Abstract
Background
The European Working Group on Sarcopenia in Older People (EWGSOP) updated in 2018 the cut-off points for low grip strength to assess sarcopenia based on pooled data from 12 British studies.
Objective
Comparison of the EWGSOP2 cut-off points for low grip strength to those derived from a large German sample.
Methods
We assessed the grip strength distribution across age and derived low grip strength cut-off points for men and women (peak mean -2.5 × SD) based on 200,389 German National Cohort (NAKO) participants aged 19–75 years. In 1,012 Cooperative Health Research in the Region of Augsburg (KORA)-Age participants aged 65–93 years, we calculated the age-standardised prevalence of low grip strength and time-dependent sensitivity and specificity for all-cause mortality.
Results
Grip strength increased in the third and fourth decade of life and declined afterwards. Calculated cut-off points for low grip strength were 29 kg for men and 18 kg for women. In KORA-Age, the age-standardised prevalence of low grip strength was 1.5× higher for NAKO-derived (17.7%) compared to EWGSOP2 (11.7%) cut-off points. NAKO-derived cut-off points yielded a higher sensitivity and lower specificity for all-cause mortality.
Conclusions
Cut-off points for low grip strength from German population-based data were 2 kg higher than the EWGSOP2 cut-off points. Higher cut-off points increase the sensitivity, thereby suggesting an intervention for more patients at risk, while other individuals might receive additional diagnostics/treatment without the urgent need. Research on the effectiveness of intervention in patients with low grip strength defined by different cut-off points is needed.
Keywords: grip strength, probable sarcopenia, European Working Group on Sarcopenia in Older People (EWGSOP), mortality, cut-off points
Key Points
Cut-off points for low grip strength from German population-based data (NAKO) were 2 kg higher than the EWGSOP2 cut-off points.
A relatively small difference between the cut-off points resulted in a large difference in the prevalence of low grip strength.
Higher cut-off points may propose intervention for more patients at risk, while others may receive intervention without the need.
Research on the effectiveness of intervention in patients with low grip strength defined by various cut-off points is needed.
Introduction
The severe loss of muscle strength with aging constitutes a detrimental factor for the health of older people. To determine the strength of an individual, handgrip strength measured with dynamometers has been established as it is suitable to indicate overall muscle strength [1, 2]. Handgrip strength has been reported to predict a multifaceted decline in various health parameters necessary to maintain daily activities such as cognition, mobility, and functional status in older people [3]. Besides functional deterioration, low handgrip strength has further been associated with an increased risk of premature death [1, 3, 4] and longer hospital stays [1, 5]. As an indicator of disease, handgrip strength represents the main component of sarcopenia [6]. Current cut-off points to identify low grip strength, which defines probable sarcopenia, as part of the sarcopenia definition for European populations were suggested by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2018 (i.e. EWGSOP2) [6] based on pooled data of 12 British studies [7]. Premised on reported comparability of normative grip strength values of the British data with other more developed regions, Dodds et al. [8] suggested that these cut-off points for low grip strength could be employed across Europe, Northern America, Australia, and Japan. Other studies reported discrepancies in grip strength between European regions [9, 10], encouraging the verification of the current cut-off points in other European countries. Most articles presenting European grip strength values and/or cut-off points for low grip strength though, reported data based on a small number of participants (because of the necessary multiple stratification by age and sex) [11–23] and/or did not include data of young adults [9, 10, 18, 19, 24]. However, young adults were recommended as the reference group for the derivation of low grip strength cut-off points [6].
In this article, we analyse the data of a large German population-based sample encompassing younger adults. Similar to other European studies, the majority of prior studies that reported German adult grip strength data either encompassed, relative to other available European data, a small number of participants [25–33] and/or were based on older individuals [28–31]. Only one previous German study calculated low grip strength cut-off points based on a younger study population (11,790 participants, aged 17–90 years) [27].
Therefore, we aimed to analyse grip strength and its distribution across age in 200,389 adults of the German National Cohort (NAKO, German: NAKO Gesundheitsstudie) aged 19–75 years and to derive cut-off points for low grip strength based on data from younger adults of the NAKO. As these cut-off points are mainly intended to define low grip strength in older people, we further aimed to compare the NAKO-derived cut-off points to the ones of the EWGSOP2 in an independent German cohort of older individuals aged 65–93 years from the Cooperative Health Research in the Region of Augsburg (KORA)-Age study using all-cause mortality since the EWGSOP recommended validation of cut-off points by their prediction of hard end-points [6].
Methods
Study sample
The NAKO is a population-based cohort study including 18 study centres across Germany. Over 205,000 men and women randomly invited from the German general population participated in the baseline examination between 2014 and 2019 [34]. General information regarding the NAKO study design and methods are described elsewhere [34–36]. We analysed grip strength data of the NAKO baseline examination after measurements for all baseline participants were completed. From the available data set of 204,916 participants, we excluded 4,527 participants due to missing, outside the measurement range or implausible (≤ 0 kg and ≥ 90 kg) grip strength values. The data set for analysis included the remaining 200,389 participants aged 19–75 years encompassing 100,640 women and 99,749 men. We did not exclude participants with diseases, as we aimed to calculate values for a general population in line with previous studies [7, 27]. Data on mortality are not yet available for the NAKO sample.
The KORA-Age study consisted of 1,079 individuals aged ≥ 65 years, who participated in the physical examination between 2008 and 2009 [4]. From the 1,079 KORA-Age participants, we excluded 10 participants with missing maximum grip strength values and 57 participants with missing values for any covariate leading to a final sample size of 1,012 participants (499 women and 513 men). Further details regarding the study sample are included in the Supplementary data.
