Abstract
The Mycobacterium tuberculosis antigen ESAT-6 has been proposed for tuberculosis immunodiagnosis. In The Gambia, 30% of community controls produced gamma interferon (IFN-γ) in response to ESAT-6. Increased proportions of responders and intensities of responses were found in household contacts. Responses that were initially low in tuberculosis patients increased after treatment. An ESAT-6 IFN-γ assay will be of limited use in the diagnosis of tuberculosis in countries where tuberculosis is endemic. Its role in contact tracing should be evaluated further.
Tuberculosis is one of the leading causes of death among adults worldwide (18). The highest incidence rates are found in sub-Saharan Africa (3, 11). As the only marker of Mycobacterium tuberculosis infection, the tuberculin skin test is widely used to confirm clinical suspicion of tuberculosis and to estimate the burden of disease in epidemiological studies (6). However, the specificity of the test is thought to be low, especially in developing countries (10, 13) where bacillus Calmette-Guérin (Mycobacterium bovis BCG) vaccination and exposure to environmental mycobacteria can lead to positive responses (4). A better marker of M. tuberculosis infection and disease is needed. ESAT-6 is a low-molecular-weight secreted antigen expressed by M. tuberculosis, but not by M. bovis BCG, and by only a limited number of environmental mycobacteria (1). It has been proposed that the immune response to ESAT-6 be used for the diagnosis of tuberculosis (1). Although an ESAT-6 skin test has not yet been developed for human use, recent studies conducted in industrialized countries have shown high in vitro gamma interferon (IFN-γ) responses to ESAT-6 in tuberculosis patients but not in healthy unexposed or BCG-vaccinated individuals (1). In Ethiopia, similar IFN-γ responses were observed in tuberculosis patients and contacts, suggesting that M. tuberculosis infection, in the absence of disease, can also induce high responses to ESAT-6 (2, 12). However, the IFN-γ response to ESAT-6 has not yet been investigated in community controls living in tuberculosis-endemic countries. This study was undertaken to evaluate whether tuberculosis patients and contacts have higher IFN-γ responses to ESAT-6 than do community controls in a tuberculosis-endemic country.
We compared the production of IFN-γ induced by ESAT-6 in tuberculosis patients, healthy household contacts, and community controls. In The Gambia, tuberculosis incidence is high, exposure to environmental mycobacteria is considered to be widespread, and BCG vaccination coverage is over 90% (4, 9, 18). Our study was nested within a large prospective household study investigating the role of environmental and genetic factors in susceptibility to tuberculosis (C. Lienhardt, O. Sow, P. Aaby, K. Manneh, V. Gomez, A. Hill, G. Del Prete, S. Bennet, J. Sillah, and P. Gustafson, 4th Int. Conf. Pathog. Mycobacterial Infect. [abstr.], p. 47, 1999). It was approved by the Gambian government and the Medical Research Council (MRC) Ethics Committee, and all subjects gave written consent to participate. Patients with sputum smear-positive pulmonary tuberculosis were enrolled at a tuberculosis clinic (Serrekunda Health Centre) before antituberculosis therapy was started. For each patient, one healthy household contact and one healthy community control were enrolled. These individuals were matched by age (10-year age bands). The tuberculosis patients had a median age of 30 years (range, 15 to 61). The contact individual had lived in the same compound as the patient for more than 3 months and was often the spouse of the patient. The community control individual had lived in the same area as the patient, had no history of tuberculosis, and had not been in known contact with an individual suffering from tuberculosis. Household contacts and community controls were examined by a physician and found to be free of signs and symptoms suggestive of tuberculosis at the time of enrolment and after 1 year of follow-up. A tuberculin skin test was done (2 TU, PPD RT23; Statens Serum Institut, Copenhagen, Denmark) and interpreted as positive when an induration equal to or greater than 10 mm was measured after 48 to 72 h. Only human immunodeficiency virus-seronegative individuals were included in the study. All study individuals had a blood sample collected at the time of enrollment. An additional blood sample was collected from patients 6 months after initiation of therapy. In total, 30 patients with tuberculosis, 28 household contacts, and 30 community controls were enrolled in the study.
