 | 0.758 | 1.669 |
| Year | |||||
|
REF | REF | REF | REF | REF |
|
1.138 | 0.206 | 0.475 | 0.798 | 1.623 |
|
1.108 | 0.211 | 0.589 | 0.764 | 1.608 |
|
1.190 | 0.240 | 0.389 | 0.801 | 1.767 |
|
0.979 | 0.203 | 0.919 | 0.652 | 1.470 |
|
0.982 | 0.202 | 0.928 | 0.656 | 1.469 |
|
1.068 | 0.241 | 0.771 | 0.686 | 1.663 |
| Constant | 0.052 | 0.019 | 0.000 | 0.025 | 0.108 |
| Physician random effect (variance of random intercepts) |
0.076 | 0.035 | 0.031 | 0.186 | |
CI, confidence interval.
Using the model’s fixed and physician random effects, we created an adjusted SSLDR by year of fellowship with 95 % CIs ( Fig. 4 ). The adjusted SSLDR for fellows for each year of the fellowship program showed significant improvement in SSLDR from 5.05 % (95 % CI: 4.24–5.86) during the first year of fellowship to 6.73 % (95 % CI: 5.24–8.22) during the third year of fellowship (95 % CI of difference: –0.033 – –0.001; P = 0.038).
Fig. 4.
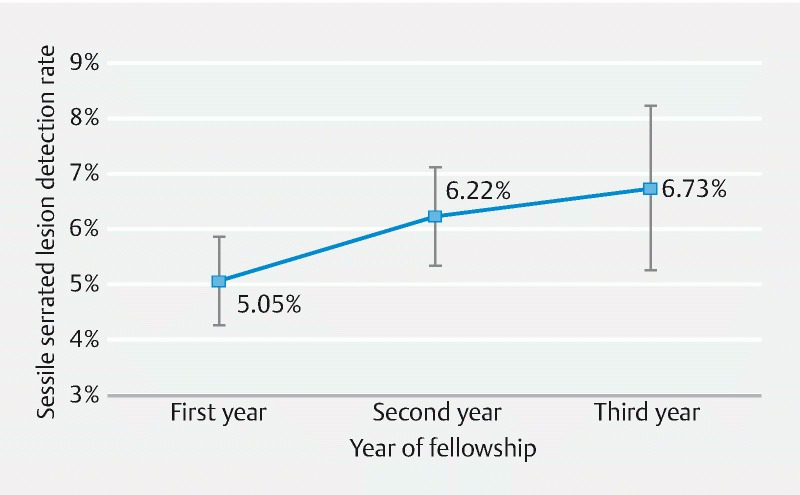
Adjusted sessile serrated lesion detection rate by fellowship year with 95 % confidence interval. Each marker represents the model-adjusted, division-level sessile serrated lesion detection rate among fellows only including its 95 % confidence interval.
Discussion
To continue the downward trajectory of CRC incidence and mortality rates, the quality, and not just the quantity, of CRC screening colonoscopies must improve. A conspicuous area for quality improvement is the proper detection and removal of serrated lesions, which account for approximately one-third of all CRC and a disproportionately high percentage of post-colonoscopy interval cancers 4 5 . However, guidelines and training for proper detection and removal of serrated lesions have proven more difficult and uniquely different than those of conventional adenomas. While the field continues to move toward determining the true prevalence of serrated lesions across diverse patient populations, a call has been made to focus on local SSLDR and use the highest rate from a unit as the local aspirational target 21 . In this study, we examined the SSLDR of a large, academic gastroenterology division from 2008 to 2020 and modeled such a local, aspirational target for SSLDR. We also assessed the progression of SSLDR among gastroenterology fellows to better understand the relationship between SSLDR and total colonoscopies performed during a multi-year fellowship.