Grip strength measurement
In the NAKO study, three grip strength measurement trials were conducted at each hand. We used the maximum grip strength value if at least two measurement values were available for at least one hand [37]. For analyses with the KORA-Age data, the maximum grip strength value of three trials of the dominant hand was used. We analysed the maximum grip strength value to ensure comparability to Dodds et al. [7] and, therefore, the EWGSOP2 low grip strength cut-off points [6]. Jamar dynamometers were used for both, NAKO and KORA-Age measurements. Details regarding the measurement procedures and devices are included in the Supplementary data.
All-cause mortality – KORA-Age
All-cause mortality was determined between the enrolment into the KORA-Age study and the end of the follow-up in 2016. Population registries inside and outside of the KORA study area were asked for the vital status of the participants. Local health authorities provided the death certificates [4].
Covariates
Sociodemographic variables, anthropometry, lifestyle, diseases, blood markers, and details regarding their data acquisition are described in the Supplementary data.
Statistical analysis
With the NAKO data, percentile curves of grip strength across age stratified for sex were created with the LMST (i.e. lambda, mu, and sigma, with Box-Cox-t) method using Box-Cox-t-orig. (BCTo) distribution [38]. Percentiles, means, and standard deviations (SD) given in the tables were calculated based on original data and not based on estimated percentile curves. Low grip strength cut-off points for men and women were calculated with the sex-specific peak mean of all ages and corresponding SD from the NAKO data using the T-score calculation (peak mean -2.5 × SD) as described by Dodds et al. [7]. We used the values rounded to the nearest integer as cut-off points in accordance with the EWGSOP2 consensus [6].
In an independent sample of older people, the KORA-Age study, we calculated the prevalence and the directly age-standardised prevalence of low grip strength (grip strength < cut-off point) for both cut-off point definitions (NAKO-derived and EWGSOP2) for the whole sample and stratified for men and women. We standardised the prevalence with the age groups (65–69, 70–74, 75–79, 80–84, 85– women: –90, men: –93) of the German population on 31 December 2008 [39]. We further calculated the rate ratio and corresponding 95% confidence interval of the NAKO-derived to EWGSOP2 prevalence of low grip strength. In a sensitivity analysis, we further calculated several T-scores (peak mean -1 × SD; -1.5 × SD; -2 × SD; -3 × SD) based on the NAKO data and the resulting prevalence of low grip strength in the KORA-Age sample.
We investigated the shape of the association of grip strength with all-cause mortality in Cox proportional hazards regression models with penalised splines stratified for men and women and fully adjusted for covariates in model 4 as detailed below. To check for potential discontinuity of the grip strength distribution in the section between the two cut-off points (EWGSOP2 and NAKO-derived), we created density plots for men and women. The association of grip strength (continuous variable) and low grip strength defined based on NAKO-derived and EWGSOP2 cut-off points with all-cause mortality was analysed using Cox proportional hazards regression models. To account for potentially confounding variables, models were adjusted as follows: model 1 was unadjusted, model 2 was adjusted for age (and sex only in the models with all participants), model 3 was additionally adjusted for physical activity, smoking, education, and body mass index (as a penalised spline term due to non-linear association with mortality), and model 4 was further adjusted for lung disease, cancer within the last three years, diabetes mellitus, heart problems or disease, neurological disease, estimated glomerular filtration rate, and albumin. Covariates for all Cox regression analyses were chosen based on stepwise backward model selection by Akaike information criterion. Variables that were available for selection and a detailed description are listed in the Supplementary data. The proportional hazards assumption was checked for all Cox proportional hazards regression models using scaled Schoenfeld residuals. There were no violations of the assumption.
We further calculated time-dependent (3-year and 6-year survival) sensitivity and specificity for all-cause mortality of EWGSOP2 and NAKO-derived cut-off points as well as the differences in sensitivity and specificity between the two cut-off points (EWGSOP2 and NAKO-derived).
All statistical analyses were performed using R, V. 4.0.5 [40]. The R packages that were used for the analyses and further details are described in the Supplementary data.
Results
Distribution of grip strength across age in the NAKO sample
Descriptive statistics of grip strength stratified by sex are listed for age groups in Table 1 and for every age individually in Supplementary Table S1, available in Age and Ageing online. The mean and SD of grip strength across age (Supplementary Table S1, available in Age and Ageing online) are illustrated for men and women separately in Supplementary Figure S1, available in Age and Ageing online.
Table 1.