Peripheral blood mononuclear cells were isolated by density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). The cells were cultured at 106 per ml in complete medium supplemented with 10% human AB serum (Sigma Chemical Co., St. Louis, Mo.). Peripheral blood mononuclear cells (2 × 105 per well) were incubated in triplicate in the presence of medium alone, purified protein derivative (PPD) (RT49; Statens Serum Institut) (10 μg/ml), ESAT-6 (10 μg/ml), or leucoagglutinin (PHA) (PHA-L; Sigma) (5 μg/ml) at 37°C in a 5% CO2 atmosphere. IFN-γ concentrations were determined in supernatants collected on day 2 (PHA wells) or day 6 (ESAT-6, PPD, and medium-only wells) using commercially available antibodies (BioSource Europe, Fleurus, Belgium) according to the recommendations of the manufacturer. Spontaneous IFN-γ production in nonstimulated wells was subtracted from IFN-γ concentrations in stimulated wells. Multiple linear regression analysis of the log-transformed responses was adjusted for age (grouped as <25, 25 to 34, 35 to 44, and >44 years), sex, ethnic group, and absence or presence of a BCG scar. A complementary analysis defined a response as an IFN-γ concentration above the mean plus 3 standard deviations of the concentration measured in nonstimulated control wells. Results were expressed as percentages of responders, and multiple logistic regression analysis was used to adjust for confounding factors. The comparison of baseline and 6-month responses was performed using the paired t test.
As shown in Table 1, 38% of the community controls had a positive skin response to tuberculin. These responses could have been induced by previous M. bovis BCG vaccination, exposure to environmental mycobacteria, or infection with M. tuberculosis. Thirty percent of the community controls had a positive IFN-γ response to ESAT-6 (Table 1). This suggests that an important proportion of the community controls had latent M. tuberculosis infection. These results are in keeping with previous estimates of infection prevalence in Africa (3). Using a sensitive IFN-γ enzyme-linked immunospot assay, Lalvani et al. recently reported results suggesting an even higher prevalence of M. tuberculosis infection in India (7). The lower sensitivity of our IFN-γ assay may have led us to underestimate the prevalence of ESAT-6 responders among the community controls. The heterogeneity of the T-cell repertoire in the population may also have affected the sensitivity of the ESAT-6 IFN-γ or skin test (14). Conversely, the possibility that some responses to ESAT-6 in the studies of both Lalvani et al. and our group may have been induced by infection with environmental mycobacteria expressing ESAT-6 cannot be excluded.
TABLE 1.
Proportions of individuals with positive IFN-γ responses to ESAT-6, PPD, and PHA and positive tuberculin skin tests
| Testa | No. of responders/total no. of individuals (%)
|
Pb | ||
|---|---|---|---|---|
| Community controls | Household contacts | Tuberculosis patients | ||
| TST | 11/29 (38) | 24/28 (86) | 27/29 (93) | <0.001 |
| PPD | 27/30 (90) | 23/28 (82) | 25/30 (83) | 0.68 |
| ESAT-6 | 9/30 (30) | 20/28 (71) | 13/30 (43) | 0.004 |
| PHA | 28/30 (93) | 27/28 (96) | 27/30 (90) | 0.03 |
TST, tuberculin skin test. TST was considered positive when the skin induration was equal to or greater than 10 mm. A positive IFN-γ response was defined as an IFN-γ concentration in the stimulated sample above the mean plus 3 standard deviations of the control samples (medium alone).
P values are based on comparisons of three groups, adjusted for age, sex, ethnic group, and BCG scar status.