We found that SSLDR increased steadily and significantly throughout our study period, from a low of 0.37 % in 2008 to a high of 7.94 % in 2020. The SSLDR from 2020 of 7.94 % should serve as the local aspirational target for the Gastroenterology Division at the University of New Mexico Health Sciences Center per Kahi & Rex’s recommendation. 21 Our SSLDRs by year are likely similar to results from other SSLDR studies when taking the year of observation into consideration. In two recent meta-analyses, the authors found lower SSLDR (estimated across all qualifying studies) than our results. However, their analyses included over a dozen SSLDR studies that were published as far back as 2010 and used even earlier data 11 20 . Our model notably estimated that the odds of detecting an SSL in 2020 were nearly 24 times higher than in 2008, controlling for all other covariates. Our pooled SSLDR (averaged across all years of our study) of 4.66 % is closer to Huang et al.’s SSLDR of 2.5 % (95 % CI: 1.5–3.8) 20 and Desai et al.’s SSLDR of 2.5 % (95 % CI: 1.8–3.4) 11 , though this pooled figure is arguably of less significance for targeting purposes than our SSLDRs observed in, say, 2019 or 2020. This finding, combined with the observation that our study’s SSLDR trend does not appear to be flattening by 2020, serves as an important consideration for policymakers and authors of future meta-analyses that the year of observation is a possible, even likely, driver of SSLDR and weighting by year ought to be considered. Our study’s local aspirational target SSLDR of 7.94 % falls well within the range of SSLDR observed in other individual studies 22 23 24 25 and is remarkably similar to the 8.1 % observed in a study that focused solely on an individual endoscopist and pathologist with notably high SSLDR 16 .
Our year indicator variable also likely serves as a proxy, catch-all measure for the true drivers of the observed, sustained improvement of SSLDR during our study period. Although this study was unable to isolate and assign singular dates for all the minor and major changes related to fellowship training, attending awareness, histopathological distinctions, or technological advancement, it is likely that each of these contributed in some part to the observed improvements in SSLDR. Indeed, studies with much narrower study periods have linked numerous endoscopic interventions to improved SSLDR 26 .
Our study also found that SSLDR for gastroenterology fellows increased significantly between the first and second year of the gastroenterology fellowship program but did not further increase in the third year. Alternatively, our initial model with all endoscopists suggested that first-year fellows are less likely to detect SSL than all other endoscopists. Although we can find no similar studies that focus solely on SSLDR among gastroenterology fellows or similar training programs, our findings generally align with other research that shows adenoma and polyp detection rates positively correlate with colonoscopy volume 24 27 28 29 30 31 . This finding highlights the importance of high-quality colonoscopy training, particularly for new gastroenterology fellows. Our results suggest that at least during a fellow’s first year of performing colonoscopy, additional “at-the-elbow” guidance by SSL-attuned attendings may be warranted.
While there have been numerous evaluations of SSLDR, few have included the total number of years and colonoscopies that we included in this study. In addition, to our knowledge, our study marks the first time that SSLDR has been studied in the context of a gastroenterology fellowship program to better understand the relationship between SSLDR and colonoscopy training during a three-year fellowship program. However, this study is not without its limitations. The lengthy study period, which served as a feature for reasons stated above, also prevented the inclusion of numerous variables. During the 13 years studied, UNM HSC and the Gastroenterology Division implemented and replaced numerous information technology systems. Changes in data standards, clinical terminology, and billing requirements resulted in a mélange of variables, few of which were available in each of the study’s 13 years. As a result, we were unable to include key indicator variables related to the patient that are known to correlate with CRC including family history, patient weight, and smoking status, among others. Additionally, due to changes in the Gastroenterology Division’s clinical software, clinical observation variables related to the colonoscopy such as withdrawal time, bowel preparation, and cecal intubation were not uniformly available or were not machine-readable. Similar changes in clinical software for the pathology department precluded us from being able to track the ever-changing composition of the pathology department and its review processes. Finally, the technologies used by the Gastroenterology Division were upgraded numerous times during the study period (e. g., zoom magnification and narrow band imaging). Many of these technologies undoubtedly improved the ability to detect and resect SSLs 26 32 . However, newer scopes and monitors were never replaced en masse, but were instead replaced on a rolling basis across months and even years in some cases. As a result, we were unable to account for these changes in the model. However, due to the continuous rotation of fellows and attendees across clinics and endoscopy suites within the division, we believe that these differing technologies were likely equally diffused across endoscopists over time. Future research should incorporate more of these patient- and technology-level variables to control for potential confounding more comprehensively.