Grip strength stratified by age groups and sex in the NAKO sample
| Age | n | Grip strength (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles | |||||||||
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | Mean (SD) | ||
| Men | |||||||||
| 19–24 | 3,140 | 32.6 | 35.8 | 41.5 | 47.3 | 53.6 | 59.5 | 63.8 | 47.6 (9.4) |
| 25–29 | 6,615 | 34.5 | 38.0 | 43.2 | 49.0 | 55.5 | 61.6 | 65.2 | 49.4 (9.4) |
| 30–34 | 5,507 | 35.8 | 39.4 | 45.0 | 50.8 | 56.8 | 62.5 | 65.9 | 50.9 (9.3) |
| 35–39 | 5,041 | 37.0 | 40.3 | 45.7 | 51.7 | 57.7 | 63.1 | 66.7 | 51.7 (9.1) |
| 40–44 | 10,670 | 36.9 | 40.4 | 45.7 | 51.3 | 57.3 | 62.9 | 66.3 | 51.5 (9.0) |
| 45–49 | 15,338 | 36.4 | 39.8 | 45.2 | 50.8 | 56.4 | 61.5 | 64.9 | 50.7 (8.7) |
| 50–54 | 14,356 | 35.3 | 38.7 | 44.0 | 49.3 | 54.9 | 60.1 | 63.2 | 49.4 (8.6) |
| 55–59 | 12,167 | 33.5 | 36.9 | 42.2 | 47.6 | 52.8 | 57.8 | 60.7 | 47.4 (8.4) |
| 60–64 | 12,598 | 32.2 | 35.2 | 40.0 | 45.3 | 50.5 | 55.3 | 58.3 | 45.2 (8.1) |
| 65–69 | 12,106 | 30.8 | 33.6 | 38.2 | 43.3 | 48.1 | 52.8 | 55.8 | 43.2 (7.7) |
| 70–75 | 2,211 | 29.2 | 31.9 | 36.3 | 41.3 | 46.3 | 50.7 | 53.6 | 41.3 (7.4) |
| All | 99,749 | 33.6 | 36.9 | 42.4 | 48.1 | 54.1 | 59.8 | 63.3 | 48.3 (9.1) |
| Women | |||||||||
| 19–24 | 3,476 | 21.2 | 23.4 | 26.8 | 30.4 | 34.1 | 37.6 | 39.6 | 30.5 (5.6) |
| 25–29 | 6,512 | 22.1 | 24.2 | 27.4 | 31.0 | 34.8 | 38.4 | 40.6 | 31.2 (5.7) |
| 30–34 | 5,572 | 22.5 | 24.7 | 28.1 | 31.9 | 35.6 | 39.0 | 41.2 | 31.9 (5.7) |
| 35–39 | 5,200 | 22.9 | 25.1 | 28.6 | 32.5 | 36.1 | 39.5 | 41.5 | 32.3 (5.7) |
| 40–44 | 10,435 | 23.1 | 25.3 | 28.6 | 32.3 | 36.1 | 39.6 | 41.7 | 32.4 (5.7) |
| 45–49 | 15,706 | 22.8 | 24.9 | 28.4 | 31.9 | 35.5 | 39.0 | 41.2 | 31.9 (5.7) |
| 50–54 | 14,746 | 21.3 | 23.4 | 27.1 | 30.6 | 34.3 | 37.5 | 39.7 | 30.6 (5.7) |
| 55–59 | 12,401 | 20.6 | 22.7 | 25.9 | 29.3 | 32.6 | 35.8 | 37.6 | 29.2 (5.3) |
| 60–64 | 12,964 | 19.9 | 21.8 | 24.8 | 28.0 | 31.3 | 34.3 | 36.3 | 28.0 (5.1) |
| 65–69 | 11,630 | 18.8 | 20.7 | 23.7 | 26.8 | 30.0 | 32.9 | 34.8 | 26.8 (4.9) |
| 70–74 | 1,998 | 18.2 | 19.9 | 23.0 | 25.9 | 28.8 | 31.4 | 33.1 | 25.8 (4.6) |
| All | 100,640 | 20.9 | 23.0 | 26.3 | 30.1 | 33.9 | 37.5 | 39.7 | 30.1 (5.8) |
Bold font indicates the highest mean of all age groups. n: number of participants, SD: standard deviation.
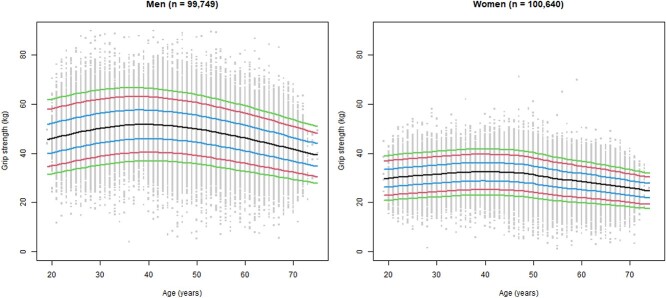
The peak mean was 52.1 kg (SD: 9.2 kg) at age 38 years and 32.5 kg (SD: 5.7 kg) at age 39 years in men and women, respectively. Considering one decimal place, the peak mean of women appeared at ages 37–40 years (Supplementary Table S1, available in Age and Ageing online). The second decimal place revealed the highest peak mean at age 39 years (32.53 kg).
The percentile curves demonstrated an increase in grip strength in the third and fourth decade of life, which appeared more pronounced in men than in women. After plateauing in the later years of the fourth decade, grip strength decreased continuously in men. The grip strength curves of women were overall flatter, the plateau was more prominent around age 40, and the decline started slightly later (Figure 1).
Figure 1.
Percentile curves of grip strength across age for men and women in the NAKO sample. The 5th (green), 10th (red), 25th (blue), 50th (black), 75th (blue), 90th (red) and 95th (green) percentiles of grip strength (kg) across age (years) are presented for men (left) and women (right). n: number of participants.
Cut-off points for low grip strength in the NAKO sample
Low grip strength cut-off points (peak mean -2.5 × SD) based on NAKO data were 29 kg (not rounded: 29.1 kg) for men and 18 kg (not rounded: 18.25 kg) for women.
Prevalence of low grip strength based on NAKO-derived and EWGSOP2 cut-off points in the KORA-Age sample
Study population characteristics of KORA-Age participants (n = 1,012) are listed in Supplementary Table S2, available in Age and Ageing online.
The prevalence of low grip strength was higher for the NAKO-derived compared to the EWGSOP2 cut-off points for all age groups in both men and women (Supplementary Figure S2, available in Age and Ageing online). After age-standardisation, the prevalence of low grip strength decreased for both NAKO-derived and EWGSOP2 cut-off points but the rate ratio between both definitions remained similar (Table 2).
Table 2.