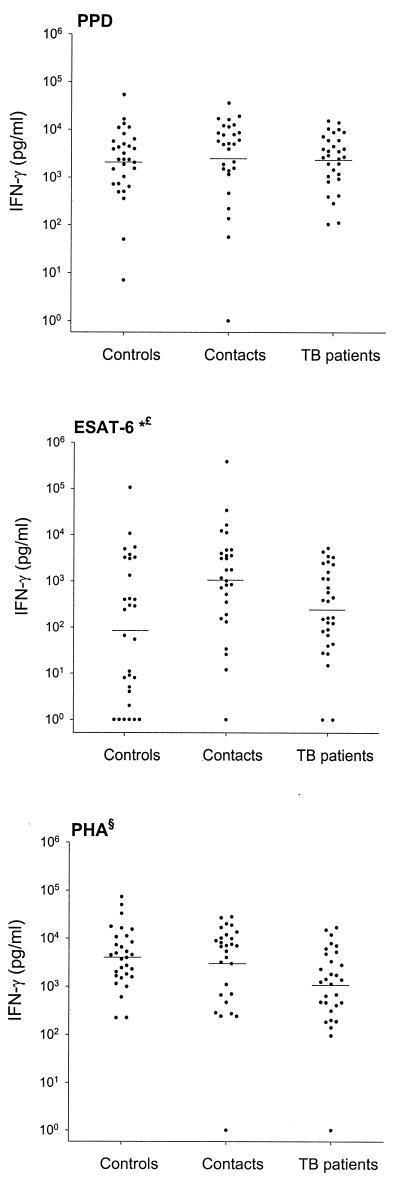
The proportions of responders to in vitro stimulation with ESAT-6 and to the tuberculin skin test were higher among household contacts than among community controls (Table 1). Household contacts also produced higher concentrations of IFN-γ in response to ESAT-6 (geometric mean [95% confidence interval], 1,049 [380 to 2,894] pg/ml) than did community controls (84 [23 to 308] pg/ml [P < 0.001]) (Fig. 1). Although we were unable to assess recent conversions, it is likely that some of the high responses to ESAT-6 and tuberculin observed among household contacts were related to recent infection with M. tuberculosis. Ulrichs et al. reported higher frequencies of lymphocytes producing IFN-γ in response to ESAT-6 in recent tuberculin skin test converters than in BCG-vaccinated healthy controls in Germany and the United States (16). Although contact tracing is recommended in countries with low tuberculosis prevalence (8), it is not frequently applied in developing countries. Prospective studies are currently under way to evaluate whether household contacts with high responses to ESAT-6 are at higher risk of developing tuberculosis disease and are therefore likely to benefit from preventive antimycobacterial therapy.
FIG. 1.
IFN-γ production induced by ESAT-6, PPD, and PHA as measured in community controls (n = 30), household contacts (n = 28), and tuberculosis patients (n = 30). Horizontal bars represent geometric means. Symbols: ∗, community controls versus household contacts (P < 0.001); £, household contacts versus tuberculosis patients (P = 0.007); §, global test of difference (P = 0.011).
The proportion of responders to the tuberculin skin test was higher among tuberculosis patients than among community controls (Table 1). In contrast, similar proportions of responders to in vitro stimulation with ESAT-6 were observed in the two groups (Table 1). The concentrations of IFN-γ induced by ESAT-6 in tuberculosis patients (235 [101 to 545] pg/ml) were similar to those in community controls (P = 0.48) and were significantly lower than those in household contacts (P = 0.007). Tuberculosis patients also produced lower concentrations of IFN-γ in response to PHA (1,033 [496 to 2,149] pg/ml) than did household contacts (2,974 [1,196 to 6,527] pg/ml) or community controls (4,020 [2,384 to 6,779] pg/ml [P = 0.011, by global test of difference]) (Fig. 1 and Table 1). In 18 patients monitored after completion of therapy, we observed significant increases in the production of IFN-γ in response to ESAT-6 (geometric mean [95% confidence interval] post- versus pretherapy, 525 [187 to 1,477] versus 238 [81 to 697] pg/ml [P = 0.05]), PPD (6,798 [3,800 to 12,162] versus 2,336 [1,165 to 4,681] pg/ml [P = 0.002]), and PHA (5,072 [2,915 to 8,826] versus 1,207 [581 to 2,506] pg/ml [P = 0.001]). These results indicate that tuberculosis disease is associated with a nonspecific defect in IFN-γ production that is improved by antimycobacterial therapy. A similar defect has been reported by several other investigators, who described a correlation with disease severity and an improvement after therapy (5, 15, 17). The defective IFN-γ production combined with the high proportion of responders among community controls could limit the use of an assay based on the production of IFN-γ induced by ESAT-6 in the immunodiagnosis of tuberculosis disease in developing countries. The high proportion of tuberculin skin test responders suggests that skin responses to mycobacterial antigens may be relatively resistant to the immunosuppression associated with tuberculosis disease. Further studies should determine the relative sensitivity of a skin test based on ESAT-6 compared to in vitro IFN-γ production in the diagnosis of tuberculosis.
