Abstract
Stomach cancer is a leading cause of cancer death. Helicobacter pylori is a bacterial gastric pathogen that is the primary risk factor for carcinogenesis, associated with its induction of inflammation and DNA damage. Dicarbonyl electrophiles are generated from lipid peroxidation during the inflammatory response and form covalent adducts with amine-containing macromolecules. 2-hydroxybenzylamine (2-HOBA) is a natural compound derived from buckwheat seeds and acts as a potent scavenger of reactive aldehydes. Our goal was to investigate the effect of 2-HOBA on the pathogenesis of H. pylori infection. We used transgenic FVB/N insulin-gastrin (INS-GAS) mice as a model of gastric cancer. First, we found that 2-HOBA is bioavailable in the gastric tissues of these mice after supplementation in the drinking water. Moreover, 2-HOBA reduced the development of gastritis in H. pylori-infected INS-GAS mice without affecting the bacterial colonization level in the stomach. Further, we show that the development of gastric dysplasia and carcinoma was significantly reduced by 2-HOBA. Concomitantly, DNA damage were also inhibited by 2-HOBA treatment in H. pylori-infected mice. In parallel, DNA damage was inhibited by 2-HOBA in H. pylori-infected gastric epithelial cells in vitro. In conclusion, 2-HOBA, which has been shown to be safe in human clinical trials, represents a promising nutritional compound for the chemoprevention of the more severe effects of H. pylori infection.
Keywords: Electrophiles, Reactive aldehyde, Oxidative damage, Gastritis, Gastric cancer, DNA damage
1. Introduction
Gastric cancer (GC) is the fourth leading cause of cancer death worldwide with over one million new cases per year [1]. The intestinal type of GC has an inflammatory etiology, which is initiated by the pathogen Helicobacter pylori. This bacterium infects half of the world’s population [2] and causes universal non-atrophic gastritis that can progress to the precancerous lesions of multifocal atrophic gastritis, intestinal metaplasia, and dysplasia, and then on to gastric adenocarcinoma [3,4]. Although eradication of H. pylori results in attenuated progression to GC [5], it does not necessarily reduce cancer risk once precancerous lesions are present [6,7]. In addition, antibiotic resistance and reinfection rates affect the efficacy of antibiotic therapies [8]. Screening upper endoscopy is a frequent strategy in some high risk regions to prevent GC, but this is far from universally applied. In this context, alternative strategies to prevent carcinogenesis may positively impact H. pylori-infected patients, especially those with precancerous lesions.
The mucosal innate immune response of the infected stomach leads to the formation of prostanoids, reactive oxygen species, and nitrogen compounds derived from the L-arginine-nitric oxide metabolic pathway. These primary molecules are highly reactive and undergo further reactions to form products of lipid peroxidation, termed dicarbonyl electrophiles. These include isolevuglandins, malondialdehyde, 4-hydroxy-nonenal, 4-oxo-nonenal, methylglyoxal, and acrolein, which is also generated by β-elimination from 3-aminopropanal [9]. These strong oxidants react principally with hard nucleophiles, such as amines present in nucleic acid bases [10,11] and lysine residues [12,13], and form irreversible covalent adducts, which may lead to changes in cell signaling, somatic genomic abnormalities [14,15] and epigenetic alterations [12]. Of importance, we have reported that gastric epithelial cells of patients with precancerous lesions exhibit high levels of nuclear adducts of isolevuglandins to lysine [16]. Moreover, we have shown that the treatment of transgenic insulin-gastrin (INS-GAS) mice with an experimental scavenger of electrophiles prevented H. pylori-induced DNA damage, somatic mutations, and development of gastric carcinoma [16].
The natural product 2-hydroxybenzylamine (2-HOBA) is derived from buckwheat seeds [17] and reacts with all electrophiles at a rate 3 orders of magnitude faster than with lysine, thus preventing adduct formation with macromolecules [18]. It is not toxic [19-21] or mutagenic [22] in mice, rats or rabbits, and protects mice from oxidative damage in models of hypertension [23] and Alzheimer’s disease [24]. In addition, two Phase 1 clinical trials have demonstrated its safety in humans [25,26]. Therefore, 2-HOBA is well positioned as a clinically available chemopreventive agent for the development of gastric cancerous lesions in patients infected with H. pylori. Hence, our goal was to test 2-HOBA in a model of gastric carcinogenesis in INS-GAS mice.
2. Materials and methods
2.1. Synthesis of 2-HOBA
2-HOBA (as the acetate salt, CAS 1206675-01-5) was obtained from TSI (China) Co., Ltd. (Shanghai, China). A commercial production lot was used (Lot SAA20200727). The purity was of the commercial lot was verified was HPLC to be > 99 %. Microbial and analytical tests were within all specification limits.
2.2. H. pylori
We used the cagA+ H. pylori strain PMSS1 [16,27]. Bacteria were maintained on Tryptic Soy agar plates containing 10 % sheep blood. For infection, bacteria were grown in Brucella broth containing 10 % FBS overnight, then diluted to an A600 nm of 0.1 in the same fresh medium, grown, harvested at the exponential phase, and resuspended into Brucella broth.