Implications for practice
SSLDR is becoming an increasingly recognized colonoscopy quality indicator making for important healthcare implications in the screening and detection rates of these lesions. It is obvious now that national benchmarks for SSLDRs need to be established. High-quality randomized prospective studies, including patient demographics, indication, standardized prep, withdrawal time, and physician training will help identify valid benchmarks eventually. Fellowship and endoscopy training programs should also continuously reappraise the evidence base for various endoscopic advances including add-on devices and endocuff vision 26 . Our study demonstrated an increasing SSLDR with over time and within a three-year fellowship experience. Previous studies have similarly shown a stepwise increase in adenoma detection rate with advancing years of gastroenterology fellowship training compared to procedures performed by an attending physician alone 30 31 . Trainee education at all levels should include the importance of detection of SSLs and their essential role in colorectal cancer pathogenesis.
Educational initiatives for new gastroenterology fellows should be implemented to improve SSLDR such as endoscopic image dataset and atlas image review, close one-to-one fellow to attending supervision, education of endoscopy nurses about SSLDRs, video tape analysis of entire colonoscopy, and mandating longer withdrawal times based on level of training 30 31 . SSLDR-specific educational initiatives should also include training of non-gastroenterology physicians performing colonoscopies, including surgeons and family physicians. Future improvement in endoscopic visualization technology along with artificial intelligence-based algorithms for SSL image base recognition will also help improve the detection of SSLs.
Acknowledgement
The authors would like to thank the following individuals for their numerous contributions to this study: Rosa Matonti, DNP, RN; Daniel De Francisco Cabral; Sergio A. Sánchez-Luna, MD, ABOM-D; David R. Martin, MD; and Richard Feddersen, MD.
Funding Statement
This work was supported by the National Cancer Institute of the National Institutes of Health, grants R01CA192967 (Mishra, PI) and 3P30CA118100-16S4 (Tomkinson PI, Mishra, PD), and the Biostatistics Shared Resource of the University of New Mexico Comprehensive cancer Center, USA
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Siegel R L, Miller K D, Fuchs H E et al. Cancer statistics, 2021. CA: Cancer J Clinicians. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . Surveillance, Epidemiology, and End Results Program – Cancer Stat Facts: Colorectal Cancer. In: Surveillance E, and End Results Program ed. 2021.
- 3.Shaukat A, Kahi C J, Burke C A. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 4.Kahi C J. Screening relevance of sessile serrated polyps. Clin Endosc. 2019;52:235. doi: 10.5946/ce.2018.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S Y, Kim T I. Serrated neoplasia pathway as an alternative route of colorectal cancer carcinogenesis. Intest Res. 2018;16:358. doi: 10.5217/ir.2018.16.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karnes W E, Johnson D A, Berzin T M et al. A polyp worth removing: a paradigm for measuring colonoscopy quality and performance of novel technologies for polyp detection. J Clin Gastroenterol. 2021;55:733–739. doi: 10.1097/MCG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J A, Bobo J K, Church T R et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol. 2017;112:1728. doi: 10.1038/ajg.2017.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L-C, Shun C-T, Hsu W-F et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol. 2017;15:872–INF. doi: 10.1016/j.cgh.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Anderson J C, Robertson D J. Serrated polyp detection by the fecal immunochemical test: an imperfect FIT. Clin Gastroenterol Hepatol. 2017;15:880–882. doi: 10.1016/j.cgh.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kim D H, Matkowskyj K A, Lubner M G et al. Serrated polyps at CT colonography: prevalence and characteristics of the serrated polyp spectrum. Radiology. 2016;280:455–463. doi: 10.1148/radiol.2016151608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai M, Anderson J C, Kaminski M et al. Sessile serrated lesion detection rates during average risk screening colonoscopy: A systematic review and meta-analysis of the published literature. Endosc Int Open. 2021;9:E610–E620. doi: 10.1055/a-1352-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rameshshanker R, Purchiaroni F, Ana W et al. PTH-039 Prevalence of sessile serrated adenomas/polyps in distal colon during screening colonoscopy/flexible sigmoidoscopy: a single bowel cancer screening experience from uk. Gut. 2017;66:A225. [Google Scholar]
