Abstract
Background
Ultra-processed food (UPF) consumption is related to increased morbidity and mortality. However, knowledge on its association with cognitive function is lacking. In this longitudinal study, we examined the associations between UPF intake and cognitive decline in older adults with type-2 diabetes (T2D).
Methods
The sample included initially nondemented T2D older adults (≥65 years), from the Israel Diabetes and Cognitive Decline study, who had complete information on nutrition at baseline and at least 3 cognitive assessments (mean follow-up 5.3 ± 1.5 years). Nutritional intake was evaluated by a validated Food-Frequency Questionnaire, and foods were categorized as UPF based on NOVA classification. Percent of calories from UPF were calculated from total caloric consumption in total and specific food groups. Mixed effect models were used to examine the link between UPF intake (top vs bottom quartiles) and change in cognitive function overall and in specific domains, adjusting for potential confounders.
Results
Of the total sample (N = 568; mean age 71.3 ± 4.5 years, 60% men), 141 consumed >31% kcal from UPF (top quartile). Greater intake of ultra-processed meat was associated with a faster decline in executive functions and global cognition (β = −0.041 ± 0.013; p = .002 and β = −0.026 ± 0.010; p = .011, respectively). Additionally, consumption of ultra-processed oils/spreads was associated with faster decline in executive functions and global cognition (β = −0.037 ± 0.014; p = .006 and β = −0.028 ± 0.010; p = .009, respectively). Total UPF consumption and UPF-derived from dairy products and bread/pastries/starch were not associated with cognitive change.
Conclusion
This study suggests that a high intake of ultra-processed meat and oils/spreads may be associated with accelerated cognitive decline in older individuals with T2D.
Keywords: Cognition, Longitudinal study, Nutrition
Dementia is a devastating clinical diagnosis that is preceded by a long prodromal phase of cognitive decline (1). To date, the efficacy of pharmacological agents to prevent or treat dementia is limited, particularly in its later stages. Projection models suggest, that even modest delays in dementia onset may lead to a substantial reduction in dementia prevalence and result in reduced social and financial burden (2). It is estimated that up to 35% of dementia cases may be due to modifiable risk factors (3) and that healthy lifestyles (eg, healthy diet and physical activity) can offset high risk of dementia due to genetic propensity (4). Hence, it is a major public health priority to identify modifiable risk factors that can slow cognitive decline and promote healthy aging (3).
The role of healthy diet in promoting cognitive health has been extensively studied, and is encouraged by most major health organizations for maintaining brain health and mitigating cognitive decline (5). Numerous macro- and micro-nutrients have been found to impact mechanisms underlying cognitive aging (6), and several lines of evidence suggest that the Dietary Approaches to Stop Hypertension (DASH) (7), the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) (8) and the Mediterranean (7,9) dietary patterns slow cognitive decline. These dietary patterns are characterized by high intake of fruits and vegetables and are low in unhealthy ingredients, including excess sodium, saturated fat, and added sugar. Particularly, these healthy diet patterns are low in ultra-processed foods (UPF) (10), which are formulations of ingredients, mostly of exclusive industrial use, that result from a series of industrial processes. UPFs are designed to create highly profitable products with low-cost ingredients, long shelf-life, and high palate. Their relative consumption is high particularly in high-income countries such as the United States (11) and the United Kingdom (12) where it exceeds 50% of total caloric intake.
It is increasingly acknowledged that UPF consumption is associated with adverse health outcomes, even independently of body weight. These include increased risk of cancer, hypertension, cardiovascular disease, type 2 diabetes (T2D) (13), depression, frailty, and all-cause mortality (14). Although UPF is thought to contribute to neurodegeneration through its effects on neuroinflammation and gut microbiome (15), the association of UPF with brain health has not been tested. It is particularly important to study the effects of UPF intake among individuals with T2D, because T2D is a strong risk factor for cognitive decline and dementia (16) and glucose control indices are greatly influenced by dietary patterns (17).
Thus, we aimed to investigate the associations of total UPF intake, and within specific food groups, with cognitive decline, among older adults with T2D who participated in the Israel Diabetes and Cognitive Decline (IDCD) cohort study.
Methods
Study sample
The IDCD study is an ongoing prospective cohort study aiming to identify the relationship of long-term diabetes characteristics with cognitive decline and dementia (18). Participants’ recruitment process started in 2010. Participants are older individuals with T2D aged 65 years and older living in the center of Israel, who were included in the diabetes registry (N = ~11 000) of the Maccabi Healthcare Services, the second-largest health maintenance organization in Israel. To be included in the diabetes registry, participants had to have at least one of the following (1): HbA1c > 7.25% (2), glucose > 200 mg/dl on 2 examinations more than 3 months apart (3), purchase of diabetic medication twice within 3 months (4), diagnosis of T2D (International Classification of Diseases [ICD9] code) by a general practitioner, internist, endocrinologist or ophthalmologist, supported by a HbA1c > 6.5% or glucose > 125 mg/dl within half a year. These criteria have been validated against records from 20 medical practice clinics (19). In addition, to be included in the IDCD, participants had to have at least 3 measurements of HbA1C, to be cognitively normal (a score of 0 on the clinical dementia rating (CDR) score) (20) at entry, and to be free of major neurological, psychiatric conditions (eg, status poststroke, schizophrenia, Parkinson’s disease) that might affect cognition. Lastly, participants were included only if they had an informant and spoke Hebrew well.
Overall, 950 attended at least 1 visit, with a maximum of 6 visits. We excluded 156 who had missing information on any of the study covariates, 209 who attended less than 3 visits and 7 who were vegetarians (consumed zero kcal from meat). In addition, we excluded 9 men and 1 women with implausible values for total calorie intake (below or above the acceptable range of 800–4 000 kcal/day for men and 500–3 500 kcal/day for women). Thus, our final sample included a total of 568 individuals.