Prevalence comparison between the NAKO-derived and EWGSOP2 cut-off points for low grip strength in the KORA-Age sample
| Low grip strength | Prevalence of low grip strength (%) | Rate ratio of NAKO-derived to EWGSOP2 prevalence (95% CI)a | Age-standardised prevalence of low grip strength (%)b | Rate ratio of NAKO-derived to EWGSOP2 age-standardised prevalence (95% CI)a | |||
|---|---|---|---|---|---|---|---|
| Yes (n) | No (n) | ||||||
| All (n = 1,012) | EWGSOP2 | 139 | 873 | 13.7 | 1.5 (1.3, 1.7) | 11.7 | 1.5 (1.3, 1.7) |
| NAKO | 209 | 803 | 20.7 | 17.7 | |||
| Men (n = 513) | EWGSOP2 | 73 | 440 | 14.2 | 1.6 (1.3, 1.9) | 10.6 | 1.6 (1.3, 1.9) |
| NAKO | 115 | 398 | 22.4 | 17.0 | |||
| Women (n = 499) | EWGSOP2 | 66 | 433 | 13.2 | 1.4 (1.1, 1.7) | 12.1 | 1.4 (1.1, 1.8) |
| NAKO | 94 | 405 | 18.8 | 17.4 | |||
Low grip strength defined based on NAKO-derived cut-off points: <29 kg for men and <18 kg for women. Low grip strength defined based on EWGSOP2 cut-off points: <27 kg for men and <16 kg for women [6]. CI: confidence interval, EWGSOP2: European Working Group on Sarcopenia in Older People 2, n: number of participants, NAKO: German National Cohort.
aEWGSOP2 is the reference group for comparison.
bAge-standardisation was performed with the German population on 31 December 2008 [39].
The T-scores of peak mean -2 × SD and -3 × SD yielded a prevalence of low grip strength of 43.0% and 11.1%, respectively compared to 20.7% with the main T-score (-2.5 × SD) (Supplementary Table S3, available in Age and Ageing online).
Association of (low) grip strength with all-cause mortality in the KORA-Age sample
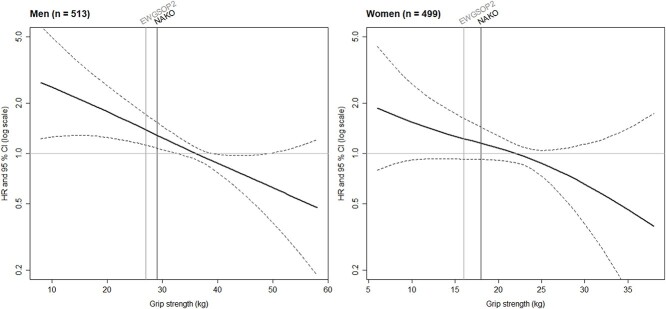
The shape of the association of grip strength with all-cause mortality was nearly linear, inverse for men and women (Figure 2).
Figure 2.
Association of grip strength with all-cause mortality by Cox regression with penalised splines in the KORA-Age sample. Solid black curve indicates the hazard ratio for all-cause mortality and dashed black curves depict the corresponding 95% confidence intervals. The reference (hazard ratio = 1) was represented by the median of the grip strength (men: 36 kg, women: 22 kg). Grey vertical line shows the cut-off point of the EWGSOP2 sarcopenia definition for low grip strength (men: 27 kg and women: 16 kg [6]) and black vertical line represents the cut-off point for low grip strength calculated based on the NAKO data (men: 29 kg and women: 18 kg). The y-axis is presented as a log scale. Cox regression models with grip strength as a penalised spline term were adjusted for body mass index (penalised spline term), age, physical activity scale for the elderly: total score, smoking, education, estimated glomerular filtration rate, albumin, lung disease (asthma, emphysema, COPD), cancer within the last three years, diabetes mellitus, heart problems or disease, and neurological disease (without stroke). CI: confidence interval, COPD: chronic obstructive pulmonary disease, EWGSOP2: European Working Group on Sarcopenia in Older People 2, HR: hazard ratio, n: number of participants, NAKO: German National Cohort.
In density plots, no discontinuity of the grip strength distribution appeared in the section between the two cut-off points (EWGSOP2 and NAKO-derived) in men and women (Supplementary Figure S3, available in Age and Ageing online).
In Cox regression models, the fully adjusted (model 4) hazard ratio (95% confidence interval) for all-cause mortality was 0.96 (0.92, 1.01) in women and 0.97 (0.94, 0.99) in men for a 1-kg increase in grip strength (Supplementary Table S4, available in Age and Ageing online). Correspondingly, the estimated decrease in all-cause mortality for a 2-kg increase in grip strength was -6% for men and -8% for women.
Out of all 1,012 participants, 23% (n = 233) died during the approximate seven years of follow-up. Employing the NAKO cut-off points, 209 individuals in the KORA-Age sample had low grip strength and 95 of them (45.5%) died. Based on the EWGSOP2 cut-off points, a total of 139 participants had low grip strength and 66 of them (47.5%) died. Due to the higher NAKO cut-off points, 70 additional participants were classified as having low grip strength as compared to the EWGSOP2 cut-off points and 29 of these 70 participants (41.4%) died. Hazard ratios of all-cause mortality were slightly (but not significantly) higher for low grip strength based on NAKO-derived compared to EWGSOP2 cut-off points (Supplementary Table S5, available in Age and Ageing online).
Low grip strength defined based on NAKO-derived cut-off points showed consistently higher sensitivity and lower specificity compared to EWGSOP2 cut-off points for all-cause mortality while the difference in sensitivity was larger than the difference in specificity (Table 3).
Table 3.