2.3. Mice, infection, and treatment with 2-HOBA
FVB/N INS-GAS mice (8–10 weeks-old) were infected by oral gavage with 109 H. pylori PMSS1 in 0.2 ml Brucella broth, two times every 2 days [16,28]. Control mice were gavaged with broth only. Mice were then fed ad libitum with the AIN-76A diet (Bio-Serv) during the time of infection [16,28]. Animals were treated with 1.45 or 4.35 mg/ml 2-HOBA-acetate, which corresponds to 1.0 and 3.0 mg/ml 2-HOBA, respectively, in the drinking water, beginning 7 days after the first infection. 2-HOBA supplementation was maintained until the end of the experiments and the solutions were changed every 3–4 days. Mice were sacrificed 56 days post-inoculation with H. pylori. Stomachs were harvested and analyzed as described [16,28]. The number of H. pylori in each stomach was determined by counting the colony forming units (CFUs) after plating serial dilutions of ground gastric tissues.
Animals were used under protocol M1800038 approved by the Institutional Animal Care and Use Committee at Vanderbilt University, and the Vanderbilt University Institutional Biosafety Committee. Procedures were performed in accordance with institutional policies, AAALAC guidelines, the AVMA Guidelines on Euthanasia, NIH regulations (Guide for the Care and Use of Laboratory Animals), and the United States Animal Welfare Act (1966).
2.4. Gastric epithelial cells
We used the human gastric epithelial cell line AGS (ATCC # CRL-1739), maintained in DMEM with 10 % FBS, sodium pyruvate, Hepes, and penicillin-streptomycin. Cells (2 × 105) were plated in 8-well Lab-Tek chamber slides (Nunc), treated with 100 μM 2-HOBA [16] for 2 h, and then infected with H. pylori PMSS1 for 24 h at a multiplicity of infection of 100 without antibiotics.
2.5. Histopathology
Longitudinal stomach segments were fixed in 10 % neutral buffered formalin and stained with hematoxylin and eosin (H&E). Histologic assessments were then performed by a gastrointestinal pathologist (M.B.P.) in a blinded manner. Acute and chronic inflammation of the antrum and the corpus regions of the stomach (0–3 for each) were determined, leading to a final 0–12 score [16,28]. Dysplasia and adenocarcinoma were diagnosed as described [16,29].
2.6. Measurement of 2-HOBA
Concentrations of 2-HOBA were determined in gastric tissues using a method that we previously described [25], with a few modifications. [2H4]-2-HOBA [30] was used as an internal standard and was added (5 ng/μl) to all standards, quality control samples, and tissue samples. Standard and quality control samples of 1 mg/ml 2-HOBA were prepared in water. Seven standard curve samples (10, 20, 100, 200, 1000, 2000, and 5000 ng/ml) were prepared with stripped human serum (GoldenWest Diagnostic, Temecula, CA), NY). In addition, three quality control samples (15, 300, and 3000 ng/ml) were prepared. Tissue samples were homogenized with the internal standard and 200 μl of ice-cold PBS, vortexed, and centrifuged at 12,000 × g for 10 min at 4 °C. The supernatants were transferred to 13 × 100 glass culture tubes and then 400 μl of acetonitrile was added to samples, standards, and controls. The tubes were then vortexed for 2 min and then centrifuged for 5 min at 4 °C. The supernatants were applied to a Solo HRP SPE column and the 2-HOBA was eluted with a 65/35 methanol/water solution and dried with a vacuum drier. The samples were reconstituted with 40 μl of LCMS-grade acetonitrile containing 0.2% formic acid and 40 μl of LCMS-grade water containing 0.2 % formic acid and 0.1 % ammonium formate. Liquid chromatography tandem mass spectrometry analysis of 2-HOBA was performed with Agilent 1290 pumps, autosampler, column oven, and degasser (Santa Clara, CA) (column: Agilent SB-C18 RRHD 1.8 μm 2.1 × 100 mm) coupled with an Agilent 6460 mass spectrometer with ESI ion source in positive mode (Santa Clara, CA). The column temperature was set to 30 °C and the flow rate was 0.3 ml/min. A gradient of 50–70 %B from 0 to 3.0 min was established by using a mobile phase A of 0.2 % formic acid and 0.1 % ammonium formate in water and mobile phase B of 0.2 % formic acid in acetonitrile. Agilent MassHunter® software (Quant) was used to integrate 2-HOBA and [2H4]-2-HOBA and quantitate 2-HOBA in tissues.
2.7. Immunostaining
Gastric tissues sections were incubated at room temperature with 3 % hydrogen peroxide in PBS to block endogenous peroxidase and blocked for 1 h in a solution of 5 % human/mouse serum and 5 % BSA in PBS. Slides were then incubated with an anti-phosphoserine 139 of H2A histone family member X (pH2AX) polyclonal antibody (1:200; Novus) overnight at 4 °C followed by 30 min at room temperature with the EnVision+, HRP (Dako). Visualization was performed using 3,3′-diaminobenzidine, and tissues were counterstained by hematoxylin. The number of positive cells were determined in a blinded manner by our GI pathologist (M.B.P.).
AGS cells were fixed in 3.7 % paraformaldehyde, washed with PBS, and blocked with Universal Protein Block (Dako) for 40 min at room temperature. Cells were then incubated with the anti- pH2AX polyclonal antibody (1:200; Novus) overnight at 4 °C followed by 1 h at room temperature with the Donkey anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (1:600; Invitrogen). Slides were mounted with VECTASHIELD HardSet™ Antifade Mounting Medium with DAPI (Fisher Scientific) and confocal images were acquired using the Cytation C10 Confocal Imaging Reader (BioTek).