Cognitive evaluation
All cognitive assessments were administered in person at the Sheba Medical Center Neuroscience unit by trained neuropsychologists. To maximize between-assessor reliability, an administration and scoring manual was in place, and new examiners viewed videotape training materials, observed experienced testers, and administered all tests to a senior neuropsychologist, by whom they were certified.
Previous studies demonstrated associations of healthy nutrition with a slower decline in global cognition as well as in various cognitive domains (21). Therefore, our outcomes consisted of global cognition as well as episodic memory, attention/working memory, semantic categorization, and executive function. The cognitive battery included 14 tests that were grouped into the 4 cognitive domains (18). Episodic memory included: (a) word list memory, word list recall, and word list recognition from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) cognitive battery; (b) Attention/working memory included the diamond cancelation and the digit span (forward and backward) tests from the Wechsler Memory Scale-Revised (WMS-R); (c) Semantic categorization included similarities, letter fluency and animal fluency tests; and (d) Executive functions included trail making test (A and B), CERAD-constructional praxis and digit symbol from the Wechsler Adult Intelligence Scale (WAIS)-Revised. Raw scores for each test were converted to z-scores using participants’ means and standard deviations (SDs), and then averaged to create scores for each cognitive domain. Additionally, a composite measure of global cognitive function (overall cognition) was created by averaging all the z-scores.
Dietary assessment
Dietary intake was assessed at baseline using a self-administered 126-item semiquantitative food frequency questionnaire (FFQ), a questionnaire specifically developed and validated for the older population in Israel (22,23). Frequencies of consumption were calculated in 9 categories (ranging from never or almost never to more than 6 servings daily), and the FFQ included a typical portion size for each item. We multiplied the portion size by the frequency of consumption in order to estimate daily consumption for each food item.
We categorized all food and beverages items included in the FFQ as UPF or not based on the NOVA classification (24). Our classification was based on a consensus made by a committee of experts at the Department of Nutrition in the Israeli Ministry of Health. Then, we calculated the daily percentage of UPF-derived calories out of total calorie intake, overall and for each of the following food groups: Oils/spreads, meat (including poultry and fish), dairy products, bread/pastries/starch, and beverages, as was previously done (25). For example, to calculate the percentage of calories derived from ultra-processed oils/spreads, we divided the number of calories consumed from ultra-processed oils/spreads items by the total calories consumed from all items in this group. The food items in each food group by processing status are listed in Supplementary Table 1. UPF intake overall and in specific food groups was adjusted for total calorie intake using the residual method (26).
Covariates
Age, sex, years of education, and smoking status were derived from the baseline assessment. HbA1c, total cholesterol levels, systolic blood pressure, and body mass index (BMI; calculated by weight [kg]/[height (m)]) were obtained from the diabetes registry database and were calculated as the means of all assessments for each participant in the MHS diabetes registry. Duration of T2D was estimated using follow-up years in the diabetes registry as a truncated surrogate (27). A physical activity index was determined by the number of various physical activities (eg, swimming, jogging or brisk walking, dancing, spinning, light exercise) performed over the previous 2 weeks using a simplified version of the Minnesota Leisure Time Activity Questionnaire (28). Total calories were calculated from the FFQ in kcal.
Statistical analysis
The data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC). p Values of <0.05 indicated significant findings. Categorical data were reported as number (%), and the association with UPF intake (%kcal of total kcal) was assessed using Chi-square tests. Continuous variables were reported as mean ± SD, and comparisons between groups of UPF intake (%kcal of total kcal) were performed using t test. UPF overall and in specific food groups was categorized such that all comparisons were made between individuals who were at the top quartile versus those who were at the 3 bottom quartiles. The cutoffs of the upper quartile were 31% for total caloric consumption, 16% for meat, 47% for dairy products, 71% for bread/pastries/starch, 34% for oils/spreads, and 46% for beverages. We used mixed-effect models with random intercepts and slopes while specifying an unstructured covariance matrix as correlation structure to assess the link between UPF consumption with change in cognitive function over time. We only considered participants’ first 4 cognitive scores because only 24 (4%) participants underwent 5 or 6 cognitive assessments. We created 2 sets of adjustments: the first models adjusted for demographic and lifestyle factors (ie, age, sex, education, current smoking, and physical activity). The second models further adjusted for cardiometabolic factors (ie, BMI, HbA1C, duration of diabetes, total cholesterol, and blood pressure). All study covariates were treated as fixed factors, and time was treated as a continuous, random factor in the mixed effect models. In a secondary analysis, we examined whether sex and obesity (BMI ≥ 30 vs <30 kg/m2) modify the associations between UPF and change in cognition. When an interaction with sex or BMI was found (p < .05), we stratified the analyses based on the effect modifier to interpret it.
Results
The total sample included 568 participants. Participants’ mean age at baseline was 71.3 ± 4.5 years and 343 (60%) were men (Table 1). Of the total sample, 381 (67%) and 187 (33%) attended 3 and 4 visits, respectively. Compared to those who were not included in the analyses, those who were included were slightly younger, were more likely to be men, were more educated, and performed more physical activity (Supplementary Table 2). For the study sample, the mean follow-up time was 5.3 ± 1.5 years (range 2–10 years), and the median time between visits was 2 years. The average consumption of total UPF intake (%kcal of total kcal) was 23.3% ± 11.0 with a range of 1.9–62.9 and upper quartile of 31.0%. Table 1 presents a comparison of baseline characteristics between participants who consumed more than 31% of their total calories from UPF (top quartile; n = 141) to those at the bottom quartiles (<31% of kcal from UPF; n = 427). There were no significant differences in the lifestyle and cardiometabolic factors tested. However, the diet of participants with high compared to low UPF intake was characterized by more calories and less consumption of protein and fruits/vegetables. During the follow-up period, global cognitive function as well as memory, language, and executive functions significantly declined, while no significant change in attention score was detected (Table 2; Supplementary Table 3).
Table 1.