Time-dependent sensitivity and specificity of EWGSOP2 and NAKO-derived cut-off points for all-cause mortality in the KORA-Age sample
| 3-year survival | 6-year survival | |||
|---|---|---|---|---|
| Sensitivity (95% CI) in % | Specificity (95% CI) in % | Sensitivity (95% CI) in % | Specificity (95% CI) in % | |
| Men (n = 513) | ||||
| EWGSOP2 low grip strength cut-off point | 32.7 (20.2, 45.4) | 87.9 (85.3, 90.3) | 30.1 (22.5, 37.2) | 90.8 (88.0, 93.1) |
| NAKO-derived low grip strength cut-off point | 51.9 (38.7, 63.1) | 80.9 (78.0, 84.5) | 45.5 (35.6, 52.4) | 84.9 (81.1, 87.9) |
| Difference (EWGSOP2 - NAKO-derived cut-off point) | -19.2 (-29.9, -8.5) | 6.9 (4.6, 9.3) | -15.4 (-21.8, -9.1) | 5.9 (3.6, 8.2) |
| Women (n = 499) | ||||
| EWGSOP2 low grip strength cut-off point | 37.5 (22.5, 53.0) | 88.0 (84.7, 90.9) | 27.3 (18.9, 40.6) | 88.9 (85.3, 91.6) |
| NAKO-derived low grip strength cut-off point | 54.2 (37.1, 72.1) | 82.9 (79.4, 86.2) | 36.4 (28.2, 52.6) | 83.8 (80.2, 87.4) |
| Difference (EWGSOP2 - NAKO-derived cut-off point) | -16.7 (-31.6, -1.8) | 5.1 (3.1, 7.0) | -9.1 (-16, -2.2) | 5.1 (3.0, 7.1) |
CI: confidence interval, EWGSOP2: European Working Group on Sarcopenia in Older People 2, n: number of participants, NAKO: German National Cohort.
Discussion
Analysing data of 200,389 adults from the German population-based study NAKO, we observed that grip strength increased in the third and fourth decade of life and declined after the fourth decade. Derived cut-off points for low grip strength were 29 kg for men and 18 kg for women, each 2 kg higher than the EWGSOP2 cut-off points. In KORA-Age, the age-standardised prevalence of low grip strength was 1.5 (95% confidence interval: 1.3, 1.7) times higher for the NAKO-derived compared to the EWGSOP2 cut-off points. The shape of the association between grip strength and all-cause mortality was nearly linear, inverse, without an indication of a clear cut-off point. The sensitivity for all-cause mortality was higher and the specificity lower for the NAKO-derived compared to the EWGSOP2 cut-off points. These findings were similar for the two investigated time points.
Distribution of grip strength across age in the NAKO sample
In line with the percentile curves of British data reported by Dodds et al. [7], we observed that grip strength increased in early adulthood and decreased progressively after the fourth decade. Irish [20] and Italian [21] percentile curves did not display such a distinct increase in grip strength in early adulthood. Comparable to our results, another German study that analysed data from the German Socio-Economic Panel (SOEP) observed an increase of the mean grip strength during the third and fourth decade of life and a decline starting in the mid-forties [27].
The age at peak mean was considerably higher for German NAKO data (men: 38 years, women: 39 years) compared to the British data (men and women: 32 years) [7]. Additionally, the peak mean was somewhat higher and the SD lower in the NAKO data (men: 52.1 ± 9.2 kg, women: 32.5 ± 5.7 kg) compared to the British sample (men: 51.9 ± 9.9 kg, women: 31.4 ± 6.1 kg) [7]. The higher SD in the British data might have resulted from the pooling of 12 different studies with various measurement protocols [7]. Presumably due to smaller sample sizes, most previous studies did not report the mean for each age, but only for age groups. The Irish study reported a peak mean of grip strength (average of the highest scores of two measurements from each hand) in men of 51.3 ± 8.5 kg (30–39 years) and in women of 32.3 ± 5.2 kg (30–39 years) [20], which were close to the NAKO results (men: 35–39 years, 51.7 ± 9.1 kg; women: 40–44 years, 32.4 ± 5.7 kg). As opposed to this, the peak mean of the Italian sample, with grip strength based on the maximum value of both hands, was distinctly lower (minimum ~4 kg) [21] compared to the present and other studies [7, 20, 25, 27]. The peak mean of Danish grip strength data (maximum of three trials of the dominant hand) [22] was ~1 kg higher in men and 2 kg higher in women than our results. Results of the German SOEP study displayed the peak mean (weighted) of the maximum value of two measurements at each hand at ages 40–44 for men (53.8 ± 9.3 kg) and women (34.5 ± 6.3 kg) [27]. These peak means were ~2 kg higher than our results and those of other European studies [7, 20]. Another German study with a small sample size (n = 769, age range 20–95 years) reported a similar peak mean for men as the SOEP data, however, only based on the right hand [25].
The NAKO-derived low grip strength cut-off points for men and women were each 2 kg higher than the EWGSOP2 cut-off points [6]. As opposed to our study and the EWGSOP2 cut-off points, other studies calculated cut-off points as 2 instead of 2.5 SDs below the sex-specific peak mean [20, 27]. Since the NAKO low grip strength cut-off points were derived within a German sample, this may imply that these might be more suitable for a German population. However, our peak mean values were closer to an Irish [20] and a British [7] study than to other German studies [25,27].
Of note, the willingness to participate was lower in younger people after the halftime of the NAKO baseline measurements [34]. It is however unlikely that this would affect the cut-off points derived from younger participants, as non-participation due to health reasons is rather unlikely for younger participants.
Prevalence of low grip strength in the KORA-Age sample
As discussed above, we identified disparities between the grip strength of different European studies and further demonstrated that relatively small changes in cut-off points led to relatively large differences in the prevalence of low grip strength. The implementation of different cut-off points in different populations would, however, decrease comparability between studies, while in clinical practice, the use of cut-off points that do not fit to the patient population could lead to misclassification. Thus, harmonisation and pooling of grip strength data from European countries may support to find suitable cut-off points for Europe.
Association of (low) grip strength with all-cause mortality in the KORA-Age sample
We observed that the shape of the association between grip strength and all-cause mortality was nearly linear, inverse. In line with our findings, other European studies of older people with larger sample sizes also observed linear inverse associations for men [41, 42] and women [41]. However, data of older Norwegians indicated that the association might have only been present below the mean of z-standardised grip strength for women [42]. The observed nearly linear association of grip strength with all-cause mortality may indicate that there is no clear cut-off point. However, cut-off points for low grip strength are necessary and reasonable for clinical practice. Of note, a linear association between disease marker and hard end-points has also been observed for other diseases with established cut-off points such as blood pressure (hypertension), which is linearly related to cardiovascular and renal diseases [43].