2.8. Analysis of mRNA levels
Total RNA was isolated using the RNeasy Mini kit (Qiagen). Reverse transcription was performed using Superscript II Reverse Transcriptase and Oligo dT (Invitrogen). mRNAs were amplified by real-time PCR using the PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) and the primers listed in Supplementary Table S1.
2.9. Statistics
The in vivo data are derived from two independent experiments, with the results combined. Figures and statistics were generated by Prism 9.4.1. Dot plots and bar graphs represent the mean ± SEM. Outliers were identified using the ROUT test (Q = 5 %) and removed from the analysis. Data that were not normally distributed according to the D′Agostino & Pearson normality test were log or square root transformed. Student’s t test was used to determine significant differences between two groups, whereas analysis of multiple groups was performed using ANOVA with the Tukey test or the Dunnett’s test. These tests were two-sided. Contingency analyses were performed by Chi-square test. For correlation, simple linear regression was used to determine the r value and P was calculated using the one-tailed Pearson test.
3. Results
3.1. 2-HOBA is bioavailable in the stomach
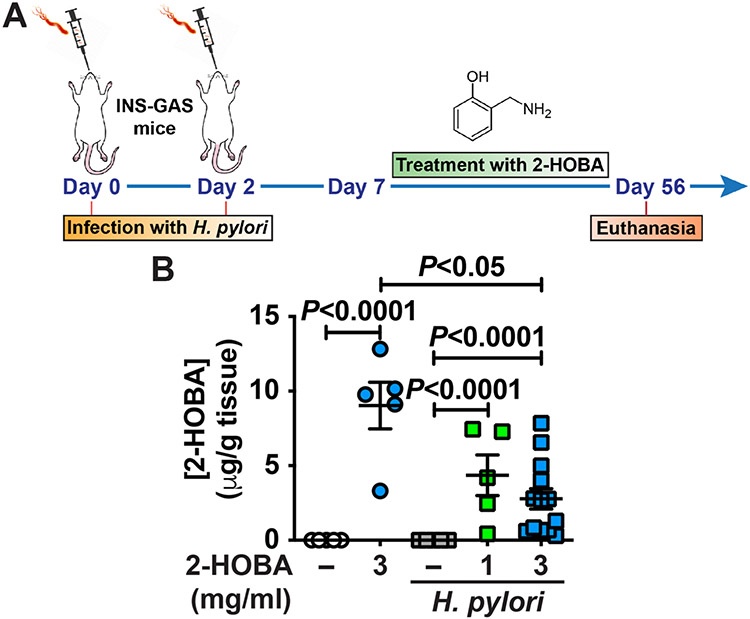
INS-GAS mice, infected or not with H. pylori, were treated with 1 mg/ml or 3 mg/ml 2-HOBA in the drinking water, according to the experimental design depicted in Fig. 1A. At the end of the experiment, we first determined the concentration of 2-HOBA by LC-MS/MS in the gastric tissue. We did not detect this compound in the stomach of uninfected or infected mice that were not treated with 2-HOBA (Fig. 1B). In contrast, 2-HOBA was found in the gastric tissues of animals that were given this scavenger drug, infected or not with H. pylori. In infected mice, we did not observe a significant difference in the levels of gastric 2-HOBA between INS-GAS mice treated with 1 mg/ml or 3 mg/ml 2-HOBA (Fig. 1B). But interestingly, the concentration was significantly decreased in H. pylori-infected mice receiving 3 mg/ml 2-HOBA compared to control animals treated with 3 mg/ml 2-HOBA.
Fig. 1.
Measurement of 2-HOBA in the gastric tissues. (A) FVB/N INS-GAS mice were infected two times, or not, with H. pylori PMSS1. Seven days after the first infection, 2-HOBA (1 mg/ml or 3 mg/ml) was given continuously in the drinking water. After 56 days, animals were euthanized, and the stomach was removed. (B) The gastric concentration of 2-HOBA was measured by LC/ESI/MS/MS. P was determined by one-way ANOVA and Tukey test. Control, n = 5; 2-HOBA (3 mg/ml), n = 5; H. pylori, n = 5; H. pylori + 2-HOBA (1 mg/ml), n = 5; H. pylori + 2-HOBA (3 mg/ml), n = 13.
These data demonstrate that a per os treatment with 2-HOBA is efficient to increase its concentration in the stomach and that 2-HOBA appears to be metabolized in the gastric tissues of H. pylori-infected mice.