Baseline Characteristics of the Total Study Sample and by Ultra-Processed Food Intake (%kcal of total kcal)
| All (N = 568) | Ultra-Processed Food Q1–Q3 (≤31%) N = 427 | Ultra-Processed Food Q4 (>31%) N = 141 | p Value | |
|---|---|---|---|---|
| Background (at baseline) | ||||
| Age (years) | 71.3 ± 4.5 | 71.2 ± 4.5 | 71.7 ± 4.4 | .206 |
| Males, n (%) | 343 (60.4) | 252 (59.0) | 91 (64.5) | .245 |
| Education (years) | 13.6 ± 3.5 | 13.5 ± 3.6 | 13.7 ± 3.5 | .621 |
| Current or pass smoking, n (%) | 332 (58.5) | 247 (57.9) | 85 (60.3) | .611 |
| Physical activity | 3.7 ± 2.2 | 3.6 ± 2.3 | 3.8 ± 2.2 | .418 |
| BMI (kg/m2) | 28.3 ± 4.2 | 28.5 ± 4.4 | 27.8 ± 3.6 | .059 |
| BMI ≥ 30 kg/m2, n (%) | 166 (29.2) | 131 (30.7) | 35 (24.8) | .185 |
| Hemoglobin A1c (%) | 6.8 ± 0.7 | 6.8 ± 0.7 | 6.7 ± 0.7 | .055 |
| Duration with diabetes (years) | 9.8 ± 4.5 | 9.7 ± 4.5 | 10.1 ± 4.4 | .382 |
| Mean total cholesterol (mg/dL) | 173.7 ± 24.0 | 174.3 ± 24.8 | 171.7 ± 21.5 | .226 |
| Systolic blood pressure (mmHg) | 134.2 ± 8.3 | 134.3 ± 8.2 | 133.8 ± 8.5 | .556 |
| Diastolic blood pressure (mmHg) | 75.5 ± 4.5 | 75.6 ± 4.5 | 75.2 ± 4.6 | .332 |
| Total calories (kcal/day) | 1 701.3 ± 505.1 | 1 672.7 ± 493.3 | 1 787.7 ± 531.8 | .019 |
| % fat (out of total calories) | 45 ± 12 | 45 ± 12 | 45 ± 11 | .984 |
| % carbohydrate (out of total calories) | 58 ± 18 | 58 ± 18 | 58 ± 16 | .631 |
| % protein (out of total calories) | 22 ± 6 | 22 ± 7 | 21 ± 6 | .006 |
| Fruits and vegetables (g/day) | 787 ± 344 | 827 ± 352 | 667 ± 289 | <.0001 |
| Fibers (g/day) | 30 ± 10 | 30 ± 11 | 29 ± 10 | .122 |
Note: BMI = body mass index.
Table 2.
The Associations Between Ultra-Processed Food Consumption (Top Quartile of %kcal vs Bottom Quartiles) and Change in Cognitive Function Over Time
| UPF (%kcal)* | Executive | Attention | Language | Memory | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Total | Time |
−0.075
(0.006) |
<.0001 | 0.000 (0.007) |
.926 |
−0.032
(0.005) |
<.0001 |
−0.188
(0.007) |
<.0001 |
−0.105
(0.005) |
<.0001 |
| UPF | 0.112 (0.072) |
.124 | 0.077 (0.081) |
.342 | 0.017 (0.080) |
.831 | 0.094 (0.071) |
.185 | 0.101 (0.067) |
.134 | |
| Time × UPF |
−0.015 (0.013) |
.254 | 0.010 (0.014) |
.469 | 0.007 (0.010) |
.483 | 0.001 (0.015) |
.917 |
−0.002 (0.010) |
.878 | |
| Meat (including poultry and fish) | Time |
−0.065
(0.007) |
<.0001 | 0.013 (0.007) |
.094 |
−0.029
(0.005) |
<.0001 |
−0.176
(0.008) |
<.0001 |
−0.096
(0.005) |
<.0001 |
| UPF | 0.157 (0.072) |
.031 | 0.087 (0.083) |
.290 | 0.089 (0.083) |
.282 | 0.043 (0.072) |
.549 | 0.127 (0.068) |
.062 | |
| Time × UPF |
−0.041
(0.013) |
.002 |
−0.023 (0.014) |
.106 | 0.003 (0.010) |
.766 |
−0.018 (0.014) |
.199 |
−0.026
(0.010) |
.011 | |
| Dairy products | Time |
−0.075
(0.006) |
<.0001 | 0.005 (0.007) |
.471 |
−0.028
(0.005) |
<.0001 |
−0.194
(0.007) |
<.0001 |
−0.108
(0.005) |
<.0001 |
| UPF | 0.043 (0.073) |
.557 | 0.038 (0.083) |
.650 |
−0.082 (0.080) |
.310 |
−0.103 (0.070) |
.145 |
−0.046 (0.067) |
.498 | |
| Time × UPF | 0.004 (0.013) |
.739 |
−0.008 (0.014) |
.599 |
−0.008 (0.010) |
.409 | 0.022 (0.014) |
.124 | 0.009 (0.010) |
.378 | |
| Bread, pastries and starch | Time |
−0.070
(0.007) |
<.0001 | 0.007 (0.007) |
.368 |
−0.036
(0.005) |
<.0001 |
−0.191
(0.007) |
<.0001 |
−0.103
(0.005) |
<.0001 |
| UPF | 0.065 (0.074) |
.381 |
−0.002 (0.084) |
.972 |
−0.068 (0.082) |
.409 | 0.018 (0.072) |
.793 | 0.016 (0.069) |
.809 | |
| Time × UPF |
−0.014 (0.013) |
.291 |
−0.004 (0.014) |
.755 | 0.018 (0.010) |
.071 | 0.013 (0.014) |
.370 | 0.002 (0.010) |
.841 | |
| Oils and spreads | Time |
−0.070
(0.006) |
<.0001 | 0.004 (0.007) |
.526 |
−0.028
(0.005) |
<.0001 |
−0.185
(0.007) |
<.0001 |
−0.099
(0.005) |
<.0001 |
| UPF | 0.102 (0.073) |
.160 | 0.031 (0.083) |
.711 |
−0.002 (0.082) |
.981 | 0.046 (0.071) |
.521 | 0.076 (0.068) |
.269 | |
| Time × UPF |
−0.037
(0.014) |
.006 | 0.002 (0.015) |
.898 |
−0.011 (0.010) |
.302 |
−0.026 (0.015) |
.079 |
−0.028
(0.010) |
.009 | |
| Beverages | Time |
−0.076
(0.007) |
<.0001 | 0.005 (0.007) |
.461 |
−0.029
(0.005) |
<.0001 |
−0.178
(0.007) |
<.0001 |
−0.101
(0.005) |
<.0001 |
| UPF |
−0.002 (0.073) |
.976 |
−0.034 (0.083) |
.679 | 0.087 (0.080) |
.277 | 0.003 (0.071) |
.965 | 0.005 (0.067) |
.931 | |
| Time × UPF |
−0.010 (0.014) |
.469 |
−0.004 (0.015) |
.775 |
−0.008 (0.010) |
.411 | 0.009 (0.015) |
.549 |
−0.005 (0.010) |
.651 |
Notes: BMI = body mass index; SE = standard error; UPF = ultra-processed food. Adjusted for sex, age, education, smoking status, physical activity, BMI, HbA1C, duration with diabetes, mean total cholesterol, and systolic blood pressure.