In contrast to skin test results, high and similar in vitro IFN-γ responses to PPD were observed in community controls, household contacts, and tuberculosis patients (Fig. 1 and Table 1). This suggests that the in vitro IFN-γ response to PPD is a more sensitive marker of mycobacterial infection than the skin test but, even more than the skin test, lacks the ability to discriminate between tuberculosis disease and infection from exposure to environmental mycobacteria. A test based on the production of IFN-γ in response to PPD will probably be of limited use in countries where tuberculosis is endemic.
Our results indicate that tuberculosis contacts have higher IFN-γ responses to ESAT-6 than do community controls in The Gambia, a country where tuberculosis is endemic. The high prevalence of ESAT-6 responses in the Gambian community could be related to a high rate of infection. Further studies should define the role of ESAT-6 immunoassays in contact tracing in tuberculosis-endemic countries. The immune suppression associated with severe tuberculosis may prevent the use of the ESAT-6 IFN-γ assay in the diagnosis of tuberculosis in developing countries. As skin responses to mycobacterial antigens may be less sensitive to immunosuppression, future studies should compare the skin and in vitro IFN-γ responses to ESAT-6.
Acknowledgments
This study was conducted within the framework of the MRC Tuberculosis Programme and a collaborative study funded by EC-DGXII, no. IC18CT980375. Johan Vekemans is supported by the Belgian Fonds National de la Recherche Scientifique.
We thank all individuals who took part in this study. The study would not have been possible without the excellent assistance of MRC tuberculosis epidemiology field workers. The collaboration of the medical staff of Serrekunda Health Centre is gratefully acknowledged.
REFERENCES
- 1.Andersen P, Munk M E, Pollock J M, Doherty T M. Review: specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 2.Demissie A, Ravn P, Olobo J, Doherty T M, Lein D, Amoudy H A, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von Reyn F, Andersen P. T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and household contacts. Infect Immun. 1999;67:5967–5971. doi: 10.1128/iai.67.11.5967-5971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality, 1990–2000. Bull W H O. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 4.Fine P. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch C, Toossi Z, Othieno C, Johnson J L, Schwander S K, Robertson S, Wallis R S, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner J J. Depressed T-cell interferon-γ responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 6.Huebner R E, Schein M F, Bass J B., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 7.Lalvani A, Nagvenkar P, Udwadia Z, Pathan A A, Wilkinson K A, Shastri J S, Ewer K, Hill A V S, Mehta A, Rodrigues C. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–477. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 8.Leitch A G. Rationalising tuberculosis contact tracing in low-prevalence areas. Respir Med. 1992;86:371–373. doi: 10.1016/s0954-6111(06)80003-x. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health and Social Welfare. Expanded Programme of Immunisation Cluster Survey. Banjul, The Gambia: Medical and Health Department, Ministry of Health and Social Welfare; 1992. [Google Scholar]
- 10.Nyboe J. The efficacy of tuberculin test. An analysis based on results from 33 countries. Bull W H O. 1960;22:5–37. [PMC free article] [PubMed] [Google Scholar]
- 11.Raviglione M C, Snider D E, Jr, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a world-wide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 12.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J C, von-Reyn F, Andersen P. Human T cell response to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 13.Rieder H L. Methodological issues in the estimation of the tuberculosis problem from tuberculosis surveys. Tuber Lung Dis. 1995;76:114–121. doi: 10.1016/0962-8479(95)90552-9. [DOI] [PubMed] [Google Scholar]
- 14.Schoel B, Gulle H, Kaufmann S H. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992;60:1717–1720. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodhi A, Gong J H, Silva C, Qian D, Barnes P F. Clinical correlates of interferon production in patients with tuberculosis. Clin Infect Dis. 1997;25:617–620. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 16.Ulrichs T, Anding P, Porcelli S, Kaufmann S H E, Munk M E. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect Immun. 2000;68:6073–6076. doi: 10.1128/iai.68.10.6073-6076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global tuberculosis control, 1999. WHO/CDS/TB/2000.275. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]