3.2. 2-HOBA dampens H. pylori pathogenesis
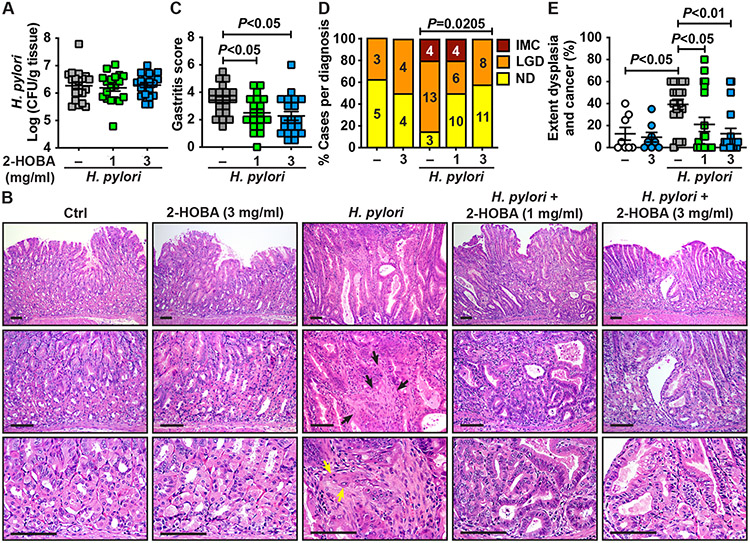
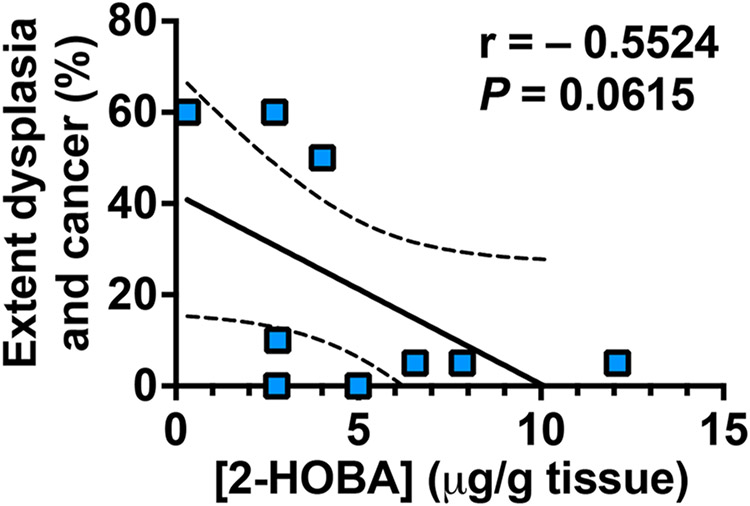
We next assessed the effect of 2-HOBA on gastric colonization, inflammation, and carcinogenesis in INS-GAS mice, which develop accelerated gastric dysplasia and intramucosal carcinoma (IMC) after H. pylori infection [16,28]. H. pylori colonization burden (Fig. 2A) was not affected by 2-HOBA treatment. As shown in the H&E staining (Fig. 2B), the gastric tissues of mice infected with H. pylori exhibited marked mucosal hyperplasia and infiltration of immune cells, both of which were less detected in mice given 2-HOBA. In addition, areas of stromal reaction and neoplastic cells infiltrating the lamina propria, which are features of IMC, were observed in infected mice, whereas low grade dysplasia (LGD) with irregular and angulated glands was found in infected animals that were given 2-HOBA (Fig. 2B). When the infiltration of immune cells was scored, we determined that gastritis in H. pylori-infected mice was significantly reduced by both concentrations of 2-HOBA (Fig. 2C). There were also significant decreases in the development of LGD and IMC in H. pylori-infected INS-GAS mice treated with the electrophile scavenger compared to infected animals not receiving the 2-HOBA compound (Fig. 2D); in mice receiving the higher dose of 3 mg/ml, there were no cases of IMC. Similarly, the extent of dysplasia and carcinoma was significantly attenuated by 2-HOBA (Fig. 2E). Lastly, 2-HOBA had no effect on the gastric tissues of uninfected mice (Fig. 2B, D, E). We then tested the hypothesis that 2-HOBA concentration in the stomach would be protective against H. pylori-induced carcinogenesis and demonstrated that gastric 2-HOBA level was inversely correlated with the extent of dysplasia and cancer (Fig. 3).
Fig. 2.
Effect of 2-HOBA on H. pylori-mediated diseases. (A) Mice were infected and treated as depicted in Fig. 1 A. After 56 days, colonization of the stomach of INS-GAS by H. pylori was assessed by serial dilution and culture. (B) Longitudinal sections were stained by H&E. The black and yellow arrows show areas of stromal reaction and epithelial neoplastic cells infiltrating the lamina propria in the stromal reaction, respectively; the scale bars represent 100 μm. (C–E) Histologic gastritis (C), the frequency of LGD and IMC in infected mice (D) and the extent of dysplasia and cancer (E) were determined by scoring H&E staining. ND, no dysplasia. P was calculated by one-way ANOVA using Tukey test (C) or Dunnett’s multiple comparisons test (E) and by Chi-square test (D). Control, n = 8; 2-HOBA (3 mg/ml), n = 8; H. pylori, n = 20; H. pylori + 2-HOBA (1 mg/ml), n = 20; H. pylori + 2-HOBA (3 mg/ml), n = 19.
Fig. 3.
Correlation plots comparing extent of dysplasia and cancer to 2-HOBA concentration in the gastric tissues. Statistical analysis was performed using the one-tailed Pearson correlation test. The dotted lines denote the 90 % confidence interval of the best-fit line. Each dot represents a mouse.
Taken together, these data indicate that the electrophile scavenger 2-HOBA reduces H. pylori-induced gastritis and is a potent inhibitor of gastric carcinogenesis, without affecting colonization.