*%kcal/day adjusted for total calories using the residual method.
Values in bold indicate p value <.05.
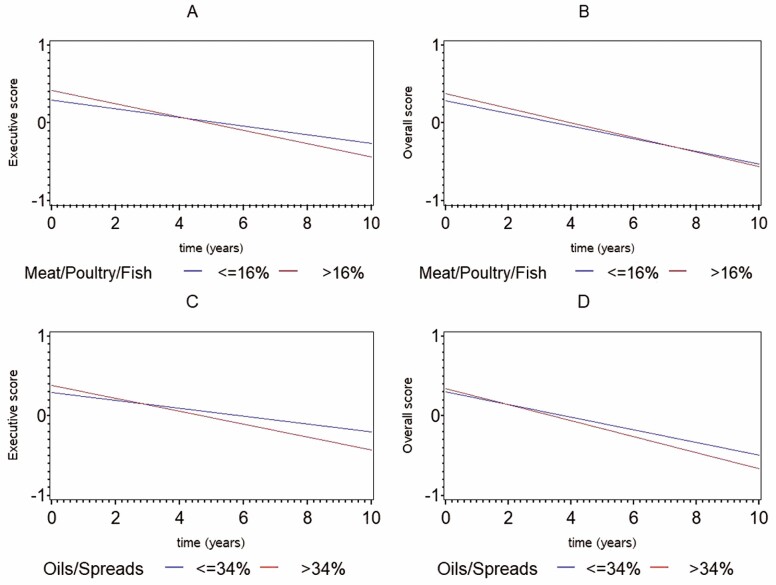
Table 2 presents the associations of time and UPFs with cognition, and the interaction between UPF and time in relation to cognitive function (ie, how total UPF intake and in specific food groups is related to changes in cognitive function over time), after adjusting for demographic, lifestyle, and cardiometabolic factors. In Supplementary Table 4 we present the same associations while adjusting for demographic and lifestyle factors only. The 2 models yielded similar results. After controlling for the larger set of covariates, higher consumption of ultra-processed meat was associated with a greater decline in executive function and global cognition independently of potential confounders (β = −0.041 ± 0.013; p = .002 and β = −0.026 ± 0.010; p = .011, respectively; Table 2 and Figure 1a and b). Moreover, those who consumed a higher percentage of calories from ultra-processed oils/spreads, demonstrated a greater decline in executive function and global cognition (β = −0.037 ± 0.014; p = .006 and β = −0.028 ± 0.010; p = .009, respectively; Table 2 and Figure 1c and d). Total UPF as well as the percentage of calories consumed from dairy products, bread/pastries/starch, and beverages were not related to cognitive change during the follow-up period (Table 2).
Figure 1.
Change in cognitive function over time by ultra-processed food intake (top vs bottom quartiles). (A) Change in executive function by ultra-processed meat/poultry/fish intake; (B) change in global cognition by ultra-processed meat/poultry/fish intake; (C) change in executive function by ultra-processed oils/spreads intake; and (D) change in global cognition by ultra-processed oils/spreads intake.
Table 3 demonstrates the associations of time, UPF, and the interaction between them (ie, how UPF is related to change in cognitive function over time) by sex and BMI group. Overall, the rate of global cognitive decline was similar in men and women and in those with high and low BMI (Table 3; Supplementary Table 3). Table 3 demonstrates significant interactions between sex and intake of ultra-processed meat and oils/spreads. In stratified models, ultra-processed meat consumption was related to greater decline in attention among women (β = −0.069 ± 0.023; p = .004) but not men (β = −0.000 ± 0.017; p = .995; p-for interaction = .018). Furthermore, ultra-processed meat consumption was associated with a greater decline in global cognition in women only (β = −0.054 ± 0.017; p = .002; p-for interaction = .009). Similarly, greater intake of ultra-processed oils/spreads was related to a greater decline in executive function (β = −0.069 ± 0.025; p = .005; p-for interaction = .006) and global cognition (β = −0.047 ± 0.018; p = .010; p-for interaction = .018) among women but not men. In addition, a statistically significant interaction was found between BMI and ultra-processed oils/spreads consumption, such that the link between ultra-processed oils/spreads and executive functions (β = −0.055 ± 0.022; p = .012; p-for interaction = .012), language (β = −0.054 ± 0.018; p = .003; p-for interaction = .034), and global cognition (β = −0.057 ± 0.019; p = .004; p-for interaction = .008) were apparent only in those with higher BMI. Lastly, we found that high consumption of UPF from dairy products was associated with a greater decline in language among those with BMI ≥ 30 kg/m2 (β = −0.051 ± 0.018; p = .005; p-for interaction = .008; Table 3).