We observed higher sensitivity and lower specificity of low grip strength for all-cause mortality for the NAKO-derived compared to the EWGSOP2 cut-off points. However, the difference in sensitivity was larger than in specificity. Thus, higher cut-off points may be more suitable in clinical practice to increase the sensitivity, i.e. to identify more patients at risk for premature death, suggesting an earlier start of intervention, while other individuals could concurrently receive additional diagnostics/treatment without the urgent need. Due to the nearly linear, inverse association between grip strength and all-cause mortality, changing the cut-off point to a lower value may easily lead to misclassification of persons at risk. According to the EWGSOP2 algorithm, in clinical practice, low grip strength ‘[…] is enough to trigger assessment of causes and start intervention’ [6]. Through the subsequent steps (i.e. assessment of muscle quality/quantity), sarcopenia can be confirmed, but if we exclude a high number of patients at the preceding step (low grip strength), then the prevalence of confirmed sarcopenia may decrease even more. Of note, the cut-off point of blood pressure to define hypertension, which also had a linear association to hard end-points, was changed after a first definition to a lower value classifying more patients into the disease group [43]. This approach though, may be conducted after evaluation if prevention and treatment programs are effective for people that have a higher grip strength than the current EWGSOP2 cut-off points. Additionally, the higher costs should be considered as intervention for more patients would increase overall health care costs.
Strengths and limitations
Strengths include foremost the sample size of the NAKO sample and its population-based origin. Furthermore, the data of the NAKO is homogeneous as all 18 study centres performed measurements according to the same protocol and with the same dynamometer type as well as combined quality control of data. Limitations include the not yet available follow-up data in the NAKO regarding outcomes and data of older age groups, prohibiting an internal assessment of the association between low grip strength and mortality. For this purpose, a different study was used, but this study had a much smaller sample size. Furthermore, the generalizability of the NAKO data could be limited due to the low response proportion [36] especially for younger people [34].
Conclusion
Cut-off points for low grip strength from German population-based data (NAKO) were 2 kg higher than the EWGSOP2 cut-off points. The relatively small difference between the cut-off points resulted in a large difference in the prevalence of low grip strength and a higher sensitivity but lower specificity for all-cause mortality of the NAKO-derived cut-off points. A higher cut-off point as suggested by the NAKO data could detect more patients at risk of premature death and thereby propose an earlier intervention, while other individuals could concurrently receive additional diagnostics/treatment without the urgent need. Future research on the effectiveness of intervention regarding hard end-points in patients with low grip strength defined by different cut-off points is crucial.
Supplementary Material
Acknowledgements
We thank all participants who took part in the NAKO study and the staff in this research program. We thank all participants for their long-term commitment to the KORA study, the staff for data collection and research data management and the members of the KORA Study Group (https://www.helmholtz-munich.de/en/epi/cohort/kora) who are responsible for the design and conduct of the study. We thank Johanna Biermann for supporting the literature research.
Contributor Information
Marie-Theres Huemer, Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH), Neuherberg, Germany.
Alexander Kluttig, Institute of Medical Epidemiology, Biometrics and Informatics, Interdisciplinary Center for Health Sciences, Medical Faculty of the Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Beate Fischer, Department of Epidemiology and Preventive Medicine, University of Regensburg, Regensburg, Germany.
Wolfgang Ahrens, Leibniz Institute for Prevention Research and Epidemiology - BIPS, Bremen, Germany.
Stefanie Castell, Department for Epidemiology, Helmholtz Centre for Infection Research, Braunschweig, Germany.
Nina Ebert, Institute for Biometrics and Epidemiology, German Diabetes Center, Leibniz Institute for Diabetes Research at Heinrich Heine University, Dusseldorf, Germany.
Sylvia Gastell, NAKO Study Center South Berlin/Brandenburg, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany.
Karl-Heinz Jöckel, Institut für Medizinische Informatik, Biometrie und Epidemiologie, Universitätsklinikum Essen, Essen, Germany.
Rudolf Kaaks, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
André Karch, Institute of Epidemiology and Social Medicine, University of Münster, Münster, Germany.
Thomas Keil, Institute of Social Medicine, Epidemiology and Health Economics, Charité - Universitätsmedizin Berlin, Berlin, Germany; Institute of Clinical Epidemiology and Biometry, University of Würzburg, Würzburg, Germany; State Institute of Health, Bavarian Health and Food Safety Authority, Erlangen, Germany.
Yvonne Kemmling, Department for Epidemiology, Helmholtz Centre for Infection Research, Braunschweig, Germany.
Lilian Krist, Institute of Social Medicine, Epidemiology and Health Economics, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Michael Leitzmann, Department of Epidemiology and Preventive Medicine, University of Regensburg, Regensburg, Germany.
Wolfgang Lieb, Institute of Epidemiology, Kiel University, Kiel, Germany.
Claudia Meinke-Franze, Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany.
Karin B Michels, Institute for Prevention and Cancer Epidemiology, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany.
Rafael Mikolajczyk, Institute of Medical Epidemiology, Biometrics and Informatics, Interdisciplinary Center for Health Sciences, Medical Faculty of the Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Ilais Moreno Velásquez, Max-Delbrueck-Center for Molecular Medicine in the Helmholtz Association (MDC), Molecular Epidemiology Research Group, Berlin, Germany.
Tobias Pischon, Max-Delbrueck-Center for Molecular Medicine in the Helmholtz Association (MDC), Molecular Epidemiology Research Group, Berlin, Germany; Max-Delbrueck-Center for Molecular Medicine in the Helmholtz Association (MDC), Biobank Technology Platform, Berlin, Germany; Berlin Institute of Health at Charité - Universitätsmedizin Berlin, Core Facility Biobank, Berlin, Germany; Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Sabine Schipf, Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany.