3.3. Reduction of inflammation by 2-HOBA
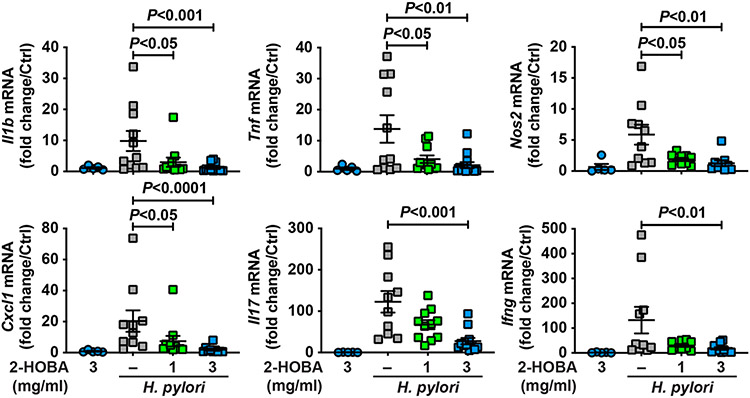
Since we observed a reduction of gastritis in H. pylori-infected mice, we assessed the expression of the genes encoding for markers of inflammation. The levels of the transcripts encoding for the innate effectors IL-1β, TNF-α, and NOS2, the chemokine CXCL1, the Th17 cytokine IL-17, and the prototype Th1 cytokine IFN-γ were significantly decreased in infected mice that were given 2-HOBA compared to infected animals not receiving this agent (Fig. 4). The rate of inhibition was similar when INS-GAS mice were treated with 1 or 3 mg/ml 2-HOBA (Fig. 4). Again, 2-HOBA had no significant effect on gene expression in uninfected mice (Fig. 4).
Fig. 4.
Effect of 2-HOBA on H. pylori-induced gastric immune response. The expression of the genes Il1b, Tnf, Nos2, Cxcl1, Il17, and Ifng was analyzed by RT-real time PCR using RNA isolated from the gastric tissues of INS-GAS mice infected or not with H. pylori ± 2-HOBA. P was determined by ANOVA and Dunnett’s test. The number of mice per group that were analyzed were: Control, n = 5; 2-HOBA (3 mg/ml), n = 5; H. pylori, n = 11; H. pylori + 2-HOBA (1 mg/ml), n = 11; H. pylori + 2-HOBA (3 mg/ml), n = 13.
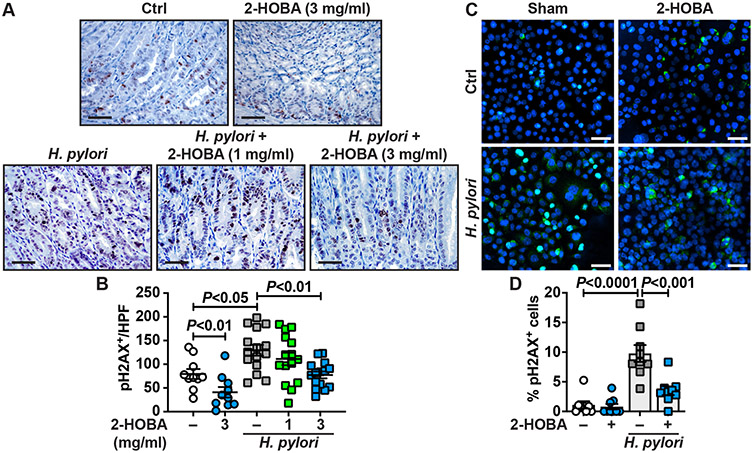
3.4. 2-HOBA reduces H. pylori-induced DNA damage
Dicarbonyl electrophiles can inflict damage to DNA by forming adducts on DNA and histones [10,12]. Using immunohistochemistry, we found that the level of pH2AX, a reliable marker of DNA damage [16, 28], was increased in the nuclei of gastric epithelial cells in INS-GAS mice infected with H. pylori compared to uninfected animals (Fig. 5A). The immunostaining was markedly reduced in infected mice that were given 3 mg/ml 2-HOBA (Fig. 5A). We then quantified the staining on multiple animals and confirmed that infection was associated with enhancement of pH2AX-positive cells, and thus with DNA damage (Fig. 5B). Interestingly, we observed that the treatment with 3 mg/ml 2-HOBA resulted in a significant reduction of pH2AX-positive cells in uninfected and infected INS-GAS mice (Fig. 5B). A slight reduction of pH2AX staining, which did not reach statistical significance, was also observed in H. pylori-infected INS-GAS mice treated with the lower dose of 1 mg/ml of 2-HOBA (Fig. 5B).
Fig. 5.
DNA damage in INS-GAS mice treated with 2-HOBA. The level of pH2AX in the gastric tissues of FVB/N INS-GAS mice ± H. pylori ± 2-HOBA was assessed by immunostaining. The images are representative of 2 mice each for the uninfected and uninfected + 3 mg/ml 2-HOBA groups, and 3 mice each for all the groups of H. pylori-infected mice. Scale bar, 50 μm. (B) The staining was quantified in a blinded manner by our GI pathologist. For each animal, the number of pH2AX-positive cells was counted in 5 high power fields (HPF; 400 ×), from the proximal corpus to the transitional mucosa of the corpus and antrum. P was calculated by one-way ANOVA and Tukey test. (C–D) The level of pH2AX in AGS cells ± H. pylori ± 2-HOBA was assessed by immunofluorescence (C); green, pH2AX; blue, DAPI; turquoise, merged images. The percentage of cells with a positive staining for pH2AX in the nucleus was determined in a blinded manner from 3 different HPFs per condition (D). The data are derived from three independent experiments. Scale bar, 50 μm.
Further, we found by immunofluorescence (Fig. 5C) and quantification (Fig. 5D) that H. pylori directly induced DNA damage in the gastric epithelial cell line AGS. When the infected cells were pre-treated with 2-HOBA, we observed a marked and significant reduction of pH2AX+ nuclei (Fig. 5C-D).