Table 3.
The Associations Between Ultra-Processed Food Consumption (Top Quartile of %kcal vs Bottom Quartiles) and Change in Cognitive Function Over Time, by Sex and BMI Group
| Executive | Attention | Language | Memory | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPF (%kcal)* | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | ||
| Meat (including poultry and fish) | Male (N = 343) |
Time |
−0.068
(0.009) |
<.0001 | 0.008 (0.010) | .427 |
−0.041
(0.007) |
<.0001 |
−0.475
(0.010) |
<.0001 |
−0.104
(0.007) |
<.0001 |
| UPF | 0.280 (0.089) |
.002 | 0.070 (0.103) | .499 | 0.133 (0.102) |
.195 | 0.065 (0.084) |
.438 | 0.159 (0.083) |
.054 | ||
| Time × UPF |
−0.037
(0.016) |
.021 | −0.000 (0.017) | .995 | 0.008 (0.012) |
.475 |
−0.013 (0.017) |
.441 |
−0.009 (0.012) |
.447 | ||
| Female (N = 225) |
Time |
−0.064
(0.012) |
<.0001 | 0.019 (0.011) | .096 |
−0.013
(0.008) |
.121 |
−0.182
(0.012) |
<.0001 |
−0.088
(0.008) |
<.0001 | |
| UPF |
−0.036 (0.125) |
.772 | 0.137 (0.136) | .314 | 0.052 (0.141) |
.710 | 0.073 (0.135) |
.591 | 0.094 (0.118) |
.428 | ||
| Time × UPF |
−0.039 (0.024) |
.102 | −0.069(0.023) | .004 † |
−0.000 (0.017) |
.958 |
−0.031 (0.025) |
.214 |
−0.054
(0.017) |
.002 ‡ | ||
| Dairy products | BMI <30 kg/m2 (N = 402) |
Time |
−0.078
(0.008) |
<.0001 | 0.008 (0.009) | .328 |
−0.036
(0.006) |
<.0001 |
−0.198
(0.008) |
<.0001 |
−0.110
(0.006) |
<.0001 |
| UPF | 0.091 (0.085) |
.284 | 0.107 (0.102) | .295 |
−0.143 (0.099) |
.149 |
−0.076 (0.083) |
.359 |
−0.024 (0.080) |
.761 | ||
| Time × UPF | 0.000 (0.015) |
.953 | −0.009 (0.017) | .575 | 0.014 (0.012) |
.233 | 0.020 (0.017) |
.237 | 0.013 (0.011) |
.272 | ||
| BMI ≥30 kg/m2 (N = 166) |
Time |
−0.066
(0.012) |
<.0001 | −0.002 (0.011) | .855 |
−0.013 (0.009) |
.184 |
−0.187
(0.014) |
<.0001 |
−0.106
(0.011) |
<.0001 | |
| UPF |
−0.016 (0.138) |
.909 | 0.003 (0.143) | .986 | 0.139 (0.141) |
.323 |
−0.113 (0.132) |
.394 | 0.011 (0.117) |
.925 | ||
| Time × UPF | 0.008 (0.022) |
.727 | 0.000 (0.021) | .998 |
−0.051
(0.018) |
.005 § | 0.020 (0.026) |
.449 |
−0.004 (0.020) |
.851 | ||
| Oils and spreads | Male (N = 343) |
Time |
−0.074
(0.008) |
<.0001 | −0.002 (0.009) | .821 |
−0.032
(0.007) |
<.0001 |
−0.185
(0.009) |
<.0001 |
−0.107
(0.007) |
<.0001 |
| UPF | 0.146 (0.091) |
.112 | 0.046 (0.109) | .672 | 0.183 (0.106) |
.084 | 0.105 (0.086) |
.222 | 0.157 (0.086) |
.071 | ||
| Time × UPF |
−0.016 (0.016) |
.311 | 0.029 (0.017) | .107 |
−0.025
(0.013) |
.048 |
−0.023 (0.018) |
.223 |
−0.017 (0.013) |
.198 | ||
| Female (N = 225) |
Time |
−0.065
(0.010) |
<.0001 | 0.011(0.010) | .275 |
−0.022
(0.008) |
.006 |
−0.186
(0.011) |
<.0001 |
−0.096
(0.008) |
<.0001 | |
| UPF | 0.007 (0.122) |
.955 | −0.048 (0.129) | .706 |
−0.337
(0.129) |
.010 |
−0.077 (0.127) |
.540 |
−0.099 (0.112) |
.377 | ||
| Time × UPF |
−0.069
(0.025) |
.005 ‖ | −0.037 (0.024) | .121 | 0.014 (0.017) |
.390 |
−0.027 (0.025) |
.279 |
−0.047
(0.018) |
.010 ¶ | ||
| BMI <30 kg/m2 (N = 402) |
Time |
−0.075
(0.007) |
<.0001 | 0.006(0.008) | .424 |
−0.032
(0.006) |
<.0001 |
−0.189
(0.008) |
<.0001 |
−0.104
(0.006) |
<.0001 | |
| UPF | 0.025 (0.086) |
.772 | −0.042 (0.105) | .688 |
−0.077 (0.102) |
.451 | 0.066 (0.087) |
.449 | 0.017 (0.083) |
.836 | ||
| Time × UPF |
−0.028 (0.016) |
.089 | 0.007 (0.019) | .722 | 0.008 (0.013) |
.531 |
−0.017 (0.018) |
.371 |
−0.018 (0.012) |
.171 | ||
| BMI ≥30 kg/m2 (N = 166) |
Time |
−0.049
(0.012) |
<.0001 | 0.000 (0.011) | .974 |
−0.013 (0.009) |
.176 |
−0.173
(0.013) |
<.0001 |
−0.090
(0.010) |
<.0001 | |
| UPF | 0.189 (0.138) |
.171 | 0.067 (0.139) | .631 | 0.111 (0.138) |
.425 |
−0.018 (0.127) |
.886 | 0.108 (0.116) |
.350 | ||
| Time × UPF |
−0.055
(0.022) |
.012 # | −0.020 (0.021) | .350 |
−0.054
(0.018) |
.003 ** |
−0.039 (0.025) |
.106 |
−0.057
(0.019) |
.004 § |
Notes: BMI = body mass index; SE = standard error; UPF = ultra-processed food. Models adjust for sex, age, education, smoking status, physical activity, BMI, HbA1C, duration with diabetes, mean total cholesterol, and systolic blood pressure. Sex and BMI were not added as covariates in models stratified by sex and BMI, respectively.