Börge Schmidt, Institut für Medizinische Informatik, Biometrie und Epidemiologie, Universitätsklinikum Essen, Essen, Germany.
Ben Schöttker, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Network Aging Research, Heidelberg University, Heidelberg, Germany.
Matthias B Schulze, Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany; Institute of Nutritional Science, University of Potsdam, Nuthetal, Germany.
Hannah Stocker, Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Network Aging Research, Heidelberg University, Heidelberg, Germany.
Henning Teismann, Institute of Epidemiology and Social Medicine, University of Münster, Münster, Germany.
Kerstin Wirkner, LIFE - Leipzig Research Centre for Civilization Diseases, University of Leipzig, Leipzig, Germany; Institute for Medical Informatics, Statistics, and Epidemiology, University of Leipzig, Leipzig, Germany.
Michael Drey, Department of Medicine IV, University Hospital, LMU Munich, Geriatrics, 80336 Munich, Germany.
Annette Peters, Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH), Neuherberg, Germany; German Center for Diabetes Research (DZD), München-Neuherberg, Germany; Chair of Epidemiology, Institute for Medical Information Processing, Biometry and Epidemiology, Medical Faculty, Ludwig-Maximilians-Universität München, Munich, Germany.
Barbara Thorand, Institute of Epidemiology, Helmholtz Zentrum München, German Research Center for Environmental Health (GmbH), Neuherberg, Germany; German Center for Diabetes Research (DZD), München-Neuherberg, Germany.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This project was conducted with data from the German National Cohort (NAKO, www.nako.de). The NAKO is funded by the Federal Ministry of Education and Research (BMBF, project funding reference numbers: 01ER1301A/B/C and 01ER1511D), federal states and the Helmholtz Association with additional financial support by the participating universities and the institutes of the Leibniz Association.The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Data collection in the KORA study is done in cooperation with the University Hospital of Augsburg. The KORA-Age project was financed by the German Federal Ministry of Education and Research (BMBF FKZ 01ET0713 and 01ET1003A) as part of the ‘Health in old age’ program. The funders played no role in design, execution, analysis, and interpretation of data, or writing of the manuscript.
Ethical Statement
Local ethics committees of all study centres approved the German National Cohort (NAKO) study. The ethics committee of the Bavarian Medical Association approved the KORA study. The NAKO and the KORA study were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from NAKO and KORA participants included in the studies.
Data Availability
The informed consent given by KORA study participants does not cover data posting in public databases. However, data are available upon request from KORA.PASST (https://helmholtz-muenchen.managed-otrs.com/external/) by means of a project agreement. Requests should be sent to kora.passt@helmholtz-muenchen.de and are subject to approval by the KORA Board. Data from the German National Cohort (NAKO) are available upon reasonable request.
References
- 1. Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care 2015; 18: 465–70. 10.1097/MCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 2. McGrath RP, Kraemer WJ, Al Snih S, Peterson MD. Handgrip strength and health in aging adults. Sports Med 2018; 48: 1993–2000. 10.1007/s40279-018-0952-y. [DOI] [PubMed] [Google Scholar]
- 3. Rijk JM, Roos PR, Deckx L, Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int 2016; 16: 5–20. [DOI] [PubMed] [Google Scholar]
- 4. Arvandi M, Strasser B, Meisinger Cet al. Gender differences in the association between grip strength and mortality in older adults: results from the KORA-age study. BMC Geriatr 2016; 16: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerr A, Syddall HE, Cooper C, Turner GF, Briggs RS, Sayer AA. Does admission grip strength predict length of stay in hospitalised older patients? Age Ageing 2006; 35: 82–4. [DOI] [PubMed] [Google Scholar]
- 6. Cruz-Jentoft AJ, Bahat G, Bauer Jet al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dodds RM, Syddall HE, Cooper Ret al. Grip strength across the life course: normative data from twelve British studies. PLoS One 2014; 9: e113637. 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing 2016; 45: 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen-Ranberg K, Petersen I, Frederiksen H, Mackenbach J, Christensen K. Cross-national differences in grip strength among 50+ year-old Europeans: results from the SHARE study. Eur J Ageing 2009; 6: 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahrenfeldt LJ, Scheel-Hincke LL, Kjærgaard S, Möller S, Christensen K, Lindahl-Jacobsen R. Gender differences in cognitive function and grip strength: a cross-national comparison of four European regions. Eur J Public Health 2019; 29: 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morlino D, Marra M, Cioffi Iet al. A proposal for reference values of hand grip strength in women with different body mass indexes. Nutrition 2021; 87–88: 111199. 10.1016/j.nut.2021.111199. [DOI] [PubMed] [Google Scholar]
- 12. Lauretani F, Russo CR, Bandinelli Set al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003; 95: 1851–60. 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 13. Bernardes SMF, Assunção A, Fujão C, Carnide F. Normative reference values of the handgrip strength for the Portuguese workers. PLoS One 2020; 15: e0236555. 10.1371/journal.pone.0236555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitsionis G, Pakos EE, Stafilas KS, Paschos N, Papakostas T, Beris AE. Normative data on hand grip strength in a Greek adult population. Int Orthop 2009; 33: 713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilsen T, Hermann M, Eriksen CS, Dagfinrud H, Mowinckel P, Kjeken I. Grip force and pinch grip in an adult population: reference values and factors associated with grip force. Scand J Occup Ther 2012; 19: 288–96. [DOI] [PubMed] [Google Scholar]
- 16. Peters MJH, Nes SI, Vanhoutte EKet al. Revised normative values for grip strength with the Jamar dynamometer. J Peripher Nerv Syst 2011; 16: 47–50. [DOI] [PubMed] [Google Scholar]