4. Discussion
Because dicarbonyl electrophiles derived from lipid oxidation can form covalent adducts with macromolecules such as histones or DNA [31,32] they represent ideal candidates to be targeted to dampen inflammation-mediated carcinogenesis. Herein, we found that the natural product 2-HOBA, which has been recognized as a potent scavenger of all electrophiles, reduces the generation of DNA damage and the formation of dysplasia and intramucosal carcinoma in H. pylori-infected INS-GAS mice. Our current data showing that 2-HOBA dampens H. pylori-mediated DNA damage in vitro in gastric epithelial cells and our previous finding that electrophile adducts colocalize with gastric epithelial cells with positive staining for pH2AX [16] support the link between generation of reactive aldehydes and DNA damage, which may ultimately lead to genomic instability and cancer.
In a previous report, we described that the electrophile scavenger 5-ethyl-2-hydroxybenzylamine (EtHOBA), a similar scavenger with an ethyl side chain, also exerts a protective effect on H. pylori-mediated gastric carcinogenesis in INS-GAS mice [16]. However, unlike 2-HOBA, EtHOBA has not been tested in human subjects. In this prior study, we used a concentration of EtHOBA of 7.5 mg/ml. In the current report, we used two lower concentrations of 2-HOBA in the drinking water, 1 and 3 mg/ml. This last concentration is the maximal amount tolerable by mice without signs of toxicity such as reduced water consumption, body weight loss, and hunched posture [30]. The no-observed-adverse-effect-level of 2-HOBA is 1000 mg/kg/day over 90 days [20,21], corresponding to 20 mg/day for a 20 g mouse. According to the reported water consumption by mice [33], our highest dosage of 3 mg/ml corresponds to a daily absorption of ~ 12 mg/mouse/day 2-HOBA, and thus was well tolerated. In addition, this dosing has been reported to prevent vascular inflammation and normalize blood pressure in mice and is not cytotoxic during a chronic treatment for 9 months [23]. Importantly, we found that 2-HOBA is detected in the gastric tissues of animals supplemented with this scavenger, supporting the likelihood that it can exert a biological function in the stomach. Intriguingly, we measured less free 2-HOBA in mice infected with H. pylori compared to uninfected animals. Since the LC-MS/MS assay detects only free 2-HOBA, we speculate that this compound had scavenged high levels of electrophiles in infected mice and was thus less detected.
Our results indicate that H. pylori-infected mice treated with 1 or 3 mg/ml 2-HOBA exhibited a significant reduction of gastritis and dysplasia versus untreated infected mice; we further observed that IMC was not observed in mice receiving 3 mg/ml 2-HOBA. This suggests that scavenging electrophiles reduces not only the formation of precancerous lesions, but also dampens the neoplastic progression. The protective effect on carcinogenesis obtained with 3 mg/ml 2-HOBA, i.e., reduction of total dysplasia by 43 % and IMC by 100 %, is comparable to the effect seen with 7.5 mg/ml EtHOBA, which reduced dysplasia by 42 % with only 1 out 30 mice with IMC [16]. Both EtHOBA [16] and 2-HOBA did not affect H. pylori burden in the stomach, indicating that these electrophile scavengers do not dampen carcinogenesis by an effect on colonization. However, we observed in the present report that both concentrations of 2-HOBA significantly inhibited H. pylori-induced gastritis, whereas EtHOBA had no effect on gastric inflammation [16]. The expression of the genes encoding for markers of the innate or T cell specific immune response was also reduced in mice receiving 2-HOBA, thus confirming the histopathology. Similarly, in murine models of hypertension [34], myocardial ischemic injury [35], atherosclerosis in hypercholesterolemic Ldlr−/− mice [36], and systemic lupus erythematosus [37], the increased expression of pro-inflammatory markers was significantly inhibited by 2-HOBA. In the context of H. pylori infection, chronic inflammation is strongly associated with increased risk for GC; therefore, a compound such as 2-HOBA that reduces both inflammation and neoplastic transformation is of great interest to treat patients.
In conclusion, our data confirm that the electrophile scavenger 2-HOBA represents a promising strategy for prevention of GC in patients infected with H. pylori, by reducing inflammation and oxidative DNA damage that may lead to mutagenesis. In contrast, the antioxidants vitamins C or E have no impact on lipid peroxidation, gastritis, and development of intestinal metaplasia or atrophy in H. pylori-infected gerbils [38]. Further, clinical trials have shown that the long-term use of use of non-enzymatic antioxidants, such as alpha-tocopherol plus beta-carotene [39], ascorbic acid and beta-carotene [7], or vitamin C and E [40] has no major effect on the occurrence of neoplastic changes of the stomach. The lack of response might be in part because of their low rate constants for reactions with free radicals [41]. In addition, such antioxidants also suppress production of other reactive oxygen species that are critical secondary messengers controlling key cellular processes. Instead, trapping highly reactive aldehydes represents a potent and selective strategy to reduce deleterious oxidative damage to macromolecules, and the protective effect of 2-HOBA in patients infected with H. pylori should now be determined in future clinical trials.