*%kcal/day adjusted for total calories using the residual method.
p-for interaction are:
†.018;
‡.009;
§ 0.008;
‖.006;
¶.018;
#.012,
**.034.
Values in bold indicate p value <.05.
Discussion
This cohort study among individuals with diabetes suggests that greater intake of ultra-processed meat is associated with a greater decline in executive function and global cognition, and that high intake of ultra-processed oils/spreads is related to a greater decline in executive function and global cognition. Further exploration revealed that ultra-processed meat, oils/spreads, and dairy products may be more strongly associated with cognitive decline particularly among females and obese individuals. Consumption of total calories from UPF overall was not associated with cognitive decline.
The average consumption of total UPF intake (% kcal of total kcal) in our sample of older Israeli adults with T2D was 23.3% ± 11.0 with range of 1.9%–62.9%. This level is somewhat low compared to estimates from samples of older adults (not necessarily with diabetes) in other high-income countries (29,30). The observed differences in UPF intake may be explained by variations in the food items included in the FFQ in various countries. However, UPF consumption in our sample was also lower compared to estimates among older adults in Israel (28%) (31), which may be expected considering the fact that the sample is comprised of individuals with diabetes who may be more aware of their nutrition.
Contrary to the growing body of evidence linking UPF consumption to various health outcomes, including cardiometabolic conditions related to cognitive decline and dementia (32), our study did not point to an association between total UPF consumption and cognitive decline. The associations between specific UPF groups (ie, meat and oils/spreads) with cognitive decline in our sample are in line with other studies showing that the Western-style dietary pattern, which is characterized by high UPF consumption, is associated with early markers of Alzheimer’s disease (33). On the other hand, high-quality diets (34) such as the Mediterranean diet, the DASH diet, and the MIND diet are inversely correlated with UPF intake and have been shown to promote brain health in multiple studies (5). Specifically, our findings imply that high consumption of ultra-processed meat and oils/spreads may be associated with cognitive aging. In line with these results, a secondary analysis from a large cohort study has demonstrated that consumption of stick margarine, an ultra-processed fat, is a strong predictor of AD mortality (35). Furthermore, in contrast to previous literature showing associations between sugary beverages consumption and AD risk (36), we did not find a relationship between ultra-processed beverages and cognitive decline. However, this inconsistency may occur due to the fact that in our study, ultra-processed beverages included items such as diet drinks and processed fruit juice in addition to sugar sweetened beverages.
Although additional evidence relating UPF intake to cognitive outcomes is lacking, several studies demonstrated a link between ultra-processed meat and fat with cardiometabolic conditions. For example, a meta-analysis of cohort studies showed evidence, that a reduction in processed meat intake of 3 servings per week is associated with a decrease, albeit small, in risk for all-cause mortality, cardiovascular mortality, stroke, myocardial infarction, and T2D (37). In addition, evidence suggests that processed meat may be linked with NAFLD and insulin resistance (38), and that consumption of margarine may be associated with higher total and cardiometabolic mortality (35). Thus, it is possible that the observed associations are mediated through these health conditions, which in turn, are closely related to cognitive health (39). In addition, because UPF meat and oils have high energy and low nutritional density, they can lead to overweight, which in turn, may confer high risk for cognitive impairment and dementia in the general population (40) and in persons with diabetes (41). In addition, there were no clinically significant differences in BMI and glycemic control indices between participants with high vs. low consumption of UPF in our sample, and these measures were controlled for in our models. Another plausible explanation for our findings is the high levels of dietary advanced glycation end products (AGEs) contained in ultra-processed meat and fat (42). According to a growing body of evidence, AGEs are implicated in AD pathogenesis (43), and dietary AGEs are associated with faster cognitive decline (44). Particularly among individuals with diabetes, high circulating levels of AGEs, which are strongly correlated with dietary AGEs (45), were associated with cognitive decline (46). In addition, observational studies suggest that excessive consumption of trans fatty acids, particularly industrial ones, are risk factors for all-cause dementia and AD (47). It is thought that excessive intake of trans fatty acids promotes atherosclerosis, chronic inflammation, and oxidative stress and impair insulin resistance and glucose control (48), processes that underlie cognitive aging. Lastly, the observed associations of ultra-processed oils/spreads and meat with cognitive decline may be mediated through changes in gut microbiota (15).
According to our findings, the cognitive domain particularly associated with ultra-processed meat and oils/spreads is executive functions. This may indicate a possible involvement of the frontal lobe, which is more prone to vascular injury (49), hence suggesting that vascular pathology may be part of the underlying mechanism (49,50). We also observed that the associations of ultra-processed meat and oils/spreads with cognitive decline is stronger among women and individuals with obesity, an observation that may require further exploration in future studies.