- 17. Werle S, Goldhahn J, Drerup S, Simmen BR, Sprott H, Herren D. Age-and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur Vol 2009; 34: 76–84. [DOI] [PubMed] [Google Scholar]
- 18. Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc 2010; 58: 1721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol 2006; 16: 554–62. [DOI] [PubMed] [Google Scholar]
- 20. Pratt J, De Vito G, Narici Met al. Grip strength performance from 9431 participants of the GenoFit study: normative data and associated factors. GeroScience 2021; 43: 2533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landi F, Calvani R, Martone AMet al. Normative values of muscle strength across ages in a 'real world' population: results from the longevity check-up 7+ project. J Cachexia Sarcopenia Muscle 2020; 11: 1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aadahl M, Beyer N, Linneberg A, Thuesen BH, Jørgensen T. Grip strength and lower limb extension power in 19–72-year-old Danish men and women: the Health2006 study. BMJ Open 2011; 1: e000192. 10.1136/bmjopen-2011-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nevill AM, Holder RL. Modelling handgrip strength in the presence of confounding variables: results from the Allied Dunbar National Fitness Survey. Ergonomics 2000; 43: 1547–58. [DOI] [PubMed] [Google Scholar]
- 24. Spruit MA, Sillen MJH, Groenen MTJ, Wouters EFM, Franssen FME. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc 2013; 14: 775.e5–775.e11. [DOI] [PubMed] [Google Scholar]
- 25. Günther CM, Bürger A, Rickert M, Crispin A, Schulz C. Grip strength in healthy caucasian adults: reference values. J Hand Surg Am 2008; 33: 558–65. [DOI] [PubMed] [Google Scholar]
- 26. Hank K, Jürges H, Schupp J, Wagner GG. Isometric grip strength and social gerontological research: results and analytic potentials of SHARE and SOEP (German). Z Gerontol Geriatr 2009; 42: 117–26. [DOI] [PubMed] [Google Scholar]
- 27. Steiber N. Strong or weak handgrip? Normative reference values for the German population across the life course stratified by sex, age, and body height. PLoS One 2016; 11: e0163917. 10.1371/journal.pone.0163917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albrecht BM, Stalling I, Bammann K. Sex- and age-specific normative values for handgrip strength and components of the Senior Fitness Test in community-dwelling older adults aged 65–75 years in Germany: results from the OUTDOOR ACTIVE study. BMC Geriatr 2021; 21: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuchs J, Busch M, Gößwald A, Hölling H, Kuhnert R, Scheidt-Nave C. Physical and cognitive capabilities among persons aged 65–79 years in Germany (German). Bundesgesundheitsbl 2013; 56: 723–32. [DOI] [PubMed] [Google Scholar]
- 30. Kemmler W, Stengel S, Kohl M. Developing sarcopenia criteria and cutoffs for an older Caucasian cohort–a strictly biometrical approach. Clin Interv Aging 2018; 13: 1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beller J, Miething A, Regidor E, Lostao L, Epping J, Geyer S. Trends in grip strength: age, period, and cohort effects on grip strength in older adults from Germany, Sweden, and Spain. SSM-Popul Health 2019; 9: 100456. 10.1016/j.ssmph.2019.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leyk D, Gorges W, Ridder Det al. Hand-grip strength of young men, women and highly trained female athletes. Eur J Appl Physiol 2007; 99: 415–21. [DOI] [PubMed] [Google Scholar]
- 33. Serafin P, Muehlemeyer C, Levchuk I, Lang K-H, Gebhardt H, Klussmann A. Physical strength of a German population sample: differences in age, gender and hand preference. Occup Ergon 2015; 12: 49–59. [Google Scholar]
- 34. Schipf S, Schöne G, Schmidt Bet al. The baseline assessment of the German National Cohort (NAKO Gesundheitsstudie): participation in the examination modules, quality assurance, and the use of secondary data (German). Bundesgesundheitsbl 2020; 63: 254–66. [DOI] [PubMed] [Google Scholar]
- 35. German National Cohort (GNC) Consortium . The German National Cohort: aims, study design and organization. Eur J Epidemiol 2014; 29: 371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peters A, Greiser KH, Göttlicher Set al. Framework and baseline examination of the German National Cohort (NAKO). Eur J Epidemiol 2022; 37: 1107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kluttig A, Zschocke J, Haerting Jet al. Measuring physical fitness in the German National Cohort—methods, quality assurance, and first descriptive results (German). Bundesgesundheitsbl 2020; 63: 312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stasinopoulos MD, Rigby RA, Bastiani FD. GAMLSS: a distributional regression approach. Stat Modelling 2018; 18: 248–73. [Google Scholar]
- 39.Statistisches Bundesamt. Statistical Yearbook 2010 for the Federal Republic of Germany including “International tables” (German). Wiesbaden: Statistisches Bundesamt (Federal Statistical Office), 2010. [Google Scholar]
- 40. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. https://www.R-project.org/. [Google Scholar]
- 41. Cai Y, Liu L, Wang J, Gao Y, Guo Z, Ping Z. Linear association between grip strength and all-cause mortality among the elderly: results from the SHARE study. Aging Clin Exp Res 2021; 33: 933–41. [DOI] [PubMed] [Google Scholar]
- 42. Strand BH, Cooper R, Bergland Aet al. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromsø study. J Epidemiol Community Health 2016; 70: 1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolz M, Cutler J, Roccella EJ, Rohde F, Thom T, Burt V. Statement from the National High Blood Pressure Education Program: prevalence of hypertension. Am J Hypertens 2000; 13: 103–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The informed consent given by KORA study participants does not cover data posting in public databases. However, data are available upon request from KORA.PASST (https://helmholtz-muenchen.managed-otrs.com/external/) by means of a project agreement. Requests should be sent to kora.passt@helmholtz-muenchen.de and are subject to approval by the KORA Board. Data from the German National Cohort (NAKO) are available upon reasonable request.