Supplementary Material
Grant support
This work was funded by NIH Grants R41CA257262 (K.T.W. and J.A. R.) P01CA116087 (K.T.W.), P01CA028842 (K.T.W.), and R01DK128200 (K.T.W.), Department of Defense Grant W81XWH-21-1-0617 (K.T.W.); Veterans Affairs Merit Review Grant I01CX002171 (K.T.W.), the Thomas F. Frist Sr. Endowment (K.T.W.), and the Vanderbilt Center for Mucosal Inflammation and Cancer (K.T.W.).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Keith T. Wilson reports financial support was provided by National Cancer Institute. John A. Rathmacher reports financial support was provided by National Cancer Institute. Keith T. Wilson reports financial support was provided by US Department of Defense. Keith T. Wilson reports financial support was provided by US Department of Veterans Affairs. Keith T. Wilson reports financial support was provided by National Institute of Diabetes and Digestive and Kidney Diseases. Keith T. Wilson reports a relationship with MTI Biotech that includes: non-financial support. Keith T. Wilson has patent #16/893,425 pending to Assignee. Keith T. Wilson and John A. Rathmacher are co-principal investigators on NIH grant R41CA257262. John Rathmacher is employed by MTI Biotech. Keith T. Wilson and Alain P. Gobert are names as inventors on US patent application 16/893.425. This patent application is still in the review process. MTI Biotech has a licensing agreement with Vanderbilt University for future use of electrophile scavenger drugs, include the 2-Hydroxybenzylamine used in the current manuscript. Dr. Wilson and Dr. Gobert are named as potential recipients of royalty payments, although none have been received to date.
Abbreviations:
- 2-HOBA
2-hydroxybenzylamine
- EtHOBA
5-ethyl-2-hydroxybenzylamine
- GC
Gastric cancer
- INS-GAS
insulin-gastrin
- pH2AX
phosphoserine 139 of H2A histone family member X
Footnotes
Declarations of interest
APG and KTW are named inventors on a patent application for the use of electrophile scavengers. In addition, APG and KTW are named on a licensing agreement between Vanderbilt University and MTI Biotech, Inc. for the future use of electrophile scavengers. All other authors have declared that no conflict of interest exists. JAR is an employee of MTI BioTech and is listed as an inventor on 2-HOBA patent applications. MTI BioTech intends to market/license 2-HOBA for commercial purposes.
CRediT authorship contribution statement
Alain P. Gobert: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Mohammad Asim: Investigation. Thaddeus M. Smith: Investigation. Kamery J. Williams: Investigation. Daniel P. Barry: Investigation. Margaret M. Allaman: Investigation. Alberto G. Delgado: Investigation. M. Blanca Piazuelo: Formal analysis, Investigation. John A. Rathmacher: Conceptualization, Formal analysis, Writing – review & editing, Funding acquisition. Keith T. Wilson: Conceptualization, Writing – review & editing, Funding acquisition.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2022.114092.
Data Availability
Data will be made available on request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J. Clin 68 (2018) 394–424. [DOI] [PubMed] [Google Scholar]
- [2].Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. , Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis, Gastroenterology 153 (2017) 420–429. [DOI] [PubMed] [Google Scholar]
- [3].Correa P, A human model of gastric carcinogenesis, Cancer Res. 48 (1988) 3554–3560. [PubMed] [Google Scholar]
- [4].Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, et al. , The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions, Gastroenterology 160 (2021) 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. , Helicobacter pylori infection and the development of gastric cancer, N. Engl. J. Med 345 (2001) 784–789. [DOI] [PubMed] [Google Scholar]
- [6].Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. , Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality, J. Natl. Cancer Inst 104 (2012) 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. , Long term follow up of patients treated for Helicobacter pylori infection, Gut 54 (2005)1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ho JJC, Navarro M, Sawyer K, Elfanagely Y, Moss SF, Helicobacter pylori antibiotic resistance in the United States between 2011 and 2021: a systematic review and meta-analysis, Am. J. Gastroenterol 117 (2022) 1221–1230. [DOI] [PubMed] [Google Scholar]
- [9].Esterbauer H, Schaur RJ, Zollner H, Chemistry and biochemistry of 4-hydroxy-nonenal, malonaldehyde and related aldehydes, Free Radic. Biol. Med 11 (1991) 81–128. [DOI] [PubMed] [Google Scholar]
- [10].Carrier EJ, Amarnath V, Oates JA, Boutaud O, Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin, Biochemistry 48 (2009) 10775–10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Galligan JJ, Rose KL, Beavers WN, Hill S, Tallman KA, Tansey WP, et al. , Stable histone adduction by 4-oxo-2-nonenal: a potential link between oxidative stress and epigenetics, J. Am. Chem. Soc 136 (2014) 11864–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carrier EJ, Zagol-Ikapitte I, Amarnath V, Boutaud O, Oates JA, Levuglandin forms adducts with histone h4 in a cyclooxygenase-2-dependent manner, altering its interaction with DNA, Biochemistry 53 (2014) 2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. , Protein-bound acrolein: potential markers for oxidative stress, Proc. Natl. Acad. Sci. USA 95 (1998) 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esterbauer H, Cytotoxicity and genotoxicity of lipid-oxidation products, Am. J. Clin. Nutr 57 (1993) 779S–785SS (discussion 85S–86S). [DOI] [PubMed] [Google Scholar]
- [15].Pollack M, Yang IY, Kim HY, Blair IA, Moriya M, Translesion DNA synthesis across the heptanone-etheno-2′-deoxycytidine adduct in cells, Chem. Res. Toxicol 19 (2006) 1074–1079. [DOI] [PubMed] [Google Scholar]