Our study has several strengths, including a well-characterized diagnosis of T2D, a plethora of directly measured, rather than self-reported potential confounders including characteristics of glucose control, and a comprehensive neuropsychological battery that permitted exploration of specific cognitive domains in addition to global cognition. The study also has several limitations: first, we found that high consumption of UPF in our sample was linked with a somewhat less healthy diet, including higher total calories and lower intake of protein and fruits/vegetables. Although total calories were controlled for in our analyses, deciphering whether the observed associations are due to possible harmful effects of ultra-processing or to the low consumption of high-quality food is difficult. Second, it should be acknowledged that while the NOVA is a valid food classification system that is commonly being used in nutrition, public health and policy research (24), there are other food processing-based classification systems (51). Therefore, the choice of classification system could affect the conclusions reached regarding UPF consumption and cognitive decline as have been shown for other health outcomes (51). Third, the study findings may be subjected to selection bias since the characteristics of the study sample differed from those of participants who were not included in the analyses. In addition, UPF consumption in the current sample was lower compared to that of other high-income countries, possibly due to nutritional education provided for individuals with diabetes. Thus, although diabetes-related characteristics were adjusted for, results cannot be extrapolated to nondiabetic older adults. The low UPF consumption in our sample compared to that of other high-income countries may also reflect variation in types of UPF food between countries (eg, canned soups are common in the United States but are rarely being consumed by Israelis), and may highlight the lack of a global consensus about UPF food items. Lastly, although we adjusted for many relevant covariates, residual confounding such as by ApoE4 genotype could also affect our results.
In conclusion, our results contribute new data to the growing body of evidence linking UPF to poor health outcomes and show that UPF, specifically of meat and oils/spreads, are associated with a faster rate of decline in global cognition, and particularly in executive function. Food processing-based classifications, particularly the NOVA system, have been established as useful markers of diet quality and have already informed public policy specifications for the promotion of healthy diets, including healthy eating guidelines and the identification of foods requiring regulatory control (52). If replicated by future investigations, these findings may lead to food policy interventions aimed at shifting consumption patterns toward less processed products in order to improve nutritional quality and therefore promote brain health.
Supplementary Material
Contributor Information
Galit Weinstein, School of Public Health, University of Haifa, Haifa, Israel.
Shiraz Vered, School of Public Health, University of Haifa, Haifa, Israel.
Dana Ivancovsky-Wajcman, Liver Unit, Department of Gastroenterology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Ramit Ravona-Springer, The Joseph Sagol Neuroscience Center, Sheba Medical Center, Ramat-Gan, Israel; Sackler School of Medicine, Tel-Aviv University, Israel.
Anthony Heymann, Sackler School of Medicine, Tel-Aviv University, Israel; Maccabi Healthcare Services, Tel-Aviv, Israel.
Shira Zelber-Sagi, School of Public Health, University of Haifa, Haifa, Israel; Liver Unit, Department of Gastroenterology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel.
Danit Rivka Shahar, Department of Public Health, Ben-Gurion University of the Negev, Beer-Sheva, Israel.
Michal Schnaider Beeri, The Joseph Sagol Neuroscience Center, Sheba Medical Center, Ramat-Gan, Israel; Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (R01 AG034087, RO1 AG051545, and R21 AG043878 to M.S.B.).
Conflict of Interest
None declared.
Author Contributions
Study concept and design: G.W., S.V., S.Z.-S., and M.S.B. Data acquisition: D.I.-W., D.R.S., A.H., and M.S.B. Data analysis: S.V. Interpretation of data: G.W., S.Z.-S., and M.S.B. Drafting of the work: G.W. All authors critically revised the manuscript for important intellectual content. All authors approved the final version and agreed to be accountable for all aspects of the work.
References
- 1. Mistridis P, Krumm S, Monsch AU, Berres M, Taylor KI. The 12 years preceding mild cognitive impairment due to Alzheimer’s Disease: the temporal emergence of cognitive decline. J Alzheimers Dis. 2015;48(4):1095–1107. doi: 10.3233/JAD-150137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. doi: 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krivanek TJ, Gale SA, McFeeley BM, Nicastri CM, Daffner KR. Promoting successful cognitive aging: a ten-year update. J Alzheimers Dis. 2021;81:871–920. doi: 10.3233/jad-201462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flanagan E, Lamport D, Brennan L, et al. Nutrition and the ageing brain: moving towards clinical applications. Ageing Res Rev. 2020;62:101079. doi: 10.1016/j.arr.2020.101079 [DOI] [PubMed] [Google Scholar]
- 7. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–1416. doi: 10.1212/WNL.0000000000000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean Diet with physical performance and cognitive function in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2021;70:101395. doi: 10.1016/j.arr.2021.101395 [DOI] [PubMed] [Google Scholar]
- 10. Baker P, Machado P, Santos T, et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21(12):e13126. doi: 10.1111/obr.13126 [DOI] [PubMed] [Google Scholar]
- 11. Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):e020574. doi: 10.1136/bmjopen-2017-020574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams J, White M. Characterisation of UK diets according to degree of food processing and associations with socio-demographics and obesity: cross-sectional analysis of UK National Diet and Nutrition Survey (2008–12). Int J Beha Nutr Phys Act. 2015;12(1):160. doi: 10.1186/s12966-015-0317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy RB, Rauber F, Chang K, et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin Nutr. 2021;40(5):3608–3614. doi: 10.1016/j.clnu.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 14. Elizabeth L, Machado P, Zinöcker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. 2020;12(7):1955. doi: 10.3390/nu12071955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez Leo EE, Segura Campos MR. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition. 2020;71:110609. doi: 10.1016/j.nut.2019.110609 [DOI] [PubMed] [Google Scholar]