- 13.Pohl H, Srivastava A, Bensen S P et al. Incomplete polyp resection during colonoscopy – results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–8.0E72. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Torlakovic E, Skovlund E, Snover D C et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Bettington M, Walker N, Clouston A et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 16.Abdeljawad K, Vemulapalli K C, Kahi C J et al. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517–524. doi: 10.1016/j.gie.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 17.Kanth P, Boylan K E, Bronner M P et al. Molecular biomarkers of sessile serrated adenoma/polyps. Clin Translat Gastroenterol. 2019;10:e00104. doi: 10.14309/ctg.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetzel J T, Huang C S, Coukos J A et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 19.Kahi C J, Hewett D G, Norton D L et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Chan P S, Pang T W et al. Rate of detection of serrated lesions at colonoscopy in an average-risk population: a meta-analysis of 129,001 individuals. Endosc Int Open. 2021;9:E472–E481. doi: 10.1055/a-1333-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahi C J, Rex D K. Sessile serrated lesions: Searching for the true prevalence. Endosc Int Open. 2021;9:E635–E636. doi: 10.1055/a-1373-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross W A, Thirumurthi S, Lynch P M et al. Detection rates of premalignant polyps during screening colonoscopy: Time to revise quality standards? Gastrointest Endosc. 2015;81:567–574. doi: 10.1016/j.gie.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IJspeert J, Nolthenius C T, Kuipers E et al. CT-colonography vs. colonoscopy for detection of high-risk sessile serrated polyps. Am J Gastroenterol. 2016;111:516–522. doi: 10.1038/ajg.2016.58. [DOI] [PubMed] [Google Scholar]
- 24.Shaukat A, Gravely A A, Kim A S et al. Rates of detection of adenoma, sessile serrated adenoma, and advanced adenoma are stable over time and modifiable. Gastroenterol. 2019;156:816–817. doi: 10.1053/j.gastro.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Buda A, De Bona M, Dotti I et al. Prevalence of different subtypes of serrated polyps and risk of synchronous advanced colorectal neoplasia in average-risk population undergoing first-time colonoscopy. Clin Translat Gastroenterol. 2012;3:e6. doi: 10.1038/ctg.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz M, Fatima R, Lee-Smith W et al. Comparing endoscopic interventions to improve serrated adenoma detection rates during colonoscopy: a systematic review and network meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2020;32:1284–1292. doi: 10.1097/MEG.0000000000001844. [DOI] [PubMed] [Google Scholar]
- 27.Qayed E, Vora R, Levy S et al. Colonoscopy procedural volume increases adenoma and polyp detection rates in gastroenterologytrainees. World J Gastrointest Endosc. 2017;9:540–551. doi: 10.4253/wjge.v9.i11.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace D, Borgaonkar M, Lougheed M. Can J Gastroenterol Hepatol; 2016. Effect of colonoscopy volume on quality indicators. [DOI] [PMC free article] [PubMed]
- 29.Zwink N, Stock C, Birkner B et al. Screening colonoscopy volume and detection of colorectal neoplasms: a state-wide study from Bavaria, Germany. Europ J Cancer Prev. 2017;26:181–188. doi: 10.1097/CEJ.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 30.Peters S L, Hasan A G, Jacobson N B et al. Level of fellowship training increases adenoma detection rates. Clin Gastroenterol Hepatol. 2010;8:439–442. doi: 10.1016/j.cgh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Rogart J N, Siddiqui U D, Jamidar P A et al. Fellow involvement may increase adenoma detection rates during colonoscopy. Am J Gastroenterol. 2008;103:2841–2846. doi: 10.1111/j.1572-0241.2008.02085.x. [DOI] [PubMed] [Google Scholar]
- 32.Aziz M, Mehta T I, Weissman S et al. Do water-aided techniques improve serrated polyp detection rate during colonoscopy? A systematic review with meta-analysis. J Clin Gastroenterol. 2021;55:520–527. doi: 10.1097/MCG.0000000000001386. [DOI] [PubMed] [Google Scholar]