- [16].Gobert AP, Boutaud O, Asim M, Zagol-Ikapitte IA, Delgado AG, Latour YL, et al. , Dicarbonyl electrophiles mediate inflammation-induced gastrointestinal carcinogenesis, Gastroenterology 160 (2021) 1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koyama M, Obata Y, Sakamura S, Identification of hydroxybenzylamines in Buckwheat Seeds (Fagopyrum esculentum Moench), Agric. Biol. Chem 35 (1971) 1870–1879. [Google Scholar]
- [18].Zagol-Ikapitte I, Amarnath V, Bala M, Roberts LJ 2nd, Oates JA, Boutaud O, Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis, Chem. Res. Toxicol 23 (2010) 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pitchford LM, Smith JD, Abumrad NN, Rathmacher JA, Fuller JC Jr., Acute and 28-day repeated dose toxicity evaluations of 2-hydroxybenzylamine acetate in mice and rats, Regul. Toxicol. Pharm 98 (2018) 190–198. [DOI] [PubMed] [Google Scholar]
- [20].Fuller JC Jr., Pitchford LM, Abumrad NN, Rathmacher JA, Subchronic (90-day) repeated dose toxicity study of 2-hydroxybenzylamine acetate in rats, Regul. Toxicol. Pharm 99 (2018) 225–232. [DOI] [PubMed] [Google Scholar]
- [21].Fuller JC Jr., Pitchford LM, Abumrad NN, Rathmacher JA, Subchronic (90-day) repeated dose oral toxicity study of 2-hydroxybenzylamine acetate in rabbit, Regul. Toxicol. Pharm 100 (2018) 52–58. [DOI] [PubMed] [Google Scholar]
- [22].Fuller JC Jr., Pitchford LM, Morrison RD, Daniels JS, Flynn CR, Abumrad NN, et al. , In vitro safety pharmacology evaluation of 2-hydroxybenzyl-amine acetate, Food Chem. Toxicol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. , Immune activation caused by vascular oxidation promotes fibrosis and hypertension, J. Clin. Invest 126 (2016) 50–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, et al. , Treatment with a gamma-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice, J. Alzheimers Dis 27 (2011) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pitchford LM, Rathmacher JA, Fuller JC Jr., Daniels JS, Morrison RD, Akers WS, et al. , First-in-human study assessing safety, tolerability, and pharmacokinetics of 2-hydroxybenzylamine acetate, a selective dicarbonyl electrophile scavenger, in healthy volunteers, BMC Pharm. Toxicol 20 (2019) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pitchford LM, Driver PM, Fuller JC Jr., Akers WS, Abumrad NN, Amarnath V, et al. , Safety, tolerability, and pharmacokinetics of repeated oral doses of 2-hydroxybenzylamine acetate in healthy volunteers: a double-blind, randomized, placebo controlled clinical trial, BMC Pharm. Toxicol 21 (2020) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Latour YL, Sierra JC, McNamara KM, Smith TM, Luis PB, Schneider C, et al. , Ornithine decarboxylase in gastric epithelial cells promotes the immunopathogenesis of Helicobacter pylori infection, J. Immunol 209 (2022) 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sierra JC, Asim M, Verriere TG, Piazuelo MB, Suarez G, Romero-Gallo J, et al. , Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis, Gut 67 (2018) 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sierra JC, Suarez G, Piazuelo MB, Luis PB, Baker DR, Romero-Gallo J, et al. , alpha-Difluoromethylornithine reduces gastric carcinogenesis by causing mutations in Helicobacter pylori cagY, Proc. Natl. Acad. Sci. USA 116 (2019) 5077–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zagol-Ikapitte IA, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, et al. , Determination of the pharmacokinetics and oral bioavailability of salicylamine, a potent gamma-ketoaldehyde scavenger, by LC/MS/MS, Pharmaceutics 2 (2010) 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Basu AK, Marnett LJ, Unequivocal demonstration that malondialdehyde is a mutagen, Carcinogenesis 4 (1983) 331–333. [DOI] [PubMed] [Google Scholar]
- [32].Davies SS, May-Zhang LS, Boutaud O, Amarnath V, Kirabo A, Harrison DG, Isolevuglandins as mediators of disease and the development of dicarbonyl scavengers as pharmaceutical interventions, Pharm. Ther 205 (2020), 107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG, Food intake, water intake, and drinking spout side preference of 28 mouse strains, Behav. Genet 32 (2002) 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. , DC isoketal-modified proteins activate T cells and promote hypertension, J. Clin. Invest 124 (2014) 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo J, Xu F, Ji H, Jing Y, Shen L, Weng X, et al. , Isolevuglandins scavenger ameliorates myocardial ischemic injury by suppressing oxidative stress, apoptosis, and inflammation, Front. Cell Dev. Biol 10 (2022), 836035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tao H, Huang J, Yancey PG, Yermalitsky V, Blakemore JL, Zhang Y, et al. , Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic Ldlr(−/−) mice, Nat. Commun 11 (2020) 4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patrick DM, de la Visitacion N, Krishnan J, Chen W, Ormseth MJ, Stein CM, et al. , Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus, JCI Insight 7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun YQ, Girgensone I, Leanderson P, Petersson F, Borch K, Effects of antioxidant vitamin supplements on Helicobacter pylori-induced gastritis in Mongolian gerbils, Helicobacter 10 (2005) 33–42. [DOI] [PubMed] [Google Scholar]
- [39].Varis K, Taylor PR, Sipponen P, Samloff IM, Heinonen OP, Albanes D, et al. , Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha-tocopherol and beta-carotene. The Helsinki Gastritis Study Group, Scand. J. Gastroenterol 33 (1998) 294–300. [DOI] [PubMed] [Google Scholar]
- [40].Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. , Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality, J. Natl. Cancer Inst 104 (2012) 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roberts LJ 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, et al. , The relationship between dose of vitamin E and suppression of oxidative stress in humans, Free Radic. Biol. Med 43 (2007) 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.