- 16. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarmento RA, Antonio JP, de Miranda IL, Nicoletto BB, de Almeida JC. Eating patterns and health outcomes in patients with type 2 diabetes. J Endocr Soc. 2018;2(1):42–52. doi: 10.1210/js.2017-00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beeri MS, Ravona-Springer R, Moshier E, et al. The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement. 2014;10(6):769–778. doi: 10.1016/j.jalz.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heymann AD, Chodick G, Halkin H, et al. The implementation of managed care for diabetes using medical informatics in a large Preferred Provider Organization. Diabetes Res Clin Pract. 2006;71(3):290–298. doi: 10.1016/j.diabres.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 20. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez L, Folch A, Rojas M, et al. Effects of nutrition on cognitive function in adults with or without cognitive impairment: a systematic review of randomized controlled clinical trials. Nutrients. 2021;13(11):3728. doi: 10.3390/nu13113728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahar D, Fraser D, Shai I, Vardi H. Development of a food frequency questionnaire (FFQ) for an elderly population based on a population survey. J Nutr. 2003;133(11):3625–3629. doi: 10.1093/jn/133.11.3625 [DOI] [PubMed] [Google Scholar]
- 23. Shai I, Rosner BA, Shahar DR, et al. Dietary evaluation and attenuation of relative risk: multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall questionnaires: the DEARR study. J Nutr. 2005;135(3):573–579. doi: 10.1093/jn/135.3.573 [DOI] [PubMed] [Google Scholar]
- 24. Monteiro CA, Cannon G, Levy R, et al. NOVA. The star shines bright. World Nutrition. 2016;7(1–3):28–38. [Google Scholar]
- 25. Fliss-Isakov N, Zelber-Sagi S, Ivancovsky-Wajcman D, Shibolet O, Kariv R. Ultra-processed food intake and smoking interact in relation with colorectal adenomas. Nutrients. 2020;12(11):3507. doi: 10.3390/nu12113507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 27. West RK, Ravona-Springer R, Schmeidler J, et al. The association of duration of type 2 diabetes with cognitive performance is modulated by long-term glycemic control. Am J Geriatr Psychiatry. 2014;22(10):1055–1059. doi: 10.1016/j.jagp.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 29. Marino M, Puppo F, Del Bo C, et al. A systematic review of worldwide consumption of ultra-processed foods: findings and criticisms. Nutrients. 2021;13(8). doi: 10.3390/nu13082778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandoval-Insausti H, Blanco-Rojo R, Graciani A, et al. Ultra-processed food consumption and incident frailty: a prospective cohort study of older adults. J Gerontol A Biol Sci Med Sci. 2020;75(6):1126–1133. doi: 10.1093/gerona/glz140 [DOI] [PubMed] [Google Scholar]
- 31. Ivancovsky-Wajcman D, Fliss-Isakov N, Webb M, et al. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021;41(11):2635–2645. doi: 10.1111/liv.14996 [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Zhang Z, Yang H, et al. Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies. Nutr J. 2020;19(1):86. doi: 10.1186/s12937-020-00604-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samuelsson J, Kern S, Zetterberg H, et al. A Western-style dietary pattern is associated with cerebrospinal fluid biomarker levels for preclinical Alzheimer’s disease―A population-based cross-sectional study among 70-year-olds. Alzheimers Dement (N Y). 2021;7(1):e12183. doi: 10.1002/trc2.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr. 2017;15(1):6. doi: 10.1186/s12963-017-0119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Zhuang P, Wu F, et al. Cooking oil/fat consumption and deaths from cardiometabolic diseases and other causes: prospective analysis of 521,120 individuals. BMC Med. 2021;19(1):92. doi: 10.1186/s12916-021-01961-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu Y, Manly JJ, Schupf N, Mayeux R. Sugary beverage consumption and risk of Alzheimer’s disease in a community-based multiethnic population. Alzheimer Dement. 2018;14(suppl 7):P645. doi: 10.1016/j.jalz.2018.06.2696 [DOI] [Google Scholar]
- 37. Zeraatkar D, Han MA, Guyatt GH, et al. Red and processed meat consumption and risk for all-cause mortality and cardiometabolic outcomes: a systematic review and meta-analysis of cohort studies. Ann Intern Med. 2019;171(10):703–710. doi: 10.7326/M19-0655 [DOI] [PubMed] [Google Scholar]
- 38. Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68(6):1239–1246. doi: 10.1016/j.jhep.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 39. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 40. Qu Y, Hu HY, Ou YN, et al. Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev. 2020;115:189–198. doi: 10.1016/j.neubiorev.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 41. West RK, Ravona-Springer R, Heymann A, et al. Waist circumference is correlated with poorer cognition in elderly type 2 diabetes women. Alzheimers Dement. 2016;12(8):925–929. doi: 10.1016/j.jalz.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–16.e12. doi: 10.1016/j.jada.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cai Z, Liu N, Wang C, et al. Role of RAGE in Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(4):483–495. doi: 10.1007/s10571-015-0233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. West RK, Moshier E, Lubitz I, et al. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev. 2014;140:10–12. doi: 10.1016/j.mad.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beeri MS, Uribarri J, Cai W, Buchman AS, Haroutunian V. Human brain and serum advanced glycation end products are highly correlated: preliminary results of their role in Alzheimer disease and type 2 diabetes. Endocr Pract. 2020;26(5):576–577. doi: 10.4158/1934-2403-26.5.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yaffe K, Lindquist K, Schwartz AV, et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77(14):1351–1356. doi: 10.1212/WNL.0b013e3182315a56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barnard ND, Bunner AE, Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging. 2014;35(suppl 2):S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 48. Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One. 2011;6(12):e29600. doi: 10.1371/journal.pone.0029600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4 [DOI] [PubMed] [Google Scholar]
- 50. Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246 [DOI] [PubMed] [Google Scholar]
- 51. Martinez-Perez C, San-Cristobal R, Guallar-Castillon P, et al. Use of different food classification systems to assess the association between ultra-processed food consumption and cardiometabolic health in an elderly population with metabolic syndrome (PREDIMED-Plus Cohort). Nutrients. 2021;13(7):2471. doi: 10.3390/nu13072471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kelly B, Jacoby E. Public Health Nutrition special issue on ultra-processed foods. Public Health Nutr. 2018;21(1):1–4. doi: 10.1017/S1368980017002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.